Abstract
Despite significant growth in the information on introduced species (NIS), agreement on their comparative fitness and effects on native (NAT) communities is heterogeneous. Hypotheses aimed at explaining their fitness and impacts are as often supported as challenged. In order to investigate whether origin is associated with fitness or impact, we reviewed 72 meta-analyses of trait differences between NAT and NIS, and impacts of NIS on NAT and the environment, covering terrestrial and aquatic organisms. Interpretation of the trends was based on overall meta-analysis conclusions, and on numbers of point estimates (individual effect sizes) within and across studies. Mixed results were found in 44% of the studies. Higher NIS fitness and significant impacts were found in 31%, and 25% concluded that significant outcomes are largely absent. Point estimates showed that non-significant outcomes (57%) are more common than significant ones. Two thirds yield mixed trait differences and impacts, or do not support the concept that NIS are more fit or have negative effects on NAT. When trait differences or impacts are significant, NAT are usually less fit, or are negatively impacted, but this trend is influenced by the fact that most surveys are based on the most damaging invasive species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, interest in the impacts of introduced species has been growing vigorously (Canning-Clode, 2015; Boltovskoy et al., 2018), fueled by the rise in new introductions across the world (Seebens et al., 2017), and by the devastating impacts of some invaders (Anonymous, 2014; Joshi, 2017; Makowski & Finkl, 2018). Invasion biology has become a hot topic in ecology, but the scientific issues involved have been strongly influenced by idiosyncratic and managerial aspects. Many scholars have become divided into two lines of thought, dramatically illustrated by the 2011 Davis vs. Simberloff debate (Davis et al., 2011; Simberloff et al., 2011). A major point in these disagreements is whether origin per se is significantly associated with the success and spread of a species. In other words, do non-native or non-indigenous species (NIS) have intrinsic differences that ultimately condition the ability of their populations to grow vigorously in density and areal extension enhancing their use of resources and influencing native (NAT) or resident members of the community or ecosystem (Rejmánek & Simberloff, 2017)?
Invasion ecology has been struggling to position itself as a distinct field with a large array of hypotheses and generalizations, often redundant (Richardson & Pysek, 2008), and underpinned by the assumption that it has unique rules which are specific to NIS (Moles et al., 2012). Most of these hypotheses have centered on attempts at explaining the mechanisms underlying the success of NIS and their impacts on resident communities and ecosystems. Among the most frequently addressed are components of the evolutionary history, such as prey naïveté (Howard et al., 2017), novel weapons (Hazlett, 2000; Callaway & Ridenour, 2004), shifting defense (Doorduin & Vrieling, 2011), genetic bottlenecks—purging of genetic load (Facon et al., 2011; Moles et al., 2012), evolution of increased competitive ability (Kelly et al., 2009; Moles et al., 2012), and Darwin’s naturalization hypothesis (Ma et al., 2016; David et al., 2017).
Some of the most frequently explored ecological hypotheses are those related to community structure, including trophic level as an invasion filter (David et al., 2017), fluctuating resource availability (Davis, 2009; González et al., 2010), resource competition (González et al., 2010), biotic resistance-invasibility vs. recipient community diversity (Levine et al., 2004; Guo et al., 2015), phylogenetic, functional, or ecological originality (David et al., 2017), disturbance, stress and “invasional meltdown” (Simberloff & Von Holle, 1999), disturbed resource-flux invasion matrix (Colautti et al., 2006); and propagule pressure (Lockwood et al., 2005). However, evidence supporting these hypotheses are as common as evidence that does not (Jeschke et al., 2012), and the fact that “each can explain at least some impacts in some situations” (Ricciardi et al., 2013) effectively precludes generalizations and casts doubts on the uniqueness of ecological interactions where NIS are involved. The significance of these theories and the debate over the association between origin and impact goes far beyond academia (e.g., ecological theory), but has also major implications for policy, management and allocation of resources for NIS research and control initiatives (Boltovskoy et al., 2018).
In the last ~ 15 years meta-analyses covering tens to hundreds of case studies have been produced using standardized protocols and objectively contrasting traits of NIS and NAT species associated with differences in their fitness and performance, and the impacts of NIS on NAT organisms, communities, and environmental variables. Taking advantage of the increasing volume of data, we examined the results of 72 meta-analytical studies in an attempt to address the above issues, summarize their findings, and assess current consensus on these issues.
Our working hypothesis is that, if most results confirm that NIS behave differently from NAT, then origin matters and the mechanisms that take place upon introduction differ from those that govern biotic interactions in uninvaded communities. On the other hand, if results are largely mixed, then the significance of origin is relative, the effects of species introductions are largely context-dependent, and the search for general rules applicable to all or most introductions is unwarranted. We center our attention on the relative frequency of the cases where NIS were found to exhibit comparatively higher fitness and performance than NAT, and on the effects of NIS on NAT and resident species, communities and/or environmental conditions of the sites invaded. Further, on the basis of a large volume of primary data we analyze the factual support of many mainstream hypotheses in invasion biology, and discuss the implications of their influence on current trends in this field.
Materials and methods
Data selection
We searched the SCOPUS database on 16 March 2019 using the following combination of terms in the abstract, title and keywords: “invasive species” OR “non-indigenous species” OR “non indigenous species” OR “non-native species” OR “alien species” OR “non native species”, combined with “meta-analysis” OR “metaanalysis”, limiting the subject area to Agricultural and Biological Sciences, Environmental Science, Biochemistry, Genetics and Molecular Biology, Earth and Planetary Sciences, and Multidisciplinary, recovering 215 documents. The literature used in these documents was backsourced and cross-referenced. A few articles that appeared in the course of this work were also checked. In total, we identified 220 potential surveys, including 217 journal articles and three book chapters (Online Resource 1). Each publication was screened for suitability for our purpose according to the following criteria:
-
(1)
Partially or totally centered on differences between traits of NIS and NAT associated with their fitness and performance, or on evaluating the impacts of NIS on NAT or resident species, communities, ecosystems, or environmental traits;
-
(2)
Meta-analyses restricted to control methods for the eradication of NIS, or to the analysis of the traits of NAT communities that modulate NIS establishment, or on NIS traits that enhance invasiveness were excluded, unless explored in the context of explicit NIS–NAT trait differences or impacts;
-
(3)
Cultured species (e.g., reforestations involving massive replacements of native plants with alien species, crop and forage organisms, including plants, pigs, cattle), as well as dogs and cats, introduced across expansive geographic regions and different from other exotic species in their persistence mechanisms, were excluded, except when assessed along with other NIS (e.g., Oduor et al., 2010; Ferreira et al., 2016). However, the wild populations of some cultured species (e.g., common carp, brown trout) were included when comparing their characteristics in their home range vs. their invasive range (e.g., Rypel, 2013);
-
(4)
In those cases where the survey was not restricted to the issues above (e.g., Montero-Castaño & Vilà, 2012; Vellend et al., 2013; Romero et al., 2015; Ferreira et al., 2016), only data on NIS–NAT trait contrasts or impacts were considered in our analyses;
-
(5)
Used meta-analytical methods in the broad sense, “vote counting” approaches used in a few surveys were not excluded (Pyšek et al., 2012; Canavan et al., 2019);
-
(6)
Included a control (usually NAT species or uninvaded communities) and a treatment (usually NIS species or invaded communities), in either observational (field) or experimental (enclosures, glasshouses, pots, mesocosms, etc.) settings;
-
(7)
Included objective estimates of the contrasts analyzed (usually cumulative effect sizes).
This screening yielded 72 articles which were retained for further analysis (Online Resource 1). The remaining 148 works were excluded because they did not satisfy one or more of the above requirements, due to methodological problems and/or data inconsistencies, because all source data were superseded by a subsequent work, and/or other reasons (Online Resource 1).
Overview of the database
Of the 72 meta-analyses surveyed, 23 contrasted traits associated with the fitness and performance of species in their home range with their invasive range, or differences between NIS and NAT (or resident) species in the same area. The effects of NIS on NAT species or communities, or on environmental variables, were assessed by 49 meta-analyses involving NIS plants and animals (Table 1; Online Resources 1, 2). In a few cases, assignment of the survey to either of these two categories (traits or effects) was not unequivocal (e.g., Bunn et al., 2015), but such instances were very few, and the ambiguities involved do not affect our general conclusions.
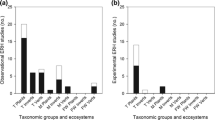
Study designs varied between surveys, but generally fell in one or more of four types for NIS–NAT trait differences, and eight for NIS impacts on NAT (Fig. 1). Comparisons were made using the “biogeographic approach” (trait differences or impacts of the same taxon in its native range vs. its invasive range), the “community approach” (NAT vs. NIS taxa in the invaded area), or both. Several, however, employed more elaborate designs, usually involving the comparison of a focal NAT in the presence of another NAT (conspecific and/or heterospecific), in the presence of a NIS, and then an assessment of the difference between the two situations. In these cases, the first two outcomes were coded as NA (not applicable), and only the difference NAT–NAT vs. NAT–NIS was assigned a value (e.g., Kuebbing & Nuñez, 2016). A few meta-analyses used more complex schemes, involving comparisons between NAT and invasive and non-invasive NIS, between invasive and non-invasive NIS, between NAT invasive elsewhere and NAT non-invasive elsewhere, etc. (Van Kleunen et al., 2010; Palacio-López & Gianoli, 2011). Because most meta-analyses did not differentiate invasive vs. non-invasive NIS, and the aim of our review was comparing traits and effects of NIS in general, invasive vs. non-invasive NIS were not differentiated, and point estimates (i.e., individual contrasts, usually effect sizes, within each meta-analysis) involving NIS only (e.g., invasive vs. non-invasive) were coded as not applicable (NA in Online Resource 3).
Schematic diagram of the general study designs used by the 72 meta-analyses surveyed comparing differences in the traits of native (NAT) and non-indigenous (NIS) species (Ta − Td), or the effects of NIS on NAT (Ea − Eg). Each meta-analysis used one or more of these designs. Point estimates where heterogeneity is explicitly indicated and significant (P < 0.05) are not included. Dark green: focal NAT species, pink hachured: NIS species (cartoons are not representative of the taxonomic identity of the organisms involved). In all cases contrasts are based on the direct or indirect evidence of the comparative response of NAT and NIS (traits), or the effects of NIS on NAT or resident organisms and/or environmental variables. Number of meta-analyses for Ea + Eb are those where some of the outcomes refer to impacts on resident organisms and environmental variables pooled. *Includes comparisons involving invasive and non-invasive NIS vs. NAT which are invasive elsewhere and NAT non-invasive elsewhere (Van Kleunen et al., 2010); **includes comparisons between NAT + non-invasive NIS vs. invasive NIS (Palacio-López & Gianoli, 2011); ***includes comparisons between uninvaded or lightly invaded sites vs. invaded sites (Ferlian et al., 2018), low and high NIS densities (Thomsen et al., 2016), sites with vs. without invasive NIS (Mollot et al., 2017), and fitness of NIS vs. NAT as hosts for resident species (Yoon & Read, 2016). See Online Resources 2 and 3 for details
Slightly over half (40) of the meta-analyses examined NIS plants, 16 investigated NIS animals, and 16 covered both plants and animals, often reporting results of pooled (i.e., plants + animals) data. In terms of habitats, terrestrial systems were analyzed in 32 works, 18 were centered on aquatic organisms (freshwater and/or marine), and 22 used data from both terrestrial and aquatic habitats (also often pooled). Freshwater and marine habitats were not treated separately because 17 meta-analyses presented outcomes for pooled data for freshwater, brackish, and/or marine habitats.
The attributes measured for assessing trait differences and impacts fell in six broad categories: abundance, species richness/diversity, fitness, environmental, other, and mixed (see Table 1 for details). Each meta-analysis measured one or more of these attributes.
Publication bias (chiefly, the under-reporting of non-significant results or disconfirming evidence) was formally checked in 42 of the surveys (funnel plots and trim and fill method, fail-safe numbers, Egger’s regression tests, correlation analyses, Fisher’s exact test, normal quantile plots, etc.). No publication bias was assumed to exist in 15 of these meta-analyses, 23 found moderate bias or bias restricted to some of the issues analyzed only, and 4 concluded that bias was present. Thirty meta-analyses did not check publication bias formally, but several of these cautioned that the data used were suspected to be biased (Online Resource 2).
Most (86%) meta-analyses used cumulative values of the effect size metric (log ratio, Hedges’ d) in order to assess NAT–NIS differences, and moderator-based analyses in random, fixed and/or mixed models. Differences and heterogeneity between effect sizes were most frequently analyzed with Q tests. Some, however, used different approaches, like ANOVA, t-tests, the proportions of significant negative and positive effects on the traits or processes analyzed with or without (two meta-analyses) further statistical testing (Online Resource 3).
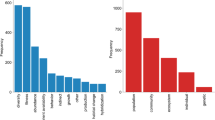
In total, 1,937 outcomes (point estimates: individual contrasts, usually based on the effect size metric) were collated (Fig. 2, Online Resource 3). Of these, 269 were not applicable; these usually only presented partial results comparing, for example, the performance of a focal NAT in isolation vs. its performance in the presence of a NAT neighbor, and in isolation vs. performance in the presence of a NIS neighbor; subsequently these were contrasted with an applicable estimate of the difference between the two situations. Outcomes with significant heterogeneity (i.e., those where results between studies varied widely suggesting that the cumulative effect size lumped dissimilar processes and responses, and whose biological meaning was therefore questionable) were reported in 142 cases comprising 7% of the total (note that not all meta-analyses estimated heterogeneity). The influence of various constraints (e.g., trophic levels, study-types, habitats, plant growth forms, etc.) on the outcomes was assessed in 36 meta-analyses; these results were tallied separately (Fig. 2).
Summary of all point estimates collated from the 72 meta-analyses surveyed. NIS–NAT T contrasts in fitness and performance between native (NAT) vs. non-indigenous (NIS) species, NIS–NAT E impacts of NIS on NAT/resident species, communities, ecosystems or the environment. Non-significant heterogeneity includes values where heterogeneity (total, between cases, and/or within cases) was non-significant, unapplicable or unspecified (heterogeneity was reported in 57% of the meta-analyses). Effects of NIS on the environment (e.g., alkalinity, chloride, pH, soil moisture, organic matter, Secchi disk depth, turbidity, sedimentation rates, suspended solids, fire regimes, etc.), largely context-dependent, are not assigned a value (positive or negative). NAT bacteria, fungi, microbes, microorganisms, decomposers are included in the environmental category. Constrains: influence of different constrains on the outcomes (e.g., trait differences or effects of NIS on NAT on island vs. mainland sites, type of study—experimental vs. observational, functional group, growth form of NIS and/or of the resident species compared; etc.). *Includes 6 outcomes of NIS on NIS effects relevant to subsequent estimates of NIS on NAT impacts. NS non-significant (P > 0.05), SIGN significant (P < 0.05). Bar graphs show relative values indicated in the adjacent boxes (absolute numbers of outcomes, in square brackets). See “Materials and methods” section for details
The 72 meta-analyses surveyed covered between 6 and 287 sources of primary data (sources used by Barrientos, 2015, were not provided by the author and could not be obtained) (Table 1, Online Resource 4). Over 97% of the sources were journal articles, the remaining 3% being books or book chapters, conference proceedings, reports, web sites, theses, and unpublished data. The degree of overlap between the sources used by different meta-analyses was generally low (overall mean < 1%). In only three (of 2,485) pairwise comparisons, the paired meta-analyses both shared > 30% of the sources of the other. However, shared sources as a proportion of those employed by one member of the pair were > 30% in 39 (of 2,485) pairwise comparisons. These overlaps are presented in detail in Online Resources 5 and 6 and discussed below.
Extraction and interpretation of the data
We analyzed the information summarized on the basis of two approaches: (1) the overall conclusions of each meta-analysis (Table 1, Online Resource 2), and (2) the outcomes of the individual comparisons (“outcomes” or “point estimates”) within and across meta-analyses (Online Resource 3).
Point estimates were collated and coded for significant (P < 0.05) and non-significant (P > 0.05) results, and, when provided, the heterogeneity of the corresponding result was noted (Online Resource 3). Codes were based on the design and the statistical approaches used by the different publications, usually cumulative effects sizes and Q tests, but occasionally correlation values, the Akaike Information Criterion, ANOVA, I2, Mann–Whitney, Z, τ2 and t-tests (Online Resource 3). Point estimates involving NIS only or NAT only were usually coded as not applicable (NA); in most of these cases subsequent comparisons evaluated differences between these interactions. However, following the rationale of this review, when the effects of NIS on other NIS were reported in the framework of NIS–NAT contrasts, the positive or null effects of NIS on other NIS were coded as negative (for NAT), whereas negative effects of NIS on other NIS were coded as positive (e.g., Oduor et al., 2010). Likewise, when the damage inflicted by NAT to NIS was stronger than that of NIS to NIS (e.g., Gonzalez-Browne et al., 2016), the outcome was coded as positive. Conversely, stronger impacts (e.g., consumption) of NIS on NAT than those of NAT on NIS were considered negative (Wood et al., 2017). Some trait differences or effects amenable to alternative interpretations were excluded from further analyses (NA). For example, Hawkes (2007) found that plants allocate more to reproduction in their introduced range than in the native range. Per se, this contrast does not necessarily imply that NIS perform better than NAT, because allocating more energy to reproduction may involve less energy for growth, competition, survival, etc. (the overall conclusion of Hawkes, 2007, was that evidence of NIS being more fit or performing better than NAT are mixed, and their competitive advantages fade with time).
Assessments based on overall meta-analysis results have the advantage of yielding a more holistic outlook, but they lack detail. On the other hand, those based on point estimates allow more in-depth analysis of the results, and the ability to tally them based on NIS and NAT type, attribute measured, and habitat. Their downside, however, is that the number of point estimates varied widely between studies, from 0 (in which case only the overall survey conclusion was used) to 123 (mean 18, excluding outcomes with significant heterogeneity). In addition, although point estimates where heterogeneity was tested and yielded significant values were excluded from our assessments, in some cases where heterogeneity should have been calculated it was not provided, which might have derived in some actually heterogeneous outcomes being misassigned to the non-heterogeneous category.
Codes were applied (and subsequently tallied) only to the point estimates explicitly presented in the meta-analyses, rather than to those that could potentially be derived from them. For example, Anton et al. (2019) concluded that in marine coastal habitats, the overall impacts of NIS on all resident species and environmental traits are negative and significant, but in island marine habitats they are not significant. These two outcomes were included in our compilation, but the probable ensuing result that the effects of NIS are different in island and mainland sites, not addressed explicitly, was not included. We also excluded a few results with overlapping 95% confidence intervals, but where no information on the significance of the difference between two cumulative effects sizes was provided. When the 95% confidence intervals of two cumulative effects sizes being contrasted do not overlap, one can safely assume that the two values are different at P < 0.05. However, if the confidence intervals do overlap, the corresponding cumulative effects sizes can differ significantly, or not.
In about half (38) of the meta-analyses, in addition to differences in NIS–NAT traits and NIS–NAT impacts, the same data were regrouped in order to assess changes in the results in response to various constraints (e.g., effects of NIS on island vs. mainland sites; type of study—experimental vs. observational; trophic position, functional group, growth form of NIS and/or of the resident species compared; etc.). When explicitly presented in the study, these results were coded denoting whether the different constraints did or did not change the result of the corresponding contrast. For example, in their analysis of the effects of NAT and NIS mutualists on plants, Aslan et al. (2012), concluded that when NIS and NAT mutualists belong to the same taxonomic group their seed dispersal effectiveness does not differ significantly, but for NIS and NAT from different groups the seed dispersal effectiveness of NIS is significantly lower. Thus, these point estimates contributed one score to the non-significant NIS–NAT effects (no difference between the seed dispersal of NIS and NAT when both belong to the same taxonomic group), one score to the significant negative impacts of NIS (NIS mutualists are significantly less effective than NAT mutualists when the two belong to different taxonomic groups), and also one score to the corresponding constraints category (taxonomic relatedness between NAT and NIS affects differences in their fitness as seed dispersers). The constraints in question were ascribed to 1 of 11 categories plus a general category pooling various miscellaneous conditions (see below and Online Resource 3).
We did not include those studies that did not center on NIS–NAT comparisons (e.g., Lamarque et al., 2011; Jeschke et al., 2012; Iacarella et al., 2015), and analyses of the explanatory variables associated with the results of the meta-analyses (e.g., Gioria et al., 2014; Ferlian et al., 2018) were usually not taken into account in this review, either. However, when the influence of explanatory variables was investigated alongside NIS–NAT contrasts in a meta-analytical context (e.g., Parker et al., 2013; Van Hengstum et al., 2014; Pintor & Byers, 2015), the corresponding results were included.
Data were extracted from tables, Appendices and/or Supplementary Materials. When unavailable from these sources, effect sizes and their confidence limits were extracted from digitized figures. Information for each point estimate from the 72 meta-analyses included the following: reference, study design (see Fig. 1), habitat, NIS and NAT involved, attribute measured, control, treatment and process assessed, statistical values (N, statistics for contrast and heterogeneity, when explicitly given), a verbal interpretation of the outcome, and its code (Online Resource 3). All point estimate codes were tallied and are presented herein as numerical counts and percentages.
For most attributes, the interpretation of the sign of the trait difference or impact (i.e., negative or positive for NAT) was fairly straightforward. We considered the outcome as negative when the diversity, abundance, reproductive success, production, etc. was lower in the presence of NIS than in their absence, or NIS performed better than NAT with respect to the trait investigated, or under the conditions examined (for example, damage by enemies) both NAT and NIS were negatively affected, but the magnitude of the impact was significantly larger for NAT than for NIS. On the other hand, the impacts of NIS on environmental traits (alkalinity, chloride, pH, etc.; see Table 1), which are not unequivocally associated with harm or benefit (Pyšek et al., 2012), were considered separately as context-dependent. Three meta-analyses included estimates of the effects of NIS on pooled NAT organisms and environmental attributes (Pyšek et al., 2012; Cameron et al., 2016; Anton et al., 2019); for the sake of simplicity, in most subsequent analyses these values (~ 3% of the total, see Fig. 2) were assigned to the NIS impacts on NAT organisms category (rather than to the effects of NIS on the environment).
A summary database was assembled with general information on each meta-analysis including the full reference, the type of process investigated (traits or effects), the NAT and NIS organisms involved, the total number of source publications used, assessment of publication bias, total number of cases analyzed, the overall conclusions of the meta-analysis, and a general assessment of whether the results support the concept that NIS possess or acquire traits that favor their competitiveness in the areas colonized or have negative effects on NAT (Table 1, Online Resource 2).
We also cross-checked all the references used in 71 meta-analyses (Online Resource 4; sources were unavailable for Barrientos, 2015) for shared sources of data (i.e., original investigations included in more than 1 meta-analysis) (Online Resource 5). Further, in these 2,485 between-meta-analyses comparisons we identified the ones where either member of the pair used ≥ 30% of the sources of the other member and evaluated the similarity between the issues assessed and the overall conclusions of each (Online Resource 6).
Analyses
Our review is based on a “vote counting” approach, which has been strongly criticized (Borenstein et al., 2009; Koricheva & Gurevitch, 2014), chiefly because vote counting does not take into account the heterogeneity involved and the magnitude of the effects. Heterogeneity was taken into account (when available), but the magnitude of the effect size was not. However, the practical meaning of the magnitude of the effect size is highly context-dependent, which precludes the use of rule of thumb cutoff values for defining “large”, “medium” and “small” effects (Durlak, 2010). Admittedly, statistical significance is not fail-proof either, because statistical significance does not necessarily mean biological significance, and the absence of significance might imply a nil effect or low study power (Borenstein et al., 2009; Ellis, 2010; Schirmel et al., 2016). Nevertheless, significance allows for a more objective assessment of the point estimates, and in many cases it was the only value informed.
The “vote counting” approach was chosen because the data collated are not independent for several reasons. First, because there is overlap between the source publications used by the 72 meta-analyses (Online Resources 5 and 6), implying that the same original results contributed to several of the surveys. Second, within each meta-analysis, many of the estimates were based on the same values regrouped on the basis of different contexts (NAT and NIS types, trophic levels, habitats, attributes measured, etc.), or different analytical approaches (e.g., Davidson et al., 2011). Further, some meta-analyses employed separate indicators for variables that are closely associated, and therefore are very likely significantly correlated (e.g., abundance, biomass and cover; species diversity and species richness). We explored the possibility of selecting the non-overlapping outcomes from each meta-analysis, but this process necessarily involved many subjective decisions, and was therefore rejected.
Independence of the data is a basic requirement for statistical analyses, chiefly because of biases associated with within-group agreement when data are not independent (Grawitch & Munz, 2004; Borenstein et al., 2009). Various techniques have been suggested to deal with this problem when comparing the results of meta-analyses. Multilevel logistic regression involves the assignment of nested levels to the outcomes and assessing statistical parameters within each level (Raudenbusch & Bryk, 2002; Barr, 2008). However, in our data the uppermost levels (the meta-analyses) are not independent (see above), and even if they were, definition of subsequent levels involves questionable decisions amenable to alternative interpretations. For example, in their assessment of the impacts of NIS vertebrate mutualists on resident plants, Aslan et al. (2012) assessed pollination, seed survival and seed germination separately. Germination clearly depends on survival, and pollination can be interpreted as yet another (higher) level of both. A multilevel approach is also hindered by the widely different processes and organisms used in the meta-analyses surveyed, which involve several hundred different combinations.
“Umbrella” or “systematic” reviews of meta-analyses (i.e., objectively pooling the results of several meta-analyses in a single study) require converting all results (e.g., odds ratios, correlations, standardized mean differences, etc.) into a common metric which allows comparison of the different values (Fusar-Poli & Radua, 2018; Allen & Walter, 2019; Zych et al., 2019). While in principle this is feasible for a fraction of the surveys used, this approach would have left out a large number of the results which informed the probability only, without specifying the value of the statistical indicator employed, as well as some that did not perform formal statistical evaluations (Pyšek et al., 2012; Canavan et al., 2019). Further, in order to meaningfully use these converted values they must all come from independent sources (i.e., the same original source data cannot be used two or more times and assessed with the same or different measures of effect size), a requirement that is not fulfilled in this survey. Using only the overall effect size of each meta-analysis only was not a viable option either because many surveys did not provide this estimate. Furthermore, when it was provided, the heterogeneity involved (when explicitly addressed) was very often highly significant implying that the outcome conveyed little value because it pooled significant and non-significant yields based on different organisms and attributes.
In short, with such limitations in the available data, application of statistical methods would only provide pretense of scientific rigor, rather than credible significance, and we therefore restricted our appraisals to assessing the overall trends (see “Discussion” section for other caveats involved). Similar approaches involving the analysis of multiple outcomes from literature data have been successfully used previously, both in some of the meta-analyses surveyed here (Pyšek et al., 2012; Canavan et al., 2019), and for summarizing NIS-related issues based on multiple sources (Jeschke et al., 2012; Dueñas et al., 2018).
Results
Trends based on overall meta-analysis conclusions
32 (44%) of the meta-analyses surveyed found that differences between the traits of NIS and NAT and the impacts of NIS on NAT are mixed and largely context-dependent. Overall support for the concept that NIS are more fit, perform better, or have significant effects on NAT was found in 22 (31%) meta-analyses, whereas the remaining 18 (25%) concluded that trait differences or significant effects are largely absent (Table 1; Fig. 3; see Online Resource 2 for details). Significant negative (for NAT) trait differences and effects were most common in terrestrial habitats (41%), and scarcest in studies where both aquatic and terrestrial habitats were included (17%) (Fig. 3). These values are based on the interpretation of the information summarized in the abstract and conclusions sections of the corresponding papers, which are almost always a balanced reflection of the actual data shown in tables, figures and appendices. However, in three cases (Montero-Castaño & Vilà, 2012; Gallardo et al., 2016; Ferlian et al., 2018) the abstract and/or conclusions suggested strong and consistent NIS–NAT trait differences or NIS impacts unsupported by the actual results of the meta-analysis, and were therefore ascribed to the “Results mixed” category (rather than to “NIS more fit than NAT or effects of NIS on NAT negative”, Fig. 3) (see Online Resource 2 for a detailed discussion of these results).
Overall conclusions of the 72 meta-analyses surveyed regarding whether or not the traits of non-indigenous (NIS)–native (NAT) species differ, or NIS have negative effects on NAT. NIS–NAT traits differences between traits of NIS and NAT or resident species associated with their fitness and performance, NIS effects on NAT: effects of NIS on NAT or resident species, communities, ecosystems or the environment. Numbers in square brackets denote numbers of meta-analyses. *Includes two meta-analyses where NIS were found to perform worse than NAT or have positive effects on the resident species (Pintor & Byers, 2015; Gonzalez-Browne et al., 2016)
Analyses of NIS–NAT trait differences and NIS impacts on NAT separately, and studies grouped by NIS type and habitat, showed generally similar patterns. In almost all cases mixed, context-dependent results accounted for around half (33–75%) of the studies. Significant (17–41%) and non-significant (0–35%) NIS–NAT trait differences or NIS impacts on NAT accounted for around one quarter each (Table 1; Fig. 3).
Meta-analyses with mixed overall results were more often based on higher numbers of primary sources (mean number of sources 86) than those that concluded NIS–NAT trait differences and NIS impacts are dominantly non-significant (mean 56) or dominantly significant (mean 66). However, these mean values did not differ statistically (ANOVA based on log-transformed values; F = 1.168, P = 0.317). Further, there also seems to be some marginal evidence that earlier surveys were more likely to conclude that NIS are more fit or have significant effects on NAT than more recent ones. When publication years are grouped by their overall conclusion (“Yes” + “Generally yes”, “Results mixed”, and “No” + “Generally no” in Table 1), the corresponding means are 2012.8, 2013.6, and 2014.9, which may suggest that overall support for the concept that NIS are more fit, perform better, or have negative effects on NAT has been declining in time. Thus far, however, these figures are not significantly different (ANOVA based on log-transformed values, F = 1.89, P = 0.159).
Trends based on point estimates
Of the 1,937 point estimates extracted from the 72 meta-analyses, 1,526 were used for our assessments. The remaining 411 were either not applicable or had significant heterogeneity (see above and Fig. 2).
Point estimates (pooled data for traits and impacts) grouped as a function of NIS and NAT type (Fig. 6), NIS and attribute measured (Fig. 7), and NIS and habitat (Fig. 8), showed large variations, but were generally in line with the results outlined above. Of the 42 combinations (bars in Figs. 6, 7, 8, excluding the overall summaries at the bottom of each figure), in 32 cases the proportions of non-significant trait differences or effects exceeded (29 cases) or equaled (3 cases) significant values (see below).
Point estimates grouped by meta-analysis
In agreement with the assessment of overall meta-analysis results (Fig. 3), assessments based on point estimates varied widely, from 100% of significant outcomes pointing at NIS higher fitness or negative effects on NAT/residents (albeit mostly based on few results, 1–5), to 100% non-significant (based on 1–21 results) (Figs. 4, 5). Overall, significant point estimates (positive and negative trait differences and impacts, including environmental effects; 43%) were less common than non-significant ones (57%), but significant negative values for NAT organisms (31%) were ~ 6 times more frequent than significant positive values (5%). Non-significant outcomes for environmental data and data where environmental variables and organisms were pooled (13%) were almost twice as common as significant outcomes (7%) (Figs. 4, 5).
Proportions of total point estimates per meta-analysis indicating non-significant (NS, P > 0.05) and significant (SIGN, P < 0.05) differences between fitness and performance of non-indigenous (NIS) and native (NAT) species. Sources are sorted in descending order of proportion of point estimates where NIS showed higher fitness than NAT. Figures in square brackets next to the reference denote total point estimates collated (in all cases point estimates where heterogeneity was explicitly addressed and significant, P < 0.05, are excluded); and whether the overall conclusion supports the concept that NIS are more fit or perform better than NAT (GN generally no, GY generally yes, M results mixed, N no, Y yes; see Table 1). *All (three) point estimates indicate non-significant NIS–NAT trait differences, but heterogeneity is significant
Proportions of total point estimates per meta-analysis indicating neutral, negative and positive impacts of non-indigenous (NIS) on native (NAT) or resident species (includes one work where effects on organisms and environmental traits were pooled), and effects of NIS on environmental variables. Sources are sorted in descending proportion of negative impacts on NAT. Square brackets next to the reference denote total point estimates collated (in all cases outcomes where heterogeneity was explicitly addressed and significant, P < 0.05, are excluded); and whether the overall conclusion supports the concept that NIS have negative effects on NAT (GN generally no, GY generally yes, M results mixed, N no, Y yes; see Table 1). NS non-significant (P > 0.05), SIGN significant (P < 0.05). *Analyses of NIS impacts including/excluding the invader from the community invaded
Across meta-analyses, NIS–NAT traits and NIS effects on NAT yielded generally similar proportions of significant and non-significant points estimates (traits: 48% non-significant, 52% significant; effects: 55% non-significant, 45% significant). Results pointing at higher fitness of NAT than NIS were few (3% for NIS plants, 13% for NIS animals), as were those with significant positive effects for NAT (5% of the total).
A salient feature of our exercise is the fact that more categorical overall conclusions, i.e., overall support, or the lack thereof, for the concept that NIS are more fit, perform better, or have negative effects on NAT (“Yes”, “Generally yes”, “No”, and “Generally no” in Table 1), were based on significantly lower numbers of point estimates than conclusions supporting the concept that NIS–NAT traits and the impacts of NIS on NAT are mixed and context-dependent (“Results mixed” in Table 1). For the former, the mean number of point estimates was 12.3, whereas for the latter it was 22.8. These values differ significantly (P = 0.023, Kruskal–Wallis test), suggesting that the more parameters are measured, the less consistent are the trait differences or impacts found. This result is in line with the fact that, across meta-analyses, higher numbers of point estimates were more likely to yield lower proportions of significant (positive, negative, or environmental) outcomes. The correlation between total point estimates and the proportion of significant values was negative and significant (Spearman’s ρ = − 0.356, P = 0.002).
Point estimates grouped by NIS type
As a function of NIS type (Fig. 6), NIS animals yielded somewhat higher proportions of significant outcomes (43%), than NIS plants (32%), and plants + animals (37%). NIS plants and NIS animals differed little in their negative effects on—or higher fitness than—resident plants and animals (40–44% of the point estimates). Positive effects were highest for NIS animals on microorganisms (20%) and plants (19%), but rare throughout (overall average 5%). Non-significant effects on environmental variables (mean 9%) were more common than significant ones (6%), with the exception of NIS plants + animals, where significant effects were 3 times more common than non-significant ones (Fig. 6).
Proportion of point estimates of the effects of non-indigenous (NIS) on native (NAT) or resident species and NIS–NAT trait differences separated by NIS type and NAT organism affected or recipient environment. Non-significant (NS, P > 0.05) and negative impacts of NIS on NAT or resident species include one meta-analysis where effects on organisms and environmental traits were pooled. Numbers in square brackets next to the reference denote total point estimates collated. Negative and positive refers to net effects or trait difference, or in relation to a comparable NAT trait or effect. Effects on the environment, which are context-dependent, are tallied separately. Microorganisms includes bacteria, fungi, microbes, decomposers. Only point estimates where heterogeneity is non-significant, unspecified or not applicable are included. NS non-significant (P > 0.05), SIGN significant (P < 0.05)
Point estimates grouped by attribute measured
Grouping the data across meta-analyses by attribute measured showed that in 13 (of 18) combinations non-significant point estimates outnumbered or equaled significant ones, but variations between combinations were large (Fig. 7). For example, the negative effects on the diversity of resident species were over 2–3 times more common for NIS plants (where they exceed the number of non-significant outcomes), than for NIS animals and NIS plants + animals. NIS animals, on the other hand, were much more frequently reported to have negative impacts on various miscellaneous traits (activity, aggression, foraging behavior, habitat use, etc., “Other” in Fig. 7), than plants and plants + animals. Non-significant effects of NIS plants or animals on environmental variables were about twice as common as significant effects; however, in studies that used pooled data for NIS plants and animals, significant effects on the environment were 3 times more common than non-significant effects.
Proportion of point estimates of non-indigenous (NIS)–native (NAT) trait differences and the effects of NIS on NAT or resident species, separated by NIS type and attribute measured (non-significant and negative impacts of NIS on NAT or resident species include one survey where effects on organisms and environmental traits were pooled). Numbers in square brackets next to the reference denote total point estimates collated. Negative and positive refer to net effects or in relation to a comparable NAT effect. Effects on the environment and those where responses of NAT and environmental variables are pooled, which are context-dependent, are tallied separately. Abundance includes biomass, cover and associated parameters. Diversity includes species or taxa richness and evenness. Environmental variables include alkalinity, ammonia, biogeochemical element pools, fluxes or ratios, chloride, decomposition, fire frequency and intensity, N2O and CH4 emission, organic matter, pH, pollution, sediment load, sedimentation rate, soil moisture, turbidity. Mixed includes combined effects on more than one of the following: abundance, biomass, production, population size, activity, decomposition, diversity, species richness, environmental traits, feeding activity, fitness and performance, foraging, habitat use, parasitism, physiology, reported impact, reproduction, survival. Other includes activity, aggression, basal respiration, enzyme activity, foraging behavior, habitat use, host preference, microbial activity, mineral and nutrient contents in tissue, parasitism, photosynthetic efficiency, production rate, decomposition rate, respiration rate, proportions of different feeding types, sex ratio, and similarity in specific composition. Only data where heterogeneity is non-significant, unspecified or not applicable are included. NS non-significant (P > 0.05), SIGN significant (P < 0.05)
Point estimates grouped by habitat
Data grouped by habitat (Fig. 8) showed that, on average, non-significant point estimates were ~ 1.3 times more common than significant ones. Higher proportions of significant than non-significant outcomes were only reported for NIS animals in terrestrial habitats, and NIS plants + animals on NAT plants + animals in aquatic habitats. In general, negative (for NAT) outcomes were more common in studies based on terrestrial and terrestrial + aquatic organisms (28–44%), than on aquatic ones (21–28%). In contrast, significant effects on environmental variables were more frequent in aquatic habitats (2–19%) than in terrestrial (0–3%) and terrestrial + aquatic (0–7%).
Proportion of point estimates of non-indigenous (NIS)–native (NAT) trait differences and the effects of NIS on NAT or resident species, separated by NIS type and habitat (non-significant and negative impacts of NIS on NAT or resident species include one meta-analysis where effects on organisms and environmental traits were pooled). Numbers in square brackets next to the reference denote total point estimates collated. Negative and positive refer to net effects or in relation to a comparable NAT effect. Effects on the environment and those where responses of NAT and environmental variables are pooled, which are context-dependent, are tallied separately. Only data where heterogeneity is non-significant, unspecified or not applicable are included. NS non-significant (P > 0.05), SIGN: significant (P < 0.05)
Changes in the results under different conditions
Thirty-eight meta-analyses estimated the influence of different constraints (different study durations, habitats, NAT/NIS trophic or functional levels, taxa or communities, etc.) on changes in the significance of the point estimates calculated (Fig. 9). Overall, non-significant point estimates were somewhat more common than significant ones (62%), but differences between constraints were high. Responses as a function of different study durations (ST in Fig. 9, based on only three outcomes), different trophic or functional levels, taxa or communities (AT in Fig. 9), and different habitats (HA in Fig. 9) showed more significant (52–67%) than non-significant values. Lowest proportions of significant outcomes (6%) were those of responses of NAT in insular vs. mainland sites (IM in Fig. 9).
Influence of various constraints on the difference in fitness and performance of non-indigenous (NIS) vs. native (NAT) or resident species, and the effects of NIS on NAT organisms or environmental variables. Numbers in square brackets next to the label denote total point estimates collated. Labels along the X-axis are: AT responses of/to different NAT/NIS trophic or functional levels, taxa or communities, GA responses of/to NAT/NIS in different geographic/biogeographic/climatic areas, GF responses of/to NAT/NIS of different growth forms (plants), HA responses of/to NAT/NIS in different habitats, IM responses of NAT/residents in insular vs. mainland sites, OF responses of/to NAT/NIS as a function of other factors (trait measured, treatment, NAT are NIS elsewhere vs. NAT are not NIS elsewhere, NIS origins, distance to nearest NIS and NIS distribution, floral color and similarity, clonality, N-fixation, life strategy and stage in plants, human land-use and disturbance, number of NIS single vs. multiple, NIS/NAT density, inclusion/exclusion of NIS in assessments of diversity/biomass of invaded community, etc.), PD responses of NAT/NIS to phylogenetic relatedness of the spp. compared, SA responses of NAT/NIS to different study-types (experimental/observational, exclosure/open, etc.), SM responses of NAT/residents to single/multiple NIS, ST responses of NAT/residents to different study durations, TI responses of NAT/residents to time since NIS introduction, US responses of NAT/NIS to different study spatial unit sizes or extents
Source overlaps
The 71 meta-analyses in our study (sources were unavailable for Barrientos, 2015) employed 4,822 unique primary sources of data (Table 1, Online Resource 4). Of these, 4,088 were used by only 1 meta-analysis, and 734 were used by 2 or more for a total of 5,203 sources.
On average, the overlap between the sources used was low (~ 1% of the sources used by any one meta-analysis were also used by any other), but in 39 (of 2,485 pairwise comparisons) the overlap was > 30% for either member of the pair. Source overlaps in excess of 30% for both members of the pair involved 20 meta-analyses. An overview of the 39 cases where the overlap was ≥ 30% for either member of the pair indicates that in 22 cases the issues addressed were different, and therefore duplication of results is unlikely. In the other 17 cases the issues addressed were similar or partially similar; in 12 of these the overall conclusions of the 2 meta-analyses were also similar, but in 5 cases they were generally different (Online Resource 6).
Discussion
Mixed and non-significant results are dominant
Our results indicate that the most frequent overall conclusion of the meta-analyses surveyed is that NIS–NAT trait differences and effects of NIS on NAT/resident species and the environment show mixed results or do not support the concept that NIS are more fit, perform better, or have negative impacts on NAT (69% for overall meta-analysis conclusions; Table 1; Fig. 3). However, the remaining third does support this tenet, and, most importantly, significant trait differences and impacts are largely dominated by results pointing at NAT lower fitness or NIS negative effects.
The general trend outlined above, however, showed some apparently contradictory results. For example, partial or total support for the concept that NIS are more fit or have negative effects on NAT ranked first in frequency for terrestrial habitats (41% of the meta-analyses), and second for aquatic ones (29%), but the proportion dropped to 17% when aquatic and terrestrial organisms were pooled (Fig. 3).
Assessments based on point estimates yielded 43% of significant outcomes, but when those referred to environmental variables and pooled organisms + environment (i.e., largely context-dependent, and therefore not univocally associated with harm or benefit) were excluded, this value dropped to 37%, of which 32% were negative for NAT, and only 5% were positive. Non-significant impacts on the environment and on organisms + environment were also more common than significant impacts.
In summary, point estimates showed large variations (Figs. 4, 5, 6, 7, 8), but the overall trends indicate that non-significant point estimates outnumber significant ones (by ~ 1.3 times for organisms, and ~ 1.5 times for environmental variables), and negative significant NIS–NAT trait differences and NIS effects on NAT are ~ 6 times more common than positive ones. Significant environmental effects were over twice as common when NIS plants and animals were considered jointly, than when they were assessed separately (Figs. 6, 7). The reduction in the diversity of resident species was particularly common for NIS plants, but substantially less frequent for NIS animals and plants + animals, whereas fitness was more often affected by NIS plants + animals than by NIS plants or NIS animals (Fig. 7). All NIS had generally fewer negative impacts in aquatic habitats than in terrestrial and terrestrial + aquatic (Fig. 8).
Given the fact that the meta-analyses surveyed are likely based chiefly on successful invasive NIS (see below), the dominance of non-significant trait differences and impacts is surprising. Even such notorious invasives as the freshwater zebra and quagga mussels (Dreissena polymorpha, D. rostriformis bugensis), ecosystem engineering organisms, crabs and crayfish, and various invasive plants yielded fewer or roughly similar numbers of non-significant and significant point estimates (Higgins & Vander Zanden, 2010; Parker et al., 2013; Leffler et al., 2014; James et al., 2015; Maggi et al., 2015; Howard et al., 2017; Guy-Haim et al., 2018, see also Devanna et al., 2011; Emery-Butcher et al., 2020; Figs. 4, 5). The few studies that specifically addressed trait or impact differences between invasive and non-invasive NIS found mixed results (Van Kleunen et al., 2010; Mollot et al., 2017), or no evidence (Palacio-López & Gianoli, 2011) of consistent dissimilarities. On the other hand, studies that examined NIS as a resource for NAT, or the participation of NIS in mutualistic interactions or indirect relationships, often unveiled positive effects (Higgins & Vander Zanden, 2010; Aslan et al., 2012; Thomsen et al., 2014; Bunn et al., 2015; James et al., 2015; Pintor & Byers, 2015; Gallardo et al., 2016; Howard et al., 2017; Ferlian et al., 2018; Zhang et al., 2019).
Origin, impact, study design, and range-expanding species
The issue of the impact of NIS can be viewed from two different perspectives: (A) Did the presence of the NIS change significantly any of the variables measured with respect to the situation prior to its arrival or in its absence? (Fig. 1Ea, Eb). And (B) Are the impacts of NIS stronger than those of equivalent NAT species? Or, alternatively, are they stronger in the NIS invasive range than in its native range? (Fig. 1Ec − Eg). Although both questions are linked, they address subtly different issues. The first situation is rather straightforward, but it involves the addition of a new species, which by default MUST have some consequences for the residents insofar as all species take space, feed and/or are fed upon, and compete for resources via direct or indirect interactions (Thomsen et al., 2015; Guiaşu, 2016). The question therefore addresses a general ecological issue, rather than the association between non-nativeness and impact.
Situation (B), on the other hand, is more specific as it effectively compares the relative performance or the impacts of NIS vs. those of NAT. As noticed by Leffler et al. (2014), “…differences in trait values [or impacts] between a native and exotic invasive species must be greater than differences [or impacts] between co-occurring natives for this difference to be ecologically meaningful…” (see also Phillips et al., 2010; Parker et al., 2013; David et al., 2017; Dickey et al., 2020). In fact, several of the major hypotheses proposed to explain the success of NIS (enemy release, evolution of increased competitive ability, empty niche, and novel weapons; see below) require an explicit comparison between native and introduced ranges (Hierro et al., 2005), or a survey design that takes this distinction into account. Ideally, in order to investigate impact as a function of origin, one should replace a NAT with a functionally identical NIS organism. This involves comparing the behavior of a species or community without and with the NAT assumed to influence it, without and with the equivalent NIS, and then assessing the difference between the two situations. This approach was attempted in a few cases (e.g., Parker et al., 2006; Salo et al., 2007; Paolucci et al., 2013; Pintor & Byers, 2015; Howard et al., 2017), but interpretations of potential differences are faced with the major problem of identifying the functionally equivalent pairs of species, and the ecologically equivalent ecosystems. In practice, most meta-analyses centered on the impacts of NIS on NAT addressed the question of impact by comparing uninvaded vs. invaded sites (Fig. 1Ea, Eb), which, from the perspective of the tenet that human-mediated origin is a relevant determinant of impact, is questionable. Indeed, if the focal NIS had arrived by its own means (rather than transported by man), would it have had different effects? In other words, does the fact that the newcomer is a NIS, rather than a natural range expansion, make a significant difference in its effects on the habitat or ecosystem investigated?
The answer to this question is elusive because most surveys that addressed the effects of biological range expansions (usually in latitude or altitude/depth; Lenoir & Svenning, 2015) framed the problem in the context of global warming, which is also a human-mediated effect. As such, although some of the processes involved differ (Essl et al., 2019), it may be interpreted as akin to biological introductions (Webber & Scott, 2012). However, as with biological invasions, the opinions on the parallels between introductions and range expansions, as well as their impacts on the native biota and the environment, differ widely. Several surveys argued that range expansions are not different from introductions, and that their impacts are also similar (Engelkes et al., 2008; Sorte et al., 2010; Hoffmann & Courchamp, 2016; Nackley et al., 2017; Pecl et al., 2017), while others considered them fundamentally different from both invasions (Wilson et al., 2016), and from “pure demographic expansions” (Excoffier et al., 2009). Further, some authors suggested that range expansions are a natural biological response to shifting environmental conditions, and should not be treated as potentially hazardous events (Davis & Watson, 2018), and even proposed human-assisted translocation of species endangered by climate change and habitat degradation (Hoegh-Guldberg et al., 2008).
Comparison of assessments of NIS effects based on the uninvaded vs. invaded site scenario (Ea in Fig. 1), with those based on more complex study designs (Ec − Eg in Fig. 1), showed some notable differences. For the uninvaded-invaded study design, overall meta-analysis conclusions point at non-significant effects in 17% of the surveys, and at mixed effects in 50%, whereas for the more elaborate designs (Ec − Eg in Fig. 1) non-significant effects were found in 40% of the meta-analyses, and mixed results in only 10%. This is in agreement with the expectation that under the uninvaded/invaded scenario fewer cases will show no effects of NIS on the residents (17%), but these effects are not necessarily unidirectional, being negative for some organisms and attributes, and positive for others (i.e., largely mixed, 50%). In contrast, when comparisons are more strictly constrained to pinpoint the effects of NIS vs. those of NAT on a focal NAT, mixed results are few (10%), but non-significant differences between the effects of NIS vs. NAT on a focal NAT are much more common (40%).
Invasion biology hypotheses
Over the last two decades, growing numbers of hypotheses and generalizations in the area of invasion biology have been proposed, including those attempting to explain invasiveness (i.e., species traits that favor their transport, establishment and subsequent dominance), and those centered on invasibility (i.e., the vulnerability of communities or ecosystems to invasions) (see reviews in Alpert et al., 2000; Hierro et al., 2005; Sax et al., 2005; Catford et al., 2009; Lamarque et al., 2011; Crystal-Ornelas & Lockwood, 2020a, b). Many of the meta-analyses surveyed are related with one or more of these hypotheses; although empirical support for most of them exists, findings differ widely among studies in line with the conclusions outlined above.
The evolution of increased competitive ability (EICA) hypothesis (Blossey & Nötzold, 1995), as well as several closely related concepts which postulate that invaders have or acquire superior competitive traits (phenotypic plasticity, novel weapons, shifting defense), were supported by four meta-analyses (Cavaleri & Sack, 2010; Van Kleunen et al., 2010; Arceo-Gómez & Ashman, 2016; Golivets & Wallin, 2018), rejected by six (Davidson et al., 2011; Palacio-López & Gianoli, 2011; Felker-Quinn et al., 2013; Parker et al., 2013; Leffler et al., 2014; Charlebois & Sargent, 2017), and yielded partial support or mixed or inconclusive results in five (Doorduin & Vrieling, 2011; Lamarque et al., 2011; Rypel, 2013; Kelley, 2014; Jauni & Ramula, 2015).
The enemy release hypothesis (NIS thrive in invaded areas because they lack coevolved enemies in their new ranges), was confirmed by Torchin et al. (2003), Liu & Stiling (2006), and Ebbs et al. (2018), but rejected by five surveys (Parker et al., 2006; Chun et al., 2010; Oduor et al., 2010; Pintor & Byers, 2015; Gonzalez-Browne et al., 2016), and two meta-analyses found mixed results (Hawkes, 2007; Meijer et al., 2016).
Prey naïveté (NAT prey do not recognize NIS consumers as being dangerous because of a lack of co-evolutionary history) was supported by Salo et al. (2007), and partially supported by Paolucci et al. (2013), but rejected by Pintor & Byers (2015) and Howard et al. (2017), who noticed that, conversely, NIS consumers might not perceive NAT prey as being edible (i.e., predator naïveté; see David et al., 2017).
The empty niche and biotic resistance hypothesis, often referred to as the diversity-invasibility hypothesis (Davis, 2009), suggests that species-poor communities are more invasible than species-rich ones because they have more empty niches receptive to NIS, which thus benefit from resources unused by the locals. This relationship, originally proposed by Elton (1958), has been the subject of numerous surveys and even opposed interpretations of the invasiveness-related effects of native species diversity. Thus, some authors proposed that more diverse communities are more resilient to invasions because their niches are more saturated (Gerhold et al., 2011; Gallien & Carboni, 2017), whereas others suggested that more diverse communities are more invasible because their current status is the result of multiple previous natural introductions (Stohlgren et al., 2003; Mizrahi et al., 2017). Three meta-analyses generally or partly confirmed this relationship (Parker et al., 2006; Korsu et al., 2010; Oduor et al., 2010), but two others did not (Leffler et al., 2014; Golivets & Wallin, 2018). Peng et al. (2019), in an exhaustive analysis of 101 publications, concluded that mean plant native-exotic richness relationships were not negative at any grain size, and that different spatial extents showed no clear patterns.
Invasional meltdown (Simberloff & Von Holle, 1999), a catchy term to describe that synergistic interactions among invaders facilitate further invasions, was recognized in two meta-analyses (Parker et al., 2006; Kuebbing & Nuñez, 2016), not found in two (Bunn et al., 2015; Cameron et al., 2016), and yielded mixed results in one (Oduor et al., 2010) (see also Devanna et al., 2011).
Because trophic relationships or, more generally, the availability of resources, are among the strongest ecological limiting factors, they have been the focus of many empirical and theoretical studies on biological invasions, and they underpin several of the hypotheses proposed (see David et al., 2017 for a review). In this context, it has been suggested that NIS impacts are highest and negative within a trophic level, but variable (and often positive) at higher trophic levels (Thomsen et al., 2014). The rationale behind this theory is quite straightforward: at the same trophic level NIS compete for resources, but themselves constitute a resource for higher trophic levels. This relationship was confirmed by some of the meta-analyses surveyed (Maggi et al., 2015; Pintor & Byers, 2015; Thomsen et al., 2016), and mixed results were found by others (Cameron et al., 2016), but the ensuing conclusion that NIS plants have more positive (and less negative) effects on NAT animals than on NAT plants does not seem to hold across studies. The negative impacts of NIS plants on NAT plants (40%), and NAT animals (44%) are very similar to those of NIS animals on NAT plants (42%) and NAT animals (41%) (Fig. 6). Thus, trophic links seem to be a major driver in some situations, but are often overridden by other variables (Penk et al., 2015).
The widely held concept that the impacts of NIS are more detrimental on islands than in continents was partially confirmed by only 1 (Vilà et al., 2011) of 4 meta-analyses that addressed this issue explicitly (Vilà et al., 2011; Paolucci et al., 2013; Qiu, 2015; Cameron et al., 2016; 1 of 16 point estimates, Fig. 9).
The theories and generalizations expressed in the invasion biology hypotheses discussed above are highly controversial (Sher & Hyatt, 1999; Colautti et al., 2006; Gerhold et al., 2011; Moles et al., 2012; David et al., 2017; Gallien & Carboni, 2017), and contain “much duplication, redundancy and reinventing the wheel” (Richardson & Pysek, 2008). Although one should not expect that any one explanation will fit all invasions (Catford et al., 2009; Ricciardi et al., 2013), these discrepancies with respect to basic tenets of the theory of biological invasions are discouraging (Moles et al., 2012). With the exception of the null “propagule pressure” hypothesis (Colautti et al., 2006; Cassey et al., 2018), which per se is rather truistic (i.e., it is obvious that large numbers of individuals and many attempts at introducing an alien species are more likely to end up in success than few attempts and few individuals), most experimental and observational studies are at least as likely to reject as to support these popular invasion biology hypotheses.
Moreover, support for these leading hypotheses has been declining over time (Jeschke et al., 2012), which may be partly explained by the underpublication of null results in the early years after a hypothesis was proposed (Mueck, 2013), and especially by the fact that the growth of empirical knowledge leads to a growing recognition of complexity and ambiguity (Davis & Chew, 2017), defying the strict bounds imposed by these attempts at establishing universal cause–effect relationships. As noticed by Hulme et al. (2013), “the more studies that are undertaken on the impacts of a particular species, the smaller the proportion of significant results found and the larger the likelihood of these being of a different sign” (see also Crystal-Ornelas & Lockwood, 2020a, b). Morales & Traveset (2009), based on 40 studies, found an overall significant negative effect of pollinator NIS on visitation rates to NAT focals, but Charlebois & Sargent (2017), using the same data expanded to 76 studies, concluded that alien/native status does not predict the outcome of pollinator-mediated interactions. This disagreement was ascribed to publication bias and experimental design, but the fact that Charlebois & Sargent (2017) doubled the number of primary sources likely also played a major role.
Several authors proposed more holistic approaches in order to reconcile discrepancies (Barney & Whitlow, 2008; Catford et al., 2009; Gurevitch et al., 2011), identify origin-related threats (Parker et al., 1999; Peterson & Vieglais, 2001; Thiele et al., 2010; Buckley et al., 2016; Gallien & Carboni, 2017), and quantify impacts (Dick et al., 2017; Bacher et al., 2018), but the implementation of those holistic approaches is hindered by the fact that most of the intervening variables that ultimately determine the success or failure of NIS, as well as their overall impacts, are unknown, and their context-dependency eludes generalizations. Thus, our results support the concept that “attempts to find traits characterizing efficient invaders in general… are bound to fail” (David et al., 2017; see also Dawson et al., 2012; Leffler et al., 2014), as are attempts to pinpoint widely applicable recipient system traits that favor invasibility. Moreover, with few notable exceptions where one or a few impacts overwhelm all others, determining the overall impacts of a NIS, even in a particular area and period of time, is usually an elusive goal (see below).
Limitations and caveats
Aside from the limitations outlined in the “Materials and methods” section, several issues may have affected our results. Some of these refer to investigations on NIS in general, while several others concern our approach and results in particular.
Although meta-analyses are widely recognized as a robust, objective method, they are not error-proof, and as most other approaches at synthesizing published information, meta-analyses based on the same or largely overlapping primary sources can lead to widely different conclusions. Some examples of such inconsistencies detected in this survey are shown in Online Resource 6, and the literature on biological invasions includes several striking examples (e.g., Morales & Traveset, 2009; Charlebois & Sargent, 2017, see above; Lortie & Callaway, 2005; Maestre et al., 2005). Aside from analytical artifacts, the choice of variables used and the complexity of the interactions involved likely play a major role in these conflicting results (Hulme et al., 2013).
Publication bias is among the most important issues in the source publications, in the meta-analyses based on them, and consequently in our results (Koricheva & Gurevitch, 2014). Around half of the meta-analyses surveyed ran formal checks of publication bias, and most of these concluded that bias was absent or minor (Online Resource 2). However, most investigations of publication bias are primarily designed to detect suspect relationships between effect size and sample size, information gaps and potentially missing studies (funnel plots, trim and fill method, fail-safe numbers) (Jennions et al., 2013; Koricheva et al., 2013). In contrast, those derived from the fact that a large proportion of the primary studies are based on the most damaging of exotic species, rather than on a random selection of NIS (as noticed in several of the meta-analyses: Hawkes, 2007; Gaertner et al., 2009; Thomsen et al., 2009; Van Kleunen et al., 2010; Lamarque et al., 2011; Aslan et al., 2012; Pyšek et al., 2012; Parker et al., 2013; Gioria et al., 2014; Thomsen et al., 2014; James et al., 2015; Gallardo et al., 2016; Thomsen et al., 2016; Charlebois & Sargent, 2017; Howard et al., 2017; Mcknight et al., 2017; Golivets & Wallin, 2018; Anton et al., 2019; see also Moles et al., 2012; Hulme et al., 2013; David et al., 2017; Guerin et al., 2018), are not objectively addressed. In fact, when attempting to assess trait differences and impacts of NIS in general (rather than those of invasive NIS, i.e., the species that reach high densities in the habitats invaded and are therefore more likely to have strong effects on the resident biota and the environment: Richardson et al., 2000; Hulme et al., 2013), this source of bias is probably the most important because invasive NIS are investigated far more often than non-invasive NIS (Radville et al., 2014; Guerin et al., 2018), a bias which is subsequently reflected in the meta-analyses based on the former. Even meta-analyses that strived to distinguish invasive from non-invasive NIS (Van Kleunen et al., 2010; Palacio-López & Gianoli, 2011; Kuebbing & Nuñez, 2016; Mollot et al., 2017) are unlikely to have circumvented this problem totally (Guerin et al., 2018), which suggests that the conclusions of the present review may overestimate the impacts of NIS in general (and, conversely, underestimate those of invasive NIS).
In our survey, separating results based on invasive and non-invasive NIS was not a viable option for two reasons. First, despite the many efforts at unifying terminology used in invasion biology, and especially at differentiating the two types of NIS (invasive vs. non-invasive) (Richardson et al., 2000; Colautti & MacIsaac, 2004; Russell & Blackburn, 2017a), no general consensus has been reached (Larson, 2007; Hodges, 2008; Guiaşu, 2016; David et al., 2017; Sagoff, 2018). Most scholars use “invasive” rather indiscriminately for any NIS (Pereyra, 2016; Boltovskoy et al., 2018; Mattingly et al., 2020), and there is no clear boundary between non-invasive and invasive, but a continuum where only the extremes can be labeled with certain confidence (based on abundance, impact, or both), whereas most NIS fall somewhere in between or behave differently throughout their invasive ranges. Second, most of the meta-analyses surveyed are based on results where invasive and non-invasive NIS were pooled. Very few addressed differences between what they interpreted as invasive and non-invasive NIS, and these arrived at dissimilar conclusions (see above).
In many observational studies, when the effects of one or a few NIS species are assessed, the results of numerical analyses usually do not take into account whether the focal NIS is the only organism that can conceivably be responsible for the differences between invaded and uninvaded sites or before/after invasion scenarios, or if these differences might respond to other NIS species that invaded the study sites in the same period, or to various other factors (e.g., human-induced changes) that may have affected the observations (Crystal-Ornelas & Lockwood, 2020a, b). For example, Higgins & Vander Zanden (2010), based on publications appeared between 1993 and 2008, evaluated the impacts of dreissenid mussels on several abiotic and biotic traits of invaded freshwater ecosystems, chiefly based on before–after invasion data from the Great Lakes (USA–Canada). However, between the 1800s and the 1990s the Great Lakes received 139 NIS (Mills et al., 1993), and 24 were established after 1993 (Sturtevant et al., 2019), many of them with reportedly important impacts (Ricciardi & MacIsaac, 2000), and the lakes were subject to considerable human-derived stress (Smith et al., 2019). The fact that the impacts reviewed are mostly based on a NIS species-by-species basis, rather than on whole ecosystem analyses, might also have obscured consistency in potential overall trends.
An important caveat of most studies dealing with the impacts of NIS, especially when measuring abundance or biomass, is whether the invader itself is included or not in the experimental (or after invasion) scenario. Some of the few surveys that analyzed the two situations found that part of the negative effects of the NIS are canceled out, and some positive effects are reinforced, when the contribution of the invader itself is included in the estimates of total standing stock and total community richness (Higgins & Vander Zanden, 2010; Thomsen et al., 2015), suggesting that invaders may substitute, rather than decrease, total biodiversity and standing stocks (Zwerschke et al., 2020).
Our analyses did not consider the magnitude of the trait differences or impacts reported (see “Materials and methods” section), and neither did we attempt to weigh the ecological importance of the contrasts, many of which are clearly different. However, their weighting would have involved highly subjective decisions. For example, in the mineral soil layer NIS earthworms increase the biomass of microbial soil biota, but in the organic soil layer they decrease it (Ferlian et al., 2018). Assigning different weights to these two results, and especially making the weighting differences reasonably proportional across studies, was not a feasible option (which, incidentally, also precludes the use of the magnitude of the effects sizes as a meaningful and consistent indicator of impact, see “Materials and methods” section).
As noticed above, when the abundance, diversity, performance, etc. of NAT were significantly lower in the presence of—or as compared with—NIS, the outcome was considered as negative, and conversely, positive outcomes were those where the result was the opposite. While this is the criterion adopted in most primary research papers and in the meta-analyses based on them, from an ecological perspective, and especially from the point of view of ecosystem services, it is a rather simplistic and potentially misguided approach, because it does not take into account whether the NAT or resident species impacted is valuable and worth preserving, or if it is a pest or nuisance organism. For example, in our analyses the reduction of phytoplankton by the zebra mussel (D. polymorpha) was included as a negative impact of NIS (Higgins & Vander Zanden, 2010). However, according to this meta-analysis, Cyanobacteria are the second most impacted taxon by the mussel, and given the fact that the impacts of toxic strains of Cyanobacteria (in particular Microcystis spp.) on other organisms, ecosystem services, and human health are invariably and often strongly deleterious (Carmichael, 1994; Merel et al., 2013), their reduction is likely a positive effect. Further, most studies analyzed the impacts of selected NIS species on resident species in general, which likely included some NIS, and therefore may have obscured interpretations of NIS–NIS interactions.
Finally, the literature in general, and most of the meta-analyses surveyed, show a strong geographical bias, with a great majority of the data coming from temperate regions of North America and Europe, whereas South America, Africa, Asia, and to a lesser degree Australia, are underrepresented (Hulme et al., 2013).
Despite these shortcomings, we contend that a comprehensive, systematic overview of the meta-analytical literature, even if unsupported by statistical testing, yields interesting insights into our current understanding of NIS–NAT trait differences and the impacts of NIS on NAT/resident species and on the environment. A major strength of this exercise is the number of primary sources covered by the meta-analyses, and particularly the fact that their selection was not based on criteria geared to support or reject either of the ideas prevailing in the field (i.e., that NIS are more fit than NAT and their impacts are negative, or that their trait differences and interactions are largely context-dependent; see Boltovskoy et al., 2018 and references therein).
Concluding remarks and future perspectives
It took three decades after Elton (1958) published his seminal book on biological invasions for this field to attract the attention of ecologists, but since the late 1980s the number of publications started increasing exponentially (Ricciardi & MacIsaac, 2008; Canning-Clode, 2015), with ~ 30,000 surveys having appeared since 1990 (Boltovskoy et al., 2018). The growing volume of information fostered efforts at establishing widely applicable rules and principles, but their success has been very limited. Every new hypothesis is supported by some empirical evidence, but subsequent endorsement is usually very heterogeneous, suggesting that impacts and trait differences are highly case-specific, depending on, among others, study spatial and temporal scales, phenotypic and genotypic variation, NIS densities, taxonomic and functional positions, methods, and locations (Hulme et al., 2013; Fig. 9).
Invasion biology is a comparatively young field of research, which might suggest that it is still in the early stages of gathering the information necessary for tuning and self-correcting its paradigms. However, disagreements are not restricted to NIS–NAT trait differences, NIS impacts, and drivers of invasiveness and invasibility, but affect such elementary concepts as “native”, “naturalized”, “introduced” and “invasive” (Richardson et al., 2000; Colautti & MacIsaac, 2004; Larson, 2007; Hodges, 2008; Thompson, 2014; Crees & Turvey, 2015; Pereyra, 2016; Russell & Blackburn, 2017a; Pereyra, 2020; Pereyra & Guiaşu, 2020), and particularly the assessment of the environmental and societal impacts of NIS (Richardson et al., 2000; Jeschke et al., 2014; Kamenova et al., 2017), which has been strongly associated with idiosyncratically opposed stands (Davis, 2006). This conflict (i.e., origin per se is or is not an attribute significantly associated with the odds of NIS becoming harmful for the recipient communities) was vividly illustrated a decade ago by the M. Davis vs. D. Simberloff debate (Davis et al., 2011; Simberloff et al., 2011), and has been resurfacing periodically in increasingly heated and often hostile discussions (see Richardson & Ricciardi, 2013; Russell & Blackburn, 2017a, b; Ricciardi & Ryan, 2018, and the rebuttals of Briggs, 2017; Crowley et al., 2017; Davis & Chew, 2017; Tassin et al., 2017; Boltovskoy et al., 2018; Guiaşu & Tindale, 2018; Sagoff, 2018; Guerin, 2019; Munro et al., 2019).
Interestingly, in ecology such disagreements are not restricted to young disciplines, but are also common in areas researched for tens to hundreds of years. For example, the long-standing assumption that biodiversity is decreasing worldwide due to human-mediated actions has been challenged by a detailed analysis of > 16,000 vegetation plots showing that temporal change in mean species diversity over periods of 5–261 years is not different from zero (Vellend et al., 2013). Latitudinal gradients in species richness have been explored since the nineteenth century in terrestrial, freshwater and marine habitats, but despite the massive volume of information available, debates are ongoing not only on the drivers that shape this relationship (including null models, such as the mid-domain effect, akin to the propagule pressure null model, see above) but also on the nature, shape, and strength of the patterns themselves (Hillebrand, 2004; Brayard et al., 2005; Tittensor et al., 2010; Kinlock et al., 2018). As with biological invasions, the likely explanation is that there is no unique explanation (Willig et al., 2003; Boltovskoy & Correa, 2017; Grady et al., 2019). Simberloff (2006) remarked that since ecology is fundamentally an idiographic science, general laws are likely to be obtained only at very large scales. Our results, however, suggest that large scales are precisely the ones which elude general rules the most. Far from fostering convergence and agreement, the growing volume of information seems to be fueling conflicting points of view (Boltovskoy et al., 2018), most probably because the inherent complexity of biological invasions restricts predictions to particular species-by-habitat interactions (Colautti et al., 2006; Davis, 2009; Moles et al., 2012).
Very few, if any, scholars deny that some NIS can and do cause extensive damage, and also most recognize the benefits conferred by some NIS (Simberloff, 2006; Thomas, 2013; Guiaşu, 2016; Ramus et al., 2017), yet we agree with David et al. (2017) that an apocalyptic vision of the impacts of invasions pervades the literature and plagues boilerplate introductory statements in journal articles such as “ecosystems are dominated by introduced species, leading to loss of biodiversity and ecosystem function” (Davis, 2009; Thompson, 2014; Warren et al., 2017; Sagoff, 2018). The fact that the purported impacts of invasive species often lack support is illustrated by a recent study of Dueñas et al. (2018). The authors mined the data on which the endangered and threatened status of the 1,363 species protected under the United States Endangered Species Act is based. They only discovered scientific evidence for 116 (8.5%) of the species, and only 6% had been found to be negatively impacted by invasive species. Our results indicate that impacts are mixed or nil in most cases, and are sometimes beneficial for the recipient communities. However, among the significant results deleterious effects and lower NAT than NIS fitness prevail. A major uncertainty in the studies with significant negative results is the extent to which they are driven by the fact that the most successful, abundant, and widespread NIS are overrepresented in the literature. Obviously, these cases are usually more relevant in terms of ecological and economic impacts. Due to their conspicuousness and importance, the species involved provide more appealing research targets, and they are usually easier to spot and investigate. However, this circumstance hampers our ability to assess the traits and impacts of NIS in general, and it fuels a lopsided outlook on the problem of biological invasions as a whole.
The implications of this bias go far beyond academia, as they are the foundation on which management actions rely. The economic costs of invasions, including direct costs and loss of ecosystem services, are an area of particular concern, as well as of a striking dearth of information. Few precise estimates have been produced, mostly local or regional in scale and restricted to one or a few species, but they are often extrapolated to introduced species in general. For example, Walsh et al. (2016), estimated the economic loss associated with reduced water clarity of Lake Mendota (USA) due to the invasion by the cladoceran Bythotrephes longimanus, arguing that “such extreme cases of economic damages call for increased investment in the prevention and control of invasive species”. However, in 2015 another invader of widespread distribution in the Great Lakes was discovered in Lake Mendota, the zebra mussel (D. polymorpha) (Hayranto, 2018), which had been predicted to significantly increase water clarity in the lake (Reed-Andersen et al., 2000), as this and other functionally similar invasive mussels (e.g., Limnoperna fortunei) did in many freshwater bodies (Boltovskoy et al., 2009; Higgins & Vander Zanden, 2010).
The most highly cited effort at producing a comprehensive assessment of the economic losses caused by biological introductions (Pimentel, 2002; Pimentel et al., 2005) has suggested staggering numbers, but these estimates were strongly criticized for their one-sided approach (e.g., the omission of the benefits of some NIS, such as crops, domesticated animals, biocontrol organisms, and ornamental plants). Subsequent more detailed estimates found Pimentel’s numbers poorly substantiated and unrealistically inflated, and many comparisons misguided (Connelly et al., 2007; Lockwood et al., 2007; Davis, 2009; Guiaşu, 2016). As noted above, although many NIS cause extensive damage worldwide, appraisals of the ecological impacts, economic, and ecosystem service losses involved are likely biased and, when impacts are mixed (as they usually are), appraisals are rarely balanced. Returning to the above example, will the negative effects of the invasive B. longimanus on the water clarity of Lake Mendota eventually be offset by the increase in water clarity caused by another invader—D. polymorpha? And, should this happen, will it be labeled as an ecosystem service (as widely accepted for bivalves in general; Vaughn, 2017), or restricted to the roster of negative impacts of this NIS? (For example, consumption of plankton thus reducing its availability to other filter-feeders, Strayer & Malcom, 2018). One-sided assessments may stem from idiosyncratic positions or narrow perspectives, but also from the fact that positive and negative NIS effects may affect NAT and NIS alike. In their meta-analysis of the effects of NIS plants on soil biota through litter and rhizosphere pathways, Zhang et al. (2019) concluded that litter- and root-based loops generate positive NIS effects on soil systems stimulating nutrient cycling, yet they remarked that this process may facilitate plant invasions.
As with native species, most NIS can (and usually do) have both beneficial and detrimental effects on the biota (including both NAT and other NIS species), the environment (Devanna et al., 2011; Nelson et al., 2017; Latombe et al., 2019), and the economy, all of which should be included in the assessments of their overall impacts. The negative impacts of the invasive bivalves D. polymorpha and L. fortunei on industrial facilities (clogging of pipes and other raw water installations: Prescott et al., 2014; Boltovskoy et al., 2015b; Gallardo & Aldridge, 2020), and their enhancement of toxic cyanobacterial blooms (Bykova et al., 2006; Knoll et al., 2008; Cataldo et al., 2012), should be weighed alongside their positive effects on water clarity (including the consumption of Cyanobacteria; Higgins & Vander Zanden, 2010; Boltovskoy et al., 2015a), and provision of food for NAT species (Thayer et al., 1997; Carlsson et al., 2009; Kornis et al., 2012; Cataldo, 2015; Paolucci & Thuesen, 2015; Cattau et al., 2016; González-Bergonzoni et al., 2020). Incidentally, D. polymorpha and L. fortunei are a vivid example of the context-dependent, often opposed, impacts of NIS on the biota and on ecosystem services. Both mussels have been reported to enhance cyanobacterial blooms. However, in North America this effect of D. polymorpha is restricted to waterbodies with moderate to low total phosphorus concentrations (< 25 µg l−1; Sarnelle, 2005; Knoll et al., 2008), while in South America L. fortunei boosts Microcystis growth at concentrations > 50–100 µg l−1; Cataldo et al., 2012). Further, the mussels’ veligers experience high mortality rates when exposed to cyanobacterial toxins (Boltovskoy et al., 2013; Boegehold et al., 2019), but their adults actively consume Cyanobacteria, including toxic strains (Dionisio Pires et al., 2007; Boltovskoy et al., 2015a), and the use of D. polymorpha has been proposed for the restoration of eutrophic lakes (Dionisio Pires et al., 2010).
Such broadly encompassing approaches are rare, and will probably remain exceptional given the complexity of the interactions involved and the difficulties in the quantification of their relative importance. Nevertheless, the recognition of the prevalence of mixed effects, and the acknowledgment of the positive ones, will greatly improve our understanding of the consequences of biological invasions and help construct a more fact-based and less idiosyncratic science.
References
Allen, M. S. & E. E. Walter, 2019. Erectile dysfunction: an umbrella review of meta-analyses of risk-factors, treatment, and prevalence outcomes. The Journal of Sexual Medicine 16: 531–541.
Alpert, P., E. Bone & C. Holzapfel, 2000. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology, Evolution and Systematics 3: 52–66.
Andreu, J. & M. Vilà, 2011. Native plant community response to alien plant invasion and removal. Management of Biological Invasions 2: 81–94.
Anonymous, 2014. Climate Change. Evidence and Causes. An Overview from the Royal Society and the US National Academy of Sciences. The National Academy of Sciences and The Royal Society.
Anton, A., N. R. Geraldi, C. E. Lovelock, E. T. Apostolaki, S. Bennett, J. Cebrian, D. Krause-Jensen, N. Marbà, P. Martinetto, J. M. Pandolfi, J. Santana-Garcon & C. M. Duarte, 2019. Global ecological impacts of marine exotic species. Nature Ecology and Evolution 3: 787–800.
Arceo-Gómez, G. & T. L. Ashman, 2016. Invasion status and phylogenetic relatedness predict cost of heterospecific pollen receipt: implications for native biodiversity decline. Journal of Ecology 104: 1003–1008.
Aslan, C. E., E. S. Zavaleta, D. Croll & B. Tershy, 2012. Effects of native and non-native vertebrate mutualists on plants. Conservation Biology 26: 778–789.
Bacher, S., T. M. Blackburn, F. Essl, P. Genovesi, J. Heikkilä, J. M. Jeschke, G. Jones, R. Keller, M. Kenis, C. Kueffer, A. F. Martinou, W. Nentwig, J. Pergl, P. Pyšek, W. Rabitsch, D. M. Richardson, H. E. Roy, W.-C. Saul, R. Scalera, M. Vilà, J. R. U. Wilson & S. Kumschick, 2018. Socio-economic impact classification of alien taxa (SEICAT). Methods in Ecology and Evolution 9: 159–168.
Barney, J. N. & T. H. Whitlow, 2008. A unifying framework for biological invasions: the state factor model. Biological Invasions 10: 259–272.
Barr, D. J., 2008. Analyzing ‘visual world’ eyetracking data using multilevel logistic regression. Journal of Memory and Language 59: 457–474.
Barrientos, R., 2015. Adult sex-ratio distortion in the native European polecat is related to the expansion of the invasive American mink. Biological Conservation 186: 28–34.
Blossey, B. & R. Nötzold, 1995. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. Journal of Ecology 83: 887–889.
Boegehold, A. G., N. S. Johnson & D. R. Kashian, 2019. Dreissenid (quagga and zebra mussel) veligers are adversely affected by bloom forming cyanobacteria. Ecotoxicology and Environmental Safety 182: 109426.
Boltovskoy, D. & N. Correa, 2017. Planktonic equatorial diversity troughs: fact or artifact? Latitudinal diversity gradients in Radiolaria. Ecology 98: 112–124.
Boltovskoy, D., A. Y. Karatayev, L. E. Burlakova, D. Cataldo, V. Karatayev, F. Sylvester & A. Mariñelarena, 2009. Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636: 271–284.
Boltovskoy, D., N. Correa, F. Bordet, V. Leites & D. Cataldo, 2013. Toxic Microcystis (cyanobacteria) inhibit recruitment of the bloom-enhancing invasive bivalve Limnoperna fortunei. Freshwater Biology 58: 1968–1981.
Boltovskoy, D., N. Correa, F. Sylvester & D. Cataldo, 2015a. Nutrient recycling, phytoplankton grazing, and associated impacts of Limnoperna fortunei. In Boltovskoy, D. (ed.), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Springer, Cham: 153–176.
Boltovskoy, D., M. Xu & D. Nakano, 2015b. Impacts of Limnoperna fortunei on man-made structures and control strategies: general overview. In Boltovskoy, D. (ed.), Limnoperna fortunei: the Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Springer, Cham: 375–393.
Boltovskoy, D., F. Sylvester & E. M. Paolucci, 2018. Invasive species denialism: sorting out facts, beliefs and definitions. Ecology and Evolution 8: 11190–11198.
Borenstein, M., L. V. Hedges, J. P. T. Higgins & H. R. Rothstein, 2009. Introduction to Meta-analysis. Wiley, Chichester.
Brayard, A., G. Escarguel & H. Bucher, 2005. Latitudinal gradient of taxonomic richness: combined outcome of temperature and geographic mid-domains effects? Journal of Zoological Systematics and Evolutionary Research 43: 178–188.
Briggs, J. C., 2017. Rise of Invasive species denialism? A response to Russell and Blackburn. Trends in Ecology and Evolution 32: 231–232.
Buckley, Y. M., J. Catford & D. Gibson, 2016. Does the biogeographic origin of species matter? Ecological effects of native and non-native species and the use of origin to guide management. Journal of Ecology 104: 4–17.
Bunn, R. A., P. W. Ramsey & Y. Lekberg, 2015. Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. Journal of Ecology 103: 1547–1556.
Bykova, O., A. Laursen, V. Bostan, J. Bautista & L. Mc Carthy, 2006. Do zebra mussels (Dreissena polymorpha) alter lake water chemistry in a way that favours Microcystis growth? Science of the Total Environment 371: 362–372.
Callaway, R. M. & W. M. Ridenour, 2004. Novel weapons: invasive success and the evolution of increased competitive ability. Frontiers in Ecology and the Environment 2: 436–443.
Cameron, E. K., M. Vilà & M. Cabeza, 2016. Global meta-analysis of the impacts of terrestrial invertebrate invaders on species, communities and ecosystems. Global Ecology and Biogeography 25: 596–606.
Canavan, S., S. Kumschick, J. J. Le Roux, D. M. Richardson & J. R. U. Wilson, 2019. Does origin determine environmental impacts? Not for bamboos. Plants, People, Planet 2019–1: 119–128.
Canning-Clode, J., 2015. General introduction. Aquatic and terrestrial biological invasions in the 21st century. In Canning-Clode, J. (ed.), Biological Invasions in Changing Ecosystems. Vectors, Ecological Impacts, Management and Predictions. De Gruyter, Berlin: 30–37.
Carlsson, N. O. L., O. Sarnelle & D. L. Strayer, 2009. Native predators and exotic prey – an acquired taste? Frontiers in Ecology and the Environment 7: 525–532.
Carmichael, W. W., 1994. The toxins of Cyanobacteria. Scientific American 270: 78–86.
Cassey, P., S. Delean, J. L. Lockwood, J. S. Sadowski & T. M. Blackburn, 2018. Dissecting the null model for biological invasions: a meta-analysis of the propagule pressure effect. PLoS Biology 16: e2005987.
Cataldo, D., 2015. Trophic relationships of Limnoperna fortunei with adult fishes. In Boltovskoy, D. (ed.), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Springer, Cham: 231–248.
Cataldo, D., A. Vinocur, I. O’Farrell, E. M. Paolucci, V. Leites & D. Boltovskoy, 2012. The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande Reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680: 25–38.
Catford, J. A., R. Jansson & C. Nilsson, 2009. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity and Distributions 15: 22–40.
Cattau, C. E., R. J. Fletcher, B. E. Reichert & W. M. Kitchens, 2016. Counteracting effects of a non-native prey on the demography of a native predator culminate in positive population growth. Ecological Applications 26: 1952–1968.
Cavaleri, M. A. & L. Sack, 2010. Comparative water use of native and invasive plants at multiple scales: a global meta-analysis. Ecology 91: 2705–2715.
Charlebois, J. A. & R. D. Sargent, 2017. No consistent pollinator-mediated impacts of alien plants on natives. Ecology Letters 20: 1479–1490.
Chun, Y. J., M. van Kleunen & W. Dawson, 2010. The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecology Letters 13: 937–946.
Colautti, R. I. & H. J. MacIsaac, 2004. A neutral terminology to define ‘invasive’ species. Diversity and Distributions 10: 135–141.
Colautti, R. I., I. A. Grigorovich & H. J. MacIsaac, 2006. Propagule pressure: a null model for biological invasions. Biological Invasions 8: 1023–1037.
Connelly, N. A., C. R. O’Neill, B. A. Knuth & T. L. Brown, 2007. Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environmental Management 40: 105–112.
Crees, J. J. & S. T. Turvey, 2015. What constitutes a ‘native’ species? Insights from the Quaternary faunal record. Biological Conservation 186: 143–148.
Crowley, S., S. Hinchliffe, S. Redpath & R. McDonald, 2017. Disagreement about invasive species does not equate to denialism: a response to Russell and Blackburn. Trends in Ecology and Evolution 32: 228–229.
Crystal-Ornelas, R. & J. L. Lockwood, 2020a. The ‘known unknowns’ of invasive species impact measurement. Biological Invasions 22: 1513–1525.
Crystal-Ornelas, R. & J. L. Lockwood, 2020b. Cumulative meta-analysis identifies declining but negative impacts of invasive species on richness after 20 years. Ecology. https://doi.org/10.1002/ecy.3082.
David, P., E. Thébault, O. Anneville, P. F. Duyck, E. Chapuis & N. Loeuille, 2017. Chapter One – impacts of invasive species on food webs: a review of empirical data. In Bohan, D. A., A. J. Dumbrell & F. Massol (eds), Advances in Ecological Research. Academic, San Diego: 1–60.
Davidson, A. M., M. Jennions & A. B. Nicotra, 2011. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecology Letters 14: 419–431.
Davis, M. A., 2006. Invasion biology 1958–2005: the pursuit of science and conservation. In Cadotte, M. W., S. M. McMahon & T. Fukami (eds), Conceptual Ecology and Invasion Biology: Reciprocal Approaches to Nature. Springer, Berlin: 35–64.
Davis, M. A., 2009. Invasion Biology. Oxford University Press, New York.
Davis, M. A. & M. K. Chew, 2017. ‘The denialists are coming!’ Well, not exactly: a response to Russell and Blackburn. Trends in Ecology and Evolution 32: 229–230.
Davis, R. A. & D. M. Watson, 2018. Vagrants as vanguards of range shifts in a dynamic world. Biological Conservation 224: 238–241.
Davis, M. A., M. K. Chew, R. J. Hobbs, A. E. Lugo, J. J. Ewel, G. J. Vermeij, J. H. Brown, M. L. Rosenzweig, M. R. Gardener, S. P. Carroll, K. Thompson, T. A. Pickett, J. C. Stromberg, P. Del Tredici, K. N. Suding, J. G. Ehrenfeld, J. P. Grime, J. Mascaro & J. C. Briggs, 2011. Don’t judge species on their origins. Nature 474: 153–154.
Dawson, W., M. Fischer & M. Kleunen, 2012. Common and rare plant species respond differently to fertilisation and competition, whether they are alien or native. Ecology Letters 15: 873–880.
Delmas, C. E. L., S. Delzon & C. J. Lortie, 2011. A meta-analysis of the ecological significance of density in tree invasions. Community Ecology 12: 171–178.
DeVanna, K. M., B. L. Bodamer, C. G. Wellington, E. Hammer, C. M. Mayer & J. M. Bossenbroek, 2011. An alternative hypothesis to invasional meltdown in the Laurentian Great Lakes region: general facilitation by Dreissena. Journal of Great Lakes Research 37: 632–641.
Dick, J. T. A., C. Laverty, J. J. Lennon, D. Barrios-O’Neill, P. J. Mensink, J. R. Britton, V. Medoc, P. Boets, M. E. Alexander, N. G. Taylor, A. M. Dunn, M. J. Hatcher, P. J. Rosewarne, S. Crookes, H. J. MacIsaac, M. Xu, A. Ricciardi, R. J. Wasserman, B. R. Ellender, O. L. F. Weyl, F. E. Lucy, P. B. Banks, J. A. Dodd, C. MacNeil, M. R. Penk, D. C. Aldridge & J. M. Caffrey, 2017. Invader Relative Impact Potential: a new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. Journal of Applied Ecology 54: 1259–1267.
Dickey, J. W. E., R. N. Cuthbert, J. South, J. R. Britton, J. Caffrey, X. Chang, K. Crane, N. E. Coughlan, E. Fadaei, K. D. Farnsworth, S. M. H. Ismar-Rebitz, P. W. S. Joyce, M. Julius, C. Laverty, F. E. Lucy, H. J. MacIsaac, M. McCard, C. L. O. McGlade, N. Reid, A. Ricciardi, R. J. Wasserman, O. L. F. Weyl & J. T. A. Dick, 2020. On the RIP: using Relative Impact Potential to assess the ecological impacts of invasive alien species. NeoBiota 55: 27–60.
Dionisio Pires, L. M., B. M. Bontes, L. Samchyshyna, J. Jong, E. Donk & B. W. Ibelings, 2007. Grazing on microcystin-producing and microcystin-free phytoplankters by different filter-feeders: implications for lake restoration. Aquatic Sciences 69: 534–543.
Dionisio Pires, L. M., B. W. Ibelings & E. van Donk, 2010. Zebra mussels as a potential tool in the restoration of eutrophic shallow lakes, dominated by toxic cyanobacteria. In Van Der Velde, G., S. Rajagopal & A. Bij De Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden: 361–372.
Doorduin, L. J. & K. Vrieling, 2011. A review of the phytochemical support for the shifting defence hypothesis. Phytochemistry Reviews 10: 99–106.
Dueñas, M.-A., H. J. Ruffhead, N. H. Wakefield, P. D. Roberts, D. J. Hemming & H. Diaz-Soltero, 2018. The role played by invasive species in interactions with endangered and threatened species in the United States: a systematic review. Biodiversity and Conservation 27: 3171–3183.
Durlak, J. A., 2010. How to select, calculate, and interpret effect sizes. Journal of Pediatric Psychology 34: 917–928.
Ebbs, E. T., E. S. Loker & S. V. Brant, 2018. Phylogeography and genetics of the globally invasive snail Physa acuta Draparnaud 1805, and its potential to serve as an intermediate host to larval digenetic trematodes. BMC Evolutionary Biology 18: 103.
Ellis, P. D., 2010. The Essential Guide to Effect Sizes. Statistical Power, Meta-analysis, and the Interpretation of Research Results. Cambridge University Press, Cambridge.
Elton, C. S., 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
Emery-Butcher, H. E., S. J. Beatty & B. J. Robson, 2020. The impacts of invasive ecosystem engineers in freshwaters: a review. Freshwater Biology 65: 999–1015.
Engelkes, T., E. Morrien, K. J. Verhoeven, T. M. Bezemer, A. Biere, J. A. Harvey, L. M. McIntyre, W. L. Tamis & W. H. van der Putten, 2008. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456: 946–948.
Essl, F., S. Dullinger, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Katsanevakis, I. Kühn, B. Lenzner, A. Pauchard, P. Pyšek, W. Rabitsch, D. M. Richardson, H. Seebens, M. van Kleunen, W. H. van der Putten, M. Vilà & S. Bacher, 2019. A conceptual framework for range-expanding species that track human-induced environmental change. BioScience 69: 908–919.
Excoffier, L., M. Foll & R. J. Petit, 2009. Genetic consequences of range expansions. Annual Review of Ecology, Evolution, and Systematics 40: 481–501.
Facon, B., R. A. Hufbauer, A. Tayeh, A. Loiseau, E. Lombaert, R. Vitalis, T. Guillemaud, J. G. Lundgren & A. Estoup, 2011. Inbreeding depression is purged in the invasive insect Harmonia axyridis. Current Biology 21: 424–427.
Felker-Quinn, E., J. A. Schweitzer & J. K. Bailey, 2013. Meta-analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecology and Evolution 3: 739–751.
Ferlian, O., N. Eisenhauer, M. Aguirrebengoa, M. Camara, I. Ramirez-Rojas, F. Santos, K. Tanalgo & M. P. Thakur, 2018. Invasive earthworms erode soil biodiversity: a meta-analysis. Journal of Animal Ecology 87: 162–172.
Ferreira, V., J. Koricheva, J. Pozo & M. A. S. Graça, 2016. A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. Forest Ecology and Management 364: 27–38.
Fusar-Poli, P. & J. Radua, 2018. Ten simple rules for conducting umbrella reviews. Evidence Based Mental Health 21: 95–100.
Gaertner, M., A. Den Breeyen, H. Cang & D. M. Richardson, 2009. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: a meta-analysis. Progress in Physical Geography 33: 319–338.
Gallardo, B. & D. C. Aldridge, 2020. Priority setting for invasive species management by the water industry. Water Research. https://doi.org/10.1016/j.watres.2020.115771.
Gallardo, B., M. Clavero, M. I. Sanchez & M. Vilà, 2016. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology 22: 151–163.
Gallien, L. & M. Carboni, 2017. The community ecology of invasive species: where are we and what’s next? Ecography 40: 335–352.
Gerhold, P., M. Pärtel, O. Tackenberg, S. M. Hennekens, I. Bartish, J. H. J. Schaminée, A. J. F. Fergus, W. A. Ozinga & A. Prinzing, 2011. Phylogenetically poor plant communities receive more alien species, which more easily coexist with natives. American Naturalist 177: 668–680.
Gioria, M., V. Jarošík & P. Pyšek, 2014. Impact of invasions by alien plants on soil seed bank communities: emerging patterns. Perspectives in Plant Ecology, Evolution and Systematics 16: 132–142.
Golivets, M. & K. F. Wallin, 2018. Neighbour tolerance, not suppression, provides competitive advantage to non-native plants. Ecology Letters 21: 745–759.
González, A. L., J. S. Kominoski, M. Danger, S. Ishida, N. Iwai & A. Rubach, 2010. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 119: 779–790.
González-Bergonzoni, I., I. Silva, F. Teixeira de Mello, A. D’Anatro, L. Bocardi, S. Stebniki, E. Brugnoli, G. Tesitore, N. Vidal & D. E. Naya, 2020. Evaluating the role of predatory fish on the invasion of the Asian golden mussel (Limnoperna fortunei) in a subtropical river. Journal of Applied Ecology. https://doi.org/10.1111/1365-2664.13573.
Gonzalez-Browne, C., M. M. Murua, L. Navarro & R. Medel, 2016. Does plant origin influence the fitness impact of flower damage? A meta-analysis. PLoS ONE 11: e0146437.
Grady, J. M., B. S. Maitner, A. S. Winter, K. Kaschner, D. P. Tittensor, S. Record, F. A. Smith, A. M. Wilson, A. I. Dell, P. L. Zarnetske, H. J. Wearing, B. Alfaro & J. H. Brown, 2019. Metabolic asymmetry and the global diversity of marine predators. Science 363: eaat4220.
Grawitch, M. J. & D. C. Munz, 2004. Are your data nonindependent? A practical guide to evaluating nonindependence and within-group agreement. Understanding Statistics 3: 231–257.
Guerin, G. R., 2019. Invoking denialism does not strengthen invasion science. Biodiversity and Conservation 28: 1939–1941.
Guerin, G. R., I. Martín-Forés, B. Sparrow & A. J. Lowe, 2018. The biodiversity impacts of non-native species should not be extrapolated from biased single-species studies. Biodiversity and Conservation 27: 785–790.
Guiaşu, R. C., 2016. Non-native Species and Their Role in the Environment: The Need for a Broader Perspective. Brill, Leiden.
Guiaşu, R. C. & C. W. Tindale, 2018. Logical fallacies and invasion biology. Biology and Philosophy 33: 34.
Guo, Q., S. Fei, J. S. Dukes, C. M. Oswalt, B. V. Iannone III & K. M. Potter, 2015. A unified approach for quantifying invasibility and degree of invasion. Ecology 96: 2613–2621.
Gurevitch, J., G. A. Fox, G. M. Wardle, D. B. Inderjit & D. Taub, 2011. Emergent insights from the synthesis of conceptual frameworks for biological invasions. Ecology Letters 14: 407–418.
Guy-Haim, T., A. Lyons Devin, J. Kotta, H. Ojaveer, M. Queirós Ana, E. Chatzinikolaou, C. Arvanitidis, S. Como, P. Magni, J. Blight Andrew, H. Orav-Kotta, J. Somerfield Paul, P. Crowe Tasman & G. Rilov, 2018. Diverse effects of invasive ecosystem engineers on marine biodiversity and ecosystem functions: a global review and meta-analysis. Global Change Biology 24: 906–924.
Hawkes, C. V., 2007. Are invaders moving targets? The generality and persistence of advantages in size, reproduction, and enemy release in invasive plant species with time since introduction. American Naturalist 170: 832–843.
Hayranto, D., 2018. Changes in the Lake Mendota Food Web Composition: Predation of Invasive Zebra Mussel (Dreissena polymorpha) Veligers by Native Water Fleas (Daphnia pulicaria), Internship Report 326. University of Madison, Center for Limnology [available on internet at https://scholarlyrepository.miami.edu/rsmas_intern_reports/326].
Hazlett, B. A., 2000. Information use by an invading species: do invaders respond more to alarm odors than native species? Biological Invasions 2: 289–294.
Hierro, J. L., J. L. Maron & R. M. Callaway, 2005. A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. Journal of Ecology 93: 5–15.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Hillebrand, H., 2004. On the generality of the latitudinal diversity gradient. The American Naturalist 163: 191–211.
Hodges, K. E., 2008. Defining the problem: terminology and progress in ecology. Frontiers in Ecology and the Environment 6: 35–42.
Hoegh-Guldberg, O., L. Hughes, S. McIntyre, D. B. Lindenmayer, C. Parmesan, H. P. Possingham & C. D. Thomas, 2008. Assisted colonization and rapid climate change. Science 321: 345–346.
Hoffmann, B. D. & F. Courchamp, 2016. Biological invasions and natural colonisations: are they that different? NeoBiota 29: 1–14.
Howard, B. R., T. W. Therriault & I. M. Côté, 2017. Contrasting ecological impacts of native and non-native marine crabs: a global meta-analysis. Marine Ecology Progress Series 577: 93–103.
Hulme, P. E., P. Pysek, V. Jarosik, J. Pergl, U. Schaffner & M. Vila, 2013. Bias and error in understanding plant invasion impacts. Trends in Ecology and Evolution 28: 212–218.
Iacarella, J. C., P. S. Mankiewicz & A. Ricciardi, 2015. Negative competitive effects of invasive plants change with time since invasion. Ecosphere 6: art123.
James, J., F. M. Slater, I. P. Vaughan, K. A. Young & J. Cable, 2015. Comparing the ecological impacts of native and invasive crayfish: could native species’ translocation do more harm than good? Oecologia 178: 309–316.
Jauni, M. & S. Ramula, 2015. Meta-analysis on the effects of exotic plants on the fitness of native plants. Perspectives in Plant Ecology, Evolution and Systematics 17: 412–420.
Jennions, M. D., C. J. Lortie, M. S. Rosenberg & H. R. Rothstein, 2013. Publication and related biases. In Koricheva, J., J. Gurevitch & K. Mengersen (eds), Handbook of Meta-analysis in Ecology and Evolution. Princeton University Press, Princeton: 207–236.
Jeschke, J., L. Gómez Aparicio, S. Haider, T. Heger, C. Lortie, P. Pyšek & D. Strayer, 2012. Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14: 1–20.
Jeschke, J. M., S. Bacher, T. M. Blackburn, J. T. Dick, F. Essl, T. Evans, M. Gaertner, P. E. Hulme, I. Kuhn, A. Mrugala, J. Pergl, P. Pysek, W. Rabitsch, A. Ricciardi, D. M. Richardson, A. Sendek, M. Vila, M. Winter & S. Kumschick, 2014. Defining the impact of non-native species. Conservation Biology 28: 1188–1194.
Joshi, R. C., 2017. Problems with the management of the golden apple snail Pomacea canaliculata: an important exotic pest of rice in Asia. In Vreysen, M. J. B., A. S. Robinson & J. Hendrichs (eds), Area-Wide Control of Insect Pests. From Research to Field Implementation. Springer, Dordrecht: 257–264.
Kamenova, S., T. J. Bartley, D. A. Bohan, J. R. Boutain, R. I. Colautti, I. Domaizon, C. Fontaine, A. Lemainque, I. Le Viol, G. Mollot, M. E. Perga, V. Ravigné & F. Massol, 2017. Chapter Three – invasions toolkit: current methods for tracking the spread and impact of invasive species. In Bohan, D. A., A. J. Dumbrell & F. Massol (eds), Advances in Ecological Research. Academic, San Diego: 85–182.
Kelley, A. L., 2014. The role thermal physiology plays in species invasion. Conservation Physiology 2: cou45.
Kelly, D. W., R. A. Paterson, C. R. Townsend, R. Poulin & D. M. Tompkins, 2009. Parasite spillback: a neglected concept in invasion ecology? Ecology 90: 2047–2056.
Kinlock, N. L., L. Prowant, E. M. Herstoff, C. M. Foley, M. Akin-Fajiye, N. Bender, M. Umarani, H. Y. Ryu, B. Şen & J. Gurevitch, 2018. Explaining global variation in the latitudinal diversity gradient: meta-analysis confirms known patterns and uncovers new ones. Global Ecology and Biogeography 27: 125–141.
Knoll, L. B., O. Sarnelle, S. K. Hamilton, C. E. H. Kissman, A. E. Wilson, J. B. Rose & M. R. Morgan, 2008. Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Canadian Journal of Fisheries and Aquatic Sciences 65: 448–455.
Koricheva, J. & J. Gurevitch, 2014. Uses and misuses of meta-analysis in plant ecology. Journal of Ecology 102: 828–844.
Koricheva, J., J. Gurevitch & K. Mengersen (eds), 2013. Handbook of Meta-analysis in Ecology and Evolution. Princeton University Press, Princeton.
Kornis, M. S., N. Mercado-Silva & M. J. Vander Zanden, 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80: 235–285.
Korsu, K., A. Huusko & T. Muotka, 2010. Impacts of invasive stream salmonids on native fish: using meta-analysis to summarize four decades of research. Boreal Environment Research 15: 491–500.
Kuebbing, S. E. & M. A. Nuñez, 2016. Invasive non-native plants have a greater effect on neighbouring natives than other non-natives. Nature Plants 2: 16134.
Lamarque, L. J., S. Delzon & C. J. Lortie, 2011. Tree invasions: a comparative test of the dominant hypotheses and functional traits. Biological Invasions 13: 1969–1989.
Larson, B. M. H., 2007. An alien approach to invasive species: objectivity and society in invasion biology. Biological Invasions 9: 947–956.
Latombe, G., S. Canavan, H. Hirsch, C. Hui, S. Kumschick, M. M. Nsikani, L. J. Potgieter, T. B. Robinson, W. C. Saul, S. C. Turner, J. R. U. Wilson, F. A. Yannelli & D. M. Richardson, 2019. A four-component classification of uncertainties in biological invasions: implications for management. Ecosphere 10: e02669.
Lee, M. R., E. S. Bernhardt, P. M. van Bodegom, J. H. Cornelissen, J. Kattge, D. C. Laughlin, U. Niinemets, J. Penuelas, P. B. Reich, B. Yguel & J. P. Wright, 2017. Invasive species’ leaf traits and dissimilarity from natives shape their impact on nitrogen cycling: a meta-analysis. New Phytologist 213: 128–139.
Leffler, A. J., J. J. James, T. A. Monaco & R. L. Sheley, 2014. A new perspective on trait differences between native and invasive exotic plants. Ecology 95: 298–305.
Lenoir, J. & J. C. Svenning, 2015. Climate-related range shifts – a global multidimensional synthesis and new research directions. Ecography 38: 15–28.
Levine, J. M., P. B. Adler & S. G. Yelenik, 2004. A meta-analysis of biotic resistance to exotic plant invasions. Ecology Letters 7: 975–989.
Liu, H. & P. Stiling, 2006. Testing the enemy release hypothesis: a review and meta-analysis. Biological Invasions 8: 1535–1545.
Lockwood, J. L., P. Cassey & T. Blackburn, 2005. The role of propagule pressure in explaining species invasions. Trends in Ecology and Evolution 20: 223–228.
Lockwood, J. L., M. F. Hoopes & M. P. Marchetti, 2007. Invasion Ecology. Blackwell, Malden.
Lortie, C. J. & R. M. Callaway, 2005. Re-analysis of meta-analysis: support for the stress-gradient hypothesis. Journal of Ecology 94: 7–16.
Ma, C., S. P. Li, Z. Pu, J. Tan, M. Liu, J. Zhou, H. Li & L. Jiang, 2016. Different effects of invader-native phylogenetic relatedness on invasion success and impact: a meta-analysis of Darwin’s naturalization hypothesis. Proceedings of the Royal Society B: Biological Sciences 283: 20160663.
Maestre, F. T., F. Valladares & J. F. Reynolds, 2005. Is the change of plant–plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. Journal of Ecology 93: 748–757.
Maggi, E., L. Benedetti-Cecchi, A. Castelli, E. Chatzinikolaou, T. P. Crowe, G. Ghedini, J. Kotta, D. A. Lyons, C. Ravaglioli, G. Rilov, L. Rindi & F. Bulleri, 2015. Ecological impacts of invading seaweeds: a meta-analysis of their effects at different trophic levels. Diversity and Distributions 21: 1–12.
Makowski, C. & C. W. Finkl (eds), 2018. Impacts of Invasive Species on Coastal Environments. Coasts in Crisis. Springer, Cham.
Mattingly, K. Z., T. A. Pelletier, J. Lanterman, D. Frevola, B. Stucke, K. Kinney, R. Schwartz, D. Spacht, G. Dixon & S. M. Hovick, 2020. Disconnects between communicated impact and ecological impact of biological invasions. BioScience. https://doi.org/10.1093/biosci/biaa003.
McCary, M. A., R. Mores, M. A. Farfan & D. H. Wise, 2016. Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: a meta-analysis. Ecology Letters 19: 328–335.
McKnight, E., E. García-Berthou, P. Srean & M. Rius, 2017. Global meta-analysis of native and nonindigenous trophic traits in aquatic ecosystems. Global Change Biology 23: 1861–1870.
Meijer, K., M. Schilthuizen, L. Beukeboom & C. Smit, 2016. A review and meta-analysis of the enemy release hypothesis in plant–herbivorous insect systems. PeerJ 4: e2778.
Merel, S., D. Walker, R. Chicana, S. Snyder, E. Baurès & O. Thomas, 2013. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International 59: 303–327.
Mills, E. L., J. H. Leach, J. T. Carlton & C. L. Secor, 1993. Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. Journal of Great Lakes Research 19: 1–54.
Mizrahi, D., S. F. Pereira, S. A. Navarrete & A. A. V. Flores, 2017. Allelopathic effects on the sun-coral invasion: facilitation, inhibition and patterns of local biodiversity. Marine Biology 164: 139.
Moles, A. T., H. Flores-Moreno, S. P. Bonser, D. I. Warton, A. Helm, L. Warman, D. J. Eldridge, E. Jurado, F. A. Hemmings, P. B. Reich, J. Cavender-Bares, E. W. Seabloom, M. M. Mayfield, D. Sheil, J. C. Djietror, P. L. Peri, L. Enrico, M. R. Cabido, S. A. Setterfield, C. E. R. Lehmann & F. J. Thomson, 2012. Invasions: the trail behind, the path ahead, and a test of a disturbing idea. Journal of Ecology 100: 116–127.
Mollot, G., J. H. Pantel & T. N. Romanuk, 2017. Chapter Two – the effects of invasive species on the decline in species richness: a global meta-analysis. In Bohan, D. A., A. J. Dumbrell & F. Massol (eds), Advances in Ecological Research. Academic, San Diego: 61–83.
Montero-Castaño, A. & M. Vilà, 2012. Impact of landscape alteration and invasions on pollinators: a meta-analysis. Journal of Ecology 100: 884–893.
Moore, T. P., K. J. Collier & I. C. Duggan, 2019. Interactions between Unionida and non-native species: a global meta-analysis. Aquatic Conservation: Marine and Freshwater Ecosystems. https://doi.org/10.1002/aqc.3040.
Morales, C. L. & A. Traveset, 2009. A meta-analysis of impacts of alien vs. native plants on pollinator visitation and reproductive success of co-flowering native plants. Ecology Letters 12: 716–728.
Mueck, L., 2013. Report the awful truth! Nature Nanotechnology 8: 693–695.
Munro, D., J. Steer & W. Linklater, 2019. On allegations of invasive species denialism. Conservation Biology 33: 797–802.
Nackley, L. L., A. G. West, A. L. Skowno & W. J. Bond, 2017. The nebulous ecology of native invasions. Trends in Ecology and Evolution 32: 814–824.
Nelson, S. B., J. J. Coon, C. J. Duchardt, J. D. Fischer, S. J. Halsey, A. J. Kranz, C. M. Parker, S. C. Schneider, T. M. Swartz & J. R. Miller, 2017. Patterns and mechanisms of invasive plant impacts on North American birds: a systematic review. Biological Invasions 19: 1547–1563.
Oduor, A. M. O., J. M. Gómez & S. Y. Strauss, 2010. Exotic vertebrate and invertebrate herbivores differ in their impacts on native and exotic plants: a meta-analysis. Biological Invasions 12: 407–419.
Oduor, A. M. O., R. Leimu, M. van Kleunen & R. Mack, 2016. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. Journal of Ecology 104: 957–968.
Palacio-López, K. & E. Gianoli, 2011. Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis. Oikos 120: 1393–1401.
Paolucci, E. M. & E. V. Thuesen, 2015. Trophic relationships of Limnoperna fortunei with larval fishes. In Boltovskoy, D. (ed.), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel. Springer, Cham: 211–229.
Paolucci, E. M., H. J. MacIsaac & A. Ricciardi, 2013. Origin matters: alien consumers inflict greater damage on prey populations than do native consumers. Diversity and Distributions 19: 988–995.
Parker, I. M., D. Simberloff, W. M. Lonsdale, K. Goodell, M. Wonham, P. M. Kareiva, M. H. Williamson, B. Von Holle, P. B. Moyle, J. E. Byers & L. Goldwasser, 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions 1: 3–19.
Parker, J. D., D. E. Burkepile & M. E. Hay, 2006. Opposing effects of native and exotic herbivores on plant invasions. Science 311: 1459.
Parker, J. D., M. E. Torchin, R. A. Hufbauer, N. P. Lemoine, C. Alba, D. M. Blumenthal, O. Bossdorf, J. E. Byers, A. M. Dunn, R. W. Heckman, M. Hejda, V. Jarošík, A. R. Kanarek, L. B. Martin, S. E. Perkins, P. Pyšek, K. Schierenbeck, C. Schlöder, R. van Klinken, K. J. Vaughn, W. T. Williams & L. M. Wolfe, 2013. Do invasive species perform better in their new ranges? Ecology 94: 985–994.
Pecl, G. T., M. B. Araujo, J. D. Bell, J. Blanchard, T. C. Bonebrake, I. C. Chen, T. D. Clark, R. K. Colwell, F. Danielsen, B. Evengard, L. Falconi, S. Ferrier, S. Frusher, R. A. Garcia, R. B. Griffis, A. J. Hobday, C. Janion-Scheepers, M. A. Jarzyna, S. Jennings, J. Lenoir, H. I. Linnetved, V. Y. Martin, P. C. McCormack, J. McDonald, N. J. Mitchell, T. Mustonen, J. M. Pandolfi, N. Pettorelli, E. Popova, S. A. Robinson, B. R. Scheffers, J. D. Shaw, C. J. Sorte, J. M. Strugnell, J. M. Sunday, M. N. Tuanmu, A. Verges, C. Villanueva, T. Wernberg, E. Wapstra & S. E. Williams, 2017. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355: 9214.
Peng, S., N. L. Kinlock, J. Gurevitch & S. Peng, 2019. Correlation of native and exotic species richness: a global meta-analysis finds no invasion paradox across scales. Ecology 100: e02552.
Penk, M., K. Irvine & I. Donohue, 2015. Ecosystem-level effects of a globally spreading invertebrate invader are not moderated by a functionally similar native. Journal of Animal Ecology 84: 1628–1636.
Pereyra, P. J., 2016. Revisiting the use of the invasive species concept: an empirical approach. Austral Ecology 41: 519–528.
Pereyra, P. J., 2020. Rethinking the native range concept. Conservation Biology 34: 373–377.
Pereyra, P. J. & R. C. Guiasu, 2020. Debate over the importance and meaning of native range in invasion biology: reply to Courchamp et al. Conservation Biology 3: 1044–1046.
Peterson, A. T. & D. A. Vieglais, 2001. Predicting species invasions using ecological niche modeling: new approaches from bioinformatics attack a pressing problem. BioScience 51: 363–371.
Phillips, B. L., G. P. Brown & R. Shine, 2010. Life-history evolution in range-shifting populations. Ecology 91: 1617–1627.
Pimentel, D. (ed.), 2002. Biological Invasions. Economic and Environmental Costs of Alien Plant, Animal, and Microbe Species. CRC Press, Boca Raton.
Pimentel, D., R. Zuniga & D. Morrison, 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52: 273–288.
Pintor, L. M. & J. E. Byers, 2015. Do native predators benefit from non-native prey? Ecology Letters 18: 1174–1180.
Powell, K. I., J. M. Chase & T. M. Knight, 2011. A synthesis of plant invasion effects on biodiversity across spatial scales. American Journal of Botany 98: 539–548.
Prescott, T. H., R. Claudi & K. L. Prescott, 2014. Impact of dreissenid mussels on the infrastructure of dams and hydroelectric power plants. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton: 244–257.
Pyšek, P., V. Jarošík, P. E. Hulme, J. Pergl, M. Hejda, U. Schaffner & M. Vilà, 2012. A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Global Change Biology 18: 1725–1737.
Qiu, J., 2015. A global synthesis of the effects of biological invasions on greenhouse gas emissions. Global Ecology and Biogeography 24: 1351–1362.
Radville, L., L. Gonda-King, S. Gómez, I. Kaplan & L. Preisser Evan, 2014. Are exotic herbivores better competitors? A meta-analysis. Ecology 95: 30–36.
Ramus, A. P., B. R. Silliman, M. S. Thomsen & Z. T. Long, 2017. An invasive foundation species enhances multifunctionality in a coastal ecosystem. Proceedings of the National Academy of Sciences of the United States of America 114: 8580–8585.
Raudenbusch, S. W. & A. S. Bryk, 2002. Hierarchical Linear Models. Applications and Data Analysis Methods. Sage Publications, Thousand Oaks.
Reed-Andersen, T., S. R. Carpenter, D. K. Padilla & R. C. Lathrop, 2000. Predicted impact of zebra mussel (Dreissena polymorpha) invasion on water clarity in Lake Mendota. Canadian Journal of Fisheries and Aquatic Sciences 57: 1617–1626.
Rejmánek, M. & D. Simberloff, 2017. Origin matters. Environmental Conservation 44: 97–99.
Ricciardi, A. & H. J. MacIsaac, 2000. Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends in Ecology and Evolution 15: 62–65.
Ricciardi, A. & H. J. MacIsaac, 2008. The book that began invasion ecology. Nature 452: 34.
Ricciardi, A. & R. Ryan, 2018. The exponential growth of invasive species denialism. Biological Invasions 20: 549–553.
Ricciardi, A., M. F. Hoopes, M. P. Marchetti & J. L. Lockwood, 2013. Progress toward understanding the ecological impacts of nonnative species. Ecological Monographs 83: 263–282.
Richardson, D. M. & P. Pysek, 2008. Fifty years of invasion ecology – the legacy of Charles Elton. Diversity and Distributions 14: 161–168.
Richardson, D. M. & A. Ricciardi, 2013. Misleading criticisms of invasion science: a field guide. Diversity and Distributions 19: 1461–1467.
Richardson, D. M., P. Pysek, M. Rejmanek, M. G. Barbour, F. D. Panetta & C. J. West, 2000. Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions 6: 93–107.
Romero, G. Q., T. Goncalves-Souza, C. Vieira & J. Koricheva, 2015. Ecosystem engineering effects on species diversity across ecosystems: a meta-analysis. Biological Reviews of the Cambridge Philosophical Society 90: 877–890.
Russell, J. C. & T. M. Blackburn, 2017a. Invasive alien species: denialism, disagreement, definitions, and dialogue. Trends in Ecology and Evolution 32: 312–314.
Russell, J. C. & T. M. Blackburn, 2017b. The rise of invasive species denialism. Trends in Ecology and Evolution 32: 3–6.
Rypel, A. L., 2013. Do invasive freshwater fish species grow better when they are invasive? Oikos 123: 279–289.
Sagoff, M., 2018. Invasive species denialism: a reply to Ricciardi and Ryan. Biological Invasions 20: 2723–2729.
Salo, P., E. Korpimaki, P. B. Banks, M. Nordstrom & C. R. Dickman, 2007. Alien predators are more dangerous than native predators to prey populations. Proceedings of the Royal Society B: Biological Sciences 274: 1237–1243.
Sarnelle, O., A. E. Wilson, S. K. Hamilton, L. B. Knoll & D. F. Raikow, 2005. Complex interactions between the zebra mussel, Dreissena polymorpha, and the harmful phytoplankter, Microcystis aeruginosa. Limnology and Oceanography 50: 896–904.
Sax, D. F., J. J. Stachowicz & S. D. Gaines (eds), 2005. Species Invasions: Insights into Ecology, Evolution and Biogeography. Sinauer Associates, Sunderland.
Schirmel, J., M. Bundschuh, M. H. Entling, I. Kowarik & S. Buchholz, 2016. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: a global assessment. Global Change Biology 22: 594–603.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Pagad, P. Pysek, M. Winter, M. Arianoutsou, S. Bacher, B. Blasius, G. Brundu, C. Capinha, L. Celesti-Grapow, W. Dawson, S. Dullinger, N. Fuentes, H. Jager, J. Kartesz, M. Kenis, H. Kreft, I. Kuhn, B. Lenzner, A. Liebhold, A. Mosena, D. Moser, M. Nishino, D. Pearman, J. Pergl, W. Rabitsch, J. Rojas-Sandoval, A. Roques, S. Rorke, S. Rossinelli, H. E. Roy, R. Scalera, S. Schindler, K. Stajerova, B. Tokarska-Guzik, M. van Kleunen, K. Walker, P. Weigelt, T. Yamanaka & F. Essl, 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8: 1–9.
Sher, A. A. & L. A. Hyatt, 1999. The disturbed resource-flux invasion matrix: a new framework for patterns of plant invasion. Biological Invasions 1: 107–114.
Simberloff, D., 2006. Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecology Letters 9: 912–919.
Simberloff, D. & B. Von Holle, 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1: 21–32.
Simberloff, D., et al., 2011. Non-natives: 141 scientists object. Nature 475: 36.
Smith, S. D. P., D. B. Bunnell, G. A. Burton, J. J. H. Ciborowski, A. D. Davidson, C. E. Dickinson, L. A. Eaton, P. C. Esselman, M. A. Evans, D. R. Kashian, N. F. Manning, P. B. McIntyre, T. F. Nalepa, A. Pérez-Fuentetaja, A. D. Steinman, D. G. Uzarski & J. D. Allan, 2019. Evidence for interactions among environmental stressors in the Laurentian Great Lakes. Ecological Indicators 101: 203–211.
Sorte, C. J. B., S. L. Williams & J. T. Carlton, 2010. Marine range shifts and species introductions: comparative spread rates and community impacts. Global Ecology and Biogeography 19: 303–316.
Sorte, C. J. B., I. Ibáñez, D. M. Blumenthal, N. A. Molinari, L. P. Miller, E. D. Grosholz, J. M. Diez, C. M. D’Antonio, J. D. Olden, S. J. Jones & J. S. Dukes, 2013. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecology Letters 16: 261–270.
Stohlgren, T. J., T. Barnett David & T. Kartesz John, 2003. The rich get richer: patterns of plant invasions in the United States. Frontiers in Ecology and the Environment 1: 11–14.
Strayer, D. L. & H. M. Malcom, 2018. Long-term responses of native bivalves (Unionidae and Sphaeriidae) to a Dreissena invasion. Freshwater Science 37: 697–711.
Sturtevant, R. A., D. M. Mason, E. S. Rutherford, A. Elgin, E. Lower & F. Martinez, 2019. Recent history of nonindigenous species in the Laurentian Great Lakes; an update to Mills et al., 1993 (25 years later). Journal of Great Lakes Research 45: 1011–1035.
Tassin, J., K. Thompson, S. P. Carroll & C. D. Thomas, 2017. Determining whether the impacts of introduced species are negative cannot be based solely on science: a response to Russell and Blackburn. Trends in Ecology and Evolution 32: 230–231.
Thayer, S. A., R. C. Haas, R. D. Hunter & R. H. Kushler, 1997. Zebra Mussel (Dreissena polymorpha) effects on sediment, other zoobenthos, and the diet and growth of adult Yellow Perch (Perca favescens) in pond enclosures. Canadian Journal of Fisheries and Aquatic Sciences 54: 1903–1915.
Thiele, J., J. Kollmann, B. Markussen & A. Otte, 2010. Impact assessment revisited: improving the theoretical basis for management of invasive alien species. Biological Invasions 12: 2025–2035.
Thomas, C. D., 2013. The Anthropocene could raise biological diversity. Nature 502: 7.
Thompson, K., 2014. Where Do Camels Belong? Why Invasive Species Aren’t All Bad. Profile Books, London.
Thomsen, M. S., T. Wernberg, F. Tuya & B. R. Silliman, 2009. Evidence for impacts of nonindigenous macroalgae: a meta-analysis of experimental field studies. Journal of Phycology 45: 812–819.
Thomsen, M. S., J. E. Byers, D. R. Schiel, J. F. Bruno, J. D. Olden, T. Wernberg & B. R. Silliman, 2014. Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Marine Ecology Progress Series 495: 39–47.
Thomsen, M. S., T. Wernberg & D. Schiel, 2015. 10. Invasions by non-indigenous species. In Crowe, T. P. & C. L. J. Frid (eds), Marine Ecosystems: Human Impacts on Biodiversity, Functioning and Services. Cambridge University Press, Cambridge: 274–331.
Thomsen, M. S., T. Wernberg, P. M. South & D. R. Schiel, 2016. Chapter 6. Non-native seaweeds drive changes in marine coastal communities around the world. In Hu, Z. M. & C. Fraser (eds), Seaweed Phylogeography. Springer, Dordrecht: 147–185.
Tittensor, D. P., C. Mora, W. Jetz, H. K. Lotze, D. Ricard, E. V. Berghe & B. Worm, 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466: 1098–1101.
Torchin, M. E., K. D. Lafferty, A. P. Dobson, V. J. McKenzie & A. M. Kuris, 2003. Introduced species and their missing parasites. Nature 421: 628–630.
van Hengstum, T., D. A. P. Hooftman, J. G. B. Oostermeijer, P. H. van Tienderen & R. Mack, 2014. Impact of plant invasions on local arthropod communities: a meta-analysis. Journal of Ecology 102: 4–11.
van Kleunen, M., E. Weber & M. Fischer, 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13: 235–245.
Vaughn, C. C., 2017. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vellend, M., L. Baeten, I. H. Myers-Smith, S. C. Elmendorf, R. Beauséjour, C. D. Brown, P. De Frenne, K. Verheyen & S. Wipf, 2013. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proceedings of the National Academy of Sciences of USA 110: 19456–19459.
Vilà, M., J. L. Espinar, M. Hejda, P. E. Hulme, V. Jarosik, J. L. Maron, J. Pergl, U. Schaffner, Y. Sun & P. Pysek, 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14: 702–708.
Walsh, J. R., S. R. Carpenter & M. J. Vander Zanden, 2016. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proceedings of the National Academy of Sciences of the United States of America 113: 4081–4085.
Warren, R. J., J. R. King, C. Tarsa, B. Haas & J. Henderson, 2017. A systematic review of context bias in invasion biology. PLoS ONE 12: e0182502.
Webber, B. L. & J. K. Scott, 2012. Rapid global change: implications for defining natives and aliens. Global Ecology and Biogeography 21: 305–311.
Willig, M. R., D. M. Kaufman & R. D. Stevens, 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annual Review of Ecology, Evolution, and Systematics 34: 273–309.
Wilson, J. R. U., P. García-Díaz, P. Cassey, D. M. Richardson, P. Pyšek & T. M. Blackburn, 2016. Biological invasions and natural colonisations are different – the need for invasion science. NeoBiota 31: 87–98.
Wood, K. A., M. T. O’Hare, C. McDonald, K. R. Searle, F. Daunt & R. A. Stillman, 2017. Herbivore regulation of plant abundance in aquatic ecosystems. Biological Reviews of the Cambridge Philosophical Society 92: 1128–1141.
Yoon, S. & Q. Read, 2016. Consequences of exotic host use: impacts on Lepidoptera and a test of the ecological trap hypothesis. Oecologia 181: 985–996.
Zhang, P., B. Li, J. Wu & S. Hu, 2019. Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecology Letters 22: 200–210.
Zwerschke, N., L. Eagling, D. Roberts & N. O’Connor, 2020. Can an invasive species compensate for the loss of a declining native species? Functional similarity of native and introduced oysters. Marine Environmental Research 153: 104793.
Zych, I., D. P. Farrington & M. M. Ttofi, 2019. Protective factors against bullying and cyberbullying: a systematic review of meta-analyses. Aggression and Violent Behavior 45: 4–19.
Acknowledgements
We are grateful to Montserrat Vilá, Julia Koricheva, Verónica Ferreira, Mads Thomsen, and Ella McKnight for fruitful discussions and for providing information on various issues associated with the interpretation of the data. The critical comments by two anonymous reviewers were very helpful for improving the original manuscript. This work was partially funded by ANPCYT-PICT 2015-2598 to DB and ANPCYT-PICT 2015-3513 to EMP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Katya E. Kovalenko, Fernando M. Pelicice, Lee B. Kats, Jonne Kotta & Sidinei M. Thomaz / Aquatic Invasive Species III
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boltovskoy, D., Correa, N.M., Burlakova, L.E. et al. Traits and impacts of introduced species: a quantitative review of meta-analyses. Hydrobiologia 848, 2225–2258 (2021). https://doi.org/10.1007/s10750-020-04378-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04378-9