Abstract
Hydrological regimes are seasonally variable in river-floodplain ecosystems. Thus, since in these environments the local and regional factors change at different temporal scales, factors structuring metacommunities might also differ over time. However, temporal dynamics of metacommunities have rarely been assessed. Here, we investigated the influence of environmental and spatial factors over time on the metacommunity structuring of periphytic ostracods in the river-floodplain system of the Upper Paraná River (Brazil). The spatial factors turned out to be more important than environmental factors, and differences in the percentage of explanation of the factors structuring ostracod metacommunities over time were significant, mainly during extreme drought period. Our results showed that the high spatial influence might be related to the low connectivity amongst environments during such extreme drought period, which can increase dispersal limitation, and consequently can increase the turnover of ostracod species throughout the region, leading to a higher beta-diversity of ostracod metacommunities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metacommunities are defined as “a set of local communities that are linked by dispersal of multiple potentially interacting species” (Leibold et al., 2004). The metacommunity approach aims to assess the role of local (e.g., environmental filtering) and regional (spatial process, e.g., dispersal limitation) factors structuring communities (Alahuhta et al., 2014). Local and regional factors can furthermore be variable at different time scales (Vanschoenwinkel et al., 2010). Consequently, the patterns of species distribution (e.g., abundance and composition variation), and the factors structuring such patterns, can differ over time (Wojciechowski et al., 2017; Bellier et al., 2014). Nevertheless, few local communities are sampled over significant time frames (Wojciechowski et al., 2017) and many studies have evaluated metacommunities with snapshot sampling (a single sampling event in time), as shown by Heino et al. (2015). Such studies assume that mechanisms influencing metacommunity structuring do not vary through time and thus frequently misrepresent the importance of temporal scales (Fernandes et al., 2014).
Riverine floodplains are excellent freshwater ecosystems to study metacommunity structure over time, owing to the presence of temporal variability in the hydrological regime, mainly related to the variation in water level (Junk & Sparks, 1989). However, this fluvial regime in itself is influenced by multiple factors, such as climatic conditions (Berri et al., 2002) associated to dam constructions (Souza Filho, 2009). In the floodplains of southern Brazil, for example, the high intensity of drought periods might be related to La Ninã phenomena (a phase of the ENSO—El Niño-Southern Oscillation—Berri et al., 2002; Grimm & Tedeschi, 2009). During flood periods, because of higher water levels, the higher connectivity amongst the habitats in floodplains (e.g., river channels, lakes, and secondary channels) decreases the spatial variability in relation to abiotic factors (environmental heterogeneity) (Thomaz et al., 2007). Inversely, during drought periods, with lower water levels, the environments are more isolated and disconnected from each other, thus increasing the abiotic variability and creating habitats with different characteristics (Junk & Sparks, 1989). In this way, hydrological regimes can have large effects on the variability in species composition (beta-diversity), because during drought periods, the replacement of species (turnover) between the environments is higher, thus decreasing similarity in community species composition (Lopes et al., 2014), when compared to flood periods (Conceição et al., 2018).
The influence of drought or flood periods has been demonstrated in several ecological communities in river-floodplain systems (see Thomaz et al., 2004). However, the variation in intensity and duration of the extreme flood or extreme drought periods has shown to have higher effects on fish (Oberdorff et al., 2001), zooplankton (Simões et al., 2013), phytoplankton (Bortolini et al., 2016), and ostracod (Conceição et al., 2018) communities.
Ostracods are small crustaceans, which abound in many aquatic and even (semi-) terrestrial ecosystems (Higuti et al., 2007; Liberto et al., 2012; Higuti & Martens, 2016). In riverine floodplains, ostracods can be found in several habitats, mainly in association with root systems of aquatic macrophytes, which serve as place of breeding, feeding and protection against predators (Higuti et al., 2007). Recent studies have evaluated the effect of environmental and spatial factors on ostracod metacommunities (Escrivà et al., 2015; Zhai et al., 2015; Castillo-Escrivà et al., 2016a, b, c, 2017; Rosati et al., 2017; Campos et al., 2018). However, only Castillo-Escrivà et al. (2017) evaluated ostracod metacommunities over time, in temporary lakes in Spain, and found little temporal influence. Thus, the temporal dynamics of ostracod metacommunities in river-floodplain systems remains poorly explored.
Here, we evaluated the factors (environmental and spatial) structuring the metacommunity of periphytic ostracods over time in the river-floodplain system of the Upper Paraná River. Based on the seasonal changes in hydrological characteristics of this ecosystem, we expected that the influence of the factors structuring ostracod metacommunities is temporally variable in extreme drought period (here defined as intense and prolonged periods of low water levels). More specifically, during extreme drought period we expected a higher influence of spatial factors (probably related to dispersal limitation), and consequently a higher beta-diversity of ostracod metacommunities.
Materials and methods
Study area
The Upper Paraná River includes approximately the first third of the Paraná River Basin with a drainage area of 891,000 km2 (Agostinho et al., 2008). It has an extensive floodplain on its west side, which is 230 km long and more than 20 km wide between the Porto Primavera Dam and the beginning of the Itaipu Reservoir. This represents the only dam-free stretch of the Paraná River in Brazilian territory (Agostinho et al., 2004).
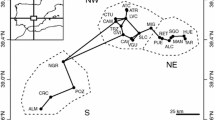
The study area are a river-floodplain system, which encompasses the main river (Paraná River), several secondary rivers (named here tributaries), and both permanently and temporarily connected lakes (Fig. 1).
Sampling and laboratory analysis
Ostracods were collected between February 2014 and May 2015, including 4 sampling periods in 2014 (summer—February, autumn—May, winter—August, and spring—November) and 2 sampling periods in 2015 (summer—February and autumn—May). No flooding event was recorded in the Upper Paraná River floodplain during the sampling periods, and the highest water levels were observed before our sampling in summer 2014 (February) (Fig. 2). On the other hand, there was an extreme drought period before our sampling in winter 2014 (August), from June 2014 to August 2014, with a duration of 65 days and an average water level of 152 cm (Fig. 2). Water level data were obtained from the LTER program (Long Term Ecological Research, site 6—http://www.peld.uem.br) developed by researchers of the Centre of Research in Limnology, Ichthyology, and Aquaculture (Nupélia) from the State University of Maringá (UEM).
We selected 15 sampling sites along the Paraná River-floodplain system, which comprise four sites along the main channel of Paraná River (1–4), five sites in tributaries (5—Baía, 6—Ivinhema1, 7—Ivinhema2, 8—Amambaí and 9—Iguatemi) and six sites in permanently connected lakes (10—Garças, 11—Xirica, 12—Ivaí, 13—São João, 14—Pavão and 15—Saraiva) (Fig. 1). In each sampling site, one to three samples of periphytic ostracods were taken, according to the presence of different macrophytes patches. Mostly we selected different patches of the floating macrophyte Eichhornia crassipes (Mart.) Solms. However, in some periods, E. crassipes disappeared (completely or partially) from some sites and we sampled in patches of Eichhornia azurea (Swartz) Kunth (or in both macrophytes species). Every patch was located in the marginal region of the environments. In the tributaries and sites in the main channel of Paraná River, these marginal regions had low influence of the water current, which allowed the growth of floating macrophytes patches. Thus, although we sampled in both lentic (open lakes) and lotic (tributaries and sites in main channel) environments, we considered the samples comparable. Between 41 and 45 samples were taken in each sampling period (e.g., autumn 2015: 15 sampling sites × 3 patches of macrophytes = 45 samples), totalling 259 samples (see Table S1).
After the patches were selected, macrophytes were removed manually from the water, immediately transferred to a plastic bucket and washed to remove the periphytic ostracods (more details about this method in Campos et al., 2017). The aerial part of the macrophytes was separated and only the submerged roots were placed in plastic bags, dried and weighed in the lab to calculate ostracod densities (dividing the total number of organisms by the dry weight of the roots). The material retained in the bucket was filtered through a hand net with 160 µm mesh size and preserved in 70° ethanol buffered with sodium tetraborate.
Samples were divided with a Folsom fractionator and ¼ of the sample was quantified to estimate densities. However, the complete sample was used to estimate richness and abundance of species, even of those that were not recorded in the subsample. Ostracods were sorted using a stereoscopic microscope and were identified down to species level using specialized literature (see Martens & Behen, 1994 and articles included therein; Rossetti & Martens, 1998; Higuti et al., 2013; Higuti & Martens, 2012a, b, 2014).
Environmental and spatial factors
We measured four chemical and physical variables, such as pH and electrical conductivity (μS cm−1) (YSI 63), dissolved oxygen (mg l−1), and water temperature (°C) (YSI 550A oximeter), which were measured in situ. These measurements were carried out during the same period of the day, to avoid problems with the daily variation, mainly in oxygen and temperature. These variables were considered “environmental factors”, and their data were log transformed for the analyses, except for pH.
We used three different methods to generate “spatial factors”. Firstly, we calculated matrices of Euclidean (overland, derived from geographical coordinates) and watercourse distances between sampling sites. These matrices (“overland” and “watercourse”) were submitted to the PCNM method (Principal Coordinates of Neighbor Matrices), for the construction of the explanatory spatial variables (eigenvectors) (Borcard & Legendre, 2002). The PCNM analysis was truncated by the minimum distance that kept all sampling sites connected (minimum spanning tree procedure; Landeiro et al., 2011). Finally, a spatial matrix was constructed considering the sampling sites connected in the watercourse, following the flow direction of the main river (Paraná River), north to south/southwest. This matrix was submitted to the asymmetric eigenvectors maps (AEM) and the generated axes (eigenvectors) were considered the explanatory spatial variables (Blanchet et al., 2008a). In the present study, the first PCNMs and AEMs generated represent larger scales of amplitude, whereas latter ones represent smaller scaling variations. We chose these three spatial methods (“overland”, “watercourse”, AEM) owing to the several ways that ostracods could passively disperse through the region (e.g., overland = carried by the wind and birds; watercourse = carried by fishes and other vertebrates; from upstream to downstream = carried by floating macrophytes or through the flow). Thus, we can also assess which of these different forms of ostracod dispersal can be affected (or not) over time by the variation on the hydrological regime (e.g., extreme drought periods).
Data analysis
We investigated the variation in relative importance (R2) of the environmental and spatial factors (using variables generated for the three spatial methods) on ostracod metacommunity structuring over time analyzing data of each season separated. We used a Partial Redundancy Analysis (pRDA) with density data matrices of ostracods, transformed following the Hellinger method (Peres-Neto et al., 2006), which is appropriate for matrices comprising large numbers of zeros (Legendre & Gallagher, 2001). Ostracod metacommunity variation was partitioned into a purely environmental component (E), a purely spatial component (S), a component explained by environmental and spatial factors combined (E∩S), and the unexplained variation (U). We used the forward selection method with two stopping rules (Blanchet et al., 2008b), in order to identify the main variables (environmental and spatial) which should be included in the analysis (P < 0.01, 999 permutations). The results were adjusted R2 values and the components (E and S) were considered significant when P < 0.05.
We evaluated the patterns of ostracod beta-diversity for each sampling period, based on multiple sites within river-floodplain system of the Upper Paraná River. We partitioned the Sørensen dissimilarity index into turnover and nestedness components, according to Baselga (2010, 2012). We used the “beta.multi’ function to calculate the multiple-site dissimilarities accounting for the spatial turnover (species replacement) and nestedness (species loss) components, such as the sum of both values. Beta-diversity measurements may help revealing the degree of differentiation of species composition amongst the sampling periods.
Possible differences in environmental heterogeneity amongst the sampling periods were investigated using the permutation test of multivariate homogeneity of group dispersion (PERMDISP, Anderson et al., 2006). We also used PERMDISP to investigate possible differences in environmental heterogeneity between the types of environment (lentic and lotic), which present different characteristics (e.g., connectivity) and might be influenced in different ways by the water level. The PERMDISP test is based on the distance between the sampling units and their group centroid, using a dissimilarity measure (Anderson et al., 2006). Thus, a great average distance of the sampling points to their centroid corresponds to a high environmental heterogeneity. The environmental heterogeneity was defined by standardized Euclidean distances of environmental factors. To test statistical differences in environmental heterogeneity (amongst the sampling periods and between lotic and lentic environments, P < 0.05), PERMDISP used an ANOVA, through 999 permutations. Analyses were performed in R 3.4 software (R Core Team, 2017), vegan (Oksanen et al., 2017), permute (Simpson et al., 2017), adespatial (Dray et al., 2018) and betapart (Baselga et al., 2018) packages.
Results
Taxonomic diversity
We recorded 40 species of ostracods, belonging to four families. Cyprididae had the highest richness (31 species), followed by Candonidae (5), Darwinulidae (3), and Limnocytheridae (1). Half of the species occurred in all sampling periods and the most abundant species were Cypricercus centrura (Klie, 1940), Cytheridella ilosvayi (Daday, 1905), and Cypretta costata (G. W. Müller, 1898) (Table 1).
Selected spatial and environmental factors
The selected environmental factors (forward selection) were variable over time and the most frequently found were water temperature and dissolved oxygen, in summer, autumn, and winter 2014 and autumn 2015 (excepted for dissolved oxygen in winter 2014) and electrical conductivity, in spring 2014 and summer and autumn 2015. Regarding the spatial factors for the “overland” method, the selected PCNMs represented broad spatial scales patterns, for all sampling periods. For the “watercourse”, the selected PCNMs were variable over time and represented broad and intermediate spatial scale patterns. For the AEM method, selected AEMs were also variable over time and indicated broad spatial scale patterns (See more in Table 2).
Factors structuring the ostracod metacommunity over time
The pRDA analysis showed a significant influence of the environmental factors in all sampling periods (except for autumn 2015, partitioned with “watercourse” matrix). Similarly, spatial factors were significant in all sampling periods for the three spatial methods analyzed (“overland”, “watercourse”, and AEM) (Fig. 3).
A considerable percentage of potential explanation for the spatial factors was found for all spatial methods (up to 34%) and these values were variable over time (“overland” = from 9% to 26%, “watercourse” = from 5 to 34% and AEM = from 4 to 21%), with higher values in the extreme drought period (winter 2014) (Fig. 3). In contrast, lower percentages of potential explanation power were observed for environmental factors, varying from 0 to 10%. The shared fraction of environmental and spatial factors was also variable over the time, from 0 to 11%. The unexplained fraction remained relatively high in all sampling periods, varying from 62 to 88% (Fig. 3).
Beta-diversity and environmental heterogeneity
Ostracod metacommunity showed high levels of beta-diversity and the variation in community composition was almost entirely attributed to turnover rather than to the nestedness component, for all sampling periods. The highest values of overall beta-diversity (Sørensen) and turnover were found in the extreme drought period (winter 2014) (Table 2).
The environmental heterogeneity amongst the sampling periods (average distance of the centroid in 2014, summer = 11.042, autumn = 12.464, winter = 10.342 and spring = 13.235; and 2015, summer = 13.085 and autumn = 11.042) and between the types of environments (lotic = 12.02 and lentic = 11.67) were not significant. (F =2.18, P = 0.06; F =0.12, P = 0.72, respectively). Thus, the environmental characteristics were similar over time (Fig. S1A) and between the types of environments (lotic and lentic) (Fig. S1B).
Discussion
Selected environmental and spatial factors
The selected abiotic variables are known to be important to ostracod metacommunities. For example, water temperature affects life history and body size of these organisms (Aguilar-Alberola & Mesquita-Joanes, 2014; Castillo-Escrivà et al., 2016a) and studies have found a correlation between ostracod distribution on the one hand and dissolved oxygen (Nagorskaya & Keyser, 2005; Higuti et al., 2017) and electrical conductivity (Liberto et al., 2012) on the other hand. Besides, the selected spatial variables (PCNMs and AEMs), representing broad-scale patterns of metacommunity variation, confirmed that the distance amongst the environments (e.g., between the upstream to downstream region) was important for the structuring of the metacommunity (e.g., degree of limiting the dispersal).
Factors structuring ostracod metacommunity over time
The spatial factors were usually more important for the ostracod metacommunity structuring than environmental factors over time. This might be related to the shared fraction between environmental and spatial factors, that was significantly high in some sampling periods, suggesting that part of the environmental gradient was spatially structured, which may have led, for example, to a decrease in the influence of environmental factors. Likewise, unexplained variation remained high in all sampling periods and other variables, which were not measured in our study, could be important for metacommunity structuring (and could increase environmental effects). For example, biological interactions (with other plants, animals, micro-organisms) and stochastic processes (communities randomly assembled) can also be responsible for the structuring of metacommunities (Chase, 2007; Nabout et al., 2009) and their effects would all be summarized in the “unexplained variability”.
We also attributed the considerable influence of spatial factors in all sampling periods to two main drivers. Firstly, the large size of the study area (approx. 200 km long and 20 km wide), which might prevent colonization of ostracods throughout the entire region, for example, from upstream to downstream region (as showed in AEM results). Secondly, aquatic macrophytes are passive disperses (here E. crassipes and E. azurea) and owing to their larger size, the dispersal range might be lower (De Bie et al., 2012). This can also be responsible for dispersal limitation in the ostracod metacommunity, since the periphytic ostracods studied here are strongly associated with root systems of these plants, which serve as shelter, place of reproduction, and foraging (Liberto et al., 2012). For example, Padial et al. (2014) showed that the spatial factors were the most important ones for structuring aquatic macrophyte communities in the Upper Paraná River floodplain. Besides, Campos et al. (2018) demonstrated the importance of aquatic macrophytes for ostracod metacommunity structuring, where macrophyte richness at a local scale influenced the dispersal of non-swimming ostracods.
The hypothesis that “everything is everywhere, but, the environment selects” predicts that low dispersal limitation of the micro-organisms is attributed to high passive dispersal and high propagule numbers (O’Malley, 2007). Although ostracods are good passive dispersers (e.g., eggs, juveniles, and adults can be carried overland by waterfowl and wind, and throughout the watercourse by fish and plants—Meisch, 2000; Brochet et al., 2010; Pereira et al., 2017), our results showed that spatial factors might also be important. Furthermore, species sorting (environmental filtering) appeared to be a poor explanative factor for ostracod metacommunity structuring (maybe related to an important environmental variable which was not measured), and this is unusual for most aquatic micro-organisms, such as periphyton (Algarte et al., 2014), phytoplankton (Padial et al., 2014) and zooplankton (Souffreau et al., 2015; Lansac-Tôha et al., 2016; Rocha et al., 2017). Recent studies have found that at least part of the structure of the ostracod metacommunities is related to spatial factors (see Zhai et al., 2015; Castillo-Escrivà et al., 2016b, c), and our results confirm this.
Since the spatial factors influencing the structuring of ostracod metacommunities were variable over time in the river-floodplain system of the Upper Paraná River, we infer that studies using snapshot samples might not always be efficient to show patterns of ostracod metacommunity structure. Furthermore, it must be emphasized that this type of sampling should be carefully evaluated in years with extreme events (e.g., extreme inundation or extreme droughts of long duration). For example, temporal analysis of ostracod community persistence and variability in a lake of the Upper Paraná River showed that this community was buffered to regular variation in water level but was significantly affected by extreme inundations (Conceição et al., 2018). Thus, in such cases, the factors structuring the metacommunity might change over time. In addition, the efficiency of snapshot samples also depends on which metacommunity is studied. For example, Wojciechowsk et al. (2017) showed that the strength of species sorting is temporally variable in phytoplankton metacommunities in reservoirs in southern Brazil. Fernandes et al. (2014) suggested that in the Brazilian Pantanal floodplain, fish metacommunity were structured by changes between dispersal limitation and environmental filtering over time. Therefore, in metacommunities in which the distribution is naturally variable over time, the factors affecting the metacommunity structuring might change over different time scales.
Beta-diversity and environmental heterogeneity
A higher percentage of potential explanation of the spatial factors was observed during the extreme drought period, which also showed the highest turnover and beta-diversity. Because connectivity amongst aquatic environments is reduced in periods of low water levels (Junk & Sparks, 1989), dispersal of ostracods species in this period is limited. Similarly, Driver & Hoeinghaus (2016) showed that prolonged drought periods in two rivers in the USA have strong effects on fish metacommunities, increasing dispersal limitation owing to the reduction of the connectivity amongst the habitats. Likewise, low connectivity amongst and between the environments during the extreme drought period might increase the turnover of species (which is confirmed by the higher influence of spatial factors, see second section of the discussion) and consequently will increase the beta-diversity in ostracod metacommunities.
River-floodplain ecosystems experience natural water level variation along the year. Furthermore, environmental heterogeneity decreases during high water levels (frequent in wet seasons) owing to increased connectivity of the environments (Thomaz et al., 2007). In the Upper Paraná River floodplain, environments become totally connected when the water level reaches 460 cm (Souza Filho, 2009). In the present study, the environmental heterogeneity was not seasonally different and this is most likely related to the relatively low ‘high water levels’ during the sampling period (see Fig. 2), which did not exceed 380 cm in the wet seasons. Thus, the variation in water level might not have been enough to change the abiotic characteristics of the environments over time.
In general, high environmental heterogeneity leads to an increase in beta-diversity (Astorga et al., 2014). Whereas the environmental heterogeneity did not change over time in the present study (and also not between types of environments), the beta-diversity was higher during the extreme drought period (winter 2014). Therefore, the variation in beta-diversity does not always follow the variation in environmental heterogeneity, which is in agreement with other studies (e.g., Bini et al., 2014; Higuti et al., 2017).
Conclusion
The present study showed significant temporal effects on ostracod metacommunity structuring, because spatial effects were variable over time. Thus, during extreme drought periods, low water levels might influence ostracod metacommunity structuring and increase the effects of spatial factors. We have corroborated our predictions, owing to the higher explanation percentage of spatial factors during (extreme) drought period, probably related to the lower connectivity and indicating possible dispersal limitation, which also led to a higher beta-diversity in ostracod metacommunities.
References
Agostinho, A. A., L. C. Gomes, S. Veríssimo & E. K. Okada, 2004. Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Reviews in Fish biology and Fisheries 14: 11–19.
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68: 1119–1132.
Aguilar-Alberola, J. A. & F. Mesquita-Joanes, 2014. Breaking the temperature-size rule: thermal effects on growth, development and fecundity of a crustacean from temporary waters. Journal of Thermal Biology 42: 15–24.
Alahuhta, J., L. B. Johnson, J. Olker & J. Heino, 2014. Species sorting determines variation in the community composition of common and rare macrophytes at various spatial extents. Ecological Complexity 20: 61–68.
Algarte, V. M., L. Rodrigues, V. L. Landeiro, T. Siqueira & L. M. Bini, 2014. Variance partitioning of deconstructed periphyton communities: does the use of biological traits matter? Hydrobiologia 722: 279–290.
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9: 683–693.
Astorga, A., R. Death, F. Death, R. Paavola, M. Chakraborty & T. Muotka, 2014. Habitat heterogeneity drives the geographical distribution of beta diversity the case of New Zeland stream invertebrates. Ecology and Evolution 4: 2693–2702.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A., 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21: 1223–1232.
Baselga, A., D. Orne, S. Villeger, J. Bortoli & F. Leprieur, 2018. betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R package version 1.5.0.
Bellier, E., V. Grøtan, S. Engen, A. K. Schartau, I. Herfindal & A. G. Finstad, 2014. Distance decay of similarity, effects of environmental noise and ecological heterogeneity among species in the spatio-temporal dynamics of a dispersal-limited community. Ecography 36: 1–11.
Berri, G. J., M. A. Ghietto & N. O. García, 2002. The influence of ENSO in the flows of the upper Paraná River of South America over the past 100 years. Journal of Hydrometeorology 3: 57–65.
Bini, L. M., V. L. Landeiro, A. A. Padial, T. Siqueira & J. Heino, 2014. Nutrient enrichment is related to two facets of beta diversity for stream invertebrates across the United States. Ecology 95: 1569–1578.
Blanchet, F. G., P. Legendre & D. Bocard, 2008a. Modelling directional spatial processes in ecological data. Ecological Modelling 215: 325–336.
Blanchet, G., P. Legendre & D. Borcard, 2008b. Forward selection of spatial explanatory variables. Ecology 89: 2623–2632.
Borcard, D. & P. Legendre, 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling 153: 51–68.
Bortolini, J. C., S. Train & L. C. Rodrigues, 2016. Extreme hydrological periods: effects on phytoplankton variability and persistence in a subtropical floodplain. Hydrobiologia 763: 223–236.
Brochet, A. L., M. Gauthier-Clerc, M. Guillemain, H. Fritz, A. Waterkeyn, Á. Baltanás & A. J. Green, 2010. Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camargue (southern France). Hydrobiologia 637: 255–261.
Campos, R., E. O. Conceição, M. B. O. Pinto, A. P. S. Bertocin, J. Higuti & K. Martens, 2017. Evaluation of quantitative sampling methods in pleuston: an example from ostracod communities. Limnologica 63: 36–41.
Campos, R., F. M. Lansac-Tôha, E. O. Conceição, K. Martens & J. Higuti, 2018. Factors affecting the metacommunity structure of periphytic ostracods (Crustacea, Ostracoda): a deconstruction approach based on biological traits. Aquatic Sciences 80: 1–16.
Castillo-Escrivà, A., J. Poquet & F. Mesquita-Joanes, 2015. Effects of environmental and spatial variables on lotic ostracod metacommunity structure in the Iberian Peninsula. Inland Waters 5: 283–294.
Castillo-Escrivà, A., J. Rueda, L. Zamora, R. Hernández, M. Del Moral & F. Mesquita-Joanes, 2016a. The role of watercourse versus overland dispersal and niche effects on ostracod distribution in Mediterranean streams (eastern Iberian Peninsula). Acta Oecologica 73: 1–9.
Castillo-Escrivà, A., L. Valls, C. Rochera, A. Camacho & F. Mesquita-Joanes, 2016b. Disentangling environmental, spatial, and historical effects on ostracod communities in shallow lakes. Hydrobiologia 789: 1–12.
Castillo-Escrivà, A., L. Valls, C. Rochera, A. Camacho & F. Mesquita-Joanes, 2016c. Spatial and environmental analysis of an ostracod metacommunity from endorheic lakes. Aquatic Sciences 78: 707–716.
Castillo-Escrivà, A., L. Valls, C. Rochera, A. Camacho & F. Mesquita-Joanes, 2017. Metacommunity dynamics of Ostracoda in temporary lakes: overall strong niche effects except at the onset of the flooding period. Limnologica 62: 104–110.
Chase, J. M., 2007. Drought mediates the importance of stochastic community assembly. Proceedings of the National Academy of Sciences 104: 17430–17434.
Conceição, E. O., J. Higuti, R. Campos & K. Martens, 2018. Effects of flood pulses on persistence and variability of pleuston communities in a tropical floodplain lake. Hydrobiologia 807: 175–188.
De Bie, T., L. Meester, L. Brendonck, K. Martens, B. Goddeeris, D. Ercken, H. Hampel, L. Denys, L. Vanhecke, K. Van der Gucht, J. Van Wichelen, W. Vyverman & S. A. J. Declerck, 2012. Body size and dispersal mode as key traits deter- mining metacommunity structure of aquatic organisms. Ecology Letters 15: 740–747.
Dray, S., D. Bauman, G. Blanchet, D. Bocard, S. Clappe, G. Guenard, T. Jombart, G. Larocque, P. Legendre, N. Madi & H. H. Wagner, 2018. adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.3-2.
Driver, L. J. & D. J. Hoeinghaus, 2016. Spatiotemporal dynamics of intermittent stream fish metacommunities in response to prolonged drought and reconnectivity. Marine and Freshwater Research 67: 1667–1679.
Fernandes, I. M., R. Henriques-Silva, J. Penha, J. Zuanon & P. R. Peres-Neto, 2014. Spatiotemporal dynamics in a seasonal metacommunity structure is predictable: the case of floodplain-fish communities. Ecography 37: 464–475.
Grimm, A. M. & R. G. Tedeschi, 2009. ENSO and extreme rainfall events in South America. Journal of Climate 22: 1589–1609.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845–869.
Higuti, J. & K. Martens, 2012a. Description of a new genus and species of Candonopsini (Crustacea, Ostracoda, Candoninae) from the alluvial valley of the Upper Paraná River (Brazil, South America). European Journal of Taxonomy 33: 1–31.
Higuti, J. & K. Martens, 2012b. On a new cypridopsine genus (Crustacea, Ostracoda, Cyprididae) from the Upper Paraná River Floodplain (Brazil). Zootaxa 38: 23–38.
Higuti, J. & K. Martens, 2014. Five new species of Candoninae (Crustacea, Ostracoda) from the alluvial valley of the Upper Paraná River (Brazil, South America). European Journal of Taxonomy 106: 1–36.
Higuti, J. & K. Martens, 2016. Invasive South American floating plants are a successful substrate for native Central African pleuston. Biological Invasions 18: 1191–1201.
Higuti, J., L. F. M. Velho, F. A. Lansac-Tôha & K. Martens, 2007. Pleuston communities are buffered from regional flood River pulses: the example of ostracods in the Paraná River floodplain, Brazil. Freshwater Biology 52: 1930–1943.
Higuti, J., I. Schön, L. Audenaert & K. Martens, 2013. On the Strandesia obtusata/elliptica lineage (Ostracoda, Cyprididae) in the alluvial valley of the upper Paraná River (Brazil), with the description of three new species. Crustaceana 86: 182–211.
Higuti, J., E. O. Conceição, R. Campos, V. G. Ferreira, J. Rosa, M. B. O. Pinto & K. Martens, 2017. Periphytic community structure of Ostracoda (Crustacea) in the river-floodplain system of the Upper Paraná River. Acta Limnologica Brasiliensia 29: e120.
Junk, W. J. & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Landeiro, V., W. E. Magnusson, A. S. Melo, H. M. V. Espírito-Santo & L. M. Bini, 2011. Spatial eigenfunction analyses in stream networks: do watercourse and overland distances produce different results? Freshwater Biology 56: 1184–1191.
Lansac-Tôha, F. M., B. R. Meira, B. T. Segovia, F. A. Lansac-Tôha & L. F. M. Velho, 2016. Hydrological connectivity determining metacommunity structure of planktonic heterotrophic flagellates. Hydrobiologia 781: 81–94.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Liberto, R., F. Mesquita-Joanes & I. César, 2012. Dynamics of pleustonic ostracod populations in small ponds on the Island of Martín García (Rio de la Plata, Argentina). Hydrobiologia 688: 47–61.
Lopes, P. M., L. M. Bini, S. A. J. Declerck, V. F. Farjalla, L. C. G. Vieira, C. C. Bonecker, F. A. Lansac-Toha, F. A. Esteves & R. L. Bozelli, 2014. Correlates of zooplankton beta diversity in tropical lake systems. PLoS ONE 9: e109581.
Martens, K. & F. Behen, 1994. A Checklist of the Recent Non-Marine Ostracods (Crustacea, Ostracoda) from the Inland Waters of South America and Adjacent Islands. Travaux Scientifiques du Musée National d’Histoire Naturelle de Luxembourg, Luxembourg 22: 1–81.
Meisch, C., 2000. Freshwater Ostracoda of Western and Central Europe. In Schwoerber, J. & P. Zwick (eds), Sußwasserfauna von Mitteleuropa 8/3. Spektrum Akademischer Verlag, Heidelberg: 522.
Nabout, C., T. Siqueira, L. M. Bini & I. D. S. Nogueira, 2009. No evidence for environmental and spatial processes in structuring phytoplankton communities. Acta Oecologica 35: 1–7.
Nagorskaya, L. & D. Keyser, 2005. Habitat diversity and ostracod distribution patterns in Belarus. Hydrobiologia 538: 167–178.
Oberdorff, T., B. Hugueny & T. Vigneron, 2001. Is assemblage variability related to environmental variability? An answer for riverine fish. Oikos 93: 419–428.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2017. vegan: Community Ecology Package. R package version 2.4-1, https://cran.r-project.org/package=vegan. Accessed 10 Nov. 2017.
O’Malley, M. A., 2007. The nineteenth century roots of ‘everything is everywhere’. Nature Reviews Microbiology 5: 647–651.
Padial, A., F. Ceschin, S. A. J. Declerck, L. De Meester, C. C. Bonecker, F. A. Lansac-Tôha, L. Rodrigues, L. C. Rodrigues, S. Train, L. F. M. Velho & L. M. Bini, 2014. Dispersal ability aetermines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9: 1–8.
Pereira, L. C., F. A. Lansac-Tôha, K. Martens & J. Higuti, 2017. Biodiversity of ostracod communities (Crustacea, Ostracoda) in a tropical floodplain. Inland Waters 7: 323–332.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625.
R Core Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org. Accessed 10 Nov. 2017
Rocha, M. P., J. Heino, L. F. M. Velho, F. M. Lansac-Tôha & F. A. Lansac-Tôha, 2017. Fine spatial grain, large spatial extent and biogeography of macrophyte-associated cladoceran communities across Neotropical floodplains. Freshwater Biology 62: 559–569.
Rosati, M., G. Rossetti, M. Cantonati, V. Pieri, J. R. Roca & F. Mesquita-Joanes, 2017. Are aquatic assemblages from small water bodies more stochastic in dryer climates?An analysis of ostracod spring metacommunities. Hydrobiologia 793: 199–212.
Rossetti, G. & K. Martens, 1998. Taxonomic revision of the Recent and Holocene representatives of the family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie 68: 55–110.
Simões, N. R., F. A. Lansac-Tôha & C. C. Bonecker, 2013. Drought disturbances increase temporal variability of zooplankton community structure in floodplains. International Review of Hydrobiology 98: 24–33.
Simpson, G. L., 2017. Permute: Functions for Generating Restricted Permutations of Data. R package version 0.9-4. https://cran.r-project.org/package=permute. Accessed 10 Nov. 2017.
Sokal, R. R. & S. L. Oden, 1978. Spatial autocorrelation in biology. 1. Methodology. Biological Journal of the Linnean Society 10: 199–228.
Souffreau, C., K. Van der Gucht, I. Gremberghe, S. Kosten, G. Lacerot, L. M. Lobão, V. L. M. Huszar, F. Roland, E. Jeppesen, W. Vyverman & L. De Meester, 2015. Environmental rather than spatial factors structure bacterioplankton communities in shallow lakes along a > 6000 km latitudinal gradient in South America. Environmental Microbiology 17: 2336–2351.
Souza Filho, E. E., 2009. Evaluation of the Upper Paraná River discharge controlled by reservoirs. Brazilian Journal of Biology 69: 707–716.
Thomaz, S. M., T. A. Pagioro, L. M. Bini, M. C. Roberto & R. R. A. Rocha, 2004. Limnological characterization of the aquatic environments and the influence of hydrometric levels. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and Its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 75–102.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Vanschoenwinkel, B., A. Waterkeyn, M. Jocqué, L. Boven, M. Seaman & L. Brendonck, 2010. Species sorting in space and time—the impact of disturbance regime on community assembly in a temporary pool metacommunity. Journal of the North American Benthological Society 29: 1267–1278.
Wojciechowski, J., J. Heino, L. M. Bini & A. A. Padial, 2017. The strength of species sorting of phytoplankton communities is temporally variable in subtropical reservoirs. Hydrobiologia 800: 31–43.
Zhai, M., O. Novácek, D. Výravský, V. Syrovátka, J. Bojková & J. Helesic, 2015. Environmental and spatial control of ostracod assemblages in the Western Carpathian spring fens. Hydrobiologia 745: 225–239.
Acknowledgements
We thank the Ministry of Science and Technology (MCT)/National Council for Scientific and Technological Development (CNPq)/Fundacão Araucária for financial support to the present project (Process: 478629/2012-5) and to the Long–Term Ecological Research - LTER. We thank the Centre of Research in Limnology, Ichthyology and Aquaculture (Nupélia) and the Graduate Program in Ecology of Inland Water Ecosystems (PEA) of the State University of Maringá (UEM) and the Academic Excellency Program (Proex)/Coordination for the Improvement of Higher Education Personnel (CAPES), USACUCAR, CORIPA, ICMBio for logistic support. We also thank André A. Padial, for the statistical support and Jaime Luiz Lopes Pereira (Nupélia), for the production of the map. R.C. and E.O.C would like to thank CAPES for granting their scholarships. Two anonymous referees suggested important improvements. The State University of Maringá (UEM, Maringá) and the Royal Belgian Institute of Natural Sciences (RBINS, Brussels) have a bilateral Memorandum of Understanding regarding collaborative Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2018_3825_MOESM1_ESM.tif
Supplementary material 1 (TIFF 2721 kb). Fig. S1 Mean values, standard deviation and standard error of the environmental heterogeneity amongst the sampling periods (A) and between the lotic and lentic environments (B)
10750_2018_3825_MOESM2_ESM.docx
Supplementary material 2 (DOCX 22 kb). Table S1 Limnological characteristics (mean and standard deviation), species of macrophytes and number of samples of each sampling site in the river-floodplain system of the Upper Paraná River. WT = water temperature, EC = electrical conductivity, DO = dissolved oxygen, Ec = Eichhornia crassipes, Ea = Eichhornia azurea. (*) = values < 0.01
Rights and permissions
About this article
Cite this article
de Campos, R., Conceição, E.O., Martens, K. et al. Extreme drought periods can change spatial effects on periphytic ostracod metacommunities in river-floodplain ecosystems. Hydrobiologia 828, 369–381 (2019). https://doi.org/10.1007/s10750-018-3825-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3825-3