Abstract
The Three Gorges Reservoir is one of the largest reservoirs in the world. Previous studies uncovered longitudinal changes in fish assemblages among riverine, transitional, and lacustrine zones, but whether they vary at small-scale habitats and how tributaries contribute to fish conservation is poorly understood. We selected three neighboring tributaries with different habitat characteristics within and among tributaries and hypothesized that the small-scale habitat heterogeneity within or among tributaries could shape spatial and temporal variation in fish assemblages. We analyzed spatial (among tributaries, pelagic versus benthic, lower versus upper reach) and seasonal patterns of fish assemblage and their relationship with the environment. Piscivorous, limnophilic, and mid-pelagic species dominated the fish assemblage in different habitats. Fish assemblage structure varied spatially between lower and upper reaches and among tributaries. Fish assemblages also varied seasonally, mainly related to environmental variability (i.e., water depth, water temperature, pH, specific conductivity, riparian slope, and vegetation coverage). Catch per unit effort was similar between pelagic and benthic habitats but exhibited horizontal and seasonal changes. The most influential environmental variables were water depth, water temperature, dissolved oxygen, and pH. Our results supported the hypothesis, and indicated that we should protect tributaries that provide important areas for fish diversity in large reservoirs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Habitat heterogeneity involves the spatial and temporal complexity of natural habitats at different scales, and it is critical to maintaining biodiversity (MacArthur & MacArthur, 1961; Kovalenko et al., 2012; Spurgeon et al., 2018; Heidrich et al., 2020). Evaluating the multidimensionality of the relationship between habitat heterogeneity and biological communities remains a priority in ecological research (Alberti & Wang, 2022; Miao et al., 2022), particularly in the face of global climate change, habitat loss, and biodiversity decline (Eddy et al., 2021). For fish fauna, habitat heterogeneity can influence fish diversity and assemblage structure at multiple temporal and spatial dimensions (López-Delgado et al., 2020), where habitat heterogeneity can be derived from channel geomorphology (e.g., lake area, river width, sediment particle size, shoreline development, and vegetation coverage; Eadie & Keast, 1984), hydrology (e.g., water depth, flow velocity; Smith & Mather, 2013), water quality, and food availability (Ye et al., 2007; Spurgeon et al., 2018). However, artificial hydrological change alters habitat heterogeneity in freshwater ecosystems, leading to habitat homogenization and potentially diminishing fish diversity (Zeng et al., 2017).
At present, 57,985 large dams (height > 15 m or height 5–15 m and impounding > 3 million m3) have been built in the world, and dam building is still growing at an annual growth rate of 2%–3% (ICOLD, 2019). How habitat changes after dam construction affect fish assemblages is a major concern for freshwater ecologists. Fish undergo different life history stages, from spawning, hatching, larvae, juvenile, to reproductive and senescent adult (Chen & Liu, 2022). Each stage requires particular environmental conditions (e.g., water velocity, water temperature, dissolved oxygen, vegetation coverage, and food availability) and fish at all stages are susceptible to damming (Toussaint et al., 2016). Dams block the migratory pathways of fishes and disrupt the hydrological conditions required for ovulation and fertilization and development of eggs, larvae, and young (Pelicice et al., 2015; Arantes et al., 2019). These combined effects on life history traits affect population persistence. Impoundment reduces flow rate and the abundance of rheophilic fish (Sá-Oliveira et al., 2015). Dam-induced changes in food availability often increase the abundance and dominance of generalist fish species (Turgeon et al., 2019). Lastly, as the riverine habitat is replaced by large areas of lentic habitat after dam regulation, the resulting habitat homogeneity can simplify fish assemblages and lead to fish diversity decline (Perônico et al., 2020). These combined impacts affect fish assemblages at a large scale, and the detrimental effect on fish biodiversity is exacerbated by the fact that the areas with greater hydropower development are generally located in river reaches with the most diverse habitat heterogeneity (e.g., headwaters, rapids) (Cheng et al., 2015).
From a metacommunity perspective, tributaries constitute an important source contributing to the maintenance of fish diversity in dammed systems (Vasconcelos et al., 2021). In large reservoirs, tributaries provide essential refuges to migratory and riverine species and also act as a natural stocking source (Da Silva et al., 2015; Vitorino Junior et al., 2016; Marques et al., 2018; Azevedo-Santos et al., 2021; Vasconcelos et al., 2021). After impoundment, tributaries retain different habitats at the local scale. In turn, fish assemblage structure is influenced by local conditions, including water depth (Mattos et al., 2014), flow regime (Yan et al., 2011), water temperature (Togaki et al., 2023), transparency (Mattos et al., 2014), dissolved oxygen (Ostrand & Wilde, 2001), substrate size (Wang et al., 2013), shelter (Mattos et al., 2014), and riparian vegetation coverage (Montag et al., 2019). The variation in these environmental variables creates small-scaled habitat heterogeneity within and among tributaries after dam regulation which support fish assemblages by providing spawning, feeding, and refuge (Szalóky et al., 2021).
The upper reach of the Yangtze River and its tributaries have diverse flow regimes and complex habitats and harbor the highest fish species richness in China (Liu & Cao, 1992). The impoundment of the Three Gorges Dam formed an enormous reservoir with a total length of 667 km and a total area of 1080 km2, which dramatically impacted aquatic habitats and fishes therein (Liao et al., 2018a). The average flow velocity of the mainstream decreased by 90% after the impoundment, and the lotic areas were greatly reduced (Yang et al., 2012), which generated a longitudinal gradient of riverine, transitional, and lacustrine habitats from the upper to lower sections of the mainstem that forms the reservoir, and from the upstream to downstream of reservoir tributaries. In addition, the artificially regulated water level altered the natural hydrological regime of the river, and diminished the hydrological heterogeneity in the reservoir (Liao et al., 2019). Based on these habitat changes, previous studies investigated alterations to the structure of fish assemblages in the TGR at large scales, including spatial comparisons among different reservoir sections (upper, middle, and lower; Liao et al., 2018a), and temporal comparisons between pre- and post-impoundment, and among different impoundment stages, from the perspectives of species richness (Gao et al., 2010; Yang et al., 2012; Liao et al., 2018a), assemblage structure (Wu et al., 2007; Gao et al., 2010; Yang et al., 2012; Zhao et al., 2015), and life history (Perera et al., 2014; Liao et al., 2018b, 2019). These studies found that habitat homogeneity simplified fish assemblages in the TGR; however, they also revealed a relatively high fish diversity in the whole reservoir due to the remaining lotic stretches at the upper section of the reservoir. Additionally to the lotic upper stretch of the TGR, > 10 tributaries flowing into the TGR create relatively high heterogeneity at a local scale (Xu et al., 2012). Nevertheless, how local habitat conditions influence fish assemblages in the TGR is still poorly understood.
To fill up this knowledge gap, our study selected three neighboring tributaries flowing into the lower section of the TGR as a model system to evaluate the influence of reservoir tributaries on fish diversity in large reservoirs. Specifically, the three tributaries constitute heterogeneous habitat characterized by variable water quality and physical characteristics within and among tributaries and relative to the reservoir. These tributaries' lower and upper reaches are affected by the TGR during the high water season (175 m, winter), while the upper reaches can partly recover riverine state during the low water period (145 m, summer). We investigated spatial and seasonal patterns in fish assemblage structure among the three tributaries and evaluated the relationship between assemblage structure and habitat characteristics. We hypothesized that the small-scale habitat heterogeneity within (gradients, habitats) or among tributaries (identity) could shape spatial and temporal variation in fish assemblages. Specifically, we aimed to: (1) test whether fish assemblage structure differed among tributaries, reaches within tributaries, and seasons; (2) identify the potentially environmental variables driving spatial and temporal changes in fish assemblage structure.
Materials and methods
Sampling sites
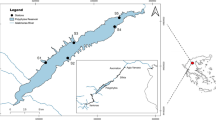
The TGR is located in the upper reach of the Yangtze River with a high fish species richness and contains numerous tributaries distributed in different reservoir sections. The Xiangxi River (X), Yuanshui River (Y), and Qinggan River (Q) are three neighboring tributaries located on the lower section of the TGR and, together with the mainstem, form a dendritic river network. The reservoir has partly impounded the three tributaries, yet different local-scale habitats with various water quality and physical characteristics remain within and between tributaries. At each tributary, we selected the lower (X-Lower, Y-Lower, and Q-Lower) and upper (X-Upper, Y-Upper, and Q-Upper) reaches to sample (Fig. 1). The X-Upper, Y-Upper, and Q-Upper reaches are about 21 km, 15 km, and 18 km from the mainstem of the TGR, while the X-Lower, Y-Lower, and Q-Lower reaches are about 0.8 km from the mainstem, and these areas are free of dams. Summary information on sampling reaches is included in Table 1.
Three Gorges Reservoir in the Yangtze River (upper right corner map) and the location of the lower and upper reaches of three reservoir tributaries flowing into the lentic section of the reservoir (center map). Abbreviations: Xiangxi River (X-Lower, X-Upper), Qinggan River (Q-Lower, Q-Upper), and Yuanshui River (Y-Lower, Y-Upper). Dotted arrows represent the flow direction of the Yangtze River
Fish sampling
We seasonally sampled fishes at the lower and upper reaches per tributary during the summer (July), autumn (October), winter (January), and spring (April) seasons between July 2020 and January 2022 (N = 36 sampling events per season; i.e., 3 rivers * 2 reaches * 3 sites * 2 repeated days). We sampled benthic and pelagic fishes using benthic and pelagic experimental multi-panel gillnets, respectively. The two gillnet types have different heights (2 and 5 m, benthic and pelagic, respectively) but have the same length (30 m) and mesh-size structure. Each gillnet consisted of 12 panels (2.5 m each) of different mesh sizes (10, 16, 20, 25, 31, 39, 48, 58, 70, 86, 110, 125 mm, stretched mesh sizes). These multimesh gillnets are comparable to CEN 2015 standard (CEN, 2015). We randomly selected three sites (≈ 500 m apart) at each sampling reach, covering different habitats, and we tied together three benthic gillnets (3*30 m = 90 m total length) and three pelagic gillnets (3*30 m = 90 m total length) and deployed them for 12 h (18:00–19:00 to 6:00–7:00) per site. We repeated our sampling at each sampling site the next day to increase capture (24 h total). The pelagic and benthic gillnets have different headropes with floats and footrope with sinkers, and we always ensured that the pelagic and benthic gillnets sampled the surface and bottom water, respectively. We deployed gillnets from the littoral to the pelagic areas with the aid of a boat driving in reverse. We estimated the depth of the benthic areas sampled by benthic gillnets based on their total length (3*30 m = 90 m) and the maximum riparian slope of sampling sites (85° angle); the benthic gillnets can sample the maximum water depth of 89.7 m.
We identified all caught fishes to species level (Ding, 1994) and measured their total length (TL), standard length (SL), and body weight (BW) to 0.1 mm, 0.1 mm, and 0.01 g, precision, respectively. We categorized each fish species based on their dietary guild (Herbivorous, detritivores, planktivorous, omnivorous, invertivores, and piscivorous; Ding, 1994), water layer (pelagic, mid-pelagic, mid-benthic, and benthic; Ding, 1994), flow guild (eurytopic, rheophilic, and limnophilic; Xiao et al., 2015), and native status (Ding, 1994; Ba & Chen, 2012; Online Resource 1). Among these, we classified fishes with superior, sub-superior, sub-inferior, and inferior mouth position as pelagic, mid-pelagic, mid-benthic, and benthic dwellers, respectively (Ding, 1994; Lund et al., 2010). We calculated catch per unit effort per sampling unit (i.e., sampling location) based on fish biomass (BPUE, g/m2 of gillnets per night) and abundance (NPUE, individuals/m2 of gillnets per night), respectively. We also calculated relative abundance or relative biomass per fish species per site (e.g., abundance per species/total abundance per site).
Habitat characterization
The highest water level at the TGR occurs from autumn to winter (from November to January), whereas the lowest occurs during summer (June and July). During the high water level season, the TGR creates a backwater effect in the tributaries, which equalizes their water level with that of the TGR. We, therefore, investigated the physical habitat characteristics of tributaries during the lowest water level period (i.e., summer, July). We established ten random transects at each sampling reach, separated by 100 m from each other. At each transect, we measured maximum water depth (MWD, m) with a Speedtech sounder, and visually defined water-level-fluctuation zones (water-land transition zone formed by fluctuating water level at an elevation ranging between 145 and 175 m above sea level; Chen et al., 2020) as natural (no artificial transformation) and artificial conditions (artificial transformation by hardening of shorelines with riprap or concrete; Lapointe et al., 2014), and estimated the vegetation cover in the riparian zone (Zhu et al., 2020). We categorized the substratum of water-level-fluctuation zones as rock, riprap, pebble, sand, and mud, recorded their respective proportions, and then grouped them into rock and sand-mud to facilitate the analysis (Eitzmann & Paukert, 2010), and we classified artificial riprap and concrete as rock substratum. We also estimated the riparian slope of each transect and calculated the average slope (degree) of each reach (Table 1). Due to the steep slopes, the substratum and vegetation coverage values are aerial proportions. We seasonally measured water transparency (WTR, m) with a Secchi disk, and quality parameters of surface water, including pH, water temperature (WTE), dissolved oxygen (DO), and specific conductivity (Con) with an YSI Pro Plus multiparameter water quality analyzer at five random transects. We also measured the vertical profile of dissolved oxygen with two-second intervals at about 0.4 m depth intervals, to a maximum depth of 94 m using an optical sensor installed on an YSI EXO2 multiparameter water quality sonde.
Data analysis
We calculated species richness, relative abundance, and relative biomass for each sampling reach by pooling data from multiple sampling sites. We measured taxonomic beta-diversity among sampling reaches based on our “reach × species” dataset using Jaccard’s dissimilarity index. We used permutated multivariate analyses of variance (PERMANOVA) to assess differences in assemblage structure between pelagic and benthic habitats among the three tributaries, the lower and upper reaches, and four seasons. We also used the PERMANOVA to analyze spatial differences in fish assemblage structure, categorized by dietary guild, water layer, and flow guild, among six sampling reaches (identified by its tributary: X-Lower, Y-Lower, Q-Lower, X-Upper, Y-Upper, and Q-Upper). We then used Non-metric multidimensional scaling (NMDS) with Bray–Curtis similarity to visualize spatial and seasonal variation in fish assemblage structure. Similarity percentages (SIMPER) tests were used to assess the contribution of fish species to the spatial and seasonal differences.
We also examined relationships between spatial and seasonal variation in fish assemblages and environmental variables using canonical correspondence analysis (CCA). The CCA selects a linear combination of environmental variables and maximizes the dispersion of the species scores (Abdel-Dayem et al., 2021). The ordination output shows patterns directly related to the environmental conditions being examined. We used the variance inflation factor (VIF) value to test collinearity among environmental variables and excluded collinear variables with VIF > 10 (Gladyshev et al., 2018); in the CCA model, we included seasonal water quality variables (i.e., MWD, WTE, DO, pH, WTR, and Con) and physical variables which are expected to be equal year round (i.e., rock substratum, slope, and vegetation coverage). We used the Monte Carlo permutation test (1000 permutations) to analyze the significance of the CCA model and its canonical axes. We calculated correlation coefficients for explanatory variables representing the importance of each variable on each axis. We excluded 14 fish species occurring at only one reach from the PERMANOVA, NMDS, SIMPER, and CCA analyses to reduce the influence of rare species (Legendre & Legendre, 1998).
We implemented linear mixed-effects model (LMM) to compare the BPUE or NPUE between pelagic and benthic assemblages (i.e., the two gillnet types) and included reach (identified by its tributary: X-Lower, Y-Lower, Q-Lower, X-Upper, Y-Upper, and Q-Upper) as a random factor to account for the lack of independence between multiple sampling events conducted in the same area (i.e., N = 237 sampling events total; 3 tributaries * 2 reaches * 2 net types * 7 seasons * 2–3 sampling sites) (Bates et al., 2015). Given the lack of difference between gillnet types, we pooled pelagic and benthic gillnets yield per sampling event and evaluated spatial and temporal differences in abundance. Specifically, we modeled BPUE or NPUE as a function of reach (identified by its tributary), season, and their interaction using a general linear model (GLM). We also modeled the BPUE or CPUE against environmental predictor variables (including MWD, WTE, DO, pH, WTR, and Con) and added reach (identified by its tributary) as random factor using a LMM. Prior to analyses, we transformed the BPUE and NPUE data by log- and logit transformations, respectively.
We conducted all statistical analyses in R (v.4.0.2, R Foundation for Statistical Computing, Vienna, Austria). We calculated the taxonomic beta-diversity using the betapart package (Baselga & Orme, 2012). We performed the regression model, main effects estimation (Type III Wald’s X2 test), model fit plots and tests, and post hoc comparisons through the lme4 (Bates et al. 2015), car (Fox & Weisberg, 2011), and emmeans packages (Lenth et al., 2022), respectively. We implemented the NMDS, CCA, PERMANOVA, and Simper analyses using the vegan package (Oksanen et al., 2020). We considered statistically significant differences at an alpha level of 0.05.
Results
We collected a total of 10,373 individuals weighing 579.9 kg and identified a total of 55 fish species at the upper and lower reaches of the three tributaries (averaged TL = 181.3 ± 1.0 mm; BW = 55.9 ± 4.2 g (mean ± S.E.)). Of these, the order Cypriniformes with three families and 38 species dominated, followed by Perciformes (three families, six species) and Siluriformes (two families, six species). The family Cyprinidae had the highest species richness (34 species), followed by Bagridae (four species), Cobitidae (three species), and Serranidae (three species). Among these, 49.1% of species inhabited benthic habitats; 60% were limnophilic species; 25.5%, 29.1%, and 21.8% were piscivorous, invertivorous, and omnivorous species, respectively; 9.1% of species (five) were non-native species (Online Resource 1). The species richness per reach ranged from 32–44 (X-Lower = 44, X-Upper = 41, Y-Lower = 32, Y-Upper = 35, Q-Lower = 34, and Q-Upper = 33, respectively).
Spatial and temporal patterns of fish assemblage structure
We observed an overarching pattern, where a few fish species accounted for most of the biomass and abundance in reservoir tributaries. Specifically, 15 species accounted for 91.2% of the biomass and 12 species made up 90.2% of the abundance. Coilia brachygnathus Kreyenberg & Pappenheim, 1908 was the most abundant species, which accounted 20.2% of the biomass and 34.6% of the abundance (Online Resource 1). The averaged value of taxonomic beta-diversity was higher when comparing reaches among tributaries (0.41 ± 0.11; lower vs. lower: 0.39 ± 0.27; upper vs. upper: 0.42 ± 0.28; lower vs. upper: 0.43 ± 0.19; mean ± SE) than when the comparisons were made between the lower and upper reaches within tributaries (0.32 ± 0.22; X-Lower vs. X-Upper, 0.31; Y-Lower vs. Y-Upper, 0.33; Q-Lower vs. Q-Upper, 0.33). Fish assemblage structure varied between the pelagic and benthic habitat (PERMANOVA, F = 5.39, P < 0.001), among the three tributaries (F = 2.66, P = 0.001; X–Y, P = 0.01; Q-X, P = 0.02; Q-Y, P = 0.55; Fig. 2a), between the lower and upper reaches (F = 2.98, P = 0.004; Fig. 2b), and among four seasons (F = 3.15, P = 0.001; all pairwise comparisons P < 0.01, except for autumn vs. winter, spring vs. summer; Fig. 2c). Coilia brachygnathus (Simper test, 26.5%), Hemiculter bleekeri Warpachowski, 1888 (11.1%), Pseudobrama simony (Bleeker, 1864) (8.7%), Culter alburnus Basilewsky, 1855 (6.5%), Hemiculter leucisculus (Basilewsky, 1855) (6.5%), Pelteobagrus nitidus (Sauvage & Dabry de Thiersant, 1874) (5.8%), Pseudolaubuca sinensis Bleeker, 1864 (4.6%), Pelteobagrus vachelli (Richardson, 1846) (4.3%), Squalidus argentatus (Sauvage & Dabry de Thiersant, 1874) (4.4%), and Saurogobio dabryi Bleeker, 1871 (3.8%) principally contributed to both spatial and temporal differences (Simper test, 82.2% in total; Fig. 2). When fish species were categorized based on their dietary guild, water layer, and flow guild, assemblage structure also varied within and among tributaries (PERMANOVA, Dietary guild: F = 0.48, P = 0.03; Water layer: F = 0.55, P = 0.01; Flow guild: F = 0.57, P = 0.01). Overall, Overall, mid-pelagic (73.4%), and limnophilic (50.9%) groups dominated the biomass, irrespective of tributaries. The Yuangshui and Qinggan have less abundance in piscivorous fish than the Xiangxi, but have more abundance in planktivorous fish. From the upper to lower reaches of the tributaries, the abundance of mid-pelagic fish decreased but that of rheophilic fish increased (Table 2).
Non-metric multidimensional scaling (NMDS) based on abundance showing changes in fish assemblage structure among tributaries (a), reaches within tributaries (b), and seasons (c). Species abbreviations: Ps_si, Pseudolaubuca sinensis; Sp_si, Spinibarbus sinensis; Co_br, Coilia brachygnathus; Cu_da, Culter dabryi; Pe_va, Pelteobagrus vachelli; Ac_ch, Acheilognathus chankaensis
The evaluated environmental factors explained 59.1% of the total variation associated with the first and second axes of the CCA (Permutation test, F = 2.29, P < 0.01; adjusted R2 = 0.35). The MWD, WTE, pH, Con, slope, and vegetation coverage significantly affected fish assemblage structure (Permutation test, all P < 0.05). The first axis summarized 38.2% of the variance (F = 7.87, P < 0.01) and was positively correlated with Con (correlation coefficient = 0.71) and WTE (0.63), influencing the abundance of C. brachygnathus and H. bleekeri; in contrast, it was negatively correlated with pH (− 0.43) and vegetation coverage (− 0.57), influencing the abundance of Parabramis pekinensis (Basilewsky, 1855) and Ctenopharyngodon idella (Valenciennes, 1844). The second axis summarized 20.9% of the variance (F = 4.31, P < 0.01) and was positively correlated with pH (0.47) and vegetation coverage (0.38), influencing the abundance of P. pekinensis, Pelteobagrus fulvidraco (Richardson, 1846), and P. nitidus; in contrast, it was negatively correlated with MWD (− 0.83) and slope (− 0.33), influencing the abundance of Leiocassis longirostris (Bleeker, 1864), Rhinogobio cylindricus Günther, 1888, Coreius heterodon (Bleeker, 1864), Acheilognathus macropterus (Bleeker, 1871), and Silurus meridionalis Chen, 1977 (Fig. 3).
Results of canonical correspondence analysis (CCA) showing the relationship between environmental variables and fish assemblage structure in the lower and upper reaches of the Xiangxi River, Qinggan River, and Yuanshui River. Variables abbreviations: DO, dissolved oxygen; WTR, water transparency; WTE, water temperature; Con, specific conductivity; MWD, maximum water depth. Species abbreviations: Pa_pe, Paralramis pekenensis; Pe_fu, Pelteobagrus fulvidraco; Pe_ni, Pelteobagrus nitidus; Cu_al, Culter alburnus; Pe_va, Pelteobagrus vachelli; Ps_sim, Pseudobrama simony; Co_br, Coilia brachygnathus; Cu_da, Culter dabryi; Cl_id, Ctenopharyngodon idella; Ps_si, Pseudolaubuca sinensis; He_le, Hemiculter leucisculus; Sq_ar, Squalidus argentatus; He_bl, Hemiculter bleekeri; Si_me, Silurus meridionalis; Ac_ma, Acheilognathus macropterus; Le_lo, Leiocassis longirostris; Rh_cy, Rhinogobio cylindricus; Co_he, Coreius heterodon; Cy_ca, Cyprinus carpio; Ar_no, Aristichthys nobilis; Cu_er, Cultrichthys erythropterus; Pr_ch, Protosalanx chinensis
Spatial and temporal comparison of CPUE
The overall BPUE was 8.96 ± 0.77 g/m2/night (mean ± SE, N = 237), which was similar between pelagic gillnets (9.31 ± 1.15 g/m2/night, N = 121) and benthic gillnets (8.6 ± 0.94 g/m2/night, N = 116; LMM, X2 = 2 × 10–4, P = 0.99). The pooled average of the BPUEs changed spatially and temporally, as indicated by a significant interaction effect between reach and season (GLM, F = 2.42, P = 0.003), and the significant effect of reach (F = 9.62, P < 0.001) and season (F = 15.65, P < 0.001; Online Resource 2). The averaged BPUE was the lowest at Y-Lower in winter (1.92 ± 0.29 g/m2/night), and the highest at Y-Upper during spring (35.33 ± 8.11 g/m2/night; Fig. 4).
Spatial and seasonal changes of the averaged (i.e., two net types) catch per unit effort based on fish biomass (BPUE) and abundance (NPUE) in the lower and upper reaches of the Xiangxi River (X-Lower, X-Upper), Qinggan River (Q-Lower, Q-Upper), and Yuanshui River (Y-Lower, Y-Upper). Values with different lowercase letters above the bar are significantly different at alpha 0.05
The overall NPUE was 0.19 ± 0.02 ind./m2/night (mean ± SE, N = 237), which was similar between the two gillnet types (benthic: 0.22 ± 0.03 ind./m2/night, pelagic: 0.16 ± 0.02 ind./m2/night; X2 = 3.22, P = 0.07). The pooled average of the NPUEs also changed spatially and temporally, but the spatial variation appears to be mediated by season (reach × season: F = 2.19, P = 0.01; reach: F = 2.08, P = 0.07; season: F = 33.6, P < 0.001; Online Resource 2). The lowest averaged NPUE was observed at the X-Upper in winter (0.05 ± 0.01 ind./m2/night), and the highest at the X-Lower in summer (0.51 ± 0.11 ind./m2/night; Fig. 4).
Both the BPUE and NPUE decreased with water level (MWD; LMM, BPUE: X2 = 30.57, P < 0.001; NPUE: X2 = 10.10, P = 0.001) and dissolved oxygen (DO, BPUE: X2 = 4.02, P = 0.04; NPUE: X2 = 5.73, P = 0.02). Meanwhile, the BPUE increased with pH (X2 = 9.71, P = 0.002), and NPUE increased with water temperature (WTE, X2 = 10.08, P = 0.002; Fig. 5).
Discussion
Classical and contemporary studies demonstrate that habitat heterogeneity influences the species assemblage structure of various animal taxa (MacArthur & MacArthur, 1961; Alberti & Wang, 2022; Meerhoff & González-Sagrario, 2022). Previous studies at the TGR demonstrated the role of large-scale habitat heterogeneity on fish assemblage structure (e.g., Liao et al., 2018a; Yang et al., 2021). The present study goes a step further to investigate whether fish assemblage structure is influenced by small-scale habitat heterogeneity and to explore how reservoir tributaries contribute to the conservation of fish diversity in large reservoirs. Our results supported the hypothesis that fish assemblage structure differed among tributaries, reaches within tributaries, and seasons. The most influential environmental variables were water depth, water temperature, dissolved oxygen, and pH, which constituted the components of habitat heterogeneity driving spatial and temporal changes in fish assemblage structure.
The Xiangxi, Qinggan, and Yuanshui are three neighboring tributaries connected with the TGR mainstem; after impoundment, flow in their lower reach is year-round static, while in the upper reach, flow is temporally static during the high water level due to a backwater effect. We found that fish assemblage structure exhibited spatial heterogeneity among the three tributaries and upper and lower reaches within tributaries and showed temporal heterogeneity among four seasons. We also found that averaged beta-diversity among tributaries was higher than that within tributaries. These findings indicate that reservoir tributaries form small-scale habitat heterogeneity through spatial variation and temporal transformation of habitat characteristics. Due to habitat heterogeneity, reservoir tributaries constitute important refuges for different fish categories. Spatial turnover across tributaries also create differences in species composition (Vitorino Junior et al., 2016), thereby maintaining diverse fish assemblages in large reservoirs. Previous studies found similar phenomena (Li et al., 2016; Pfauserová et al., 2022) and argued for protecting tributary habitats and constructing artificial habitats to improve habitat heterogeneity in reservoirs (Lin et al., 2014; Marques et al., 2018). Small-scale habitat heterogeneity among reservoir tributaries and between reaches within tributaries, together with longitudinal habitat heterogeneity among the lentic, transitional, and lotic sections (Liao et al., 2018a), likely contributes to maintaining the high fish diversity observed at the regional scale. Species richness in the surveyed reservoir tributaries accounts for 88.7% (55 out of 62 species) of the total richness in the lower section of the TGR (Liao et al., 2018a).
Understanding which environmental factors contribute to the aforementioned small-scale heterogeneity and how they interact to support biodiversity is crucial for freshwater fish conservation. In this study, a mix of water quality and physical stream attributes, chiefly water depth, water temperature, pH, specific conductivity, riparian slope, and vegetation coverage, were found to be drivers of fish assemblage variation. First, we found that fishes producing sinking eggs (e.g., Pelteobagrus spp.; Ding, 1994) or grass-attached eggs (e.g., Cyprinus carpio Linnaeus, 1758, Paralramis pekenensis (Basilewsky, 1855), C. alburnus, and Culter dabryi (Bleeker, 1871); Ding, 1994) preferred habitats in the upper reaches. The upper reaches recover to a riverine state with decreased water levels during spring and summer, and at the same time, terrestrial plants also recover rapidly at the water-level-fluctuation zones, except for the Xiangxi. This consistency suggests that the upper reaches provide important spawning structure for these fish groups (Hladík & Kubecka, 2004; Marshall et al., 2015). Second, our results also revealed that fishes producing rock-attached eggs (e.g., L. longirostris, S. meridionalis) and drifting eggs (e.g., P. sinensis, H. bleekeri, and S. argentatus) were more abundant in the lower reaches, which is likely because the lower reaches have deeper water and contain mixed substratum with abundant rocks, providing important reproduction and nursery grounds for these fish groups (Probst et al., 2009; Szalóky et al., 2021).
The TGR has water-level-fluctuation zones with a total shoreline length of 5426 km and an area affected by drawdown events of 350 km2, which are mainly covered with herbaceous plants such as Bermuda grass, Cynodon dactylon (Linnaeus) Persoon, and annual species like Xanthium sibiricum Patrin ex Widder (Dou et al., 2023). The upper reaches of the tributaries have shallower water depth and higher vegetation coverage in the water-level-fluctuation zones; these areas attracted more herbivorous species such as C. idella and P. pekenensis, suggesting that vegetation provided a food subsidy to these fishes (Felden et al., 2021) and enhanced structural complexity with increasing water level (Norris et al., 2020). A previous study revealed a similar phenomenon, i.e., riparian plants and flooded vegetation were important food sources for fishes in the Daning River, a tributary of the TGR (Deng et al., 2018). In addition, some lotic fish species (e.g., R. cylindricus, C. heterodon), that only spawn in the riverine mainstem upstream of the TGR, were commonly sampled at the lower reaches, suggesting that the confluence areas of tributaries with the reservoir provide important feeding and nursery ground for lotic fish species (Moreira et al., 2022). The water temperature mainly affected the abundance of common small-bodied fish populations (e.g., C. brachygnathus and H. bleekeri), which preferred warmer habitat in the upper reaches during summer. These species are zooplanktivorous fish, and in turn, zooplankton biomass was higher during spring and summer (spring: 1.40 ± 0.34 mg/L; summer: 0.65 ± 0.13; autumn: 0.22 ± 0.09; winter: 0.04 ± 0.01; C. Liao, unpublished information) and in the upper reaches (upper: 0.98 ± 0.26; lower: 0.42 ± 0.09 mg/L; C. Liao, unpublished information) of the three tributaries. These lines of evidence suggest that upstream summer migration may be related with behavioral traits of fish seeking optimal thermal conditions for spawning and feeding (Togaki et al., 2023).
We also found that fish abundance exhibited spatial and seasonal variation. Gillnets are commonly used to estimate fish resources, yet capture can be influenced by factors such as mesh size (Loisl et al., 2014) and diel variation in fish activity (Wegscheider et al., 2020). In this study, we sampled fish using multimesh gillnets comparable to CEN 2015 standard (CEN, 2015), and standardized the sampling time (night) and duration to ensure comparability across reaches and seasons. To the best of our knowledge, it is the first time that such comparable multimesh gillnet sampling approach was used to survey fish assemblages in the TGR, rather than studying fish assemblages based on fisher's catches (Gao et al., 2010; Yang et al., 2012; Wei et al., 2021). Interestingly, we obtained similar averaged CPUEs between pelagic and benthic gillnets, suggesting that the benthic and pelagic habitats have similar fish abundance in the three tributaries. This can be explained by several reasons. The benthic dissolved oxygen often affects benthic fish abundance (Prchalová et al., 2008); commonly, in deep reservoirs, dissolved oxygen is relatively low below the thermocline and leads to a low benthic fish abundance compared to the pelagic layer (Järvalt et al., 2005; Prchalová et al., 2008). In these reservoir tributaries, the dissolved oxygen was > 6 mg/L all the way down to a depth of 50 m (unpublished information), which may stem from high water transparency and rapid water exchange rate (> 10 times per year) therein (Lu & Yang, 2020). Regardless of the mechanisms originating high dissolved oxygen, this dissolved oxygen concentration is sufficient to sustain benthic fishes year-round (Wen et al., 2022), Meanwhile, the benthic fish faunas were dominated by omnivorous and invertivores fishes (e.g., S. dabryi, S. argentatus, P. vachelli, and P. nitidus), and the abundant detritus, zoobenthos, and shrimps therein can provide abundant food resources to maintain relatively high benthic fish abundance (Liao et al., 2019).
Spatial (e.g., horizontal and vertical, upper and lower reaches) and temporal (e.g., seasonal, diel) variation in fish abundance is common in reservoirs, which may be induced by matching environmental change, as well as by fish morphological adaptations and behavior (i.e., littoral vs. pelagic) (Riha et al., 2022). We found that the averaged fish BPUE and NPUE exhibited seasonal and spatial changes in the three reservoir tributaries, among which the main influencing environmental variables were water depth, water temperature, dissolved oxygen, and pH. First, water depth decreases from the lower to the upper reach; in turn, we found that BPUE and NPUE tended to increase from the lower to the upper reaches of the tributaries, and the shallowest Y-Upper reach yielded the highest BPUE (averaged 20.53 g/m2/night) during our study. Other studies found similar spatial patterns and attributed such findings to abundant food resources and suitable habitats for feeding and spawning activities in shallow areas (Prchalová et al., 2008, 2009). The spatial differences in phytoplankton (lower: 3.63 ± 1.21 mg/L; upper: 12.37 ± 4.12 mg/L; C. Liao, unpublished information) and zooplankton biomass (lower: 0.42 ± 0.09 mg/L; upper: 0.98 ± 0.26; C. Liao, unpublished information) in our study supported this assumption; shallower habitats support greater autochthonous primary productivity and therefore, greater consumer fish biomass. The second factor, water temperature, changed along with seasonal fluctuations in water depth at the TGR, where water level declines from winter (175 m) to summer (145 m). During spring and summer, water temperature increased (Liao et al., 2018a), and most fish species increased their feeding activity and swimming speed, which increased the BPUE and NPUE specially in the upper reaches (Specziár et al., 2013). Dissolved oxygen and pH also are considered to be important factors influencing fish abundance (Matthews et al., 2004; Khalsa et al., 2021). In our study, dissolved oxygen and pH were at a suitable range for fishes; thus, the observed gradients in water depth, water temperature, and other factors most likely influenced fish abundances. Meanwhile, we acknowledge some shortcomings during our fish sampling; we should have measured the deployed depth of each benthic gillnet, and deployed other gillnets in the water layers between those sampled by the pelagic and benthic gillnets. These strategies would allow future studies investigate changes in fish assemblages at different depths.
Conclusion
In conclusion, the present study demonstrated that fish assemblage structure differed among tributaries, reaches within tributaries, and seasons due to the heterogeneity in water quality and physical stream attributes. The findings from this study revealed that reservoir tributaries in the TGR have abundant fish both in pelagic and benthic habitats, and fish abundance varied spatially and temporally driven by water quality and physical variables at a small scale. Our results also improve scientific understanding of how small-scale habitat heterogeneity enhances spatial and temporal variation of fish assemblage structure. Based on our findings, local habitat heterogeneity is critical for conserving fish diversity in large reservoirs coupled with large-scale heterogeneity among the lotic, transitional, and lentic zones.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used in this study are available from the corresponding author on reasonable request.
References
Abdel-Dayem, M. S., M. R. Sharaf, J. D. Majer, M. K. Al-Sadoon, A. S. Aldawood, H. M. Aldhafer & G. M. Orabi, 2021. Ant diversity and composition patterns along the urbanization gradients in an arid city. Journal of Natural History 55: 2521–2547.
Alberti, M. & T. Z. Wang, 2022. Detecting patterns of vertebrate biodiversity across the multidimensional urban landscape. Ecology Letters 25: 1027–1045.
Arantes, C. C., D. B. Fitzgerald, D. J. Hoeinghaus & K. O. Winemiller, 2019. Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Current Opinion in Environmental Sustainability 37: 28–40.
Azevedo-Santos, V. M., V. S. Daga, F. M. Pelicice & R. Henry, 2021. Drifting in a free-flowing river: distribution of fish eggs and larvae in a small tributary of a neotropical reservoir. Biota Neotropica. https://doi.org/10.1590/1676-0611-bn-2021-1227.
Ba, J. W. & D. Q. Chen, 2012. Invasive fishes in three gorges reservoir area and preliminary study on effects of fish invasion owing to impoundment. Journal of Lake Sciences 24: 185–189 (In Chinese with English Abstract).
Baselga, A. & C. D. L. Orme, 2012. betapart: an R package for the study of beta diversity. Methods in Ecology and Evolution 3: 808–812.
Bates, D., M. Maechler, B. M. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48.
CEN (European Committee for Standardization), 2015. Water Quality-Sampling of Fish with Multi-mesh Gillnets (EN 14757), Belgium, Brussels:
Chen, X. & B. Liu, 2022. Life history and early development of fishes. Biology of Fishery Resources. https://doi.org/10.1007/978-981-16-6948-4_3.
Chen, F. Q., M. Zhang, Y. Wu & Y. W. Huang, 2020. Seed rain and seed bank of a draw-down zone and their similarities to vegetation under the regulated water-level fluctuation in Xiangxi River. Journal of Freshwater Ecology 35: 57–71.
Cheng, F., W. Li, L. Castello, B. R. Murphy & S. G. Xie, 2015. Potential effects of dam cascade on fish: lessons from the Yangtze River. Reviews in Fish Biology and Fisheries 25: 569–585.
Da Silva, P. S., M. C. Makrakis, L. E. Miranda, S. Makrakis, L. Assumpção, S. Paula, J. H. P. Dias & H. Marques, 2015. Importance of reservoir tributaries to spawning of migratory fish in the upper Paraná River. River Research and Applications 31: 313–322.
Deng, H. T., Y. Li, M. D. Liu, X. B. Duan, S. P. Liu & D. Q. Chen, 2018. Stable isotope analysis reveals the importance of riparian resources as carbon subsidies for fish species in the Daning River, a tributary of the Three Gorges Reservoir. China. Water 10: 1233.
Ding, R. H., 1994. The Fishes of Sichuan, Sichuan Publishing House of Science and Technology, Chengdu, China, China: ((In Chinese)).
Dou, W. Q., W. T. Jia, J. H. Zhang, X. M. Yi, Z. F. Wen, S. J. Wu & M. H. Ma, 2023. Research progress of vegetation status, adaptive strategies and ecological restoration in the water-level fluctuation zones of the Three Gorges Reservoir. Chinese Journal of Ecology 42: 208–218. (In Chinese with English Abstract)
Eadie, J. M. A. & A. Keast, 1984. Resource heterogeneity and fish species diversity in lakes. Canadian Journal of Zoology 62: 1689–1695.
Eddy, T. D., V. W. Lam, G. Reygondeau, A. M. Cisneros-Montemayor, K. Greer, M. L. D. Palomares, J. F. Bruno, Y. Ota & W. W. Cheung, 2021. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4: 1278–1285.
Eitzmann, J. L. & C. P. Paukert, 2010. Longitudinal differences in habitat complexity and fish assemblage structure of a Great Plains River. The American Midland Naturalist 163: 14–32.
Felden, J., I. González-Bergonzoni, A. M. Rauber, M. da Luz Soares, M. V. Massaro, R. Bastian & D. A. Reynalte-Tataje, 2021. Riparian forest subsidises the biomass of fish in a recently formed subtropical reservoir. Ecology of Freshwater Fish 30: 197–210.
Fox, J. & S. Weisberg, 2011. An R companion to applied regression, 2nd ed. Thousand Oaks, Sage:
Gao, X., Y. Zeng, J. W. Wang & H. Z. Liu, 2010. Immediate impacts of the second impoundment on fish communities in the Three Gorges Reservoir. Environmental Biology of Fishes 87: 163–173.
Gladyshev, M. I., N. N. Sushchik, A. P. Tolomeev & Y. Y. Dgebuadze, 2018. Meta-analysis of factors associated with omega-3 fatty acid contents of wild fish. Reviews in Fish Biology and Fisheries 28: 277–299.
Heidrich, L., S. Bae, S. Levick, S. Seibold, W. Weisser, P. Krzystek, P. Magdon, T. Nauss, P. Schall, A. Serebryanyk, S. Wöllauer, C. Ammer, C. Bässler, I. Doerfler, M. Fischer, M. M. Gossner, M. Heurich, T. Hothorn, K. Jung, H. Kreft, E. D. Schulze, N. Simons, S. Thorn & J. Müller, 2020. Heterogeneity–diversity relationships differ between and within trophic levels in temperate forests. Nature Ecology & Evolution 4: 1204–1212.
Hladík, M. & J. Kubecka, 2004. The effect of water level fluctuation on tributary spawning migration of reservoir fish. International Journal of Ecohydrology and Hydrobiology 4: 449–457.
ICOLD (International Commission on Large Dams), 2019. Number of Dams by Country Members. http://www.icold-cigb.org/.
Järvalt, A., T. Krause & A. Palm, 2005. Diel migration and spatial distribution of fish in a small stratified lake. Hydrobiologia 547: 197–203.
Khalsa, N. S., K. P. Gatt, T. M. Sutton & A. L. Kelley, 2021. Characterization of the abiotic drivers of abundance of nearshore Arctic fishes. Ecology and Evolution 11: 11491–11506.
Kovalenko, K. E., S. M. Thomaz & D. M. Warfe, 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685: 1–17.
Lapointe, N. W. R., 2014. Effects of shoreline type, riparian zone and instream microhabitat on fish species richness and abundance in the Detroit River. Journal of Great Lakes Research 40: 62–68.
Legendre, P. & L. Legendre, 1998. Numerical Ecology, Elsevier, New York:
Lenth, R. V., P. Buerkner, M. Herve, J. Love, F. Miguez, H. Riebl & H. Singmann, 2022. emmeans: estimated marginal means, aka least-squares mean. R Package Version 1(7): 3.
Li, X., Y. R. Li, L. Chu, R. Zhu, L. Z. Wang & Y. Z. Yan, 2016. Influences of local habitat, tributary position, and dam characteristics on fish assemblages within impoundments of low-head dams in the tributaries of the Qingyi River, China. Zoological Research 37: 67–74.
Liao, C. S., S. B. Chen, S. S. De Silva, S. B. Correa, J. Yuan, T. L. Zhang, Z. J. Li & J. S. Liu, 2018a. Spatial changes of fish assemblages in relation to filling stages of the Three Gorges Reservoir, China. Journal of Applied Ichthyology 34: 1293–1303.
Liao, C. S., S. B. Chen, Z. Q. Guo, S. W. Ye, T. L. Zhang, Z. J. Li, B. R. Murphy & J. S. Liu, 2018b. Species-specific variations in reproductive traits of three yellow catfish species (Pelteobagrus spp) in relation to habitats in the Three Gorges Reservoir, China. Plos One 13: e0199990.
Liao, C. S., S. B. Chen, S. B. Correa, W. Li, T. L. Zhang & J. S. Liu, 2019. Impoundment led to spatial trophic segregation of three closely related catfish species in the Three Gorges Reservoir, China. Marine and Freshwater Research 71: 750–760.
Lin, J. Q., Q. D. Peng, J. Ren, H. X. Bai & L. Zhao, 2014. Similarity analysis of fish habitats between the Chishui River and downstream reaches of the Jinsha River. Freshwater Fisheries 44: 93–99 (In Chinese with English Abstract).
Liu, J. K. & W. X. Cao, 1992. Fish resources of the Yangtze River basin and the tactics for their conservation. Resources and Environment in the Yangtze Valley 1: 17–23 (In Chinese with English Abstract).
Loisl, F., G. Singer & H. Keckeis, 2014. Method-integrated fish assemblage structure at two spatial scales along a free-flowing stretch of the Austrian Danube. Hydrobiologia 729: 77–94.
López-Delgado, E. O., K. O. Winemiller & F. A. Villa-Navarro, 2020. Local environmental factors influence beta-diversity patterns of tropical fish assemblages more than spatial factors. Ecology 101: e02940.
Lu, L. & P. Yang, 2020. Runoff characteristics in the Downstream of the Three Gorges Reservoir Dam from 1956 to 2017. Ecology and Environmental Monitoring of Three Gorges 5: 55–60 (In Chinese with English Abstract).
Lund, S. S., F. Landkildehus, M. Søndergaard, T. L. Lauridsen, S. Egemose, H. S. Jensen, F. Andersen, L. S. Johansson, M. Ventura & E. Jeppesen, 2010. Rapid changes in fish community structure and habitat distribution following the precipitation of lake phosphorus with aluminium. Freshwater Biology 55: 1036–1049.
MacArthur, R. H. & J. W. MacArthur, 1961. On bird species diversity. Ecology 42: 594–598.
Marques, H., J. H. P. Dias, G. Perbiche-Neves, E. A. L. Kashiwaqui & I. P. Ramos, 2018. Importance of dam-free tributaries for conserving fish biodiversity in Neotropical reservoirs. Biological Conservation 224: 347–354.
Marshall, S. M., T. Espinoza & A. J. Mcdougall, 2015. Effects of Water Level Fluctuations on Spawning Habitat of an Endangered Species, the Australian Lungfish (Neoceratodus Forsteri). River Research and Application 31: 552–562.
Matthews, W. J., K. B. Gido & F. P. Gelwick, 2004. Fish assemblages of reservoirs, illustrated by Lake Texoma (Oklahoma–Texas, USA) as a representative system. Lake and Reservoir Management 20: 219–239.
Mattos, T. M., M. R. Costa, B. C. T. Pinto, J. L. Borges & F. G. Araújo, 2014. To what extent are the fish compositions of a regulated river related to physico-chemical variables and habitat structure? Environmental Biology of Fishes 97: 717–730.
Meerhoff, M. & M. D. L. Á. González-Sagrario, 2022. Habitat complexity in shallow lakes and ponds: importance, threats, and potential for restoration. Hydrobiologia 849: 3737–3760.
Miao, B. G., Y. Q. Peng, D. R. Yang, B. Guénard & C. Liu, 2022. Diversity begets diversity: low resource heterogeneity reduces the diversity of nut-nesting ants in rubber plantations. Insect Science 29: 932–941.
Montag, L. F., K. O. Winemiller, F. W. Keppeler, H. Leão, N. L. Benone, N. R. Torres, B. S. Prudente, T. O. Begot, L. M. Bower, D. E. Saenz, E. O. Lopez-Delgado, Y. Quintana, D. J. Hoeinghaus & L. Juen, 2019. Land cover, riparian zones and instream habitat influence stream fish assemblages in the eastern Amazon. Ecology of Freshwater Fish 28: 317–329.
Moreira, M. F., A. Peressin & P. S. Pompeu, 2022. Small rivers, great importance: refuge and growth sites of juvenile migratory fishes in the upper Sao Francisco Basin, Brazil. Fisheries Management and Ecology. https://doi.org/10.1111/fme.12595.
Norris, D. M., H. R. Hatcher, M. E. Colvin, G. Coppola, M. A. Lashley & L. E. Miranda, 2020. Assessing establishment and growth of agricultural plantings on reservoir mudflats. North American Journal of Fisheries Management 40: 394–405.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2020. vegan: Community Ecology Package. R package version 2.5.7
Ostrand, K. G. & G. R. Wilde, 2001. Temperature, dissolved oxygen, and salinity tolerances of five prairie stream fishes and their role in explaining fish assemblage patterns. Transactions of the American Fisheries Society 130: 742–749.
Pelicice, F. M., P. S. Pompeu & A. A. Agostinho, 2015. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries 16: 697–715.
Perera, H. A. C. C., Z. J. Li, S. S. De Silva, T. L. Zhang, J. Yuan, S. W. Ye, Y. G. Xia & J. S. Liu, 2014. Effect of the distance from the dam on river fish community structure and compositional trends, with reference to the Three Gorges Dam, Yangtze River, China. Acta Hydrobiologica Sinica 38: 438–445.
Perônico, P. B., C. S. Agostinho, R. Fernandes & F. M. Pelicice, 2020. Community reassembly after river regulation: rapid loss of fish diversity and the emergence of a new state. Hydrobiologia 847: 519–533.
Pfauserová, N., M. Brabec, O. Slavík, P. Horký, V. Žlábek & M. Hladík, 2022. Effects of physical parameters on fish migration between a reservoir and its tributaries. Scientific Reports 12: 8612.
Prchalová, M., J. Kubečka, M. Vašek, J. Peterka, J. Seďa, T. Jůza, M. Říha, O. Jarolím, M. Tušer, M. Kratochvíl, M. Čech, V. Draštík, J. Frouzová & E. Hohausová, 2008. Distribution patterns of fishes in a canyon-shaped reservoir. Journal of Fish Biology 73: 54–78.
Prchalová, M., J. Kubečka, M. Čech, J. Frouzová, V. Draštík, E. Hohausová, T. Juza, M. Kratochvíl, J. Matěna, J. Peterka, M. Ríha, M. Tušer & M. Vašek, 2009. The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecology of Freshwater Fish 18: 247–260.
Probst, W. N., S. Stoll, L. Peters, P. Fischer & R. Eckmann, 2009. Lake water level increase during spring affects the breeding success of bream Abramis brama (L.). Hydrobiologia 632: 211–224.
Říha, M., R. Rabaneda-Bueno, I. Jarić, A. T. Souza, L. Vejřík, V. Draštík, P. Blabolil, M. Holubová, T. Jůza, K. Gjelland, P. Rychtecký, Z. Sajdlová, L. Kočvara, M. Tušer, M. Prchalová, J. Seďa & J. Peterka, 2022. Seasonal habitat use of three predatory fishes in a freshwater ecosystem. Hydrobiologia 849: 3351–3371.
Sá-Oliveira, J. C., J. E. Hawes, V. J. Isaac-Nahum & C. A. Peres, 2015. Upstream and downstream responses of fish assemblages to an eastern Amazonian hydroelectric dam. Freshwater Biology 60: 2037–2050.
Smith, J. M. & M. E. Mather, 2013. Beaver dams maintain fish biodiversity by increasing habitat heterogeneity throughout a low gradient stream network. Freshwater Biology 58: 1523–1538.
Specziár, A., Á. I. György & T. Erős, 2013. Within-lake distribution patterns of fish assemblages: the relative roles of spatial, temporal and random environmental factors in assessing fish assemblages using gillnets in a large and shallow temperate lake. Journal of Fish Biology 82: 840–855.
Spurgeon, J. J., M. A. Pegg, P. Parasiewicz & J. Rogers, 2018. Diversity of river fishes influenced by habitat heterogeneity across hydrogeomorphic divisions. River Research and Applications 34: 797–806.
Szalóky, Z., V. Füstös, B. Tóth & T. Erős, 2021. Environmental drivers of benthic fish assemblages and fish-habitat associations in offshore areas of a very large river. River Research and Applications 37: 712–721.
Togaki, D., M. Inoue & K. Ikari, 2023. Seasonal habitat use by warmwater fishes in a braided river, southwestern Japan: effects of spatiotemporal thermal heterogeneity. Ichthyological Research 70: 91–100.
Toussaint, A., N. Charpin, S. Brosse & S. Villéger, 2016. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Scientific Reports 6: 22125.
Turgeon, K., C. Turpin & I. Gregory-Eaves, 2019. Dams have varying impacts on fish communities across latitudes: a quantitative synthesis. Ecology Letters 22: 1501–1516.
Vasconcelos, L. P., D. C. Alves, L. F. da Camara & L. Hahn, 2021. Dams in the Amazon: the importance of maintaining free-flowing tributaries for fish reproduction. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 1106–1116.
Vitorino Junior, O. B., R. Fernandes, C. S. Agostinho & F. M. Pelicice, 2016. Riverine networks constrain β-diversity patterns among fish assemblages in a large Neotropical river. Freshwater Biology 61: 1733–1745.
Wang, W. J., L. Chu, C. Si, R. Zhu, W. H. Chen, F. M. Chen & Y. Z. Yan, 2013. Spatial and temporal patterns of stream fish assemblages in the Qiupu Headwaters National Wetland Park. Zoological Research 34: 417–428 (In Chinese with English Abstract).
Wegscheider, B., T. Linnansaari, C. C. Wall, M. D. Gautreau, W. A. Monk, R. Dolson-Edge, K. M. Samways & R. A. Curry, 2020. Diel patterns in spatial distribution of fish assemblages in lentic and lotic habitat in a regulated river. River Research and Applications 36: 1014–1023.
Wei, N., Y. Zhang, F. Wu, Z. W. Shen, H. J. Ru & Z. H. Ni, 2021. Current status and changes in fish assemblages in the Three Gorges Reservoir. Resources and Environment in the Yangtze Valley 30: 1858–1869 (In Chinese with English Abstract).
Wen, G., S. Wang, R. H. Cao, C. C. Wen, M. Yang & T. L. Huang, 2022. A review of the formation causes, ecological risks and water quality responses of metalimnetic oxygen minimum in lakes and reservoirs. Journal of Lake Sciences 34: 711–726 (In Chinese with English Abstract).
Wu, Q., X. B. Duan, S. Y. Xu, C. X. Xiong & D. Q. Chen, 2007. Studies on fishery resources in the Three Gorges Reservoir of the Yangtze River. Freshwater Fisheries 37: 70–75 (In Chinese with English Abstract).
Xiao, Q., Z. Yuan, H. Y. Tang, P. X. Duan, X. Q. Wang, T. Y. Xiao & X. Y. Liu, 2015. Species diversity of fish and its conservation in the mainstream of the lower reaches of Wu River. Biodiversity Science 23: 499–506 (In Chinese with English Abstract).
Xu, Y. Y., Q. H. Cai, M. L. Shao & X. Q. Han, 2012. Patterns of asynchrony for phytoplankton fluctuations from reservoir mainstream to a tributary bay in a giant dendritic reservoir (Three Gorges Reservoir, China). Aquatic Sciences 74: 287–300.
Yan, Y. Z., X. Y. Xiang, L. Chu, Y. J. Zhan & C. Z. Fu, 2011. Influences of local habitat and stream spatial position on fish assemblages in a dammed watershed, the Qingyi stream, China. Ecology of Freshwater Fish 20: 199–208.
Yang, S. R., X. Gao, M. Z. Li, B. S. Ma & H. Z. Liu, 2012. Interannual variations of the fish assemblage in the transitional zone of the Three Gorges Reservoir: persistence and stability. Environmental Biology of Fishes 93: 295–304.
Yang, Z., X. J. Pan, L. Hu, W. Xu, Y. Jin, N. Zhao, Q. Yang, X. J. Chen & H. Liu, 2021. Effects of upstream cascade dams and longitudinal environmental gradients on variations in fish assemblages of the Three Gorges Reservoir. Ecology of Freshwater Fish 30: 503–518.
Ye, S. W., Z. J. Li & W. X. Cao, 2007. Species composition, diversity and density of small fishes in two different habitats in Niushan Lake. Chinese Journal of Applied Ecology 18: 1589–1595 (In Chinese with English Abstract).
Zeng, L., L. Zhou, D. L. Guo, D. H. Fu, P. Xu, S. Zeng, Q. D. Tang, A. L. Chen, F. Q. Chen, Y. Luo & G. F. Li, 2017. Ecological effects of dams, alien fish, and physiochemical environmental factors on homogeneity/heterogeneity of fish community in four tributaries of the Pearl River in China. Ecology and Evolution 7: 3904–3915.
Zhao, S. S., S. W. Ye, S. G. Xie & F. Cheng, 2015. The current situation of fishery resources in the Xiangxi River of the Three Gorges Reservoir and advices on the management. Acta Hydrobiologica Sinica 39: 973–982 (In Chinese with English Abstract).
Zhu, K. W., Y. C. Chen, S. Zhang, B. Lei, Z. M. Yang & L. Huang, 2020. Vegetation of the water-level fluctuation zone in the Three Gorges Reservoir at the initial impoundment stage. Global Ecology and Conservation 21: e00866.
Acknowledgements
This study was funded by the Earmarked Fund for China Agriculture Research System (CARS-45), the National Natural Science Foundation of China (32102798), and the China Postdoctoral Science Foundation (2020M672448). S.B. Correa was supported by the Forest and Wildlife Research Center of Mississippi State University, USA (MISZ-081700).
Funding
The Earmarked Fund for China Agriculture Research System, CARS-45, Chuanbo Guo, The National Natural Science Foundation of China, 32102798, Chuansong Liao, The China Postdoctoral Science Foundation, 2020M672448, Chuansong Liao, Byrd Polar and Climate Research Center, Ohio State University, MISZ-081700, Sandra Bibiana Correa.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by LC, YS, ZD, YJ, SBC, WF, ZC, FL, GC, and LJ. The first draft of the manuscript was written by LC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to this article.
Ethical approval
The manuscript has not been published or is under consideration for publication elsewhere, in whole or in part.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, C., Ye, S., Zhai, D. et al. Tributaries create habitat heterogeneity and enhance fish assemblage variation in one of the largest reservoirs in the world. Hydrobiologia 850, 4311–4326 (2023). https://doi.org/10.1007/s10750-023-05306-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05306-3