Abstract
Context
Primates are an important component of biodiversity in tropical regions. However, many studies on the effects of habitat change on primates ignore the relative influence of landscape composition and configuration.
Objectives
This study addresses the question: how important are landscape-scale forest area and composition relative to patch-scale (1–1080 ha) and site-scale (transect of 1 km) habitat variables for the occupancy and abundance of four primate species in the Colombian Llanos.
Methods
Using a randomly stratified survey design, 81 fragments were surveyed for primate occupancy and abundance. We used zero-inflated models to test the relative influence of landscape-scale, patch-scale and site-scale variables on occupancy and abundance for each species. A 95% confidence set of models was constructed using the cumulative Akaike weight for each model and the relative importance of each set of variables calculated for each primate species.
Results
Occupancy was determined by a combination of site-scale, patch-scale and landscape-scale variables but this varied substantially among the primate species.
Conclusion
Our study highlights the importance of managing primates at a range of scales that considers the relative importance of site-, patch- and landscape-scale variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deforestation and forest fragmentation continues at an alarming rate in the tropics (FAO 2011; Hansen et al. 2013). Understanding how this drives the spatial distribution of wildlife populations is important for their conservation and management, especially in tropical areas where the greatest losses are occurring (Fahrig 2001; McAlpine et al. 2006; Fisher and Lindenmayer 2007; Elith and Leathwick 2009; Guisan et al. 2013; Arroyo-Rodriguez and Fahrig 2014). Species’ distributions are influenced not only by the characteristics of individual habitat patches, but also by the composition and spatial configuration of the surrounding landscape (McGarial and McComb 1995; Guisan et al. 2007; Elith and Leathwick 2009). Understanding the contribution of landscape-level processes to species’ distributions require a multi-scaled approach (Oja et al. 2005; McAlpine et al. 2006; Wiens 2009), but this has often been ignored in studies of primate species in the tropics (Arroyo-Rodriguez et al. 2013b; Arroyo-Rodriguez and Fahrig 2014). Quantifying the response of primate species to landscape variables relative to the more commonly studied site/patch level variables is therefore a key research priority.
Primates are an important component of biodiversity and ecosystem function in many tropical regions. However, they are under threat from habitat loss and fragmentation (Mittermier and Oates 1985; Rylands et al. 2008; Schipper et al. 2008). Nonetheless, most studies focus on the effects of site and patch-scale variables on primates and have ignored the influence of landscape composition and configuration (Harcourt and Doherty 2005; Arroyo-Rodriguez et al. 2013a; Benchimol and Peres 2013; Arroyo-Rodriguez and Fahrig 2014; Carretero-Pinzón et al. 2015). Only a few studies have included landscape-scale (100–1000 ha) variables to predict the occurrence of primate species and demographic change (Anzures-Dadda and Manson 2007; Escobedo-Morales and Mandujano 2007; Arroyo-Rodriguez et al. 2008, 2013b; Pyritz et al. 2010; Thornton et al. 2011). This is a critical limitation because species’ responses to landscape change are influenced by the scale at which this occurs, that change processes are ultimately multi-scaled in nature, and vary from species to species (Eigenbrod et al. 2008; Thornton et al. 2011; Martin and Fahrig 2012; Arroyo-Rodriguez et al. 2013b; Smith et al. 2013). For example, Thornton et al. (2011) and Arroyo-Rodriguez et al. (2013b) applied a multiscale approach to evaluate primate species’ responses to habitat loss and fragmentation. Thornton et al. (2011) found that habitat fragmentation strongly affected Geoffroy’s spider monkey (Ateles geoffroyi) in Guatemala, within a 500 m buffer distance from the forest fragment edge. On the other hand, Arroyo-Rodriguez et al. (2013b) found that populations of the black howler monkey (Alouatta pigra), in Mexico, were primarily affected by changes in patch-scale attributes rather than landscape-scale metrics in a 500 ha landscape. These conflicting results indicate the need for comparisons across multiple species in the same landscape to disentangle the responses of different species from variation among study sites (Carretero-Pinzón et al. 2015).
In Colombia, the main drivers of deforestation are human population growth and migration, infrastructure projects, palm oil plantations, agriculture and cattle ranching (Etter et al. 2006, 2008; Fedepalma 2014; Ecopetrol 2015). Orinoquia (an area of 388,101 km2 in size) is a region of Colombia with high rates of conversion of natural savannas and the degradation of gallery forest and lowland rain forest (Etter et al. 2008). This region is part of the Orinoco River catchment (Domínguez 1998), and is an important area for primate biodiversity. In Orinoquia, the main drivers of habitat loss and fragmentation are similar to those in the rest of Colombia and includes the cultivation of illegal crops (Armenteras et al. 2009, 2013; Castiblanco et al. 2013). The region supports from 2 to 10 primate species depending on the vegetation composition and structure, including the endemic dusky titi monkey (Callicebus ornatus), Brumback’s night monkey (Aotus brumbacki) and the Colombian squirrel monkey (Saimiri cassiquiarensis albigena) (Defler 2010). Therefore, this region provides an excellent opportunity to simultaneously understand the responses of multiple primate species to landscape and site/patch variables. Further, studies evaluating the effects of habitat loss and fragmentation on primates in Orinoquia are scarce and limited to density estimates of populations in forest fragments (Wagner et al. 2009; Carretero-Pinzón 2013a) and behavioral studies of species living in forest fragments (Zarate and Stevenson 2014). Better understanding of the influence of landscape change on the region’s diverse primate community is therefore critical for their conservation.
This study addressed the question: how important is landscape-scale forest area and configuration relative to patch-scale and site-scale habitat variables for the occupancy and abundance of four primate species in the Colombian Llanos. This question was addressed by using count data from four diurnal primate species in 81 fragments and zero-inflated generalised linear models. We found that the abundance and occupancy of the primate species studied are influenced by a combination of site, patch and landscape variables, with their relative importance varying with the species.
Methods
Study area
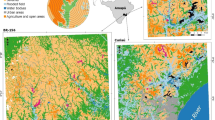
The study was conducted in the Los Llanos bioregion (sensu Lasso et al. 2010), near the town of San Martin in the Colombian Orinoquia (Fig. 1a). The Llanos is characterized by lowland alluvial terraces and plains, dissected by rivers originating in the Andes or in the upland savannahs and draining into the Orinoco River (Lasso et al. 2010). The vegetation is dominated by flooded and dryland savannas, gallery forest associated with drainage lines and lowland rainforest (Lasso et al. 2010). There are five primate species living sympatrically in the region: red howler monkey (Alouatta seniculus), dusky titi monkey (Callicebus ornatus), black-capped capuchin (Sapajus apella fatuellus), Colombian squirrel monkey (Saimiri cassiquiarensis albigena) and Brumback’s night monkey (Aotus brumbacki) (Carretero-Pinzón 2013a). We focus on the first four species because logistic and time constraints available did not make it possible to survey the nocturnal species (A. brumbackii) present in the study area.
Survey design
Site selection A randomly stratified survey design (Rogerson 2010) based on forest fragment size and the proportion of forest surrounding each patch at a 1000 m buffer distance were used to select potential sites for primate and vegetation surveys (Fig. 1b). The design was based on a land cover map derived from a mosaic of Landsat 7 ETM images from 2000 (www.earthexplorer.usgs.gov) at a 30 m spatial resolution using a supervised classification with ArcMap 10.1 (ESRI ArcGIS 10).
Four classes of land cover were identified (crops, forest, pastures and water). The classified map was then used to stratify each forest patch by size (3 classes: 1–50, 51–100 and 101–1000 ha) and the percentage of forest cover surrounding the fragments at a 1000 m buffer radius (3 classes: 0–33, 34–66 and >0.67%). The selection of the largest fragment size category (101–1000 ha) was chosen to be consistent with Marsh (2003). The minimum fragment size considered (1 ha) was based on the smallest fragment in which any of the primate species had been observed previously in the study area (Carretero-Pinzón Pers. Obs.). The buffer distance was based on an intermediate value within the range of the dispersal distances of the target primate species (200–3000 m; Crockett 1996, 1998; Arroyo-Rodriguez and Dias 2010; Defler 2010; Carretero-Pinzon, unpublished data). A combination of forest fragment size and the percentage of forest cover surrounding the fragments (9 classes, Table 1) were then used to randomly select 10 sites per class with sites widely distributed across the study region. Spatial autocorrelation among fragments was minimised by selecting fragments at least 1 km apart.
Landowners were contacted to obtain permission for data collection in the selected survey sites. Selected sites more than 60 km from the focal area of San Martin and which were near areas of conflict (i.e. guerrillas) were eliminated for logistical and security reasons. The final set of selected fragments was then evaluated in the field for minimum canopy height. Fragments with canopies less than 10 m in height were considered regenerating or regrowth forest and were not included in the study, as we focused on primary forest. All sites within “forest” fragments that were identified as palm oil plantations were eliminated and verified in the field by direct observation. All the pre-selected areas that were eliminated were replaced by fragments in similar categories. For consistency, these were located at least 1 km from fragments already sampled. A total of 81 fragments were surveyed within all the combinations of classes present (Online Appendix 1).
One transect was located in each fragment, except for the largest fragment (1080 ha) where three transects were surveyed. Transect direction was randomly chosen and where possible, transects were straight, although in fragments with irregular shapes, the direction varied according to the fragment form.
Primate surveys
Count data were collected by recording every primate group and individual of each group observed along a transect during each survey. Field data collection was conducted between August 2013 and May of 2014 (this period includes the wet and dry season for the study area). Counts were conducted from 0600 to 1100 h and again at 1330 to 1630 h on the same day, and repeated on consecutive days. Each transect was surveyed three to six times, with a minimum of three surveys per transect. Surveys were not conducted in heavy rain. Transects were walked at approximately 0.5 km/h and when a primate group was visually detected, a minimum of 15 min were taken to count the group members and determine group composition (number of males, females and immature). The time of detection and the coordinates of the location of each observation based on a GPS reading were also recorded. All observations and species identifications were aided by binoculars, and primate species’ classification followed Defler (2010), Ruiz-Garcia and Castillo (2016) and Mittermeier et al. (2013). Primate surveys were carried out by the first author.
Vegetation surveys
Vegetation surveys were conducted in four 10 × 50 m plots, located every 250 m along the length of each transect. In each plot, all trees with a diameter at breast height (DBH) >10 cm were recorded to species level. Species identifications were based on vegetative and reproductive material using the guide “Guia de frutos de La Macarena” (Stevenson et al. 1998) as well as expert identification by Francisco Castro (Botanist of Los Llanos University). For each tree, the DBH and the presence or absence of fruits, flowers and young leaves during survey time was recorded. Finally, the percentage of canopy cover and canopy height were recorded from one single point every 200 m along the transect. Presence of natural fence rows (defined as tree-lines used to separate adjoining pastures, Carretero-Pinzón et al. 2010) and the type of land use directly adjacent to the patch were recorded by direct observation for each fragment. The types of land use in the matrix adjacent to each forest fragment were categorised as pastures (including introduced pastures or natural savannahs) or plantations (crops and palm oil plantations, alone or combined with exotic pastures). Vegetation surveys were conducted by the first author with the assistance of Francisco Castro.
Variable selection
A range of ecologically relevant site-scale, patch-scale and landscape-scale variables were selected, based on a primate literature review (Table 1). Four site-scale variables were selected that were canopy cover, canopy height, number of food trees and number of trees with fruits. The variable, number of food trees, was based on each primate species’ diet (see Table 2 for sources of this information). Four patch-scale variables were also selected: patch size, patch shape index (a measure of complexity of the patch shape, see Table 1 for calculations), presence of natural fence rows and the type of matrix adjacent to the patch. The presence of natural fence rows and the type of matrix were considered in our analysis as patch variables because they were measured directly adjacent to the focal fragment, rather than being reflective of the broader landscape. The percentage of forest cover and patch density were selected as respective measures of landscape composition and configuration. Site-scale variables were measured based on the vegetation plot data aggregated along each transect. Patch-scale variables were measured for the whole patch in which a transect was located. Landscape-scale variables were measured at five buffer distances (500, 1000, 2500, 3000 and 3500 m) surrounding each forest fragment. The range of buffer sizes were chosen in relation to the range of recorded dispersal distances available in the literature for the species. These dispersal distances range from a dispersal distance of 500 m for Callicebus to a dispersal dispersal distance of >3000 m for Alouatta (Crockett 1998, Carretero-Pinzon, unpublished data). No information was available on the dispersal distances for the other species. Although we recognise the considerable uncertainty in these dispersal estimates, our choices of buffer size do likely broadly capture the range of dispersal scales for the study species. Prior to the calculation of patch- and landscape-scale variables, the mapping of the selected fragments was improved using a more recent forest and non-forest map of Colombia produced by IDEAM (2014) which is derived from satellite images captured in 2010.
Statistical Analysis
To model the occupancy and abundance of each species, we used zero-inflated Poisson generalized linear models (Lambert 1992; Martin et al. 2005; Zuur et al. 2009; Rhodes 2015). These models allow for the simultaneous modelling of an occupancy component and an abundance component, given occupancy, and are ideally suited for dealing with zero-inflated count data (Martin et al. 2005; Rhodes 2015). Each species was modelled separately and the response variable was the number of individuals observed per transect. Note that the modelled counts are indices of abundance rather than true abundances because we do not account explicitly for detection error. They are therefore interpreted as such. Ignoring detectability essentially assumes that detectability is constant among sites and this may be an issue if detectability actually varies strongly among sites. However, given that forest structural characteristics do not vary strongly among sites, except for some variation in canopy height and closure, we do not expect this to be a major issue. Nonetheless, this may be a limitation of the approach we took. The study area is a part of the piedmont and dissected savannas found in this region, with an uneven topography (Lasso et al. 2010), that can be a factor for the varied canopy height observed in our study sites. We modelled the occupancy and abundance components of the model as functions of the site, patch and landscape variables with variation in sampling effort controlled for in the models as an offset (Zeileis et al. 2008). We formulated a range of alternative hypotheses about the effects of landscape, patch and site scale variables on the occupancy and abundance and tested the support for these based on an information theoretic approach (Burnham and Anderson 2002). To reduce the number of models to a manageable level, we first determined the best buffer size at which to represent the landscape variables for each species. To do this, we modelled the occupancy and abundance of each primate species with only the landscape variables measured at each buffer size separately. We chose the buffer size for each species that had the lowest AIC. Then, we constructed alternative models of the site, patch and landscape variables using landscape variables measured at the chosen buffer size. We constructed our alternative models based on all combinations of site, patch and landscape variables, but keeping variables within a scale grouped together. We assumed that the same variables influence occupancy and abundance in each model. For each species, we also included a null model that included no predictor variables. For each species this resulted in a consideration of 8 models.
All statistical analyses were performed using the R software (www.r-project.org) and the package pscl (Zeileis et al. 2008). We ranked all models for each species according to their AIC values and calculated their Akaike weights (Burnham and Anderson 2002). For each species, a 95% confidence set of models was constructed using the cumulative Akaike weight for each model, starting with the highest and adding the next model until the cumulative sum of weights exceeded 0.95 (Burnham and Anderson 2002). In addition, the average relative importance of each set of variables (site, patch and landscape) was calculated to evaluate the importance of each set of variables for explaining the occupancy and abundance of each species. In order to calculate the relative importance, we controlled for the number of variables by taking the importance of the set of variables rather than each variable separately and then summing the relative importances (see Rhodes et al. 2009).
Finally, to test for spatial autocorrelation in the final model residuals, we created spline correlograms of the residuals of the highest ranked model for each species using the ncf package in R (Bjørnstad 2013). The spline correlograms estimated spatial correlation using a smoothed spline with 95% confidence intervals calculated by bootstrapping (Bjørnstad and Falck 2001). Splines that are centred on zero indicate spatial randomness (i.e., the data are spatially independent), while splines where the 95% confidence intervals do not overlap zero indicate spatial autocorrelation (Bjørnstad and Falck 2001).
Results
All four species were observed in 22% of the patches surveyed with only 1% of the patches surveyed having no primate species present. A total of 271 dusky titi monkeys, 627 howler monkeys, 1092 black-capped capuchin monkeys and 2799 Colombian squirrel monkeys were observed, including adults and immature (sub-adults, juveniles and infants).
Ranking of explanatory variables
The 95% confidence sets of models showed some model uncertainty with one or two models within the 95% confidence set for all primate species studied (Table 2). However, in general the best model was well supported with Akaike weights >0.8 [∆i (wi)]: dusky titi monkeys: 7.05 (0.95); black-capped capuchins 5.3 (0.98); red howler monkeys: 3.02 (0.99); Colombian squirrel monkeys: 17.35 (0.99), and all substantially better than the null model. The difference between the best model and the null model for all species range from 21.67 to 229.36 (∆i: dusky titi monkeys: 21.67; black-capped capuchins 60.54; red howler monkeys: 83.83; Colombian squirrel monkeys: 229.36) indicating very little support for the null models. The best model explaining the occupancy and abundance of Colombian squirrel monkeys, red howler monkeys, and black-capped capuchins contained the variables at the site, patch and landscape scales (500 m buffer for Colombian squirrel monkeys and red howler monkeys and 1000 m buffer for black-capped capuchins). For dusky titi monkeys, the best model explaining occupancy and abundance contained the variables at the site and landscape scales (1000 m buffer). There was no evidence of spatial autocorrelation in the model residuals for any of the best models (Online Appendix 2).
The relative importance of the site-scale and patch-scale variables was high relative to the landscape-scale variables for red howler monkeys, black-capped capuchins and Colombian squirrel monkeys. On the other hand, landscape-scale variables showed a high importance relative to site-scale and patch-scale variables for dusky titi monkeys (Fig. 2).
Effect of explanatory variables
The occupancy and abundance of primate species were significantly influenced by some variables at all spatial scales, but with some differences among species (data based only in the best model for all species, Fig. 3). For red howler monkeys, variable patch density and canopy height had significant effects on occupancy, variables canopy height, canopy cover, presence of natural fence rows, patch density and patch shape index had significant effects on abundance. For black-capped capuchins canopy height and the number of food trees had significant effects on occupancy, while the variables canopy cover, number of food trees, matrix, presence of natural fence rows and patch density had significant effects on abundance. For Colombian squirrel monkeys, percentage of forest cover variable had significant effect on occupancy, variables canopy height, number of trees with fruits, patch shape index, presence of natural fence rows and the percentage of forest cover had significant effects on abundance. For dusky titi monkeys, the percentage of forest cover had significant effect on occupancy, variable number of trees with fruits had significant effects on abundance. Fragment size was no significant for any of the primate species studied here.
The direction of the effect of the explanatory variables also varied among species (Fig. 3). For red howler monkey, occupancy was influenced positively by patch density, while their abundance was negatively influenced by canopy height and the patch shape index. Dusky titi monkeys’ occupancy was positively influenced by the percentage of forest cover at the landscape scale, while the number of trees with fruits had a positive effect on the abundance of this species. The occupancy of black-capped capuchin was negatively influenced by canopy height and the number of food trees, while its abundance was positively influenced by canopy cover, number of food trees and matrix and negatively influenced by presence of natural fence rows and patch density. The percentage of forest cover at the landscape-scale had a positive effect on Colombian squirrel monkey occupancy. The abundance of this species was also negatively influenced by the presence of natural fence rows and the percentage of forest cover in the landscape and positively influenced by canopy height, the number of trees with fruits and the patch shape index.
Discussion
In this study, we applied a multiscale approach to assess the importance of landscape-scale variables relative to site-scale and patch-scale variables for four primate species in the Colombian Llanos. The main contribution is the identification of key spatial scales at which habitat variables influence primate occupancy and abundances and the variation in the influence of these scales among species. It highlights the importance of site-scale and patch-scale variables for three of the studied species (red howler monkeys, black-capped capuchins and Colombian squirrel monkeys), but quite differently, landscape-scale variables for dusky titi monkeys in the same study area. The multiscale approach allowed us to disentangle the relative importance that variables measured at different spatial scales have on primate species occupancy and abundance, improving our understanding on how primate species are distributed. The importance of spatial scale on the distribution and abundance of primate species has been highlighted by other authors (Thornton et al. 2011; Arroyo-Rodriguez et al. 2013b), but few studies have empirically tested this importance. Our study demonstrates the need for primate studies to focus at multiple spatial scales of analysis to better understand how site, patch and landscape-scale variables affect species occupancy and abundance for different species to make informed conservation and management decisions.
Our finding that the relative importance of the site, patch and landscape scales is different for each species is likely related to specific life-history strategies of those species. For example, red howler monkeys, black-capped capuchins and Colombian squirrel monkeys are able to use the matrix and disperse through it across a diverse range of habitats (Defler 2010; Carretero-Pinzón 2013a, b, pers. obs.). Therefore, these species are likely to be less influenced by the landscape context than their specific habitat requirements at patch- and site-scales. In contrast, the dusky titi monkey is a more restricted species in terms of dispersal movements through the matrix (only a few anecdotic observations of this species crossing pastures apparently to disperse have been noted, Carretero-Pinzón unpublished data). Therefore, variables describing the structure of the landscape, such as percentage of forest cover, are more significant because it will restrict their dispersal. Nonetheless, data on dispersal patterns for the species studied here are scarce, except for red howler monkeys (Crockett 1996, 1998). This is because dispersal events are difficult to observe in primate species. Below we expand on pattern-process-scale inferences for the species studied here.
Site-scale processes
The study highlights the importance of site-scale variables in explaining primate occupancy and abundance. Our site-scale variables are related to forest structural attributes such as canopy height and canopy cover and measures of resource availability during the survey such as number of trees with fruits. However, the influence of these variables varied substantially among species. For example, a negative effect of canopy height on the occupancy of red howler (non-significant) and black-capped capuchin monkeys (significant) was unexpected. Canopy height has been used as a measure of habitat quality for species of Alouatta (A. palliata, Anzures-Dadda and Manson 2007; A. pigra: Pozo-Montuy et al. 2008). However, this interpretation may not be true for other types of forest which have different canopy heights and structures. The negative effect observed in our study may be related to the variable canopy height of Colombian Llanos forests (ranging from 10 to 25 m in height), which may not necessarily relate to habitat quality, but rather to other features such as topography and forest composition (Lasso et al. 2010). While the positive effects of the number of trees with fruits showed for dusky titi monkeys and Colombian squirrel monkeys emphasise the importance of this resource availability in the abundance of these endemic taxa in fragmented areas. For the Colombian squirrel monkey, the importance of resource availability on activity patterns and home range use has been previously observed (Carretero-Pinzón 2016). This study found that forest fragment was used depending on fruit production available on that fragment at that time (Carretero-Pinzón et al. 2016). Also, Data for dusky titi monkeys is limited (Ospina 2006). This variation on site-scale effects on primate species occupancy and abundance highlight the importance of forest structure and resource availability within forest fragments, depending on species traits. For example, medium and large species such as black-capped capuchins and red howler monkeys seem to be more affected by forest structure, whilst small body size species such as dusky titi monkeys and Colombian squirrel monkeys seem to be more effected by resource availability variables.
Patch-scale processes
An interesting result was that fragment size did not have a significant effect for any of the species studied, contrary to the findings of other studies (Anzures-Dadda and Manson 2007; Cristobal-Azkarate and Arroyo-Rodriguez 2007; Arroyo-Rodriguez et al. 2008; 2013b). The species studied here are a subset of all primate species present in the Llanos. They are typical of naturally fragmented gallery forest of the Colombian and Venezuelan Llanos and adapted to other different habitats (Carretero-Pinzon and Defler 2016). Their adaptation to edge habitats may explain their persistence in anthropogenic forest fragments typical of the study region and the limited role of fragment size. On the other hand, the significant negative effect of the presence of natural fence rows on black-capped capuchins and Colombian squirrel monkeys’ abundances can be related with a reduction of dispersal opportunities for these two species which use these landscape elements. This finding highlights the importance that matrix elements such as natural fence rows have on some primate species distributions in these fragmented landscapes. This finding has been reported previously by other authors who note the use of natural fence rows for dispersal movements (Zunino et al. 2007; Asensio et al. 2009; Pozo-Montuy and Serio-Silva 2007; Carretero-Pinzón et al. 2010).
Landscape-scale processes
The significant effect of the landscape-scale percentage forest cover, for dusky titi monkeys and Colombian squirrel monkeys and patch density for black-capped capuchins and red howler monkeys highlights the need to also consider the landscape-scale in understanding the spatial distribution and abundance of some primate species. This is particularly important for the dusky titi monkey, an endemic species to the study area, whose small body size may limit its ability to successfully disperse in fragmented landscapes where there is a higher risk of domestic dog predation. The influence of percentage of landscape-scale forest cover for another primate species has been reported by Arroyo-Rodriguez et al. (2008). They compared landscapes with different compositions, identifying a positive relationship between the total amount of forest and the occurrence of Mexican mantled howlers (Alouatta palliata mexicana). In our study, the strong positive influence of patch density on the red howler monkeys’ occupancy and negative influence on black-capped capuchins’ abundance also highlights the importance of considering the spatial configuration of the landscape for species persistence. The black-capped capuchin, as for other species of capuchins, is an opportunistic species that uses the matrix and has a varied diet and can utilities a range of habitat types. However, this adaptability may also make it susceptible to a higher predation risk while exploiting fragmented resources in the matrix (=Sapajus spp.; Chiarello 2003; Fragaszy et al. 2004a, c; Pyritz et al. 2010). Therefore, more fragmented landscapes with a higher density of patches may have a higher risk of predation. Although a high predation risk is also true for red howler monkeys, their large body size as well as their minimizing energy strategy (reduced daily travel distance and a diet dependent on a higher proportion of leaves in forest fragments, Arroyo-Rodriguez and Dias 2010), and their suggested use of different fragments and matrix landscape elements to supplement their diet (Pozo-Montuy et al. 2012) could explain the positive effect of patch density in this species occupancy in our study area.
Implications for conservation
This study highlights the importance of considering multiple spatial scales for the conservation and management of species of primates in highly fragmented forest landscapes. Our findings also highlight the value of jointly applying landscape ecology and spatial distribution modelling to inform management actions for species that persist in fragmented tropical forest landscapes. It emphasises the need to focus conservation actions on avoiding habitat loss and increasing the amount of suitable habitat available in the landscape to increase the probability of occupancy of the dusky titi monkeys and Colombian squirrel monkeys. There also needs to be an increase in within-patch habitat quality to increase species abundance. This is particularly important for the two endemic species (the dusky titi monkey and the Colombian squirrel monkey) because their distributions occur in areas where habitat loss is increasing (Carretero-Pinzón et al. 2009, 2013; Carretero-Pinzón 2013b). For example, maintaining and increasing percentage of forest cover is an important for conserving the dusky titi monkey while also increasing resource availability within forest patches. For the Colombian squirrel monkeys, variables at all scales need to be considered to manage its abundance and occupancy. Some specific actions that could be beneficial for these two species are the implementation of reforestation and regenerating projects, to increase the amount and connectivity of suitable habitat in the landscape.
References
Anderson J, Rowcliffe JM, Cowlishaw G (2007) Does the matrix matter? A forest primate in a complex agricultural landscape. Biol Conserv 135:212–222
Anzures-Dadda A, Manson RH (2007) Patch and landscape-scale effects on howler monkey distribution and abundance in rainforest fragments. Anim Conserv 10:69–76
Armenteras D, Rodriguez N, Renata J (2009) Are conservation strategies effective in avoiding the deforestation of the Colombian Guyana Shield? Biol Conserv 142:1411–1419
Armenteras D, Rodriguez N, Renata J (2013) Landscape dynamics in northwestern Amazonia: an assessment of pastures, fire and illicit crops as drivers of tropical deforestation. PLoS ONE 8(1):e54310. doi:10.1371/journal.pone.005431
Arroyo-Rodriguez V, Cuesta-del Moral E, Mandujano S, Chapman CA, Reyna-Hurtado R, Fahrig L (2013a) Assessing habitat fragmentation effects on primates: the importance of evaluating questions at the correct scale. In: Marsh LK, Chapman CA (eds) Primates in fragments: ecology and conservation, developments in primatology: progress and prospects. Springer, New York, pp 13–28
Arroyo-Rodriguez V, Dias PA (2010) Effects of habitat fragmentation and disturbance on howler monkeys: a review. Am J Primatol 72:1–16
Arroyo-Rodriguez V, Fahrig L (2014) Why is a landscape perspective important in studies of primates? Am J Primatol 76(10):901–909
Arroyo-Rodriguez V, González-Perez IM, Garmendia A, Solà M, Estrada A (2013b) The relative impact of forest patch and landscape attributes on black howler populations in the fragmented Lacandona rainforest, Mexico. Landscape Ecol 28:1717–1727
Arroyo-Rodriguez V, Mandujano S, Benitez-Malvido J (2008) Landscape Attributes Affecting Patch Occupancy by Howler Monkeys (Alouatta palliata mexicana) at Los Tuxtlas, Mexico. Am J Primatol 70:69–77
Asensio N, Arroyo-Rodríguez V, Dunn J, Cristóbal-Azkarate J (2009) Conservation value of landscape supplementation for howler monkeys living in forest patches. Biotorpica 41:768–773
Beltran ML (2005) Estrategias ecológicas e influencia de la dominancia social en la adquisición de alimento en monos aulladores (Alouatta seniculus) en San Martin (Meta- Colombia). Undergraduate thesis, Universidad de Los Andes, Bogotá, Colombia
Benchimol M, Peres CA (2013) Anthropogenic modulators of species-area relationships in Neotropical primates: a continental-scale analysis of fragmented forest landscapes. Divers Distrib 19:1339–1352
Bjørnstad ON (2013) Spatial nonparametric covariance functions. http://onb.ent.psu.edu/onb1/R
Bjørnstad ON, Falck W (2001) Nonparametric spatial covariance functions: estimation and testing. Environ Ecol Stat 8:53–70
Blair ME, Melnick DJ (2012) Scale-dependent effects of a heterogeneous landscape on genetic differentiation in the Central American squirrel monkey (Saimiri oerstedii). PLoS ONE 7(8):e43027. doi:10.1371/journal.pone.0043027
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York
Carretero-Pinzón, X (2000) Un estudio ecológico de Saimiri sciureus y su asociación con Cebus apella, en la Macarena, Colombia. Undergraduate thesis. Pontificia Universidad Javeriana, Bogotá, Colombia
Carretero-Pinzón X (2008) Efecto de la disponibilidad de recursos sobre la ecología y comportamiento de Saimiri sciureus albigena en fragmentos de bosque de galería, San Martín (Meta – Colombia). Masters thesis, Pontificia Universidad Javeriana, Bogotá, Colombia
Carretero-Pinzón X (2013a) An eight-year life history of a primate community in the Colombian Llanos. In: Marsh LK, Chapman CA (eds) Primates in fragments: complexity and resilience, developments in primatology: progress and prospects. Springer, New York, pp 159–182
Carretero-Pinzón X (2013b) Population density and habitat availability of Callicebus ornatus, a Colombian endemic titi monkey. In: Defler TR, Stevenson PR, Bueno ML, Guzman DC (eds) Especies de Primates Colombianos en Peligro de Extinción. Panamericana, Bogotá, pp 160–169
Carretero-Pinzon X, Defler TR (2016) Primates and flooded forest in the Colombian Llanos. In: Barnett AA, Matsuda I, Nowak K (eds) Primates in flooded habitats: ecology and conservation. Cambridge University Press, Cambridge
Carretero-Pinzón X, Defler TR, McAlpine CA, Rhodes JR (2015) What do we know about the effect of patch size on primate species across life history traits? Biodivers Conserv. doi:10.1007/s10531-015-1028-z
Carretero-Pinzón X, Defler TR, Ruíz-García M (2013) Conservation status of Saimiri sciureus albigena, an endemic subspecies of squirrel monkeys. In: Defler TR, Stevenson PR, Bueno ML, Guzman DC (eds) Especies de Primates Colombianos en Peligro de Extinción. Editorial Panamericana, Bogotá, pp 243–252
Carretero-Pinzón X, Defler TR, Ruiz-García M (2016) How does the Colombian squirrel monkey cope with habitat fragmentation? strategies to survive in small fragments. In: Ruiz-García M, Shostell JM (eds) Evolutionary biology and conservation of the neotropical primates. Nova Publishers, New York, pp 491–506
Carretero-Pinzón X, Ruíz-García M, Defler TR (2009) The Taxonomy and Conservation Status of Saimiri sciureus albigena: a squirrel monkey endemic to Colombia. Primate Conserv 24:59–64
Carretero-Pinzón X, Ruíz-García M, Defler TR (2010) Uso de cercas vivas como corredores biológicos por primates en los llanos orientales. In: Pereira V, Stevenson PR, Bueno ML, Nassar-Montoya F (eds) Primatología en Colombia: Avances al principio del milenio. Fundación Universitaria San Martin, Bogotá, pp 91–98
Castiblanco C, Etter A, Aide TM (2013) Oil palm plantations in Colombia: a model of future expansion. Environ Sci Policy 27:172–183
Chapman CA, Onderdonk DA (1998) Forest without primates: primate/plant codependency. Am J Primatol 45:127–141
Chapman CA, Wasserman MD, Gillespie TR, Speirs ML, Lawes MJ, Saj TL, Ziegler TE (2006) Do food availability, parasitism and stress have synergistic effects on red colobus populations living in forest fragments? Am J Phys Anthropol 131:525–534
Chiarello AG (2003) Primates of the Brazilian Atlantic forest: the influence of forest fragmentation on survival. In: Marsh LK (ed) Primates in fragments: ecology and conservation. Kluwer Academic/Plenum, Washington, pp 63–78
Cristobal-Azkarate J, Arroyo-Rodriguez V (2007) Diet and activity pattern of howler monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: effects of habitat fragmentation and implications for conservation. Am J Primatol 69:1013–1029
Crockett CM (1996) The relation between red howler monkey (Alouatta seniculus) troop size and population growth in two habitats. In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptative radiation of neotropical primates. Kluwer Academic/Plenum, Washington, DC
Crockett CM (1998) Conservation biology of Alouatta. Int J Primatol 19:549–578
Defler TR (2010) Historia Natural de los Primates Colombianos. Universidad Nacional de Colombia, Bogotá
Domínguez C (1998) La gran Cuenca del Orinoco. In: Domínguez C (ed) Colombia Orinoco. Fondo Fen, Bogotá, pp 39–67
Ecopetrol (2015) http://www.ecopetrol.com.co/especiales/Sustainability-report-2014/espanol/principal/nuestra-cadena-de-valor/produccion. Accessed 22 Oct 2015
Eigenbrod F, Hecnar SJ, Fahrig L (2008) The relative effect of road traffic and forest cover on anuran populations. Biol Conserv 141:35–46
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Syst 40:677–697
Escobedo-Morales LA, Mandujano S (2007) Conservación del mono aullador en la reserva de la biosfera Los Tuxtlas, Veracruz: un enfoque metapoblacional. Mongr terc milenio 6:131–140
Escudero SP (2005) Patrón de actividad, recorridos diarios y dieta de Alouatta seniculus en fragmentos de bosque de galería San Martín Meta. Undergraduate thesis, Pontificia Universidad Javeriana, Bogota, Colombia
Etter A, McAlpine C, Phinn S, Pullar D, Possingham H (2006) Characterizing a tropical deforestation wave: a dynamic spatial analysis of a deforestation hotspot in the Colombian Amazon. Glob Change Biol 12:1409–1420
Etter A, McAlpine C, Possingham H (2008) Historical patterns and drivers of landscape change in Colombia since 1500: a regionalized spatial approach. Ann Assoc Am Geogr 98(1):2–23
Fahrig L (2001) How much habitat is enough? Biol Conserv 100:65–74
FAO (2011) State of the World’s forests 2011. Food and Agriculture Organization of the United Nations, Rome
Fedepalma (2014) Anuario Estadistico 2014: la agroindustria de la palma de aceite en Colombia y en el mundo: 2009–2013. Javegraf, Bogotá
Fisher J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280
Forman RTT, Godron M (1986) Landscape ecology. Wiley, New York
Fragaszy D, Izar P, Visalberghi E, Ottoni EB, Gomes de Oliveira M (2004a) Wild capuchin monkeys (Cebus libidinosus) use anvils and stone pounding tools. Am J Primatol 64:359–366
Fragaszy DM, Visalberghi E, Fedigan LM (2004b) Behavioral ecology: How do capuchins make a living? In: Fragaszy DM, Visalberghi E, Fedigan LM (eds) The complete capuchin: The biology of the genus Cebus. Cambridge University Press, Cambridge, pp 36–54
Fragaszy DM, Visalberghi E, Fedigan LM (eds) (2004c) The complete capuchin: The biology of the genus Cebus. Cambridge University Press, Cambridge
Gómez-Posada C (2012a) Dieta y comportamiento alimenticio de un grupo de mico maicero Cebus apella de acuerdo a la variación en la oferta de frutos y artrópodos, en la Amazonía colombiana. Acta Amazonica 43(3):363–372
Gómez-Posada C (2012b) Patrón de actividad y de alimentación de un grupo aprovisionado de Cebus apella en un bosque húmedo tropical (Meta, Colombia). Boletín Científico Centro de Museos 13(1):49–62
Guisan A, Graham CH, Elith J, Huettmann F, Species Disribution Modelling Goup NCEAS (2007) Sensitivity of predictive species distribution models to change in grain size. Divers Distrib 13:332–340
Guisan A, Tingley R, Baumgartner JB, Naujokaitis-Lewis I, Sutcliffe PR, Tulloch AIT, Regan TJ, Brotons L, McDonald-Madden E, Mantyka-Pringle C, Martin TG, Rhodes JR, Maggini R, Setterfield SA, Elith J, Schwartz MW, Wintle BA, Broennimann O, Austin M, Ferrier S, Kearney MR, Possingham HP, Buckey YM (2013) Predicting species distributions for conservation decisions. Ecol Lett 16:1424–1435
Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman V, Goetz SJ, Loveland TR, Kommareddy A, Egorov A, Chini L, Justice CO, Townshend JRG (2013) High-resolution global maps of 21st-century forest cover change. Science 342:850–853
Harcourt AH, Doherty DA (2005) Species-area relationships of primates in tropical forest fragments: a global analysis. J Appl Ecol 42(4):630–637
IDEAM (2014) Mapa de cambio de bosque Colombia – Área continental (Escala Fina LANSAT) Periodo 2005-2010. República de Colombia, Instituto de Hidrología, meteorología y estudios ambientales, Bogotá, Colombia
Lambert D (1992) Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 34:1–14
Lasso CA, Usma JS, Trujillo F, Rial A (eds) (2010) Biodiversidad de la cuenca del Orinoco: bases científicas para la identificación de áreas prioritarias para la conservación y uso sostenible de la biodiversidad, Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, WWF Colombia, Fundación Omacha, Fundación La Salle e Instituto de Estudios de la Orinoquía (Universidad Nacional de Colombia), Bogotá D.C., Colombia
Marsh LK (ed) (2003) The nature of fragmentation. In: Primates in fragments: ecology in conservation. Kluwer Academic/Plenum Publishers, New York, pp 1–10
Martin AE, Fahrig L (2012) Measuring and selecting scales of effect for landscape predictors in species-habitat models. Ecol Appl 22(8):2277–2292
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham H (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol Lett 8:1235–1246
McAlpine CA, Rhodes JR, Callaghan JG, Bowen ME, Lunney D, Mitchell DL, Pullar DV, Possingham HP (2006) The importance of forest area and configuration relative to local habitat factors for conserving forest mammals: a case study of koalas in Queensland, Australia. Biol Conserv 132:153–165
McGarial K, McComb WC (1995) Relationships between landscape structure and breeding birds in the Oregon Coast Range. Ecol Monogr 65(3):235–260
Mittermeier RA, Rylands AB, Wilson DE (2013) Handbook of the mammals of the world, vol. 3, Primates. Lynx, Barcelona
Mittermier RA, Oates JJ (1985) Primate diversity: the world’s top countries. Primate Conservation 5:41–48
Oja T, Alamets K, Pärnamets H (2005) Modelling bird habitat suitability based on landscape parameters at different scales. Ecol Indic 5(4):314–321
Ospina MJ (2006) Comparación de los patrones comportamentales de Callicebus cupreus ornatus durante dos épocas estacionales en un fragmento de bosque de galería, en San Martin (Meta). Undergraduate thesis, Pontificia Universidad Javeriana, Bogota, Colombia
Pozo-Montuy G, Serio-Silva JC (2007) Movement and resource use by a group of Alouatta pigra in a forest fragment in Balancan, Mexico. Primates 48:102–107
Pozo-Montuy G, Serio-Silva JC, Bonilla-Sanchez YM, Bynum N, Landgrave R (2008) Current status of the habitat and population of the black howler monkey (Alouatta pigra) in Balancan, Tabasco, Mexico. Am J Primatol 70:1169–1176
Pozo-Montuy G, Serio-Silva JC, Chapman CA, Bonilla-Sanchez YM (2012) Resource use in a landscape matrix by an arboreal primate: evidence of supplementation in black howlers (Alouatta pigra). Int J Primatol 34:714–731
Pyritz LW, Büntgr ABS, Herzog SK, Kessler M (2010) Effects of habitat structure and fragmentation on diversity and abundance of primates in tropical deciduous forests in Bolivia. Int J Prrimatol 31:796–812
Ramos J (2007) Comparación de la cantidad y el tipo de semillas dispersadas por Cebus apella y Alouatta seniculus en un bosque fragmentado, San Martin, Meta. Undergraduate thesis, Universidad de Los Andes, Bogota, Colombia
Rhodes JR (2015) Mixture models for overdispersed data. In: Fox GA, Negrete-Yankelevich S, Sosa VJ (eds) ecological statistics: contemporary theory and application. Oxford University Press, Oxford, pp 285–309
Rhodes JR, McAlpine CA, Zuur AF, Smith GM, Ieno EN (2009) GLMM applied on the spatial distribution of koalas in a fragmented landscape. In: Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (eds) Mixed effects models and extensions in ecology with R. Springer, New York, pp 469–492
Rogerson PA (2010) Statistical methods for geography: a student’s guide, 3rd edn. SAGE, London
Ruiz-Garcia M, Castillo MI (2016) Temporal evolutionary splits among Cebus taxa: the positive and negative use of Cebus and Sapajus and possible diversification scenarios. In: Ruiz-Garcia M, Shostell J (eds) Phylogeny, Molecular Population Genetics, Paleoprimatology and Evolutionary Biology of Neotropical Primates. Nova Science Publisher, New York
Rylands AB, Williamson EA, Hoffmann M, Mittermeier RA (2008) Primate surveys and conservation assessments. Oryx 42(3):313–314
Santamaria M (2005) The effect of home range reduction on the ecology of Red Howler Monkeys in Central Amazonia. Dissertation, Cambridge University
Schipper J, Chanson JS, Chiozza F, Cox NA, Hoffmann M, Katariya V, Lamoreux J, Rodrigues ASL, Stuart SN, Temple HJ, Baillie J, Boitani L, Lacher Jr TE, Mittermeier RA et al. (2008) The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science 322:225–230
Smith AG, McAlpine CA, Rhodes JR, Lunney D, Seabrook L, Baxter G (2013) Out on a limb: habitat use of a specialist folivore, the koala, at the edge of its range in a modified semi-arid landscape. Landscape Ecol 28:415–426
Stevenson PR, Quiñones MJ, Castellanos MC (1998) Guía de los frutos de los bosques del Río Duda La Macarena, Colombia. Giro Editores, Bogotá
Thornton DH, Branch LC, Sunquist ME (2011) The relative influence of habitat loss and fragmentation: do tropical mammals meet the temperate paradigm? Ecol Appl 21(6):2324–2333
Torres JA (2005) Historia natural del maicero café (Cebus apella) y patrones de asociación interespecifica con el mono ardilla (Saimiri scciureus albigena) en un bosque fragmentado (San Martín, Meta). Undergraduate thesis, Universidad de Los Andes, Bogotá, Colombia
Wagner M, Castro F, Stevenson PR (2009) Habitat characterization and population status of the dusty titi monkey (Callicebus ornatus) in fragmented forest, Meta, Colombia. Neotropical Primates 16(1):18–24
Wieczkowski J (2004) Ecological correlates of abundance in the Tana mangabey (Cercocebus galeritus). Am J Primatol 63:125–138
Wiens JA (2009) Landscape ecology as a foundation for sustainable conservation. Landscape Ecol 24:1053–1065
Zarate DA, Stevenson PR (2014) Behavioral ecology and interindividual distance of woolly monkeys (Lagothrix lagothricha) in a rainforest fragment in Colombia. In: Defler TR, Stevenson PR (eds) The woolly monkey, developments in primatology: progress and prospects. Springer, New York, pp 227–245
Zeileis A, Kleiber C, Jackman S (2008) Regression Models for Count Data in R. J Stat Softw 27(8). http://www.jstatsoft.org/v27/i08/
Zunino GE, Kowalewski MM, Oklander L, Gonzaléz V (2007) Habitat framntation and population size of the black and gold howler monkeys (Alouatta caraya) in a semideciduous forest in Northern Argentina. Am J Primatol 69:966–975
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Zero-truncated and zero-inflated models for count data. In: Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (eds) Mixed effect models and extensions in ecology with R. Springer, New York, pp 261–293
Acknowledgements
This research was supported by funding from the Australian Research Council Centre of Excellence for Environmental Decisions and IPRS and UQCent scholarships from The University of Queensland also supported the first author. This research met the Animal Ethics Regulations from The University of Queensland (AEC No. GPEM/096/13). The first author wishes to thank the local people and landowners who supported the fieldwork.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carretero-Pinzón, X., Defler, T.R., McAlpine, C.A. et al. The influence of landscape relative to site and patch variables on primate distributions in the Colombian Llanos. Landscape Ecol 32, 883–896 (2017). https://doi.org/10.1007/s10980-017-0493-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-017-0493-z