Abstract
The predicted relationship between home-range size and group mass in primates developed by Clutton-Brock and Harvey (1977) has proved extremely robust in describing the use of space by most primate species. However, mandrills (Mandrillus sphinx) are now known to have an extreme group mass in the wild, far larger than that of the species used originally to generate that relationship, and so it was unknown whether this relationship would be robust for this species. We investigated the home-range size and use of a wild horde of ca. 700 mandrills in Lopé National Park, Gabon, using radiotelemetry. The total area the horde used over a 6-yr period [100% minimum convex polygon (MCP)] was 182 km2, including 89 km2 of suitable forest habitat. Mandrills used gallery forests and isolated forest fragments with high botanical diversity far more intensively that the continuous forest and completely avoided savanna and marsh. Peeled polygons and fixed kernel contours revealed multiple centres of use, with the horde spending more than half its time in <10% of the total documented range, typical of a frugivore using a patchy environment. Home-range size and internal structure varied considerably between years, but total home range fitted the predicted relationship between group mass and home range size, despite being an outlier to the dataset. We discuss the conservation implications of the species’ space requirements, in light of current pressures on land use in their range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of food resources in time and space is recognized to be of great importance in determining home-range size and structure in primates (Clutton-Brock and Harvey 1977; Di Bitetti 2001; Hladik 1975; Olupot et al. 1997; Terborgh 1983). Positive correlations between home-range area and group mass in primates (Clutton-Brock and Harvey 1977), based on empirical data for a selection of species, have been shown to be robust in estimating range use by other primates, with a tendency for frugivores/omnivores to have larger ranges than folivores, and terrestrial species to have larger ranges than arboreal species (Clutton-Brock and Harvey 1977; Milton and May 1976). Other factors that may also influence home-range use include the location of specific resources, i.e., sleeping sites and watering holes (Altmann 1974; Barton et al. 1992; Chapman 1988; Hamilton 1982) and avoidance of areas with a high risk of predation (Cowlishaw 1997) or fecal contamination (Freeland 1980). Studies of ranging behavior facilitate understanding of a species’ foraging strategies, key resources, niche adaptations, and social organization (Boinski 1987; Clutton-Brock 1977; Defler 1996; Kaplin 2001) and provide vital information for conservation management (Singleton and van Schaik 2001).
The ranging behavior of mandrills (Mandrillus sphinx, Linnaeus 1758), a forest primate found in the rain forests of western central Africa (Fig. 1, inset), is of particular interest. Mandrills are now known to live in the largest permanent social groups, termed hordes, recorded for any nonhuman primate (Abernethy et al. 2002) and are also among the largest of the cercopithecine primates; thus group mass for mandrills is extremely high, probably the highest of all the primates.
Data from the species in the wild were not available when the theoretical relationship between mass and range was established and so it is unknown whether the relationship will be robust for the extreme group mass of this species. If empirical data from mandrills do not fit the relationship for group mass and range size, it might be concluded that in fact the shape of the relationship is fundamentally different but that this was not exposed by the smaller spread of group sizes initially used, or that other factors come into play at these extreme horde sizes, such as high levels of parasite transmission, sleep site saturation, or patch size limits to foraging.The social grouping, habitat requirements and ranging behavior of mandrills have previously been scantily documented, due to great difficulties in locating, observing, and following groups in dense forest, though some basic knowledge has accumulated through a handful of studies in the last 30 yr (Harrison 1988; Hoshino et al. 1984; Jouventin 1975; Lahm 1986; Rogers et al. 1996; Sabater Pi 1972). Mandrills travel and forage at ground level, but also feed and sleep high in the canopy (Harrison 1988), consuming a broad, omnivorous diet of fruits, seeds, invertebrates, and a variety of vegetative plant parts (Harrison 1988; Hoshino 1985; Lahm 1986; Rogers et al. 1996). Ripe fruit forms a major component of the diet (Harrison 1988; Hoshino 1985; Lahm 1986; Rogers et al. 1996), so the distribution of fruit is likely to have a strong influence on ranging behavior. Previous estimates of mandrill home-range size, ranging from 30 to 50 km2, were made by extrapolation from short follows of small groups of mandrills (Harrison 1988; Hoshino et al. 1984; Jouventin 1975).
Mandrills are classified as Vulnerable on the IUCN Red List of threatened species due to the major threats of habitat loss/degradation and hunting for their meat (IUCN 2009; Oates and Butynski 2008a), yet the habitat requirements and key resources needed by mandrills remain largely guesswork and their conservation cannot be well planned, nor likely to succeed, until their ecology is better known. A greater understanding of mandrill ecology may also facilitate conservation of drills (Mandrillus leucophaeus, F. Cuvier 1807), the only other members of the genus. Drills are classified as Endangered (IUCN 2009; Oates and Butynski 2008b) and cited as one of the highest priority primate species for conservation in Africa (Oates 1996).
Radio-collaring of a horde of mandrills in the north of Lopé National Park, Gabon, where human densities have been very low for at least the past century (Oslisly 2001) and where hunting has been prevented since 1982, has enabled us to collect systematically new data on the movements of an undisturbed mandrill horde over a number of years and thus to document empirically the annual or multiyear home-range size or structure for a known number of individuals and group mass.
We set out to describe the home-range use by this horde between July 1998 and October 2004. Our specific objectives were to determine the size, shape, and internal structure of the horde’s home range and to relate these characters to the horde size and mass and habitat heterogeneity of the range. From existing theory we expect mandrill hordes to occupy a large total home range (Clutton-Brock and Harvey 1977), but to use space in a highly uneven manner, with strong seasonal/annual differences in use patterns, as fruit abundance in tropical forests generally shows considerable spatial and temporal variation (Chapman et al. 1999, 2005; Terborgh 1983; Tutin and White 1998; van Schaik et al. 1993).
Methods
Study Area
We conducted research at the Station d’Études des Gorilles et Chimpanzés, SEGC (0°12′S, 11°36′E) in Lopé National Park, Gabon. Annual rainfall was 1474 mm (SE ± 44.8, SEGC: 1984–2004) with a long dry season between mid-June and mid-September. Temperatures varied little throughout the year (total range 19.9–34.5°C: SEGC: 1984–2004), with the coolest temperatures in July and August.
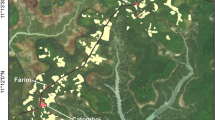
Most of Lopé NP is rain forest, with mature closed-canopy forest in the south and west, grading into younger Marantaceae forests further north (Fig. 1). A heterogeneous landscape of ancient savannas and forest fragments exists along the park’s northern and eastern boundaries (White 2001; White and Abernethy 1997), including the northern half of our study area (Fig. 2). Gallery forests extend along watercourses and moist valley bottoms in the savannas, and small, isolated forest fragments (bosquets) are found on most savannas hilltops. Botanical studies of this forest-savanna mosaic reveal distinct forest types differing in age, structure, and floristic composition from the continuous forest types and from each other (Léal 2004; Tutin et al. 1997; Ukizintambara et al. 2007; White 1995, 2007).
The Focal Horde
The focus of our study was a horde of ca. 700 (650–750) mandrills in the forest-savanna mosaic, in the northeast of Lopé NP (Fig. 2). We radio-collared a total of 14 females and young males (permanent members of the horde) between 1998 and 2003. Thirteen adult males were also collared for other study aims; the number of collared individuals present in any month varied from 1 to 7 (mode = 2). Whenever possible, we took film recordings of the entire horde as it crossed open areas, which enabled us to collect reliable data on group size. These films (N = 57) show that the horde had between 650 and 750 members during our study period, the precise number varying due to births, deaths, and the semipermanent membership of mature and submature males. Mandrills differ from most other group-living primates in that mature, breeding-age males are not permanent members of the horde (Abernethy et al. 2002) and thus only females and juveniles (<5 yr) were representative of the horde movements.
We estimated the mass of the focal horde based on the recorded mean group size of 700 individuals, and using the average group age-sex composition published in Abernethy et al. (2002). We recorded adult body masses of 30.4 (25–36) kg for males (N = 11), and 8.9 (7–11) kg (N = 10) for adult females (Abernethy and White 2010) during the captures for radio collaring. Adult body masses used in group mass calculations were those empirically measured during this study, and we took juvenile body masses from measurements of captive mandrills (Wickings and Dixson 1992: Table I).
Capture, Immobilization, and Collaring Procedures
We set up hides in narrow portions of gallery forest, along trails frequently used by mandrills. As the mandrill horde approached, we visually evaluated individuals for potential immobilization, excluding >5-yr-old males, <4-yr-old juveniles, and females with infants. We darted the individuals with a CO2-powered, variable pressure rifle, using 3-ml plastic darts (Dan-Inject US or Telinject USA). Both systems use air pressure in the syringe and a silicone sleeve on the needle that acts both as a rubber stop and prevents injection of the drug until the needle penetrates the skin. We used Telazol® for the initial darting. Postdarting travel was <50 m, and in most cases we located the subjects ≤15 min of darting.
Immediately upon capture, we ensured normal respiration and heart rate in the anesthetized individual. We then gave each individual a complete physical examination that included notation of any observed or palpable abnormalities; examination of oral cavity, eyes, and ears; auscultation of heart and lungs; and recording of body temperature and heart and respiratory rates at intervals throughout the anesthetic procedure. We located the dart wound and treated it with a topical antibiotic ointment.
If the health assessment showed that the subject was fit and uninjured, we fitted radio-transmitter collars immediately and carried out body measurements if anesthesia allowed. Our radio transmitters for females weighed ca. 0.9–1.2% of the body mass of adult female mandrills; well within 2% suggested limits (Amlaner and MacDonald 1980). Details of our mandrill collar designs are available from the manufacturers (www.telonics.com or www.ATS.com).
We placed the subject in a cloth bag for recovery from anesthesia. When the subject became active in the bag, we left the bag beside an easily climbed tree, within earshot of the horde, and at dusk, so that the horde was certain not to travel for a further 12 h. We watched the newly collared individual from >20 m (limit of forest visibility), until it emerged from the bag and climbed into the tree. We checked all subjects early the next day to ensure that no problems had arisen and they were travelling with the horde. More details on anesthesia and other medical care concerns are described by Karesh et al. (1998).
Radiotelemetry Data
We located the horde by radio-tracking collared individuals with a Telonics hand-held metal H antenna and portable receiver, using triangulation (Kenward 2001). We based point locations on ≥3 bearings and confirmed the horde’s location and group spread via visual/auditory contact whenever possible.
Following radio-collared individuals from this focal horde has revealed that the horde sometimes splits into 2–4 subgroups, which can range independently for some time (White 2007). We followed each subgroup when the horde was divided, in order to document the ranging of all horde members. However, we could reliably follow continuously only subgroups containing a radio-collared individual. We did not include encounters with unknown groups in the analysis, as it was not possible to determine whether these were subgroups of the focal horde or members of a neighboring horde; however, we encountered no unknown groups outside the 100% MCP range of the focal group as calculated from the telemetry data. We encountered initially unknown groups within the focal horde’s home range in 12.8% of weeks during the study, but on all but 2 occasions (in the same week), these mandrills later rejoined a group containing a radio-collared individual and were thus deemed to be of the original horde. We never encountered additional groups on days when all of the known horde members were already accounted for in the radio-collared subgroups.
On 1 predetermined day per week, where logistically possible, we measured day journeys. The horde was tracked from sleep site to sleep site, which equates to a daylight travel time of 10.5 h (0700–1730 h) year round, as the site is so close to the equator.
Accuracy
We took multiple bearings on the radio signal, until they converged on a point, or a polygon of <0.25 km2 (gallery forests) or <1 km2 (continuous forest block), the center of which we took as the group’s location. Group spread of mandrill hordes can be several hundred meters, so we considered this level of error associated with polygon locations on 1 collared individual, negligible in determining group positions. Whenever possible, we confirmed the group’s location by observation or audition of the mandrills, which enabled us to locate the group center to <50 m accuracy on a UTM projected field map with 100-m gridlines, or by GPS position of the observer. Such cross-checks also confirmed the general accuracy of our triangulation skills.
Bias and Autocorrelation
Ranging locations will be autocorrelated (locations at time t are dependent on an individual’s location at time t–1), but a basic assumption of most home-range estimators is that locations used in the data set are independent (De Solla et al. 1999; Harris et al. 1990; Kernohan et al. 2001; Otis and White 1999; White and Garrott 1990). As we documented the horde cross their entire range in 2 d on several occasions, and recorded a day journey of >5 km (approximate home range radius) on 30% of days (N = 168), we considered points >48 h apart as independent. To investigate overall home-range use, we removed point locations from the original data set to obtain a maximum frequency of 1 location for the horde/each subgroup per week (data set 1). This corresponded to the minimum frequency with which we located mandrills throughout the entire study period, allowing an analysis of equivalent data across the entire 6 yr. To investigate interannual variation in home-range use, we removed point locations from the original data set to obtain a maximum frequency of 1 location for the horde/each subgroup every 2 d (data set 2). This gave the larger sample size needed to investigate interannual home-range use, while still minimizing autocorrelation. However, periods when we located mandrills less frequently are inevitably under-represented in this data set, causing some bias. For data set 1, we selected point locations from the original data set by taking the point location obtained each Tuesday (the originally predetermined data collection day), or as soon as possible thereafter. For data set 2, we took the first point location obtained each week and then point locations every second day thereafter.
Home-Range Estimators
The concept that animals generally confine their activities to a definable area of the landscape is well established, but there is no single accepted definition of a home range. Animals occasionally make forays far from the area in which they are usually concentrated, and different home-range estimators vary in the importance they attach to these outlying points (Harris et al. 1990; Kernohan et al. 2001; White and Garrott 1990). If such forays are included within the proposed home range, then large areas not actually used by the animal may also be included within the convex polygon, overestimating total home-range size. Therefore, it may be desirable to exclude outlying points from what is considered to be the animal’s home range (Kernohan et al. 2001; White and Garrott 1990).
We used 3 home-range estimators: the MCP (Mohr 1947); the peeled polygon (Kenward 1985), and fixed kernel contours (Silverman 1986; Worton 1989). We chose the MCP because it is has been most widely used and therefore facilitates comparison between studies (Harris et al. 1990; Seaman et al. 1999). However, including all locations makes the method highly sensitive to outliers, often causing home-range size to be overestimated. The MCP gives no information about the intensity of range use (Harris et al. 1990; Kenward 2001; White and Garrott 1990). We added the peeled polygon analysis to investigate internal use of the range. Peeled polygons remove a proportion of the outliers, then use the remaining data to form a new polygon, giving a more accurate estimate of frequently used home range, which varies less with sample size. By gradually peeling away outliers, the internal structure of the home range is revealed. We used the harmonic mean outlier removal method to create 95%, 70%, and 50% frequentation peeled polygons. Finally, we used fixed-kernel contours (FKC), which, unlike the polygon methods, is able to define multiple centers of activity, yet it still benefits from being nonparametric and less sensitive to autocorrelated data and outlying data points, compared with other available home range estimators (Kernohan et al. 2001). FKC use the probability of finding an animal at any particular location on a plane (Hayne 1949; Jenrich and Turner 1969; Van Winkle 1975), by placing a kernel (a probability density) around each point location. Contours connecting areas of equal density are then constructed to describe the internal structure of the home range (Kernohan et al. 2001; Seaman and Powell 1996). We used the 95% probability contour as the outer home-range boundary (from which we calculated FKC home-range area), and the 70% and 50% probability contours to describe internal home-range structure. We computed all home-range estimates via the Animal Movement Analyst Extension (AMAE) to ESRI’s ArcView GIS, version 3.2.
We calculated annual FKC home ranges for each of the 6 yr, based on data set 2.
Statistical Analysis
We bootstrapped cumulative subsets of 50 points from the original data set, with 100 replicates between each interval, to determine the adequacy of our sample size. Estimated home-range area ceased to increase significantly after 300 point locations, confirming that our smaller data set, data set 1 (N = 438) was sufficiently robust to estimate overall home-range area over the study period.
We used the MRPP test (multi-response permutation procedure; Mielke and Berry 1982, 2001) to compare the interannual distribution of point locations, in addition to visual comparison of the utilisation distributions. The MRPP test is a nonparametric test, analogous to a 1-way ANOVA, which makes no assumptions about the shape of the underlying distribution. It compares the intragroup average pairwise distance between locations with the average distance that would have resulted from all other possible combinations of the data. We conducted the test via BLOSSOM statistical software (Cade and Richards 2001).
Results
The frequency with which we located the mandrills varied over the 6-yr period (Table II) due to logistical constraints. Throughout the entire 6 yr, we located the horde at least once a week, providing standardized data on the horde’s movements. Figure 2 shows the standardised locations we obtained during the study.
Overall Home-Range Size and Structure
We found that our estimates of the total area used by the focal horde varied considerably depending on method and dataset used (Table III, Figs. 3 and 4). Using all available points, the overall MCP home-range area was 182 km2; however, this decreased by 10% to 164 km2, when we used only the weekly standardized locations in data set 1, indicating the sensitivity of the MCP method to sample size and outlier points (Fig. 3). We found no significant relationship between the number of collars present in any month and the number of locations we obtained (N = 76 months, R 2 = 0.23, p = 0.128, ns).
Both the MCP and FKC home-ranges include large areas of savanna, a habitat not used by the mandrills, and thus overestimate the area of suitable habitat supporting this horde. We refined the home range estimates by manually removing the area of savanna from the polygons, and this approximately halved both the MCP and FKC estimates of the total home-range area (Table III).
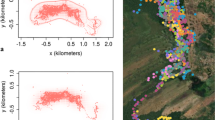
The FKC method (Fig. 4) was more sensitive than the peeled polygons (Fig. 3) in its ability to reveal the internal structure of the mandrills’ home range. Both showed that mandrills used the northern forest fragments far more intensively than the continuous forest; however, we could identify 4 centers of activity (the areas enclosed by the 50% probability contours) via the FKC method, 2 of which lay outside the single peeled polygon core area. The internal structure calculated via the FKC method emphasizes how unevenly the mandrills used their home range; the area covered by the 50% probability contour indicates that the mandrills spent half of their time in an area covering <10% of their home range.
Group Mass and Range Size
We show the predicted relationship between home-range size and population group mass for primates, published by Clutton-Brock and Harvey (1977) in Fig. 5. We have added data from this study (Mandrillus points) using the home-range area estimated via the FKC method, as this gives the most comparable estimate of the area regularly used by the focal horde to the rest of the dataset in the figure. We show both the total area enclosed by the 95% probability contour (118 km2, point 1) and the forested area only (46 km2, point 2), as the large area of savanna may inflate the estimate of home-range area truly required by this horde and comparable to the ranges measured for other species. It is clear that both estimates of home-range area for the focal horde fit the predicted relationship between home-range size and group mass for frugivorous primates.
Home-range area plotted against population group weight for different primate genera. (Redrawn from Clutton-Brock and Harvey 1977, p. 13, with permission from Blackwell Scientific). Two data points for Mandrillus are added. Point 1 shows the total 95% FKC area and point 2 shows the forested area within the 95% FKC range, both plotted against the measured group mass of 4852 kg. Note: Population group size is the number of individuals that regularly associate together and share a common home range. Population group mass is the total mass of a typical group. Separate regression lines are shown for frugivores (top) and folivores (bottom).
Group Size and Day Journey Length
We could accurately record subgroup size (film record) and day journey length for only 17 days during the study period, all within the 2003 period when the team was sufficiently large to allow a day journey to be followed as well as a hide set up for filming. We recorded only 4 day journeys for known subgroups in the wet season, and 13 in the dry season. There was no significant correlation between day journey length and group size for this sample set (DJL = 4.94 ± 1.9 km, range 1.96–10.2 km; R 2 = 0.0085, p = 0.464, ns), though the small sample size disallows any robust conclusions from this.
Interannual Variation in Home-Range Use
We found highly significant interannual differences in home range size, using each of our data sets for the whole years 1999–2004 (data set 1, N = 409 locations MRPP δ = –18.21, p < 0.0001; data set 2, N = 815, MRPP δ = –35.66, p < 0.0001). Using the FKC analysis, we found that both home-range size and structure varied between years (Table IV); the total area used in some years was more than double that of others. However, the focal horde ranged within the same overall area during each of the 6 yr, indicating that mandrills recognize a fixed home range over many years, as opposed to showing a more nomadic use of the landscape (Fig. 6). The main difference in home-range area between years was in the extent to which mandrills used the continuous forest in the south of their range. The variation in annual range size was not explained by the annual number of locations made (R 2 = 0.232, p = 0.112) and is likely to be biologically significant.
Discussion
Mandrill spatial needs for their group mass are very similar to those of other forest frugivorous primates, their uniqueness being in the extreme social group size in which they associate, which may require them to roam over such large areas to maintain group unity. The range size documented for Mandrillus in this study conforms to the relationship derived by Clutton-Brock and Harvey (1977), which illustrates remarkable consistency in the spatial needs per unit biomass of primate species groups, fine-tuned by their diet type.
The large total area used by the mandrills, their highly uneven use of space. and the annual differences in home-range use are all consistent with a species whose movements are dictated primarily by the distribution of a spatiotemporally patchy food resource, in this case ripe fruit. Similar patterns of home-range use have been recorded in other primates whose ranging behavior is, at least in part, related to the abundance and distribution of fruit resources. Such primates have been found to use their home ranges in a highly uneven manner, spending a disproportionately large amount of time concentrated in relatively small areas, with multiple centers of activity scattered throughout their home ranges, or changes in the intensity with which they use different areas of their home range each season (Chapman 1988; Defler 1996; Kaplin 2001; Mitani 1989; Olupot et al. 1997; Terborgh 1983; Wallace 2006). However, the extremely large group size of mandrills is somewhat atypical of frugivorous species exploiting patchy resources and more typical of species exploiting widely available resources, such as the folivorous colobus species, or the granivorous baboons (Barton et al. 1992; Clutton-Brock 1977; Milton and May 1976). Other frugivorous species with high group masses and range sizes tend to be those with large body masses, but relatively small group sizes (apes, baboons). The mandrills’ use of rich but spatiotemporally variable fruit patches across a large area enables individuals to benefit from group-living, while minimizing competition for food, which has been a limiting factor for group cohesion in other rain forest species with similar group masses (Tutin et al. 1991).
The mandrills’ heavy use of the forest fragments, with centers of activity located within the forest-savanna mosaic in all years (Fig. 6), indicate that these forests probably contain resources of key importance to the horde. Botanical data indicate that the forests throughout the hordes’ home range are highly heterogeneous, and a number of different vegetation types have been identified (Ukizintambara et al. 2007; White 1995, 2007). The isolated forest patches in the savannah mosaic are almost all of anthropic origin, originating from villages inhabited in the last 2500 yr (Oslisly 2001), whereas the fingers of gallery forest and a few of the valley bottom fragments are of natural origin, in areas of high humidity by watercourses or marshes (White and Abernethy 1997). Both sorts of fragment have higher tree diversity than the continuous forest, and the anthropic bosquet fragments are relatively rich in fruit trees useful to primates (Ukizintambara et al. 2007; White 2007). For example, oil palms (Elaeis guineensis) are aseasonal fruiting trees, with fruit recorded in all months at Lopé (Tutin and White 1998; White 2007), and may provide staple food for mandrills (Rogers et al. 1996; White 2007). Oil palm regeneration is completely prevented by elephant grazing, and thus, at Lopé, mature oil palms exist only where villages were sited in the last 2 centuries (White 1995) and densities are much higher in the northern savannas than in the continuous forests of the south (White 1995). The mosaic habitat to the north also has a high proportion of edge habitat, where higher densities of species such as guava, zingibers, and Psychotrias (Ukizintambara et al. 2007), which are preferred fruits for mandrills (White 2007), occur than in the other forest types. The high diversity of the savanna mosaic forest types means that food availability here is likely to be high in more months than in continuous forest types. Further investigation of the fine-scale distribution of preferred mandrill foods within the range is required to determine the individual importance of different species or fruit guilds to determining seasonal or annual mandrill range use.
At present, we do not know whether the home-range area of the focal horde at Lopé is typical of Mandrillus sphinx, as ours is the only study to measure empirically both group mass and home-range use in this species in the wild. We compared our data to a previous study that attempted to estimate the home-range size of mandrills (Hoshino et al. 1984), conducted over a period of 27 mo in SW Cameroon. The authors reported that they often located 2 or 3 groups simultaneously within their 25-km2 study area, and they believed that 4 groups had their ranges within this area. They estimated group sizes to range from 15 to 95 individuals, but measured only the home-range size of C group (thought to contain 15 individuals) by direct observation as ≥5 km2. The other groups ranged outside the study area, so the authors could not determine their home ranges directly. The authors estimated minimum home-range areas of 11.7 km2, 23 km2, and 28.5 km2 for these 3 groups, based on the equation R = 0.3 N, where R is home-range area in km2 and N is total group size, derived from their observations of C group. The authors reported that visibility in the forest was poor (<20 m; similar to that at Lopé) and that group sizes were estimated from sightings/audition of adult males and the assumed ratio of adult males to females (1 M:13.9 F), as well as counts of all visible individuals. These methods are now considered unreliable for estimating mandrill group size, as adult males are not permanent members of the horde, and the number of males present in a horde changes over time and does not have a simple relationship to female numbers as previously assumed (Abernethy et al. 2002). The authors also refer to groups as 1-male and multimale groups, suggesting that the number of adult males may have been used to assign group identity, which would again be unreliable, as males move in and out of groups and change between groups (Abernethy et al. 2002; White 2007). From our study, it seems possible that the different groups observed in the study by Hoshino et al. (1984) were in fact temporary subgroups of 1 large horde, occupying the whole 25-km2 study area and beyond, and that membership (even existence) of the 4 subgroups could potentially have changed during the study. If this is the case, then the Hoshino horde may have numbered ≥226 individuals, roaming over >25 km2 of habitat. Hoshino et al. (1984) do not suggest that any of the 25-km2 study area contains unsuitable habitat, and so this figure is comparable to use by 700 mandrills of ≥77.4 km2 of forest, which is surprisingly similar to the Lopé estimates for the suitable habitat in the 100% MCP (89 km2). This similarity could lend weight to the stability of the group mass-home range size relationship for Mandrillus, although the question marks over the true group sizes and masses that Hoshino studied mean that we must treat this range estimate with caution. Group size and consequent home-range area are likely to vary throughout the mandrills’ geographic range, in relation to habitat quality differences.
The very large home-range area used by the horde is of great practical interest in conservation planning for African rain forests. The demonstration that use of space by the Lopé mandrill horde falls close to the expected relationship suggests that estimates of space requirements necessary for conservation planning may be made with more confidence for Mandrillus groups of known mass. Conservation managers in the past may well have underestimated both the group sizes and the space needs of mandrills, being unwilling to make estimates so far above the top requirements of other rain forest species.
Forests throughout central Africa have been severely impacted by slash-and-burn agriculture, commercial logging, infrastructure development, mining, and fuel-wood extraction (Wilkie and Laporte 2001; WRI Global Forest Watch 2009), with Cameroon and Equatorial Guinea estimated to have lost over half of their ancestral closed-canopy forest already (Sayer et al. 1992). Primates throughout central Africa continue to be threatened by growth in the commercial bushmeat trade (Bowen-Jones and Pendry 1999; Chapman et al. 2006; Fa et al. 1995; Walsh et al. 2003), which is often facilitated by access roads for logging operations (Nasi et al. 2008). Mandrills have probably disappeared from some areas most heavily affected by habitat loss and associated hunting (southern Cameroon, northwestern Gabon), and with such large ranges, there are probably very few remaining hordes that have not been impacted in part of their original home range by logging, mining, or civil road building operations. Drills in heavily hunted areas of Cameroon, Nigeria, and Equatorial Guinea and mandrills in Equatorial Guinea and Cameroon have been surviving in much smaller social groups than the presumed original hordes (Astaras et al. 2008; Gadsby et al. 1994; Hoshino et al. 1984; Wild et al. 2005). Mandrillus spp. may be able to adapt in the short term to life in much smaller social groups, and these small groups in the same habitat probably use smaller home ranges, in line with the predicted relationship. However, the long-term effects on the species ecology of these social changes remain to be seen.
References
Abernethy, K. A., & White, L. J. T. (2010). Mandrillus sphinx. In T. Butynski, D. Happold, & J. Kingdon (Eds.), Mammals of Africa. San Francisco: University of California Press, in press.
Abernethy, K. A., White, L. J. T., & Wickings, E. J. (2002). Hordes of mandrills (Mandrillus sphinx): Extreme group size and seasonal male presence. Journal of Zoology (London), 258, 131–137.
Altmann, S. A. (1974). Baboons, space, time and energy. American Zoologist, 14, 221–248.
Amlaner, C. J., Jr., & Macdonald, D. W., eds. (1980). A handbook on biotelemetry and radiotracking. Proceedings of an international conference on telemetry and radio tracking in biology and medicine. Oxford: Pergamon.
Astaras, C., Muhlenberg, M., & Waltert, M. (2008). Note on drill (Mandrillus leucophaeus) ecology and conservation status in Korup National Park, Southwest Cameroon. American Journal of Primatology, 70, 306–310.
Barton, R. A., Whiten, A., Strum, S. C., Byrne, R. W., & Simpson, A. J. (1992). Habitat use and resource availability in baboons. Animal Behaviour, 43, 831–844.
Boinski, S. (1987). Habitat use by squirrel monkeys (Saimiri oerstedi) in Costa Rica. Folia Primatologica, 49, 151–167.
Bowen-Jones, E., & Pendry, S. (1999). The threat to primates and other mammals from the bushmeat trade in Africa, and how this threat could be diminished. Oryx, 33, 233–246.
Cade, B. S., & Richards, J. D. (2001). User manual for BLOSSOM statistical software. Fort Collins: Midcontinent Ecological Science Centre, US Geological Survey.
Chapman, C. A. (1988). Patterns of foraging and range use by three species of neotropical primates. Primates, 29, 177–194.
Chapman, C. A., Wrangham, R. W., Chapman, L. J., Kennard, D. K., & Zanne, A. E. (1999). Fruit and flower phenology at two sites in Kibale National Park, Uganda. Journal of Tropical Ecology, 15, 189–211.
Chapman, C. A., Chapman, L. J., Struhsaker, T. T., Zanne, A. E., Clark, C. J., & Poulsen, J. R. (2005). A long-term evaluation of fruiting phenology: Importance of climate change. Journal of Tropical Ecology, 21, 31–45.
Chapman, C. A., Lawes, M. J., & Eeley, H. A. C. (2006). What hope for African primate diversity? African Journal of Ecology, 44, 116–133.
Clutton-Brock, T. H. (1977). Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes. London: Academic Press.
Clutton-Brock, T. H., & Harvey, P. H. (1977). Primate ecology and social organisation. Journal of Zoology (London), 183, 1–29.
Cowlishaw, G. (1997). Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. Animal Behaviour, 53, 667–686.
De Solla, S. R., Bonduriansky, R., & Brooks, R. J. (1999). Eliminating autocorrelation reduces biological relevance of home range estimates. The Journal of Animal Ecology, 68, 221–234.
Defler, T. R. (1996). Aspects of the ranging pattern in a group of wild woolly monkeys (Lagothrix lagothricha). American Journal of Primatology, 38, 289–302.
Di Bitetti, M. S. (2001). Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. Journal of Zoology (London), 253, 33–45.
Fa, J. E., Juste, J., Delval, J. P., & Castroviejo, J. (1995). Impact of market hunting on mammal species in Equatorial Guinea. Conservation Biology, 9, 1107–1115.
Freeland, W. J. (1980). Mangabey (Cercocebus albigena) movement patterns in relation to food availability and faecal contamination. Ecology, 61, 1297–1304.
Gadsby, E. L., Jenkins, P. D., Jr., & Feistner, A. T. C. (1994). Coordinating conservation for the drill (Mandrillus leucophaeus): Endangered in forest and zoo. In P. J. S. Olney, G. M. Mace, & A. T. C. Feistner (Eds.), Creative conservation. Interactive management of wild and captive animals (pp. 39–454). London: Chapman and Hall.
Hamilton, W. J. I. (1982). Baboon sleep site preferences and relationships to primate grouping patterns. American Journal of Primatology, 3, 41–53.
Harris, S., Cresswell, W. J., Forde, P. G., Trewhella, W. J., Woollard, T., & Wray, S. (1990). Home-range analysis using radio-tracking data—a review of problems and techniques particularly as applied to the study of mammals. Mammal Review, 20, 97–123.
Harrison, M. J. S. (1988). The mandrill in Gabon's rain forest—ecology, distribution and status. Oryx, 22, 218–228.
Hayne, D. W. (1949). Calculation of size of home range. Journal of Mammalogy, 30, 1–18.
Hladik, C. M. (1975). Ecology, diet, and social patterning in Old and New World primates. In R. H. Tuttle (Ed.), Socio-ecology and psychology of primates (pp. 3–36). The Hague: Mouton.
Hoshino, J. (1985). Feeding ecology of mandrills (Mandrillus sphinx) in Campo Animal Reserve, Cameroon. Primates, 26, 248–273.
Hoshino, J., Mori, A., Kudo, H., & Kawai, M. (1984). Preliminary report on the grouping of mandrills (Mandrillus sphinx) in Cameroon. Primates, 25, 295–307.
IUCN (2009). 2009 IUCN Red List of Threatened Species. www.iucnredlist.org.
Jenrich, R. I., & Turner, F. B. (1969). Measurement of non-circular home range. Journal of Theoretical Biology, 22, 227–237.
Jouventin, P. (1975). Observations sur la socio-ecologie du mandrill. Terre et Vie, 29, 439–532.
Kaplin, B. A. (2001). Ranging behavior of two species of guenons (Cercopithecus lhoesti and C. mitis doggetti) in the Nyungwe Forest Reserve, Rwanda. International Journal of Primatology, 22, 521–548.
Karesh, W. B., Wallace, R. B., Painter, R. L. E., Rumiz, D., Braselton, W. E., Dierenfeld, E. S., et al. (1998). Immobilization and health assessment of free-ranging black spider monkeys (Ateles paniscus chamek). American Journal of Primatology, 44, 107–123.
Kenward, R. E. (1985). Ranging behaviour and population dynamics in grey squirrels. In R. M. Sibly & R. H. Smith (Eds.), Telemetric studies of vertebrates (pp. 319–330). London: Academic Press.
Kenward, R. E. (2001). A manual for wildlife radio tagging. San Diego: Academic Press.
Kernohan, B. J., Gitzen, R. A., & Millspaugh, J. J. (2001). Analysis of animal space use and movements. In J. J. Millspaugh & J. M. Marzluff (Eds.), Radio tracking and animal Populations (pp. 125–166). San Diego: Academic Press.
Lahm, S. A. (1986). Diet and habitat preference of Mandrillus sphinx in Gabon—implications of foraging strategy. American Journal of Primatology, 11, 9–26.
Léal, M. E. (2004). The African rain forest during the Last Glacial Maximum, an archipelago of forests in a sea of grass. PhD thesis Wageningen University, Wageningen, Netherlands.
Mielke, P. W., Jr., & Berry, K. J. (1982). An extended class of permutation techniques for matched pairs. Communications in Statistics A—Theory and. Methods, 11, 1197–1207.
Mielke, P. W., Jr., & Berry, K. J. (2001). Permutation methods: A distance function approach. New York: Springer-Verlag.
Milton, K., & May, M. L. (1976). Body weight, diet and home range area in primates. Nature, 259, 459–462.
Mitani, M. (1989). Cercocebus torquatus—Adaptive feeding and ranging behaviors related to seasonal fluctuations of food resources in the tropical rain-forest of southwestern Cameroon. Primates, 30, 307–323.
Mohr, C. O. (1947). Table of equivalent populations of North American small mammals. American Midland Naturalist, 37, 223–449.
Nasi, R., Brown, D., Wilkie, D., Bennett, E., Tutin, C., van Tol, G., et al. (2008). Conservation and use of wildlife-based resources: The bushmeat crisis. CBD Technical Series no. 33. Montreal: Secretariat of the Convention on Biological Diversity and Bogor and Center for International Forestry Research.
Oates, J. F. (1996). African Primates: Status survey and Conservation Action Plan, Revised Edition. Gland, Switzerland: IUCN/Species Survival Commission (SSC), Primate Specialist Group.
Oates, J. F., & Butynski, T. M. (2008a). Mandrillus sphinx. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.2. www.iucnredlist.org.
Oates, J. F., & Butynski, T. M. (2008b). Mandrillus leucophaeus. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.2. www.iucnredlist.org.
Olupot, W., Chapman, C. A., Waser, P. M., & Isabirye Basuta, G. (1997). Mangabey (Cercocebus albigena) ranging patterns in relation to fruit availability and the risk of parasite infection in Kibale National Park, Uganda. American Journal of Primatology, 43, 65–78.
Oslisly, R. (2001). The history of human settlement in the middle Ogooue´ valley (Gabon). Implication for the environment. In W. Webber, L. J. T. White, A. Vedder, & L. Naughton-Treves (Eds.), African rain forest ecology & conservation (pp. 101–118). New Haven: Yale University Press.
Otis, D. L., & White, G. C. (1999). Autocorrelation of location estimates and the analysis of radiotracking data. Journal of Wildlife Management, 63, 1039–1044.
Rogers, M. E., Abernethy, K. A., Fontaine, B., Wickings, E. J., White, L. J. T., & Tutin, C. E. G. (1996). Ten days in the life of a mandrill horde in the Lopé Reserve, Gabon. American Journal of Primatology, 40, 297–313.
Sabater Pi, J. (1972). Contribution to the ecology of Mandrillus sphinx Linnaeus 1758 of Rio Muni (Republic of Equatorial Guinea). Folia Primatologica, 17, 304–319.
Sayer, J. A., Harcourt, C. S., & Collins, N. M. (1992). The conservation atlas of tropical Forests: Africa. London: Macmillan.
Seaman, D. E., & Powell, R. A. (1996). An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology, 77, 2075–2085.
Seaman, D. E., Millspaugh, J. J., Kernohan, B. J., Brundige, G. C., Raedeke, K. J., & Gitzen, R. A. (1999). Effects of sample size on kernel home range estimates. Journal of Wildlife Management, 63, 739–747.
Silverman, B. W. (1986). Density estimation for statistics and data analysis. London: Chapman and Hall.
Singleton, I., & van Schaik, C. P. (2001). Orangutan home range size and its determinants in a Sumatran swamp forest. International Journal of Primatology, 22, 877–911.
Terborgh, J. (1983). Five New World Primates: A study in comparative ecology. Princeton, NJ: Princeton University Press.
Tutin, C. E. G., & White, L. J. T. (1998). Primates, phenology and frugivory: Present, past and future patterns in the Lopé reserve, Gabon. In: D. M. Newberry, H. H. T. Prins, & N. Brown (Eds.), Dynamics of tropical communities. 37th Symposium of the British Ecological Society (pp. 309–337). Oxford: Blackwell Science.
Tutin, C. E. G., Fernandez, M., Rogers, M. E., Williamson, E. A., & McGrew, W. G. (1991). Foraging profiles of sympatric gorillas and chimpanzees in the Lopé Reserve, Gabon. Philosophical Transactions of the Royal Society, London (B), 334, 179–186.
Tutin, C. E. G., White, L. J. T., & Mackanga-Missandzou, A. (1997). The use by rain forest mammals of natural forest fragments in an equatorial African savanna. Conservation Biology, 11, 1190–1203.
Ukizintambara, T., White, L., Abernethy, K., & Thébaud, C. (2007). Gallery forests versus bosquets: Conservation of natural fragments at Lopé National Park in central Gabon. African Journal of Ecology, 45, 476–482.
van Schaik, C. P., Terborgh, J. W., & Wright, S. J. (1993). The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics, 24, 353–377.
Van Winkle, W. (1975). Comparison of several probabilistic home-range models. Journal of Wildlife Management, 39, 118–123.
Wallace, R. B. (2006). Seasonal variations in black-faced black spider monkey (Ateles chamek) habitat use and ranging behaviour in a southern Amazonian tropical forest. American Journal of Primatology, 68, 313–332.
Walsh, P. D., Abernethy, K. A., Bermejo, M., Beyersk, R., De Wachter, P., Akou, M. E., et al. (2003). Catastrophic ape decline in western equatorial Africa. Nature, 422, 611–614.
White, L. J. T. (1992). Vegetation history and logging damage; effects on rain forest mammals in the Lopé Reserve. Gabon: Doctoral thesis, University of Edinburgh, Edinburgh.
White, L. J. T. (1995). Vegetation study of Lopé reserve. Gabon: Unpublished report, ECOFAC.
White, L. J. T. (2001). Forest-savanna dynamics and the origins of Marantaceae forest in Central Gabon. In W. Weber, L. J. T. White, A. Vedder, & L. Naughton-Treves (Eds.), African rain forest ecology and conservation: An interdisciplinary perspective (pp. 165–182). New Haven and London: Yale University Press.
White, E. C. (2007). Ecology of Mandrillus sphinx: Ranging, diet and social structure of a mandrill horde in Lopé National Park. Gabon: Doctoral thesis, University of Exeter, Cornwall, U.K.
White, L. J. T., & Abernethy, K. (1997). A guide to the vegetation of the Lopé Reserve, Gabon. Libreville, Gabon: ECOFAC.
White, G. C., & Garrott, R. A. (1990). Analysis of wildlife radio-tracking data. San Diego: Academic Press.
Wickings, E. J., & Dixson, A. F. (1992). Development from birth to sexual maturity in a semi-free- ranging colony of mandrills (Mandrillus sphinx) in Gabon. Journal of Reproduction and Fertility, 95, 129–138.
Wild, C., Morgan, B. J., & Dixson, A. (2005). Conservation of drill populations in Bakossiland, Cameroon: Historical trends and current status. International Journal of Primatology, 26, 759–773.
Wilkie, D. S., & Laporte, N. T. (2001). Forest area and deforestation in Central Africa. Current knowledge and future directions. In W. Weber, L. J. T. White, A. Vedder, & L. Naughton-Treves (Eds.), African rain forest ecology and conservation: An interdisciplinary perspective (pp. 119–139). New Haven and London: Yale University Press.
Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology, 70, 164–168.
WRI Global Forest Watch. Interactive Forest Atlas series. (2009). www.wri.org/gfw
Acknowledgments
We thank the Ministère des Eaux et Forêts and the Agence National des Parcs Nationaux (ANPN, formerly CNPN) for permission to work in Lopé National Park. In particular, we thank the late Dr. Alphonse Mackanga-Missandzou, M. Simon Angoué and M. Jean Tondangoye, and M. Augustin Mihindou for support at Lopé as Chefs de Brigade de Faune 1998–2002, and from 2003, M. Joseph Ngowou as National Park conservator. We thank the Centre International de Recherches Médicales de Franceville (CIRMF) for long-term core funding of SEGC, from where the study was carried out, and for support to K. Abernethy. The Wildlife Conservation Society Field Veterinary Program (1998–2003) provided support to W. B. Karesh and M. D. Kock, and Edith McBean and the Disney Foundation (1998–2000) provided project grants to K. Abernethy and L. White via the WCS Africa Program. The Welcome Trust provided support to T. Ukizintambara and the Natural Environment Research Council (NERC), U.K. provided studentship (NER/S/A/2002/12024) and fieldwork expenses grant to E. C. White, via the University of Stirling. We thank ECOFAC II (Gabon) and WCS Gabon for logistical support in the field, and Martial Bouassa, Jean-Gael Emptaz-Collomb, Tom Gilbert, Philipp Henschel, Olivier Houé, Miguel Léal, Ludovic Momont, Jules Penze, Peter Ragg, Trish Reed, Paul Telfer, Caroline Tutin, Peter Walsh, and E. Jean Wickings for their assistance in the field, in particular capturing mandrills for radio-collaring. We also thank David Bryant and 2 anonymous reviewers for useful comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
White, E.C., Dikangadissi, JT., Dimoto, E. et al. Home-range Use by a Large Horde of Wild Mandrillus sphinx . Int J Primatol 31, 627–645 (2010). https://doi.org/10.1007/s10764-010-9417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9417-3