Abstract
The invasive bivalve Limnoperna fortunei (Dunker 1857) was introduced in South America in 1991, with the first occurrence in Brazil in 1998. In the Iguassu River, the species was recorded in 2001; however, it is unknown how it was introduced and spread. Adults and larvae were sampled in Iguassu and Paraná Rivers, and the genetic profiles were compared. The species was absent in the upper reaches and only larvae were found, in low densities, in intermediary reaches. The L. fortunei populations from the lower Iguassu River presented no genetic differentiation among themselves, suggesting strong connectivity, and were significantly different from the Paraná River populations, most likely because of Iguassu Falls. Furthermore, the results suggest that the Paraná River represents the source of propagules to the Iguassu River. Generally, no significant differences were observed between the genetic structure inferred from adults and larvae. Only the population from the Iguassu National Park, a lotic environment, differed from the remaining subpopulations. The characterization of genetic profile using larval stages of L. fortunei populations was satisfactory, and represents an important protocol for studying the population genetics of aquatic species with planktonic larval stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of exotic aquatic species into a new environment can occur naturally or accidentally and is usually associated with human activity (Mills et al. 1993; Drake and Bossenbroek 2004). The invasive bivalve, the golden mussel Limnoperna fortunei (Dunker 1857), was introduced in South America in 1991, possibly from ballast water of merchant ships in the River Río de la Plata estuary (Darrigran and Pastorino 1995). The species rapidly expanded its distribution to the upper portions of the Paraná River Basin at a rate of approximately 240 km/year (Darrigran and Ezcurra de Drago 2000; Boltovskoy et al. 2006; Darrigran and Mansur 2009). It was first reported in Brazil in 1998 in the upper Paraguay River Basin near Caceres City, Mato Grosso State (Darrigran and Mansur 2009). In the same year, the golden mussel was also reported from the delta of the Jacuí River, Rio Grande do Sul State (Mansur et al. 2003). The dispersion and extensive proliferation of the golden mussel in the invaded environs has caused serious environmental impacts (Mansur et al. 1999; Darrigran 2002; Mansur et al. 2003; Darrigran and Damborenea 2005, 2011; Gazulha et al. 2012) and significant economic losses to hydroelectric power plants in South America (Darrigran 2002; Darrigran and Damborenea 2005; Belz 2006; Darrigran and Mansur 2009).
The first record of L. fortunei in the Iguassu River, a tributary of the Paraná River, is from late 2001 in the region of the upper Iguassu River Basin from two points near Curitiba City, the capital of Paraná State (Takeda et al. 2003). In April of the same year, the species was recorded for the first time at the Itaipu Hydroelectric Power Plant (HPP), Paraná River (Zanella and Marenda 2002). There were no records of the golden mussel along most of the Iguassu River, suggesting independent introductions (Takeda et al. 2003). In 2005, larvae of L. fortunei were detected in the final stretch of the Iguassu River, downstream from Iguassu Falls, and in the Paraná River at low density (3–218 larvae/m3) (Pestana et al. 2008). Despite the natural barrier to golden mussel dispersion into the upstream reaches of the Iguassu River formed by the Iguassu Falls, activities associated with the introduction of sand from contaminated river systems is blamed for the quick spread of this mussel in the basin (Belz et al. 2012).
Until 2005, surveying for larvae of L. fortunei was performed by screening plankton samples with a stereoscopic microscope and actively searching for adult specimens attached to substrates. Since 2006, however, a molecular surveying method proposed by Pie et al. (2006) (see also Boeger et al. 2007; Darrigran et al. 2009) was added to the repertoire of survey protocols. Using these protocols, Pestana et al. (2010) reported the presence of L. fortunei larvae in all sites sampled along the Iguassu River. Additionally, adult specimens of L. fortunei were also detected in the Salto Caxias HPP Reservoir in the lower Iguassu River in 2005 (Belz et al. 2012).
Molecular markers were used in previous studies to elucidate the invasion history of the golden mussel in South America (Zhan et al. 2012). This technology offers insights on the origins, the number of introductions during the invasion process, the population structure, and the invasion pathways (Zhan et al. 2012; Paolucci et al. 2014). According to Zhan et al. (2012), molecular studies on L. fortunei populations in South America revealed that the dispersion occurred by “jumps”, mediated by small boats that encompass the main vector of propagation of this invader from the River Río de la Plata Estuary to the upper Paraná Basin. Thus, molecular markers and their respective analytical tools can be useful for retrieving the invasion history and aspects of population structure of this invasive species among small tributary rivers in South America.
Bivalve larvae are often used in molecular surveys of species (e.g., Claxton and Boulding 1998; Pestana et al. 2010; Ludwig et al. 2014), in monitoring and population studies (e.g., Cataldo and Boltovskoy 2000; Santos et al. 2005; Darrigran et al. 2007; Pestana et al. 2008; Neto et al. 2012), and in tests of tolerance to different control treatments (e.g., Cataldo et al. 2005; Angonesi et al. 2008; Perepelizin and Boltovskoy 2011). However, planktonic larvae are often not considered in population genetic studies of the species. However, larvae are often more easily available than adult specimens of L. fortunei and may represent a more extensive sample because the larval pool may represent the offspring of many, perhaps even more distant, groups of adults within a specific environ. Thus, in this study, we: (1) evaluate the use of data of larval stages of L. fortunei to study the genetic profile of populations, (2) which are combined with data from adult populations to test hypotheses on the recent history of spreading of the species in the Iguassu River. Among the hypotheses tested are the origin of the Iguassu populations from the populations detected in the medium Paraná River, geographically the closest area with established populations of the golden mussel in the region, and the dispersal process. We postulate, based on Belz et al. (2012), that introduction is likely associated with human activities and low propagule pressure, which should result in reduced genetic diversity of established populations. We also test the proposal of single vs multiple introduction of the golden mussel in the Iguassu River by evaluating the genetic structure of the population established therein. If multiple introductions in distinct portions of the river occurred, genetic diversity should increase towards the upper stretches of the river. Single or limited introductions with dispersion within the river should result in non-structured populations whenever the species is known to occur.
Methods
Sampling sites, DNA extraction and mtDNA COI sequencing
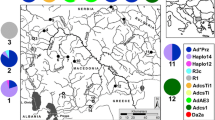
Samples were collected at eight sites along the Iguassu River, Paraná State, Brazil, covering the lower, medium, and upper reaches (Table 1; Fig. 1). These sampling sites were determined based on positive records of putatively established adult populations and/or larvae of L. fortunei (Fig. 1). These sampling sites include both lentic and lotic stretches of the Iguassu River: (1) Iguassu National Park (PQ) (lotic); (2) Governor José Richa (Salto Caxias) HPP (CX) (lentic); (3) Salto Osorio HPP (OS) (lentic); (4) Salto Santiago HPP (ST) (lentic); (5) Governador Ney Aminthas de Barros Braga (Salto Segredo) HPP (SG) (lentic); (6) Bento Munhoz da Rocha Netto (Foz do Areia) HPP (FA) (lentic); (7) City of Porto Amazonas (PA) (lotic) and (8) Piraquara Dam II (PR) (lentic). Additionally, samples from the Itaipu HPP (IT) Reservoir (lentic), which is located upstream from the mouth of the Iguassu River in the Paraná River, were obtained to test the hypothesis that these older populations were the origin of propagules to the recently colonized sites of the Iguassu River (Table 1; Fig. 1). This site/river is the most like candidate as source for propagules for the establishment of the population in the Iguassu River due to geographical proximity and previous studies of potential dispersion vectors (Belz et al. 2012). The Paraná River is separated from the remaining sampling sites by the Iguassu Falls, a natural geographical barrier, on the border of Brazil and Argentina (Fig. 1).
Sampling sites (gray boxes) along Iguassu (PQ, Iguassu National Park; CX, Salto Caxias HPP; OS, Salto Osório HPP; ST, Salto Santiago HPP; SG, Salto Segredo HPP; FA, Foz do Areia HPP; PA, Porto Amazonas City; PR, Piraquara II Dam) and Paraná (IT, Itaipu HPP) Rivers in Paraná State, Brazil. White boxes indicate the presence of L. fortunei only by molecular detection and black boxes indicate historic records of adult specimens only. Pie charts indicate mtDNA COI haplotype (colors) frequencies for L. fortunei in adults (A) and larvae (L) populations (acronyms as in Table 1). The number of sampled specimens are indicated within each pie-chart (N). Star indicates the position of the Iguassu Falls, and arrows, the flow direction of the rivers
Adult specimens were collected manually from being encrusted in algae, aquatic plants, drifting twigs and small recreation boats. Adults were also collected within the cooling systems of power plants. Otherwise, at all sites, plankton samples were taken with 64-μm-mesh plankton nets, filtering approximately 4000 l of water, as recommended by Tschá et al. (2012). All samples were preserved in 70 % alcohol and subsequently taken to the laboratory for further processing.
All samples were collected during known reproductive periods of L. fortunei in the region (Pestana et al. 2008; Boltovskoy et al. 2009). Adults and larvae samples were collected in IT and OS sites in December 2012, and in CX and PQ in March 2012. In the intermediary stretches of the river, in ST, plankton samples were collected monthly from January until December 2013; in SG, monthly from January 2012 until December 2013; and in FA, in March 2013. In the upper Iguassu River, the plankton samples were collected in October 2012 in PA and in December 2012 in PR.
In the laboratory, plankton samples were analyzed using a molecular technique according to Pie et al. (2006) and Boeger et al. (2007) to detect L. fortunei larvae. A subsample of each sample was filtered, the whole DNA was extracted, and subjected to a PCR with primers species specific for the golden mussel. For positive samples, larvae were separated individually in microtubes for subsequent molecular processing. Genomic DNA of an individual larva was extracted using the WLB (worm lysis buffer) protocol adapted from Waeyenberge et al. (2000). A fragment of the mtDNA COI (cytochrome c oxidase subunit I) gene was amplified by polymerase chain reactions (PCR) using universal primers (LCO1490 and HCO2198 of Folmer et al. 1994). Reactions were performed in a total volume of 25 µl, containing 2 nM MgCl2, 0.4 mM dNTPs, 1X buffer, 1.25 U of AmpliTaq DNA polymerase (Life Technologies®) and 0.5 nM of each primer. The temperature conditions of the PCR for the mtDNA COI amplification consisted of initial denaturation at 95 °C for 5 min, followed by 35 cycles of 30 s at 92 °C, 30 s at 51 °C, 30 s at 72 °C and the final extension at 72 °C for 2 min. The PCR products were electrophoresed in a 1.5 % agarose gel and visualized under UV-light to confirm amplification of the target fragment. Subsequently, the PCR products were purified using PEG (polyethylene glycol). A total of 25 µl of PEG was added to PCR products and incubated at 37 °C for 30 min. Each sample was centrifuged for 20 min (14,000 rpm), and the supernatant was discarded. Two cycles of ethanol washes were performed, adding 125 µl of 80 % ethanol, centrifuging for 2 min (14,000 rpm) and discarding the supernatant. Residual ethanol was discarded. DNA was resuspended in 15 µl of distilled water. After purification, the amplified fragments were sequenced with the same PCR primers. The sequencing reactions were performed with the Big DyeTerminator v3 kit (Applied Biosystems®) and subsequently purified with SephadexTM G-50 medium (GE HealthcareBio-Sciences AB®). The samples were sequenced in an ABI 3130 automatic sequencer (Applied Biosystems®). Sequences were edited and aligned manually using Geneious® 6.1.2 (Biomatters; available at http://www.geneious.com/). Finally, each obtained consensus sequence was compared to reference L. fortunei sequences available at GenBank using the BLASTn tool (http://www.ncbi.nlm.nih.gov/BLAST/) to confirm the identity and their respective haplotypes.
Data analysis
The number of haplotypes (n), haplotype diversity (h) and nucleotide diversity (π) were estimated using DnaSP 5.0 (Librado and Rozas 2009). The genetic structure within and between sampled populations was tested by analysis of molecular variance (AMOVA) implemented in ARLEQUIN v3.5.1.2 (Schneider et al. 2000). The same software was used to test differences in haplotype frequencies among sampled populations. These tests were performed comparing two groups: (1) PQ, CX and OS sites and (2) PQ, CX, OS and IT sites. Subsequently, the genetic differentiation was evaluated by paired values of Gst (Nei 1973) and Jost’s D (Jost 2008), calculated using the packages seqinr (Charif and Lobry 2007) and ape (Paradis et al. 2004) in R version 3.1 (R Development CoreTeam 2005) (see Pennings et al. 2011). Bonferroni corrections were applied to correct the significance level for multiple comparisons. Mann–Whitney U test, calculated in PAST 3.08 (Hammer et al. 2001), and values of Gst and Jost’s D, calculated with the R-script of Pennings et al. (2011) were used to verify genetic differentiation between L. fortunei populations of adults and larvae in IT, PQ and CX. Tajima’s D and Fu’s F tests, implemented in DnaSP software, were used to verify whether the fragment is under selection or drift. Values of Gst and Jost’s D were also calculated to compare the present genetic profile of the IT population with that previously reported by Zhan et al. (2012). The haplotype composition of the sample of Zhan et al. (2012) from IT was reconstructed based on haplotype frequencies inferred from their pie chart (their Fig. 1), and the sample size reported.
Results
From all eight sites sampled, only four yielded adult specimens in sufficient numbers to proceed with further analysis (IT, PQ, CX and OS). Larval specimens were available solely for the sites IT, PQ and CX. In ST, SG and FA sites, no adults were found; only larvae, probably at very low densities, were detected solely by the molecular detection protocol. In PR and PA, no specimens were recovered with any method.
Sequences from 186 adults and larvae resulted in an alignment 490-bp long. Eleven polymorphic sites and four haplotypes, all reported previously by Zhan et al. (2012) and Paolucci et al. (2014), were detected (Lfm02, Lfm03, Lfm04 and Lfm05; GenBank references HQ843795–HQ843798). The haplotype diversity (h) ranged from 0.1376 to 0.6833, and the nucleotide diversity (π) ranged from 0.00084 to 0.00506 (Table 1). Haplotype Lfm03 was the most frequent in all populations, followed by Lfm05, except in the larvae population of PQ. Haplotype Lfm02 was found in only IT and PQ larvae populations. Lfm04 was exclusive for the IT site (Table 1). Attempts to obtain sequences of the species-specific amplicon obtained from ST, SG and FA using the molecular protocol of detection (see Materials and methods) were unsuccessful.
For adults, the IT population showed significant genetic structure when compared to the PQ, CX and OS populations (Table 2, Table 3). The Fst values for adults suggest moderate structure (Table 2). A similar pattern was observed for the larval populations, but AMOVA resulted in a marginally non-significant Fst value (0.03835; P = 0.06155) (Table 2). The IT population shows the highest diversity of haplotype values among all sampled sites (Table 1). No significant genetic structuring, considering Bonferroni correction (P < 0.016 for larvae and P < 0.008 for adults) was observed for larvae and adults from all populations sampled in the Iguassu River (PQ, CX and OS) (Table 2). Furthermore, these populations presented no significantly different Gst and Jost’s D values (Table 3).
Paired comparisons of haplotype frequencies (Mann–Whitney U test, Gst, Jost’s D) indicate that adult and larval populations from the same site are not significantly different (P < 0.016, after Bonferroni correction) (Table 4). However, qualitatively, there is a difference in the PQ population. Whereas both adult and larval populations have the same dominant haplotype (Lfm03), the second most frequent haplotype in the larval population is Lfm02 (haplotype Lfm05 is absent), and in adults, the second most frequent is haplotype Lfm05 (haplotype Lfm02 is absent) (Fig. 1). Both tests of neutrality, Tajima’s D and Fu’s F resulted in non-significant values for all populations (P > 0.05).
Differences in haplotype composition were observed between the present and past (Zhan et al. 2012) populations sampled in IT. Haplotypes Lfm1 and Lfm6 have been detected by Zhan et al. (2012) but are not present in our study. However, despite these qualitative differences, Gst (0.17) and Jost’s D (0.21) do not support significant differences (P = 1.00 for both Gst and Jost’s D) between the genetic profile of the populations sampled in the present study and by Zhan et al. (2012).
Discussion
Patterns of introduction and dispersion
The absence of adults and larvae in samples from the upper reaches of the Iguassu River contrasts with previous reports of the presence of L. fortunei in this area (see Takeda et al. 2003; Pestana et al. 2010). The current absence of the species suggests that the golden mussel was introduced in the area but the initial populations did not successfully establish. However, the positive results for the molecular scrutiny of larvae of L. fortunei in the intermediary reaches of the river, with simultaneous negative results of visual and microscopic detection of adults and larvae, respectively, also provide insights on the dynamics of invasion for this portion of the river. Indeed, this stretch of the Iguassu River, including some upstream sites, was known previously to present low densities of larvae in plankton samples since 2007, at least according to Pestana et al. (2010). Thus, the present and past results strongly suggest that established populations exist in this portion of the Iguassu River but are likely not large enough to allow detection. Nevertheless, in the lower reaches of the Iguassu River, adult populations of the golden mussel, particularly those downstream of OS, are large and have been characterized genetically.
The difference in haplotype composition between the present study and that reported by Zhan et al. (2012) does not appear to be associated with sample size. During this study, 53 individuals were sampled from this site (both adults and larvae), whereas the previous studies sampled only 32 adult individuals, each. While one haplotype presented low frequency in the IT population reported by Zhan et al. (2012) (Lfm06), the second (Lfm01) was relatively more prevalent in that study. Thus, the origin of these differences in haplotype composition is not evident at the moment, but could represent a dramatic shift in the genetic profile of the population that deserves subsequent scrutiny.
The genetic differences detected between IT and the remaining sites studied herein appears to be a consequence of the natural geographical barrier formed by Iguassu Falls and/or the mode of introduction of the invasive species into the Iguassu River system. The genetic profile of all evaluated sites from the Iguassu River above Iguassu Falls includes a subset of those haplotypes present in the IT population, indicating the possibility that these established golden mussel populations originated from the medium Paraná River as suggested by Belz et al. (2012).
There is neither genetic structuring nor significant differences in haplotype composition and frequency among sampled adult populations of the lower Iguassu River. This indicates that the introduction of the golden mussel in this river (at least in the middle to lower reaches) is the result of an extremely limited number of introduction events (i.e., low propagule pressure). Indeed, the observed general haplotypic homogeneity among sampled populations strongly suggests a single or very few introductions upstream with downstream dispersion promoted by flow. All adult populations from the sampled sites within the lower Iguassu River consist of only the haplotypes Lfm03 and Lfm05.
The scenario suggested above strongly suggests the invasion of the Iguassu River by few propagules and subsequent dispersion by natural (downstream flow) and human-associated vectors, which include the transportation of contaminated sand from invaded areas and sport fishing. Among the dispersion vectors most likely involved in the introduction of L. fortunei in the lower Iguassu River is the introduction of contaminated sand from invaded areas (Belz et al. 2006, 2012). For instance, sand from the Paraná River is often used in the construction of artificial beaches at reservoirs within the Iguassu River (Belz et al. 2012). The low haplotypic diversity observed in populations of the Iguassu River likely resulted from founder effect (associated with the low propagule pressure in the invaded areas). Indeed, low propagule pressure is supported by Belz (2006). Despite an extensive survey, this author detected only two living individuals of L. fortunei, after inspection of 32 sand-transport trucks. Additionally, both aquaculture and sport fishing are common in reservoirs and associated watersheds in the region, indicating the possibility of introduction and/or dispersion by these vectors in the area as suggested by Belz et al. (2012) and Zhan et al. (2012). A more complex process associated with the introduction of L. fortunei in the Iguassu River (i.e., many independent introductions) should result in a higher diversity and a reticulated pattern of distribution of the genetic profile of populations within the basin.
The only contradictory evidence to the proposed scenario is the presence of the Lfm02 haplotype in the larval population of the golden mussel in PQ, the most lotic site sampled. The same haplotype, although present in IT, was not detected in larvae or adults from all remaining study sites in the Iguassu River (including adults of PQ). This result may reflect a recent invasion process, most likely upstream of PQ but downstream from CX (see also discussion on sweepstake reproductive success below). Since this is a lotic stretch of the river, collection from this site likely includes mostly larvae that originated from adult populations established further upstream, carried away from the original location of introduction by the river flow.
Larvae as a proxy for population genetics
Because there was no significant genetic differentiation between adult and larval populations based on the mtDNA COI gene for the golden mussel in most sites, the use of larvae as a proxy for genetic population studies of L. fortunei populations is partially supported by this study. The collection of plankton samples is faster, simpler, and requires less effort (in time and man-power) than the collection of adult specimens (e.g., Tschá et al. 2012). The combined use of the specific molecular marker for detection and identification of larvae of the golden mussel (Boeger et al. 2007) and the subsequent genetic characterization of individual or pooled larvae (see Giessler and Wolinska 2013 for compatible protocol) should expedite and simplify laboratory processing. Protocols for population genetics using new-generation sequencing (NGS) technology will likely become the technique of choice since it can be applied in sequencing or genotyping individual or pooled larvae (see McCormack et al. 2013).
Alternatively, the use of NGS methods with environmental DNA should also represent an important procedure in characterizing local populations of aquatic organisms that have at least one planktonic stage, such as L. fortunei. For instance, methods of sequence capture (or “target enrichment”) (see Gnirke et al. 2009; Jones and Good 2016) or sequence-specific amplifications (as suggested by Shokralla et al. 2012), may be applied directly to pooled DNA of plankton samples. Procedures to analyze the genetic structure of organisms from pooled DNA approaches are already available (e.g., Giessler and Wolinska 2013). The present relatively elevated costs of NGS protocols are likely to reduce in the near future and are prone to become the method of choice for molecular-ecology studies. Whatever the protocol utilized, targeting larvae — rather than or along with adult specimens — should be considered both for ease of sampling and for the information obtained.
In many cases, larvae represent a more comprehensive sample of local populations, especially in places where adults occur in small numbers and/or are present at great depths, which can be aggravated during flood periods. Thus, overall, the protocol proposed herein represents an important strategy for producing rapid empiric data on which to base decisions on control, management, and monitoring of this and other invasive species with planktonic larval stages.
However, targeting solely larval stages of L. fortunei needs caution. First, sweepstake reproductive success (see Hedgecock and Pudovkin 2011) may induce differences in the genetic profiles of adult and larval populations. In the present study, however, the lack of significant difference in the genetic composition of the larval and adult samples at most locations analyzed herein indicates that this is not the case for L. fortunei. Second, in certain areas, larvae are not always present, as a consequence of variation in the reproductive activity throughout the year. Third, depending on the size of the larval stages of the goal species, there may be a limited amount of DNA extract available for procedures that require more reactions, such as genotyping with microsatellites (but see Christian et al. 2007; Baggio et al. 2011). Fourth, an adequate strategy for sampling plankton should be employed to avoid bias, because of the possibility of uneven distribution of the local genetic variability in the plankton based on differences in spawning activity among local clusters of adult specimens. Additionally, comparisons of results derived from larvae and adults using more variable markers should provide additional tests for the proposed use of larvae as an efficient proxy for the genetic profile of adult populations.
Care must exist in the interpretation of the results as well. The qualitative difference between the genetic structure of larvae and adults of the Iguassu National Park (PQ) is associated with the presence of haplotype Lfm02, detected solely in the sample of larvae. Similarly, in this site, the haplotype Lfm05 was detected in only the adult population. Thus, these results further indicate additional limitations in the use of the proposed protocol. In a lotic habitat, sampled plankton likely bears mostly larvae that originated from adult populations of the golden mussel established in stretches above that sampled point. Thus, the use of larvae as a proxy for the genetic profile of the local adult population should consider this lack of spatial and temporal synchrony when sampling is performed in lotic environments.
Whereas the above-described limitations deserve attention when population genetic analyses are based solely on larval specimens, the proposed method provides complementary insights into the structure and genetic distribution of populations above the sampled point in lotic systems. For instance, larvae collected from one point downstream potentially incorporate information on the genetic structure of all upstream populations. This would confer an advantage for prospective studies of L. fortunei in large geographic areas, especially in areas under risk of invasion or that have been recently invaded. One can rapidly access the genetic profile of the invasive population in the new area and identify the source(s) of propagules if genetic information of populations of the surrounding contaminated areas is known. Ideally, more detailed genetic studies should incorporate information on both samples of adults and larvae.
References
Angonesi LG, Rosa NG, Bemvenuti CE (2008) Tolerance to salinities shocks of the invasive mussel Limnoperma fortunei under experimental conditions. Iheringia Sér Zoo 98:66–69
Baggio RA, Pil MW, Boeger WA, Patella LA, Ostrensky A, Pie MR (2011) Genetic evidence for multiple paternity in the mangrove land crab Ucides cordatus (Decapoda: Ocypodidae). Mar Biol Res 7:520–524
Belz CE (2006) Risk analysis bioinvasion by Limnoperna fortunei (Dunker1857): a model for the basin of the river Iguaçu, Paraná (in Portuguese). Federal University of Paraná. Thesis Ph.D., Graduate Program in Zoology
Belz CE, Darrigran G, Mader-Netto OS, Boeger WA, Junior PRR (2012) Analysis of four dispersion vectors in inland waters: the case of the invading bivalves in South America. J Shellfish Res 31:777–784
Boeger W, Pie MR, Falleiros RM, Ostrensky A, Darrigran G, Dreher Mansur MC, Belz CE (2007) Testing a molecular protocol to monitor the presence of golden mussel larvae (Limnoperna fortunei) in plankton samples. J Plankton Res 29:1015–1019
Boltovskoy D, Correa N, Cataldo D, Sylvester F (2006) Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Rio de la Plata watershed and beyond. Biol Invasions 8:947–963
Boltovskoy D, Sylvester F, Otaegui A, Leites F, Cataldo DH (2009) Environmental modulation of reproductive activity of the invasive mussel Limnoperna fortunei: implications for antifouling strategies. Austral Ecol 34:719–730
Cataldo D, Boltovskoy D (2000) Yearly reproductive activity of Limnoperna fortunei (Bivalvia) as inferred from the occurrence of its larvae in the plankton of the lower Paraná River and the Río de la Plata estuary (Argentina). Aquat Ecol 34:307–317
Cataldo D, Boltovskoy D, Hermosa JL, Canzi C (2005) Temperature-dependent rates of larval development in Limnoperna fortunei (bivalvia: mytilidae). J Molluscan Stud 71:41–46
Charif D, Lobry JR (2007) SeqinR 1.0-2: a contributed package to the R project for statistical computing devoted to biological sequences retrieval and analysis. In: Bastola U, Porto M, Romano HE, Vendruscolo M (eds) Structural approaches to sequence evolution: molecules, networks, populations. Springer, New York, pp 207–232 (Biological and Medical Physics, Biomedical Engineering)
Christian AD, Monroe EM, Asher AM (2007) Methods of DNA extraction and PCR amplification for individual freshwater mussel (Bivalvia: Unionidae) glochidia, with the first report of multiple paternity in these organisms. Mol Ecol 7:570–573
Claxton WT, Boulding EG (1998) A new molecular technique for identifying field collections of zebra mussel (Dreissena polymorpha) and quagga mussel (Dreissena bugensis) veliger larvae applied to eastern Lake Erie, Lake Ontario, and Lake Simcoe. Can J Zool 76:194–198
Darrigran G (2002) Potential impact of filter-feeding invaders on temperate inland fresh water environments. Biol Invasions 4:145–156
Darrigran G, Damborenea C (2005) A South American bioinvasion case history: Limnoperna fortunei (Dunker1857), the golden mussel. Am Malacol Bull 20:105–112
Darrigran G, Damborenea C (2011) Ecosystem engineering impacts of Limnoperna fortunei in South America. Zool Sci 28:1–7
Darrigran G, Ezcurra de Drago I (2000) Invasion of the exotic freshwater mussel Limnoperna fortunei (Dunker1857) (Bivalvia: Mytilidae) in South America. Nautilus 114:69–73
Darrigran G, Mansur MCD (2009) Introduction and spread of Limnoperna fortunei (in Portuguese). In: Darrigran G, Damborenea C (eds) Introduction to biology of invasions: the golden mussel in South America: biology, spread, impact, prevention and control (in Portuguese). Cubo Editora, São Carlos, pp 89–110
Darrigran G, Pastorino G (1995) The recent introduction of Asiatic bivalve, Limnoperna fortunei (Mytilidae) into South America. Veliger 38(2):183–187
Darrigran G, Damborenea C, Greco N (2007) Freshwater invasive bivalves in man-made environments: a case study of larvae biology of Limnoperna fortunei in a hydroelectric power plant in South America. Ambio 36:575–579
Darrigran G, Boeger W, Damborenea CI, Maroñas MI (2009) Evaluation of sampling and analysis techniques for early detection of Limnoperna fortunei (Mytilidae) in limit areas of its distribution. Braz J Biol 69:979–980
Drake J, Bossenbroek J (2004) The potential distribution of zebra mussels in the United States. Bioscience 54:931–941
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Marine Biol Biotechnol 5:294–299
Gazulha V, Mansur MCD, Cybis LF, Azevedo SMFO (2012) Grazing impacts of the invasive bivalve Limnoperna fortunei (Dunker1857) on single-celled, colonial and filamentous cyanobacteria. Braz J Biol 72:33–39
Giessler S, Wolinska J (2013) Capturing the population structure of microparasites: using ITS-sequence data and a pooled DNA approach. Mol Ecol Resour 13:918–928
Gnirke A, Melnikov A, Maguire J, Rogov P, LeProust EM, Brockman W, Fennell T, Giannoukos G, Fisher S, Russ C, Gabriel S (2009) Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol 27:182–189
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Available via DIALOG http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed 13 May 2001
Hedgecock D, Pudovkin AI (2011) Sweepstakes reproductive success in highly fecund marine fish and shellfish: a review and commentary. Bull Marine Sci 87:971–1002
Jones MR, Good JM (2016) Targeted capture in evolutionary and ecological genomics. Mol Ecol 25:185–202
Jost L (2008) GST and its relatives do not measure differentiation. Mol Ecol 17:4015–4026
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Ludwig S, Tschá MK, Patella R, Oliveira AJ, Boeger WA (2014) Looking for a needle in a haystack: molecular detection of larvae of invasive Corbicula clams. Manag Biol Invasions 5:143–149
Mansur MCD, Richinitti LMZ, Santos CP (1999) Limnoperna fortunei (Dunker 1857), invasive bivalve, Guaiba Bay, Rio Grande do Sul, Brazil (in Portuguese). Biociencias 7:147–150
Mansur MCD, Santos CP, Darrigran G, Heydrich I, Callil CT, Cardoso FR (2003) First qualitative and quantitative data of the golden mussel, Limnoperna fortunei (Dunker) in Delta Jacuí, Lake Guaíba and Patos Lagoon, Rio Grande do Sul, Brazil and some aspects of their invasion in the new environment. Rev Bras Zool 20:75–84
McCormack JE, Hird SM, Zellmer AJ, Carstens BC, Brumfield RT (2013) Applications of next-generation sequencing to phylogeography and phylogenetics. Mol Phylogenet Evol 66:526–538
Mills EL, Leach JH, Carlton JT, Secor CL (1993) Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. J Great Lakes Res 19:1–54
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci 70:3321–3323
Neto RM, Zeni TO, Ludwig S, Horodesky A, Girotto MVF, Castilho-Westphal A, Ostrensky A (2012) Influence of environmental variables on the growth and reproductive cycle of Crassostrea (Mollusca, Bivalvia) in Guaratuba Bay, Brazil. Invertebr Reprod Dev 57:208–218
Paolucci EM, Sardin P, Sylvester F, Perepelizin PV, Zhan A, Ghabooli S, Cristescu ME, Oliveira MD, MacIsaac HJ (2014) Morphological and genetic variability in an alien invasive mussel across an environmental gradient in South America. Limnol Oceanogr 59:400–412
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Pennings PS, Achenbach A, Foitzik A (2011) Similar evolutionary potentials in an obligate ant parasite and its two host species. J Evol Biol 24:871–886
Perepelizin PV, Boltovskoy D (2011) Thermal tolerance of Limnoperna fortunei to gradual temperature increase and its applications for biofouling control in industrial and power plants. Biofouling 27:667–674
Pestana D, Pie MR, Ostrensky A, Boeger WA, Andreoli C, Franceschi F, Lagos P (2008) Seasonal variation in larval density of Limnoperna fortunei (Bivalvia, Mytilidae) in the Iguassu and Paraná Rivers, in the region of Foz do Iguassu, Paraná, Southern Brazil. Braz Arch Biol Technol 51:607–612
Pestana D, Ostrensky A, Tschá MK, Boeger WA (2010) Prospecting of invasive mussel Limnoperna fortunei (Dunker 1857) in the main water bodies of the state of Paraná, Brazil (in Portuguese). Pap Avulsos Zool 50:553–559
Pie MR, Boeger WA, Patella L, Falleiros R (2006) A fast and accurate molecular method for the detection of larvae of the golden mussel Limnoperna fortunei (Mollusca: Mytillidae) in plankton samples. J Molluscan Stud 72:218–219
Santos CP, Würdig NL, Mansur MCD (2005) Larval stage of the mussel Limnoperna fortunei (Dunker) (Mollusca, Bivalvia, Mytilidae) in the lake Guaiba basin, Rio Grande do Sul, Brazil. Rev Bras Zool 22:702–708
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver. 2000: a software for population data analysis. Evol Bioinform Online 1:47–50
Shokralla S, Spall JL, Gibson JF, Hajibabaei M (2012) Next-generation sequencing technologies for environmental DNA research. Mol Ecol 2:1794–1805
Takeda AM, Mansur MCD, Fujita DS, Bibian JPR (2003) Occurrence of invasive species golden mussel, Limnoperna fortunei (Dunker 1857) in two small reservoirs near Curitiba, PR. Acta Biol Leopold 25:251–254
Tschá MK, Patella R, Ostrensky A, Boeger WA (2012) The molecular method of prospecting the golden mussel (in Portuguese). In: Mansur MCD, Santos CP, Pereira D, Paz ICP, Zurita MLL, Rodriguez MTR, Nehrke MV, Bergonci PEA (eds) Limnic invasive molluscs in Brazil: biology, prevention and control (in Portuguese). Redes Editora, Porto Alegre, pp 143–148
Waeyenberge L, Ryss A, Moens M, Pinochet J, Vrain TC (2000) Molecular characterisation of 18 Pratylenchus species using rDNA restriction fragment length polymorphism. Nematology 2:135–142
Zanella O, Marenda LD (2002) Occurrence of Limnoperna fortunei in Itaipu Hydropower Central. In: 5 Latin-American congress os malacology, São Paulo. Resumes. Butantan Institute/Institute of Biosciences, USP, São Paulo, p 41 (in Portuguese)
Zhan A, Perepelizin PV, Ghabooli S, Paolucci E, Sylvester F, Sardiña P, Cristescu ME, MacIsaac HJ (2012) Scale-dependent post-establishment spread and genetic diversity in an invading mollusc in South America. Divers Distrib 18:1042–1055
Acknowledgments
The authors thank the Companhia Paranaense de Energia—COPEL and Tractebel Energia, Eletric Utilities at which services and R and D projects were conducted, regulated by the Agência Nacional de Energia Elétrica—ANEEL and that provided data to develop this research. Additionally, the authors thank D. Agostinho from Itaipu Binacional Hidroeltric Power Plant for providing samples, Companhia de Saneamento do Paraná—SANEPAR for authorizing the study at Piraquara II Dam and for all field support, colleagues from the Institutos Lactec for collecting samples and colleagues from LEMPE for helping with the molecular protocols and methodology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Toshifumi Minamoto.
Rights and permissions
About this article
Cite this article
Borges, P.D., Ludwig, S. & Boeger, W.A. Testing hypotheses on the origin and dispersion of Limnoperna fortunei (Bivalvia, Mytilidae) in the Iguassu River (Paraná, Brazil): molecular markers in larvae and adults. Limnology 18, 31–39 (2017). https://doi.org/10.1007/s10201-016-0485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-016-0485-8