Abstract
The impacts of biological invasions remain poorly known for some habitats, regions and taxa. To date, there has been no comprehensive effort to review and synthesize the impacts of invasive mollusc species in South America. In this paper, we provide a synoptic view on what is known on documented socio-ecological impacts of aquatic no-native mollusc species (NNMS) and transplanted mollusc species (TMS) from South America. An expert group involving malacologists and taxonomists from different countries, the “South America Alien Molluscs Specialists” (eMIAS), shared and summarized the scientific literature, databases, and published and unpublished information on confirmed impacts of NNMS and TMS in South America. Three broad categories, non-mutually exclusive were used as a framework: “Environmental/Biodiversity impacts”, “Economic and social effects”, and “Human health impacts”. Some 21 NNMS and seven TMS have documented impacts on at least one of those three categories. We encourage targeting the less known areas of research, such as economic valuation of human health (and veterinary) impacts attributable to NNMS or TMS and expand our knowledge of environmental impacts for the species listed in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans are completely dependent on the goods and services provided by Earth ecosystems, such as food, water, disease management, climate regulation and even for the intrinsic value it provides such as spiritual fulfilment and aesthetic enjoyment (Millennium Ecosystem Assessment, 2005). In the last 50 years, humans have changed ecosystems faster and more extensively than in any other comparable period in human history, in large part to meet humans’ demands for ecosystem services. The harmful effects from that practice are causing a persistent decline in the ability of ecosystems to provide such services (Millennium Ecosystem Assessment, 2005).
Biological invasions are a significant aspect of the Anthropocene (Campinha et al., 2015; Pyšek et al., 2017) and a constant threat to biodiversity (IPBES, 2019). Humanity has introduced thousands of species to areas outside their native ranges, and while most of these fails to establish viable populations, invasive non-native species have been traditionally identified as one of the main drivers of biodiversity loss worldwide, but their impacts on ecosystem services, sustainable development, and human well-being are poorly quantified and understood (IPBES, 2019). Further, the magnitude of the threat to endangered species is still controversial, due to a scarcity of empirical data and a high degree of uncertainty (Gurevitch & Padilla, 2004; Dueñas et al., 2018). This knowledge gap is more pronounced for some regions and taxa.
The impacts of invasive species have been studied, and reviewed more often for temperate latitudes of the Northern hemisphere in comparison to the Southern hemisphere, for terrestrial rather than aquatic ecosystems, and for plants and insects (together accounting for two-thirds of the studies), in comparison with other taxa (Pyšek et al., 2008). Molluscs, the second most diverse metazoan phylum (Darrigran et al., 2020) are no exception, and there is a direr situation concerning non-native mollusc species (NNMS) in aquatic environments. Molluscs account for only 5% of global studies, and South America is among the regions with fewer studies concerning this topic (Speziale et al., 2012; Thomsen et al., 2014). For example, Thomsen et al. (2014) reported only 10 studies that quantified impacts of 13 aquatic non-native species from a review of 259 papers published between 1972 and 2012, but no information on aquatic molluscs seems to be included. Similarly, a recent paper addressing the economic cost of biological invasions worldwide (Diagne et al., 2021) found that 79% of the information regarding impacts was gathered form studies performed in North America, Oceania and Europe. These biases affect our understanding and management of this pressing issue.
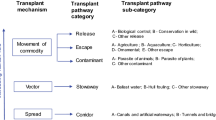
Non-native aquatic molluscs play important roles in the ecosystems where they are introduced (e.g., as consumers, competitors, hosts or prey). Despite their potential environmental importance, the distribution patterns of NNMS in South America and their entry points have only recently been documented by Darrigran et al. (2020), who listed 86 NNMS distributed in 152 (out of 189) terrestrial, freshwater and marine ecoregions of South American continent. Of those, 30 were aquatic (16 in freshwater and 14 in marine environments). More recently, 20 aquatic transplanted mollusc species (TMS), i.e., native mollusc species introduced deliberately or accidentally beyond their natural range, were recognised in South America (Darrigran et al., 2022).
To date, there have been no comprehensive efforts to review and synthesise the impacts of NNMS and TMS in South American ecosystems, and thus a synoptic picture on the impacts of NNMS and TMS in the region is still lacking. One of the underlying reasons is the greater attention given to Corbicula fluminea (Müller, 1774) and Limnoperna fortunei (Dunker, 1857), which have been the subject of numerous studies and important reviews (Penchaszadeh, 2005; Darrigran & Damborenea, 2006, 2009; Dreher Mansur et al., 2012; Boltovskoy, 2015a). In this work, a synthesis of the known impacts documented in South America for all registered NNMS and TMS is presented. Both C. fluminea and L. fortunei are included, without claiming an exhaustive review of all published information. Such synthesis aims to provide a better understanding of the present situation on the continent and grant insights for future monitoring and policies, including limiting new introductions. Therefore, in this study we synthesize and provide examples of socio-economic effects and environmental impacts of marine and freshwater NNMS and TMS in South America, highlighting avenues for future research.
Materials and methods
An expert group involving malacologists and taxonomists from different countries of South America (Argentina, Brazil, Chile, Ecuador, Peru, Uruguay, Venezuela), the “South American Alien Molluscs Specialists'' (eMIAS; https://emiasgroup.wixsite.com/emias), reviewed and shared scientific literature (including “grey” literature), collection data, databases and experiences on the subject through a virtual forum. Additionally, the group compiled published information on confirmed impacts of non-native mollusc species (NNMS) and transplanted mollusc species (TMS) in South America. The list of NNMS and TMS was presented in previous contributions of the eMIAS (Darrigran et al., 2020, for NNMS; Darrigran et al., 2022, for TMS). Each contributor provided information based on published evidence and/or research experience according to their expertise, familiar taxa and region. The database on species and impacts was completed with a literature search on Scopus and Google Scholar, with an open search period. Keywords used in the search strategy include “species name,” and “impacts” in English, Spanish and Portuguese, identifying those publications relevant to the current study, according to the criteria stated below.
Definitions
We define non-native mollusc species (NNMS) in South America as species introduced outside their natural geographical range through human action, that are able to maintain a self-sustaining population (Darrigran et al., 2020). Transplanted mollusc species (TMS) are defined as species native to South America that underwent changes in their natural distribution within the continent, either through human action or due to human–induced environmental factors (Darrigran et al., 2022). In our discussion, if a given species has an evident impact on the environment and human well-being and livelihoods, it is dubbed an “invasive species” (irrespectively of being a NNMS or a TMS). Cryptogenic species sensu Carlton (1996) were not considered.
In the present study, an impact is considered to be a measurable change in the state of a given indicator of an invaded ecosystem, which can be attributed to non-native or transplanted species (Ricciardi, 2003). This definition of impact includes any change in ecological or ecosystem properties but takes no position on whether a given impact is positive or negative value (Jeschke et al., 2014). Therefore, only the effects on human well-being and livelihoods caused by invasive species are considered either as positive or negative. Examples of ecosystem impacts include increased risk of extinction of native species, changes in the genetic composition of native populations, modification of the phylogenetic and functional diversity of invaded communities and food webs, changes in the productivity of ecosystems, nutrient cycling and pollutants (e.g., Pyšek et al., 2020). We acknowledge, however, that ecosystem impacts can also directly or indirectly affect human well-being (e.g., Martinez-Juarez et al., 2015) and that species redistributions itself may impair economic development, livelihoods, food security, human health and culture (Pecl et al., 2017).
There are several frameworks to assess the impacts or effects of aquatic non-native species (e.g., Dextrase & Mandrak, 2006; Everard et al., 2009; Thomsen et al., 2014; Doherty-Bone et al., 2019; Pyšek et al., 2020), which can be grouped into three broad and non-mutually exclusive categories: “Environmental impacts or Ecological impacts” or “Biodiversity impacts” (i.e., impacts on “wild” populations, communities, species or ecosystems), “Economic and social effects” and “Human health effects”. The latter two pertain to different dimensions of human well-being and, although effects in human health can also be considered Economic and Social impacts, we maintained these two categories separated (cf. Martuzzi, 2005; Ebi et al., 2006; Zeimes et al., 2012; Pedersen et al., 2014).
Herein, we focused on the documented impacts and effects of invasive species in South America, not considering possible risks and potential threats. Therefore, studies reporting range expansion and first records of a given species in a certain area were not considered if they lack significant observations on local impacts, even though those studies often include a list of potential impacts based on what is known from elsewhere. Thus, we did not consider impacts that have been reported from other continents where the same species has been introduced. When available, some experimental results were included, although we did not necessarily affirm that the reported interactions are occurring in nature.
For the category of environmental impacts, it may be argued that just by the arrival of a NNMS or TMS there is a modification of the biogeographic distribution of native taxa, causing a change in several community-level attributes, such as local species composition, diversity and evenness of local communities. In this study, we focused mainly on conspicuous changes in local community structure driven by the abundance of NNMS and TMS, and/or their incorporation into food webs. These effects may be particularly relevant in human-modified ecosystems invaded by bivalves (Burlakova et al., 2022). Other documented impacts may include changes on abiotic conditions directly attributable to the presences of NNMS or TMS, or genetic interaction with local species (e.g., hybridization). Correlational evidence for some impacts was accepted as a “documented” impact, but these cases clearly deserve further experimental analysis to elucidate the underlying mechanisms or to confirm cause-effect relationships.
Socio-economic effects include direct and indirect monetary costs associated with the action of invasive species (Adelino et al., 2021; Diagne et al., 2021; Burlakova et al., 2022). For example, reduction or loss of profits due to the effect of mollusc-borne parasites in domestic cattle may be particularly difficult to quantify or even estimate. We thus considered primarily those reports highlighting the interaction of NNMS and TMS species with economic activities. Furthermore, some species were introduced for the development of commercial aquaculture, and some accidentally introduced species may also be commercially exploited.
Some NNMS can cause the spread of new and/or existing diseases acting as vectors of pathogens. In the public health category, we were more liberal, so any reports documenting the presence of a human or veterinary parasite or pathogen in a NNMS or TMS in South America were considered. Other potential effects include allergic reaction, ingestion of toxins, loss of aesthetical value or mechanical harms of several sorts (Mazza et al., 2013). Clearly, public health effects further include an economic dimension, which should be considered elsewhere.
Results
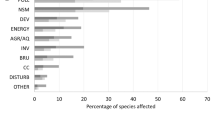
The information on confirmed impacts and effects of NNMS and TMS in South America is synthesised in Tables 1 and 2. A total of 28 mollusc species was documented as having impacts in South America, 21 of them are NNMS (nine freshwater and 12 marine) and seven are TMS (two freshwater and five marine). All marine TMS are bivalves, Leiosolenus aristatus (Dillwyn, 1817), Mytella strigata (Hanley, 1843), Mytilopsis trautwineana (Tryon 1866), Argopecten purpuratus (Lamarck, 1819) and Tawera elliptica (Lamarck, 1818) which cause economic and social effects. A. purpuratus is included on the basis of being transplanted for commercial aquaculture. In the freshwater environment, Anodontites trapesialis (Lamarck, 1819) causes economic and social effects. On the other hand, a species of gastropod, Pomacea canaliculata (Lamarck, 1822) has documented impacts on the three categories (Biodiversity impacts, Economic and social effects, Human health effects). However, this species can also exhibit positive effects as a potential control of the NNMS Physa acuta Draparnaud, 1805. Altogether, negative effects are more commonly documented than positive effects. Finally, several NNMS are listed in more than one category (see Tables 1 and 2). In the marine environment, nine NNMS cause Biodiversity impacts, nine Economic and social effects and two Human health effects. In freshwater, seven NNMS cause Biodiversity impacts, seven Economic and social effects, while three affects Human health.
Discussion
Documented impacts on biodiversity
Marine ecosystems
There are 14 aquatic NNMS that were intentionally introduced to develop commercial marine aquaculture (Darrigran et al., 2020). However, only two of these species (the abalones Haliotis discus Reeve, 1846 and Haliotis rufescens Swainson, 1822) are not established in natural environments, and they have been highlighted as a threat to native and cultured species as a vector that facilitates the spread of boring polychaetes (Moreno et al., 2006; Diez et al., 2011). The remaining 12 species are currently distributed in coastal environments along South America, where they are at least modifying species composition and relative abundances within communities, which can be viewed as a primary impact on biodiversity. For some species, [e.g., Perna viridis (Linnaeus, 1758), Isognomon bicolor (Adams, 1845), Magallana gigas (Thunberg, 1793) [= Crassostrea gigas (Thunberg, 1793)] and Eualetes tulipa (Rousseau in Chenu, 1843)] there are studies quantifying densities or abundances, and that provide a description of community structure after the arrival of NNMS. Often, those species that increase the heterogeneity of native environments (e.g., M. gigas reefs on mudflats) cause shifts in the occurrence and abundances of associated species (Melo et al., 2010; Ludwig et al., 2011; Mendez et al., 2015), thus increasing alpha diversity at a local scale. Studies on some other encrusting, hard-bottom species (e.g., I. bicolor) have likewise been carried out, and most include occurrence reports and abundance estimates (Ignacio et al., 2010; Dias et al., 2013; Agostini & Ozorio, 2016; Oricchio et al., 2019).
Other impacts at the functional level include the incorporation of NNMS in local food webs. For example, Rapana venosa (Valenciennes, 1846) seems to be an important food item for Loggerhead turtle, Caretta caretta (Linnaeus 1758), in the Río de la Plata estuary (Carranza et al., 2010a). Another example is I. bicolor that causes changes of food habit in the gastropod Stramonita haemastoma (Linnaeus, 1758), which fundamentally preyed on the mussel Perna perna (Linnaeus, 1758), native species according to Darrigran et al. (2020), before the arrival of I. bicolor (López et al., 2010). There are other interactions reported, such as the massive fouling of R. venosa on green sea turtles Chelonia mydas (Linnaeus, 1758) (Lezama et al., 2013), although the effects on individual fitness are yet to be confirmed. Similarly, due to the predatory role and high local abundances of R. venosa, this species could be significantly affecting some ecological properties of their intertidal habitat, such as mussel coverage on rocky bottoms (Carranza et al., 2010b), but no studies have quantified the extension of this presumably environmental impact.
Freshwater ecosystems
Reports of biodiversity impacts of NNMS/TMS are available for only eight species in freshwater environments. One of the best studied species is the golden mussel, Limnoperna fortunei, which is the most aggressive aquatic invasive in South America. The rapid spread of L. fortunei populations in hydrographic basins have been attributed to human-mediated dispersal (Belz et al., 2012; Boltovskoy, 2015b; Borges et al., 2017; Ludwig et al., 2021).
Populations of L. fortunei are found on virtually any natural hard surface available (e.g., logs, water vegetation, and compact sandy silt), as well as any artificial structure and substrate (e.g., walls, piers, pipes, glass, nylon) (Darrigran & Damborenea, 2005). De Lucía et al. (2023) recommend conservation efforts given the constant advance of urbanization, with environmental impact studies prior to coastal reforms, and implementation of density control strategies for L. fortunei in protected areas. Considering the serious problems that it causes, it is astonishingly overlooked by society and governments in South America. The golden mussel modifies environmental conditions of invaded South American inland freshwater environments, altering both abiotic and biotic variables affecting ecosystem services, with large environmental and socio-economic impacts (Darrigran & Damborenea, 2011; Boltovskoy & Correa, 2015). Impacts of L. fortunei are difficult to interpret due to the multiple interactions with the biotic and abiotic components and their dynamics and to the regional environmental conditions. So, the impacts are variable in the medium and long terms, and in both local and regional scale. The impacts and the effects are reflected in the high number of publications. Boltovskoy (2015a) and Burlakova et al. (2022) summarized the scale and variety of the environmental impacts and economic and human well-being effects caused by the golden mussel. In this contribution we only have addressed the most conspicuous effects, such as fouling on native molluscs and other macroinvertebrates (including Anodontites trapesialis and C. fluminea (Darrigran, 2002), the crabs Trichodactylus borellianus Nobili, 1896 (Rojas Molina & Williner, 2013) and Aegla platensis Schmitt, 1942, and the gastropod Pomacea canaliculata (Darrigran & Damborenea, 2005; Silva et al., 2021a), impacts on benthic communities, fish communities, bioaccumulation of metals, impacts in water column, nutrient cycling, and on plankton communities and cyanobacteria blooms (Table 1). In summary, L. fortunei is a very effective ecosystem engineer, altering both the structure and function of the ecosystem (Darrigran & Damborenea, 2011; Boltovskoy, 2015a).
Four NNMS of the genus Corbicula were recorded in South America [C. fluminea, C. largillierti (Philippi, 1844), C. fluminalis (Müller, 1774) and Corbicula sp.] (Mansur et al., 2011). Among these species, C. fluminea causes a severe impact on the environment. This species invaded ecosystems around the world, being present between 39° South and 53° North. In less than 100 years, it has invaded all continents except Antarctica, being one of the most successful invasive species in aquatic ecosystems (Crespo et al., 2015). In the hydrographic basins of South America, the macroinvertebrates assemblages are mainly impacted by displacement and reduction of available habitat (Darrigran et al., 2020; Labaut et al., 2021). Thus, like the golden mussel, C. fluminea often plays a role of ecosystem engineer, causing physical disruptions wherever it establishes and changing the structure of macroinvertebrate benthic communities (Reshaid et al., 2017). Labaut et al. (2021) observed that on the Limay River, in the Argentinean Patagonia, C. fluminea impacts the abundance of some taxa, due to the competition for resources in a low productivity ecosystem. The faeces and pseudo-faeces of C. fluminea deposited on the sediment enrich their organic content. However, they compete for food with benthic macroinvertebrates. Sites invaded by C. fluminea showed a tendency towards homogenization of species and functional composition (Labaut et al., 2021). However, in other cases, the evidence for competitive displacement of native species is not always strong. Clavijo and Carranza (2014), analysing the correlation between the critical reduction of the distribution of the native Cyanocyclas spp. and the spread of Corbicula in Uruguay, proposed the interplay between a) the direct adverse effect of interspecific competition with the Asiatic clam, and/or b) the degradation of environmental conditions leading to the disappearance of the native species and their replacement by opportunistic species. Both hypotheses should be regarded as extremes of a continuum, with several intermediate scenarios likely to coexist.
Reproductive studies offer a solid basis for predictive trends of the invasion of populations of C. fluminea. The reproductive features (Ludwig et al. 2014; Pigneur et al., 2014; Cao et al., 2017) facilitate the survival of C. fluminea from Venezuela (10°10’S—63°30’W) to Patagonia Argentina (39°28′S—68°58′W) (Labaut et al., 2021), being present in about half of the South American freshwater ecoregions (Darrigran et al., 2020). The rapid spread of C. fluminea in South America has involved humans as vectors, either transporting individuals in the bilge water of crafts, with or as fish bait, in dredged river sand, as juveniles attached to boat hulls, and by aquarium hobbyists (McMahon, 2000; Belz et al., 2012; Labaut, 2021).
Other NNMS freshwater species with reported impacts in South America are Melanoides tuberculata (Müller, 1774) and the New Zealand mud snail Potamopyrgus antipodarum (Gray, 1843). In Brazil, M. tuberculata has negatively affected native populations of Pomacea lineata (Spix in J. A. Wagner, 1827) in Rio de Janeiro state, Biomphalaria glabrata (Say, 1818) in Minas Gerais and Rio de Janeiro states, Biomphalaria straminea (Dunker, 1848) in Minas Gerais, and Aylacostoma tenuilabris (Reeve, 1860) in the Tocantins River, Goiás (Guimarães et al., 2001; Giovanelli et al., 2002; Fernandez et al., 2003). Similarly, Collado et al. (2019) reported correlational evidence of competitive displacement of native gastropods by P. antipodarum in Chile. Interactions of native species with NNMS or TMS are also worth evaluating. Maldonado & Martin (2019) experimentally evaluated the effects of Pomacea canaliculata, Melanoides tuberculata and Physa acuta on native snails [Heleobia parchappii, (d'Orbigny, 1835), Biomphalaria peregrina (d'Orbigny, 1835), and Chilina parchappii (d'Orbigny, 1835)], showing negative interactions including reduced fecundity in P. acuta and B. peregrina, although the NNMS M. tuberculata was not affected by P. canaliculata. Thus, the impact of P. canaliculata in recently colonised regions of South America deserves further attention.
Documented socio-economic effects
Marine ecosystems
So far, there are few documented negative effects on economic activities by NNMS in South American marine ecosystems. The mytilids Mytella strigata and the false mussels Mytilopsis spp. have been reported to produce a trophic imbalance in culture pools, decreasing production in shrimp farming as well as fouling in some structures (Aldridge et al., 2008; Lodeiros et al., 2019, 2021). Similarly, the boring TMS Leiosolenus aristatus caused damage to shells of the cultured scallop Nodipecten nodosus (Linnaeus, 1758), producing serious scars, deformations and even death in a marine farm in São Paulo state (Brazil; Simone & Gonçalves, 2006). Additionally, Talonostrea talonata Li & Qi, 1994 [= Crassostrea talonata (Li & Qi, 1994)] may outcompete Crassotrea tulipa (Lamarck, 1819) [= Crassostrea gasar (Lamarck, 1819)], being a nuisance species in oyster culture (Cavaleiro et al., 2019). Finally, the vermetid Eualetes tulipa fouls power plant turbines in Venezuela (Miloslavich & Penchaszadeh, 1992).
On the other hand, positive economic return is associated with commercial cultures of Haliotis discus and Haliotis rufescens (Flores-Aguilar et al., 2007; Castilla & Neill, 2009; SUBPESCA, 2021) and the Pacific oyster Magallana gigas (Furse et al., 2004; dos Santos & Costa, 2016; Martínez-García et al., 2021). This kind of introductions for commercial aquaculture often presents positive social effects such as direct income, increased employment and associated research. In this line, the development of experimental aquaculture may also be considered as a positive effect associated with the green mussel Perna viridis in Venezuela, since it provides new employment opportunities for local researchers and workers (Acosta-Balbás et al., 2019).
Another interesting effect to be more carefully analysed is the claim that NNMS act as vectors of boring polychaetes. Once marine species are introduced to new areas for aquaculture, their associated epibionts can also be accidentally introduced. This may pose a risk both to the economic activity and the native biodiversity, since non-native epibionts may be able to exploit new native hosts (e.g., Kuris & Culver, 1999). This effect could change population and community composition and dynamics (Grosholz et al., 2000), but this phenomenon remains poorly understood in South America. However, Moreno et al. (2006) pointed out that aquaculture activities may be the primary introduction vector for boring polychaete species in Chile. Similarly, spionid polychaetes heavily parasitize and destroy the shell of the invading Rapana venosa in Uruguay (A. Carranza, unpublished), and in certain areas it may be exerting some control of the invader species. However, the identity and biogeographic origin of the polychaete species involved is hard to elucidate.
Freshwater ecosystems
Limnoperna fortunei easily invades water transfer tunnels and attaches to tunnel walls and structures with extremely high density, resulting in biofouling and being responsible for negative effects on hydropower generation, water quality, and damages in man-made structures (Adelino et al., 2021). The effect on turbine components occurs by hydro-abrasion; the abrasiveness of the golden mussel shell was compared with that of silicon carbide (SiC) and the wear mechanisms acting on the SiC tests are the same as for the mussels (de Castro et al., 2019). Additionally, the consequences of the establishment of L. fortunei also include reduction in pipe diameter or outright blockage of pipes, water contamination by massive mortality of individuals, and obstruction of cooling systems (Darrigran, 2010; Boltovskoy, 2015a). Rebelo et al. (2018) estimated that the cost of monitoring and maintenance due to golden mussel fouling in the infrastructure of hydroelectric power plants in Brazil ranges between USD 6.9 and 8 million annually, and the economic losses in that country due to the stoppage of a turbine are in the order of USD 120 million a year. For Argentina, Duboscq-Carra et al. (2021) indicated a cost of around USD 2 million from three reports on management, while Haubrock et al. (2022) reported a total of USD 40.5 million between 2001 and 2020 for South America.
In contrast with the effects of Corbicula fluminea reported from North America (McMahon, 2000), in South America the only known report come from a hydroelectric power station in the Rio Grande do Sul state, Brazil, where it fouled heat exchangers in 1988 (dos Santos et al., 2012).
Another socio-economic issue is reported for Anodontites trapesialis, a TMS whose larvae heavily parasitize some fish cultures in South America (Silva-Souza & Eiras, 2002; Felipi & Silva-Souza, 2008; Agudo-Padrón, 2019). Furthermore, the mollusc-borne fluke Philophthalmus gralli Mathis and Leger, 1910 (Digenea, Philophthalmidae; hosted by Melanoides tuberculata) can infect poultry causing profit loss (Pinto & de Melo, 2010). Well-documented direct economic effects of Pomacea spp. in rice cultures has also been reported (Wiryareja & Tjoe-Awie, 2006; Agudo-Padrón et al., 2010; Horgan et al., 2014a, b; Correoso Rodriguez et al., 2017).
Pseudosuccinea columella (Say, 1817) and Galba truncatula (O. F. Müller, 1774) are vectors for the trematodes Fasciola hepatica Linnaeus, 1759 (Digenea, Fasciolidae) and Cotylophoron cotylophorum (Fischoeder, 1901) (Digenea, Paramphistomidae), which can infect domestic cattle, resulting in deteriorated condition of infected individuals and consequent economic losses (Ueta, 1980; Heinzen et al., 1994; Mas-Coma et al., 2001; Salazar Jaramillo et al., 2006; Lopez et al., 2008; Prepelitchi & Wisnivesky-Colli, 2013).
Effects on public health
Marine ecosystems
No public health issues or even risks are reported associated with most marine NNMS. The only exception pertains to the sea slug Pleurobranchaea maculata (Quoy & Gaimard, 1832), which can carry neurotoxins that affect human and domestic animals (Bökenhans et al., 2019). The presence of the bacteria Xenohaliotis californiensis and the probable presence of Bonamia sp. in abalone cultures is also worth noting (Campalans & Lohrmann, 2009).
Freshwater ecosystems
At least four NNMS can be hosts of pathogen parasites that cause human diseases. The liver fluke Fasciola hepatica, that causes human fasciolosis, has been reported in the lymnaeid snails Pseudosuccinea columella (Ueta, 1980; Heinzen et al., 1994; Salazar Jaramillo et al., 2006; Prepelitchi & Wisnivesky-Colli, 2013) and Galba truncatula (Mas-Coma et al., 2001; Esteban et al., 2002). In the Bolivian Altiplano, where endemic fasciolosis has been reported since 1984, the transmission to humans appears to be linked with the ingestion of aquatic plants infected with metacercariae, and the prevalence of the disease is correlated with the presence of snails (Marcos et al., 2006; Parkinson et al., 2007). Genetic evidence from individuals of Fasciola hepatica and G. truncatula suggest a recent introduction from Europe (Mas-Coma et al., 2001), and concomitantly, prevalence and intensity of human fasciolosis in the northern Bolivian Altiplano are the highest reported to date.
The freshwater gastropod Melanoides tuberculata can also act as an intermediate host of the trematode Centrocestus formosanus Nishigori, 1924 (Digenea, Heterophyidae) (Hernández et al., 2003; Velásquez et al., 2006; Pinto et al., 2018), which can infect humans through ingestion of raw or undercooked parasitized fish, causing gastric pain and indigestion accompanied by diarrhea (Chai et al., 2013). However, there are no reported cases in South America.
In 2008, the presence of Angiostrongylus cantonensis (Chen, 1935) (Nematoda, Angiostrongylidae) was reported for the first time in Ecuador, as well as the first cases of an emerging disease caused by the larval stage, eosinophilic meningitis. Several authors have highlighted the apple snail Pomacea canaliculata as an intermediate host of A. cantonensis in Ecuador (Solórzano Álava et al., 2014; Correoso Rodriguez et al., 2017; Thiengo et al., 2017). In 2015 an experimental infection of P. canaliculata with Angiostrongylus vasorum (Baillet, 1866), which infects the heart and pulmonary artery of domestic and wild canids was reported (Mozzer et al., 2015).
On the other hand, Schistosoma mansoni Sambon, 1907 (Digenea, Schistosomatidae) is a blood fluke causing schistosomiasis in humans, depending on Planorbidae snails as intermediate hosts. This tropical disease is largely neglected but ranks amongst the most prevalent in humans: in 2021, the World Health Organisation reported 236.6 millions of people diagnosed with schistosomiasis in Africa, the Middle East, the Caribbean, Brazil, Venezuela and Suriname. In this case, Marisa cornuarietis (Linnaeus, 1758) has been regarded as a biological control of schistosomiasis vectors, thus providing an example of a positive impacts of a NNMS in the Public Health dimension.
Finally, the New Zealand mud snail Potamopyrgus antipodarum is another NNMS known to host parasites of veterinary and human health relevance, such as Sanguinicola sp. (Bacteria), Paracardicoloides yamagutii Martin, 1974 (Digenea, Aporocotylidae) and Notocotylus gippyensis (Beverley-Burton, 1958) (Digenea, Notocotylidae) (Hine, 1978; Morley, 2008), but no study has yet analysed their prevalence in South America.
Concluding remarks
Twenty-eight NNMS and TMS are known to have documented impacts and effects on at least one of the three dimensions here considered. Given that South America is a large and heterogeneous continent, it is unclear how impacts or effects (positive or negative) of a NNMS or TMS can be distributed along a species distribution range. However, this contribution provides a synoptic view of the literature at a continental scale, and thus can be useful to direct future research priorities. The first interesting fact emerging from our study, is that 70% of all NNMS from marine and freshwater habitats in South America [30 species according to Darrigran et al., (2020)] had documented impacts and effects compared with only 41% of all TMS [14 species according to Darrigran et al., (2022)]. Thus, the overall impact of NNMS exceeds that of TMS, and/or alternatively, there may be a bias towards documenting impacts of exotic, well known invasive species. This putative bias should be further investigated, since there are known biases towards reporting negative over positive effects (e.g., Boltovskoy et al., 2021, 2022). This provides interesting avenues for new research, and to disentangle if this perceived pattern is correlated with biological reality or a publication bias. Notice, however, that we were not able to compare the relative magnitude of these impacts and effects. Besides, impacts have different levels of certainty. Studies reporting correlational evidence were often included as an impact (e.g., Clavijo & Carranza, 2018), in particular when direct quantitative estimates were lacking. Further work should focus on a deeper analysis of these claimed or suggested impacts. Finally, there is not a clear relationship between the direct impact of NNMS and TMS in aquatic environments of South America and losses in native biodiversity, in line with previous work suggesting that the main threat drivers are habitat loss, overharvesting and habitat disturbances (e.g., Dueñas Gurevitch et al., 2018; Gurevitch & Padilla, 2004).
Except for costs associated with the control of Limnoperna fortunei, quantified direct economic effects are scarce in the available data and literature. Our results provide an underestimation of the environmental impacts of NNMS and TMS in South America, due to both underreporting and the often-considerable lag between first record, identification and communication of new NNMS and TMS (Pires Teixeira & Creed, 2020). Among the ecosystem services recognised (Millennium Ecosystem Assessment, 2005), the results of this work show the alterations caused by NNMS and TMS in South America directly on provisioning, regulation, and supporting services (Tables 1 and 2), but do not often consider the cultural services of ecosystems. However, there is evidence that indicates that both directly (e.g., injuries caused in bathers by mussel colonies in recreational waterbodies) and indirectly (e.g., enhancing cyanobacterial blooms), NNMS and TMS can affect recreational, aesthetic, and spiritual services.
The effective control of established invasive species remains a pressing challenge for most South American ecosystems. If this control is not achieved, it is very likely that the dispersal of species mediated by humans will cause the breakdown of biogeographic barriers and that, not only climate, but also to some extent, socio-economic relations will define biogeography in an era of global change (Campinha et al., 2015). A lot of work remains to be done concerning the impact of NNMS and TMS in South America. In this vein, it is worth noting that the listed impacts and positive or negative effects for all established categories may be based on a single study for a given region. We encourage targeting less explored areas of research, such as economic valuation of human health (and veterinary) effects attributable to NNMS/TMS, and expanding the knowledge of environmental impacts for all the species listed here. We hope that this review will help direct efforts of the research community in South America and beyond to achieve a multidisciplinary approach in investigating the socio-ecological effects of biological invasions in aquatic habitats.
Data availability
The information necessary to replicate this study is present in the manuscript.
References
Acosta, V., A. Prieto & C. Lodeiros, 2006. Índice de condición de los mejillones Perna perna y Perna viridis (Bivalvia: Mytilidae) bajo un sistema suspendido de cultivo en la Ensenada de Turpialito, Golfo de Cariaco, Venezuela. Zootecnia Tropical 24: 177–192.
Acosta-Balbás, V., C. Lodeiros, J. Mendoza-Hill & J. M. Mazón-Suástegui, 2019. Tropical mussels Perna perna and P. viridis (Bivalvia: Mytilidae): bottom or suspended culture? Aquaculture 512: 734298.
Adelino, J. R. P., G. Heringer, C. Diagne, F. Courchamp, L. D. B. Faria & R. D. Zenni, 2021. The economic costs of biological invasions in Brazil: a first assessment. Neobiota 67: 349–374.
Agostini, V. O. & C. P. Ozorio, 2016. Colonization record of Isognomon bicolor (Mollusca: Bivalvia) on pipeline monobuoys in the Brazilian south coast. Marine Biodiversity Records 9: 84.
Agudo-Padrón, A. I., 2019. The giant native freshwater mussel/naiad Mycetopodidae Anodontites trapesialis (Lamarck, 1819), an emerging invasive plague in fish culture farms of Santa Catarina State/ SC and other localities in Southern Brazil: new geographical records and brief revision. FMCS Newsletter Ellipsaria 21: 36–38.
Agudo-Padrón, A. I., J. V. D. Oliveira & T. F. S. D. Freitas, 2010. Ocorrência de moluscos em culturas de arroz irrigado (Oryza sativa L.) no Rio Grande do Sul, RS, Brazil. Informativo Sociedade Brasileira De Malacologia 41: 9–13.
Aldridge, D. C., M. Salazar, A. Serna & J. Cock, 2008. Density-dependent effects of a new invasive false mussel, Mytilopsis trautwineana (Tryon 1866), on shrimp, Litopenaeus vannamei (Boone 1931), aquaculture in Colombia. Aquaculture 281: 34–42.
Bazterrica, M. C., F. J. Hidalgo, C. Rumbold, A. M. Casariego, M. L. Jaubet, M. Merlo, I. César, M. Provenzal, M. Addino, P. J. Barón & S. Obenat, 2022. Macrofaunal assemblages structure three decades after the first report of the invasive Crassostrea gigas reefs in a soft-intertidal of Argentina. Estuarine, Coastal and Shelf Science 5: 107832.
Belz, C. E., G. Darrigran, O. S. Mäder Netto, W. A. Boeger & P. J. Ribeiro, 2012. Analysis of four dispersion vectors in inland waters: the case of the invading bivalves in South America. Journal of Shellfish Research 31: 777–784.
Belz, C. E., L. R. L. Simone, N. SilveiraJúnior, R. A. Baggio, M. D. V. Gernet & C. J. Birckolz, 2020. First record of the Mediterranean mussel Mytilus galloprovincialis (Bivalvia, Mytilidae) in Brazil. Papéis Avulsos De Zoologia. https://doi.org/10.11606/1807-0205/2020.60.07.
Besen, M. A. & N. Garcia Marengoni, 2021. Bioaccumulation of metals and evaluation of golden mussels encrusted on different screens of net cages. Boletim Do Instituto De Pesca 47: e624.
Bökenhans, V., J. E. Fernández Alfaya, G. Bigatti & A. Averbuj, 2019. Diet of the invasive sea slug Pleurobranchaea maculata in Patagonian coastal waters. New Zealand Journal of Zoology 46: 87–94.
Boltovskoy, D., 2015a. Limnoperna Fortune. The Ecology Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel, Invading Nature, Cham:
Boltovskoy, D., 2015b. Distribution and Colonization of Limnoperna fortunei: Special Traits of an Odd Mussel. In Boltovskoy, D. (ed), Limnoperna Fortunei The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Invading Nature, Cham: 301–311.
Boltovskoy, D. & N. Correa, 2015. Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia 746: 81–95.
Boltovskoy, D., N. Correa, D. Cataldo & F. Sylvester, 2006. Dispersion and impact of invasive freshwater bivalves: Limnoperna fortunei in the Río de la Plata watershed and beyond. Biological Invasions 8: 947–963.
Boltovskoy, D., A. Karatayev, L. Burlakova, D. Cataldo, V. Karatayev, F. Sylvester & A. Mariñelarena, 2009. Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636: 271–284.
Boltovskoy, D., N. Correa, F. Bordet, V. Leites & D. Cataldo, 2013. Toxic Microcystis (Cyanobacteria) inhibit recruitment of the bloom-enhancing invasive bivalve Limnoperna fortunei. Freshwater Biology 58: 1968–1981.
Boltovskoy, D., N. Correa, F. Sylvester & D. Cataldo, 2015. Nutrient recycling, phytoplankton grazing, and associated impacts of Limnoperna fortunei. In Boltovskoy, D. (ed), Limnoperna Fortune. The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Invading Nature, Cham: 153–176.
Boltovskoy, D., N. M. Correa, L. E. Burlakova, A. Y. Karatayev, E. V. Thuesen, F. Sylvester & E. M. Paolucci, 2021. Traits and impacts of introduced species: a quantitative review of meta-analyses. Hydrobiologia 848: 2225–2258.
Boltovskoy, D., R. Guiaşu, L. Burlakova, A. Karatayev, M. A. Schlaepfer & N. Correa, 2022. Misleading estimates of economic impacts of biological invasions: including the costs but not the benefits. Ambio 51: 1786–1799.
Bonelli, A. G., C. B. Giachetti, A. J. Jaureguizar & A. C. Milessi, 2016. First report of predation by a small shark on the invasive rapa whelk Rapana venosa (Valenciennes, 1846) in Argentinean waters. BioInvasions Records 5: 169–172.
Borges, P. D., S. Ludwig & W. A. Boeger, 2017. Testing hypotheses on the origin and dispersion of Limnoperna fortunei (Bivalvia, Mytilidae) in the Iguassu River (Paraná, Brazil): molecular markers in larvae and adults. Limnology 18: 31–39.
Breves-Ramos, A., A. O. R. Junqueira, H. P. Lavrado, S. H. G. Silva & M. A. G. Ferreira-Silva, 2009. Population structure of the invasive bivalve Isognomon bicolor on rocky shores of Rio de Janeiro State (Brazil). Journal of the Marine Biological Association of the United Kingdom 90: 453–459.
Brugnoli, E., J. Clemente, L. Boccardi, A. Borthagaray & F. Scarabino, 2005. Golden mussel Limnoperna fortunei (Bivalvia: Mytilidae) distribution in the main hydrographical basins of Uruguay: update and predictions. Anais Da Academia Brasileira De Ciencias 77: 235–244.
Brugnoli, E., J. Clemente, G. Riestra, L. Boccardi & A. I. Borthagaray, 2006. Especies acuáticas exóticas en Uruguay: situación, problemática y manejo. In Menafra, R., L. Rodríguez-Gallego, F. Scarabino & D. Conde (eds), Bases para la conservación y el manejo de la costa uruguaya Vida Silvestre (Sociedad Uruguaya para la Conservación de la Naturaleza), Montevideo: 351–362.
Burlakova, L. E., A. Y. Karatayev, D. Boltovskoy & N. M. Correa, 2022. Ecosystem services provided by the exotic bivalves Dreissena polymorpha, D. rostriformis bugensis, and Limnoperna fortunei. Hydrobiologia. https://doi.org/10.1007/s10750-022-04935-4.
Campalans, M. & K. Lohrmann, 2009. Histological survey of four species of cultivated molluscs in Chile susceptible to OIE notifiable diseases. Revista De Biología Marina y Oceanografía 44: 561–569.
Campinha, C., F. Essl, H. Seebens, D. Moser & H. M. Pereira, 2015. The dispersal of alien species redefines biogeography in the Anthropocene. Science 348: 1248–1251.
Cao, L., C. Damborenea, P. E. Penchaszadeh & G. Darrigran, 2017. Gonadal cycle of Corbicula fluminea (Bivalvia: Corbiculidae) in Pampean streams (Southern Neotropical Region). PLoS ONE 12: e0186850.
Carlton, J. T., 1996. Biological invasions and cryptogenic species. Ecology 77: 1653–1655.
Carranza, A., A. Estrades, F. Scarabino & A. Segura, 2010a. Loggerhead turtles Caretta caretta (Linnaeus, 1758) preying on the invading gastropod Rapana venosa (Valenciennes, 1846) in the Río de la Plata Estuary. Marine Ecology 32: 142–147.
Carranza, A., C. de Mello, A. Ligrone, S. González, P. Píriz & F. Scarabino, 2010b. Observations on the invading gastropod Rapana venosa in Punta del Este, Maldonado Bay, Uruguay. Biological Invasions 12: 995–998.
Castilla, J. C. & P. E. Neill, 2009. Marine bioinvasions in the Southeastern Pacific: status, ecology, economic Impacts, conservation and management. In Rilov, G. & J. A. Crooks (eds), Biological Invasions in Marine Ecosystems Springer, Berlin: 439–457.
Cataldo, D., 2015. Trophic relationships of Limnoperna fortunei with adult fishes. In Boltovskoy, D. (ed), Limnoperna fortune. The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Invading Nature - Springer Series in Invasion Ecology, New York: 231–248.
Cataldo, D. H., D. Boltovskoy, J. Stripeikis & M. Pose, 2001. Condition index and growth rates of field caged Corbicula fluminea (Bivalvia) as biomarkers of pollution gradients in the Paraná river delta (Argentina). Aquatic Ecosystem Health and Management 4: 187–201.
Cataldo, D., A. Vinocur, I. O’Farrell, E. Paolucci, V. Leites & D. Boltovskoy, 2012a. The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680: 25–38.
Cataldo, D., I. O’Farrell, E. Paolucci, F. Sylvester & D. Boltovskoy, 2012b. Impact of the invasive golden mussel (Limnoperna fortunei) on phytoplankton and nutrient cycling. Aquatic Invasions 7: 91–100.
Cavaleiro, N. P., C. Lazoski, C. R. Tureck, C. M. R. Melo, V. S. Do Amaral, B. J. Lomovasky, T. M. Absher & A. M. Solé-Cava, 2019. Crassostrea talonata, a new threat to native oyster (Bivalvia: Ostreidae) culture in the Southwest Atlantic. Journal of Experimental Marine Biology and Ecology 511: 91–99.
Chai, J. Y., W. M. Sohn, T. S. Yong, K. S. Eom, D. Y. Min, M. Y. Lee, H. Lim, B. Insisiengmay, B. Phommasack & H. J. Rim, 2013. Centrocestus formosanus (Heterophyidae): human infections and the infection source in Lao. Journal of Parasitology Research 99: 531–536. https://doi.org/10.1645/12-37.1.
Clavijo, C., 2014. Diversidad de Corbiculidae (Mollusca: Bivalvia) en Uruguay. Tesis de Maestría. Universidad de la República Facultad de Ciencias Maestría en Ciencias Biológicas PEDECIBA Subárea Zoología. 122 pp.
Clavijo, C. & A. Carranza, 2018. Critical reduction of the geographic distribution of Cyanocyclas (Cyrenidae: Bivalvia) in Uruguay. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 1249–1252. https://doi.org/10.1002/aqc.2941.
Collado, G. A., M. A. Vidal, K. P. Aguayo, M. A. Méndez, M. A. Valladares, F. J. Cabrera, L. Pastenes, D. E. Gutiérrez Gregoric & N. Puillandre, 2019. Morphological and molecular analysis of cryptic native and invasive freshwater snails in Chile. Scientific Reports 9: 7846.
Correoso Rodriguez, M., E. Espinoza & M. C. Rodríguez, 2017. Pomacea canaliculata in Ecuador: a recent pest with multiple implications, pp. 257–291. In: Joshi R. C., Cowie R. H. & Sebastian L. S. (eds.) Biology and Management of Invasive Apple Snails. Philippine Rice Research Institute (PhilRice), Nueva Ecija.
Costa, J. I., M. I. E. Martins & D. M. M. R. Ayroza, 2018. Impact of control of the golden mussel on the production costs of tilapia bred in net cages. Boletim Do Instituto De Pesca 44: 110–115.
Crespo, D., M. Dolbeth, S. Leston, R. Sousa & M. A. Pardal, 2015. Distribution of Corbicula fluminea (Muller, 1774) in the invaded range: a geographic approach with notes on species traits variability. Biological Invasions 17: 2087–2101.
Darrigran, G., 2002. Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biological Invasions 4: 145–156.
Darrigran, G., 2010. Summary of the distribution and impact of the golden mussel in Argentina and neighbouring countries, pp 389–396. In: Claudi R. & Mackie G. (eds.) Monitoring and Control of Aquatic Invasive Molluscs in Freshwater Systems. Taylor and Francis Group, LLC. NW, USA.
Darrigran, G. & C. Damborenea, 2005. A bioinvasion history in South America. Limnoperna fortunei (Dunker, 1857), the golden mussel. American Malacological Bulletin 20: 105–112.
Darrigran, G. & C. Damborenea, 2006. Bio-invasión del mejillón dorado en el continente americano. Editorial de la Universidad Nacional de La Plata (EDULP).
Darrigran G. & C. Damborenea, 2009. Introdução a biologia das Invasões o Mexilhão Dourado na América do Sul: biologia, dispersão, impacto, prevenção e controlo. CUBO editora AES Tiete. São Carlos SP. 248 pp.
Darrigran, G. & C. Damborenea, 2011. Ecosystem engineering impact of Limnoperna fortunei in South America. Zoological Science 28: 1–7.
Darrigran, G. & G. Pastorino, 1995. The recent introduction of a freshwater Asiatic bivalve, Limnoperna fortunei (Mytilidae) into South America. The Veliger 38: 171–175.
Darrigran, G., S. M. Martin, B. Gullo & L. Armendariz, 1998. Macroinvertebrados associated with Limnoperna fortunei (Dunker, 1857) (Pelecipoda, Mytilidae) in Río de la Plata, Argentina. Hydrobiologia 367: 223–230.
Darrigran, G., C. Damborenea & N. Greco, 2007. An evaluation pattern for antimacrofouling procedures: Limnoperna fortunei larvae study in a hydroelectric power plant in South America. Ambio A Journal of the Human Environment 36: 575–579.
Darrigran, G., I. Agudo-Padrón, P. Baez, C. Belz, F. Cardoso, A. Carranza, G. Collado, M. Correoso, M. G. Cuezzo, A. Fabres, D. E. Gutiérrez Gregoric, S. Letelier, S. Ludwig, M. C. Mansur, G. Pastorino, P. E. Penchaszadeh, C. Peralta, A. Rebolledo, A. Rumi, S. Santos, S. Thiengo, T. Vidigal & C. Damborenea, 2020. Non-native mollusks throughout South America: emergent patterns in an understudied continent. Biological Invasions 22: 853–871.
Darrigran, G., I. Agudo-Padrón, P. Baez, C. Belz, F. Cardoso, G. A. Collado, M. Correoso, M. G. Cuezzo, C. Damborenea, A. Fabres, M. A. Fernandez, S. R. Gomes, D. E. Gutiérrez Gregoric, S. Letelier, C. Lodeiros, S. Ludwig, M. C. Mansur, S. Narciso, G. Pastorino, P. Penchaszadeh, A. C. Peralta, A. Rebolledo, A. Rumi, R. B. Salvador, S. Santos, P. Spotorno, S. Thiengo, T. Vidigal & A. Carranza, 2022. Species movements within biogeographic regions: Exploring the distribution of transplanted molluscs species in South America. Biological Invasions. https://doi.org/10.1007/s10530-022-02942-z.
de Castro, A. L. P., R. O. P. Serrano, M. A. Pinto, G. H. T. Á. da Silva, L. de Andrade Ribeiro, E. M. de Faria Viana & C. B. Martinez, 2019. Case study: Abrasive capacity of Limnoperna fortunei (golden mussel) shells on the wear of 3 different steel types. Wear 438–439: 202999.
de Lucía, M., G. Darrigran & D. Gutiérrez Gregoric, 2023. The most problematic freshwater invasive species in South America, Limnoperna fortunei (Dunker, 1857), and its status after 30 years of invasion. Aquatic Sciences 85: 5.
Dextrase, A. J. & N. E. Mandrak, 2006. Impacts of alien invasive species on freshwater fauna at risk in Canada. Biological Invasions 8: 13–24.
Diagne, C., B. Leroy, A. C. Vaissière, R. E. Gozlan, D. Roiz, I. Jarić, J. M. Salles, C. J. Bradshaw & F. Courchamp, 2021. High and rising economic costs of biological invasions worldwide. Nature 592: 571–576.
Dias, T. L. P., E. L. S. Mota, A. I. Gondim, J. M. Oliveira, E. F. Rabelo, S. M. de Almeida & M.L. Christoffersen, 2013. Isognomon bicolor (C. B. Adams, 1845) (Mollusca: 527 Bivalvia): first record of this invasive species for the States of Paraíba and Alagoas and new records for other localities of Northeastern Brazil. Check List 9: 159–611
Diez, M. E., V. I. Radashevsky, J. M. Orensanz & F. Cremonte, 2011. Spionid polychaetes (Annelida: Spionidae) boring into shells of molluscs of commercial interest in northern Patagonia, Argentina. Italian Journal of Zoology 78: 497–504.
do Amaral, V. S., L. R. Simone, F. T. de S. Tâmega, E. Barbieri, S. H. Calazans, R. Coutinho & P. Spotorno-Oliveira, 2020. New records of the non-indigenous oyster Saccostrea cucullata (Bivalvia: Ostreidae) from the southeast and south Brazilian coast. Regional Studies in Marine Science 33: 100924
Doherty-Bone, T. M., A. M. Dunn, F. L. Jackson & L. E. Brown, 2019. Multi-faceted impacts of native and invasive alien decapod species on freshwater biodiversity and ecosystem functioning. Freshwater Biology 64: 461–473.
dos Santos, A. A. & S. W. d. Costa, 2016. Síntese Informativa da Maricultura 2015. Empresa de Pesqouisa Agropecuária e Extensão Rural de Santa Catarina (EPAGRI) and Centro de Desenvolvimento em Agricultura e Pesca (Cedap) 8.
dos Santos S. B., S. C. Thiengo, M. A. Fernandez, I. C. Miyahira, I. C. B. Gonçalves, R. de F. Ximenes, M. C. D. Mansur & D. Pereira, 2012. Espécies de moluscos límnicos invasores no Brasil, pp 25–49. In: Mansur M. C. D. et al (org.). Moluscos límnicos invasores no Brasil: biologia, prevencao e controle. Redes Editora, Porto Alegre.
Dreher Mansur, M.C., C. Pinheiro dos Santos, D. Pereira, I.C. Padula Paz, M.L. Leite Zurita, M.T. Raya Rodriguez, M. Vilar Nehrke, P.E. Aydos Bergonci (org), 2012. Moluscos Límnicos Invasores no Brasil. Biologia, prevenção, controle. Redes Editora. Porto Alegre. 412pp.
Duchini, D., D. Boltovskoy & F. Sylvester, 2018. The invasive freshwater bivalve Limnoperna fortunei in South America: multiannual changes in its predation and effects on associated benthic invertebrates. Hydrobiologia 817: 431–446.
Duboscq-Carra, V. G., R. D. Fernandez, P. J. Haubrock, R. D. Dimarco, E. Angulo, L. Ballesteros-Mejia, C. Diagne, F. Courchamp & M. A. Nuñez, 2021. Economic impact of invasive alien species in Argentina: a first national synthesis. NeoBiota 67: 329–348.
Dueñas Gurevitch, M. A., H. J. Ruffhead, N. H. Wakefield, P. D. Roberts, D. J. Hemming & H. Diaz-Soltero, 2018. The role played by invasive species in interactions with endangered and threatened species in the United States: a systematic review. Biodiversity and Conservation 27: 3171–3183.
Ebi, K. L., R. S. Kovats & B. Menne, 2006. An approach for assessing human health vulnerability and public health interventions to adapt to climate change. Environmental Health Perspectives 114: 1930–1934.
Esteban, J. G., C. González, M. D. Bargues, R. Angles, C. Sanchez, C. Náquira & S. Mas-Coma, 2002. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Tropical Medicine International Health 7: 339–348.
Everard, M., J. Gray, V. Wilkins-Kindemba & I. G. Cowx, 2009. Impacts of invasive species on ecosystem services: The case of the signal crayfish (Pacifastacus leniusculus). Environmental Law and Management 21: 250–259.
Felipi, P. G. & A. T. Silva-Souza, 2008. Anodontites trapesialis (Lamarck, 1819): um bivalve parasito de peixes de água doce. Semina: Ciências Agrârias 29: 895–904.
Fernandez, M. A., S. C. Thiengo & M. F. Boaventura, 2001. Gastrópodes límnicos do Campus de Manguinhos, Fundação Oswaldo Cruz, Rio de Janeiro, RJ. Revista Da Sociedade Brasileira De Medicina Tropical 34: 279–282.
Fernandez, M. A., S. C. Thiengo & L. R. L. Simone, 2003. Distribution of the introduced freshwater snail Melanoides tuberculatus (Gastropoda: Thiaridae) in Brazil. The Nautilus 117: 78–82.
Flores-Aguilar, R. A., A. Gutiérrez, A. Ellwanger & R. Searcy-Bernal, 2007. Development and current status of abalone aquaculture in Chile. Journal of Shellfish Research 26: 705–711.
Furse, K., J. Gallo, M. Noriega & C. Ramos, 2004. Cultivo y comercialización de ostras del tipo Crassostrea gigas. Universidad San Ignacio de Loyolo, Peú. https://repositorio.usil.edu.pe/server/api/core/bitstreams/b3b9a929-3bec-43bc-8306-3d5c64cc1c3d/content
García, M. & L. Montalto, 2006. Los peces depredadores de Limnoperna fortunei en los ambientes colonizados. In: Darrigran G. & Damborenea C. (eds.) Bioinvasion del mejillón dorado en el continente americano. Edulp La Plata: 113–129.
Giberto, D. A., A. Schiariti & C. S. Bremec, 2011. Diet and daily consumption rates of Rapana venosa (Valenciennes, 1846) (Gastropoda: Muricidae) from the Ro de la Plata (Argentina-Uruguay). Journal of Shellfish Research 30: 349–358.
Giovanelli, A., C. L. P. A. C. D. Silva, L. Medeiros & M. C. D. Vasconcellos, 2002. The molluscicidal activity of Niclosamide (Bayluscide WP70®) on Melanoides tuberculata (Thiaridae), a snail asssociated with habitats of Biomphalaria glabrata (Planorbidae). Memórias Do Instituto Oswaldo Cruz 97: 743–745.
González-Bergonzoni, I., F. Teixeira-de Mello, N. Vidal, A. D’Anatro & M. Masdeu, 2010. Reappearance and diet of juvenile armado catfish (Pterodoras granulosus) in Lower Uruguay River (Rio Negro, Uruguay). Boletín De La Sociedad Zoológica Del Uruguay 19: 42–46.
Grosholz, E. D., G. M. Ruiz, C. A. Dean, K. A. Shirley, J. L. Maron & P. G. Connors, 2000. The impacts of a nonindigenous marine predator in a California bay. Ecology 81: 1206–1224.
Guimarães, V. & J. Barbujiani Sígolo, 2008. Detecção de contaminantes em espécie bioindicadora (Corbicula fluminea) - Rio Ribeira de Iguape – SP. Química Nova 31: 1696–1698.
Guimarães, C. T., C. P. de Souza & D. de M. Soares, 2001. Possible competitive displacement of planorbids by Melanoides tuberculata in Minas Gerais, Brazil. Memórias Do Instituto Oswaldo Cruz 96: 173–176.
Gurevitch, J. & D. K. Padilla, 2004. Are invasive species a major cause of extinctions? Trends in Ecology and Evolution 19: 470–474.
Haubrock, P. J., R. N. Cuthbert, A. Ricciardi, C. Diagne & F. Courchamp, 2022. Economic costs of invasive bivalves in freshwater ecosystems. Diversity and Distributions 28: 1010–1021.
Heinzen, T., O. Castro, C. Pepe & A. Ibarburu, 1994. Lymnaea columella como hospedero intermediario de Fasciola hepatica en Uruguay. XXII Jornadas Uruguayas de Buiatría, Centro Médico Veterinario de Paysandú, Uruguay.
Hermes-Silva, S., J. Ribolli, S. D. Ávila-Simas, E. Zaniboni-Filho, G. F. M. Cardoso & A. P. D. O. Nuñer, 2021. Limnoperna fortunei-Updating the geographic distribution in the Brazilian watersheds and mapping the regional occurrence in the Upper Uruguay River basin. Biota Neotropica 21: e20201175
Hernández, L. E., M. T. Díaz & A. K. Bashirullah, 2003. Description of different developmental stages of Centrocestus formosanus (Nishigori, 1924) (Digenea: Heterophyidae). Revista Científica FCV-LUZ 13: 285–292.
Hine, P. M., 1978. Distribution of some parasites of freshwater eels in New Zealand. New Zealand Journal of Marine and Freshwater Research 12: 179–187.
Horgan, F., M. Felix, D. Portalanza, L. Sanchez, W. Rios, E. F. Simón, J. Wither, C. Andrade & E. Espin, 2014a. Responses by farmers to the apple snail invasion of Ecuador’s rice fields and attitudes toward predatory snail kites. Crop Protection 62: 135–143.
Horgan, F. G., A. M. Stuart & E. P. Kudavidanage, 2014b. Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecologica 54: 90–100.
Ignacio, B. L., L. M. Julio, A. O. R. Junqueira & M. A. G. Ferreira-Silva, 2010. Bioinvasion in a Brazilian Bay: Filling Gaps in the Knowledge of Southwestern Atlantic Biota. PLoS ONE 5: e13065.
IPBES, 2019. Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. In: Brondizio E. S., Settele J., Díaz S. & Ngo H. T. (eds.) IPBES secretariat, Bonn, Germany. 1148 pages.
Jeschke, J. M., S. Bacher, T. M. Blackburn, J. T. A. Dick, F. Essl, T. Evans, M. Gaertner, P. E. Hulme, I. Kühn, A. Mrugała, J. Pergl, P. Pyšek, W. Rabitsch, A. Ricciardi, D. M. Richardson, A. Sendek, M. Vilà, M. Winter & S. Kumschick, 2014. Defining the impact of non-native species. Conservation Biolology 28: 1188–1194.
Kuris, A. M. & C. S. Culver, 1999. An introduced sabellid polychaete pest of cultured abalone and its potential spread to other California gastropods. Invertebrate Biology 118: 391.
Labaut, Y., 2021. Proceso de Invasión de Corbicula fluminea (Müller, 1774) en la Patagonia Argentina. Tesis Doctoral, Facultad de Ciencias Naturales y Museo de la Universidad Nacional de La Plata.
Labaut, Y., P. A. Macchi, F. M. Archuby & G. Darrigran, 2021. Homogenization of macroinvertebrate assemblages and asiatic clam Corbicula fluminea invasion in a river of the arid Patagonian Plateau Argentina. Frontiers in Environmental Science 9: 728620.
Lanfranconi, A., E. Brugnoli & P. Muniz, 2013. Preliminary estimates of consumption rates of Rapana venosa (Gastropoda, Muricidae); a new threat to mollusk biodiversity in the Río de la Plata. Aquatic Invasions 8: 437–442.
Lezama, C., A. Carranza, A. Fallabrino, A. Estrades, F. Scarabino & M. López-Mendilaharsu, 2013. Unintended backpackers: Biofouling of the invasive gastropod Rapana venosa on the green turtle Chelonia mydas in the Río de la Plata Estuary, Uruguay. Biological Invasions 15: 483–487.
Lodeiros, C., N. González-Henríquez, J. Cuéllar-Anjel, D. Hernández-Reyes, C. Medina-Alcaraz, J. Quinteiro & M. Rey-Méndez, 2019. Invasion of the dark false mussel in shrimp farms in Venezuela: species identification and genetic analysis. BioInvasions Records 8: 838–847.
Lodeiros, C., D. Hernández-Reyes, J. M. Salazar, M. Rey-Méndez & N. González-Henríquez, 2021. First report of the mussel Mytella strigata (Hanley, 1843) in the Venezuelan Caribbean from an invasion in a shrimp farm. Latin American Journal of Aquatic Research 49: 531–537.
Lopez, L., J. Romero & L. Velásquez, 2008. Aislamiento de Paramphistomidae en vacas de leche y en el hospedador intermediario (Lymnea truncatula y Lymnea columella) en una granja del trópico alto en el occidente de Colombia. Revista Colombiana De Ciencias Pecuarias 21: 9–18.
López, M. S. & R. Coutinho R, C. E. L. Ferreira & G. Rilov, 2010. Predator–prey interactions in a bioinvasion scenario: differential predation by native predators on two exotic rocky intertidal bivalves. Marine Ecolology Progress Series 403: 101–112.
Ludwig, S., R. Patella, S. Stoiev, G. Castilho-Westphal, M. V. Girotto & A. Ostrensky, 2011. A molecular method to detect and identify the native species of southwestern Atlantic Crassostrea (Mollusca: Ostreidae). Zoologia (curitiba) 28: 420–426.
Ludwig, S., M. K. Tschá, R. Patella, A. J. Oliveira & W. A. Boeger, 2014. Looking for a needle in a haystack: molecular detection of larvae of invasive Corbicula clams. Management of Biological Invasions 5: 143–149.
Ludwig, S., E. H. R. Sari, H. Paixão, L. C. Montresor, J. Araújo, C. F. A. Brito, G. Darrigran, A. R. Pepato, T. H. D. A. Vidigal & C. B. Martinez, 2021. High connectivity and migration potentiate the invasion of Limnoperna fortunei (Mollusca: Mytilidae) in South America. Hydrobiologia 848: 499–513.
Maldonado, M. A. & P. R. Martín, 2019. Dealing with a hyper-successful neighbor: effects of the invasive apple snail Pomacea canaliculata on exotic and native snails in South America. Current Zoology 65: 225–235.
Mansur M. C. D., C. P. Santos & M. V. Nehrke, 2011. Corbiculidae na América do Sul, espécies nativas e invasoras, dispersão e a situação das pesquisas no Brasil (Mollusca: Bivalvia), pp. 324–335. In: Fernandez M. A., Santos S. B., Pimenta A. D. & Thiengo S. C (eds.) 2011. Tópicos em malacologia, ecos do XIX EBRAM. Sociedade Brasileira de Malacologia, Rio de Janeiro.
Marcos, L., V. Maco, F. Samalvides, A. Terashima, J. E. Espinoza & E. Gotuzzo, 2006. Risk factors for Fasciola hepatica infection in children: a case-control study. Transactions of the Royal Society of Tropical Medicine and Hygiene 100: 158–166.
Marengoni, N. G., E. S. Klosowski, K. P. Oliveira, A. P. S. Chambo & A. C. Gonçalves, 2013. Bioaccumulation of heavy metals and nutrients in the golden mussel of the reservoir of the Itaipu Binational Hydroelectric power plant. Química Nova 36: 359–363.
Martínez-García, M. F., J. L. Ruesink, J. M. Grijalva-Chon, C. Lodeiros, J. A. Arreola-Lizárraga, E. de la Re-Vega, A. Varela-Romero & J. Chávez-Villalba, 2021. Socioecological factors related to aquaculture introductions and production of Pacific oysters (Crassostrea gigas) worldwide. Reviews in Aquaculture. https://doi.org/10.1111/raq.12615.
Martinez-Juarez, P., A. Chiabai, T. Taylor & S. Q. Gómez, 2015. The impact of ecosystems on human health and well-being: a critical review. Journal of Outdoor Recreation and Tourism 10: 63–69.
Martuzzi, M., 2005. Science, policy, and the protection of human health: A European perspective. Bioelectromagnetics 26: S151–S156.
Mas-Coma, S., I. R. Funatsu & M. D. Bargues, 2001. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 123: 115–127.
Mazza, G., E. Tricario, P. Genovesi & F. Gherardi, 2013. Biological invaders are threats to human health: an overview. Ethology, Ecology & Evolution 26: 112–129.
McMahon, R. F., 2000. Invasive characteristics of the freshwater bivalve, Corbicula fluminea. In Claudi, R. & J. H. Leach (eds), Nonindigenous freshwater organisms: vectors, biology, and impacts Lewis, Washington DC: 315–343.
Melo, C. M. R., F. C. Silva, C. H. A. M. Gomes, A. M. Solé-Cava & C. Lazoski, 2010. Crassostrea gigas in natural oyster banks in southern Brazil. Biological Invasions 12: 441–449.
Mendez, M. M., E. Schwindt, A. Bortolus, A. Roche, M. Maggioni & M. Narvarte, 2015. Ecological impacts of the austral-most population of Crassostrea gigas in South America: a matter of time? Ecological Research 30: 979–987.
Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-being: Synthesis, Island Press, Washington, DC:
Miloslavich, P. & P. E. Penchaszadeh, 1992. Reproductive biology of Vermetus sp. and Dendropoma corrodens (Orbigny, 1842): Two vermetid gastropods from the Southern Caribbean. The Veliger 35: 78–88.
Moreno, R. A., P. E. Neill & N. Rozbaczylo, 2006. Native and non-indigenous boring polychaetes in Chile: a threat to native and commercial mollusc species. Revista Chilena De Historia Natural 79: 263–278.
Morley, N. J., 2008. The role of the invasive snail Potamopyrgus antipodarum in the transmission of trematode parasites in Europe and its implications for ecotoxicological studies. Aquatic Sciences 70: 107–114.
Mozzer, L. R., A. L. Coaglio, R. M. Dracz, V. M. A. Ribeiro & W. S. Lima, 2015. The development of Angiostrongylus vasorum (Baillet, 1866) in the freshwater snail Pomacea canaliculata (Lamarck, 1822). Journal of Helminthology 89: 755–759.
Oliva, D. & L. R. Durán, 2012. Cultivo de almejas, una alternativa para la diversificación de la acuicultura de pequeña escala en Chile. AQUA 159: 88–89.
Oricchio, F. T., A. C. Marques, E. Hajdu, F. B. Pitombo, F. Azevedo, F. D. Passos, L. M. Vieira, S. N. Stampar, R. M. Rocha & G. M. Dias, 2019. Exotic species dominate marinas between the two most populated regions in the southwestern Atlantic Ocean. Marine Pollution Bulletin 146: 884–892.
Paolucci, E. M. & E. V. Thuesen, 2015. Trophic relationships of Limnoperna fortunei with larval fishes, pp 211–229. In: Boltovskoy D. (ed.) Limnoperna fortunei. The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Invading Nature - Springer Series in Invasion Ecology 10
Paolucci, E. M., D. H. Cataldo, C. M. Fuentes & D. Boltovskoy, 2007. Larvae of the invasive species, Limnoperna fortunei (Bivalvia), in the diet of fish larvae in the Parana River. Hydrobiologia 589: 219–233.
Parkinson, M., S. M. O’Neill & J. P. Dalton, 2007. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiology & Infection 135: 669–674.
Pecl, G. T., M. B. Araújo, J. D. Bell, J. Blanchard, T. C. Bonebrake, I. C. Chen & S. E. Williams, 2017. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355(6332): 9214.
Pedersen, U. B., N. Midzi, T. Mduluza, W. Soko, A. S. Stensgaard, B. J. Vennervald, S. Mukaratirwa & T. K. Kristensen, 2014. Modelling spatial distribution of snails transmitting parasitic worms with importance to human and animal health and analysis of distributional changes in relation to climate. Geospatial Health 8: 335–343.
Penchaszadeh, P. E. (coord.), 2005. Invasores: Invertebrados exóticos en el Río de la Plata y región marina aledaña. Eudeba, Buenos Aires.
Penchaszadeh, P. E., G. Darrigran, C. Angulo, A. Averbuj, M. Brögger, A. Dogliotti & N. Pírez, 2000. Predation of the invasive freshwater mussel Limnoperna fortunei (Dunker, 1857) by the fish Leporinus obtusidens Valenciennes, 1846 (Anostomidae) in the Río de la Plata, Argentina. Journal Shellfish Research 19: 229–231.
Pereira, D., M. C. Dreher Mansur, L. D. S. Duarte, A. Schramm de Oliveira, D. Mansur Pimpão, C. Tasso Callil, C. Ituarte, E. Parada, S. Peredo, G. Darrigran, F. Scarabino, C. Clavijo, G. Lara, I. C. Miyahira, M. T. Raya Rodriguez & C. Lasso, 2013. Bivalve distribution in hydrographic regions in South America: historical overview and conservation. Hydrobiologia 735: 15–44.
Pigneur, L. M., E. Etoundi, D. C. Aldridge, J. Marescaux, N. Yasuda & K. Van Doninck, 2014. Genetic uniformity and long-distance clonal dispersal in the invasive androgenetic Corbicula clams. Molecular Ecology 23: 5102–5116.
Pinto, H. A. & A. L. de Melo, 2010. Melanoides tuberculata as intermediate host of Philophthalmus gralli in Brazil. Revista Do Instituto De Medicina Tropical De São Paulo 52: 323–327.
Pinto, H. A., N. Q. Gonçalves, D. López-Hernandez, E. A. Pulido-Murillo & A. L. Melo, 2018e. The life cycle of a zoonotic parasite reassessed: experimental infection of Melanoides tuberculata (Mollusca: Thiaridae) with Centrocestus formosanus (Trematoda: Heterophyidae). PLoS ONE 13: e0194161.
Pires Teixeira, L. M. & J. C. Creed, 2020. A decade on an updated assessment of the status of marine non-indigenous species in Brazil. Aquatic Invasions 15: 30–43.
Prepelitchi, L. & C. Wisnivesky-Colli, 2013. Fasciola hepatica: epidemiología y control en la región noreste de Argentina. In: Salomon O. & Rumi A. (eds.) Moluscos de Interés Sanitario en la Argentina. Ministerio de Salud de la Nación – INMET: 54–83.
Pyšek, P., D. M. Richardson, J. Pergl, V. Jarošík, Z. Sixtová & E. Weber, 2008. Geographical and taxonomic biases in invasion ecology. Trends in Ecology and Evolution 23: 237–244.
Pyšek, P., J. Pergl, F. Essl, B. Lenzner, W. Dawson, H. Kreft, P. Weigelt, M. Winter, J. Kartesz, M. Nishin, L. A. Antonova, J. F. Barcelona, F. J. Cabezas, D. Cárdenas, J. Cárdenas-Toro, N. Castańo, E. Chacón, C. Chatelain, S. Dullinger, A. L. Ebel, E. Figueiredo, N. Fuentes, P. Genovesi, Q. J. Groom, L. Henderson, S. Inderjit, A. Kupriyanov, S. Masciadri, N. Maurel, J. Meerman, O. Morozova, D. Moser, D. Nickrent, P. M. Nowak, S. Pagad, A. Patzelt, P. B. Pelser, H. Seebens, W. Shu, J. Thomas, M. Velayos, E. Weber, J. J. Wieringa, M. P. Baptiste & M. van Kleunen, 2017. Naturalized alien flora of the world: species diversity, taxonomic and phylogenetic patterns, geographic distribution and global hotspots of plant invasion. Preslia 89: 203–274.
Pyšek, P., P. E. Hulme, D. Simberloff, S. Bacher, T. M. Blackburn, J. T. Carlton, W. Dawson, F. Essl, L. C. Foxcroft, P. Genovesi, J. M. Jeschke, I. Kühn, A. M. Liebhold, N. E. Mandra, L. A. Meyerson, A. Pauchard, J. Pergl, H. E. Roy, H. Seebens, M. van Kleunen, M. Vilà, M. J. Wingfield & D. M. Richardson, 2020. Scientists’ warning on invasive alien species. Biological Reviews of the Cambridge Philosophical Society. 95: 1511–1534.
Rebelo, M. F., L. F. Afonso, J. A. Americo, L. da Silva, J. L. Neto, F. Dondero & Q. Zhang, 2018. A sustainable synthetic biology approach for the control of the invasive golden mussel (Limnoperna fortunei). PeerJ Preprints: e27164v3.
Resende, M. F. d., C. B. Martinez & T. Vidigal, 2014. Interferências provocadas pela infestação de mexilhões-dourados (Limnoperna fortunei) sobre bombas centrífugas. Congreso Latinoamericano XII de Hidrogeología y XXVI de hidráulica - 25 a 30 de Agosto de 2014, Santiago de Chile.
Reshaid, Y., L. Cao, F. Brea, M. O. Blanche, S. Torres & G. Darrigran, 2017. Variation in the distribution of Corbicula species (Mollusca: Bivalvia: Corbiculidae) after 25 years of its introduction in the Río de la Plata, Argentina. Zoologia 34: 1–6.
Reyna, P. B., M. L. Ballesteros, M. L. Albá, L. Bertrand, M. González, K. S. B. Miglioranza, M. Tatián & A. C. Hued, 2019. A multilevel response approach reveals the Asian clam Corbicula largillierti as a mirror of aquatic pollution. Science of the Total Environment 692: 175–187.
Reyna, P. B., M. L. Alba, F. A. Rodríguez, M. Gonzalez, C. Pegoraro, A. C. Hued, M. Tatian & M. L. Ballesteros, 2021. What does the freshwater clam, Corbicula largillierti, have to tell us about chlorothalonil effects? Ecotoxicology and Environmental Safety 208: 111603.
Ricciardi, A., 2003. Predicting the impacts of an introduced species from its invasion history: an empirical approach applied to zebra mussel invasions. Freshwater Biology 48: 972–981.
Rojas Molina, F. & S. J. de Paggi, 2008. Zooplankton in the Paraná River floodplain (South America) before and after the invasion of Limnoperna fortunei (Bivalvia). Wetlands 28: 695–702.
Rojas, Molina F. & V. Williner, 2013. First record of the non-indigenous mussel Limnoperna fortunei (Bivalvia, Mytilidae) as an epibiont of the crab Trichodactylus borellianus (Decapoda, Trichodactylidae). Crustaceana 86: 682–692.
Rojas Molina, F., J. C. Paggi & M. Devercelli, 2010. Zooplanktophagy in the natural diet and selectivity of the invasive mollusk Limnoperna fortunei. Biological Invasions 12: 1647–1659.
Rojas Molina, F. & S. B. José de paggi & D. F. Frau, 2012. Impacts of the Invading Golden Mussel Limnoperna fortunei on Zooplankton: A Mesocosm Experiment. Zoological Studies 51: 733–744.
Rojas Molina, F. R., S. B. José de Paggi & J. C. Paggi, 2015. Impacts of Limnoperna fortunei on Zooplankton, pp. 177–190. In: Boltovskoy D. (ed.) Limnoperna fortunei. The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Invading Nature - Springer Series in Invasion Ecology 10.
Salazar Jaramillo, L., V. Estrada & L. E. Velásquez, 2006. Effect of the exposure to Fasciola hepatica (Trematoda: Digenea) on life history traits of Lymnaea cousini and Lymnaea columella (Gastropoda: Lymnaeidae). Experimental Parasitology 114: 77–83.
Sardiña, P., D. Cataldo & D. Boltovskoy, 2008. The effects of the invasive mussel, Limnoperna fortunei, on associated fauna in South American freshwaters: importance of physical structure and food supply. Fundamental and Applied Limnology 173: 135–144.
Sardiña, P., E. Chaves & M. Marchese, 2011. Benthic community responses to invasion by the golden mussel, Limnoperna fortunei Dunker: biotic homogenization vs environmental driving forces. Journal of the North American Benthological Society 30: 1009–1023.
Silva, I., E. Brugnoli, C. Clavijo, A. D'Anatro, D. E. Naya, F. T. de Mello, G. Tesitore, I. González-Bergonzoni, 2021a. Interacciones entre el mejillón dorado y macroinvertebrados bentónicos nativos del Río Uruguay. Innotec: e573-e573.
Silva, I., D. Naya, F. T. de Mello, A. D’Anatro, G. Tesitore, C. Clavijo & I. González-Bergonzoni, 2021b. Fish vs. Aliens: Predatory fish regulate populations of Limnoperna fortunei mitigating impacts on native macroinvertebrate communities. Hydrobiologia 848: 2281–2301.
Silva Bertão, A. P., R. V. V. Leite, A. Horodesky, M. R. Pie, T. L. Zanin, O. S. M. Netto & A. Ostrensky, 2021. Ecological interactions between invasive and native fouling species in the reservoir of a hydroelectric plant. Hydrobiologia 848: 5169–5185.
Silva-Souza, A. T. & J. C. Eiras, 2002. The histopathology of the infection of Tilapia rendalli and Hypostomus regani (Osteichthyes) by Lasidium larvae of Anodontites trapesialis (Mollusca, Bivalvia). Memórias Do Instituto Oswaldo Cruz 97: 431–433.
Simone, L. R. L. & E. P. Gonçalvez, 2006. Anatomical study on Myoforceps aristatus, an invasive boring bivalve in S.E. Brazilian coast (Mytilidae). Papeis Avulsos De Zoologia 46: 57–65.
Solórzano Álava, L. F., L. Martini Robles, H. Hernández Álvarez, J. Sarracent Pérez, J. Muzzio Aroca & L. R. Rivero, 2014. Angiostrongylus cantonensis: un parásito emergente en Ecuador. Revista Cubana De Medicina Tropical 66: 20–33.
Speziale, K. L., S. A. Lambertucci, M. Carrete & J. L. Tella, 2012. Dealing with non-native species: What makes the difference in South America? Biological Invasions 14: 1609–1621.
Spotorno-Oliveira, P., R. Pereira Lopes, A. Larroque, D. Monteiro, P. Dentzien-Dias & F. de Souza Tâmega, 2020. First detection of the non-indigenous gastropod Rapana venosa in the southernmost coast of Brazil. Continental Shelf Research 194: 1–10.
SUBPESCA, 2021. Informe sectorial de Pesca y Acuicultura. https://www.subpesca.cl/portal/618/w3-article-112811.html.
Sylvester, F. & P. Sardiña, 2015. Relationships of Limnoperna fortunei with Benthic Animals, pp 191–210. In: Boltovskoy D. (ed.) Limnoperna fortunei. The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Invading Nature - Springer Series in Invasion Ecology 10.
Sylvester, F., D. Boltovskoy & D. Cataldo, 2007a. The invasive bivalve Limnoperna fortunei enhances benthic invertebrate densities in South American floodplain rivers. Hydrobiologia 589: 15–27.
Sylvester, F., D. Boltovskoy & D. Cataldo, 2007b. Fast response of freshwater consumers to a new trophic resource: predation on the recently introduced Asian bivalve Limnoperna fortunei in the lower Paraná River, South America. Austral Ecology 32: 403–415.
Thiengo, S., H. Pinto, A. Mattos & M. Fernandez, 2017. Helminths parasitizing species of Pomacea in South America (Caenogastropoda; Ampullariidae). In: Santanna B. S. & Hattori G. Y. (eds.) Amazonian Apple Snails, Nova Science Publishers, New York.
Thomsen, M. S., T. Wernberg, J. D. Olden, J. E. Byers, J. F. Bruno, B. R. Silliman & D. R. Schiel, 2014. Forty years of experiments on aquatic invasive species: Are study biases limiting our understanding of impacts? NeoBiota 22: 1–22.
Ueta, M., 1980. Ocorrência de infecção natural de Fasciola hepatica Linnaeus, 1758 em Lymnaea columella Say, 1817, no Vale do Paraíba, SP, Brasil. Revista Saude Publica 14: 230–233.
Velásquez, L. E., J. C. Bedoya, A. Areiza & I. Vélez, 2006. Primer registro de Centrocestus formosanus (Digenea: Heterophyidae) en Colombia. Revista Mexicana De Biodiversidad 77: 119–121.
Villafranca, S. & M. Jiménez, 2006. Comunidad de moluscos asociados al mejillón verde Perna viridis (Mollusca: Bivalvia) y sus relaciones tróficas en la costa norte de la península de Araya, Estado Sucre. Venezuela. Revista De Biología Tropical 54(Suppl. 3): 135–144.
von Brand, E., A. Abarca, G. E. Merino & W. Stotz, 2016. Scallop fishery and aquaculture in Chile: A history of developments and declines. In Shumway, S. E. & G. J. Parsons (eds), Scallop, biology, ecology, aquaculture and fisheries Elsevier, Oxford: 1047–1072.
Westfall, K. M. & J. P. A. Gardner, 2013. Interlineage Mytilus galloprovincialis Lmk. 1819 hybridization yields inconsistent genetic outcomes in the Southern hemisphere. Biological Invasions 15: 1493–1506.
Wiryareja, S. & J. R. Tjoe-Awie, 2006. Golden apple snail: its occurrence and importance in Suriname’s rice ecosystem, pp. 337- 342. In: Joshi R. C. & Sebastian L. S. (eds.) Global Advances in Ecology and Management of Golden Apple Snails, Philippines Rice Research Institute, Nueva Ecija, Philippines.
Zbawicka, M., M. I. Trucco & R. Wenne, 2018. Single nucleotide polymorphisms in native South American Atlantic coast populations of smooth shelled mussels: hybridization with invasive European Mytilus galloprovincialis. Genetics Selection Evolution 50: 1–14.
Zeimes, C. B., G. E. Olsson, C. Ahlm & S. O. Vanwambeke, 2012. Modelling zoonotic diseases in humans: comparison of methods for hantavirus in Sweden. International Journal of Health Geographics 11: 39.
Acknowledgements
Partial financial support was received by GD-CD-DEGG from Agencia Nacional de Promoción Científica y Tecnológica (PICT-2019-01417); GD from Universidad Nacional de La Plata (UNLP 11/ H949 and 11/N927); GD-DEGG from Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 1966) and TV from Serra do Facão Energia S.A. (SEFAC) [06899-2912/2016].
Funding
Funding was provided by Fondo para la Investigación Científica y Tecnológica (Grant No. PICT2019-01417), Consejo Nacional de Investigaciones Científicas y Técnicas (Grant No. PIP 1966).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject discussed in this manuscript.
Additional information
Handling editor: Luis Mauricio Bini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carranza, A., Agudo-Padrón, I., Collado, G.A. et al. Socio-environmental impacts of non-native and transplanted aquatic mollusc species in South America: What do we really know?. Hydrobiologia 850, 1001–1020 (2023). https://doi.org/10.1007/s10750-023-05164-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05164-z