Abstract
Despite advances in phytoplankton ecology through functional approaches, little is known about functional traits highlighting metacommunity processes. Our aim was to highlight and compare the influence of spatial and local environmental factors in a phytoplankton metacommunity, in a subtropical shallow lake system, based on taxonomic composition and different functional trait measures. Environmental filtering significantly explained metacommunity variation in most approaches. The spatial signal found could be interpreted as mass effects, given the scale of the present study, and was also a significantly driver of community variability. Among the functional measures, Morphology-Based Functional Groups revealed a stronger influence of the pure environmental component. Although the taxonomic approach helped capture variability in the local environment in a reliable way, it also showed the highest residual variance. Phytoplankton volume significantly captured both local and spatial processes under low residual variance, which may make it a promising functional trait for metacommunity studies. Our findings demonstrated that the drivers of phytoplankton metacommunity may be differently captured by taxonomic and functional measures, so that the approaches can eventually give more or less weight to the environmental and/or spatial signals. We thus recommend the use of taxonomic and functional approaches in metacommunity studies in a complementary way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal is a fundamental process in ecology and evolution, influencing demographic rates, colonization success, speciation, extinction, and, in some cases, even in the geographical distribution of species (Sharma et al., 2007). In microbial ecology, dispersal processes were overlooked until recently (Guo et al., 2019), because it was generally assumed that passive dispersal was so high that microorganisms (smaller than 1 mm) had no geographical barrier (Finlay, 2002) and that environmental control was the only factor controlling communities. To date, however, it is recognized that microorganisms also exhibit biogeographic patterns (Horner-Devine et al., 2007; Naselli-Flores & Padisák, 2016; Ribeiro et al., 2018a) and that microbial communities can be structured not only by environmental factors but also by other processes such as historical contingencies, ecological drift, and dispersal (Martiny et al., 2006; Vellend et al., 2014; Zhou & Ning, 2017). Therefore, the metacommunity framework, in which dispersal between local communities is considered a key factor to understand and explain regional biodiversity patterns (Leibold et al., 2004; Cottenie 2005; Vilmi et al., 2017), can be applied in microbial studies (Wojciechowski et al., 2017; Ribeiro et al., 2018b; Bortolini et al., 2019).
Since dispersal is distant dependent (Kristiansen, 1996; Ng et al., 2009), it can be expected a larger influence of dispersal limitation over a broad spatial scale. At smaller scales, the high dispersal rates homogenize communities determining a spatial influence which may be related to mass effects (Heino et al., 2015). At adequate dispersal rates, species are susceptible to the environmental filtering, being sorted to the favorable habitats (species sorting) (Leibold et al., 2004; Heino et al., 2015). However, dismantling the influence of dispersal is often not straightforward, as spatial significance highlight both limited and high dispersal rates (Ng et al., 2009). Addressing this problem, some authors have succeeded in extracting the influence of limited and high dispersal in metacommunities using analysis at different spatial scales (Ng et al., 2009), while others managed to direct address the dispersal mechanism through ecological hypothesis based on functional traits and their relation with dispersal processes (De Bie et al., 2012; Padial et al., 2014; Guo et al., 2019). However, for microorganisms that are assumed to have high dispersal rates, the question arises as to the appropriate functional trait to capture the impact of dispersal on the arrangement of these communities.
A functional trait is any morphological, physiological, or phenological feature measurable at the individual level, which impacts fitness (Violle et al., 2007). Using traits to group distinct organisms in a functional classification facilitates the understanding of patterns and processes along environmental, spatial, and temporal gradients (Litchman & Klausmeier, 2008; Soininen et al., 2016; Leruste et al., 2018). Then, there are different types of traits. For instance, at individual level, response traits vary in response to the environmental variability; meanwhile, the effect traits reflect the effects of the individuals on the environmental conditions, communities, or in the ecosystems’ properties (Violle et al., 2007). The trait-based approach may detect patterns that are not explicit when using classic species identification (Huszar et al., 2015; Vilmi et al., 2017). For phytoplankton, the most considered functional traits are the morphological (e.g., size, form of life, mucilage), the physiological (e.g., mixotrophy, resting stages, nitrogen fixation), and the behavioral traits (e.g., flagella, aerotopes) (Litchman & Klausmeier, 2008). Based on that, several measures and grouping systems have been proposed so far and, depending on the selected traits, some measures may differently capture community assembly drivers (Guo et al., 2019; Weithoff & Beisner, 2019). The Morphology-Based Functional Group system (MBFG) proposed by Kruk et al. (2010) and enhanced by Reynolds et al. (2014), which included a new group to the original proposal, groups phytoplankton species into eight groups based on morphological (cell volume, surface area, maximum linear dimension, presence of mucilage, and siliceous exoskeletal structure), physiological (presence of heterocytes), and behavioral traits (presence of flagella and aerotopes), and have presented strong relation to environmental variation (Huszar et al., 2015; Salmaso et al., 2015; Xiao et al., 2018). Another grouping system, much less used nowadays, that may also track environmental variation, is the life strategy system (CSR—competitive, stress-tolerant, and ruderal strategies) proposed by Reynolds (1988), based on the concepts introduced by Grime (1977) for terrestrial vegetation. Here, phytoplankton species are sorted into categories based on relations between morphological traits (surface area, volume, and maximum linear dimension) which refer to nutrient uptake, light harvesting, growth rates, loss rates (sinking and grazing) in an environmental spectrum of habitat productivity (nutrient availability), and habitat duration (water column mixing depth and euphotic zone). Then, in this perspective, functional approaches may be useful if correctly applied in the research context, and the misuse of functional classifications, i.e., ignoring the applicability of the functional approach used and the ecological role of the traits, can lead to serious mistakes in interpreting ecological processes underlying community organization (Padisák et al. 2009; Salmaso et al., 2015; Weithoff & Beisner, 2019).
As most phytoplankton researchers keep working with different functional approaches to uncover community variation (Soininen et al., 2016; Vilmi et al., 2016, 2017; Xiao et al., 2018; Bortolini et al., 2019) or comparing functional and taxonomic approaches (Huszar et al., 2015; Wojciechowski et al., 2017; Leruste et al., 2018), few have tried to access community variation using the functional traits themselves to test their hypothesis. By doing so, it may be possible to track a direct role of the functional trait in the community structuring over environmental and spatial gradients (e.g., Crossetti & Bicudo 2008; Crossetti et al., 2018; Wu et al., 2018; Guo et al., 2019). In these sense, some master traits were highlighted for further ecological tests (Litchman & Klausmeier, 2008; Iatskiu et al., 2018; Weithoff & Beisner, 2019), such as the body size, because of its direct relation to nutrient acquisition, growth rates, reproduction, sedimentation rates, grazing susceptibility (Reynolds, 2006; Litchman & Klausmeier, 2008; Litchman et al., 2010; Iatskiu et al., 2018), grazer pressure (for a review on the topic see Pančić & Kiørboe, 2018), and dispersal capability (Jenkins et al., 2007; De Bie et al., 2012). From this perspective, size, estimated, for example, through species’ maximum linear dimension (MLD) or cell volume, could be considered response traits, as they can be associated with the species performance and to the responses to environmental factors such as resources and disturbances. The same may be expected from the functional CRS and MBFG groupings.
On the phytoplankton metacommunity perspective, recent findings demonstrated that species identification (taxonomic approach) explained a higher variance in community data at the large spatial scale than the functional grouping systems (Xiao et al., 2018) and that species composition and its functional groups are mostly shaped by environmental variation (Huszar et al, 2015). Despite comparisons between taxonomic approaches and functional groupings, recent studies have shown that the influence of spatial and environmental variation in phytoplankton metacommunities may be trait dependent (Guo et al., 2019). Then, given the lack of studies integrating taxonomic, multiple, and unique functional trait grouping responses to environmental and spatial variations, in the present study, our goal was to highlight and compare differences in the influence of spatial (assuming dispersal) and local environmental variation in a phytoplankton metacommunity, using a taxonomic approach (Sp), functional grouping systems (MBFG and CSR), and the unique functional traits volume and maximum linear dimension as size measures, in a fourteen subtropical shallow lakes’ system (58 km long), in south Brazil. These functional measures were chosen since they are easily found in studies being related to environmental conditions, but little is known about their relationship with spatial processes. Furthermore, we selected two functional groupings that used different classification criteria, as MBFG system which has mainly morphometrical and structural criteria for classification, while the CSR system is mainly based on functional (growth), morphological, and morphometric criteria (Salmaso et al., 2015). By doing so, we hope to contribute to the discussion upon using different approaches (single traits or functional grouping systems) to access biodiversity patterns in microorganisms, as well as to encourage the interpretation of the ecological role of traits in phytoplankton functional classifications. We expected that both local environmental and spatial influence would be better accessed by functional classifications than the taxonomic approach. In addition, given the importance of the body size to the species’ performance and its direct relation to several ecological functions, we expected that this trait would better summarize the spatial influence and environmental filters than the other functional measures on the variance of the studied phytoplankton metacommunity.
Material and methods
Study area and sampling
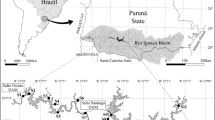
The study was conducted in 14 coastal lakes located within the Tramandaí River Basin at the northern and medium coast of Rio Grande do Sul state - southern Brazil (Fig. 1). These lakes are shallow freshwater water bodies with different types of interconnections and a single link to the ocean (Guimarães et al., 2014). They vary widely in shape and size and were formed between Pleistocene and Holocene (Schwarzbold & Schäfer, 1984; Schäfer et al., 2017). Also, these lakes are highly influenced by northeast and southwest winds (Bohnenberger et al., 2018), in a humid subtropical climate with hot summers (Cfa; Alvares et al., 2013). Furthermore, many of these coastal lakes have been suffering the effects of increasing regional urbanization, promoting a trophic increase that can be related to waste water pollution (Pedrozo & Rocha, 2007).
In this study, 14 coastal lakes with distinct trophic status and with maximum linear distance of 58 km between lakes were sampled in February of 2018, during austral summer period. In each lake, we collected samples for biotic and abiotic analyses at the subsurface of the water column at three sampling stations, comprising equidistance of 10 m in the pelagic zone, resulting in 42 samples. We also obtained the geographical coordinates at each sampling station using a Garmin Etrex 10 GPS (Online Appendix A).
The abiotic sampling encompasses 23 environmental variables measured both in situ and in laboratory. The temperature of the water (T), electrical conductivity (Cond), dissolved oxygen (DO), and pH were measured in situ by means of a multiparameter probe (Manta 2 Water-Quality; Eureka, Austin, TX, USA). In addition, in field we also measured depth (Depth) and water transparency (SD) (using a Secchi disk). Water was sampled for analysis in laboratory of total suspended, fixed and volatile solids (TSS, FSS, and VSS, respectively), soluble reactive silicon (Sil), total nitrogen (TN), total dissolved nitrogen (TDN), total ammonia nitrogen (N-NH3+, N-NH4+), nitrate (N-NO3–), nitrite (N-NO2–), total phosphorus (TP), and soluble reactive phosphorus (SRP), following APHA (2012). We also obtained the dissolved inorganic nitrogen (DIN) by summing N-NH3+, N-NH4+, N-NO3–, and N-NO2–. Total carbon (TOC), dissolved organic carbon (DOC), dissolved inorganic carbon (DIC), and particulate organic carbon (POC) were evaluated using TOC V equipment (Shimadzu VCPH). Finally, color (i.e., absorbance at 450 nm) was measured with Digimed DM-COR colorimeter (Digimed Instrumentação Analítica, São Paulo, SP, Brazil).
Phytoplankton quantification followed the Utermöhl method (1958) and Lund et al. (1958) for settling time and quantification accuracy (95%). The biovolume (mm3 l–1) was considered the abundance measure of phytoplankton species found in the 42 samples, based on the product of the density (individuals l−1) and the average volume of each species (µm3) obtained from the measurements of at least 20 individuals (whenever possible) in each sample, using approximate geometric shapes closest to the cellular form (Hillebrand et al., 1999; Fonseca et al., 2014; Calvacante et al., 2018). All phytoplankton species were identified at the lowest taxonomic classification possible, following the available literature (Komárek and Fott, 1983; Castro et al., 1991; Xavier, 1994; Azevedo et al., 1996; Bicudo et al., 2003; Bicudo, 2004; Araújo & Bicudo 2006; Fernandes & Bicudo, 2009; Hentcheke & Torgan, 2010; Silva et al., 2010; Silva et al., 2013; Bicudo & Menezes, 2017).
Biotic datasets
We used traditional taxonomic composition and four functional measures based on biovolume data from the 42 sample units. The taxonomic composition (Sp) included the estimated biovolume of 169 phytoplankton species registered in all the sampling stations. The functional measures were divided in multiple trait grouping classifications and unique trait categories. For the multiple trait grouping system, we used MBFG groups according to Kruk et al. (2010) and Reynolds et al. (2014), where the species were sorted into eight groups: I—small organism with high S/V; II—small flagellated organisms with siliceous exoskeletal structures; III—large filaments with aerotopes; IV—organisms of medium size lacking specialized traits; V—unicellular flagellates of medium to large size; VI—non-flagellated organisms with siliceous exoskeletons; VII—large mucilaginous colonies; and VIII—nitrogen-fixing cyanobacteria. We also classified phytoplankton species in CSR multiple trait grouping system (Reynolds, 1988, 2006), as C—invasive opportunists/competitors; R—disturbance-tolerant/ruderals; S—Acquisitive/stress-tolerant; and SS—chronic stress-tolerant. As unique trait categories, we used volume (Vol), estimated by the mean volume obtained for each species, and the maximum linear dimension (MLD), estimated by the mean greatest linear dimension of the individual for each species. Then, phytoplankton species were classified in four volume categories, following Crossetti et al. (2018), and in four maximum linear dimension categories, as described by Sieburth et al. (1978), where I—volume < 10 µm3; II—volume between 11 and 1000 µm3; III—volume between 1001 and 10,000 µm3; IV—volume > 10,000 µm3; picophytoplankton—MLD = 0.2 to 2 µm; nanophytoplankton—MLD = 2 to 20 µm; microphytoplankton—MLD = 20 to 200 µm; and mesophytoplankton—MLD = 200 to 2000 µm.
After classification of species based on MBFG, CSR, Vol, and MLD (Online Appendix B), we then summed the species biovolume at each functional group/category to construct four functional biotic response datasets. Before analyses, all biotic datasets were Hellinger transformed (Legendre & Legendre, 2012), in order to reduce the influence of common species and to avoid biased data, thus allowing the use of linear multivariate methods (Peres-Neto et al., 2006).
Environmental and spatial variables
All 23 environmental variables were used to construct an environmental explanatory matrix (E) (Online Appendix A), which was standardized to compare the variables with distinct scales (Legendre & Legendre, 2012). We also tested the collinearity of these variables, maintaining only variables with variance inflation factor (VIF) less than 20 (ter Braak & Smilaurer, 2012). Hence, after selection, 12 environmental variables were retained: Depth, SD, T, pH, DO, Cond, Sil, TSS, TP, SRP, DIC, and DIN (Table 1), which were used in the following analysis.
We did not assume directionality in the spatial processes in the metacommunity of the studied lakes. Although the lakes are connected, previous study on phytoplankton beta diversity in this system demonstrated that the influence of connectivity is negligible (Costa et al., 2020). In addition, even though there are predominant wind directions recognized for the studied region, their behavior and influence within these lakes can be quite unpredictable in short temporal scales, and can vary in intensity and direction within a single day (Cardoso & Motta-Marques, 2003). Then, for the spatial matrix, we used distance-based Moran’s Eigenvector Maps (dbMEMs, Dray et al., 2012; Legendre & Legendre, 2012), to generate spatial variables representing geographical positions at different spatial scales. dbMEMs were calculated from the geographical coordinates of the 42 sampling stations, assuming geographical distances based on soil and not on hydrological connections. This assumption was made considering that in our small study area highly influenced by wind, phytoplankton distribution will not be limited by aquatic connectivity, since species can disperse through air (Kristiansen, 1996; Finlay, 2002; Sharma et al., 2007; Incagnone et al., 2015). This procedure generated 41 spatial variables, 6 with positive spatial autocorrelation (Moran’s I), and 35 with negative spatial autocorrelation. Since species spatial patterns may be described as aggregated (individuals tend to be close together) for positive spatial autocorrelation or regularity (individuals tend to avoid each other) for negative spatial autocorrelation (Dray et al., 2012), and that dispersal may be an underlying mechanism in these spatial variables, we test the significance of both positive and negative spatial variables using ANOVA with 999 permutations, after a redundancy analysis (RDA) with all 5 biotic datasets as response. Since the negative spatial variables did not significantly explain community variability, we used only positive spatial autocorrelation as explanatory variables (S) for further analysis.
Furthermore, we divided our positive dbMEMs into submodels corresponding to different spatial scales (Dray et al., 2012; Legendre & Legendre, 2012). By concentrating most of the spatial variation, the first eigenvector usually describes broad spatial scales, while the last eigenvectors (with lower eigenvalues) describe fine spatial structures. Since we obtained 6 positive MEMs, we classified MEM 1–3 into broad spatial scale (Sb) and MEM 4–6 into fine spatial scales (Sf).
Lastly, we implemented a forward selection procedure with double stopping criteria (Legendre & Legendre, 2012) to only select spatial variables (MEMs) that significantly explained the variance in each biotic dataset. Therefore, for each response matrix (biotic dataset) distinct MEMs were selected for representing broad and fine spatial scales, as spatial explanatory matrices (S) (Table 1).
Data analysis
The relative importance of local environmental and spatial variables in the structuring of our biotic datasets was assessed by partial redundancy analyses (pRDA) in association with variation-partitioning procedures (Borcard et al., 1992; Legendre & Legendre, 2012). This analysis decomposes the total variance into fractions that indicate the relative importance of the pure environmental (E|S) and pure spatial (S|E) components, as well the influence of unique broad and fine spatial scales, shared fractions, and the unexplained variation (Residuals). The analysis was performed separately for each of the five response matrices, using the environmental variables (E) and the selected MEM (S), decomposed into broad (Sb) and fine (Sf) spatial scales. The explained variation at each fraction was estimated using adjusted R2 values (Peres-Neto et al., 2006) and the significance of the pure fractions was tested through an ANOVA, with 999 permutations (Legendre & Legendre, 2012).
All analyses were performed in the R environment (R Core Team, 2019) using the following packages: adespatial (Dray et al., 2019) to construct spatial variables (dbMEMs) and to perform the forward selection procedures; vegan (Oksanen et al., 2019) for the multivariate analyses and VIF selection; and ggplot2 (Wickham et al., 2019) to plot the data.
Results
A total of 169 phytoplankton species were identified. Dolichospermum sp. 2 (mean biovolumen=42 = 51.0 mm3 l−1), Melosira varians C. Agardh (46.3 mm3 l−1), Ceratium furcoides (Levander) Langhans (9.4 mm3 l−1), Desmodesmus armatus var. bicaudatus (Guglielmetti) E. H. Hegewald (9.0 mm3 l−1), and Microcystis aeruginosa (Kützing) Kützing (6.8 mm3 l−1) were abundant throughout the sampling. Only Plagioselmis lacustris (Pascher & Ruttner) Javornicky, Synechococcus spp., and Synechocystis spp. occurred in all sampling stations. Overall, larger species of nitrogen-fixing cyanobacteria (Group VIII—MBFG), disturbance-tolerant species (Group R—CSR), species with greater length (Mesophytoplankton—MLD = 200 to 2000 µm) and with higher volume (Category IV—Vol > 10,000 µm3) attained a higher biomass, over other multiple trait groups and unique trait categories (Fig. 2, Online Appendix C).
The variance partitioning revealed that the phytoplankton metacommunity was influenced by both pure environmental (E) and spatial (S) components, varying according to the biotic dataset used (Table 2, Fig. 3). The fine spatial scale (Sf) showed a higher contribution to the S component than the broad spatial scale (Sb), for most of the measures used (Fig. 4). We also found that the joint effect of environmental and spatial components called spatially structured environment (E + S) had a higher explanatory power for most of the analysis and between functional groups/categories.
The taxonomic composition matrix (Sp) was significantly explained by all explanatory variables, showing a higher influence of the pure environmental component (adjusted R2 = 0.2241, P < 0.001). This approach had also the highest value of unexplained variation comparing all other functional approaches (residuals = 0.6652).
The MBFG system revealed a higher influence of the pure environmental component (adjusted R2 = 0.2615, P < 0.001) compared to the other functional measures. However, the spatial component also significantly predicted community data, regarding broad spatial scales (adjusted R2 = 0.0667, P < 0.005). Comparing other approaches, MBFG was the only in which broad spatial scale (Sb) was the main contributor of the spatial component (Fig. 4). When analyzing between groups, most of the MBFG groups was significantly explained by both environmental and spatial (as broad and fine scales) components (Table 2; Fig. 3), with exception of the Group III (large filaments with aerotopes) which was almost unexplained by our variables (residuals = 0.9202). While some groups were significantly explained only by the environmental variables measured at the local scale (Groups IV, V, and VII), others were highly influenced by spatial components at different scales (Groups I and VI). Group II (small flagellated organisms with siliceous exoskeletal structures) was significantly explained by local environmental variables and fine spatial scale; meanwhile, the local environment was also significant for Group VIII variability (nitrogen-fixing cyanobacteria) and by broad spatial scale component.
Differently from MBFG and Sp approach, grouping species in CSR displayed a stronger influence of spatial component at fine scale, which was the only component that was significantly explained (adjusted R2 = 0.2275, P < 0.001). When analyzed between groups (Fig. 3), fine spatial scale continued to explain data variation in Group C (Invasive opportunists/competitors) and R (Disturbance-tolerant/ruderals) (P < 0.005, for both). Yet, environmental component also became influential for Group C (adjusted R2 = 0.1833, P < 0.05). Group S (Acquisitive/stress-tolerant) was unexplained by any variable, showing a higher residual value = 0.7356. Species belonging to Group SS (Chronic stress-tolerant) were not found in the sampling stations and, therefore, were not considered in the analysis.
MLD categorization displayed similar results as the CSR grouping system, revealing the influence of spatial component, as fine scale (adjusted R2 = 0.0653, P < 0.05) in the metacommunity. Environmental component was not influential, and fine-scale spatial significance was found only for the smaller length categories, such as Picophytoplankton (MLD = 0.2 to 2 µm) (adjusted R2 = 0.0806, P < 0.05) and Nanophytoplankton (MLD = 2 to 20 µm) (adjusted R2 = 0.1370, P < 0.005). The data variation of the bigger length categories (Microphytoplankton and Mesophytoplankton) were not explained by our variables (Table 2). However, for Mesophytoplankton (MLD = 200 to 2000 µm) the spatially structured environment (E + S) component could explain data variability, as it showed an adjusted R2 = 0.5611.
Lastly, Vol approach enabled to explain more variance in community data comparing to all other approaches (Table 2; Fig. 3), resulting in a lower residual value (residuals = 0.3424). Using Vol phytoplankton metacommunity variance was significantly explained by all components of data variation at both large and fine spatial scales, showing a higher influence of the pure environmental component (adjusted R2 = 0.1772, P < 0.001). Fine-scale spatial variance showed a higher influence in structuring the community through volume than broad spatial scale (Fig. 4). All volume categories revealed significance with local environment and spatial (at different scales) components, with exception of the larger category (IV—Vol > 10,000 µm3), which was explained only by the fine-scale spatial component (Table 2; Fig. 3).
By comparing approaches, the results demonstrated that the influence of local environmental and spatial variables in structuring metacommunities were better ascertained by Vol, MBFG, and by Sp (Fig. 3). From these, volume categorization displayed a higher spatial signal, and MBFG functional groupings showed a higher influence of local environmental variables. Taxonomic composition (Sp) was less explained by our explanatory variables than functional measures, such as Vol and MBFG. We highlight that for Vol, MBFG, and Sp, the major metacommunity driver was the environmental component, with also a significant influence of spatial component at different scales.
When accessing differences between groups from distinct functional measures, the following groups and categories were better predicted by local environmental and spatial variables than the others functional measures: Groups II (Small flagellated organisms with siliceous exoskeletal structures) and VIII (Nitrogen-fixing cyanobacteria) of MBFG; Categories I (volume < 10 µm3), II (volume between 11 and 1000 µm3), and III (volume between 1001 and 10,000 µm3) of volume categorization; and Group C (Invasive opportunists/competitors) from CSR multiple functional trait grouping. From these, CSR Group C revealed a higher influence of spatial variables, and Category III of Vol displayed higher local environmental influence than other categories/groups, despite MBFG having an overall higher environmental influence (Table 2).
Discussion
Phytoplankton metacommunity in the studied area was explained by both local environmental and spatial components, i.e., local environmental variables are important for the variance of the phytoplankton, at the same time that spatial distances may also contribute to the overall organization of the phytoplankton of the studied lakes’ system. However, environmental and spatial signs were differently evidenced regarding the functional measures used.
By comparing the classical taxonomic approach with multiple functional trait grouping (MBFG and CSR) and with unique functional trait categorizations (Vol and MLD), we expected that the latter would be, comparatively, more explained by the local environmental and spatial variables. As functional approaches group species with similar ecological characteristics (Padisák et al., 2009), they would better summarize the environmental and spatial influence than species as individual entities (Huszar et al., 2015; Leruste et al., 2018). The present results showed that the overall MBFG measure displayed a higher influence of environmental variables than taxonomic approach (Sp) and fine spatial signal was better captured by the overall Vol and CRS variations than Sp. In turn, Sp was better predicted by local environmental variables than overall MLD, Vol, and CSR. Also, Sp and Vol were the only approaches to access significant influence of both broad and fine spatial variables.
Recently, Xiao et al. (2018) demonstrated that, for phytoplankton metacommunity of three Chinese lakes regions (distance range between lakes of 1285–3232 km), species-based classification revealed better spatial processes than functional approaches, including MBFG, pointing out that, despite common responses of species within functional groups, species-specific approach comprises dispersal abilities, mechanisms, and strategies that cannot be neglected in phytoplankton patterns at spatial scales. On the other hand, Huszar et al. (2015) demonstrated that species composition and MBFG were influenced by local environment, but spatial influence on species were not seen in a large subtropical Brazilian river basin (1150 km). Interestingly, Sp approach had the higher residual value in the present study. The low explanatory power of Sp may reflect the large multiplicity of factors that affects phytoplankton species variation (Scheffer et al., 2003; Wojciechowski et al., 2017). Despite the scale differences observed between our results and that of Huszar et al. (2015), the taxonomic approach significantly captured the spatial signal and should not be ruled out in phytoplankton metacommunity studies. The lack of other comparative studies in this regard suggests the need for further research, especially comprising different spatial scales.
Among the functional measures here tested, the overall Vol variability was the only to be significantly sensible to both local environmental and spatial variables (fine and broad scales). This may be a result of the strong association of the trait volume, a direct measurement of size, with both environmental and dispersal factors (De Bie et al., 2012; Iatskiu et al., 2018; Leruste et al., 2018). Since volume is also a functional trait used in MBFG (Kruk et al., 2010), the divergences observed between MBFG and Vol measures, especially regarding Sf component, may be related to the influence of other traits in MBFG classification, which are highly related to environmental conditions, such as presence of mucilage and aerotopes (Litchman & Klausmeier, 2008). Further, MBFG multiple functional trait grouping is known to be a better predictor of larger scale variations (Salmaso et al., 2015), which may also explain the absence of significance at fine-scale spatial component. Regarding the spatial signal, the overall CSR approach presented the highest explanation power in fine scale, which may indicate the dispersal-related ability comprised in the relation between the morphological traits (surface, volume ratio, and maximum linear dimension) considered in this functional metric. The non-significant variance of MLD to local environmental component, in turn, may have occurred due to the spatial structure of local environmental variables. This functional measure reflected most the influence of spatially structured environment variables (E + S). Besides, the maximum linear dimension of the organisms may not capture most of the environmental variability when used by itself, being rather used to track grazing susceptibility (e.g., Cardoso et al., 2019).
For phytoplankton, the small cell size and also the enormous abundance of vegetative cells and propagules allows a huge passive dispersal potential (Finlay, 2002; Sharma et al., 2007; De Bie et al., 2012; Incagnone et al., 2015). Dispersal is distant dependent (Kristiansen, 1996; Ng et al., 2009) and a gradual increase in dispersal limitation with body size in aquatic passive dispersers was already seen (De Bie et al., 2012).Within MBFG, we found fine-scale spatial significance for small body-related Groups I (small organism with high S/V) and II (small flagellated organisms with siliceous exoskeletal structures. In addition, we found spatial signal (broad and fine scales) for the Groups VIII (Nitrogen-fixing cyanobacteria) and VI (non-flagellated organisms with siliceous exoskeletons—diatoms), which are also usually associated to environmental variability (Reynolds 2006; Vilmi et al., 2016). For cyanobacteria (MBFG—Groups III, VII and VIII), Ribeiro et al. (2018b) found that, at broader spatial scales, the community distribution was mainly structured by spatial (assumed dispersal limitation, considering a larger scale—220 km) and spatially structured environment components, whereas other studies indicated dispersal limitation occurring only at continental scales (Martiny et al., 2006; Izaguirre et al., 2015). Although we do not assume dispersal limitation because of the small scale of our study area, the results showed that nitrogen-fixing cyanobacteria reflected the influence of both environmental and dispersal processes even at small geographical extents. Regarding the diatoms (MBFG—Group VI), much of the species’ observed in the present study are meroplankton because of the hydrodynamics of the lakes’ system and the high wind influence (Online Appendix B). The previous study on planktic and surface sediment diatoms within the same reservoirs evidenced the influence of pure space in the planktic diatoms in terms of traits and species and pure environment in terms of traits based on morphology, meanwhile the surface sediment diatoms recorded spatial signal only with species and morphology-based traits and the environmental signal with species matrix (Zorzal-Almeida et al., 2017). Within the CSR system, the small species categories C (invasive opportunists/competitors) and R (disturbance-tolerant/ruderals) showed significant fine scale spatial signals, whereas the larger organisms of S (acquisitive/stress-tolerant) were not explained by neither spatial nor local environmental variables. For smaller species in group C (invasive opportunists/competitors), we found spatial and environmental significance, which is reasonable since these species are resource constrained (Reynolds, 2006; Salmaso et al., 2015). In group R (disturbance-tolerant/ruderals) species generally need water turbulence to be suspended. Since that is a common feature in our study area (Bohnenberger et al., 2018), the lack of environmental signal may be caused by the absence of environmental constraint for this group, while the spatial signal may indicate that dispersal processes do overcome environmental filtering, at least for group R (disturbance-tolerant/ruderals) in our study area.
As found for CSR, MLD smaller-sized categories picophytoplankton (0.2 to 2 µm) and nanophytoplankton (2 to 20 µm) displayed fine-scale spatial significant sensitivity. However, for the larger species categories microphytoplankton (20 to 200 µm) and mesophytoplankton (200 to 2000 µm), the results did not show significance with any explanatory variable. The absence of environmental influence for larger microorganisms may be caused by their higher in-cell nutrient storage capacity, lower metabolic rate, and greater resistance to predation, all factors that assist organism to withstand environmental constraints (Litchman et al., 2010; Iatskiu et al., 2018; Pančić & Kiørboe, 2018).
Lastly, regarding the Vol categories I (volume < 10 µm3), II (volume between 11 and 1000 µm3), and IV (volume > 10,000 µm3) showed fine spatial scale significance, while groups II and III (volume between 1001 and 10,000 µm3) were significantly explained by broad spatial scale. In this metric, only the larger species group IV (volume > 10,000 µm3) showed no significance with the local environmental component, yet it presented the higher significance with the Sf component. As larger organisms may have higher tolerances to environmental constraints (Iatskiu et al., 2018), which undermine explanation power of environmental variables, one may wonder if in a gradual size increase, the environmental filtering is replaced by dispersal limitation for the larger organisms (here > 10,000 µm3), even in our small study area.
In a general view, based on the presented results, the spatial signal found for the overall functional measures can be interpreted as high dispersal effects or mass effects (Heino et al., 2015; Vilmi et al., 2017), given the scale comprised in the present study. As spatial signals in phytoplankton are found at larger geographical scales and commonly interpreted as limited dispersal (Huszar et al., 2015; Izaguirre et al., 2015; Soininen et al., 2016; Moresco et al., 2017; Ribeiro et al., 2018b; Wu et al., 2018; Xiao et al., 2018), our findings do imply that dispersal processes are significant drivers of phytoplankton metacommunity variation even at smaller spatial scales. Since dispersal mechanisms were historically ignored factors structuring microorganism metacommunities (Huszar et al., 2015; Incagnone et al., 2015; Padisák et al., 2016), our results contribute to the actual understanding that microorganism community may be structured by other processes, rather than environmental factors alone (Beisner et al., 2006; Martiny et al., 2006; Vellend et al., 2014; Zhou & Ning, 2017).
Summing up, we evaluated different influences of community drivers not only through using distinct approaches but also when analyzing data variation in between groups/categories of the same functional measure. We showed that both spatial and environmental are important drivers of community variation in a small-scale area, but their influence can be tracked differently depending on the approach used, as seem by Guo et al. (2019). In our study, taxonomic composition displayed better influence of environmental and spatial components than some functional approaches and should not be neglected in phytoplankton community assembly studies. Our findings also indicated that, by accessing community through functional traits that are highly responsive to environmental filtering and dispersal, studies can uncover different aspects of the metacommunity driving factors. Considering functional measures, Vol and MBFG seemed to be fitting classifications to uncover phytoplankton structuring factors, because of its higher explanatory power and sensitivity to spatial and environmental signals. However, when deconstructing the overall classifications and evaluating the functional groups (usually composed by multiple traits), it may not be easy to relate the ecological mechanisms of interest in metacommunity studies. Using size traits (MLD and Vol) to access dispersal processes improved our spatial signal, as all functional measures used were related to morphology and displayed spatial significant sensitivity at least in one scale. Volume displayed the best results, being the only trait measure of the present study that was significantly sensitive to both environmental and spatial signals (broad and fine). It also presented the higher explanation power of the variables comparing to all other approaches, resulting in a lower residual value, indicating to be a promising functional trait in metacommunity studies, and should be better explored in further studies. In conclusion, all functional measures evaluated might be considered complementary, as they were all, in some way, evidenced the community drivers. Therefore, to provide a direct and more conceive answers to patterns observed in nature we need to evaluate and understand the functional relevance of the metric used, as well the relationship of the traits to local environmental variables and dispersal processes in phytoplankton metacommunity studies. As future perspectives, comparative approaches upon functional classifications and species composition must be encouraged in different spatial scales to strengthen the understanding of these measures in the context of microorganism metacommunities assembly.
References
Alvares, C. A., J. L. Stape, P. C. Sentelhas, J. L. De Moraes Gonçalves & G. Sparovek, 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22(6): 711–728.
Association, American Public Health. & – APHA, 2012. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington, DC:
Araújo, A. C. & E. M. Bicudo, 2006. Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas, 22: Zygnemaphyceae (gêneros Actinotaenium, Cosmarium e Heimansia). Hoehnea 33(2): 219–237.
Azevedo, M. T. P., N. M. C. Nogueira & C. L. Sant’Anna, 1996. Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas, 8: Cyanophyceae. Hoehnea 23(1): 1–38.
Beisner, B. E., P. R. Peres-Neto, E. S. Lindström, A. Barnett & M. L. Longhi, 2006. The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87: 2985–2991. https://doi.org/10.1890/0012-9658(2006)87[2985:TROEAS]2.0.CO;2.
Bicudo, C. E. M., 2004. Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP Algas, 18: Chlorophyceae (Volvocales). Revista Brasileira de Botânica 27(1): 85–102.
Bicudo, C. E. M., D. C. Bicudo, C. Ferragut, M. R. M. Lopes & P. R. Pires, 2003. Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas, 17: Chrysophyceae. Hoehnea 30(2): 127–153.
Bicudo, C. & M. Menezes, 2017. Gêneros de algas de águas continentais do Brasil (chave para identificação e descrições). São Carlos: RiMa, 3º ed, 554p.
Bohnenberger, J. E., F. Schneck, L. O. Crossetti, M. S. Lima & D. Motta-Marques, 2018. Taxonomic and functional nestedness patterns of phytoplankton communities among coastal shallow lakes in southern Brazil. Journal of Plankton Research 40: 555–567. https://doi.org/10.1093/plankt/fby032.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055.
Bortolini, J. C., P. R. L. Silva, G. Baumgartner & N. C. Bueno, 2019. Response to environmental, spatial, and temporal mechanisms of the phytoplankton metacommunity: comparing ecological approaches in subtropical reservoirs. Hydrobiologia 830: 45–61. https://doi.org/10.1007/s10750-018-3849-8.
Calvacante, K. P., V. Becker & L. S. Cardoso, 2018. A proxy for estimating the cell volume of Ceratium furcoides (Dinophyceae): basis for monitoring Brazilian reservoirs. Lakes and Reservoirs 23: 168–171. https://doi.org/10.1111/lre.12211.
Cardoso, L. S., D. M. Faria, L. O. Crossetti & D. Da Motta Marques, 2019. Phytoplankton, periphyton, and zooplankton patterns in the pelagic and littoral regions of a large subtropical shallow lake. Hydrobiologia 831: 119–132. https://doi.org/10.1007/s10750-018-3729-2.
Cardoso, L. S. & D. Da Motta-Marques, 2003. Rate of change of the phytoplankton community in Itapeva Lake (North Coast of Rio Grande do Sul, Brazil), based on the wind driven hydrodynamic regime. Hydrobiologia 497: 1–12. https://doi.org/10.1023/A:1025449202083.
Castro, A. A. J., C. E. M. Bicudo & D. C. Bicudo, 1991. Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas 2: Cryptophyceae. Hoehnea 18(1): 87–106.
Cottenie, K., 2005. Integrating environmental and spatial processes in ecological community dynamics. Ecology Letters 8: 1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x.
Costa, A. P. T., L. O. Crossetti, S. M. Hartz, F. G. Becker, L. U. Hepp, J. E. Bohnenberger, M. S. Lima, T. Guimarães & F. Schneck, 2020. Land cover is the main correlate of phytoplankton beta diversity in subtropical coastal shallow lake. Aquatic Ecology 54: 1015–1028. https://doi.org/10.1007/s10452-020-09790-w.
Crossetti, L. O., L. M. Freitas-Teixeira, J. L. Bohnenberger, U. H. Schulz, L. R. Rodrigues & D. Motta-Marques, 2018. Responses of the phytoplankton functional structure to the spatial and temporal heterogeneity in a large subtropical shallow lake. Acta Limnologica Brasiliensia. https://doi.org/10.1590/S2179-975X7217.
De Bie, T., L. De Meester, L. Brendonck, K. Martens, B. Goddeeris, D. Ercken, H. Hampel, L. Denys, L. Vanhecke, K. Van der Gucht, J. Van Wichelen, W. Vyverman & S. A. J. Declerck, 2012. Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecology Letters 15: 740–747. https://doi.org/10.1111/j.1461-0248.2012.01794.x.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, T. Jombart, G. Larocque, P. Legendre, N. Madi & H. H. Wagner, 2019. adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.3-7.
Dray, S., R. Pélissier, P. Couteron, M. J. Fortin, P. Legendre, P. R. Peres-Neto, E. Bellier, R. Bivand, F. G. Blanchet, M. De Cáceres, A. B. Dufour, E. Heegaard, T. Jombart, F. Munoz, J. Oksanen, J. Thioulouse & H. H. Wagner, 2012. Community ecology in the age of multivariate multiscale spatial analysis. Ecological Monographs 82: 257–262. https://doi.org/10.1890/11-1183.1.
Fernandes, S. & C. E. M. Bicudo, 2009. Criptógamos do Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas, 26: Chlorophyceae (famílias Chlorococcaceae e Coccomyxaceae). Hoehnea 36(1): 173–191.
Finlay, B. J., 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063. https://doi.org/10.1126/science.1070710.
Fonseca, B. M., C. Ferragut, A. Tucci, L. O. Crossetti, F. Ferrari, D. C. Bicudo, C. L. Sant’Anna & C. E. M. Bicudo, 2014. Biovolume de cianobactérias e algas de reservatórios tropicais do Brasil com diferentes estados tróficos. Hoehnea 41(1): 9–30. https://doi.org/10.1590/S2236-89062014000100002.
Grime, J. P., 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111(982): 1169–1194.
Guimarães, T. F. R., S. M. Hartz & F. G. Becker, 2014. Lake connectivity and fish species richness in southern Brazilian coastal lakes. Hydrobiologia 740: 207–217. https://doi.org/10.1007/s10750-014-1954-x.
Guo, K., N. Wu, C. Wang, D. Yang, Y. He, Y. Luo, Y. Chai, M. Duan, X. Huang & T. Riis, 2019. Trait dependent roles of environmental factors, spatial processes and grazing pressure on lake phytoplankton metacommunity. Ecological Indicators 103: 312–320. https://doi.org/10.1016/j.ecolind.2019.04.028.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. L. Valanko & M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60(5): 845–869. https://doi.org/10.1111/fwb.12533.
Hentcheke, G. S. & L. C. Torgan, 2010. Desmodesmus and Scenedesmus (Scendesmaceae, Sphaeropleales, Chlorophyceae) in aquatic environments from the Coastal Plain of Rio Grande do Sul. Brazil. Rodriguésia 61(4): 585–601.
Hillebrand, H., D. Dürseken, D. Kirschiel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Horner-Devine, M. C., J. M. Silver, M. A. Leibold, B. J. M. Bohannan, R. K. Colwell, J. A. Fuhrman, J. L. Green, C. R. Kuske, J. B. H. Martiny, G. Muyzer, L. Øvreås, A. L. Reysenbach & V. H. Smith, 2007. A comparison of taxon co-occurrence patterns for macro- and microorganisms. Ecology 88: 1345–1353. https://doi.org/10.1890/06-0286.
Huszar, V. L. M., J. C. Nabout, M. Appel, J. B. O. Santos, D. S. Abe & L. H. S. Silva, 2015. Environmental and not spatial processes (directional and non-directional) shape the phytoplankton composition and functional groups in a large subtropical river basin. Journal of Plankton Research 37: 1190–1200. https://doi.org/10.1093/plankt/fbv084.
Incagnone, G., F. Marrone, R. Barone, L. Robba & L. Naselli-Flores, 2015. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750: 103–123. https://doi.org/10.1007/s10750-014-2110-3.
Iatskiu, P., V. M. Bovo-Scomparin, B. T. Segovia, L. F. M. Velho, M. J. Lemke & L. C. Rodrigues, 2018. Variability in mean size of phytoplankton in two floodplain lakes of different climatic regions. Hydrobiologia 823: 135–151. https://doi.org/10.1007/s10750-018-3702-0.
Izaguirre, I., J. F. Saad, M. Romina-Schiaffino, A. Vinocur, G. Tell, M. L. Sánchez, L. Allende & R. Sinistro, 2015. Drivers of phytoplankton diversity in patagonian and antarctic lakes across a latitudinal gradient (2150 km): the importance of spatial and environmental factors. Hydrobiologia 764: 157–170. https://doi.org/10.1007/s10750-015-2269-2.
Jenkins, D. G., C. R. Brescacin, C. V. Duxbury, J. A. Elliott, J. A. Evans, K. R. Grablow, M. Hillegass, B. N. Lyon, G. A. Metzger, M. L. Olandese, D. Pepe, G. A. Silvers, H. N. Suresch, T. N. Thompson, C. M. Trexler, G. E. Williams, N. C. Williams & S. E. Williams, 2007. Does size matter for dispersal distance? Global Ecology and Biogeography 16: 415–425. https://doi.org/10.1111/j.1466-8238.2007.00312.x.
Komárek, J. & B. Fott, 1983. Das Phytoplankton des Süβwassers (Systematik und Biologie). Sttutgart: E. Schweizerbart’sche Verlagsbuchhandlung (Nägele u. obermiller).
Kristiansen, J., 1996. Dispersal of freshwater algae – a review. Hydrobiologia 336: 151–157.
Kruk, C., V. L. M. Huszar, E. T. H. M. Peeters, S. Bonilla, L. Costa, M. Lürling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627. https://doi.org/10.1111/j.1365-2427.2009.02298.x.
Legendre, P. & L. Legendre, 2012. Numerical Ecology, 3rd ed. Elsevier, Oxford:
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x.
Leruste, A., S. Villéger, N. Malet, R. De Wit & B. Bec, 2018. Complementarity of the multidimensional functional and the taxonomic approaches to study phytoplankton communities in three Mediterranean coastal lagoons of different trophic status. Hydrobiologia 815: 207–227. https://doi.org/10.1007/s10750-018-3565-4.
Litchman, E. & C. A. Klausmeier, 2008. Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution, and Systematics 39: 615–639. https://doi.org/10.1146/annurev.ecolsys.39.110707.173549.
Litchman, E., P. T. Pinto, C. A. Klausmeier, M. K. Thomas & K. Yoshiyama, 2010. Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia 653: 15–28. https://doi.org/10.1007/s10750-010-0341-5.
Lund, J. W. G., C. Kipling & E. D. LeCren, 1958. The invert microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11: 143–170.
Martiny, J. B. H., B. J. M. Bohannan, J. H. Brown, R. K. Colwell, J. A. Fuhrman, J. L. Green, M. C. Horner-Devine, M. Kane, J. A. Krumins, C. R. Kuske, P. J. Morin, S. Naeem, L. Øvreås, A. L. Reysenbach, V. H. Smith & J. T. Staley, 2006. Microbial biogeography: putting microorganisms on the map. Nature Reviews Microbiology 4: 102–112. https://doi.org/10.1038/nrmicro1341.
Moresco, G. A., J. C. Bortolini, J. D. Dias, A. Pineda, S. Jati & L. C. Rodrigues, 2017. Drivers of phytoplankton richness and diversity components in Neotropical floodplain lakes, from small to large spatial scales. Hydrobiologia 799(1): 203–215. https://doi.org/10.1007/s10750-017-3214-3.
Naselli-Flores, L. & J. Padisák, 2016. Blowing in the wind: how many roads can a phytoplanktont walk down? A synthesis on phytoplankton biogeography and spatial processes. Hydrobiologia 764(1): 303–313. https://doi.org/10.1007/s10750-015-2519-3.
Ng, I. S. Y., C. M. Carr & K. Cottenie, 2009. Hierarchical zooplankton metacommunities: distinguishing between high and limiting dispersal mechanisms. Hydrobiologia 619: 133–143. https://doi.org/10.1007/s10750-008-9605-8.
Oksanen, J. F., G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2019. vegan: Community Ecology Package. R Package Version 2.5-6.
Padial, A. A., F. Ceschin, S. A. J. Declerck, L. De Meester, C. C. Bonecker, F. A. Lansac-Tôha, L. Rodrigues, L. C. Rodrigues, S. Train, L. F. M. Velho & L. M. Bini, 2014. Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9: 1–8. https://doi.org/10.1371/journal.pone.0111227.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19. https://doi.org/10.1007/s10750-008-9645-0.
Padisák, J., G. Vasas & G. Borics, 2016. Phycogeography of freshwater phytoplankton: traditional knowledge and new molecular tools. Hydrobiologia 764: 3–27. https://doi.org/10.1007/s10750-015-2259-4.
Pančić, M. & T. Kiørboe, 2018. Phytoplankton defence mechanisms: traits and trade-offs. Biological Reviews 93(2): 1269–1303. https://doi.org/10.1111/brv.12395.
Pedrozo, S. C. & O. Rocha, 2007. Environmental quality evaluation of lakes in the Rio Grande do Sul Coastal Plain. Brazilian Archives of Biology and Technology 50: 673–685. https://doi.org/10.1590/S1516-89132007000400013.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625. https://doi.org/10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2.
R Core Team, 2019. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject.org.
Reynolds, C. S., 1988. Functional morphology and the adaptive strategies of freshwater phytoplankton. In Sandgren, C. D. (ed), Growth and reproductive strategies of freshwater phytoplankton Cambridge University Press, Cambridge: 388–433.
Reynolds, C. S., 2006. The ecology of phytoplankton, Cambridge University Press, Cambridge:
Reynolds, C. S., J. A. Elliott & M. A. Frassl, 2014. Predictive utility of trait-separated phytoplankton groups: a robust approach to modeling population dynamics. Journal of Great Lakes Research 40: 143–150. https://doi.org/10.1016/j.jglr.2014.02.005.
Ribeiro, K. F., L. Duarte & L. O. Crossetti, 2018a. Everything is not everywhere: a tale on the biogeography of cyanobacteria. Hydrobiologia 820: 23–49. https://doi.org/10.1007/s10750-018-3669-x.
Ribeiro, K. F., C. M. da Rocha, D. de Castro, L. R. Rodrigues & L. O. Crossetti, 2018b. Distribution and coexistence patterns of phytoplankton in subtropical shallow lakes and the role of niche-based and spatial processes. Hydrobiologia 814(1): 233–246. https://doi.org/10.1007/s10750-018-3539-6.
Salmaso, N., L. Naselli-Flores & J. Padisák, 2015. Functional classifications and their application in phytoplankton ecology. Freshwater Biology 60: 603–619. https://doi.org/10.1111/fwb.12520.
Schäfer, A., R. Lanzer & L. Scur, 2017. Atlas Socioambiental do Município de Osório. Caxias do Sul, RS: Educs, 237p.
Scheffer, M., S. Rinaldi, J. Huisman & F. J. Weissing, 2003. Why plankton communities have no equilibrium: solutions to the paradox. Hydrobiologia 491: 9–18. https://doi.org/10.1023/A:1024404804748.
Schwarzbold, A. & A. Schäfer, 1984. Gênese e morfologia das lagoas costeiras do Rio Grande do Sul, Brasil. Amazoniana 9: 87–104.
Sharma, N. K., A. K. Rai, S. Singh & R. M. Brown Jr., 2007. Airborne algae: their present status and relevance. Journal of Phycology 43: 615–627. https://doi.org/10.1111/j.1529-8817.2007.00373.x.
Sieburth, J. M. N., V. Smetacek & J. Lenz, 1978. Pelagic ecosystem structure: heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnology and Oceanography 23: 1256–63.
Silva, A. M., T. A. V. Ludwig, P. I. Tremarin & I. S. Vercellino, 2010. Diatomáceas perifíticas em um sistema eutrófico brasileiro (Reservatório do Iraí, estado do Paraná). Acta Botânica Brasílica 24(4): 997–1016. https://doi.org/10.1590/S0102-33062010000400015.
Silva, S. M. A., J. C. Cabreira, J. G. Voos & E. A. Lobo, 2013. Species richness of the genera Trachelomonas and Strombomonas (pigmented Euglenophyceae) in a subtropical urban lake in the Porto Alegre Botanical Garden, RS. Brazil. Acta Botanica Brasilica 27(3): 526–536. https://doi.org/10.1590/S0102-33062013000300010.
Soininen, J., A. Jamoneau, J. Rosebery & S. I. Passy, 2016. Global patterns and drivers of species and trait composition in diatoms. Global Ecology and Biogeography 25: 940–950. https://doi.org/10.1111/geb.12452.
ter Braak, C. J. F. & P. Smilauer, 2012. Canoco reference manual and user's guide: software for ordination, version 5.0. Ithaca USA: Microcomputer Power.
Utermöhl, H., 1958. Zur Vervolkomnung der quantitative Phytoplankton-Methodik. Mitteilungen der Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Vellend, M., D. S. Srivastava, K. M. Anderson, C. D. Brown, J. E. Jankowski, E. J. Kleynhans, N. J. B. Kraft, A. D. Letaw, A. A. M. Macdonald, J. E. Maclean, I. H. Myers-smith, A. R. Norris & X. Xue, 2014. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 0: 1–11. https://doi.org/10.1111/oik.01493.
Vilmi, A., S. M. Karjalainen, S. Hellsten & J. Heino, 2016. Bioassessment in a metacommunity context: Are diatom communities structured solely by species sorting?. Ecological Indicators 62: 86–94. https://doi.org/10.1016/j.ecolind.2015.11.043.
Vilmi, A., K. T. Tolonen, S. M. Karjalainen & J. Heino, 2017. Metacommunity structuring in a highly-connected aquatic system: effects of dispersal, abiotic environment and grazing pressure on microalgal guilds. Hydrobiologia 790: 125–140. https://doi.org/10.1007/s10750-016-3024-z.
Violle, C., M.-L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel, & E. Garnier, 2007. Let the concept of trait be functional!. Oikos 116: 882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x.
Weithoff, G. & B. E. Beiner, 2019. Measures and approaches in trait-based phytoplankton community ecology—from freshwater to marine ecosystems. Frontiers in Marine Science. 6: 40. https://doi.org/10.3389/fmars.2019.00040.
Wickham, H., W. Chang, L. Henry, T. L. Pedersen, K. Takahashi, C. Wilke, K. Woo & H. Yutani, 2019. ggplot2: create elegant data visualisations using the grammar of graphics. R Package Version 3(2): 1.
Wojciechowski, J., J. Heino, L. M. Bini & A. A. Padial, 2017. The strength of species sorting of phytoplankton communities is temporally variable in subtropical reservoirs. Hydrobiologia 800(1): 31–43. https://doi.org/10.1007/s10750-017-3245-9.
Wu, N., Y. Qu, B. Guse, K. Makarevičiūtė, S. To, T. Riis & N. Fohrer, 2018. Hydrological and environmental variables outperform spatial factors in structuring species, trait composition, and beta diversity of pelagic algae. Ecology and Evolution 8: 2947–2961. https://doi.org/10.1002/ece3.3903.
Xavier, M. B., 1994. Criptógamos do Parque Estadual das Fontes Do Ipiranga, São Paulo, Sp. Algas, 5: Euglenophyceae (Euglenophyceae Pigmentadas). Hoehnea 21(1/2): 47–73.
Xiao, L. J., Y. Zhu, Y. Yang, Q. Lin, B. P. Han & J. Padisák, 2018. Species-based classification reveals spatial processes of phytoplankton meta-communities better than functional group approaches: a case study from three freshwater lake regions in China. Hydrobiologia 811: 313–324. https://doi.org/10.1007/s10750-017-3502-y.
Zhou, J. & D. Ning, 2017. Stochastic community assembly: does it matter in microbial ecology? Microbiology and Molecular Biology Reviews 81: 1–32. https://doi.org/10.1128/mmbr.00002-17.
Zorzal-Almeida, S., J. Soininen, L. M. Bini & D. C. Bicudo, 2017. Local environment and connectivity are the main drivers of diatom species composition and trait variation in a set of tropical reservoirs. Freshwater Biology 62: 1551–1563. https://doi.org/10.1111/fwb.12966.
Acknowledgements
We thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for the scholarship granted for the first author.
Author information
Authors and Affiliations
Contributions
For the development of this research paper, LVC conducted the data sampling and analysis and contributed in the manuscript writing and revising. KFR conducted the data sampling and analysis and manuscript review. LOC conceived and implemented the research, coordinated all laboratory activities, and contributed in the data analysis, manuscript writing, and revising.
Corresponding author
Ethics declarations
Conflict of interest
This study was elaborated with a scholarship granted for the first author by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Viktória B-Béres, Luigi Naselli-Flores, Judit Padisák & Gábor Borics / Trait- Based Approaches in Micro-Algal Ecology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stela, L.V., Ribeiro, K.F. & Crossetti, L.O. Functional and taxonomic approaches differently highlight local and spatial processes in phytoplankton metacommunities. Hydrobiologia 851, 785–800 (2024). https://doi.org/10.1007/s10750-023-05374-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05374-5