Abstract
The Pacific oyster Crassostrea gigas is one of the most invasive species worldwide. This oyster has a preponderant ecological role in the invaded environments, for example structuring the benthic community through the provision of micro-habitats. Twenty-five years after its introduction in Argentina, the species is colonizing new areas along the coast, extending northwards and southwards its local distribution. In this study, we provide the first ecological characterization of the southern-most population of C. gigas; where the composition, density, richness and diversity of the macroinvertebrate assemblages associated with zones with oysters were compared with zones where it is absent at four different times of the year. Additionally, the main epibionts taxa settled on the oyster shells were studied. Our results showed differences in the assemblage composition between zones. However, these differences were not consistent throughout the year. Furthermore, density, richness and diversity were higher in the zones with oysters only in one of the surveys and the parameters did not differ between zones in the remaining months. Moreover, the majority of oysters were used as settlement substrate by the sessile common species present in the area. Thus, our work provides new information about the ecology of C. gigas in recently invaded areas that enhance our understanding of the role that facilitation plays in physically stressful ecosystems and the importance that density and time since the invasion may have in the engineering effects of the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species affect natural ecosystems in several ways. For example, changing ecosystem processes (Carlton 1999; Hoffmeister et al. 2005), generating a loss of biodiversity (Vitousek et al. 1997; Sala et al. 2000), introducing diseases and parasites (Hallegraeff 1998; Torchin et al. 2002), altering habitats (Schwindt et al. 2001; Crooks 2002) and modifying community functioning (Byers 1999; Grosholz et al. 2000). Specifically, facilitation by invasive species plays an important role in physically stressful ecosystems (Crooks 2002; Sousa et al. 2009). Many marine invasive plants (Bruno 2000; Brusati and Grosholz 2006; Neira et al. 2006), macroalgae (Strong et al. 2006; Byers et al. 2010; Irigoyen et al. 2011) and invertebrate species (Schwindt et al. 2001; Sousa et al. 2009; Sellheim et al. 2010) have profound architectural consequences on the ecosystem structure where they arrive. Examples from the literature suggest that introduced ecosystem engineers that increase habitat complexity or heterogeneity tend to change the abundances and/or species richness (Crooks 2002). Furthermore, several introduced species not only facilitate the survival of native species but also support the establishment and spread of other invaders (Simberloff and Von Holle 1999; Simberloff 2006). Thus, while invasive species are considered among the top-five threats to native biodiversity (Vitousek et al. 1997; Sala et al. 2000), their arrival in the environments could lead to an increase in local richness.
Oyster introductions probably began as early as the seventieth century, when the so-called Portuguese oyster (Crassostrea angulata) arrived in Europe from Asia (Carlton 1999). Overall, oysters have been introduced and established permanently in at least 30 countries outside their native ranges (Carlton 1999; Ruesink et al. 2005; Padilla 2010). The Pacific oyster Crassostrea gigas, a species native to Japan, is currently the most widely bivalve under aquaculture around the world and has been extensively introduced for cultivation (Orensanz et al. 2002; Ruesink et al. 2005; Padilla 2010). The Pacific oyster continues invading shores around the world, and is currently an invader in most of the temperate zones and some tropical areas worldwide (Ruesink et al. 2005; Padilla 2010). Introduced oysters have innumerable ecological impacts and do not only simply replace native oyster species (Ruesink et al. 2005). Oysters are considered autogenic engineer species because they change the physical structure, complexity and heterogeneity of the environment through their own structure (Jones et al. 1997; Ruesink et al. 2005; Sousa et al. 2009). The species is also an allogenic engineer since can decrease turbidity, increase light penetration in the water column and affect near bed flows and sedimentation processes (Ruesink et al. 2005). By suspension feeding, they influence energy flow and nutrient cycling at the scale of entire estuaries. Not only invasive oysters behave as engineers species; the eastern oyster Crassostrea virginica also exhibits the ability to form reefs and generate different effects on local communities in North America, where this species is native (Meyer and Townsend 2000; Shervette and Gelwick 2008). The transformations generated by oysters, both native and invasive, affect the associated species composition and their relative abundances since the interstices between oysters provide refuge for other species, and shells can be used as a substratum for the settlement of algae and invertebrates (Meyer and Townsend 2000; Ruesink et al. 2005).
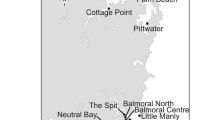
In South America, Crassostrea gigas was introduced to Chile and Perú in the Pacific Ocean, and to Brazil and Argentina in the Atlantic (Orensanz et al. 2002). In Argentina, its introduction dates back to 1982 when oysters from Coquimbo (Chile) were transplanted to Anegada Bay (~40ºS, Fig. 1) with cultivation purposes (Orensanz et al. 2002). After a few months, the culture was abandoned and the oysters were left in the field (Orensanz et al. 2002). Fifteen years later, the settlement of oysters increased explosively and densities reached up to 120 recruits m−2 in the same area where they were introduced (Orensanz et al. 2002). After this main introduction, installations of other cultivation areas in the coast of Argentina were authorized between 1998 and 1999 (Orensanz et al. 2002). Currently, only a small population of C. gigas has being detected in the marshes of Samborombón Bay (~36ºS, Fig. 1) possibly derived from one of these new cultivation areas (Giberto et al. 2012, but see Lomovasky et al. 2014). Since 2010, C. gigas has expanded its range to the north (~38ºS, dos Santos and Fiori 2010, Fig. 1) and south (~41ºS, Roche et al. 2010, Fig. 1) of the main introduction area. Thus, the invasion by this species could be reaching a new peak of expansion after a lag time in its invasion process (Crooks 2005). Although some ecological studies were performed (Escapa et al. 2004; Giberto et al. 2012; Croce and Parodi 2012), little is known about the effects of this species on native communities and, as often occurs, there is no information of how this species behaves in recently invaded areas. Indeed, this study is the first to investigate the potential impacts of C. gigas at the beginning of the establishment of a new population. The aim of the study is to provide the first ecological characterization of the southern-most population of C. gigas and to discuss possible facilitation processes of local fauna associated with its recent invasion. To do this, we compare the composition, density, richness and diversity of the macroinvertebrate assemblages in zones with and without C. gigas and we repeat this in four times throughout one year. The main taxa of epibionts on C. gigas and their cover are also provided. Considering that density of a population is one of the key factors scaling ecosystem engineering impacts, we hypothesize that the effect generated by the presence of oysters in recent invaded environments will be less evident than that recorded in areas where the species has spent several decades established and populations reached higher densities.
Materials and methods
Study site
The study was performed in a fine sandstone rocky shore of the central Argentinean Patagonia, El Cóndor (41º03′S, 62°49′W, Fig. 1) where the tidal regime is semidiurnal with a mean amplitude of 4 m. The region is characteristic of the arid steppe, with predominant winds from the southwest, annual precipitation of 200 mm and mean annual temperature of 12 °C (Paruelo et al. 1998). In this rocky shore, Crassostrea gigas was recently found forming the austral-most population since its first introduction in the southwestern Atlantic coast with mean density of 0.11 ind m−2 (Roche et al. 2010). The distribution of the oysters in the shore, mostly solitary individuals, is concentrated in the middle intertidal where the mytilid Brachidontes rodriguezii and the barnacles Amphibalanus improvisus and Balanus glandula are the dominant species.
Crassostrea gigas as habitat-forming species
In order to compare the composition, density, richness and diversity of macroinvertebrates in areas with and without C. gigas (hereafter with oyster and without oyster zones) macrofaunal samples were collected. Fourteen samples per zone (10 cm × 10 cm) were obtained randomly at the middle intertidal of the rocky shore. Surveys were performed at four different times of the year: November 2009, April 2010, September 2010 and February 2011 to see if the effect of oysters was consistent throughout the year. In the field, the samples were collected separated by at least one meter, and interspersed in the middle intertidal and throughout the entire study sites. Each benthic sample was scraped off the surface, bagged, labelled and stored. In the laboratory, samples were sieved using a 0.5 mm mesh. The organisms retained on the mesh were fixed in 4 % formalin and preserved in 70 % ethanol. All organisms were identified to the lowest taxonomic level possible under a dissecting stereo microscope (80×), using updated taxonomic keys and the invertebrate reference collection from the CENPAT (http://www.cenpat-conicet.gob.ar/). A voucher of the taxa collected was deposited in the General Invertebrate Collection (CNP-INV). Moreover, we requested the assistance of taxonomic specialists for some particular taxa. Afterwards, total density (individuals 100 cm−2), richness and Shannon diversity (Shannon and Weaver 1949) were calculated for each sample.
To determine if there were significant differences in invertebrate community composition between zones and months data were statistically analyzed using a permutational analysis of variance (PERMANOVA) with Primer 6 software (Primer-E, Plymouth; v.6.1.7) (Anderson et al. 2008). PERMANOVA compares the F statistics to a distribution generated by multiple random permutations of the analyzed data, thus liberating it from the formal assumptions of traditional ANOVA (Anderson 2001; Anderson et al. 2008). PERMANOVA model was performed considering zones (with and without) as a fixed factor and sampling months (4 levels) as a random factor (9999 permutations). Pairwise post hoc tests were performed for each month to identify where the differences occurred. The abundance of all invertebrate species was square-root transformed in order to down-weight the most abundant species. A similarity percentage analysis (SIMPER) was used to determine the taxa responsible for the differences between groups. To visualise multivariate patterns in benthic assemblages, non-metric multidimensional scaling (nMDS) was used as an ordination method. PERMANOVA was performed using a Bray–Curtis similarity matrix with a dummy variable. The PERMANOVA routine creates a non-parametric, permutational analogue of ANOVA when applied to univariate data (Anderson 2001; Anderson et al. 2008). Thereby, PERMANOVA models were also used to determine if there were significant differences in density, richness and diversity (9999 permutations). Pairwise post hoc tests were performed for each month to identify differences between zones for the three variables.
Fouling on Crassostrea gigas
Epibionts on C. gigas were registered in each of the four sampling months (n = 14 oysters per month, except for April n = 10). The oyster size (maximum length × maximum width) was measured to the nearest mm with digital calliper (precision ± 0.01). The epibiotic organisms settled on the oyster shells were identified to the lowest taxonomic level possible under a dissecting stereo microscope (80×) and then the percentage cover of sessile organisms was estimated. Mobile organisms identified on the shells were also registered and counted. Differences in the percentage cover among oysters collected in different months were evaluated with a one way fixed ANOVA (Zar 1999). Normality and homogeneity of variance assumptions were evaluated with Kolmogorov–Smirnov and Levene tests, respectively. Parametric correlation analysis was performed to evaluate the relationship between the percentage cover and the size of the oyster (Zar 1999).
Results
Crassostrea gigas as habitat-forming species
A total of 27 macroinvertebrate taxa were found in the surveys (Table 1). The most abundant taxa for both zones were the mytilids (Aulacomya atra, Brachidontes rodriguezii and Mytilus sp.). Multivariate analysis showed that the invertebrate assemblage of the zones with oysters was different from zones without oysters (Fig. 2; Table 2). In MDS representation, samples from the same zone were more closely related to each other (Fig. 2). Besides, samples from the zones with oysters presented an average similarity of 74 % and those from the oyster zone 60 %. The average dissimilarity between the two zones was close to 40 % (Table 2). SIMPER analysis also showed that the taxa responsible for these differences changed throughout the year, being impossible to determine which of the taxa characterized each zone (Table 2). For example, the exotic barnacle Balanus glandula was more abundant in with oyster zone in November and September but the opposite result was found for April and February (Table 2). Furthermore, mytilids were the taxa that contributed mostly to the observed differences in assemblage structure between zones, regardless of the zone considered (SIMPER, top 40 % for all the surveys; Table 2); thus they were excluded in the density comparisons and their abundances analyzed separately. Community composition, density, richness and diversity differed significantly between zones; PERMANOVA detected a significant interaction between the factors Zones and Months showing that the differences between zones were not consistent over time and changed during the course of the study (Table 3). Pairwise comparisons shsowed that assemblage composition significantly differed between zones in the 4 months (Table 4). Density, richness, and diversity, instead, were higher in with oyster zone than in without oyster zone in November (Fig. 3; Table 4). Density of mytilids differed significantly between zones (PERMANOVA: pseudo-f zone = 2.42, P > 0.05; pseudo-f month = 5.28, pseudo-f zone×month = 3.47, P < 0.05), being higher in the without oyster zone in April and September (pairwise comparisons P < 0.05).
Mean (±SD) values of density (a), richness (b) and Shannon Diversity Index (c) of macroinvertebrates associated to the zones with oysters (black) and without oysters (white) for each month. Asterisks indicate significant differences between zones. Since mytilids account for about 90 % of the total abundance in both zones, they were excluded in the density comparisons
Fouling on Crassostrea gigas
Over 90 % of the studied oysters showed epibiotic organisms. The size distribution of the oysters ranged from 10 to 60 cm2. Ten taxa of sessile organisms (corresponding to four species of bivalves, two barnacles, two hydrozoans and two bryozoans) and five of mobile ones (three polychaetes, one decapod and one insect larvae) were registered. The most abundant taxa were the mytilids and the barnacles Balanus glandula and Amphibalanus improvisus. The mean percentage cover was 34.8 (SD = 34.4). There were no significant differences in the percentage cover among months (F = 2.06, df = 3, P > 0.05) and the size of the oysters was not significantly correlated with the epibionts percentage cover (P > 0.05).
Discussion
After three decades since the first introduction of Crassostrea gigas in Argentina, the species expanded its range to two new locations, El Cóndor (Roche et al. 2010, this study) the southern-most and Blanca Bay (dos Santos and Fiori 2010) the northern-most area. In our study, the invertebrate assemblage of the zones with oysters was different from zones without oysters. These differences in the community composition were not consistent throughout the year of study and the taxa that contributed to the identity of the zones also changed during the study. Density, richness, and diversity were higher in the oyster zone only in one of the surveys. In addition, oyster shells provide substratum for the settlement of several species, mostly mytilids and barnacles. Together, the results show that the presence of the invasive oyster C. gigas would not change markedly natural community patterns. However, the presence of oysters facilitated the establishment of some species probably by providing shelter and settlement sites. Since the invasion of oysters in the studied rocky shore is a recent phenomenon, the influence of the species in the environment could increase in the near future along with an increase in the population density. In this way, the potential effects of C. gigas could become more important if repeated recruitments occur and could resemble the effect of the species registered for other locations (Escapa et al. 2004; Padilla 2010).
Temporal dynamics in the invasion process, as well as the timing of many invasion-related events, are hard to predict (Crooks and Soulé 1999; Simberloff 2003; Crooks 2005). For example, the time course of invasions often includes long initial periods of relative inactivity followed by drastic changes in invader dynamics (Crooks 2005). This phenomenon of lag times has become an increasingly recognized aspect of invasion biology (Crooks and Soulé 1999; Simberloff 2003; Crooks 2005; Bortolus 2008). This kind of time lags can be found throughout the invasion process, including in the arrival, establishment, and in the impacts generated by invaders. For example, populations often grow exponentially in the early phases of invasion, and this gives rise to an inherent lag (Crooks 2005). Such is the case of Crassostrea gigas invasion in Argentina. After its introduction in 1982, the species colonized the area at low densities and fifteen years later, settlement and densities increased explosively (Orensanz et al. 2002). Now, after 30 years the species has shown the capacity of expansion outside the original area of introduction, establishing populations to the north and the south (dos Santos and Fiori 2010 and Roche et al. 2010, respectively). Thereby, density of C. gigas in Argentina varies radically throughout its current introduced range (Fig. 1). In the first invaded areas (Anegada Bay) population reached average densities of 300 ind m−2 (Borges 2006); however in new invaded areas (El Cóndor) mean density is 0.11 ind m−2 (Roche et al. 2010).
The density of a population is one of the key factors scaling ecosystem engineering impact; at low abundance, species are unlikely to cause community or ecosystem level change (Borthagaray and Carranza 2007; Padilla 2010; Harley and O’Riley 2011). In a detailed review of the engineering effect of Crassostrea gigas, Padilla (2010) highlighted the fact that the potential effects of oysters clearly depend on its population densities. For example, in rocky shores dominated by mussels (like our study site) oysters at low densities can produce an overgrowth of benthic species, generate local impacts on diversity and increase diversity of species sensitive to stress. However, at higher densities, oysters could also displace mussels, increase sedimentation, increase local diversity and alter species composition (Padilla 2010). Therefore, the major differences in density between early and newly invaded areas registered for Argentina could be explaining why the oysters in El Cóndor did not modify sharply natural community patterns. In Anegada Bay, the first invaded intertidal in Argentina, the dense population of C. gigas has led to the creation of shallow intertidal reefs (Borges 2006). In this system, the abundance of several invertebrate species is highest within the reefs created by the oysters and, consequently, the density and rate of foraging of shorebirds is greatest in these areas, probably due to an increase in preys supply (Escapa et al. 2004). Also, Anegada Bay reefs provide microhabitats for macroalgal settlement (Croce and Parodi 2012). In Samborombón Bay, oysters are grouped forming small reefs that, unlike Anegada Bay, are made up by small sizes individuals (shell height up to 37 mm, Giberto et al. 2012). Crustaceans, bivalves and other invertebrates are associated to these reefs (Giberto et al. 2012). Given the low density of oysters, reef habitats are still absent in our study site and could explain the differences in the effect generated by oysters found between Anegada Bay or Samborombón Bay and El Cóndor. Nevertheless, oysters at El Cóndor offer a new settlement site for other organisms, such as mussels and barnacles, which in turn create micro-habitats for other benthic organisms (Prado and Castilla 2006; Sellheim et al. 2010; Sueiro et al. 2011). In this manner, differences in the time of establishment of the populations of C. gigas in Argentina have generated a density gradient between different invaded sites. This gradient, allow us to understand the complex relationship between the density of oysters and the effect exerted on communities, resulting in greater impacts in places where oyster populations are denser and longer time since invasion.
It should be noted that some other environmental variation between Anegada Bay and El Cóndor, such as the exposure of the coast or bottom type, could be determining Crassostrea gigas behavior and should be considered and analyzed in future research. In this sense, how oysters would affect the benthic community in the new invaded area of El Cóndor is difficult to predict. In addition, the effect generated by oysters may vary depending on the presence of other engineering species (Padilla 2010). In general, the extremely high stress imposed by weather conditions in Patagonian rocky shores results in the dominance of habitat-forming species, mainly coralline algal turfs and mussel beds (Liuzzi and López Gappa 2008; Silliman et al. 2011). Mussels were dominant in our samples and we found differences in mussel densities between with oyster and without oyster zones in April and September. However, the increments in mussel densities were not consistent correlated with changes in the abundances of the other members of the invertebrate assemblage, and neither would influence the results found. Regardless of this, further investigation should focus on the potential interactive effects of oysters and mussels. Moreover, it is known that abiotic and biotic factors jointly contribute to the risk of proliferation of invasive species (Case 1990; Alpert et al. 2000; Ruesink 2007). In this respect, during the field surveys we observed that fine sediment load was considerably high (up to 500 g 100 cm−2). Since oysters are filter-feeders, the suspension of sediment could be affecting, indirectly and negatively, the feeding activity and therefore their survival. Alternatively, density of oyster’s larvae can be reduced due to predation by filter-feeders (Pechenik et al. 2004; Porri et al. 2008; Troost 2010). In fact, oysters in particular, appear to consume large numbers of larvae, including those of their own species (Pechenik et al. 2004; Troost 2010). Predation by filter-feeders present in the area, as mytilids and the own C. gigas, could be an important source of variation in settlement success of oysters and possibly will affect population density. Additional studies are needed to evaluate how the different factors are conditioning the establishment and proliferation of the new population of C. gigas.
In densely populated marine environments like rocky shores, competition for space is critical (Connell 1961; Jackson 1977), thus the advantage of colonizing new surfaces is probably the major benefit of epibiosis (Wahl 1989). In El Cóndor, more than 90 % of Crassostrea gigas individuals presented incrusted organisms mainly composed by sessile taxa. Among the most abundant organisms settled on oyster shells, we found the barnacles Balanus glandula and Amphibalanus improvisus. This association between the presence of exotic oysters and the barnacles deserves special attention since B. glandula is an invasive species and A. improvisus a cryptogenic one for Argentina (Orensanz et al. 2002). In the same way, it has been observed that the invasive filamentous algae Polysiphonia morrowii (Raffo et al. 2014) recruits on C. gigas reefs in Anegada Bay (Croce and Parodi 2012). Both cases illustrate how invasive species are able to facilitate the colonization of other exotic species (Simberloff and Von Holle 1999; Simberloff 2006). As a result, ecological consequences of this kind of facilitation process could be severe (Simberloff and Von Holle 1999; Simberloff 2006; Sellheim et al. 2010) and should be studied in detail. Furthermore, several advantages and disadvantages may occur as a result of epibiosis, for both substrata organism and epibiont species (Wahl 1989, 2008). In the case of C. gigas, the settlement of large epibionts may increase the risk of dislodgement, reduce growth, increase the susceptibility to predation or affect feeding activities of oysters (Buschbaum and Reise 1999; Thieltges 2005a; da Gama et al. 2008). On the other hand, water-retaining epibionts can slow down oyster desiccation or even camouflage them from predators (Thieltges 2005b; Ramsby et al. 2012). Thereby, performing experimental studies of the potential effects of epibiosis could be useful to have a complete scenario of the impact of C. gigas on the communities.
To summarize, in the last years Crassostrea gigas started a new period of expansion in its invasion in Argentina. Our study suggests that the recent arrival of the invasive oyster in El Cóndor has not yet changed intensely the benthic community. Given the engineering activities commonly exhibited by the species, possible habitat alteration may occur in the short term if the density of the oyster increases. Taking into account that the population density in El Cóndor is still low, temporal and spatial monitoring becomes critical and allows this system to be an excellent opportunity to study the performance of an invader and its progressive effects. Such studies will help to focus concerns about how invaders face abiotic and/or biotic constrains. Environmental managers, decision makers and government authorities should exploit and benefit from the early detection of this invasion expansion as to prevent the spread of biological invasions and monitor their effects.
References
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3:52–66
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Borges ME (2006) Ecología de las ostras en ambientes del sur bonaerense: cultivo y manejo de sus poblaciones. PhD thesis, Universidad Nacional del Sur
Borthagaray AI, Carranza A (2007) Mussels as ecosystem engineers: their contribution to species richness in rocky littoral community. Acta Oecol 31:243–250
Bortolus A (2008) Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. AMBIO J Hum Environ 37:114–118
Bruno JF (2000) Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology 81:1179–1192
Brusati ED, Grosholz ED (2006) Native and introduced ecosystem engineers produce contrasting effects on estuarine infaunal communities. Biol Invasions 8:683–695
Buschbaum C, Reise K (1999) Effects of barnacle epibionts on the periwinkle Littorina littorea (L.). Helgol Mar Res 53:56–61
Byers JE (1999) The distribution of an introduced mollusc and its role in the long-term demise of a native confamilial species. Biol Invasions 1:339–352
Byers JE, Wright JT, Gribben PE (2010) Variable direct and indirect effects of a habitat-modifying invasive species on mortality of native fauna. Ecology 91:1787–1798
Carlton JT (1999) Molluscan invasions in marine and estuarine communities. Malacologia 41:439–454
Case TJ (1990) Invasion resistance arises in strongly interacting species-rich model competition communities. Proc Natl Acad Sci 87:9610–9614
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
Croce ME, Parodi ER (2012) Seasonal dynamic of macroalgae in intertidal pools formed by beds of Crassostrea gigas (Mollusca, Bivalvia) on the north Patagonian Atlantic coast. Bot Mar 55:49–58
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
Crooks JA (2005) Lag times and exotic species: the ecology and management of biological invasions in slow-motion. Ecoscience 12:316–329
Crooks JA, Soulé ME (1999) Lag times in population explosions of invasive species: causes and implications. In: Sandlund OT, Schei PJ, Viken A (eds) Invasive species and biodiversity management. Kluwer Academic Press, Dordrecht, pp 103–125
da Gama BA, de Santos RPA, Pereira RC (2008) The effect of epibionts on the susceptibility of the red seaweed Cryptonemia seminervis to herbivory and fouling. Biofouling 24:209–218
dos Santos EP, Fiori SM (2010) Primer registro sobre la presencia de Crassostrea gigas (Thunberg, 1793) (Bivalvia: Ostreidae) en el estuario de Bahía Blanca (Argentina). Comun Soc Malacol Urug 9:245–252
Escapa M, Isacch JP, Daleo P, Alberti J, Iribarne O, Borges M, dos Santos EP, Gagliardini DA, Lasta M (2004) The distribution and ecological effects of the introduced pacific oyster Crassostrea gigas (Thunberg, 1793) in Northern Patagonia. J Shellfish Res 23:765–772
Giberto DA, Bremec CS, Schejter L, Escolar M, Souto V, Schiariti A, Romero MV, Dos Santos EP (2012) La ostra del pacífico Crassostrea gigas (Thunberg, 1793) en la provincia de Buenos Aires: reclutamientos naturales en Bahía Samborombón. Rev Investig Desarro Pesq 21:21–30
Grosholz ED, Ruiz GM, Dean CA, Shirley KA, Maron JL, Connors PG (2000) The impacts of a nonindigenous marine predator in a California bay. Ecology 81:1206–1224
Hallegraeff GM (1998) Transport of toxic dinoflagellates via ships’ ballast water: bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar Ecol Prog Ser 168:297–309
Harley CDG, O’Riley JL (2011) Non-linear density-dependent effects of an intertidal ecosystem engineer. Oecologia 166:531–541
Hoffmeister TS, Vet LEM, Biere A, Holsinger K, Filser J (2005) Ecological and evolutionary consequences of biological invasion and habitat fragmentation. Ecosystems 8:657–667
Irigoyen AJ, Trobbiani G, Sgarlatta MP, Raffo MP (2011) Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasions 13:1521–1532
Jackson JBC (1977) Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am Nat 111:743–767
Jones CG, Lawton JH, Shachak M (1997) Positive and negative effects of organisms as physical engineers. Ecology 78:1946–1957
Liuzzi MG, López Gappa J (2008) Macrofaunal assemblages associated with coralline turf: species turnover and changes in structure at different spatial scales. Mar Ecol Prog Ser 363:147–156
Lomovasky BJ, Alvarez G, Addino M, Montemayor DI, Iribarne O (2014) A new non-indigenous Crassostrea species in Southwest Atlantic salt marshes affects mortality of the cordgrass Spartina alterniflora. J Sea Res 90:16–22
Meyer DL, Townsend EC (2000) Faunal utilization of created intertidal eastern oyster (Crassostrea virginica) reefs in the southeastern United States. Estuaries 23:34–45
Neira C, Grosholz ED, Levin LA, Blake R (2006) Mechanisms generating modification of benthos following tidal flat invasion by a Spartina (alterniflora × foliosa) hybrid. Ecol Appl 16:1391–1404
Orensanz JM, Schwindt E, Pastorino G, Bortolus A, Casas G, Darrigran G, Elías R, López Gappa JJ, Obenat S, Pascual M, Penchaszadeh P, Piriz ML, Scarabino F, Spivak ED, Vallarino EA (2002) No longer a pristine confine of the world ocean—a survey of exotic marine species in the Southwestern Atlantic. Biol Invasions 4:115–143
Padilla DK (2010) Context-dependent impacts of a non-native ecosystem engineer, the Pacific oyster Crassostrea gigas. Integr Comp Biol 50:213–225
Paruelo JM, Beltrán A, Jobbágy E, Sala OE, Golluscio RA (1998) The climate of Patagonia: general patterns and controls on biotic processes. Ecol Austral 8:85–101
Pechenik JA, Blanchard M, Rotjan R (2004) Susceptibility of larval Crepidula fornicata to predation by suspension-feeding adults. J Exp Mar Biol Ecol 306:75–94
Porri F, Jordaan T, McQuaid CD (2008) Does cannibalism of larvae by adults affect settlement and connectivity of mussel populations? Estuar Coast Shelf Sci 79:687–693
Prado L, Castilla JC (2006) The bioengineer Perumytilus purpuratus (Mollusca: Bivalvia) in central Chile: biodiversity, habitat structural complexity and environmental heterogeneity. J Mar Biol Assoc UK 86:417–421
Raffo MP, Geoffroy A, Destombe C, Schwindt E (2014) First record of the invasive red alga Polysiphonia morrowii Harvey (Rhodomelaceae, Rhodophyta) on the Patagonian shores of the Southwestern Atlantic. Bot Mar 57:21–26
Ramsby B, Massaro A, Marshall E, Wilcox T, Hill M (2012) Epibiont–basibiont interactions: examination of ecological factors that influence specialization in a two-sponge association between Geodia vosmaeri (Sollas, 1886) and Amphimedon erina (de Laubenfels, 1936). Hydrobiologia 687:331–340
Roche MA, Narvarte MA, Maggioni M, Cardón R (2010) Monitoreo de la invasión de la ostra cóncava Crassostrea gigas en la costa norte de Río Negro: estudio preliminar. IV Reunión Binacional de Ecología, Buenos Aires
Ruesink JL (2007) Biotic resistance and facilitation of a non-native oyster on rocky shores. Mar Ecol Prog Ser 331:1–9
Ruesink JL, Lenihan HS, Trimble AC, Heiman KW, Micheli F, Byers JE, Kay MD (2005) Introduction of non-native oysters: ecosystem effects and restoration implications. Ann Rev Ecol Evol Syst 36:643–689
Sala OE, Chapin FS III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Leroy Poff N, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Schwindt E, Bortolus A, Iribarne O (2001) Invasion of a reef-builder polychaete: its direct and indirect impacts on the native benthic community structure. Biol Invasions 3:137–149
Sellheim K, Stachowicz JJ, Coates RC (2010) Effects of a nonnative habitat-forming species on mobile and sessile epifaunal communities. Mar Ecol Prog Ser 398:69–80
Shannon CE, Weaver W (1949) The mathematical theory of communications. University of Illinois Press, Urbana
Shervette VR, Gelwick F (2008) Seasonal and spatial variations in fish and macroinvertebrate communities of oyster and adjacent habitats in a Mississippi estuary. Estuar Coast 31:584–596
Silliman BR, Bertness MD, Altieri AH, Griffin JN, Bazterrica MC, Hidalgo FJ, Crain CM, Reyna MV (2011) Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS One 6:e24502
Simberloff D (2003) Eradication—preventing invasions at the outset. Weed Sci 51:247–253
Simberloff D (2006) Invasional meltdown six years later: important phenomenon, unfortunate metaphor, or both? Ecol Lett 9:912–919
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Sousa R, Gutiérrez JL, Aldridge DC (2009) Non-indigenous invasive bivalves as ecosystem engineers. Biol Invasions 11:2367–2385
Strong JA, Dring MJ, Maggs CA (2006) Colonisation and modification of soft substratum habitats by the invasive macroalga Sargassum muticum. Mar Ecol Prog Ser 321:87–97
Sueiro MC, Bortolus A, Schwindt E (2011) Habitat complexity and community composition: relationships between different ecosystem engineers and the associated macroinvertebrate assemblages. Helgol Mar Res 65:467–477
Thieltges DW (2005a) Impact of an invader: epizootic American slipper limpet Crepidula fornicata reduces survival and growth in European mussels. Mar Ecol Prog Ser 286:13–19
Thieltges DW (2005b) Benefit from an invader: American slipper limpet Crepidula fornicata reduces star fish predation on basibiont European mussels. Hydrobiologia 541:241–244
Torchin ME, Lafferty KD, Kuris AM (2002) Parasites and marine invasions. Parasitology 124:137–151
Troost K (2010) Causes and effects of a highly successful marine invasion: case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. J Sea Res 64:145–165
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Wahl M (1989) Marine epibiosis I. Fouling and antifouling: some basic aspects. Mar Ecol Prog Ser 58:175–189
Wahl M (2008) Ecological lever and interface ecology: epibiosis modulates the interactions between host and environment. Biofouling 24:427–438
Zar JH (1999) Biostatistical analysis. Prentice-Hall Inc, New Jersey
Acknowledgments
We are particularly grateful to Kelo Camarero and Raúl Cardón for their kind help and support in the field surveys. We thank F. Scarabino (gastropods), R. Elías (pycnogonids) and M.P. Raffo (hydrozoans and bryozoans) for their essential assistance with taxonomic identifications and S. Flaherty for assisting us with the English. We are grateful to two anonymous reviewers for their constructive comments and suggestions that greatly improved the manuscript. The study was financially supported by PID 371 (Agencia Nacional de Promoción Científica y Tecnológica) and PIP CONICET 089 and 508 (to ES), FONCyT-PICT # 2206 (to AB and ES). Special thanks to the environmental authorities of Río Negro for allowing us to work in the area.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mendez, M.M., Schwindt, E., Bortolus, A. et al. Ecological impacts of the austral-most population of Crassostrea gigas in South America: a matter of time?. Ecol Res 30, 979–987 (2015). https://doi.org/10.1007/s11284-015-1298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1298-7