Abstract
We assessed the spatial extent at which the species-landscape relationship is strongest (i.e. the scale of effect—SE) on primate occurrence (Alouatta belzebul, Saguinus midas, Saimiri sciureus, and Sapajus apella and Cebus olivaceus, the last two considered together in the analysis) and species richness and evaluated which landscape, patch, and human variables influence primate distribution in a savanna ecosystem in Brazil. We used nested buffers to measure the landscape attributes, and used these data to assess the SE of the species-landscape relationships. We explored the relative contributions of landscape, patch, and human variables to species richness and occurrences by using Generalized Linear Mixed Models and logistic regression. We found that the SE did not differ between primates, but did between two regions with different matrix composition. At the landscape level, occurrence of all species was higher as the distance to the nearest block of continuous forest decreased, but was lower as the amount of water bodies and anthropogenic cover in the matrix increased. The occurrence of S. apella, C. olivaceus and A. belzebul was positively related to forest cover, and all species but A. belzebul had higher occurrence in taller forest. The occurrence of S. apella, C. olivaceus and A. belzebul decreased closer to the city, and S. apella and C. olivaceus presence increased with the number of residents. Richness was negatively related to the number of residents and anthropogenic cover, but positively to forest height. We concluded that conservation planning for primates should follow a “functional landscape” perspective, by maintaining higher forest cover and minimizing the anthropogenic alterations in the matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest loss and the area occupied by human-modified landscapes are increasing rapidly around the world due to deforestation, wildfire, forestry and agriculture (Melo et al. 2013; Curtis et al. 2018). This has resulted in increasingly fragmented landscapes, where forest remnants are reduced and disconnected (Arroyo-Rodríguez and Fahrig 2014), being surrounded by matrices of non-natural landscape components, such as agricultural fields, roads and human settlements (Anderson et al. 2007; Laurance et al. 2009; Tee et al. 2018). Understanding how such landscape components affect species within those fragments is crucial for the formulation of robust conservation plans (Fahrig and Merriam 1994; Arroyo-Rodríguez and Fahrig 2014). Furthermore, an understanding of both the spatial extent at which the species-landscape relationship is strongest (i.e. the scale of effect) (Arroyo-Rodríguez and Fahrig 2014) and how other features related to human presence (e.g. hunting) affect the species are necessary to build a more holistic knowledge of how animal communities are affected in human-modified landscapes (Cardillo et al. 2004; Urquiza-Haas et al. 2009).
Primates are among the most threatened animals globally, and currently many species inhabit human-modified landscapes (Estrada et al. 2017; Galán-Acedo et al. 2019a). They are sensitive to climate change and are threatened by land-use changes (Graham et al. 2016; Estrada et al. 2017; Calle-Rendón et al. 2018). Primates play important roles in sustaining ecological processes such as seed dispersal, and as such, in the maintenance of a high diversity of forest plants (Chapman et al. 2013; Andresen et al. 2018). Some species are important for human populations as food, pets and medicines, and for aspects related to myth, folklore, magic, and religion (Cormier 2006; Parathian and Maldonado 2010). From a landscape perspective (see Fahrig 2005), primate occurrence and richness are positively related to both higher forest cover and matrix permeability (Benchimol and Peres 2014; Carretero-Pinzón et al. 2017; Galán-Acedo et al. 2019b), and additional spatial components, such as distance to the nearest fragment negatively affect the ratio of adult females to adult males (Puig-Lagunes et al. 2016). Some patch characteristics such as fragment size and forest height positively affect both primate occurrence and richness (Michalski and Peres 2005; Arroyo-Rodríguez et al. 2008; Boyle and Smith 2010; Benchimol and Venticinque 2014; Gouveia et al. 2014; da Silva et al. 2015; Puig-Lagunes et al. 2016; Calle-Rendón et al. 2019), but others such as irregular fragment shape negatively affect primate occurrence and population structure (Arroyo-Rodríguez et al. 2008; Puig-Lagunes et al. 2016), as in such fragments edge effects drive environmental changes related to vegetation structure (Laurance et al. 1998, 2006) and composition (Liu et al. 2019), affecting the availability of food for primates. Indeed, although some species are negatively affected by these changes (e.g. Ateles paniscus), others may benefit from it (e.g. Alouatta macconnelli) (Lenz et al. 2014). Furthermore, while variables related to human presence, such as distance to the city and hunting pressure, have been shown to negatively affect primate occurrence (Arroyo-Rodríguez et al. 2008; Urquiza-Haas et al. 2009), the effects of human density on primate distributions are complex, and not yet well-understood.
A large part of the world’s human population lives within tropical savanna ecosystems (Scholes and Archer 1997). These ecosystems represent mosaics of forest patches occurring in a non-forested matrix (Furley 1999). Historical and continued human use of these areas has led to many highly modified ecosystems (Scholes and Archer 1997). The Savannas of Amapá, in the far north of Brazil, covers an area of approximately 10,000 km2, and is the least protected complex of Amazonian savannas, being currently highly threatened by the expansion of large-scale agriculture (Carvalho and Mustin 2017; Hilário et al. 2017; Mustin et al. 2017). Eight primate species are present in this ecosystem (Aotus infulatus, Saguinus midas, Saimiri sciureus, Pithecia pithecia, Cebus olivaceus, Sapajus apella, Alouatta macconnelli and Alouatta belzebul) and one of them (A. belzebul) is listed globally (Valença-Montenegro et al. 2019) and nationally (Valença-Montenegro et al. 2012) as threatened (Vulnerable). Amapá’s savannas are under-studied (Carvalho and Mustin 2017; Mustin et al. 2017), and few studies have previously related species richness and abundance of mammals—including primates—to conversion of savannas into anthropogenic environments (but see Coelho et al. 2014; Piña et al. 2019). The Savannas of Amapá are naturally patchy, containing patches of riparian forests, immersed in a matrix of savannas and flooded fields, and particularly in the south of the state, they are being increasingly replaced by anthropogenic cover. This spatial configuration presents an opportunity to study the effects of patch-level attributes, landscape composition and human activities on non-human primates, via a landscape perspective. Most of the knowledge about the effect of landscape composition on primates comes from studies in anthropogenically fragmented landscapes, and little is known about these processes in naturally patchy landscapes. Moreover, due to the cultural and ecological roles played by primates, using them as a model could help in the conservation of naturally patchy landscapes (Estrada et al. 2017; Galán-Acedo et al. 2019c), including the Savannas of Amapá.
Here, we address two key questions. Firstly, we seek to understand the scale of effect (SE) of landscape composition on primate occurrence and primate species richness in the Savannas of Amapá. The SE is “the spatial extent within which the landscape affects a population” (Arroyo-Rodriguez and Fahrig 2014), and as such, we will test if the amount of forest cover and matrix attributes affect primates over short or long distances. Due to accelerated land use change over large areas in the Savannas of Amapá, a better comprehension of the spatial extent over which such changes affect primates will allow us to identify whether land use change will actually impact populations (i.e. occurs within the SE radius), allowing for better species and habitat management plans to prevent population losses. Thus, we expect that (i) the SE will increase with increasing species’ home range size because species with larger home ranges interact with the environment over large spatial extents (Miguet et al. 2016; Galán-Acedo et al. 2018); and (ii) the SE in more disturbed areas will be lower than in less disturbed areas, because in disturbed areas, movements are disrupted due to alterations in the matrix and primates are forced to depend on resources from the focal patch (Galán-Acedo et al. 2018). Our second key question concerns the relative importance of landscape attributes, patch characteristics, and human factors in driving patterns of primate species richness and occurrence in the Savannas of Amapá. We predict that, in terms of landscape attributes, forest cover and savanna area will be positively related to primate occurrence and species richness, as forests represent habitat for feeding and reproduction, whereas savannas may be more suitable for primate dispersal than water bodies (e.g. rivers and flooded fields) and anthropogenic cover (e.g. urban areas, roads and agricultural fields) (Benchimol and Peres 2013; Carretero-Pinzón 2013; Garmendia et al. 2013; Carretero-Pinzón et al. 2017; Galán-Acedo et al. 2019a). We also expect that the distance to the nearest large block of continuous forest will be negatively related to primate occurrences and species richness, as such forested areas may act as sources (Lawes et al. 2000; Naranjo and Bodmer 2007). In terms of patch-level characteristics, we predict that both larger patches and taller forests will have higher occurrences and species richness of primates (Benchimol and Peres 2014; Gouveia et al. 2014; Calle-Rendón et al. 2019), as taller forests may allow vertical niche segregation between primates and both taller forests and larger forest areas may represent higher resource availability. We further predict that patches that are more irregularly shaped will have higher rates of occurrence of primates with a higher proportion of arthropods in their diets because such patches have more edge effect (Murcia 1995). Finally, in terms of human factors, since human population density is related to the loss of some biodiversity components (Thompson and Jones 1999; Cardillo et al. 2004; Urquiza-Haas et al. 2009), we predict that overall primate richness and rates of occurrence of larger primates will be lower in landscapes with higher numbers of residents, due to increased hunting and clearing of native vegetation (Laurance et al. 2002; Urquiza-Haas et al. 2009). As a second proxy for these types of disturbances, we also used distance to the capital city, predicting that patches closer to the city will be less species rich and have lower rates of occurrence of larger primates, as hunting pressure will be greater than in more isolated patches (Silvestre et al. 2020), and the patches are likely to be more highly disturbed (Michalski and Peres 2005).
Methods
Study area
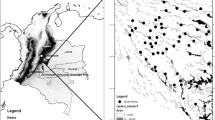
We conducted this study in three locations (Curiaú, Pedreira and BR-156) in a savanna ecosystem in the Brazilian state of Amapá, in the northeastern part of the Amazon (Fig. 1). The savanna complex in this region is often referred to as the “Cerrado of Amapá” or “Savannas of Amapá” (here after Savannas of Amapá—SOA). The climate in this region is wet and hot, the annual mean temperature is 26.5 °C and average annual precipitation is 2570 mm, with a dry season between August and November (Tavares 2014). The SOA are characterized by a mosaic of natural, open, grassy areas with sparse shrub and tree cover, and flooded fields, interspersed with patches of riparian forest and non-natural areas such as commercial plantations of maize, soybean and manioc (Hilário et al. 2017; Mustin et al. 2017). Currently, the SOA is inhabited by colonists and Quilombolas (the descendants of escaped African slaves, who have special land rights in Brazil). This region has been occupied for centuries, and as such, forest patches in this landscape are disturbed, in part as a result of timber extraction to build settlements (Lima 2003). However, one of the main drivers of land conversion in the SOA is to make way for agricultural plantations (Hilário et al. 2017; Mustin et al. 2017). The increase in area planted with soybeans in recent years was higher in the municipality of Macapá, where the Curiaú and Pedreira locations are situated. BR-156 is situated in the municipality of Santana, where the area planted with soybeans is lower compared with Macapá (Hilário et al. 2017). For that reason, we assumed that the anthropogenic disturbance is higher in Curiaú and Pedreira than in BR-156.
The three study locations (Pedreira, Curiaú and BR-156) in a savanna ecosystem in the state of Amapá (Brazil), in the northeastern part of the Amazon. Buffers with dotted lines represent the forest patches that were not included in the analyses to test the second prediction about the scale of effect, nor in the analysis of species richness
Study species
There are seven diurnal primate species that have part of their distributions in the SOA, but there are no published data on their specific ecology in the SOA. However, studies from other parts of their range show that both the weeper capuchin (Cebus olivaceus) and the brown capuchin (Sapajus apella) are omnivorous, groups present a home ranges between 270 and 320 ha (Zhang 1995; Miller 1996), and population densities range between 6 and 55 individuals/km2 (Freese and Oppenheimer 1981). The squirrel monkey (Saimiri sciureus) is mainly insectivorous-frugivorous, groups have a home range varying from 65 to 280 ha (Mittermeier and Roosmalen 1981; Lima and Ferrari 2003; Defler 2010), and population densities range between 16 and 528 individuals/km2 (Baldwin and Baldwin 1981). The red-handed tamarin (Saguinus midas) is the smallest primate in the SOA, is mainly insectivorous, group home range size is 31–42 ha (Mittermeier and Roosmalen 1981; Day and Elwood 1999), and population densities range between 2 and 30 individuals/km2 (Snowdon and Soini 1988). The Guiana red howler (Alouatta macconnelli) and the red-handed howler (Alouatta belzebul) are the largest primate species in the SOA, their home ranges vary between 6–45 and 9–18 ha, respectively (Bonvicino 1989; Pinto et al. 2003; Boubli et al. 2008). Howler monkeys are folivorous-frugivorous (Mittermeier and Roosmalen 1981; Julliot and Sabatier 1993; Pinto et al. 2003). Population density of these Alouatta species are unknown in their distributional range, however, A. seniculus (a close species to A. macconnelli, Cortés-Ortiz et al. 2003) is present in forest patches of a savanna ecosystem in the Orinoco basin in densities between 23 and 54 individuals/km2 (Defler 2010). Meanwhile, A. belzebul seems to be common in some areas of continuous forest from the Amazon and less common in the Atlantic Forest, where less than 500 individuals survive in some forest fragments, with no information about the species in savanna areas (Valença-Montenegro et al. 2019). The white-faced saki (Pithecia pithecia) is mainly a seed predator and its group home range size is the smallest among the species in the SOA, approximately 8–9 ha (Mittermeier and Roosmalen 1981; Oliveira et al. 1985). Population densities range between 1 and 13 individuals/km2 (Buchanan et al. 1981).
Primate survey
Across a total area of approximately 2300 km2, we selected 70 forest patches by using images from Google Earth Pro (version 7.3.2.5776). We produced maps of these forest patches, their access roads and any nearby buildings, which were then printed for use in the field. Between October 2017 and February 2018, we visited the buildings marked on the map to carry out interviews with local inhabitants and gather information about the occurrence of primate species. We were able to conduct 77 interviews that provided information on primate presence in 43 forest patches (13 patches in Pedreira, 17 in Curiaú and 13 in BR-156) across a total area of approximately 1700 km2. In some cases, interviews were not possible either because houses marked on the map were not inhabited, locals declined to participate in the interview, or the buildings marked on the map were not actually houses. During the interviews, we used the map as a reference to identify the forest patch of interest. We then showed participants photographs of the 10 primate species known to be present in the state of Amapá (Alouatta belzebul, Alouatta macconnelli, Ateles paniscus, Chiropotes sagulatus, Pithecia pithecia, Cebus olivaceus, Sapajus apella, Saimiri sciureus, Aotus infulatus, and Saguinus midas) (Silva et al. 2013) and one Neotropical primate species not present in the study region (Callithrix jacchus from the Atlantic Forest and Caatinga), included as a control (Bezerra et al. 2018). Participants were asked to identify which species they had seen in that forest patch.
Interviews with locals have been commonly used to gather information about occurrence of several mammal species, including primates, and are especially useful in large areas (Michalski and Peres 2005; Urquiza-Haas et al. 2009; Martínez-Marti et al. 2016; Camino et al. 2020). Moreover, interviews and methods based on local knowledge (e.g. locally-based surveys) have a higher detection probability than standard methods such as transects and camera traps and represent a useful and cost-effective approach (Camino et al. 2020). However, to validate the data on primate presence and absence obtained in the interviews, we performed playback sessions in at least 30% of forest patches in each study location (9 in Curiaú, 9 in Pedreira and 4 in BR-156) between July and December 2018.
We established transects of 800 m in length in each forest patch using Google Earth Pro, standardizing the sampling effort according to the patch area: area < 25 ha (2 transects), 25 < area < 50 ha (3 transects), 50 < area < 100 ha (4 transects) and area > 100 ha (5 transects). Five playback stations were established along each transect at intervals of 200 m. We broadcast a playback session from each station, consisting of a sequence of vocalizations of seven diurnal primates in the following order: A. belzebul, S. midas, P. pithecia, A. macconnelli, S. sciureus, S. apella and C. olivaceus. Vocalizations of each species were broadcast for 3 min, except Alouatta genus, which was broadcast for 7 min because the vocalizations of these species are longer than those of other species (Drubbel and Gautier 1993). Playbacks of vocalizations were followed by a 7-min interval without playback which was used to listen for any response (except Alouatta genus, where the interval was 8 min). Playback sessions began at 07:00 h, 09:00 h, 10:30 h, 15:10 h, and 16:40 h. We broadcast vocalizations of Alouatta only at the first and the last playback station of each transect (07:00 h and 16:40 h), because they generally vocalize at sunrise and sunset (Drubbel and Gautier 1993; Oliveira and Ades 2004). Between playback sessions, the distance between stations was walked slowly (40 min), either following our own transect in a Global Positioning System (Garmin eTrex 20) or using human and cattle paths, to detect any primate occurrences and to check the forest floor for primate fecal matter. In eight of the 43 forest patches it was not possible to establish transects of 800 m in length due to patch size and shape, and in these cases we performed just two or three playback sessions per day (i.e. transects of 200 m and 600 m in length respectively).
The data from the playback sessions was only used to “correct” the interview data in two cases: (1) where species identified as absent in the interviews were recorded as present using playback (N = 1); and (2) where species presence or absence was recorded as unknown during the interview (because the interviewee was unsure), in which case presence was considered to be established by the playback data (N = 2). Three opportunistic confirmations of presence (made outside of playbacks) were used to evaluate the veracity of the interview data. Specifically, two presences of A. belzebul (a vocalization from Curiaú and a sighting from Pedreira) and one of S. sciureus (a sighting in Curiaú). Finally, as participants frequently reported both C. olivaceus and S. apella to be present in patches in which only one was recorded during playback, records of the presence of both species were joined. As such, absences of C. olivaceus and S. apella were only recorded in those patches in which participants reported both species as absent, and where neither species was recorded during playback.
The vocalizations used were obtained from Emmons et al. (1997). In the case of A. macconnelli, the vocalization used was of A. seniculus, as until recently these taxa were considered to be a single species (Boubli et al. 2008). The vocalizations were edited to standardize the total playback time, and also to reduce the background noise not related to the primate vocalizations. Each vocalization was saved as an MP3 file and broadcast using a Max Print 601205-3 speaker (frequency range: 50 to 20,000 Hz, output power: 100 RMS). Before carrying out the playback sessions, we tested the speaker and established that the maximum distance at which we could still hear the vocalizations was approximately 100 m. The same person carried out all of the playback sessions to avoid biases in species detection.
Landscape composition
We adopted a patch-landscape approach (Arroyo-Rodríguez and Fahrig 2014) in which response variables are measured in a focal patch, and landscape attributes are measured within a specific radius (buffer) from the focal patch. For each of the 43 forest patches we generated 10 nested buffers using QGIS (Version 2.14.9-Essen). The smallest landscape size was a buffer of 300 m radius (28 ha landscape), and the largest landscape size was a buffer of 1200 m radius (452 ha landscape), as larger radii would have led to overlapping landscapes around the different forest patches. The smallest landscape created is larger than the home range size reported for at least two primate species present in the study site (P. pithecia and A. belzebul), and the largest landscape is larger than the home range reported for the species with the largest home ranges in the study site (C. olivaceus and S. apella). We nested eight additional buffers between the smallest and largest buffers, at intervals of 100 m: 400 m (50.3 ha landscape), 500 m (78.5 ha), 600 m (113.1 ha), 700 m (153.9 ha), 800 m (201.1 ha), 900 m (254.5 ha), 1000 m (314.2 ha) and 1100 m (380.1 ha).
We carried out a supervised classification for each study location using Landsat 8 OLI images from 2017 and 2018 at a 30 m spatial resolution, retrieved from the U.S. Geological Survey (https://earthexplorer.usgs.gov/). We used the Semi-Automatic Classification Plugin (Version 5.4.2) in QGIS (Version 2.14.9-Essen), combining bands 2 to 8. We separated the area into four general attributes of landscape composition: (1) forest cover (FC), which included forest environments and palm corridors; (2) savanna (Sav), including some cleared areas used for cattle ranching which were not distinguishable from savannas areas; (3) water bodies (WB), including rivers, lakes and flooded fields; and (4) anthropogenic cover (AC), including urban areas, roads, open areas (e.g. bare ground) and agricultural fields (mainly soybeans, maize and manioc plantations). We used a total of 2122 points based on field observations and from Google Earth Pro images to make the classification in the three regions, and used between 28 and 38% of those points to validate the classification. Overall, classification accuracy was between 89 and 98%.
We calculated the distance between each forest patch and the nearest block of forest considered as a possible source of species (DF). To do so, we first identified in Google Earth the blocks of continuous forest that could be sources of individuals, based on the information on presence of primates gathered in the interviews with locals. We then drew a polygon of each block, exported the polygon into QGIS, and calculated the Euclidean distance between each forest patch and the nearest block of forest. Although DF is not a landscape attribute according to the landscape perspective, it was included as landscape predictor since it is representing a measure of the space in the landscape.
Patch characteristics
We imported the forest patches delineated in Google Earth Pro into QGIS and calculated the patch area (PA). We then calculated a shape index (SI) as:
where p and PA are the perimeter and patch area, respectively, in meters (Carretero-Pinzón et al. 2017). Higher values of SI represent forest patches that are more irregular and a value of 2 represents a forest patch that is perfectly circular. We calculated forest height in each playback station by using the difference between X-band and P-band from Synthetic-Aperture Radar (SAR) images of 2.5 m spatial resolution, obtained from the Secretaria de Estado do Meio Ambiente (State’s Secretariat of the Environment) of Amapá. We then used the average among points to calculate the forest height (FH) of each patch.
Human factors
We used a shapefile based on data from the 2010 census, obtained from the Instituto Brasileiro de Geografia e Estatística (Brazilian Institute of Geography and Statistics) (IBGE 2019). We used the nested buffers from the landscape evaluation to extract the values of number of residents (NR) in each buffer. Additionally, we calculated the distance by road of each forest patch to Macapá (DC), the most populated city and capital of the state of Amapá, by delineating in Google Earth the roads using the ‘path tool’. DC was used as a proxy of hunting frequency (Silvestre et al. 2020).
Statistical analysis
All analyses were performed in R (version 3.5.1) (R Core Team 2018). We calculated the Cohen’s kappa coefficient to measure the concordance of presence-absence data between interviews and playbacks, by using the ‘irr’ package (Gamer et al. 2012). Concordance was high for A. belzebul (Kappa = 0.83) and P. pithecia (Kappa = 1), and moderate for S. midas (Kappa = 0.529). Although concordance was low for S. sciureus (Kappa = 0.188), and C. olivaceus and S. apella (Kappa = 0.253), there were no false negatives for these species (i.e. an interview participant reporting an absence when a playback reported a presence) (Table S1). Indeed, there was only one false negative (for S. midas). As such, we are confident in the reliability of the interviews to inform primate occurrence in the study area, because such differences between interviews and playback likely reflect low detectability of those species using playback, rather than a low quality of information provided by the interview participants. Once a species is detected, its presence is assured, while it may take a number of surveys without detection to be sure about the absence of a species. Therefore, it is more common to have false absences (i.e. imperfect detection or false negatives) than false presences (i.e. false positives). Considering this, we think that playback sessions may have missed some species, but these species may have been detected by locals, as they have spent far more time within the forest patches. Moreover, no participant reported the presence of the species used as a control. While species richness was modeled using all reported species, we only used the data of A. belzebul, S. midas, S. sciureus, and C. olivaceus and S. apella (together) to model occurrence, as A. macconnelli and P. pithecia were only present in one and six forest patches, respectively.
We used an ANOVA to test for significant differences in landscape attributes (FC, Sav, WB and AC) among regions using the amount calculated in the largest radius (1200 m). Following Jackson and Fahrig (2012), we identified the spatial extent that maximized the strength of the relationship (i.e. the scale of effect) between primate richness and occurrence and landscape attributes and only one human factor measured using a landscape perspective (NR). We used pairwise Generalized Linear Models (GLM) to relate primate occurrence (binomial distribution) and species richness (Poisson distribution) to each attribute of landscape composition (FC, WB, Sav and AC) and NR (transformed as ln[1 + NR]) in a specific radius. The scale of effect was considered to be the radius of the buffer for which the AIC (Akaike’s information criterion) value of the model was lowest (Jackson and Fahrig 2015).
To answer our first question about the scale of effect, we calculated the ΔAIC for each model by computing the difference between the AIC of each model and the AIC of the most supported model (i.e. the model with the lowest AIC). We considered that models with ΔAIC < 2 could have the same empirical support, and where all models had ΔAIC < 2, we inferred that there was no definable scale of effect (Gestich et al. 2018). Then we plotted the radius of each landscape against the ΔAIC of forest cover, considering the occurrence models of all species (prediction 1), and against the ΔAIC of forest cover considering models of species richness (prediction 2). In this second prediction, pairwise GLMs were implemented for each location separately (BR-156, Pedreira and Curiaú). For these models, we selected only 11 forest patches per location to increase the spatial separation between Pedreira and Curiaú, as these two locations were 3.8 km apart from each other, thus we excluded four landscapes at the northern limit of Curiaú. We also excluded an additional six patches for which the presence of some species was unknown (i.e. the interviewee could not confirm the presence of the species) (Fig. 1). After this process, the distance between the closest forest patches of Curiaú and Pedreira was 11 km.
To answer our second question, we used a multimodel inference approach to assess the relative effect of each predictor on each response variable (Burnham and Anderson 2002). For species occurrence data we fitted logistic regression models using Firth Logistic Regression from the ‘logistf’ package (Heinze et al. 2018), as data exploration revealed problems of perfect separation (Heinze and Schemper 2002). Then we assessed the effect of the attributes of landscape composition (FC, WB, Sav, and AC measured at the scale of effect, and DF), patch characteristics (FH, ln[PA], and SI) and human factors (ln[1 + NR] measured at the scale of effect, and DC) on occurrence of each species through full models. As we detected differences in the SE among locations (see results concerning our second prediction in “Results” section), we used Generalized Linear Mixed Models (GLMM) to assess the effect of landscape composition (FC, WB, Sav, and ln[1 + AC], and DF), patch characteristics (FH, ln[PA], and SI) and human factors (ln[1 + NR] and DC) on species richness. We implemented the GLMM using the ‘lme4’ package (Bates et al. 2018) with the Laplace approximation (Bolker et al. 2009), and each location (Curiaú, Pedreira and BR-156) as a random factor. The SE used in this GLMM were those detected in each location in the evaluation of the second prediction related to our first question. Additionally, we assessed the effect of the random factor (i.e. the effect of the location) plus fixed factors (i.e. landscape attributes, patch characteristics and human factors) on species richness using the conditional R2 (Nakagawa and Schielzeth 2013). We used the ‘MuMIn’ package (Bartoń 2018) to obtain a set of models for the occurrence of each species and species richness and ranked them according to the AICc, considering only those models with ΔAICc < 2. Variance inflation factors (VIF) were calculated for each model using the package ‘car’ (Fox et al. 2018). If a VIF > 3 was detected, then we ran a new analysis with the ‘subset’ argument to exclude models with collinear variables. The performance of each logistic regression from the set of models was assessed with the area under the ROC curve (AUC) using the ‘pROC’ package (Robin et al. 2019). We checked for spatial autocorrelation in the residuals of all models with ΔAICc < 2 through Moran’s I correlograms in the package ‘ncf’ (Bjørnstad and Cai 2018). We obtained the Akaike weights (wi) of each model in the set of models and hence, the relative importance of each predictor variable (i.e. the sum of the weights: ∑wi). We used a threshold of ∑wi = 0.4 to decide whether a predictor was important or not (Burnham 2015). Additionally, we calculated the average relative importance of each group of variables (landscape, patch and human) for the occurrence of each species, and for species richness (Carretero-Pinzón et al. 2017).
Results
Difference in landscape composition between locations
We found that anthropogenic, forest and savanna cover were significantly different between the three locations (ANOVA: p = 0.008, p = 0.02 and p = 0.03, respectively), however the post hoc analysis showed that the differences were only significant between BR-156 and Curiaú (Fig. S1). Anthropogenic cover was higher in Curiaú (mean 86.3 ± 80 SD) and lower in Pedreira (mean 44.7 ± 37.9 SD) and BR-156 (mean 18.5 ± 17.3 SD) (Fig. S1). The average forest cover in BR-156 was approximately 30% higher than forest cover in both Curiaú and Pedreira (Fig. S1). Savanna cover from BR-156 was 34% and 40% higher than in Pedreira and Curiaú, respectively (Fig. S1). Patch size did not differ significantly between localities, and the mean area was 38 ± 32.3 ha, 46.6 ± 51.6 ha and 57.5 ± 63.4 ha in BR-156, Curiaú and Pedreira, respectively.
Primate surveys
Alouatta belzebul was recorded in 48% of the patches, and C. olivaceus and S. apella in 57%, and were present mainly in Pedreira and BR-156. Saguinus midas was recorded in 64% of the patches and was present mainly in BR-156 and Curiaú. Saimiri sciureus was present in 61% of patches and occupied the three locations in similar proportions. Pithecia pithecia and A. macconnelli were present only in the BR-156 and occupied six and one patch respectively. Species richness ranged between zero and five species per patch. The location BR-156 had the highest mean species richness (mean 3.9 ± 1.5 SD) followed by Pedreira and Curiaú (mean 2.3 ± 1.2 SD and mean 1.8 ± 1.4 SD, respectively).
Scale of effect of landscape composition on primate occurrences and species richness
We found that the scale of effect (SE) varied widely across the buffer radius (Fig. 2). There was no clear positive relationship between home range size and the SE (first prediction). The SE for occurrence of A. belzebul (species with smallest home range in our analysis) varied from 500 to 800 m, while the SE for C. olivaceus and S. apella (species with the largest home range) varied from 500 to 1100 m. For S. sciureus, the SE was plausible at distances between 300 and 1100 m and there was no definable SE for S. midas (Fig. 2a). Finally, the SE of forest cover on species richness in the most disturbed location (Curiaú, between 800 and 1200 m) was higher than the SE of the least disturbed location (BR-156, between 300 and 500 m), which was contrary to our second prediction (Fig. 2b).
Association between landscape size (radius in a circular landscape) and difference in Akaike’s Information Criterion (ΔAIC) between forest cover and two response variables: a primate occurrence, and b species richness. ΔAIC was obtained from generalized linear models. The range of scale of effect is indicated with a gray horizontal line (Ab: A. belzebul; Cap: Capuchins (C. olivaceus and S. apella); Ss: S. sciureus; Sm: S. midas; Cur: Curiaú; BR: BR-156; and Ped: Pedreira). Dotted horizontal line indicate the limit in which models are considered to have equivalent support. Black symbols indicate ΔAIC ≥ 2
Relative importance of landscape attributes, patch characteristics and human factors on primate occurrence and species richness
Models showed that occurrence of all species but S. sciureus may be explained by landscape, patch and human variables (Table S2). For C. olivaceus and S. apella, A. belzebul, and S. midas all models in the set of models were significant and the AUC ranged between 0.90 and 0.98, but for S. sciureus models were not significant and AUC ranged between 0.76 and 0.77 (Table S2). Landscape attributes were more important than patch characteristics and human factors in explaining the occurrence of all species, however, human factors were more important than landscape attributes and patch characteristics for species richness (Fig. 3).
Average relative importance for each group of variables (landscape, patch and human) for four primate species and species richness in a Brazilian Amazonian savanna. For S. midas and A. belzebul only one variable related to human factors was present in the set of models, impeding the calculation of the average. Capuchins are C. olivaceus and S. apella
At the landscape level, both the probability of occurrence of all species and species richness were lower where landscapes had higher anthropogenic cover (∑wi = 1 for occurrence of all species and ∑wi = 48 for species richness—Fig. 4), and the probability of occurrence of all species was also lower where the matrix contained higher proportions of water bodies (∑wi = 1 for all species—Fig. 4). The probability of occurrence of A. belzebul (∑wi = 1) and S. midas (∑wi = 0.85) were lower where landscapes had larger areas of savanna (Fig. 4). Forest cover was positively related to the occurrence of S. sciureus (∑wi = 1), C. olivaceus and S. apella (∑wi = 0.68), and A. belzebul (∑wi = 1) (Fig. 4). The distance to the nearest block of forest was negatively related to the occurrence of all species (∑wi = 0.85 for S. midas, ∑wi = 1 for S. sciureus, ∑wi = 0.72 for C. olivaceus, S. apella, and A. belzebul—Fig. 4). At the patch level, forest height was important and positively related to species richness (∑wi = 0.48) and occurrence of S. midas (∑wi = 1), S. sciureus (∑wi = 0.79), and C. olivaceus and S. apella (∑wi = 0.63) (Fig. 4), but was negatively related to A. belzebul (∑wi = 0.71) (Fig. 4). Saimiri sciureus and A. belzebul had a higher probability of occurrence in more irregularly shaped patches (∑wi = 0.8 and ∑wi = 0.45, respectively—Fig. 4). In terms of human factors, distance from the city was important and positively related to the occurrence of S. sciureus, A. belzebul, and C. olivaceus and S. apella, but was negatively related to the occurrence of S. midas (∑wi = 1 for all species—Fig. 4). Species richness was lower (∑wi = 0.94) and the probability of occurrence of C. olivaceus and S. apella higher (∑wi = 0.4) in areas with more residents (Fig. 4).
Relative Importance of landscape attributes, patch characteristics and human factors for four primate species (capuchins are C. olivaceus and S. apella) and species richness in a Brazilian Amazonian savanna. Numbers within bars of landscape attributes and NR are the scale of effect. Note that for species richness the scale of effect depends on each locality (Ped: Pedreira; Cur: Curiaú; and BR: BR-156). Symbols indicate whether the relationship is positive (+) or negative (–). Predictors are anthropogenic cover (AC), water bodies (WB), forest cover (FC), savanna (Sav), distance to block of forest (DF), patch area (PA), forest height (FH), shape index (SI), distance to city (DC), and number of residents (NR). Vertical line is the threshold (∑wi = 0.4) above which the variable is considered to be an important correlate of primate occurrences and richness
Eleven models of species richness had empirical support (Table S3). Fixed factors explained the same variation as the fixed and random factors together (i.e. marginal R2 = conditional R2) in all models in the set of models (Table S3). In fact, values of the variance for the random intercept (\(\widehat{d}\)) were zero or close to zero (Table S3). This means, there is no effect of the locality in explaining species richness when considering landscape, patch and human variables together. However, when considering only one landscape attribute (e.g. anthropogenic cover or forest cover), the random factor (i.e. the locality) and the fixed factor (i.e. the landscape attribute) together explain a higher variation in species richness (i.e. conditional R2 > marginal R2), but these models had low empirical support.
Discussion
To the best of our knowledge, this study represents the first assessment of patterns of occurrence and species richness of Neotropical primates from a naturally patchy landscape to include human factors alongside landscape attributes and patch characteristics. Our key findings include that, while landscape variables, particularly matrix composition, are the most important correlates of occurrence of all species, the number of residents is the most important correlate of species richness. The probability of occurrence of the large-bodied primates (A. belzebul, and C. olivaceus and S. apella) also decreased with increasing proximity to the state capital, Macapá. Beyond this, we found that C. olivaceus and S. apella, A. belzebul, and S. midas were less frequent in patches in Curiaú, the most disturbed region, than in the other two regions, and that P. pithecia and A. macconnelli were restricted to BR-156, the least disturbed region, where overall species richness was also higher. Taken together, these results seem to indicate that anthropogenic use and disturbance are influencing the primate community in the Savannas of Amapá. Indeed, the proportion of anthropogenic cover (urban areas, roads, bare ground and agricultural fields) in the landscape was found to be an important predictor, and negatively related to species richness and the probability of occurrence of all primate species. Given the precipitous increase in anthropogenic cover in the region in recent years, and the likely continuing trend towards the expansion of large-scale agriculture (Hilário et al. 2017; Mustin et al. 2017), our results have important implications for the conservation of Neotropical primates in the Savannas of Amapá.
Scale of effect
The only factor that influenced SE was the location, which is likely due to variation in disturbance levels, with SE of forest cover being higher in Curiaú (more disturbed) than in BR-165 (least disturbed). This result may indicate that in Curiaú primates use habitat further away from the focal patch and that as such, they need to travel larger distances than in the less disturbed regions. This result is consistent with another study regarding Neotropical primates where the SE was larger in the most disturbed region and where animal movements among fragments seemingly follow metapopulation dynamics (Galán-Acedo et al. 2018). From an ecological perspective, these results suggest that the primate community in the SOA may be modulated by a metacommunity dynamic, such that a set of local communities are interacting through dispersal among patches (Livingston et al. 2013). At the species level (i.e. metapopulation dynamics), similar processes have been suggested to occur for Alouatta palliata in a highly fragmented landscape in Mexico (Galán-Acedo et al. 2018), as many individuals were recorded switching between fragments to obtain resources (Anzures-Dadda and Manson 2007; Galán-Acedo et al. 2018). In another savanna ecosystem from Colombia, four primate species have been recorded using live fences to move between patches (2010), and indeed this could also be occurring in the SOA. Given these results, conservation planning for primates in the SOA must take into account locality when considering forest cover, as the SE of forest cover varied between localities. However, when other variables are included, differences in SE between localities disappear, suggesting that conservation planning does not need to be carried out in separate regional units, unless strong changes occur in the landscape. That being said, the smaller proportion of anthropogenic cover in BR-156 compared with Pedreira and Curiaú might call for different conservation priorities between the localities. Specifically, avoiding the conversion of savannas into agricultural fields, such as has been anticipated in the Zoneamento Socioambiental do Cerrado do Amapá (Socioenvironmental Zoning of the Savannas of Amapá) (Hilário et al. 2017), is urgent to maintain primate species richness in the BR-156. While this is also crucial in the more highly disturbed location, there the primate community could also benefit from further conservation actions such as increasing connectivity using live fences. In addition, species home range size had no effect on the SE. This may reflect the importance of other spatio-temporal processes, such as migration and source-sink dynamics, in determining species occurrences (Jackson and Fahrig 2015; Galán-Acedo et al. 2018), as has previously been suggested for Atelids in Mexico (Galán-Acedo et al. 2018).
Influence of landscape scale processes on primates
Anthropogenic cover was the only important landscape correlate of the probability of occurrence of all primate species, as well as overall species richness. Roads, agricultural fields and human settlements denote hostile, less permeable and low quality matrices that act to increase isolation of primates in the forest patches (Michalski and Peres 2005). However, according to our results, the effects of anthropogenic cover operate mostly over short distances (scale of effect up to 400 m). This may indicate that primate dispersal might be affected not only by the anthropogenic cover in and of itself (i.e. roads, agriculture and human settlements), but also by other human–environment interactions that may be taking place, such as persecution from people and domestic animals (Michalski and Peres 2005), and degradation of forest vegetation (Lewis et al. 2015).
While anthropogenic cover was an important correlate for all species, forest cover was only important for the larger primates (A. belzebul, C. olivaceus and S. apella). In general, studies in fragmented landscapes have shown that forest cover is important not only for primates (Benchimol and Peres 2013; Carretero-Pinzón et al. 2017), but also for bats, carnivores, rodents, marsupials and forest specialist birds (Carrara et al. 2015; Arroyo-Rodríguez et al. 2016; Melo et al. 2017; Rabelo et al. 2019). This is consistent with the habitat amount hypothesis which posits that species richness (or occurrence) increases as the patch size and the natural habitat surrounding the patch increase (Fahrig 2013). Our study indicates that the same pattern is true for patchy non-fragmented environments, indicating that similar mechanisms are probably operating. This reinforces the significance of forests as reservoirs of biodiversity and highlights the importance of maintaining forest patches to support primate communities and the ecological services they provide (Chapman et al. 2013; Estrada et al. 2017; Andresen et al. 2018). Although deforestation is not a common process in the SOA, wildfires are common there (Mustin et al. 2017) and could reduce the extension and the quality (reducing forest height, tree diversity, increasing lianas and pioneer species) of the forest patches (Hoffman et al. 2003) with negative consequences for primates and other species.
At the landscape level, and contrary to our expectations, the probability of occurrence of two primate species declined with increasing savanna cover, which may suggest that the savannas are not very permeable for these species. Indeed, savannas generally seem to represent poor structural connectivity for mammals (Piña et al. 2019). Additionally, and consistent with our prediction, water bodies have a negative effect on the occurrence of all primate species in the SOA. Large seasonal lakes (mainly found in Curiaú) may reduce primate movements between forest patches because of the physical impediment, but also due to disturbances caused by fishing and rearing of African buffalo. Small dams are frequently made to provide water for cattle, and to be used for fish farming and recreation, which may also increase disturbance levels, acting as deterrents to primates.
Finally, the large block of forest that surrounds the SOA may act as source of individuals of all primate species as occurrence rate is higher as distance to the block decrease. The mainland-island model from metapopulation theory assumes that the mainland (the block of forest) is a source of individuals that migrate to habitat islands (the forest patches) (Hanski and Gyllenberg 1993). Patterns of occurrence of some mammal species have been consistent with this theory (Lawes et al. 2000). This has implications for the conservation of wild populations since such blocks of forest, when protected, can act as a source of species in the landscape (Naranjo and Bodmer 2007). The state of Amapá is the most protected in Brazil, and its protected areas generally encompass continuous forest. It is possible that immigration to forest patches is necessary to keep viable primate populations in the SOA and thereby, effort must be made to optimize landscape connection in order to keep the movements of fauna through riparian forest, palm corridors and forest patches.
Influence of patch scale processes on primates
For all but A. belzebul, probability of occurrence was higher in patches where the forest is taller, which is a reflection of higher habitat quality in these patches (Anzures-Dadda and Manson 2007; Gouveia et al. 2014; Carretero-Pinzon et al. 2017; Piña et al. 2019), and possibly of vertical stratification of the primate community (Peres 1993). The fact that A. belzebul is more likely to be found in patches where the forest is not so tall does not however mean that this species prefers low-quality habitat, but rather likely reflects the importance of other environmental variables (e.g. soils) in influencing forest characteristics related to high-quality habitat, such as fruit production. In the Colombian Llanos, occupancy of both Alouatta seniculus and Sapajus apella has been shown to decrease with increasing forest height, which it has been suggested reflects the importance of topography and forest composition, rather than canopy height, in determining habitat quality for these species (Carretero-Pinzon et al. 2017). Indeed, our results show that at the patch level, the probability of occurrence of A. belzebul is related to patch shape, increasing with irregularity of the patch. Alouatta belzebul has a relatively high proportion of leaves in its diet (Pinto et al. 2003), and irregular forest patches have a higher edge proportion, leading to edge effects that include modified plant species composition in the patch border (Liu et al. 2019), ultimately increasing the representation of plant species with higher nutrient content (Poorter and Bongers 2006), with obvious benefits to A. belzebul. These findings are consistent with another Amazonian areas where Alouatta prefers forest edges (in fragmented forests) and river borders (in continuous forest) instead of the forest interior (Peres 1997; Lenz et al. 2014). However, such results may depend on site context or even depend on species specificity, since another Alouatta species (A. palliata) did not present preference for either edge or interior environments in Costa Rica (Bolt et al. 2018; Johnson et al. 2020). Similarly, we found higher occurrence of S. sciureus, a species with a higher proportion of arthropods in its diet, in irregular forest patches. However, models of this species were not reliable, which may suggest that other factors not considered in this study, such as site level variables (e.g. number of trees with fruits, Carretero-Pinzón et al. 2017), are more important correlates of the occurrence of S. sciureus.
Influence of human processes on primates
The probability of occurrence of the largest bodied primates in the SOA increased with distance from the most populated city (Macapá). The occurrence of Alouatta genus has also been shown to be positively correlated with distance to the city in forest fragments in the southern Amazon (Michalski and Peres 2005). This pattern may reflect increased hunting pressure on large bodied mammals, which are often preferred by hunters (Jerozolimski and Peres 2003). In the SOA, hunting is a common activity closer to the city (Silvestre et al. 2020). Indeed, eight interviewees indicated that primates were among their target hunted species during the data collection for this study, with a focus on the larger species (A. belzebul 50%, A. macconnelli 12.5%, and C. olivaceus and S. apella 37.5%). Moreover, two interviewees told us they had eaten A. belzebul during the three weeks prior to the interview. In contrast to this pattern for the larger primates, and contrary to our prediction, we found that the probability of occurrence of S. midas—the smallest primate—increased with proximity to the city. This could reflect a pattern of reduced inter-specific competition as larger-bodied species are absent from these patches (likely due to hunting), meaning that smaller species can occupy the fragments (Peres and Dolman 2000), though this has not been assessed specifically in the SOA.
While the occurrence of most species is not related to the number of residents in the landscape, we found that overall species richness is lower where the number of residents is higher, and that the probability of occurrence of C. olivaceus and S. apella is actually higher in more densely populated areas. Human population density has previously been related to low probability of occurrence of mammals and low species richness (Parks and Harcourt 2002; Urquiza-Haas et al. 2009). The number of residents is a driver of biodiversity loss mainly at small scales, reducing species richness, and it is associated with other human activities such as road kills, persecution and low habitat quality (Urquiza-Haas et al. 2009). In terms of C. olivaceus and S. apella, their increased occurrence may reflect increased resource availability as areas with higher numbers of residents are associated with more shifting cultivation of manioc and small-scale fruit plantations, and Cebus and Sapajus can feed actively on such crops (Freitas et al. 2008; Spagnoletti et al. 2017).
Conservation implications and future directions
Amazonian savannas, including the SOA, are being cleared for large-scale agribusiness plantations at a fast pace, before conservationists and researchers are truly able to characterize their biodiversity and ecosystem processes, and as such, understanding their role for biodiversity conservation (Carvalho and Mustin 2017). As we found no difference in the scale of effect between primate species, conservation strategies for primates in this landscape can focus on the primate community as a whole, rather than on particular species. Given that landscape attributes are the most important correlates of primate occurrence in the SOA, conservation actions for primate populations should follow a “functional landscape” perspective by maintaining both higher forest cover and structural connectivity (see Melo et al. 2013). Possible strategies to increase the conservation value of forest fragments in the SOA for primates include protecting against the spread of large-scale agriculture and infrastructure projects, the planting of live fences to promote dispersal throughout the landscape, and reduction of disturbance associated with water bodies via fishing, pastoralism and recreational activities. These actions can, in theory, be achieved in collaboration with private landowners or through the establishment of protected areas. Conservation strategies for primates, and even other animals, outside of protected areas could include planning for their sustainable use through agreements of use with local communities, and enhancing connectivity through protection of native vegetation on private properties, including individual trees that may be used as stepping-stones in agricultural fields, and tree-lines or palm corridors that act as elements of structural connectivity between forest patches across crop fields and non-forest environments. The establishment of protected areas may be particularly important in landscapes such as the SOA, as forest patches are currently surrounded by extensive areas of natural environments (e.g. savannas), but anthropogenic cover is increasing quickly (Mustin et al. 2017). Planning and implementation of potential new protected areas must take into account both the biodiversity value of the SOA, and also their importance for the well-being, livelihoods and traditions of local communities, and the process through which such areas are planned must be open, transparent, participatory and respectful of local land and resource use rights and customary tenure.
References
Anderson J, Rowcliffe JM, Cowlishaw G (2007) Does the matrix matter? a forest primate in a complex agricultural landscape. Biol Conserv 135(2):212–222
Andresen E, Arroyo-Rodríguez V, Ramos-Robles M (2018) Primate seed dispersal: old and new challenges. Int J Primatol 39(3):443–465
Anzures-Dadda A, Manson RH (2007) Patch- and landscape-scale effects on howler monkey distribution and abundance in rainforest fragments. Anim Conserv 10(1):69–76
Arroyo-Rodríguez V, Fahrig L (2014) Why is a landscape perspective important in studies of primates? Am J Primatol 76(10):901–909
Arroyo-Rodríguez V, Mandujano S, Benítez-Malvido J (2008) Landscape attributes affecting patch occupancy by Howler Monkeys (Alouatta palliata mexicana) at Los Tuxtlas, Mexico. Am J Primatol 70(1):69–77
Arroyo-Rodríguez V, Rojas C, Saldaña-Vázquez RA, Stoner KE (2016) Landscape composition is more important than landscape configuration for Phyllostomid bat assemblages in a fragmented biodiversity hotspot. Biol Conserv 198:84–92
Baldwin JD, Baldwin JI (1981) The Squirrel Monkeys, genus Saimiri. In: Coimbra-Filho AF, Mittermeier RA (eds) Ecology and behavior of Neotropical primates. Academia Brasileira de Ciências, Rio de Janeiro, pp 277–330
Bartoń K (2018) MuMIn: multi-model inference. R package version 1.42.1. https://cran.r-project.org/web/packages/MuMIn/index.html. Accessed 24 January 2019
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B et al (2018) lme4: linear mixed-effects models using ‘eigen’ and S4. R package version 1.1–19. https://cran.r-project.org/web/packages/lme4/index.html. Accessed 21 January 2019
Benchimol M, Peres CA (2013) Anthropogenic modulators of species–area relationships in Neotropical primates: a continental-scale analysis of fragmented forest landscapes. Divers Distrib 19(11):1339–1352
Benchimol M, Peres CA (2014) Predicting primate local extinctions within “real-world” forest fragments: a pan-Neotropical analysis. Am J Primatol 76(3):289–302
Benchimol M, Venticinque EM (2014) Responses of primates to landscape change in Amazonian land-bridge islands–a multi-scale analysis. Biotropica 46(4):470–478
Bezerra B, Bicca-Marques J, Miranda J, Mittermeier RA, Oliveira L, Pereira D, Ruiz-Miranda C et al (2018) Callithrix jacchus. The IUCN Red List of Threatened Species 2018: e.T41518A17936001. https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T41518A17936001.en. Accessed 11 July 2019
Bjørnstad ON, Cai J (2018) ncf: spatial covariance functions. R package version 1.2–6. https://cran.r-project.org/web/packages/ncf/index.html. Accessed 26 Dec 2018
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24(3):127–135
Bolt LM, Schreier AL, Voss KA, Sheehan EA, Barrickman NL, Pryor NP, Barton MC (2018) The influence of anthropogenic edge effects on primate populations and their habitat in a fragmented rainforest in Costa Rica. Primates 59(3):301–311
Bonvicino CR (1989) Ecologia e comportamento de Alouatta belzebul (Primates: Cebidae) na Mata Atlântica. Rev Nordestina Biol 6(2):149–179
Boubli J-P, Di Fiore A, Mittermeier RA (2008) Alouatta macconnelli. The IUCN Red List of Threatened Species 2008: e.T40642A10347360. https://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T40642A10347360.en. Accessed 11 July 2019
Boyle SA, Smith AT (2010) Can landscape and species characteristics predict primate presence in forest fragments in the Brazilian Amazon? Biol Conserv 143(5):1134–1143
Buchanan DB, Mittermeier RA, van Roosmalen MGM (1981) The Saki Monkeys, genus Pithecia. In: Coimbra-Filho AF, Mittermeier RA (eds) Ecology and behavior of Neotropical primates. Academia Brasileira de Ciências, Rio de Janeiro, pp 391–417
Burnham KP (2015) Multimodel inference: understanding AIC relative variable importance values. Colorado State University. https://sites.warnercnr.colostate.edu/kenburnham/wp-content/uploads/sites/25/2016/08/VARIMP.pdf. Accessed 3 July 2019
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Calle-Rendón BR, Hilário RR, de Toledo JJ (2019) Effect of site attributes and matrix composition on Neotropical primate species richness and functional traits: a comparison among regions. Diversity 11(5):83
Calle-Rendón BR, Moreno F, Hilário RR (2018) Vulnerability of mammals to land-use changes in Colombia’s post-conflict era. Nat Conserv 29:79–92
Camino M, Thompson J, Andrade L, Cortez S, Matteucci SD, Altrichter M (2020) Using local ecological knowledge to improve large terrestrial mammal surveys, build local capacity and increase conservation opportunities. Biol Conserv 1:108450
Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM (2004) Human population density and extinction risk in the world’s carnivores. PLoS Biol 2(7):e197
Carrara E, Arroyo-Rodríguez V, Vega-Rivera JH, Schondube JE, de Freitas SM, Fahrig L (2015) Impact of landscape composition and configuration on forest specialist and generalist bird species in the fragmented Lacandona rainforest, Mexico. Biol Conserv 184:117–126
Carretero-Pinzón X (2010) Uso de cercas vivas como corredores biológicos por primates en los Llanos Orientales. In: Pereira-Bengoa V, Stevenson PR, Bueno ML, Nassar-Montoya F (eds) Primatología en Colombia: avances al principio del milenio. Fundación Universitaria San Martín, Bogotá, pp 91–97
Carretero-Pinzón X (2013) An eight-year life history of a primate community in the Colombian Llanos. In: Marsh LK, Chapman CA (eds) Primates in fragments: complexity and resilience. Springer, New York, pp 159–182
Carretero-Pinzón X, Defler TR, McAlpine CA, Rhodes JR (2017) The influence of landscape relative to site and patch variables on primate distributions in the Colombian Llanos. Landsc Ecol 32(4):883–896
Carvalho WD, Mustin K (2017) The highly threatened and little known Amazonian savannahs. Nat Ecol Evol 1:0100
Chapman CA, Bonnell TR, Gogarten JF, Lambert JE, Omeja PA, Twinomugisha D, Wasserman MD et al (2013) Are primates ecosystem engineers? Int J Primatol 34(1):1–14
Coelho M, Juen L, Mendes-Oliveira NA (2014) The role of remnants of Amazon savanna for the conservation of Neotropical mammal communities in eucalyptus plantations. Biodivers Conserv 23(13):3171–3184
Cormier L (2006) A preliminary review of Neotropical primates in the subsistence and symbolism of indigenous lowland South American peoples. Ecol Environ Anthropol 2(1):14–32
Cortés-Ortiz BE, Rico C, Rodríguez-Luna E, Sampaio I, Ruiz-García M (2003) Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol Phylogenet Evol 26(1):64–81
Curtis PG, Slay CM, Harris NL, Tyukavina A, Hansen MC (2018) Classifying drivers of global forest loss. Science 361(6407):1108–1111
da Silva LG, Ribeiro MC, Hasui E, da Costa CA, da Cunha RGT (2015) Patch size, functional isolation, visibility and matrix permeability influences. Neotropical primate occurrence within highly fragmented landscapes. PLoS ONE 10(2):e0114025
Day RT, Elwood RW (1999) Sleeping site selection by the Golden-handed Tamarin Saguinus midas midas: the role of predation risk, proximity to feeding sites, and territorial defence. Ethology 105(12):1035–1051
Defler TR (2010) Historia natural de los primates Colombianos. Universidad Nacional de Colombia and Conservación Internacional, Bogotá
Drubbel RV, Gautier J-P (1993) On the occurrence of nocturnal and diurnal loud calls, differing in structure and duration, in Red Howlers (Alouatta seniculus) of French Guyana. Folia Primatol 60:195–209
Emmons LH, Whitney BM, Ross DL (1997) Sounds of Neotropical rainforest mammals: an audio field guide. Library of natural sounds (CD), Cornell Laboratory of Ornithology.
Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, DiFiore A, Nekaris KAI et al (2017) Impending extinction crisis of the world’s primates: why primates matter. Sci Adv 3(1):e1600946
Fahrig L (2005) When is a landscape perspective important? In: Wiens JA, Moss MR (eds) Issues and perspectives in landscape ecology. Cambridge University Press, Cambridge, pp 3–10
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40(9):1649–1663
Fahrig L, Merriam G (1994) Conservation of fragmented populations. Conserv Biol 8(1):50–59
Fox J, Weisberg S, Price B, Adler D, Bates D, Baud-Bovy G, Bolker B et al (2018) car: Companion to Applied Regression. R package version 3.0-2. https://cran.r-project.org/web/packages/car/index.html. Accessed 24 Jan 2019
Freese CH, Oppenheimer JR (1981) The Capuchin Monkeys, genus Cebus. In: Coimbra-Filho AF, Mittermeier RA (eds) Ecology and behavior of Neotropical primates. Academia Brasileira de Ciências, Rio de Janeiro, pp 331–390
Freitas CH, Setz EZF, Araújo ARB, Gobbi N (2008) Agricultural crops in the diet of bearded Capuchin Monkeys, Cebus libidinosus Spix (Primates: Cebidae), in forest fragments in southeast Brazil. Rev Bras Zool 25(1):32–39
Furley PA (1999) The nature and diversity of Neotropical savanna vegetation with particular reference to the Brazilian cerrados. Global Ecol Biogeogr 8(3–4):223–241
Galán-Acedo C, Arroyo-Rodríguez V, Andresen E, Arregoitia LV, Vega E, Peres CA, Ewers RM (2019a) The conservation value of human-modified landscapes for the world’s primates. Nat Commun 10:152
Galán-Acedo C, Arroyo-Rodríguez V, Cudney-Valenzuela SJ, Fahrig L (2019b) A global assessment of primate responses to landscape structure. Biol Rev 94(5):1605–1618
Galán-Acedo C, Arroyo-Rodríguez V, Estrada A, Ramos-Fernández G (2018) Drivers of the spatial scale that best predict primate responses to landscape structure. Ecography 41(12):2027–2037
Galán-Acedo C, Arroyo-Rodríguez V, Estrada A, Ramos-Fernández G (2019c) Forest cover and matrix functionality drive the abundance and reproductive success of an endangered primate in two fragmented rainforests. Landsc Ecol 34(1):147–158
Gamer M, Lemon J, Fellows I, Singh P (2012) irr: various coefficients of interrater reliability and agreement. R package version 0.84. https://cran.r-project.org/web/packages/irr/index.html. Accessed 26 Dec 2018
Garmendia A, Arroyo-Rodríguez V, Estrada A, Naranjo EJ, Stoner KE (2013) Landscape and patch attributes impacting medium- and large-sized terrestrial mammals in a fragmented rain forest. J Trop Ecol 29(4):331–344
Gestich CC, Arroyo-Rodríguez V, Ribeiro MC, da Cunha RGT, Setz EZF (2018) Unraveling the scales of effect of landscape structure on primate species richness and density of Titi Monkeys (Callicebus nigrifrons). Ecol Res 34(1):150–159
Gouveia SF, Villalobos F, Dobrovolski R, Beltrão-Mendes R, Ferrari SF (2014) Forest structure drives global diversity of primates. J Anim Ecol 83(6):1523–1530
Graham TL, Matthews HD, Turner SE (2016) A global-scale evaluation of primate exposure and vulnerability to climate change. Int J Primatol 37(2):158–174
Hanzki I, Gyllenberg M (1993) Two general metapopulation models and the core-satellite species hypothesis. Am Nat 142(1):17–41
Heinze G, Schemper M (2002) A solution to the problem of separation in logistic regression. Stat Med 21(16):2409–2419
Heinze G, Ploner M, Dunkler D, Southworth H (2018) logistf: firth’s bias-reduced logistic regression. R package version 1.23. https://cran.r-project.org/web/packages/logistf/index.html. Accessed 19 July 2019
Hilário RR, de Toledo JJ, Mustin K, Castro IJ, Costa-Neto SV, Kauano EE, Eilers V et al (2017) The fate of an Amazonian savanna: government land-use planning endangers sustainable development in Amapá, the most protected Brazilian state. Trop Conserv Sci 10:1–8
Hoffmann WA, Orthen B, do Nascimento TKV (2003) Comparative fire ecology of tropical savanna and forest trees. Funct Ecol 17(6):720–726
IBGE (2019) Censo Demográfico 2010: IBGE. https://mapasinterativos.ibge.gov.br/grade/default.html. Accessed 15 June 2019
Jackson HB, Fahrig L (2012) What size is a biologically relevant landscape? Lands Ecol 27(7):929–941
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr 24(1):52–63
Jerozolimski A, Peres CA (2003) Bringing home the biggest bacon: a cross-site analysis of the structure of hunter-kill profiles in Neotropical forests. Biol Conserv 111(3):415–425
Johnson ET, Benítez ME, Fuentes A, McLean CR, Norford AB, Ordoñez JC, Beehner JC, Bergman TJ (2020) High density of white-faced capuchins (Cebus capucinus) and habitat quality in the Taboga Forest of Costa Rica. Am J Primatol 82(2):e23096
Julliot C, Sabatier D (1993) Diet of the Red Howler Monkey (Alouatta seniculus) in French Guiana. Int J Primatol 14(4):527–550
Laurance WF, Ferreira L, Rankin-de Merona J, Laurance SGW (1998) Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79(6):2032–2040
Laurance WF, Albernaz AKM, Schroth G, Fearnside PM, Bergen S, Venticinque EM, da Costa C (2002) Predictors of deforestation in the Brazilian Amazon. J Biogeogr 29(5–6):737–748
Laurance WF, Nascimento HEM, Laurance SG, Andrade AC, Fearnside PM, Ribeiro JEL (2006) Rain forest fragmentation and the proliferation of successional trees. Ecology 87(2):469–482
Laurance WF, Goosem M, Laurance SGW (2009) Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 24(12):659–669
Lawes MJ, Mealin PE, Piper SE (2000) Patch occupancy and potential metapopulation dynamics of three forest mammals in fragmented Afromontane forest in south Africa. Conserv Biol 14(4):1088–1098
Lenz BB, Jack KM, Spironello WR (2014) Edge effects in the primate community of the biological dynamics of forest fragments project, Amazonas, Brazil. Am J Phys Anthropol 155(3):436–446
Lewis SL, Edwards DP, Galbraith D (2015) Increasing human dominance of tropical forests. Science 349(6250):827–832
Lima EM, Ferrari SF (2003) Diet of a free-ranging group of Squirrel Monkeys (Saimiri sciureus) in eastern Brazilian Amazonia. Folia Primatol 74(3):150–158
Lima P (2003) Antropización, dinámicas de ocupación del territorio y desarrollo en la Amazonía brasileña: el caso del estado de Amapá. Dissertation, Universitat Autònoma de Barcelona
Liu J, Coomes DA, Hu G, Liu J, Yu J, Luo Y, Yu M (2019) Larger fragments have more late-successional species of woody plants than smaller fragments after 50 years of secondary succession. J Ecol 107(2):582–659
Livingston G, Philpott SM, Rodriguez AM (2013) Do species sorting and mass effects drive assembly in tropical agroecological landscape mosaics? Biotropica 45(1):10–17
Martínez-Martí C, Jiménez-Franco MV, Royle JA, Palazón JA, Calvo JF (2016) Integrating occurrence and detectability patterns based on interview data: a case study for threatened mammals in Equatorial Guinea. Sci Rep 6:33838
Melo FPL, Arroyo-Rodríguez V, Fahrig L, Martínez-Ramos M, Tabarelli M (2013) On the hope for biodiversity-friendly tropical landscapes. Trends Ecol Evol 28(8):462–468
Melo GL, Sponchiado J, Cáceres NC, Fahrig L (2017) Testing the habitat amount hypothesis for South American small mammals. Biol Conserv 209:304–314
Michalski F, Peres CA (2005) Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol Conserv 124(3):383–396
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landsc Ecol 31(6):1177–1194
Miller LE (1996) The behavioral ecology of wedge-capped capuchin monkeys (Cebus olivaceus). In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptive radiations of Neotropical primates. Springer, New York, pp 271–288
Mittermeier RA, Roosmalen MGM (1981) Preliminary observation on habitat utilization and diet in eight Surinam monkeys. Folia Primatol 36(1–2):1–39
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10(2):58–62
Mustin K, Carvalho WD, Hilário RR, Costa-Neto SV, Silva CR, Vasconcelos IM, Castro IJ et al (2017) Biodiversity, threats and conservation challenges in the Cerrado of Amapá, an Amazonian savanna. Nat Conserv 22:107–127
Nakagawa S, Schielzeth HA (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Method Ecol Evol 4(2):133–142
Naranjo EJ, Bodmer RE (2007) Source–sink systems and conservation of hunted ungulates in the Lacandon Forest. Mexico Biol Conserv 138(3–4):412–420
Oliveira DAG, Ades C (2004) Long-distance calls in Neotropical primates. An Acad Bras Ciênc 76(2):393–398
Oliveira JMS, Lima MG, Bonvincino C, Ayres JM, Fleagle JG (1985) Preliminary notes on the ecology and behavior of the Guianan Saki (Pithecia pithecia, Linnaeus 1766, Cebidae, Primate). Acta Amazonica 15(1–2):249–263
Parathian HE, Maldonado AM (2010) Human–nonhuman primate interactions amongst Tikuna people: perceptions and local initiatives for resource management in Amacayacu in the Colombian Amazon. Am J Primatol 72(10):855–865
Parks SA, Harcourt AH (2002) Reserve size, local human density, and mammalian extinctions in U.S. protected areas. Conserv Biol 16(3):800–808
Peres CA (1993) Structure and spatial organization of an Amazonian terra firme forest primate community. J Trop Ecol 9(3):259–276
Peres CA (1997) Effects of habitat quality and hunting pressure on arboreal folivore densities in Neotropical forests: a case study of howler monkeys (Alouatta spp.). Folia Primatol 68(3–5):199–222
Peres CA, Dolman PM (2000) Density compensation in Neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122(2):175–189
Pinto ACB, Azevedo-Ramos C, Carvalho O (2003) Activity patterns and diet of the howler monkey Alouatta belzebul in areas of logged and unlogged forest in Eastern Amazonia. Anim Biodiv Conserv 26(2):39–49
Piña TEM, Carvalho WD, Rosalino LMC, Hilário RR (2019) Drivers of mammal richness, diversity and occurrence in heterogeneous landscapes composed by plantation forests and natural environments. Forest Ecol Manag 449:117467
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87(7):1733–1743
Puig-Lagunes AA, Canales-Espinosa D, Rangel-Negrín A, Dias PAD (2016) The influence of spatial attributes on fragment occupancy and population structure in the Mexican Mantled Howler (Alouatta palliata mexicana). Int J Primatol 37(6):656–670
Rabelo RM, Aragón S, Bicca-Marques JC, Nelson BW (2019) Habitat amount hypothesis and passive sampling explain mammal species composition in Amazonian river islands. Biotropica 51(1):84–92
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/. Accessed 22 Oct 2018
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M et al (2019) pROC: Display and Analyze ROC Curves. R package version 1.15.0. https://cran.r-project.org/web/packages/pROC/index.html. Accessed 19 July 2019
Scholes RJ, Archer SR (1997) Tree-grass interactions in savannas. Annu Rev Ecol Syst 28:517–544
Silva CR, Martins ACM, Castro IJ, Bernard E, Cardoso EM, Lima DS, Gregorin R et al (2013) Mammals of Amapá state, eastern Brazilian Amazonia: a revised taxonomic list with comments on species distributions. Mammalia 77(4):409–424
Silvestre SM, Calle-Rendón BR, de Toledo JJ, Hilário RR (2020) Drivers of hunting in the savannas of Amapá: implications for conservation. Oryx. https://doi.org/10.1017/S0030605319000085
Snowdon CT, Soini P (1988) The Tamarins, genus Saguinus. In: Mittermeier RA, Rylands AB, Coimbra-Filho A, Fonseca GAB (eds) Ecology and behavior of neotropical primates. World Wildlife Fund, Washington, pp 223–298
Spagnoletti N, Cardoso TCM, Fragaszy D, Izar P (2017) Coexistence between humans and Capuchins (Sapajus libidinosus): comparing observational data with farmers’ perceptions of crop losses. Int J Primatol 38(2):243–262
Tavares JPN (2014) Características da climatologia de Macapá-AP. Caminhos Geogr 15(50):138–151
Tee SL, Samantha LD, Kamarudin N, Akbar Z, Lechner AM, Ashton-Butt A, Azhar A (2018) Urban forest fragmentation impoverishes native mammalian biodiversity in the tropics. Ecol Evol 8(24):12506–12521
Thompson K, Jones A (1999) Human population density and prediction of local plant extinction in Britain. Conserv Biol 13(1):185–189
Urquiza-Haas T, Peres CA, Dolman PM (2009) Regional scale effects of human density and forest disturbance on large-bodied vertebrates throughout the Yucatán Peninsula, Mexico. Biol Conserv 142(1):134–148
Valença-Montenegro MM, Carvalho A, Cortes-Ortíz L, Fialho M, Jerusalinsky L, Melo F, Mittermeier RA, Ravetta A, Régis T, Talebi M, Veiga LM (2019) Alouatta belzebul. The IUCN Red list of threatened species 2019: e.T39957A17925370. https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T39957A17925370.en. Accessed 19 March 2020
Valença-Montenegro MM, Fialho MS, Carvalho AS, Ravetta AL, Régis T, de Melo FR, Veiga LM (2012) Avaliação do risco de extinção de Alouatta belzebul (Linnaeus, 1766) no Brasil. Processo de avaliação do risco de extinção da fauna brasileira. ICMBio. https://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/lista-de-especies/7171-mamiferos-alouatta-belzebul-guariba-de-maos-ruivas.html. Accessed 11 July 2019
Zhang S-Y (1995) Activity and ranging patterns in relation to fruit utilization by Brown Capuchins (Cebus apella) in French Guiana. Int J Primatol 16(3):489–507
Acknowledgements
This study has been supported by the Conservation Leadership Programme (02327917), The Rufford Foundation (22322-1), Idea Wild (CALLBRAZ0916), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES: scholarships to RRH—process: 88881.314420/2019-01—and BRCR). The authors are extremely grateful to local people who agreed to participate in the study and provided the data used in this study. We also thank the local communities in the Savannas of Amapá for logistical support in the field. We thank an anonymous reviewer for the comments, which significantly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stephen Garnett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Calle-Rendón, B.R., de Toledo, J.J., Mustin, K. et al. Drivers of primate richness and occurrence in a naturally patchy landscape in the Brazilian Amazon. Biodivers Conserv 29, 3369–3391 (2020). https://doi.org/10.1007/s10531-020-02028-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-02028-z