Abstract
Hydroelectric expansion has affected the functioning of most tropical rivers in the world. However, community reassembly in Neotropical impoundments remains poorly understood, because long-term studies are incipient. In this context, we evaluated temporal changes in fish diversity before and after the construction of Peixe Angical Dam, Upper Tocantins River, Amazon Basin. Specifically, we investigated changes in taxonomic (richness, abundance and species composition) and functional diversity (trait richness and composition) over a period of a decade. Fish assemblages were sampled at five sites along the river, between 2004 and 2014 (before and after river regulation). As predicted, taxonomic and trait richness declined in the impoundment, and the composition shifted over the years, with increasing dissimilarity between sites. We also observed significant changes in species abundance and trait composition. However, changes were abrupt and a novel community state emerged after the fifth year of the impoundment. After this period, richness and abundance tended to stabilize at low levels, with different species/trait composition when compared to the pre-damming period. Our results indicate that major changes in community structure occurred during the first years of the impoundment, with relevant losses in taxonomic and functional diversity, along with a rapid change towards a new state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large dams modify important characteristics of river systems in tropical regions, affecting biodiversity patterns in these species-rich ecosystems (Winemiller et al., 2016). The formation of huge lentic areas (reservoir) changes several environmental filters that regulate the structure of local assemblages, related mainly to hydrological, geomorphological and limnological conditions (Poff et al., 1997; Agostinho et al., 2016). In addition, physical and ecological barriers (the dam and the impoundment) restrict the movement of organisms through the main river and lateral channels (Pelicice et al., 2015; Rahel & McLaughlin, 2018), affecting dispersal mechanisms and metacommunity dynamics. Combined, these impacts translate into species deletions, replacements and demographic changes, with direct implications to the structure and functioning of aquatic communities (Santos et al., 2018a).
Fish diversity is particularly affected, because fish are vagile organisms with a wide range of behaviors and requirements (Toussaint et al., 2016). New environmental filters and dispersal constraints select for specific functional traits, usually related to sedentary behavior, trophic/reproductive opportunism and/or adaptation to live in lentic conditions (Angulo-Valencia et al., 2016; Santos et al., 2017). A common pattern is the loss of rheophilic species, since these organisms depend on migration and/or fluvial heterogeneity to complete their life cycles (Agostinho et al., 2016). In Neotropical rivers, studies have consistently reported community changes below and above large dams (e.g., Petesse & Petrere Jr., 2012, Araújo et al., 2013, Lima et al., 2016). Moreover, recent studies have also revealed changes in functional diversity (e.g., Santos et al., 2017; Lima et al., 2018). Functional traits are key components of biodiversity, since they determine how species respond to the environment and its related environmental filters (Cadotte et al., 2011; Logez et al., 2013; Mouillot et al. 2013; Kraft et al., 2015). In this sense, functional traits are pivotal to understand community reassembly in regulated rivers, especially because fish assemblages in impoundments are subsets of richer assemblages found in pristine conditions (Petesse & Petrere Jr., 2012; Santos et al., 2017), where species are selected or removed according to their traits (Arantes et al., 2019).
Long-term studies suggest that fish assemblages stabilize in another state after some decades (Mol et al., 2007; Orsi & Britton, 2014; Schork & Zaniboni-Filho, 2017; Loures & Pompeu, 2018), although other processes (e.g., dam operation, species invasions) may induce variations in community structure. However, trends about reservoir aging, community assembly and stabilization remain poorly known in Neotropical impoundments (e.g., Loures & Pompeu, 2018). Most studies are constrained to compare fish diversity before and after the disturbance (e.g., Araújo et al., 2013; Lima et al., 2016; Cella-Ribeiro et al., 2017), or to investigate only the impoundment (e.g., Terra et al., 2010; Sá-Oliveira et al., 2016; Schork & Zaniboni-Filho, 2017; Pelicice et al., 2018). Moreover, trends in functional diversity remain poorly investigated (e.g., Santos et al., 2017). For example, we do not know when (years after impoundment) and how (gradual or abrupt) functional losses and shifts occur, and whether changes in taxonomic diversity are coupled with changes in functional diversity. This information is crucial to improve predictions about community change and diversity losses following the construction of large impoundments in species-rich ecosystems.
Neotropical rivers have been subjected to dramatic hydroelectric development (Agostinho et al., 2016). In South America, most basins are affected by river regulation, including cascades of dams along the main channel and tributaries (Petesse & Petrere Jr., 2012; Loures & Pompeu, 2018; Pelicice et al., 2018; Santos et al., 2018a). Hydropower expansion has reached the Amazon basin (Castello et al., 2013; Winemiller et al., 2016); the Tocantins River, in particular, is currently regulated by several large dams (Araújo et al., 2013, Lima et al., 2016). This river, consequently, may serve as a model to understand the effects of large impoundments in Amazonian tributaries. In this context, we evaluated temporal changes in fish diversity before and after the construction of Peixe Angical Dam, Upper Tocantins River, to understand community reassembly in the impoundment. Specifically, we investigated changes in taxonomic (richness, abundance and species composition) and functional diversity (trait richness and composition) over a decade. We hypothesized that the impoundment would induce considerable changes in community structure, as short-term studies have indicated (Lima et al., 2016, 2018), causing a continuous loss of taxonomic and trait diversity over the years. In this sense, we predicted strong shifts in assemblage structure after river regulation, with a progressive decline in taxonomic and trait diversity. Moreover, considering evidence reported by other studies, where novel stable assemblages took a long time to emerge (decades after river regulation; Agostinho et al., 2008; Loures & Pompeu, 2018), we further predicted that local assemblages do not reach a new stable state within the first decade of the impoundment.
Materials and methods
Study area
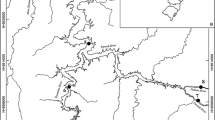
The Tocantins River is a major tributary of the Amazon system. The basin has an area of ca. 760,000 km2, and the river flows through 2500 km from its sources in central Brazil to its mouth in the Lower Amazon River, near Marajó Island. The precipitation regime is seasonal, with a well-defined wet period extending from October to April. It is a clear-water river (Secchi depth > 150 cm) with an average annual discharge between 10,000 and 11,000 m3/s. The geomorphological characteristics of the Tocantins River (e.g., slope) favored the implantation of hydroelectric plants. At present, the main channel is regulated by seven large dams, which changed the natural flow regime, created large impoundments and stabilized the water level in many segments (Aráujo et al., 2013; Angulo-Valencia et al., 2016; Lima et al., 2016). The upper section is the most affected by river regulation, with five dams in the mainstream, and several small ones in the tributaries. Most dams were built in the last 15 years, and several others are planned (Winemiller et al., 2016).
The study took place in the upper section of the basin, in the area affected by Peixe Angical hydroelectric plant (Fig. 1), Tocantins State, Brazil. The dam created a large impoundment of 294 km2 and ca. 70 km. It is a run-of-the-river hydropower dam; construction was completed in June 2006. Peixe Angical Dam is located in the middle of a long cascade of hydropower dams (Agostinho et al., 2009), with the São Salvador Dam upstream and the Lajeado Dam downstream.
Fish sampling
Fish assemblages were sampled at five sites along the river (extent ca. 80 km), covering the whole stretch affected by the impoundment (i.e., fluvial, transition and lacustrine zones of the reservoir). All sites were located upstream from the dam. Sites 1 and 2 were 4 and 20 km distant from the dam, respectively (lacustrine zone). Sites 3 and 4 were 33 and 43 km distant from the dam, respectively (transition zone). Site 5 was located 74 km upstream of the dam (fluvial zone).
Fish sampling took place in five different periods between 2004 and 2014 (120 months). The first period occurred prior to dam construction (Pre: months 1 to 12), carried out monthly between October 2004 and September 2005. The remaining periods occurred after river regulation: in the first year of the impoundment (Post-1st: months 25 to 36), monthly between October 2006 and September 2007; during the fifth year (Post-5th: months 77 to 88), monthly between February 2011 and January 2012; the sixth year (Post-6th: months 90 to 100), quarterly between February 2012 and January 2013; and the seventh year (Post-7th: months 105 to 115), quarterly between June 2013 and May 2014.
We used a set of gill nets (2.4 to 16 cm between adjacent knots) to capture fish of different sizes. At each site, nets remained deployed for 24 h and were checked at 8:00 a.m., 4:00 p.m. and 10:00 p.m. Fishing effort was standardized in all sampling occasions. In the laboratory, all fish were identified and biological variables were measured: total length (cm), standard length (cm), weight (g), sex and reproductive stage (some data were not used in this study). Stomachs were kept in 70% ethanol for a dietary analysis. Voucher specimens are deposited in the zoological collection at the Universidade Federal de Goiás (Campus Samambaia, Goiânia, GO) and in the fish collection of the Laboratório de Ictiologia Sistemática at the Universidade Federal do Tocantins (Campus Porto Nacional, TO).
Functional traits
Based on Hoeinghaus et al. (2007), Cunico et al. (2011) and Vitorino Jr. et al. (2016), we built a matrix with all species (rows) and their respective functional traits (columns). We considered as traits morphology (size, shape and swimming type), feeding (resources consumed), reproduction (fertilization, embryo development and migration), and habitat preference, totaling 8 traits and 35 trait states (Online Resource 1). The definition of ecological trait followed Violle et al. (2007), where “a trait is any morphological, physiological or phenological feature measurable at the individual level”. In this sense, ecological traits affect individual fitness via its effects on species performance (i.e., survival, growth or reproduction). Our set of traits (i.e., morphology, feeding, reproduction and habitat preference) covers these dimensions and, therefore, portrays species ability to respond to environmental variation. We were able to assign virtually all traits to each species; traits with no information were left empty in the matrix. For some traits (i.e., feeding, habitat), more than one trait-state could be assigned for each species (Online Resource 2).
Body size corresponded to maximum total length (cm) and individuals were classified in five length classes: < 10 cm; 10 to 20 cm; 21 to 30 cm; 31 to 40 cm; 41 to 50 cm; > 50 cm. Maximum sizes were obtained from FishBase (Froese & Pauly, 2016) and extensive literature searches. Species were also classified according to seven body shapes (anguilliform, elongated, fusiform/cylindrical, dorsoventral compression, lateral compression, rounded with dorsoventral compression and rounded with lateral compression) and five swimming types (anguiliform, carangiform, labriform, rajiform and gymnotiform) (adapted from Pough et al., 2008; Helfman et al., 2009). In relation to feeding behavior, we used diet composition to assign species to five different trait states: detritivore, algivore, herbivore, invertivore and piscivore. Stomachs were analyzed by stereoscopic microscopy and the contents were identified to the lowest taxonomic level. For each food resource, volume (ml) was measured using graduated cylinders. Resources consumed were then grouped into classes: detritus/sediment, algae, plants, invertebrates and fish, calculating the percentage volume of each class in the diet. To assign species into feeding trait states, we considered the predominance of resource classes in the diet (> 20%); when more than one resource predominated, the species was assigned to a combination of feeding trait states (e.g., invertivore-piscivore). For species with no stomach samples, we consulted Fishbase (Froese & Pauly, 2016) and published papers (Mérona et al., 2001; Melo et al., 2004; Mérona & Rankin-de-Mérona, 2004; Pereira et al., 2007).
Regarding reproductive behavior, species were classified according to fertilization (internal or external), embryo development (internal or external), parental care (present or absent) and migratory behavior (sedentary, short-distance migrant and long-distance migrant) (following Neuberger et al., 2009). Finally, species were classified according to habitat preference: benthic (associated to the bottom), pelagic (associated to the water column) or structure-dependent (associated with submerged structures). Habitat preference was assigned based on empirical knowledge and consultation with ichthyologists that work in the Amazon Basin.
Data analysis
We investigated spatial and temporal variations in taxonomic and functional diversity over a decade, considering months (1 to 115) and sites (1 to 5) as factors. For some analysis (i.e., non-metric multidimensional scaling), monthly/quarterly samples were combined within sites and periods (Pre, Post-1st, Post-5th, Post-6th, Post-7th) to remove seasonal variation. Other analysis combined all samples within periods (i.e., abundance rank, top 20 species) to focus on temporal dynamics.
To evaluate taxonomic diversity, we considered three metrics: species richness, abundance and composition. Richness is the total number of species recorded in each site/month. Abundance is the total number of individuals captured in each site/month; we did not calculate capture per unit effort because sampling was standardized over the study. Composition is the set of species registered in each site/month. To evaluate functional diversity, we considered trait richness and composition. To calculate both functional diversity components, first we used the trait matrix to construct a functional dendrogram (cophenetic correlation coefficient = 0.802) based on species dissimilarity (Gower distance), using agglomerative hierarchical clustering analysis (UPGMA linkage method). To measure trait richness, we calculated the functional diversity metric (Petchey & Gaston, 2006), where local richness is the total branch length of the functional dendrogram, considering species occurring in each sample. As trait richness is usually dependent on species richness (Mouchet et al., 2010), we used null models to calculate expected trait richness controlling for the effect of local species richness. We used an independent swap algorithm (Gotelli, 2000) to generate 999 random assemblages, swapping species occurrences among sites but keeping fixed the species richness and abundance distribution within sites. Then, we calculated the expected trait richness value as standardized effect size (SES, i.e., difference between the observed value and the mean of null values, divided by standard deviation of null values).
We used two-way analysis of variance (ANOVA) to test the effect of months, sites and their interaction on taxonomic (abundance and richness) and functional diversity (trait richness). Due to the absence of true replication in our samples (temporal autocorrelation), we used the method proposed by Legendre et al. (2010). The analyses used the algorithm Model 5, and the statistical significance was calculated through 999 permutations.
We used the unified framework proposed by Cardoso et al. (2014) to quantify taxonomic and functional dissimilarities, based on Jaccard dissimilarity. Based on these calculated dissimilarities, we applied non-metric multidimensional scaling (NMDS) to investigate variations in taxonomic and functional composition over periods and among sites. Functional dissimilarity was based on the functional dendrogram mentioned above. Additionally, we investigated variation in trait and species abundance. To investigate variation in trait composition and abundance, we calculated the community-weighted mean (CWM; Fu et al., 2014) for each trait-state for each sample, using species mean trait values and species relative abundance. The effect of months and sites on CWM values was tested by two-way ANOVA (the same protocol described above). To investigate variation in species abundance and dominance over periods, we calculated species abundance ranks (Whittaker plots). For this, we considered the species rank observed in Pre, and plotted their total abundance against Post periods. We also investigated abundance trends considering the top 20 species recorded before river regulation (most abundant in Pre), plotting their total abundance against periods. We conducted the same investigation for migratory fishes, because they are iconic and usually impacted by impoundments (Hoeinghaus et al., 2009).
All analyses were run using the statistical package R 2.13.1 (R Development Core Team, 2011). We used the FD package (Laliberté et al., 2014) to calculate functional diversity metrics (trait richness and CWM), and the BAT package to calculate indexes of taxonomic and functional dissimilarity (Cardoso et al., 2018). For the factorial ANOVA, we used the adespatial package (Dray et al., 2016), and for the ordinations, the vegan package (Oksanen et al., 2018).
Results
During the study, we sampled 31,949 individuals belonging to 209 fish species (Online Resource 1). We recorded 21.03% of all fishes (146 species) in the Pre period, 65% in the Post-1st (149 species), 8.42% in the Post-5th (89 species), 11.16% in the Post-6th (59 species) and 3.75% in the Post-7th period (67 species).
Two-way ANOVA indicated significant variations in abundance, species richness and functional richness among months and sites (Table 1). Fish abundance increased in Post-1st, especially at sites near the dam; other Post periods showed low values at all sites (Fig. 2a). Higher values of species and functional richness were recorded in Pre and Post-1st months (Fig. 2b, c), with lower values in subsequent months. No clear spatial trend was observed, excepting for functional richness in Post-1st, where higher values were recorded at sites close to the dam. Functional richness, when controlling for the effect of species richness (Fig. 2d), showed no significant variation over months and among sites.
Monthly variation in fish diversity along the Tocantins River (4 to 74 km from the dam), before and after the construction of Peixe Angical Dam (2004 to 2014). Diversity was measured as fish abundance (a), species richness (b), trait richness (c) and trait richness controlling for the effect of species richness (d). The dashed vertical line indicates the construction of the dam. Months 1 to 12: before river regulation (October 2004 to September 2005); months 25 to 36: the first year of the impoundment (October 2006 to September 2007); months 77 to 88: the fifth year (February 2011 to January 2012); months 90 to 100: the sixth year (February 2012 to January 2013); months 105 to 115: the seventh year (June 2013 and May 2014)
We observed relevant spatiotemporal variation in taxonomic composition (NMDS stress = 0.106). Strong composition dissimilarity was observed among periods, as evidenced by axis 1; spatial dissimilarity (along the river corridor) increased in the Post periods, as shown by both axes (Fig. 3a). A similar pattern characterized functional composition (NMDS stress = 0.218). Main differences were observed among periods, with increasing spatial dissimilarity in Post periods (Fig. 3b). Periods were separated along axis 1, but Post-6th and 7th overlapped.
Non-metric multidimensional scaling (NMDS) applied to investigate variations in taxonomic (a) and functional (b) composition in fish assemblages along the Tocantins River, before and after the construction of Peixe Angical Dam. The line connecting sites represents the river corridor (1 = downstream; 5 = upstream)
Two-way ANOVA indicated significant spatial and temporal variation in several CWM traits (Online Resource 3). CWM values showed declining, increasing or oscillatory trends over months (Online Resource 4). Main declining trends were observed for anguiliform, gymnotiform, detritivore, invertivore and no parental care; main increasing trends characterized maximum length, rounded with lateral compression, algivore, piscivore, parental care and sedentary; other traits oscillated, such as carangiform, algivore/detritivore/herbivore and benthic, in addition to several traits related to feeding behavior.
We observed strong changes in species abundance ranks over periods (Fig. 4), indicating that assemblage structure changed in the impoundment. The main species in Pre were not the same in the Post periods, except for Hemiodus unimaculatus (Fig. 4). The most abundant species in Pre were Hypostomus sp. 7, Caonotropus labyrinthicus (Kner, 1858), Curimata acutirostris Vari & Reis, 1995, Geophagus altifrons Heckel, 1840 and Rhaphiodon vulpinus Spix & Agassiz, 1829; these species (and others) declined severely after the impoundment. In Post periods, some species increased in abundance, such as Argonectes robertsi Langeani, 1999, Serrasalmus rhombeus (Linnaeus, 1766) and Plagioscion squamosissimus (Heckel, 1840). A total of 63 species were recorded only after river regulation, but most were sporadic, with low abundance in all periods (< 10 individuals, or restricted to < 2 periods). Others became more abundant, however, such as Satanoperca jurupari (Heckel, 1840), Serrassalmus maculatus Kner, 1858, Metynnis hypsauchen (Müller & Troschel, 1844), Squaliforma emarginata (Valenciennes, 1840), Geophagus proximus (Castelnau, 1855) and Metynnis lippincotiannus (Cope, 1870).
Species abundance ranks before (Pre) and after (Post-1st to -7th) the construction of Peixe Angical Dam (S = total species richness). The rank is based on the sequence observed in Pre, maintaining species positions over periods. The most abundant species in each period are indicated. Anuc = Ageneiosus nuchalis; Arob = Argonectes robertsi; Bcuv = Boulengerella cuvieri; Cacu = Curimata acutirostris; Clab = Caenotropus labyrinthicus; Cpiq = Cichla piquiti; Galt = Geophagus altifrons; Huni = Hemiodus unimaculatus; Hyp7 = Hypostomus sp. 7; Mdic = Moenkhausia dichroura; Mhyp = Metynnis hypsauchen; Psqu = Plagioscion squamosissimus; Raf = Roeboides affinis; Rvul = Raphiodon vulpinus; Srho = Serrassalmus rhombeus
Considering the most abundant species before river regulation (top 20 in Pre), many increased in abundance in the Post-1st period, with a subsequent decline (Fig. 5a, b); others declined progressively over periods (Fig. 5c, d). Only two species did not decline, and oscillated over the years (P. squamosissimus and S. rhombeus). Analyzing migratory fishes, abundance declined consistently over periods, considering either total abundance (Fig. 6a) or the top 10 species (Fig. 6b, c). Some species, however, showed a peak of abundance in the post-1st period.
Abundance of migratory species over periods (total number of individuals), considering all species (a) and the top 10 recorded prior to river regulation (b, c). Argonectes robertsi is among the top 10, but it was not plotted due to scale incompatibility (Pre = 88; Post-1st = 2063; Post-5th = 201; Post-6th = 55; Post-7th = 89). Numbers above the line indicate species richness in each period
Discussion
Fish diversity patterns along the Tocantins River changed significantly after the construction of the Peixe Angical Dam. Other studies in the basin have reported short-term changes (Araújo et al., 2013; Lima et al., 2016), but the present study investigated community assembly during the first decade of the impoundment. As predicted, we observed shifts in assemblage structure, with a loss of taxonomic and functional diversity. However, contrary to our prediction (i.e., progressive changes), diversity declined rapidly in the first 5 years. Species richness, abundance and trait diversity tended to stabilize in a different level after the fifth year, indicating a novel community state. Therefore, our results support the notion that large dams cause significant changes to the structure of Amazonian fish assemblages (Santos et al., 2018b), and they also indicate that major changes occur during the first years of the impoundment, with relevant losses in taxonomic and functional diversity, and rapid development toward a new state.
All diversity metrics declined after river regulation. Fish abundance, however, increased during the first year of the impoundment, but dropped to very low levels after the fifth year. This sudden increase may be associated with the trophic upsurge period, when resource availability favors opportunistic species in the flooded area (Petrere Jr., 1996; Turgeon et al., 2016). Alternatively, higher abundance may result from the sudden flooding of different habitats (e.g., lagoons, streams), which increases habitat connectivity and pools disparate populations within the impounded area (Agostinho et al., 2016). However, in subsequent years, richness and abundance declined in all sites along the river, indicating that the new conditions were unsuitable for most species; this pattern has been observed worldwide (e.g., Agostinho et al., 2008; Lenhardt et al., 2009; Branquinho & Brito, 2016; Mazumder et al., 2016; Turgeon et al., 2016). Furthermore, species losses were congruent with the decrease in trait diversity, and our null model analysis showed no variation in trait diversity when species richness was accounted for. In this sense, species losses, as commonly reported in regulated rivers, must be linked to the loss of traits (e.g. Santos et al., 2017; Lima et al., 2018). The null model analysis also indicated that the temporal decrease in trait diversity was not so intense, probably because remaining species maintained a poll of traits. However, although few traits were completely lost, we reported significant changes in trait composition and abundance over years. For example, we recorded 27 migratory species: 23 before river regulation, and 12 in the seventh year of the impoundment. Total abundance declined considerably (87%), and several species, abundant before the impoundment, had their abundance reduced by 90% or disappeared completely. Species able to colonize the impoundment must have specific traits to cope with lentic waters and the loss of riverine habitats (Gomes & Miranda, 2001; Pelicice et al., 2015; Angulo-Valencia et al., 2016). Limnophilic species or those with greater behavioral flexibility, such as sedentary fish or r-strategists, usually colonize the impoundment. On the other hand, species with more specialized niches, such as fishes that rely on long-distance migration, specific habitats or display rheophilic behavior, have their populations reduced (Barthem et al., 1991; Hoeinghaus et al., 2009; Schork & Zaniboni-Filho, 2017; Santos et al., 2018b). Therefore, river regulation created altered assemblages in the impoundment, with lower species richness, trait diversity and abundance.
Taxonomic and functional composition shifted over the years, with increasing dissimilarity between sites. In addition, species abundance and trait frequency changed considerably over these periods. Abundant species in the pre-disturbance phase virtually disappeared after river regulation, such as Hypostomus sp. 7, C. labyrinthicus, C. acutirostris, G. altifrons and R. vulpinus. In contrast, abundant species in the impoundment were mainly sedentary and lentic-adapted, such as S. rhombeus and P. squamosissimus. It is worth noting that few species became abundant in the impoundment, and none developed high abundance levels. Large impoundments create new environmental gradients (Agostinho et al., 2008), associated with different flow conditions, geomorphology and limnology. These changes affect alpha and beta diversity patterns by changing species sorting and dispersal mechanisms (Araújo et al., 2013; Pelicice et al., 2015; Vitorino Jr. et al., 2016). Environmental filters in the impoundment eliminate species with certain traits, but this process may depend on other factors, such as reservoir position in the basin and cumulative effects (Santos et al., 2017). In addition, large impoundments are long and heterogeneous (lentic to lotic gradients), offering opportunities for different traits along the main axis. In fact, the CWM analysis revealed that the abundance of some traits declined after river regulation (e.g., detritivore, invertivore and no parental care), while others increased in the impoundment (e.g., rounded with lateral compression, algivore, piscivore, parental care and sedentary). In addition, we observed the formation of spatial gradients (e.g., increase of fusiform in upstream sites, or decline of dorsoventral compression in downstream sites) and oscillation trends for several traits. In this sense, specific environmental filters of each zone of the impoundment (lentic to lotic) probably selected for specific traits, a pattern commonly reported in large Neotropical impoundments (Okada et al., 2005; Oliveira et al., 2005; Terra et al., 2010). It is worth noting that traits represent niche dimensions linked to species performance; therefore, they determine how species respond to environmental conditions (Mouillot et al., 2013) and consequently mediate changes in diversity.
The emergence of different community structure is the norm in regulated Neotropical rivers (Oliveira et al., 2005; Terra et al., 2010; Araújo et al., 2013), but community reassembly remains poorly understood. Long-term studies, especially those that track community changes, are incipient (e.g., Mol et al., 2007; Hoeinghaus et al., 2009; Schork & Zaniboni-Filho, 2017; Loures & Pompeu, 2018). Short-term changes (pre vs. post comparisons) have been reported (e.g., Agostinho et al., 2007), and a few comparative studies suggest that a new community state, with simplified structure, emerges after decades (Agostinho et al., 1999; Mol et al., 2007). Our study, however, showed that this process might be faster. We provide evidence that major changes in taxonomic and functional diversity occurred during the first 5 years of the impoundment. After this period, metrics tended to stabilize at low levels, with different compositions when compared to the pre-damming period. Therefore, our results show that diversity losses occurred rapidly in this species-rich ecosystem (288 species catalogued in the study area; Soares et al., 2009). This process is probably related to the fact that Neotropical fishes evolved mainly in lotic systems (Pelicice et al., 2015), so the selection imposed by the impoundment is severe. It may explain the low fish species richness commonly reported in Brazilian impoundments (ca. < 40 species; Agostinho et al., 2007), with assemblages typically composed of sedentary species. Therefore, the presence of several rheophilic fishes in Neotropical river systems may explain why fish diversity is so sensitive to hydraulic alterations. In this scenario, significant losses of taxonomic and functional diversity should constitute a general pattern in species-rich ecosystems subjected to river regulation, at both local (i.e., stretches) and regional (i.e. sub-basins) scales. Future studies should investigate how impoundments select specific traits and functional groups (e.g., Santos et al., 2017), as a means of improving predictions about fish diversity in impounded areas, particularly to predict future Amazonian scenarios and functional homogenization at global scales.
River regulation is a global process causing the loss of freshwater biodiversity and ecosystem functions (Pringle et al., 2000; Nilsson et al., 2005). Hydropower development in South America is a matter of great concern, because Neotropical freshwater fishes are disproportionately diverse (Leveque et al., 2008; Toussaint et al., 2016; Vitule et al., 2017). The regulation of major Amazonian tributaries (e.g., Tocantins, Madeira, Tapajós, Xingu), in particular, will affect global diversity patterns, if we consider that these regional watersheds are characterized by extraordinary fish diversity and endemism (Winemiller et al., 2016). One important point is that diversity losses are inevitable in impounded areas (Pelicice et al., 2015; Agostinho et al., 2016), so the maintenance of fish diversity in the Amazon Basin and other large tropical rivers will depend on the preservation of natural fluvial ecosystems (e.g., stretches, tributaries or whole basins) and their processes. This approach conflicts with hydropower production, so future energy development must balance environmental costs with economic gains (Winemiller et al., 2016), as a means of preserving fish diversity in the large rivers of the world.
References
Agostinho, A. A., L. E. Miranda, L. M. Bini, L. C. Gomes, S. M. Thomaz & H. I. Suzuki, 1999. Patterns of colonization in Neotropical reservoirs, and prognoses on aging. In Tundisi, J. G. & M. Straskraba (eds), Theoretical Reservoir Ecology and its Applications. Backhuys Publishers, Leiden: 227–265.
Agostinho, A. A., L. C. Gomes & F. M. Pelicice, 2007. Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil. EDUEM, Maringá.
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68: 1119–1132.
Agostinho, C. S., F. M. Pelicice & E. E. Marques, 2009. Reservatório de Peixe Angical: Bases ecológicas para o manejo da ictiofauna. RiMa Editora, São Carlos.
Agostinho, A. A., L. C. Gomes, N. C. L. Santos, J. C. G. Ortega & F. M. Pelicice, 2016. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fisheries Research 173: 26–36.
Angulo-Valencia, M. A., A. A. Agostinho, H. I. Suzuki, K. D. G. Luz-Agostinho & C. S. Agostinho, 2016. Impoundments affect fish reproduction regardless of reproductive strategy. Lakes and Reservoirs: Research and Management 21: 362–374.
Arantes, C. C., D. B. Fitzgerald, D. J. Hoeinghaus & K. O. Winemiller, 2019. Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Current Opinion in Environmental Sustainability 37: 28–40.
Araújo, E. S., E. E. Marques, I. S. Freitas, A. L. Neuberger, R. Fernandes & F. M. Pelicice, 2013. Changes in distance decay relationships after river regulation: similarity among fish assemblages in a large Amazonian river. Ecology of Freshwater Fish 22: 543–552.
Barthem, R. B., M. C. L. B. Ribeiro & M. Petrere Junior, 1991. Life strategies of some long-distance migratory catfish in relation to hydroelectric dams in the Amazon Basin. Biological Conservation 55: 339–345.
Branquinho, A. A. & D. Brito, 2016. Impact of dams on global biodiversity: a scienciometric Analysis. Neotropical Biology and Conservation 11(2): 101–109.
Cadotte, M. W., K. Carscadden & N. Mirotchnik, 2011. Beyond species: functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology 48: 1079–1087.
Cardoso, P., F. Rigal, J. C. Carvalho, M. Fortelius, P. A. V. Borges, J. Podani & D. Schmera, 2014. Partitioning taxon, phylogenetic and functional beta diversity into replacement and richness difference components. Journal of Biogeography 41: 749–761.
Cardoso, P., F. Rigal & J. C. Carvalho, 2018. BAT: Biodiversity Assessment Tools. R Package Version 1.6.0.
Castello, L., D. G. McGrath, L. L. Hess, M. T. Coe, P. A. Lefebvre, P. Petry, M. N. Macedo, V. F. Reno & C. C. Arantes, 2013. The vulnerability of Amazon freshwater ecosystems. Conservation Letters 6: 217–229.
Cella-Ribeiro, A., C. R. C. Doria, J. Dutka-Gianelli, H. Alves & G. Torrente-Vilara, 2017. Temporal fish community responses to two cascade run-of-river dams in the Madeira River, Amazon basin. Ecohydrology 10: e1889.
Cunico, A. M., J. D. Allan & A. A. Agostinho, 2011. Functional convergence of fish assemblages in urban streams of Brazil and the United States. Ecological Indicators 11: 1354–1359.
Dray, S., G. Blanchet, D. Borcard, G. Guenard, T. Jombart, G. Larocque, P. Legendre & H. H. Wagner, 2016. adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.0-4.
Froese, R. & D. Pauly, 2016. FishBase. FAO, Rome. http://fishbase.org.
Fu, H., J. Zhong, G. Yuan, L. Ni, P. Xie & T. Cao, 2014. Functional traits composition predict macrophytes community productivity along a water depth gradient in a freshwater lake. Ecology and Evolution 4(9): 1516–1523.
Gomes, L. C. & L. E. Miranda, 2001. Riverine characteristics dictate composition of fish assemblages and limit fisheries in reservoirs of the Upper Paraná River Basin. Regulated Rivers: Research & Management 17: 67–76.
Gotelli, N. J., 2000. Null model analysis of species co-occurrence patterns. Ecology 81: 2606–2621.
Helfman, G. S., B. B. Collette & D. E. Facey, 2009. The Diversity of Fishes, 2nd ed. Wiley-Blackwell, Oxford.
Hoeinghaus, D. J., K. O. Winemiller & J. S. Birnbaum, 2007. Local and regional determinants of stream fish assemblage structure: inferences based on taxonomic vs. functional groups. Journal of Biogeography 34: 324–338.
Hoeinghaus, D. J., A. A. Agostinho, L. C. Gomes, F. M. Pelicice, E. K. Okada, J. D. Latini, E. A. L. Kashiwaki & K. O. Winemiller, 2009. Effects of river impoundment on ecosystem services of large tropical rivers: embodied energy and market value of artisanal fisheries. Conservation Biology 23: 1222–1231.
Kraft, N. J. B., P. B. Adler, O. Godoy, E. C. James, S. Fuller & J. M. Levine, 2015. Community assembly, coexistence and the environmental filtering metaphor. Functional Ecology 29: 592–599.
Laliberté, E., P. Legendre & B. Shipley, 2014. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12.
Legendre, P., M. De Cáceres & D. Bocard, 2010. Community surveys through space and time: testing the space-time interaction in the absence of replication. Ecology 91: 262–272.
Lenhardt, M., G. Markovic & Z. Gacic, 2009. Decline in the index of biotic integrity of the fish assemblage as a response to reservoir aging. Water Resources and Management 23: 1713–1723.
Leveque, C., T. Oberdorff, D. Paugy, M. L. J. Stiassny & P. A. Tedesco, 2008. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595: 545–567.
Lima, A. C., C. S. Agostinho, D. Sayanda, F. M. Pelicice, A. M. V. M. Soares & K. A. Monaghan, 2016. The rise and fall of fish diversity in a Neotropical river after impoundment. Hydrobiologia 763(1): 207–221.
Lima, A. C., D. Sayanda, C. S. Agostinho, A. L. Machado, A. M. V. M. Soares & K. A. Monaghan, 2018. Using a trait-based approach to measure the impact of dam closure in fish communities of a Neotropical River. Ecology of Freshwater Fish 27: 408–420.
Logez, M., P. Bady, A. Melcher & D. Pont, 2013. A continental-scale analysis of fish assemblage functional structure in European Rivers. Ecography 36: 80–91.
Loures, R. C. & P. S. Pompeu, 2018. Long-term study of reservoir cascade in south-eastern Brazil reveals spatio-temporal gradient in fish assemblages. Marine & Freshwater Research 69(12): 1983–1994.
Mazumder, D., R. J. Williams, L. Wen, N. Saintilan & C. T. Walsh, 2016. Impoundment constraint of fish niche diversity in a temperate Australian river. Hydrobiologia 771: 195–206.
Melo, C. E., F. A. Machado & V. Pinto-Silva, 2004. Feeding habits of fish from a stream in the savanna of Central Brazil, Araguaia Basin. Neotropical Ichthyology 2(1): 37–44.
Mérona, B. & J. Rankin-de-Mérona, 2004. Food resource partitioning in a fish community of the Central Amazon floodplain. Neotropical Ichthyology 2(2): 75–84.
Mérona, B., G. M. Santos & R. G. Almeida, 2001. Short term effects of Tucurui Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environmental Biology of Fishes 60: 375–392.
Mol, J. H., B. Mérona, P. E. Ouboter & S. Sahdew, 2007. The fish fauna of Brokopondo Reservoir, Suriname, during 40 years of impoundment. Neotropical Ichthyology 5: 351–368.
Mouchet, M. A., S. Villéger, N. W. H. Mason & D. Mouillot, 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology 24: 867–876.
Mouillot, D., N. A. J. Graham, S. Villéger, N. W. H. Mason & D. R. Bellwood, 2013. A functional approach reveals community responses to disturbances. Trends in Ecology & Evolution 28(3): 167–177.
Neuberger, A. L., E. E. Marques, C. S. Agostinho & F. M. Pelicice, 2009. Variações espaciais na atividade reprodutiva de peixes na área de influência do reservatório de Peixe Angical. In Agostinho, C. S., F. M. Pelicice & E. E. Marques (eds), Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. RiMa Editora, São Carlos: 59–68.
Nilsson, C., C. A. Reidy, M. Dynesius & C. Revenga, 2005. Fragmentation and flow regulation of the world’s large river systems. Science 308: 405–408.
Okada, E. K., A. A. Agostinho & L. C. Gomes, 2005. Spatial and temporal gradients in artisanal fisheries of a large Neotropical reservoir, the Itaipu Reservoir, Brazil. Canadian Journal of Fisheries and Aquatic Sciences 62: 714–724.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2018. Vegan: Community Ecology Package. R Package Version 2.5-3.
Oliveira, E. F., C. V. Minte-Vera & E. Goulart, 2005. Structure of fish assemblages along spatial gradients in a deep subtropical reservoir (Itaipu Reservoir, Brazil-Paraguay border). Environmental Biology of Fishes 72: 283–304.
Orsi, M. L. & J. R. Britton, 2014. Long-term changes in the fish assemblage of a Neotropical hydroelectric reservoir. Journal of Fish Biology 84: 1964–1970.
Pelicice, F. M., P. S. Pompeu & A. A. Agostinho, 2015. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries 16: 697–715.
Pelicice, F. M., V. M. Azevedo-Santos, A. L. H. Esguícero, A. A. Agostinho & M. S. Arcifa, 2018. Fish diversity in the cascade of reservoirs along the Paranapanema River, southeast Brazil. Neotropical Ichthyology 16(2): e170150.
Pereira, P. R., C. S. Agostinho, R. J. Oliveira & E. E. Marques, 2007. Trophic guilds of fishes in sandbank habitats of a Neotropical river. Neotropical Ichthyology 5(3): 399–404.
Petchey, O. L. & K. J. Gaston, 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9: 741–758.
Petesse, M. L. & M. Petrere Jr., 2012. Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tietê river basin, Brazil. Ecological Engineering 48: 109–116.
Petrere Jr., M., 1996. Fisheries in large tropical reservoirs in South America. Lakes and Reservoirs: Research and Management 2: 111–133.
Poff, N. L., J. D. Allan, M. B. Bain, J. R. Karr, K. L. Prestegaard & B. D. Richter, 1997. The natural flow regime. BioScience 47: 769–784.
Pough, F. H., C. M. Janis & J. B. Heiser, 2008. A vida dos vertebrados. 2ª edição. Atheneu Editora, São Paulo.
Pringle, C. M., M. C. Freeman & B. Freeman, 2000. Regional effects of hydrologic alterations on riverine macrobiota in the New World: tropical-Temperate comparisons. BioScience 50(9): 807–823.
R Development Core Team, 2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. www.r-project.org.
Rahel, F. J. & R. L. McLaughlin, 2018. Selective fragmentation and the management of fish movement across anthropogenic barriers. Ecological Applications 28(8): 2066–2081.
Santos, N. C. L., H. S. Santana, J. C. G. A. Ortega, R. M. Dias, L. F. Stegmann, I. M. S. Araújo, W. Severi, L. M. Bini, L. C. Gomes & A. A. Agostinho, 2017. Environmental filters predict the trait composition of fish communities in reservoir cascades. Hydrobiologia 802: 245–253.
Santos, N. C. L., E. Garcia-Berthou, J. D. Dias, T. M. Lopes, I. P. Affonso, W. Severi, L. M. Bini, L. C. Gomes & A. A. Agostinho, 2018a. Cumulative ecological effects of a Neotropical reservoir cascade across multiple assemblages. Hydrobiologia 819: 77–91.
Santos, R. E., R. M. Pinto-Coelho, R. Fonseca, N. R. Simões & F. B. Zanchi, 2018b. The decline of fisheries on the Madeira River, Brazil: the high cost of the hydroelectric dams in the Amazon Basin. Fisheries Management and Ecology 25: 380–391.
Sá-Oliveira, J. C., V. J. Isaac, A. S. Araújo & S. F. Ferrari, 2016. Factors structuring the fish community in the area of the Coaracy Nunes hydroelectric reservoir in Amapá, northern Brazil. Tropical Conservation Science 9(1): 16–33.
Schork, G. & E. Zaniboni-Filho, 2017. Structure dynamics of a fish community over ten years of formation in the reservoir of the hydroelectric power plant in upper Uruguay River. Brazilian Journal of Biology 77(4): 710–723.
Soares, A. B., F. M. Pelicice, P. H. F. Lucinda, A. Akama & C. S. Agostinho, 2009. Diversidade de peixes na área de influência da barragem de Peixe Angical, antes e após a formação do reservatório. In Agostinho, C. S., F. M. Pelicice & E. E. Marques (eds), Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. RiMa Editora, São Carlos: 15–27.
Terra, B. F., A. B. I. Santos & F. G. Araújo, 2010. Fish assemblage in a dammed tropical river: an analysis along the longitudinal and temporal gradients from river to reservoir. Neotropical Ichthyology 8(3): 599–606.
Toussaint, A., N. Charpin, S. Brosse & S. Villéger, 2016. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Scientific Reports 6: 22125.
Turgeon, K., C. T. Solomon, C. Nozais & I. Gregory-Eaves, 2016. Do novel ecosystems follow predictable trajectories? Testing the trophic surge hypothesis in reservoirs using fish. Ecosphere 7(12): e01617.
Violle, C., M. L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel & E. Garnier, 2007. Let the concept of trait be functional! Oikos 116: 882–892.
Vitorino Jr., O. B., R. Fernandes, C. S. Agostinho & F. M. Pelicice, 2016. Riverine networks constrain b-diversity patterns among fish assemblages in a large Neotropical river. Freshwater Biology 61: 1733–1745.
Vitule, J. R. S., A. A. Agostinho, V. M. Azevedo-Santos, V. S. Daga, W. R. T. Darwall, D. B. Fitzgerald, F. A. Frehse, D. J. Hoeinghaus, D. P. Lima-Junior, A. L. B. Magalhães, M. L. Orsi, A. A. Padial, F. M. Pelicice, M. Petrere Jr., P. S. Pompeu & K. O. Winemiller, 2017. We need better understanding about functional diversity and vulnerability of tropical freshwater fishes. Biodiversity and Conservation 26: 757–762.
Winemiller, K. O., P. B. McIntyre, L. Castello, E. Fluet- Chouinard, T. Giarrizzo, S. Nam, I. G. Baird, W. Darwall, N. K. Lujan, I. Harrison, M. L. J. Stiassny, R. A. M. Silvano, D. B. Fitzgerald, F. M. Pelicice, A. A. Agostinho, L. C. Gomes, J. S. Albert, E. Baran, M. Petrere-Juńior, C. Zarfl, M. Mulligan, J. P. Sullivan, C. C. Arantes, L. M. Sousa, A. A. Koning, D. J. Hoeinghaus, M. Sabaj, J. G. Lundberg, J. Armbruster, M. L. Thieme, P. Petry, J. Zuanon, G. T. Vilara, J. Snoeks, C. Ou, W. Rainboth, C. S. Pavanelli, A. Akama, A. van Soesbergen & L. Saenz, 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351: 128–129.
Acknowledgements
We thank colleagues from the Núcleo de Estudos Ambientais (Universidade Federal do Tocantins) for their assistance in field and laboratory work (2004–2007 period), Biota for providing data (2011–2014 period), and Enerpeixe S.A. for financial funding. CNPq provided a scholarship for P.B.P. and a research grant for F.M.P. We thank Luis M. Bini (Editor) and anonymous reviewers for providing comments and corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luis Mauricio Bini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perônico, P.B., Agostinho, C.S., Fernandes, R. et al. Community reassembly after river regulation: rapid loss of fish diversity and the emergence of a new state. Hydrobiologia 847, 519–533 (2020). https://doi.org/10.1007/s10750-019-04117-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04117-9