Abstract
Phytoplankton communities respond rapidly to environmental selection at a given locality, and they are also shaped by spatial processes at certain scales. The extracted effect of environmental selection and spatial processes may vary if different classification approaches are applied to phytoplankton and the spatial scales. In this study, summer phytoplankton of 43 lakes from three major lake regions in China were investigated and phytoplankton were classified with three approaches: taxonomy of species, habitat template-based functional groups, and morphology-based functional groups. Within a single lake region, the pattern of phytoplankton meta-community was significantly explained by environmental but not by spatial variables. In a larger scale covering all the three lake regions, both environmental and spatial variables explained the variance of the phytoplankton community, but the spatial variables were detected only by classification with species, and only the environmental variables were detected with phytoplankton functional groups. This study revealed that although vegetative populations of different species sorted into the same functional group have common response to environmental changes, their dispersal abilities, mechanisms, and strategies might differ markedly and therefore the species-specific approach cannot be disregarded when studying phytoplankton patterns at spatial scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplankton can easily be dispersed or transported by agents such as rivers, streams, wind, or animals, and the human vector also contributes to their dispersal (Incagnone et al., 2015; Padisák et al., 2016). Thus, phytoplankton communities in separated water bodies are usually connected as a general meta-community via dispersal (Leibold et al., 2004). In a given water body, phytoplankton species are strongly selected by environmental conditions, e.g., physical and chemical conditions, and grazing (Reynolds, 2006). Strong passive dispersal allows phytoplankton to track changes in environmental conditions (Leibold & Miller, 2004; Leibold et al., 2004). Considering the strong environmental filtering in phytoplankton, the species-sorting and mass effects paradigms were used to explain the structure of phytoplankton meta-community according the dispersal rate (Bergström et al., 2008; Vanormelingen et al., 2008; Shoemaker & Melbourne, 2016). Meta-community theory is useful to answer “the paradox of phytoplankton,” and answer questions such as how to recognize the effect of environmental filtering and dispersal in phytoplankton community.
To better identify the variables and their effects of environmental selection, three widely used classifications have been developed: functional groups (FG) as described by Reynolds et al. (2002), morpho-functional groups by Salmaso & Padisák (2007), and the morphology-based functional groups (MBFG) by Kruk et al. (2010). The FG groups (coda) were described along with their habitat templates, which is based on carbon- and nutrient supply, mixing regime and grazing resistance (Padisák et al., 2009), and the functional traits of MBFG focused on cell morphology and later habitat properties were allocated to each group mainly combining transparency, nitrogen, and temperature (Kruk et al., 2010; Kruk & Segura, 2012). As studies with no exception supported that the critical environmental variables can be more easily identified for phytoplankton communities when they are classified with functional groups (e.g., Hu et al., 2013, 2017; Huszar et al., 2015), these approaches became widespread in phytoplankton studies. However, exploring the importance of the spatial scale has started only recently (Naselli-Flores & Padisák, 2016).
Phytoplankton is characterized with small sizes and can easily be transported. So far, dispersal limitation of phytoplankton was detected only on large spatial scales and only large spatial distance explained the compositional differences in lakes (Vyverman et al., 2007; Stomp et al., 2011; De Bie et al., 2012; Rojo et al., 2016). The similarity of phytoplankton communities tends to increase with the distance decay (Nekola & White, 1999). In a natural water body, phytoplankton community is composed by many species with different dispersal abilities. For example, the endemism in flagellates has been repeatedly reported (Wołowski et al., 2013; Cavalcante et al., 2016). The traits used to classify the functional groups are relevant mostly to the response in nutrients, light, and grazing resistance (Salmaso et al., 2015). The differences in dispersal ability have not been incorporated into the current classification of functional groups yet.
Considering the high correlation between functional traits and environment conditions, it is expected that classifications by functional groups would show strong environmental selection but much less effect of dispersal. To confirm the expectation, we conducted a survey of phytoplankton assemblages, environmental variables, and the spatial information of 43 lakes in three major lake regions in China in summer of 2012 (20 July–20 August). The phytoplankton was grouped as taxonomy of species, functional groups (FG; Reynolds et al., 2002; Padisák et al., 2009), and morphological-based functional groups (MBFG; Kruk et al., 2010).
Materials and methods
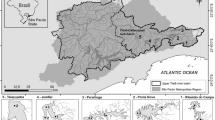
Most lakes in China are located on the west plateau, in the middle and lower reaches of the Yangtze River (middle-east of China) and on the northeast plain. In the present study, 21 lakes (A1–A21) were located on Yunnan plateau (lake region A); the distance between the lakes ranges in 0.34–540 km, average distance is 222 km; 9 lakes (B1–B9) in the lower reaches of the Yangtze River (lake region B), with a distance between the lakes ranging from 21 to 546 km, average distance is 255 km; 13 lakes (C1–C13) on the northeast plain of China (lake region C), the distance between the lakes ranges in 2–679 km, average distance is 307 km; and the distance between the lakes from different regions ranges in 1285–3232 km, average distance is 2353 km (Fig. 1). All the lakes were sampled in summer of 2012 from 20 July to 20 August. The water samples were collected at center of the lake in small lakes (area < 1 km2), and in pelagic zone of large lakes (area > 1 km2). Whole water column samples were taken if water depth (Depth) was lower than 10 m, and whole euphotic layer was sampled in lakes deeper than 10 m. Subsamples were taken for phytoplankton analyses, chlorophyll-a, nutrients, and total suspended solids (TSS) simultaneously. Water temperature (WT), pH, conductivity (Con), and turbidity (Tur) were measured at 1 m depth with Yellow Spring Instrument (YSI). Transparency was measured as Secchi depth (SD). The geographic variables such longitude (Lon), latitude (Lat), and altitude (Alt) and the depth of lakes were also recorded. Nutrients (total nitrogen—TN; total phosphorus—TP; soluble reactive phosphorus—SRP; and total dissolved nitrogen—TDN) were determined following the Chinese National Standards for water quality (similar to those of American Public Health Association, 1989).
One-liter samples were preserved with 1% Lugol for phytoplankton counting. Phytoplankton identification and enumeration were carried out under an inverted microscope (at 400 magnification) in 10-ml sedimentation chambers (Utermöhl, 1958). At least 400 settling units were counted, and the size of each counted algal cell was measured. The biovolume was calculated according to Hillebrand et al. (1999), with assuming a specific gravity of 1 mg mm−3. Phytoplankton were classified in three ways: species and two kinds of functional groups: FG and MBFG.
Analysis of variance (ANOVA) was used to test the difference of each environmental variable in lakes between regions, and 1,000 times’ bootstrap method was used to get the same sample size in the ANOVA analysis. The environmental matrix contained 11 variables (Depth, SD, WT, TP, SRP, TN, TDN, pH, Con, TSS, Tur), and was standardized before the analyses. Phytoplankton community matrices were constructed for three classifications (species, FG, and MBFG), and phytoplankton biomass was then transformed with “Hellinger” (Borcard et al., 2011). Minimum squared-error criterion was used in cluster analysis. The geographical distance (surface distance on earth), the dissimilarity (Bray–Curtis) of phytoplankton community and the gradient of environment (Euclidean) between lakes were calculated, and the relationships between them were tested with Mantel test. The β diversity was calculated and partitioned to species variance (SCBD) among sites and local contributions (LCBD) in β diversity (Legendre & De Cáceres, 2013). The spatial structure was modeled with Moran’s eigenvector map (MEM) to yield MEM variables (V i , i = 1, 2, 3,…, n). Redundancy analysis (RDA) was used to test the relationship between phytoplankton community matrix and environmental matrix, spatial matrix, and the significant variables were detected with forward selection (10,000 permutations). Significant environmental variables and spatial variables were retained for variation partitioning analysis in order to test the interaction of the significant variables of environmental and spatial matrixes on phytoplankton community. In the multivariate analyses, datasets were set by two scales: each dataset for one lake region, and a combined dataset that included all lakes in all the three lake regions. All calculations were carried out in R platform version 3.0.0 (Borcard et al., 2011; R Core Team, 2014).
Results
Physical and chemical conditions
The altitude of lakes was above 1500 m in lake region A, below 77 m in lake region B, and between 125 m and 660 m in lake region C. The environmental conditions were different between the three regions, and six environmental variables showed significant differences (ANOVA, n = 1,000, P < 0.05) between the three regions: water depth (Depth), transparency (SD), pH, turbidity (Tur), total suspended solid concentration (TSS), and total phosphorus concentration (TP). The plateau lakes (in lake region A) were characterized with lower temperature and nutrient concentrations compared to lakes in Lake Regions B and C. The average environmental gradient order (Euclidean distance) in the three regions was as follows: A (3.7) > C (3.5) > B (2.6). The 43 lakes were grouped to five clusters according to the 11 environmental variables (Fig. 2).
Phytoplankton biomass and richness
Altogether 165 species, 26 FGs and 7 MBFGs were observed in all the samples. The biomass of phytoplankton ranged from 0.01 to 29.2 mg l−1 in the 43 lakes. Phytoplankton biomass was significantly lower (ANOVA, n = 1,000, P < 0.05) in lake region A than it in lake region B and C. The dominant species in the 43 lakes and the groups (the first in brackets is FG, the second is MBFG) included the following: Aulacoseira granulata (Ehrenberg) Simonsen (B; VI), Aulacoseira granulata var. angustissima (Otto Müller) Simonsen (B; VI), Cyclotella meneghiniana Kützing (B; VI), Gloeocapsa sp. (TC; VII), Limnothrix planctonica (Woloszynska) Meffert (S1; III), Microcystis aeruginosa (Kützing) Kützing (M; VII), Pseudanabaena sp. (MP; III), Botryococcus braunii Kützing (F; VII), Chlorella cf. pyrenoidosa (H. Chick) (X3; I), Chlorococcum sp. (MP; I), Coelastrum sphaericum Nägeli (F; IV), Palmella mucosa Kützing (F; VII), Cosmarium sp. (N; IV), Peridinium gatunense Nygaard (L O ; V), Peridinium sp. (L O ; V), Ceratium hirundinella (O.F. Müller) Dujardin (L O ; V), Strombomonas ensifera (Daday) Deflandre (W2; V), and Euglena viridis (O.F.Müller) Ehrenberg (W1; V). The lakes could be grouped to four or five groups at the height of 1.5 in cluster analysis with classification of the two functional groups, but only two groups at height of 1.5 in cluster analysis with species (Fig. 3).
The dominant MBFGs were as follows: I, III, IV, VI, V, VII, and the 43 lakes were distinctly grouped into four groups when species were pooled to MBFGs. Group VI is characterized by non-flagellated organisms with siliceous exoskeletons, and dominated mostly in lakes at elevated trophic states (TP > 0.08 mg l−1 and/or SD < 1.5 m). Group VII (large mucilaginous colonies) dominated in lakes at lower trophic state (depth < 8.5 m and TP < 0.08 mg l−1). Group V (unicellular flagellates of medium to large size) dominated the deeper lakes at lower trophic states (lakes with TP < 0.08 mg l−1 and depth > 8.5 m or SD > 1.5 m). The shallow lakes with high level of total suspended solids and turbidity hosted diverse MBFGs (Fig. 3).
The dominant FGs were as follows: B, F, L O , L M , MP, N A , P, S1, Tc, W2, X3, Y, and the 43 samples were distinctly grouped also into four groups. The codon P, mostly Aulacoseira granulata, dominated the lakes with elevated trophic states (TP > 0.08 mg l−1 and/or SD < 1.5 m). The codon B was mostly contributed by Cyclotella and dominated the lakes with lower trophic status (depth > 8.5 m and/or SD > 1.5 m) than codon P. The codon Tc, included mostly Gloeocapsa, dominated in shallow lakes with abundant macrophytes (depth < 8.5 m). The shallow lakes with high level of total suspended solids and turbidity hosted diverse FGs, too (Fig. 3).
The average species number was 31 species per sampled community. A significant difference (ANOVA, n = 1,000, P < 0.05) was found in species richness of local phytoplankton communities between the three meta-communities: 20 species in lake region A, 28 in lake region B, 52 in lake region C. After being grouped into FGs and MBFGs, the numbers of FGs and MBFGs in each local community were substantially lower than species richness, and the biomass and occurrence of FGs and MBFGs were higher than the species (Fig. 4).
Phytoplankton biomass and richness of phytoplankton community. a The phytoplankton biomass in each lakes; b phytoplankton richness of species, FGs, and MBFGs in each lakes; c average biomass of each taxonomy units (each species, FGs and MBFGs) in meta-community; d the occurrence of each taxonomy units (each species, FG and MBFG) in meta-community. A, B, C were the datasets of the three lake regions; T was the combined dataset including all of the 43 lakes. The bottom and upper of box are the first and the third quartile; the dark line in the box is average; dashed line is 1.5 times quartile, the line of the top of dashed line is outlier truncation, and the circle means outliers
There were 49 species, which were observed only in one of the three regions, and most of them belonged to Chlorophyta (mainly from Chlorococcales—15 species and Desmidiales—10 species). In percentage, 30% of species belonging Chlorophyta, Bacillariophyceae, and Dinophyta and 55% of Euglenophyta species could only be observed in one of the three regions. There were 25 species observed only once in all of the 43 samples. In contrast, most FG and all of MBFG could be observed in all of the three regions (Fig. 5). It means the distribution of FGs and MBFGs showed a more homogenous distribution in the three regions than most of species.
Phytoplankton community dissimilarity
The average dissimilarity between local phytoplankton communities was quite high within each lake region. For example, it was 0.82 in lake region A, 0.70 in lake region B, 0.63 in lake region C when the community was described as species. The dissimilarity was lower for FG and MBFG. For partitioning β diversity, the difference in dissimilarity attributed to the difference in SCBD. There was a significant difference between species, FGs and MBFGs in biomass variance among lakes. Most of species biomass variances were low, and the biomass variance of FGs and MBFGs among lakes was significantly higher than that of species (Fig. 6).
Phytoplankton community dissimilarity. a Phytoplankton community dissimilarity in the three lake regions and in the combined dataset; b LCBD of β diversity in the three lake regions and in the combined dataset; c SCBD of β diversity in the three lake regions and in the combined dataset. A, B, C were the datasets of the three lake regions, and T was the combined dataset. The bottom and upper of box are the first and the third quartile; the dark line in the box is average; dashed line is 1.5 times quartile; the line of the top of dashed line is outlier truncation, and the circles mean outliers
In Mantel test, the environmental gradient but not the geographic distance explained significantly the variance of dissimilarity in single region datasets, while both the environmental gradient and the geographic distance contributed significantly to the variance of community dissimilarity in the combined dataset covering all the three regions (Table 1).
Redundancy analysis of phytoplankton communities
In the redundancy analysis (RDA), environmental variables significantly (P < 0.05) explained the compositional variation of phytoplankton community, and no any significant spatial variable was selected in any of the regions. In lake region B, classification by FG and MBFG detected the strongest relationship between environment and phytoplankton community, and conductivity was the significant variable explaining the variation of phytoplankton community.
For the combined dataset covering all the three regions, several environmental variables explained significantly (P < 0.05) phytoplankton community variance, but the component of significant variables was different between the three classifications (Table 2). Twenty positive MEM variables were constructed in Moran’s eigenvector map (MEM), three of them, V1 (\(R_{\text{adj}}^{2}\) = 0.03, F = 2.5, P = 0.005), V2 (\(R_{\text{adj}}^{2}\) = 0.09, F = 1.8, P = 0.04), and V18 (\(R_{\text{adj}}^{2}\) = 0.07, F = 2.4, P = 0.006) explained significantly phytoplankton community classified with species. V1 and V2 both were broad scale variables. The significant spatial variables could explain 11% variance of phytoplankton communities and mostly did not induced by environmental variables (Fig. 7).
Variation in the sites scores of each significant MEM variable (V1, V2, and V18) and partial analysis of significant environment variables and the three significant spatial variables (MEM variables) on the variance of phytoplankton communities classified with species. An overlaid map of studied region is included in the first graph; the larger black or whiter square means the higher positive or negative absolute values for a site, respectively. In partial analysis, a represents environmental variables
Discussion
This study showed that phytoplankton meta-communities were determined mainly by the environment and the spatial processes had effect only at the large spatial scale covering all the three lake regions. The observable effect of environmental and spatial variables in shaping phytoplankton community varied with the used phytoplankton classifications: species, FG, and MBFG.
The classification of phytoplankton by functional groups was developed to better describe the phytoplankton assemblage response to environmental forcing (Reynolds et al., 2002; Salmaso & Padisák, 2007; Kruk et al., 2010) and have been widely applied in description and analysis of phytoplankton patterns. In multivariate analyses, including this study, a higher portion of variance of the phytoplankton communities was repeatedly explained by using any of three functional approaches (Kruk et al., 2011; Hu et al., 2013; Salmaso et al., 2015) than when phytoplankton species or higher taxonomic units were used.
There are dozens of phytoplankton species in natural water bodies but only few of them become dominants because of environmental filtering (Titman, 1976; Tilman et al., 1981; Huisman & Weissing, 1999, 2000). The non-dominant species contribute to the redundancy of the phytoplankton community, and the use of FGs and MBFGs reduces this redundancy (Salmaso et al., 2015; Kruk et al., 2016). In the present study, the total species was up to 165 but the average species number of the single lakes was only 31. The average number of FGs and MBFGs of the investigated lakes were 10 and 6, respectively, and the dissimilarity between phytoplankton assemblages became lower when FGs and MBFGs were used, because of the high occurrence of some FGs and MBFGs.
The distribution and abundance (or biomass) of species in space increased the dissimilarity of phytoplankton communities with the increasing scale. β diversity is a primary indicator presenting variance of communities and it can be partitioned into species variance (SCBD) and local contributions (LCBD) (Legendre & De Cáceres, 2013). The SCBD of both FGs and MBFGs for biomass variance were higher than that for the SCBD of species, while the LCBD were quite similar. It means FGs and MBFGs could more sensitively catch the variance of community than species along the same environmental gradient.
Although the altitude, longitude, and latitude of the three regions were distinct, lakes within the same region experienced similar climate conditions. Therefore, e.g., temperature should be not a critical factor for explaining the pattern of summer phytoplankton community in a single region. The multivariate analysis demonstrated that nitrogen and conductivity were the critical variables in explaining the pattern of phytoplankton meta-community in a single regional scale. The same environmental variables were identified as explanatory variables for the phytoplankton meta-community with the three classifications. The phytoplankton communities of most lakes were dominated by codon B in lake region A characterized by lower temperature and nutrients (nitrogen and phosphorus) than in the other regions. This dominance is consistent with the general description (Padisák et al., 2009) that codon B is typical for lakes with moderate trophic states and low temperatures. Codon P, which prefers higher trophic states (Padisák et al., 2009), was dominant in lake region B and C.
Both FGs and MBFGs could better capture the variation of community along the environmental gradient than species (Reynolds et al., 2002; Padisák et al., 2009; Kruk et al., 2010). In larger scale (combined dataset), transparency and conductivity were the most significant explaining variables for variance of phytoplankton grouped as FGs, and only total dissolved nitrogen explained significantly the variance of phytoplankton community grouped as MBFGs. This result indicates that selection of environmental variables depends not only on the pooling method (here FGs and MBFGs) applied but it might depend on the spatial scale as well.
Although environmental variables significantly explained the variance of species, FGs, and MBFGs, the spatial process (dispersal or transportation) also affect the distribution and abundance of phytoplankton (Bergström et al., 2008; Xiao et al., 2016), especially along large scales (Smith et al., 2005; Vyverman et al., 2007). In the present study, the spatial elements modeled with Moran’s eigenvector map (MEM) based on latitude (Lat) and longitude (Lon) and with Redundancy analysis (RDA), spatial variables significantly explained phytoplankton community at large scale covering three lake regions but not in a single region.
According to Legendre (1993), the spatial variance of phytoplankton community can be explained by spatial gradient of environment (true gradient) and spatial autocorrelation (false gradient). If the spatial variance can be explained by spatial distance, the spatial heterogeneity of phytoplankton must exist (Legendre, 1993; Blanchet et al., 2011). In this study, the distribution of MBFGs was highly homogenous in each of the three regional communities (all MBFGs occurred in all the three regions). The MEM analysis did not select any significant spatial variable explaining the phytoplankton community variance at the regional scale. The distribution of FGs was slightly heterogeneous in single regional meta-communities and between the three regions, but none of the spatial variables were significant, which means the heterogeneity cannot be attributed to spatial distance.
In contrast, most species distributions were highly heterogeneous with low biomass and occurrence and dozens species occurred only in one of the three regions. The distinctive species mostly belonged to Chlorophyta, especially to Chlorococcales and Desmidiales, and most of Euglenophyta species occurred only in one of the three regions. Therefore, the species occurring in high spatial heterogeneity were the important elements in shaping the spatial pattern of phytoplankton community. The MEM analysis showed that large-scale spatial variables significantly explained the variance of phytoplankton community, and they mostly did not induce by environmental variables.
The use of functional groups (Reynolds et al., 2002; Salmaso & Padisák, 2007; Kruk et al., 2010) in phytoplankton advanced greatly our understanding of phytoplankton responses to environmental forcing. Of the three available FG groupings, only the one described by Reynolds et al. (2002) requires obligatorily species-specific identifications, the other two (Salmaso & Padisák, 2007; Kruk et al., 2010), at least in theory, can be applied without almost any knowledge in taxonomy since the specimens occurring in the microscope can be sorted to groups largely by their morphologies. The use functional groups were facilitated by the implementation of the EU water framework directive (WFD; European Commission, 2000), which is aimed at identifying the main human pressures threatening ecological status of surface waters. The so far only direct application of the FG grouping for WFD purposes (Padisák et al., 2006) is based on the FG system by Reynolds et al. (2002) and was not meant to replace species-based documentation of phytoplankton. A quite recent work on functional traits of attached diatoms (Tapolczai et al., 2017) also highlights the necessity of species-specific identifications. This analysis of phytoplankton communities of 43 lakes located in three distant eco-regions of China clearly approved that species-specific approach cannot be neglected when analyzing spatial processes shaping phytoplankton distributions. Another recent study (Várbíró et al., 2017) analyzed the shape of species–area relationship (also a spatial issue) and concluded that species–area relationships are modified by functional redundancy. Thus, spatial issues have to be explored in detail and by a number of different case studies for getting a clear overview in usefulness of species- or functional-based approaches. In a wider view, according to the Baas-Becking (1934) hypothesis, for such small organisms as phytoplankton the “everything is everywhere but environment selects” paradigm applies. If this hypothesis were fully true, we would not find endemics as we do, we would not experience invasions as we do and we would not track adaptive radiation, as we do. So, spatial processes do matter even in phytoplankton. The question is the scale! This paper did show an example at a quite wide spatial scale and many more case studies will be necessary to explore this issue in detail.
In conclusions, the structure of phytoplankton meta-community is strongly organized by the environmental selection, and spatial variables become important only at large scales but not within single lake regions. The functional groups (FG) and morphology-based functional groups (MBFG) successfully identified the environmental filtering on phytoplankton communities but failed in describing the relationship between spatial variables and phytoplankton community. In exploring the effect of spatial distance, analysis of data with high taxonomical resolution might be more promising than use of functional groupings.
References
American Public Health Association, 1989. Standard methods for the examination of water and wastewater. American Water Works Association and Water Pollution Control Federation, Washington, DC.
Bass-Becking, L. G. M., 1934. Geobiologie of inleiding tot de milieukunde. WP Van Stockum and Zoon, The Hague, The Netherlands.
Bergström, A. K., C. Bigler, U. Stensdotter & E. S. Lindström, 2008. Composition and dispersal of riverine and lake phytoplankton communities in connected systems with different water retention times. Freshwater Biology 53: 2520–2529.
Blanchet, F. G., P. Legendre, R. Maranger, D. Monti & P. Pepin, 2011. Modelling the effect of directional spatial ecological processes at different scales. Oecologia 166: 357–368.
Borcard, D., F. Gillet & P. Legendre, 2011. Numerical ecology with R. Springer, New York.
Cavalcante, K. P., L. D. S. Cardoso, R. Sussella & V. Becker, 2016. Towards a comprehension of Ceratium (Dinophyceae) invasion in Brazilian freshwaters: autecology of C. furcoides in subtropical reservoirs. Hydrobiologia 771: 265–280.
De Bie, T., L. L. De Meester, L. Brendonck, K. Martens, B. Goddeeris, D. Ercken, H. Hampel, L. Denys, L. Vanhecke, K. Van der Gucht, J. Van Wichelen, W. Vyverman & S. A. J. Declerck, 2012. Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecology Letters 15: 740–747.
European Commission, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities 327: 1–72.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hu, R., B. P. Han & L. Naselli-Flores, 2013. Comparing biological classifications of freshwater phytoplankton: a case study from South China. Hydrobiologia 701: 219–233.
Hu, R., D. Xueke, L. Peng, B. P. Han & L. Naselli-Flores, 2017. Phytoplankton assemblage in a complex system of interconnected reservoirs: the role of water transport in dispersal. Hydrobiologia 800: 17–30.
Huisman, J. & F. J. Weissing, 1999. Biodiversity of plankton by species oscillations and chaos. Nature 402: 407–410.
Huisman, J. & F. J. Weissing, 2000. Reply: coexistence and resource competition. Nature 407: 694.
Huszar, V. L. M., J. C. Nabout, M. O. Appel, J. B. O. Santos, D. S. Abe & L. H. S. Silva, 2015. Environmental and not spatial processes (directional and non-directional) shape the phytoplankton composition and functional, groups in a large subtropical river basin. Journal of Plankton Research 660: 1190–1200.
Incagnone, G., F. Marrone, R. Barone, L. Robba & L. Naselli-Flores, 2015. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750: 103–123.
Kruk, C. & A. M. Segura, 2012. The habitat template of phytoplankton morphology-based functional groups. Hydrobiologia 698: 191–202.
Kruk, C., V. L. M. Huszar, E. T. H. M. Peeters, S. Bonilla, L. Costa, M. Lürling, C. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Kruk, C., E. T. H. M. Peeters, E. H. Van Nes, V. L. M. Huszar, L. S. Costa & M. Scheffer, 2011. Phytoplankton community composition can be predicted best in terms of morphological groups. Limnology and Oceanography 56: 110–118.
Kruk, C., A. M. Segura, L. S. Costa, G. Lagerot, S. Kosten, E. T. H. M. Peeters, V. L. M. Huszar, N. Mazzeo & M. Scheffer, 2016. Functional redundancy increases towards the tropics in lake phytoplankton. Journal of Plankton Research 39: 518–530.
Legendre, P., 1993. Spatial autocorrelation: trouble or new paradigm? Ecology 74: 1659–1673.
Legendre, P. & M. De Cáceres, 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16: 951–963.
Leibold, M. A. & T. E. Miller, 2004. From metapopulations to metacommunities. In Hanski, I. & O. E. Gaggiotti (eds), Ecology, genetics and evolution of metapopulations. Academic Press, San Diego: 133–150.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Naselli-Flores, L. & J. Padisák, 2016. Blowing in the wind: how many roads can a phytoplankton walk down? A synthesis on phytoplankton biogeography and spatial process. Hydrobiologia 764: 303–313.
Nekola, J. C. & P. S. White, 1999. The distance decay of similarity in biogeography and ecology. Journal of Biogeography 26: 867–878.
Padisák, J., G. Borics, I. Grigorszky & É. Soróczki-Pintér, 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: the assemblage index. Hydrobiologia 553: 1–14.
Padisák, J., L. O. Crossetti & L. Naselli-flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Padisák, J., G. Vasas & G. Borics, 2016. Phycogeography of freshwater phytoplankton: traditional knowledge and new molecular tools. Hydrobiologia 764: 1–25.
R Core Team, 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Reynolds, C. S., 2006. The ecology of phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rojo, C., F. Mesquita-Joanes, J. S. Monrós, J. Armengol, M. Sasa, F. Bonilla, R. Rueda, J. Benavent-Corai, R. Picula & M. M. Segura, 2016. Hydrology affects environmental and spatial structuring of microalgal metacommunities in tropical Pacific coast wetlands. PLoS ONE 11: e0149505.
Salmaso, N. & J. Padisák, 2007. Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112.
Salmaso, N., L. Naselli-Flores & J. Padisák, 2015. Functional classifications and their application in phytoplankton ecology. Freshwater Biology 60: 603–619.
Shoemaker, L. G. & B. A. Melbourne, 2016. Linking metacommunity paradigms to spatial coexistence mechanisms. Ecology 97: 2436–2446.
Smith, V. H., B. L. Foster, J. P. Grover, R. D. Holt, M. A. Leibold & F. deNoyelles, 2005. Phytoplankton species richness scales consistently from laboratory microcosms to the world’s oceans. Proceedings of the National Academy of Sciences of the United States of America 102: 4393–4396.
Stomp, M., J. Huisman, G. G. Mittelbach, E. Litchman & C. A. Klausmeier, 2011. Large-scale biodiversity patterns in freshwater phytoplankton. Ecology 92: 2096–2107.
Tapolczai, K., A. Bouchez, C. Stenger-Kovács, J. Padisák & F. Rimet, 2017. Taxonomy- or trait-based ecological assessment for tropical rivers? Case study on benthic diatoms in Mayotte island (France, Indian Ocean). Science of the Total Environment 607–608: 1293–1303.
Tilman, D., M. Mattson & S. Langer, 1981. Competition and nutrient kinetics along a temperature gradient: an experimental test of a mechanistic approach to niche theory. Limnology and Oceanography 26: 1020–1033.
Titman, D., 1976. Ecological competition between algae: experimental confirmation of resource-based competition theory. Science 192: 463–465.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodi. Mitteilungen der Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Várbíró, G., J. Görgényi, B. Tóthmérész, J. Padisák, É. Hajnal & G. Borics, 2017. Functional redundancy modifies SAR for freshwater phytoplankton. Ecology and Evolution 7: 9905–9913.
Vanormelingen, P., K. Cottenie, E. Michels, K. Muylaert, W. Vyverman & L. De Meester, 2008. The relative importance of dispersal and local processes in structuring phytoplankton communities in a set of highly interconnected ponds. Freshwater Biology 53: 2170–2183.
Vyverman, W., E. Verleyen, K. Sabbe, K. Vanhoutte, M. Sterken, D. A. Hodgson, D. G. Mann, S. Juggins, B. V. De Vijver, V. Jones, R. Flower, D. Roberts, V. A. Chepurnov, C. Kilroy, P. Vanormelingen & A. De Wever, 2007. Historical processes constrain patterns in global diatom diversity. Ecology 88: 1924–1931.
Wołowski, K., M. Poniewozik & P. L. Walne, 2013. Pigmented euglenophytes of the genera Euglena, Euglenaria, Lepocinclis, Phacus and Monomorphina from the southeastern United States. Polish Botanical Journal 58: 659–685.
Xiao, L. J., R. Hu, L. Peng, L. M. Lei, Y. Feng & B. P. Han, 2016. Dissimilarity of phytoplankton assemblages in two connected tropical reservoirs: effects of water transportation and environmental filtering. Hydrobiologia 764: 127–138.
Acknowledgements
We acknowledge the Grants from National Basic Research Program of China (No: 2012CB956100) and the Science and Technology Project of Guangdong Province (No: 2013B08050022). Partial support was provided by the Hungarian National Research, Development and Innovation Office (NKFIH-120595) and by the GINOP –2.3.2–15–2016–00019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Xiao, LJ., Zhu, Y., Yang, Y. et al. Species-based classification reveals spatial processes of phytoplankton meta-communities better than functional group approaches: a case study from three freshwater lake regions in China. Hydrobiologia 811, 313–324 (2018). https://doi.org/10.1007/s10750-017-3502-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3502-y