Abstract
With persistent degradation of tropical forests creating fragmented landscapes, the study of patterns of primate responses to habitat changes is of increasing conservation relevance. We modeled primate abundance in the Udzungwa Mountains of Tanzania through a landscape approach, i.e., one that includes a representative range of discrete forest blocks. The area is internationally recognized for biological endemism and is a primate hotspot in Africa. We targeted three predominantly arboreal monkeys: Udzungwa red colobus (Procolobus gordonorum), Peters’ Angola colobus (Colobus angolensis palliatus), and the Tanzania Sykes’ monkey (Cercopithecus mitis monoides). In each of the four forests (12–522 km2 in size), we counted primate groups along a grid of line transects (267 km walked) and sampled canopy trees in vegetation plots along the same transects (N = 408) to derive structural and floristic forest parameters and proxies of human impact. We found that elevation and the percentage of climber coverage on trees consistently emerged as significant predictors of primate abundance for all three species in spite of their differences in feeding habits, with a negative effect of elevation and a positive effect of climber coverage. This pattern held despite large variations in elevation, forest habitat, and human disturbance across the four forests surveyed. We conclude that arboreal primates in the Udzungwas are dependent on lowland and medium-elevation forests (ca. 300–1200 m a.s.l.) and show considerable resilience to moderate forest disturbance. However, agricultural intensification causes rapid forest degradation, with detrimental effects on primates that need to be prevented through increased protection and community conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global changes, local overexploitation of natural resources, and habitat destruction are causing unparalleled rates of species and population loss (Brook et al. 2008; Busch and Hayward 2009; Fahrig 2003). Among mammals, nonhuman primates have the highest rate of threatened species per family (Schipper et al. 2008), and the most common threats to them are hunting and habitat degradation. While direct hunting eventually leads to population extinction (Fa et al. 2005), habitat degradation caused by human activities, e.g., logging, pole and firewood collection, creates smaller forest patches and causes secondary vegetation to take over primary, old growth forest. This reinforces the scientific and conservation relevance of studying how animal populations are able to persist in rapidly changing, naturally heterogeneous or disturbed habitats (Chapman and Lambert 2000; Henle et al. 2004; Laurance et al. 2012). However, determining general patterns of associations between animal abundance and habitat characteristics remains difficult, as a wealth of studies show that a complex array of species- and site-specific factors may mask the understanding of general patterns (Henle et al. 2004; Onderdonk and Chapman 2000). In this context, arboreal primates are an excellent model group because of their dependence on closed-canopy forest while being seemingly highly resilient in variably degraded tropical forests (Chapman et al. 2000; Johns and Skorupa 1987).
Primate resilience to disturbance may depend on a complex interplay of factors, such as body size and ecological, demographic, and behavioral traits (Cardillo et al. 2005, Cowlishaw and Dunbar 2000; Fa et al. 2005; Onderdonk and Chapman 2000). For example, other factors being equal, species that do not have a specialized diet are more likely to survive in habitats modified by humans, whereas species that rely on specific food trees are more likely to suffer in degraded forests (Bracebridge et al. 2012; Johns and Skorupa 1987). For tree dwellers in particular, a number of studies have shown a dependence on old growth forest, and hence their vulnerability in suboptimal habitats (Marsh 2003; Plumptre and Johns 2001; Rovero and Struhsaker 2007; Struhsaker 1997).
Here, we present a case study modeling responses of primate abundance to habitat in a complex landscape, the Udzungwa Mountains of Tanzania. The area is part of the Eastern Arc, an internationally recognized region for biological diversity and endemism (Rovero et al. 2014), including primates (Rovero et al. 2009). We focused on the three arboreal, forest-dwelling primate species occurring in the target forests: the endemic Udzungwa red colobus (Procolobus gordonorum), the Peters’ Angola colobus (Colobus angolensis palliatus), and the Tanzania Sykes’ monkey (Cercopithecus mitis monoides) (Davenport et al. 2013).
Although a few ecological studies of Udzungwa primates are available, they are based mainly on regression analysis limited to single forest blocks (and hence treating each population separately), and/or are based on count data from a small set of line transects (usually three or four) covering only a small portion of the overall forest area, with a bias toward the lower elevations (Marshall 2007; Rovero and Struhsaker 2007; Rovero et al. 2012). Here, instead, we collected data using a grid of line transects that comprehensively covered each target forest, achieving a much larger sampling coverage than earlier studies. Previous studies have indicated that Tanzania Sykes’ monkeys cope better in secondary, degraded habitat than colobines, which tend to prefer more mature forest habitat. They also reported on the heavy impact of hunting on both colobines at one particular unprotected site (Uzungwa Scarp). A pattern of decreasing abundance with elevation also emerged from other studies (Barelli et al. 2014; Marshall et al. 2005). However, we still lack a general understanding of primate responses to the full variation of forest habitats at the landscape scale. Overall, our objective was to assess how habitat type and human disturbance affect primate abundance, predicting that habitat type, elevation, as well as protection level have an impact on primate abundance. In particular, we predict that primate abundance would be higher 1) in continuous, less patchy or degraded forests than in fragmented ones; 2) at mid- to high-elevation levels where vegetation is of better quality; and 3) in forests with effective protection on the ground.
Finally, the present study also aimed to contribute to the understanding of the spatial dynamics of threatened species in very heterogeneous landscapes, which is relevant, in turn, to the provision of conservation recommendations.
Methods
Study Area
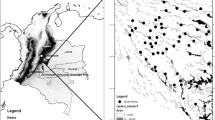
The Udzungwa Mountains (7°40′ S to 8°40′ S and 35°10′ E to 36°50′ E; Fig. 1) extend over 19,000 km2 (Platts et al. 2011) and represent a mosaic of moist forest blocks of variable sizes (from 12 km2 to >500 km2) that are under the influence of the east-blowing Indian Ocean winds, interspersed in a matrix of naturally drier and/or habitat modified through agriculture, settlements, and logging. Rainfall in the moister forest slopes ranges from 2000 to 2500 mm/yr, concentrated in two periods: November–December and March–May. Overall, elevation spans from 270 (Kilombero Valley in the eastern side) to 2576 m a.s.l. (Mount Luhomero).
Map of the Udzungwa Mountains showing the four forest blocks surveyed (MW = Mwanihana; MG = Magombera; MT = Matundu; US = Uzungwa Scarp). The borders of the Udzungwa Mountain National Park (UMNP) are highlighted in white Reprinted with permission from Araldi et al. 2014.
The four forests selected represent a wide contrast in habitat type, elevation gradient, and protection level (Table I).
-
1)
Magombera (MG) is a small, remnant ground-water and evergreen lowland forest patch which is highly isolated (Fig. 1) from other forest blocks and surrounded by intensive agriculture and settlements (Marshall 2008).
-
2)
Matundu (MT) is inside UMNP and well protected, i.e., periodic patrols by the park rangers; it is mainly a lowland deciduous and semi-evergreen forest (Marshall 2007);
-
3)
Mwanihana forest (MW) is part of the Udzungwa Mountains National Park (UMNP; Fig. 1) and also well protected; it is a forest escarpment with forest zones from lowland deciduous to montane evergreen forest (Lovett et al. 2006);
-
4)
Uzungwa Scarp (US) is 150 km to the southwest of MW, is a Forest Reserve, and lacks effective law enforcement on the ground. Primate disturbance occurs in the form of both pole and timber cutting, which alter the canopy and understory structure, but also in form of traditional hunting, which particularly impacts colobine monkeys (Rovero et al. 2012). The forest habitat is similar to MW.
Target Species
Peters’ Angola colobus monkeys live in one-male/multifemale groups, they are typically arboreal and feed mainly on mature leaves (Bocian 1997; Fimbel et al. 2001). Udzungwa red colobus monkeys live in multimale/multifemale groups, are predominantly arboreal, and feed preferably on young leaves (Struhsaker 2010). Tanzania Sykes’s monkeys live in one-male/multifemale groups, use both low- and high-canopy strata, and feed predominantly on fruits (Rovero et al. 2009).
Vervets (Chlorocebus pygerythrus) and yellow baboons (Papio cynocephalus) are also present in the area; however, they mainly live in deciduous woodland and/or marginal areas of the forests (Rovero et al. 2009).
Primate Counts
One team of two trained field assistants collected data during two time periods (July 2011–February 2012 and August–November 2012). The team was always the same and counted primate groups, spending a maximum of 10 min for each group (Araldi et al. 2014) along line transects, evenly spread throughout the four selected forests, according to a randomly placed and predesigned grid conceived for a density estimation study (for details on transects’ preparation see Araldi et al. 2014). Transects were ca. 2 km long (length that encompasses the home range of target species (1–3 ha on average; Rovero et al. 2009)), spaced 1 km apart (along both longitude and latitude) and oriented north–south. Not all transects could be completely walked because of obstacles, such as cliffs or dense bamboo vegetation. In MW (transects: N = 43; total km walked = 70.8) and MT (N = 39; total km walked = 70.5) predesigned grids were strictly followed and we walked each transect once. US, owing to logistic limitations and paucity of observations, the 24 transects established were walked twice (total km walked = 82.6). For MG forest, which is a very small forest patch, we adapted the grid by orienting transects along the west–east direction, shortening them to 1.5 km, and walking them twice (N = 15; total km walked = 42.7). Most transects did not require any prior vegetation cutting, except for some lower elevation transects that crossed secondary, regenerating vegetation. Transects were walked by a team of two assistants at an average speed of about 1–1.5 km/h, starting in the early morning (between 07:00 and 07:30 h) according to cloud cover and sunrise time, and completed in 2–3 h, depending on vegetation density (average: 2 h 53 min; range: 1 h 2 min–4 h 33 min). In case of rain or poor visibility, transects were not walked or temporarily interrupted. We measured the length walked by using a hip chain, and we used a compass to keep a constant direction. On observing a primate group, we recorded the following data: time, species, number of individuals seen, observer’s position from the starting point of the transect (measured with a hip chain), position, and elevation recorded by using a hand-held GPS unit (Garmin Map60 CSx).
Vegetation Sampling
To assess the relationship between primate abundance and habitat parameters, we established four squared vegetation plots, 25 × 25 m, centered on the line transect and placed every 500 m along transects (at 250, 750, 1250, and 1750 m from the starting point). Within each plot we counted, measured, and identified (by involving the same local experts to ensure consistency) all tree and woody climber species above 10 cm of diameter at breast height (DBH) by using a DBH measuring tape. In addition, we visually estimated the extent of canopy cover, above each plot, by using five classes: completely open canopy (0%), canopy partly open (up to 25%), half open (50%), semiclosed (up to 75%), and completely closed (100%). Similarly, we visually estimated the coverage of climbers on trees in terms of proportion of volume of the canopy, using five classes: no climbers in the plot (0%), few climbers in the plot (up to 25%), plot half-full of climbers (50%), plot semifull of climbers (up to 75%), and plot completely full of climbers (100%).
Assessment of Human Disturbance
While walking line transects, we also recorded signs of human presence along a width of ca. 5 m centred on the transect line, under three categories: 1) “cutting signs” whenever we encountered cut poles (<15 cm) and cut trees (>15 cm in DBH), as well as signs of machete cuts on the bark’s surface. 2) Under “human signs” we included all recent and old paths or trails made by humans, sites where pit sawing had been carried out or charcoal was produced, as well as signs of recent and old poacher camps. 3) Finally, we recorded the incidence of “animal snares” that were removed after observation and their presence reported to the headquarters of the respective park authority. While this task was done by the second observer of the field team, a number of least-detectable signs of disturbance may have been missed; however, we took this measurement consistently, and it is adequate for deriving an index of general disturbance.
Data Analyses
We analyzed survey data separately for each primate species, relating, for the regression analysis, each primate group encountered to the closest vegetation plot surveyed and controlling for the effort (meters walked) by splitting transects in equal portions of 500 m centered on plots. For each plot we derived several habitat parameters, including human impact, as potential predictors of primate abundance: 1) tree species richness (total number of tree species, including climbers); and 2) tree diversity indices. To measure tree diversity we used the Shannon entropy index (Bolliger et al. 2005; Shannon 1948), standardized such that the largest possible value was = 1 (Shannon diversity = 1 – Σ(p i × log(p i ))/log(n) where pi is the relative abundance of cases), and the Simpson diversity index (Simpson 1949), the reversed dominance (1 – Σ(p i 2) derived from the Simpson dominance index (Σ(p i 2)); as a proxy of potential food available (size of trees that bear food) and forest structure, we included into the model 3) MBA [mean basal area (BA) derived from DBH and expressed in m2 through the formula BA = (DBH/200)2 × π (Hédl et al. 2009)] and TBA (total basal area); 4) canopy cover; and 5) percentage of climbers. As vegetation variables are influenced by elevation, we also considered 6) altitude as a predictor variable. As a proxy of human impact we used 7) the summed encounter rates of cutting signs, human activities, and snares. We lumped the scores for these three categories both because the number of signs for some categories was too small, i.e., up to 80% of transects with no signs, to be used as a predictor, and to minimize the number of predictors. As these latter variables were available only per transect, they were identical for all four plots along each transect.
Before the analysis, we inspected the distributions of each predictor and transformed them, when necessary, to achieve a more symmetrical distribution. Specifically, we log-transformed both human impact and MBA; we square-root transformed percentage of climbers, Simpson’s index, and TBA. Shannon index was first square-root transformed after we reversed it (max Shannon – Shannon), then standardized to a minimum of 0 and finally reversed again. We then checked whether a principal components analysis (PCA) or factor analysis (FA) would allow removing potential redundancy among the predictors. Because only few larger correlations were found, we subjected two sets of these to separate PCAs/FAs. First, we ran a PCA with MBA and TBA, justified by the high correlation between them (Pearson’s ρ = 0.78) as well as the Kaiser–Meyer–Olkin measure of sampling adequacy (0.5) and Bartlett’s test of sphericity (χ2 = 384, df = 1, P < 0.001; McGregor 1992). This PCA revealed one component with an eigenvalue of 1.33, explaining 89% of the total variance. Loadings of both variables on this component were 0.70. Second, we ran a FA with varimax rotation with Shannon’s index, Simpson’s index, and tree species richness, again justified by large correlations (Simpson and Shannon: –0.77, Simpson and tree species richness: –0.75, Shannon and tree species richness: 0.27) as well as by Bartlett’s test of sphericity (χ2 = 177, df = 3, P < 0.001) and the Kaiser–Meyer–Olkin measure of sampling adequacy (0.37). The reason for conducting a FA for the second set of variables was that it allows a rotation of the derived factors, a means to achieve easier interpretable loadings (Quinn and Keough 2002). The resulting single factor had an eigenvalue of 2.14, explaining 71% of the total variance. Loadings of the variables were 0.77 (Shannon), –1.00 (Simpson), and 0.75 (tree species richness). In all further analyses therefore, we used the two derived scores as predictors: basal area score representing MBA and TBA, and tree score representing both diversity indices and tree species richness. The largest and average absolute correlations between all other pairs of variables were 0.51 and 0.16, respectively.
We analyzed the effects of predictors on the number of primate group encounters per species and plot using generalized linear models (GLMs: McCullagh and Nelder 2008) applied separately for the three primates species. The models built for all species were identical and included canopy cover, percentage of climbers, tree score, basal area score, altitude, human impact, and forest (factor with four levels). Because we assumed that altitude may have a nonlinear effect, i.e., relatively larger numbers of primates at intermediate altitudes, we also included squared altitude in the models. We also expected that the effect of human impact on monkey abundance differed between forests, namely that our measure of human impact would have a particularly strong effect in unprotected forests. Hence, we included the interaction between human impact and forest block into the model. Before running the GLMs, we z-transformed all predictors (except forest) to a mean of 0 and a standard deviation of 1 (Aiken and West 1991; Schielzeth 2010). To account for variation in the effort taken per transect, we included the number of meters walked per transect segment (log-transformed) as an offset term (in case transects were walked twice, we doubled the effort). Because the response variable was a count, we fitted the models using a Poisson error distribution and a log link function. Overdispersion did not appear problematic (range of dispersion parameters: 0.96–1.14; Gelman and Hill 2007). The realized sample size for all three models was 408 plots.
Because the response variables (primate counts) may be autocorrelated, the assumption of independent residuals could have been violated, i.e., residuals closer in space being more similar than those placed more distant from each other. Hence, we decided to explicitly incorporate spatial autocorrelation using the same approach as in Fürtbauer et al. (2011). However, we kept the autocorrelation term only in the model for Tanzania Sykes’ monkey because it appeared insignificant for red colobus monkeys (P = 0.45) and it did not reveal an interpretable result for Peters’ Angola colobus, probably owing to too many plots lacking the species and absence of spatial autocorrelation.
As a global test of the impact of all predictors, we compared the full model with the respective null model (Forstmeier and Schielzeth 2011) comprising only the offset term (and potentially the autocorrelation term) using a likelihood ratio test (Dobson 2002). We first checked for the significance of the interaction between forest and human impact (using a likelihood ratio test comparing the full model with the respective reduced model lacking the interaction but comprising all other terms present in the full model) and removed it when it was nonsignificant (P > 0.1) to infer about the respective main effects (Zar 1999). We also removed squared altitude in case it did not reveal significance to infer about the effect of altitude unsquared.
To check for validity and stability of the model, we inspected various diagnostics. Although leverage values showed some data points to be potentially influential, DFBη values did not reveal any problem. Similarly, collinearity did not appear to be problematic (largest variance inflation factor, as determined from models lacking the interaction and squared altitude = 1.73; Field 2005; Quinn and Keough 2002).
We conducted all analyses using R (version 3.0.1, R Core Team 2013). We conducted the PCA using the function prcomp and the FA by using the function factanal. We fitted the Poisson models using the function glm, while we assessed the diagnostics of the validity of PCA/FA by using the function paf of the package rela (Chajewski 2009). We conducted likelihood ratio tests using the function ANOVA with the argument test set to Chisq.
Ethical Note
We conducted the study without direct contact or interaction with any of three monkey species surveyed and under permission of the Tanzania Commission for Science and Technology (COSTECH), Tanzania Wildlife Research Institute (TAWIRI), and Tanzania National Parks (TANAPA). Our data collection procedure adhered to the legal requirements and complied with the laws governing wildlife research in Tanzania.
Results
We accomplished a sampling effort of 266.6 km walked, corresponding to a total of 160 transects (30, 39, 43, and 48 in MG, MT, MW, and US, respectively) and 408 vegetation plots surveyed (29, 143, 151, and 85 in MG, MT, MW, and US, respectively). The relative abundance of colobine monkeys varied highly across forests, with highest abundance in MW, MG, and MT, in this order, and much lower abundance in US (Fig. 2). On the contrary, Tanzania Sykes’ monkeys had much more similar abundances across the forests, with highest values in US and similar values in the other three forests (Fig. 2).
Encounter rates of Peters’ Angola colobus, Udzungwa red colobus, and Tanzania Sykes’ monkeys in four forests (MG = Magombera; MW = Mwanihana; MT = Matundu; US = Uzungwa Scarp) of the Udzungwa Mountains National Park, Tanzania, studied in two time periods (July 2011–February 2012 and August–November 2012). Shown are medians (bold black horizontal lines), quartiles (boxes), and percentiles (2.5% and 97.5%) of the observed encounter rates. The area of the gray dots corresponds to the sample size (larger area if the relative number of encounters is higher) of groups encountered along transects (y-axis), per monkey species and forest (x-axis). The blue crosses show the fitted values and the corresponding 95% confidence intervals.
We encountered 90 groups of Peters’ Angola colobus monkeys (MG: 18, MW: 35, MT: 30, US: 7) in all four forests. The predictors (Fig. 3) had a clear impact on the number of encounters (likelihood ratio test comparing full and null model: χ2 = 59.74, df = 13, P < 0.001). Neither the interaction between forest and human impact (χ2 = 2.9, df = 3, P = 0.408) nor squared altitude (estimate + SE = 0.06 + 0.26, z = 0.22, P = 0.829) was significant. After the removal of these two effects we found that Peters’ Angola colobus were more abundant where there were more climbers and were less common at higher altitudes (Table II). Encounter rates for Peters’ Angola colobus also differed between the four forests (χ2 = 14.28, df = 3, P = 0.003; Fig. 2), with the species being more abundant in MW and MG. None of the other terms in the model revealed significance (Table II).
Variables used as potential predictors of primate counts in four different forests in the Udzungwa Mountains of Tanzania (MG = Magombera; MT = Matundu; MW = Mwanihana; US = Uzungwa Scarp) during two time periods (July 2011–February 2012 and August–November 2012). Values are medians (horizontal lines), quartiles (boxes), and percentiles (2.5 and 97.5%, vertical lines).
We encountered 102 groups of Udzungwa red colobus (MG: 20, MW: 40, MT: 34, US: 8) in the four forests sampled. Similarly to Peters’ Angola colobus, the full null model comparison was significant (χ2 = 63.78, df = 13, P < 0.001), indicating that the predictors (Fig. 2) clearly influenced the number of encounters. As for the Peters’ Angolacolobus monkey, we found no significant interaction between forest and human impact (χ2 = 1.96, df = 3, P = 0.58) and also no strong effect of squared altitude (estimate + SE = 0.01 + 0.27, z = –1.7, P = 0.09). After removal of these two terms we found results comparable to those obtained for the Peters’ Angola colobus: Udzungwa red colobus abundance increased with increasing percentage of climbers and decreased with increasing altitude (Table II). The abundance differed between forests (χ2 = 14.83, df = 3, P = 0.002), being highest in MW (Fig. 2). None of the other terms in the model were significant (Table II).
We scored 126 Tanzania Sykes’ groups (MG: 22, MW: 28, MT: 50, US: 26) across the four forests. As for the other two monkey species, the full null comparison was significant (χ2 = 36.32, df = 13, P = 0.001). Again, we found no significant interaction between forest and human impact (χ2 = 2.66, df = 3, P = 0.446) and also no obvious impact of squared altitude (estimate + SE = 0.01 + 0.22, z = –0.74, P = 0.461), thus, we removed these terms. The final model revealed no differences between forests (χ2 = 1.16, df = 3, P = 0.762). However, as for the other two species, also Tanzania Sykes’ monkeys were more abundant when percentage of climber increased and less abundant with increasing altitude (Table II). None of the other terms were significant (Table II).
Discussion
Among the suite of habitat and disturbance predictors considered across four forests in the Udzungwa Mountains, elevation and percentage of climbers consistently emerged as significant covariates of three arboreal primate abundance. While primate–habitat association modeling has been the subject of previous studies for this renowned primate diversity hotspot (Marshall 2007; Rovero and Struhsaker 2007; Rovero et al. 2012), by achieving a sampling coverage representative of the entire habitat variation within the landscape, we determined abundance predictors of much more general relevance than in earlier studies. This, in turn, allows inference into how the rapid changes in the area may impact primate populations.
Our method of sampling the arboreal vegetation differed from that in previous studies, as we measured discrete and squared plots regularly distributed along the transects, rather than an elongated and continuous plot along the transect line. Owing to the large number and diffuse placing of transects we surveyed, it was logistically unfeasible for us to measure the arboreal vegetation along the entire transect length. Nevertheless, we considered our design sound for studying forest-wide patterns of associations between primates and their habitats.
That all three forest monkeys preferred lower to medium elevations may partly reflect the fact that MG, a floodplain remnant forest, as well as MT, a lowland forest, both have a relative high abundance of primates. Interestingly however, this pattern also held for MW and US where, despite the much wider elevation range, primate abundances were still greatest at lower-to-mid elevation (this study; Barelli et al. 2014). Ecological constraints, such as low fruit productivity at higher elevation zones, may explain the lower abundance of Tanzania Sykes’ monkeys, which mainly feed on fruits (Butynski 1990; Rovero et al. 2009). Colobines are predominantly folivorous, but the high preference for young and more digestible leaves, which is especially shown by the target red colobus species (as well as others, e.g., Procolobus rufomitratus: Ryan et al. 2013), may have driven their preference for low elevation, semideciduous forest zones where there is a relatively higher rate of production of new leaves (Lovett 1993). In turn, our results imply that these monkeys did not prefer the old-growth forest interiors with a mature forest structure, i.e., larger basal area, and higher tree species’ richness and diversity (Barelli et al. 2014; Lovett et al. 2006). Indeed, most of MT, MG, and the lower elevation zones of both MW and US forests are currently predominantly covered by secondary, regenerating zones (owing to logging activity in the past and current disturbance for MG and US) that are intermingled with closed canopy areas of deciduous and semideciduous forest. What instead emerged is a consistent preference for forest areas that are variably disturbed, or where past logging has left a patchy canopy cover. In this respect, our results seem to contrast with previous findings showing that primate abundance is positively related to tree basal area and species richness (Marshall 2007; Rovero and Struhsaker 2007). However, these studies were related to primate counts along a limited set of line transects placed only at low-to-medium elevation within single forests and therefore were not able to assess differences across the whole habitat gradient in the area.
The preference for lower elevations is concordant with the result that the climber coverage of trees was the second most important driver of abundance. In tropical forests, climbers grow faster and are more abundant in lowland, disturbed, and regenerating areas (Bakayoko et al. 2011; Schnitzer and Bongers 2011; Schnitzer and Carson 2010). Although climbers certainly predominate in areas that have been logged in the past, the steep terrain morphology also results in areas with naturally-broken canopy, which may explain the widespread but moderate occurrence of climbers. Very high coverage by climbers is more typical of areas logged in the past and currently regenerating, a condition that is likely avoided by primates, as shown by Marshall (2007). This study revealed that >50% climber coverage negatively affected primate abundance in MT, a forest that is currently well protected by being part of the national park but was formerly heavily logged. The increasing presence of elephants (Loxodonta africana) especially in lowland forests such as MT may facilitate persistence or even increase of climber coverage and hence modulate optimality of primate habitat. In general, however, the moderate cover we recorded overall (generally around 20% or less; Fig. 3) matches accounts on the important role that woody vines have in forest regeneration and contribution to tree species’ diversity, particularly in the tropics (Gerwing et al. 2006; Schnitzer and Bongers 2002). Indeed, the importance of climber coverage for primates was also reported by Rovero and Struhsaker (2007), who found positive associations between primate abundance and proportion of trees with climbers for both Tanzania Sykes’ monkeys and Peters’ Angola colobus in MW forest. Moreover, Udzungwa monkeys also rely on climbers for a portion of their diet (Steel 2012). Climbers may represent fallback food, as already shown for a number of primate species, e.g., Colobus vellerosus (Wong et al. 2006), Hylobates albibarbis (Vogel et al. 2009), howlers (Dunn et al. 2012), Presbytis rubicunda (Hanya and Bernard 2012), and Udzungwa monkeys also rely on climbers for a portion of their diet (Steel 2012).
The finding that canopy cover was nonsignificant, in relation to the abundance of Peters’ Angola colobus, matches the results from a previous study in MW (Rovero and Struhsaker 2007). This also supports the above considerations that preference for areas with moderate climber coverage does not imply avoidance of closed-canopy areas by typically arboreal colobus with a predominantly folivourous diet. However, the lack of significance of basal area score, which is more directly indicative of mature, interior, and higher elevation forest habitat, supports the preceding statements above, i.e., that interior mature forest is not an optimal habitat. Equivalent considerations apply to the lack of significance for tree species diversity scores, as floristic diversity peaks at medium-to-high elevations of the forests (Barelli et al. 2014; Lovett et al. 2006).
The complex and intermingled effects of predictors on primate abundance are especially evident with human impact. Human impact is currently highest in US and second highest in MG; however, the pattern of primate abundance in these two forests was very different. The surprisingly high relative abundance of colobines in MG is likely a compression effect, as observed elsewhere (Nowak and Lee 2013; Siex and Struhsaker 1999), and likely caused by the rapid shrinking and isolation that the forest has undergone over the last three decades (Marshall 2008). Since the late 1960s, when the TAZARA railroad was built through this forest, most of the original cover has been cleared and marketable timber removed (Rodgers et al. 1979). In contrast, in US the very low abundance of colobines, which declined rapidly in the last decade, has been explained by hunting (Rovero et al. 2012). Although we did not record hunting directly, in this forest the human impact we measured appeared to be a reliable proxy of the various forms of human encroachment.
A wealth of studies has been conducted on primates’ ability to cope with habitat fragmentation (Onderdonk and Chapman 2000; review in Marsh and Chapman 2013). They broadly indicate that there are few generalizations that work across primate taxa to predetermine success or failure in fragments, hence warning against making generalizations and suggesting that responses may be site specific. Nevertheless, several studies converge on the importance of facilitating range expansion and connectivity between insulated subpopulations. Consequently, the understanding of drivers of primates’ resilience in suboptimal habitat is of critical ecological and conservation importance. For example, kipunjis (Rungwecebus kipunji) in southwestern Tanzania are mainly associated with closed-canopy forest habitat at mid to lower elevations, showing marked tolerance for fragmented forests with secondary and regenerating areas that tended to dominate at lower elevation and along edges (Bracebridge et al. 2012). These results partially mirror ours, and similarly stress that the persistence of these species in suboptimal habitat is critical to conservation management as it will determine their potential range expansion and connectivity of populations across degraded habitat. Similarly, Onderdonk and Chapman (2000) suggested that the ability of primates in Kibale forest, Uganda, to live in forest patches is related to their ability to persist in forest edges. For colobines, in particular, this is likely related to their dietary preference for secondary growth that includes climbers, matching our findings and interpretation.
Conclusions and Conservation Recommendations
Udzungwa arboreal primates appear generally dependent on lowland and medium elevation forests that have undergone a long and complex history of human disturbance. The surprisingly similar model results among the three target species are remarkable when considering their different feeding habits (especially between Tanzania Sykes’ monkeys and colobines), and allow for the development of generalized conservation recommendations for these primates. The target species are resilient to moderate forest disturbance (and/or adapted to naturally heterogeneous forest cover), as shown by studies on similar species (Chapman et al. 2000; Mammides et al. 2009; Plumptre and Johns 2001). However, in addition to the increased hunting in the insulated US forest, human pressure on these areas has escalated, particularly because the large, floodplain Kilombero Valley is currently under progressive saturation due to intensive agriculture. This is a pan-tropical phenomenon that is emerging as a top threat to biodiversity in the African tropical forest zone (Linder 2013). Mechanized rice and sugar cane production is intensifying in the Kilombero valley due to large investments (http://www.sagcot.com), which will inevitably drive the already elevated human density to further increase (National Bureau of Statistics 2013), hence rising the demand for firewood and timber.
In this scenario, the consequences on lowland forest habitat may soon become detrimental for primates. Therefore we recommend that environmental protection strategies be implemented in parallel with these developments. These include, on the one hand, capacity building and increased funding for the effective protection of the Forest Reserves and Nature Reserves by the Tanzania Forest Service, and on the other hand, maintenance and/or restoration of floodplain forest remnants and potential dispersal corridor fragments, such as Magombera, along with scaling-up the efforts to provide local communities with adequate alternatives to firewood collection through tree planting, agro-forestry, and energy-saving cooking technology.
References
Aiken, L. S., & West, S. G. (1991). Multiple regression: Testing and interpreting interactions. Newbury Park, CA: SAGE.
Araldi, A., Barelli, C., Hodges, K., & Rovero, F. (2014). Density estimation of the endangered Udzungwa red colobus (Procolobus gordonorum) and other arboreal primates in the Udzungwa Mountains using systematic distance sampling. International Journal of Primatology, 35, 941–956.
Bakayoko, A., Martin, P., Chatelain, C., Traore, D., & Gautier, L. (2011). Diversity, family dominance, life forms and ecological strategies of forest fragments compared to continuous forest in Southwestern Côte d'Ivoire. Candollea, 66, 255–262.
Barelli, C., Gallardo Palacios, J. F., & Rovero, F. (2014). Variation in primate abundance along an elevational gradient in the Udzungwa Mountains of Tanzania. In N. Grow, S. Gursky-Doyen, & A. Krzton (Eds.), High-altitude primates. Developments in primatology: Progress and prospects (pp. 211–226). New York: Springer Science+Business Media.
Bocian, C. M. (1997). Niche separation of black-and-white colobus monkeys (Colobus angolensis and C. guereza) in the Ituri Forest. Ph.D. dissertation, City University of New York.
Bolliger, J. (2005). Simulating complex landscapes with a generic model: Sensitivity to qualitative and quantitative classifications. Ecological Complexity, 2, 131–149.
Bracebridge, C. E., Davenport, T. R., & Marsden, S. J. (2012). The impact of forest disturbance on the seasonal foraging ecology of a critically endangered african primate. Biotropica, 44, 560–568.
Brook, B. W., Sodhi, N. S., & Bradshaw, C. J. A. (2008). Synergies among extinction drivers under global change. Trends in Ecology & Evolution, 23, 453–460.
Busch, D. S., & Hayward, L. S. (2009). Stress in a conservation context: A discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biological Conservation, 142, 2844–2853.
Butynski, T. M. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in high- and low-density subpopulations. Ecological Monographs, 60, 1–26.
Cardillo, M., Mace, G. M., Jones, K. E., Bielby, J., Bininda-Emonds, O. R. P., Sechrest, W., et al. (2005). Multiple causes of high extinction risk in large mammal species. Science, 309, 1239–1241.
Chajewski, M. (2009). rela: Scale item analysis. R package version 4.1.
Chapman, C. A., Balcomb, S. R., Gillespie, T. R., Skorupa, J. P., & Struhsaker, T. T. (2000). Long-term effects of logging on African primate communities: A 28-year comparison from Kibale National Park, Uganda. Conservation Biology, 14, 207–217.
Chapman, C. A., & Lambert, J. E. (2000). Habitat alteration and the conservation of African primates: Case study of Kibale National Park, Uganda. American Journal of Primatology, 50, 169–185.
Cowlishaw, G., & Dunbar, R. I. M. (2000). Primate conservation biology. Chicago: University of Chicago Press.
Davenport, T. R. B., Nowak, K., & Perkin, A. (2013). Priority primate areas in Tanzania. Oryx, 48, 39–51.
Dobson, A. J. (2002). An introduction to generalized linear models. Boca Raton, FL: Chapman & Hall/CRC.
Dunn, J. C., Asensio, N., Arroyo-Rodríguez, V., Schnitzer, S., & Cristóbal-Azkarate, J. (2012). The ranging costs of a fallback food: Liana consumption supplements diet but increases foraging effort in howler monkeys. Biotropica, 44, 705–714.
Fa, J. E., Ryan, S. F., & Bell, D. J. (2005). Hunting vulnerability, ecological characteristics and harvest rates of bushmeat species in afrotropical forests. Biological Conservation, 121, 167–176.
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34, 487–515.
Field, A. (2005). Discovering statistics using SPSS. London: SAGE.
Fimbel, C., Vedder, A., Dierenfeld, E., & Mulindahabi, F. (2001). An ecological basis for large group size in Colobus angolensis in the Nyungwe Forest, Rwanda. African Journal of Ecology, 39, 83–92.
Forstmeier, W., & Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behavioural Ecology and Sociobiology, 65, 47–55.
Fürtbauer, I., Mundry, R., Heistermann, M., Schülke, O., & Ostner, J. (2011). You mate, I mate: macaque females synchronize sex not cycles. PLoS ONE, e26144.
Gelman, A., & Hill, J. (2007). Data analysis using regression and multilevel/hierarchical models. Cambridge, U.K.: Cambridge University Press.
Gerwing, J. J., Schnitzer, S. A., Burnham, R. J., Bongers, F., Chave, J., DeWalt, S. J., et al. (2006). A standard protocol for liana censuses. Biotropica, 38, 256–261.
Hanya, G., & Bernard, H. (2012). Fallback foods of red leaf monkeys (Presbytis rubicunda) in Danum Valley, Borneo. International Journal of Primatology, 33, 322–337.
Hédl, R., Svátek, M., Dancak, M., Rodzay, A. W., Salleh, A. B. M., & Kamariah, A. S. (2009). A new technique for inventory of permanent plots in tropical forests: A case study from lowland dipterocarp forest in Kuala Belalong, Brunei Darussalam. Blumea, 54, 124–130.
Henle, K., Lindenmayer, D. B., Margules, C. R., Saunders, D. A., & Wissel, C. (2004). Species survival in fragmented landscapes: Where are we now? Biodiversity Conservation, 13, 1–8.
Johns, A. D., & Skorupa, J. P. (1987). Responses of rain-forest primates to habitat disturbance: A review. International Journal of Primatology, 8, 157–191.
Laurance, W. F., Useche, D. C., Rendeiro, J., Kalka, M., Bradshaw, C. J. A., et al. (2012). Averting biodiversity collapse in tropical forest protected areas. Nature, 489, 290–294.
Linder, J. M. (2013). African primate diversity threatened by “new wave” of industrial oil palm expansion. African Primates, 8, 25–38.
Lovett, J. C. (1993). Eastern Arc moist forest flora. In J. C. Lovett & S. K. Wasser (Eds.), Biogeography and ecology of the rain forests of Eastern Africa (pp. 33–55). New York: Cambridge University Press.
Lovett, J. C., Marshall, A. R., & Carr, J. (2006). Changes in tropical forest vegetation along an altitudinal gradient in the Udzungwa Mountains National Park, Tanzania. African Journal of Ecology, 44, 478–490.
Mammides, C., Cords, M., & Peters, M. K. (2009). Effects of habitat disturbance and food supply on population densities of three primate species in the Kakamega Forest, Kenya. African Journal of Ecology, 47, 87–96.
Marsh, L. K. (2003). Primates in fragments: Ecology and conservation. New York: Plenum Press.
Marsh, L. K., & Chapman, C. (2013). Primates in fragments. Complexity and resilience. Developments in primatology: Progress and prospects. New York: Springer Science+Business Media.
Marshall, A. R. (2007). Disturbance in the Udzungwas: responses of monkeys and trees to forest degradation. Ph.D. dissertation, University of York, Ann Arbor, MI.
Marshall, A. R. (2008). Ecological report on Magombera forest. Unpublished report to WWFTPO. Retrieved from http://www.easternarc.or.tz/MagomberaEcologicalReport2008.pdf
Marshall, A. R., Topp-Jørgensen, J. E., Brink, H., & Fanning, E. (2005). Monkey abundance and social structure in two high-elevation forest reserves in the Udzungwa Mountains of Tanzania. International Journal of Primatology, 26, 127–145.
Marshall, A. R., Jørgensbye, H. I. O., Rovero, F., Platts, P. J., White, P. C. L., & Lovett, J. C. (2010). The species-area relationship and confounding variables in a threatened monkey community. American Journal of Primatology, 72, 325–36.
McCullagh, P., & Nelder, J. A. (2008). Generalized linear models. London: Chapman and Hall.
McGregor, P. K. (1992). Quantifying responses to playback: One, many, or composite multivariate measures? In P. K. McGregor (Ed.), Playback and studies of animal communication (pp. 79–96). New York: Plenum Press.
National Bureau of Statistics. (2013). 2012 Population and housing census. Ministry of Finance, United Republic of Tanzania. Retrieved from http://www.ocgs.go.tz/sensa/PDF/Census%20General%20Report%20-%2029%20March%202013_Combined_Final%20for%20Printing.pdf
Nowak, K., & Lee, P. C. (2013). Status of Zanzibar red colobus and Sykes's monkeys in two coastal forests in 2005. Primate Conservation, 27, 65–73.
Onderdonk, D. A., & Chapman, C. A. (2000). Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology, 21, 587–611.
Platts, P. J., Burgess, N. D., Gereau, R. E., Lovett, J. C., Marshall, A. R., McClean, C. J., et al. (2011). Delimiting tropical mountain ecoregions for conservation. Environmental Conservation, 38, 312–324.
Plumptre, A. J., & Johns, A. G. (2001). Changes in primate communities following logging disturbance. In R. A. Fimbel, J. G. Robinson, & A. Grajal (Eds.), The cutting edge: Conserving wildlife in logged tropical forests (pp. 71–92). New York: Columbia University Press.
Quinn, G. P., & Keough, M. J. (2002). Experimental designs and data analysis for biologists. Cambridge, U.K.: Cambridge University Press.
R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rodgers, W. A., Homewood, K. M., & Hall, J. B. (1979). An ecological survey of Magombera forest reserve. Unpublished report, University of Dar es Salaam, Tanzania.
Rovero, F., Marshall, A. R., Jones, T., & Perkin, A. (2009). The primates of the Udzungwa Mountains: Diversity, ecology and conservation. Journal of Anthropological Sciences, 87, 93–126.
Rovero, F., Menegon, M., Fjeldså, J., Collett, L., Doggart, N., Leonard, C., Norton, G., Owen, N., Perkin, A., Spitale, D., Ahrends, A., & Burgess, N. D. (2014). Targeted vertebrate surveys enhance the faunal importance and improve explanatory models within the Eastern Arc Mountains of Kenya and Tanzania. Diversity and Distributions, 20, 1438–1449.
Rovero, F., Mtui, A., Kitegile, A. S., & Nielsen, M. R. (2012). Hunting or habitat degradation? Decline of primate populations in Udzungwa Mountains, Tanzania: An analysis of threats. Biological Conservation, 146, 89–96.
Rovero, F., & Struhsaker, T. T. (2007). Vegetative predictors of primate abundance: Utility and limitations of a fine-scale analysis. American Journal of Primatology, 69, 1242–1257.
Ryan, A. M., Chapman, C. A., & Rothman, J. M. (2013). How do differences in species and part consumption affect diet nutrient concentrations? A test with red colobus monkeys in Kibale National Park, Uganda. African Journal of Ecology, 51, 1–10.
Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution, 1, 103–113.
Schipper, J., Chanson, J. S., Chiozza, F., Cox, N. A., Hoffmann, M., et al. (2008). The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science, 322, 225–230.
Schnitzer, S. A., & Bongers, F. (2002). The ecology of lianas and their role in forests. Trends in Ecology and Evolution, 17, 223–230.
Schnitzer, S. A., & Bongers, F. (2011). Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecology Letters, 14, 397–406.
Schnitzer, S. A., & Carson, W. P. (2010). Lianas suppress tree regeneration and diversity in treefall gaps. Ecology Letters, 13, 849–857.
Shannon, C. (1948). A mathematical theory of communication. Bell System Technical Journal, 27, 379–423.
Siex, K. S., & Struhsaker, T. T. (1999). Colobus monkeys and coconuts: A study of perceived human–wildlife conflicts. Journal of Applied Ecology, 36, 1009–1020.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688.
Steel, R. I. (2012). The effects of habitat parameters on the behavior, ecology, and conservation of the Udzungwa red colobus monkey (Procolobus gordonorum). Ph.D. dissertation, Duke University.
Struhsaker, T. T. (1997). Ecology of an African rain forest: Logging in Kibale and the conflict between conservation and exploitation. Gainesville: University Press of Florida.
Struhsaker, T. T. (2010). The red colobus monkeys: Variation in demography, behavior, and ecology of endangered species. Oxford: Oxford University Press.
Vogel, E. R., Haag, L., Mitra-Setia, T., van Schaik, C. P., & Dominy, N. J. (2009). Foraging and ranging behavior during a fallback episode: Hylobates albibarbis and Pongo pygmaeus wurmbii compared. American Journal of Physical Anthropology, 140, 716–726.
Wong, S. N. P., Saj, T. L., & Sicotte, P. (2006). Comparison of habitat quality and diet of Colobus vellerosus in forest fragments in Ghana. Primates, 47, 365–373.
Zar, J. H. (1999). Biostatistical analysis. Upper Saddle River, NJ: Prentice Hall.
Acknowledgments
We thank the Tanzania Commission for Science and Technology (COSTECH), Tanzania Wildlife Research Institute (TAWIRI), and Tanzania National Parks (TANAPA) for granting us permission to conduct the study (Costech Permits No. 2011-85-NA-2011-33; 2011-84-NA-2011-33; 2011-351-NA-2011-68; 2011-346-NA-2011-183). We are also grateful to the warden and staff of Udzungwa Mountains National Park, the Tanzanian field assistants for their valuable assistance throughout the study, and to J. F. Gallardo Palacios for conducting and managing most of the field work. We also thank S. Tenan, three anonymous reviewers, and the editor-in-chief Joanna Setchell for helpful comments on previous versions of the manuscript. Financial support was provided by the Provincia Autonoma di Trento and the EU (Marie Curie Actions COFUND, postdoctoral grant to C. Barelli), Rufford Small Grants Foundation (1033-C to F. Rovero), Idea Wild (to A. Araldi), and the German Primate Centre (DPZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barelli, C., Mundry, R., Araldi, A. et al. Modeling Primate Abundance in Complex Landscapes: A Case Study From the Udzungwa Mountains of Tanzania. Int J Primatol 36, 209–226 (2015). https://doi.org/10.1007/s10764-015-9815-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-015-9815-7