Abstract
Large rivers in tropical regions can experience strong variations of abiotic factors owing to the occurrence of flood pulses. Both man-made and natural floods can cause pulses with varying intensity and duration. Here, we test the hypothesis that ostracod communities in the pleuston of floating plants are persistent during regular pulses and more variable during extreme floods. Ostracod communities were monitored in the Manezinho Backwater of the Upper Paraná River floodplain (Brazil) for 83 months (seven hydrologic cycles). Flood pulses directly influenced the abiotic variables, which in turn were correlated with the species composition and abundance of ostracods. Variability in the species composition differentiated only between the limnophase of cycle 4 (L4: 2009) and the subsequent extreme flood of cycle 5 (P5: 2009–2010). The longer duration and higher intensity of the extreme flood can increase the exchange of organisms through the aquatic connections between the lake and the river and can potentially trigger the hatching of dry-resistant eggs, increasing variability in ostracod species composition. The absence of significant differences in the species composition variability between the other succeeding periods shows that these communities are persistent and buffered to the effects of natural pulses of short durations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrological (flood) pulses can cause important environmental variations in floodplain systems. Floods can be the main controller of the abiotic factors, which subsequently influence the species composition. Species developed life history strategies that allow their persistence in disturbance regime due to seasonality of the pulses (Bunn & Arthington, 2002). Besides, the flood increases the connectivity between the aquatic environments that allows the recruitment of new individuals in populations, but also facilitates the colonization of species that potentially can change the dynamics of the communities, making them more variable (Bunn & Arthington, 2002).
Neiff (1990) defines two phases in flooding cycles: potamophases (flood periods) during which horizontal flows of living organisms, debris and sediments are pushed sideways from the riverbed towards laterally situated lakes and channels, and limnophases (drought periods) during which these elements are drained back toward the main riverbed. These well-defined periods cause changes in patterns of distribution and species abundance of floodplain lake communities (Junk & Sparks, 1989; Thomaz et al., 2007). In addition to regular, moderate pulses, more irregular and larger flood pulses can also occur. In the Upper Paraná River Floodplain, for example, such extreme floods occur on average once a year (Thomaz et al., 2004). Floodplains are therefore convenient aquatic systems to study causes and consequences of variability in community structure.
High floods, and even more so extreme floods, will homogenize floodplain biodiversity through increased habitat similarity, which in turn is (in part) owing to decreased variation in limnological factors (Thomaz et al., 2007). During periods of low water levels, the landscape will become more heterogeneous through increased isolation of habitats. This will result in an increase of variability in the species composition amongst the different habitats (Bozelli et al., 2015).
The natural dynamics of flood pulses in floodplains has been strongly influenced by the construction of man-made dams. In such dams, water release to downstream habitats is controlled by man and results in irregular floods downstream of such reservoirs (Souza Filho, 2009).
Another, more natural, factor that causes interannual variations in seasonal fluctuations in the flood levels is the precipitation variation caused by the El Niño-Southern Oscillation phenomenon. The alternating El Niño and La Niña episodes are caused by anomalous warming and cooling of the waters of the Central and East Equatorial Pacific Ocean and can in addition be intensified or weakened by the difference in atmospheric pressure between the Central and West Pacific, the so-called South Oscillation index (SO; Cera & Ferraz, 2015). Occurrences of El Niño of greater amplitude can cause extreme floods around the world (Ward et al., 1999, 2014). In Brazil, for example, this phenomenon can cause intense drought in the north and higher precipitation in the south during the summer season (Cera & Ferraz, 2015).
Effects of limnophases and potamophases have already been studied in several aquatic communities. Cichlid assemblages are persistence during interannual variations of water level, showing two equilibrium points, one in the early limnophase and the other in the early potamophase (Lourenço et al., 2012). Phytoplankton species richness and abundance are more variable in periods of normal floods due to environmental fluctuations. On the other hand, in extreme limnophases (drought) these communities are persistence and less variable (Bortolini et al., 2016). The limnophase increase temporal dissimilarity of zooplankton community, whereas the potamophase maintains a lower variability of environmental characteristics, sustaining a lower temporal variability of the community (Simões et al., 2013). The effects of fluctuation in water level on macroinvertebrates are more often studied in streams. In general, macroinvertebrate communities show greater persistence in cases where flow conditions remained relatively constant (Scarsbrook, 2002; Collier, 2008).
Pleuston communities, however, have thus far rarely been investigated. Higuti et al. (2007) postulated that the origin of pleuston in general, and its associated communities, e.g., ostracods, might be an adaptation to buffer the limiting effects of flood pulses. Indeed, extreme flood pulses, causing for example an increase in water level of 6 m or more overnight, might cause sediments to become anoxic. This is a danger for obligate benthic communities, but pleuston in root systems of floating plants, typical of South American floodplains, will not be impacted as these plants simply follow the rising water levels. Given that ostracods can abound in pleuston (Higuti & Martens, 2016), they are an excellent model system to study effects of flood pulses on community structure.
Here, we investigate the effects of flood pulses on the variability of the species composition in ostracod communities over seven hydrological cycles (potamophase and limnophase periods). We analyze the variability over subsequent periods (P1, L1, P2, L2,…,P7, L7) and between interperiods (non-subsequent periods—Fig. 1). We test the hypothesis that ostracod communities are largely persistent during regular pulses and are more variable during extreme floods. We expected that: (i) variation in abiotic factors will occur as a result of the oscillation of the flood pulses and will in its turn influence the ostracod community structure, (ii) the variability of the ostracod species composition will increase over time after a potamophase (high flood levels) and (iii) more environmentally tolerant species will occur in potamophase periods, as compared to in limnophase periods.
Materials and methods
Study area
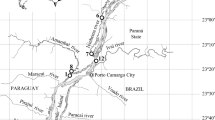
The Upper Paraná River Floodplain (c 230 km long and c 20 km wide) has a hydrological regime consisting of flood pulses, alternating with periods of low water levels (Junk & Sparks, 1989). This floodplain is the last dam-free stretch of the entire Paraná River, where the 25 man-made reservoirs located into its basin affect 70% of the total length of the river. The floodplain includes areas that are vital to biodiversity conservation (Ward et al., 1999; Agostinho et al., 2000). The intensity of extreme precipitation events, such as El Niño episodes, in southeastern South America has had significant impacts on the Paraná River flow rate (Grimm & Tedeschi, 2009).
One lentic backwater, named Manezinho, was monitored in the Upper Paraná River Floodplain for 7 years (from 2004 to 2005 and from 2007 to 2013). It is permanently connected to the main Paraná River by a narrow channel of 1 m long and 3 m wide. The backwater is located on the right bank of the Mutum Island and has a length of approximately 100 m, an area of c 0.1 ha, a perimeter of 2,330 m, and a mean depth of 2.1 m (Edvard et al., 2000). Samples were collected in three patches of Eichhornia crassipes (Mart.) Solms (considered as replicates in this study), along the backwater with station S1 situated furthest in the backwater, S2 in the center and S3 situated close to the entrance of the backwater (Fig. 2).
Sampling, sorting, and identification of ostracods
Samples were taken monthly, from March 2004 to January 2005 and from February 2007 to February 2013 in Manezinho Backwater, totalling 83 months of sampling. From each macrophyte stand (S1–3), three individual E. crassipes plants were transferred to a plastic bucket (Campos et al., 2017). The roots of E. crassipes were separated and washed in the bucket to remove the ostracods, and the aerial part of the plant was discarded. The washed roots were placed in plastic bags and later were dried and weighed. The material resulting from washing was filtered through a hand net with 160 μm mesh size, and the samples were preserved in 70° ethanol buffered with sodium tetraborate.
The physical and chemical variables of the water were measured in situ before the collection of ostracods. The water temperature (WT, °C) and dissolved oxygen (DO, mg l−1) were measured by YSI 550A oximeter, pH and electrical conductivity (EC, μS cm−1) using the YSI Model 63 apparatus. Water level data were obtained by the Long-Term Ecological Research project (site 6—http://www.peld.uem.br/peld-nivel_fluviometrico.htm) of the Nucleus of Research in Limnology, Ichthyology and Aquaculture (Nupélia) from the State University of Maringá (UEM).
In the laboratory, the samples were divided using a Folsom fractioner, 1/4 of the sample was used for quantification of ostracods, while the entire sample was used to quantify species richness. We used a Gehaka BK 3000 semi-analytical balance to weigh the roots of E. crassipes. Ostracods were sorted using a stereoscopic microscope and were identified down to species level using specialized literature (see Martens & Behen, 1994 and articles included; Rossetti & Martens, 1998; Higuti & Martens, 2012a, b, 2014; Higuti et al., 2013). The density of ostracods was calculated and estimated in relation to the dry weight of E. crassipes roots (ind g−1 DW).
Classification of data
The water level data were classified according to the hydrological cycles, limnophases (drought periods), and potamophases (flood periods), to facilitate interannual comparisons. For the selection of the periods, we considered both the starting month and the month that marked its end (Table 1). The reference water level of the Paraná River of 350 cm (Souza Filho, 2009) was used to classify the limit between the two hydrological periods: limnophase <350 cm and potamophase ≥350 cm.
Data analysis
The limnophase amplitude (number of days represented by the water level <350 cm) and potamophase amplitude (number of days represented by the water level ≥350 cm) was calculated using the software PULSO (Neiff & Neiff, 2003). The water level values were obtained as the means of values from 6 days preceding sampling. According to Thomaz et al. (2004), the covariation between limnological variables and the water level is higher when considering the temporal average that preceeds the sampling. Thus, Spearman’s correlation was carried out to test the influence of water levels on the limnological variables, such as WT, EC, pH, and DO.
The (dis)similarity (Bray–Curtis index) in ostracod species composition was analyzed between the hydrological periods. The first two axes of the principal coordinate analysis (PCoA) were retained, in accordance with the criteria of the Broken-Stick method, and submitted to a one-way ANOVA. Both axes of the PCoA were used to test the influence of limnological factors on the ostracod community structure through Spearman’s correlation.
The variability, or inversely the persistence, of the species composition of ostracod communities between subsequent periods and interperiods (see Fig. 1), was evaluated through the dispersion homogeneity test—PERMDISP (Anderson et al., 2006) using a density matrix. The test is based on the distances between the sample units for the centroid of the group, using a dissimilarity measure, within a dimensional space calculated by PCoA. The analysis was based on a dissimilarity matrix obtained using the Bray–Curtis index. Thus, higher mean Bray–Curtis distances suggest greater variability in ostracod species composition. The graphs were plotted according to each of the hydrological cycle (limnophase and potamophase). The level of significance between the limnophase and potamophase periods was analyzed using a permutation test for homogeneity of multivariate dispersions with 999 permutations. Significant differences were distinguished through a Tukey test. Spearman’s correlation was used to evaluate the relationship between the PERMDISP values of each hydrological period with increasing time.
We have also checked for possible correlations between variability and duration of each period and between variability and number of samples within each period using Spearman’s and Pearson’s correlations.
Potential differences in variability between the three stations within the backwater were assessed with PERMDISP analyses, first with data per station for all seven cycles combined, and second with data per station for each cycle individually.
The analysis of indicator value (IndVal, Dufrene & Legendre, 1997) was carried out to test the potential of persistent ostracod species in limnophase (drought) and potamophase (flood) periods. A density data matrix of the ostracod species and the respective previously established category (hydrological periods) was used in each sampled month. This method consists in the identification of an index for each species i in each site group j (IndVal ij ) calculated through the expression: IndVal ij = A ij × B ij × 100; A ij = Nindividuals ij /Nindividuals j . B ij = Nperiods ij /Nperiods j , A ij represents a measure of specificity and the elements of the formula are constituted by Nindividuals ij [mean number of density of species i in the samples of group j (periods)], Nindividuals j (sum of mean values of density of species i over all groups), A ij is maximum when species i is found only in cluster j, B ij is the measure of fidelity and the elements of the formula are Nperiod ij (number of samples from grouping j where species i is present), Nperiod j (total number of samples in group j), B ij is maximum when species i is found in all samples of cluster j. The quantities A and B are combined by multiplication because they represent independent information on species distributions (results are expressed as percentage). The Monte Carlo test was used to test the significance of the IndVals of the species.
Analyses were performed using software R 3.3.1 (R Development Core Team, 2016) through the vegan (Oksanen et al., 2016), permute (Simpson, 2016) and lattice (Sarkar, 2008) packages. The species indicator test was processed in PC-Ord 6.0 software (McCune & Mefford, 1999). Analyses of variance and correlations were carried out with the program Statistica 7.1 (StatSoft, Inc., 2005).
Results
Abiotic variables
Figure 3 shows the variation of the water levels for all data series. During the monitoring, seven hydrological cycles were classified in limnophases (drought periods) and potamophases (flood periods, Table 1). The most intense drought period occurred in the first cycle (2004, 250.3 cm ± 28.8), the highest variation of the drought period was in the cycle 3 (2008–2009, 259.8 cm ± 37.6) and duration in the cycle 7 (2012–2013 for 374 days). The higher average value for the flood period was in the cycle 2 (2007, 591.0 cm ± 0.1) and the highest variation and duration of the high-water level was in the fifth cycle (2009–2010, 514.7 cm ± 100.1 for 130 days).
The results of Spearman’s correlation between water level and limnological variables showed a positive and significant relation to WT (r = 0.39, P = 0.000), EC (r = 0.33, P = 0.000) and pH (r = 0.20, P = 0.001), evidencing an increment of these variables values when the water level was increased. DO also showed a positive trend with the increase of the water level, however, the correlation was not significant (r = 0.08, P = 0.205, see also plots in Fig. S1). The mean values and standard deviation of the limnological variables for the seven hydrological cycles are shown in Table 1, during the period from March 2004 to February 2005 and from February 2007 to February 2013.
Composition and variability of the ostracod communities
The first two retained axes of PCoA showed significant differences in the species composition between hydrological periods (Axis 1: F = 2.011, P = 0.000 and Axis 2: F = 2.697, P = 0.001). Spearman’s correlation between scores of axes of the PCoA and abiotic variables showed negative relations with WT (Axis 1: r = −0.27, P = 0.000, Axis 2: r = −0.24, P = 0.000), EC (Axis 1: r = −0.21, P = 0.000) and water level (Axis 2: r = −0.13, P = 0.004), indicating higher similarity of the species composition with increase of the values these variables mainly in the potamophases (Fig. 4A, B, D, E). On the other hand, ostracods composition was positively correlated with pH (Axis 1: r = 0.14, P = 0.024, Fig. 4C).
The dispersion homogeneity test (PERMDISP), used to investigate the variability, or inversely, persistence of the species composition of communities, showed significant differences between potamophase and limnophase periods (F = 8.47, P = 0.00). The variability in species composition during the potamophases was always much smaller than in the limnophase, except in the potamophase period of cycle 5 (2009–2010) and to a lesser degree in cycle 6 (2011–2012, Fig. 5).
Multivariate permutation PCoA diagram showing the variation in the ostracod species composition in both phases (potamophase and limnophase) of the seven hydrological cycles. The lines indicate the maximum dispersion of sample units for each hydrological period. The center from where the lines radiate represents the respective centroid group
No correlation was observed between community variability and duration of each period and between community variability and number of samples within each period. Results of Spearman’s correlation for limnophase periods: variability and number of samples (P = 0.64), variability and duration of periods (P = 0.70). Results of Pearson’s correlation for potamophase periods: variability and number of samples (P = 0.62), variability and duration of periods (P = 0.62).
The community variability, measured as the average distance of the sample units for the centroid, was highest in the limnophase periods of cycle 5 (2010–2011, 0.47) and cycle 7 (2012–2013, 0.46), and the potamophase of cycle 5 (2009–2010, 0.43), while the lowest mean distance was in the potamophase period of the first cycle (2005, 0.11), followed by the potamophase of cycle 4 (2009, 0.20) and the potamophase of cycle 2 (2004, 0.22, Fig. 5). Figure 6 shows that the values of the dispersion measure increased significantly over time, as evidenced by the Spearman’s correlation (r = 0.62, P = 0.017).
Mean distance of the sample units to the centroid evaluated through the dispersion homogeneity test (PERMDISP) in each hydrological cycle. The X-axis shows the number of months of the start of each hydrological cycle in relation to the end of the first limnophase period (2004). The numbers (1–7) represent the hydrological cycles, their durations are described in Table 1
The results of the Tukey test showed, during the subsequent periods, that ostracod communities were persistent since the potamophase of cycle 1 (2004) to the limnophase of cycle 4 (2009); and the limnophase of cycle 5 (2010–2011) to the limnophase of cycle 7 (2012–2013). The community was variable only in the potamophase of cycle 5 (2009–2010), which comprised an extreme flood with long duration. Considering interperiods, we observed variability of composition after the extreme flood (cycle 5, Table 2).
The PERMDISP analyses of the variability of species composition in the three stations for all cycles combined showed that there were no significant differences between the three stations (F = 0.4407, P = 0.6441, Fig. S2). Similar analyses for the seven cycles separately showed similar absence of significant differences, except for cycle 4 (Fig. S3).
Indicator species
In total, 34 ostracod species were recorded belonging to four families. Cyprididae was the most numerous family, with 22 species, followed by Candonidae, Darwinulidae, and Limnocytheridae. Diaphanocypris meridana (Furtos, 1936), Strandesia lansactohai Higuti & Martens, 2013, Bradleystrandesia trispinosa (Pinto & Purper, 1965), Cypricercus centrura (Klie, 1940), Cypretta costata (G. W. Müller, 1898), Cabelodopsis hispida (Sars, 1901), Cytheridella ilosvayi (Daday, 1905), Alicenula serricaudata (Klie, 1935), Vestalenula pagliolii (Pinto & Kotzian, 1961), and Penthesilenula brasiliensis (Pinto & Kotzian, 1961) were recorded in all hydrological periods. Strandesia variegata (Sars, 1901) occurred exclusively in the limnophase period of the fifth cycle (2010–2011), Strandesia sp. 7 n.sp. only in the limnophase period of the fourth cycle (2009) and Physocypria sp. 2 n.sp. in the limnophase period of the sixth cycle (2011–2012, Table S1).
Results of the species indicator test (IndVal) showed that D. meridana, Strandesia mutica, C. centrura, Chlamydotheca iheringi, Cypridopsis cf. vidua sp. 2, “Cypridopsis” sp. 1 n.gen. n.sp., “Cypridopsis” sp. 2 n.gen. n.sp., C. hispida, C. ilosvayi, A. serricaudata, V. pagliolii, and P. brasiliensis were indicative of potamophase periods, which means that these species were persistent in the flood periods (Table 3). No indicator species were found for the limnophase periods.
Discussion
Abiotic variables
In the Upper Paraná River Floodplain, the water level during the limnophase period may change from few days to several months throughout the year, as for example in 2004 and 2012–2013 (cycles 1 and 7, respectively). The same was true for the potamophase period in 2009–2010 (cycle 5). The occurrence of several oscillations of the flood pulses is mostly associated with the operation of man-made dams that regulate and alter the natural hydrological regime of the Paraná River (Thomaz et al., 2004; Souza Filho, 2009). Also, the natural variations in precipitation caused by El Niño play a role. In this way, the dynamics of the abiotic variables (local scale) are closely linked to the variations of the flood pulses (regional scale), as evidenced by the Spearman’s correlations (see above), and consequently alter the composition of ostracod communities (see below).
Extreme floods in South America, especially in southern Brazil, are associated with the El Niño phenomenon, which leads to irregular flood pulses in river–floodplain systems (Grimm & Tedeschi, 2009; Ward et al., 2010, 2014; Räsänen & Kummu, 2013). In the 2009–2010 (cycle 5), for example, the higher waters level in the Paraná River were directly linked to the occurrence of an El Niño event. This was only one of a series of marked El Niño events since the beginning of the twenty first century (Yu & Kim, 2013) which caused extreme climatic anomalies around the world (Lee et al., 2010; Seager et al., 2010; Kim et al., 2011; Ratnam et al., 2012). In addition, several dams upstream of the Upper Paraná River Floodplain released higher volumes of water and, together with the El Niño event, this caused the extreme flood (CPTEC, 2016) during the potamophase of hydrological cycle 5 (2009–2010).
Composition and variability of ostracod communities
Changes in species composition were observed between potamophase and limnophase periods, because of the homogenization effects of the higher water levels on ostracod communities during the potamophases. These homogenizing effects lead to increase of the similarity of species composition of the ostracod communities. Organisms can develop morphological, physiological, and phenological adaptations to natural flood pulses, with recurrent intervals and frequencies, thus reducing potential negative effects of such regular flood pulses (Junk & Wantzen, 2007). Such adaptations will also buffer effects of flood pulses when they are regulated by man-made dams (Robinson & Uehlinger, 2006). In addition, “normal” flooding increases habitat similarity by minimizing resource variation (Thomaz et al., 2007) and, in response, causing decreasing community structure variability under relatively homogenous and constant environmental conditions (Bengtsson et al., 1997). Flood pulses usually occur during summer and the accompanying higher temperatures might accelerate the life cycle of ostracods (Smith et al., 2015). In the potamophase periods during the present study, the wider range of the limnological variables (e.g., temperature, conductivity and pH) contributed to the increase of similarity in species composition (see Fig. 4) and consequently to the decrease of the species composition variability (see Fig. 5). However, an increase of the ostracod species composition variability was observed in the extreme flood with longer duration (the potamophase of cycle 5). This is unexpected as such extreme floods will further augment the exchange of organisms through the aquatic connections between lakes and rivers. It is possible that extreme floods may trigger the hatching of dry-resistant eggs (see discussion of “Indicator species” section). In this way, the persistence of the community structure between successive periods from the potamophase of cycle 1 to the limnophase of cycle 4 may be associated with lower intensity of the disturbances, which do not significantly interfere with the ostracod community structure.
We have found that ostracod species composition variability within periods in both limnophases and potamophases are not correlated to neither the duration of, nor to the number of samples within these periods, which means that comparisons between these units are valid.
Some periods of the hydrological cycles were discrete, with a short duration or little variation, as in the floods of 2009 and 2012 (cycles 4 and 7, respectively). Higuti et al. (2007) argued that free-floating macrophytes accompany the water level and this does not become a limiting factor in the density change of the ostracod communities. Indeed, during such discrete events, ostracod abundances did not change significantly in the Manezinho Backwater, indicating buffering and high persistence of community structure. Other studies have demonstrated similar persistence of community structure in several aquatic groups, such as macroinvertebrates (Scarsbrook, 2002; Collier, 2008), phytoplankton (Schneck et al., 2011), and cichlid fish (Lourenço et al., 2012), indicating resistance to natural disturbances of short durations (see also “Introduction” section). In contrast, planktonic crustacean communities in a Polish oxbow lake experienced a marked decrease in densities with increasing water levels during the potamophases (Napiórkowski & Napiórkowska, 2017).
Other differences in the variability in the ostracod species composition between interperiods, such as the potamophase of cycle 1 (2005) and the limnophase of cycle 5 (2010–2011, Table 2), were also owing to longer duration of the flood, in this case caused by a sequence of successive floods and especially by the extreme flood (potamophase of cycle 5). Studies of other aquatic communities have demonstrated the increase in the variability of community structure over time owing to greater fluctuation of environment conditions, as fish (Oberdorff et al., 2001) and macroinvertebrates (Brown et al., 2006; Bêche & Resh, 2007).
Ecological disturbance
Since 2008, the Manezinho Backwater was increasingly colonized by a native submerged macrophyte (e.g., Egeria najas Planch.) while the stands of E. crassipes dwindled by the middle of 2013, and few individuals of E. crassipes remained in this backwater. This is why the present monitoring program had to be stopped in that year. Propagules of E. najas established in the backwater in 2008, and possibly interfered in the variability of species composition during its colonization, as shown by the reduced variability in cycle 4 (see Fig. 6). Studies developed in lentic environments, e.g., reservoirs, showed that E. najas occur between 2 and 4 m deep, with a growth coefficient between 0.022 and 0.082 day−1 and a doubling time between 8.5 and 31.5 days, with aquatic radiation and turbidity being the main limiting factors for its expansion (Thomaz et al., 2003). This species can achieve a large standing biomass and competition between macrophytes and/or habitat preference of some ostracods (Mormul et al., 2010) may have also contributed to the decrease in the variability of species composition in cycle 4. An increase of composition variability was observed between 2008–2009 (cycle 4) and 2009–2010 (cycle 5, see Fig. 6), indicating the effects of the extreme flood pulses and minimizing the effects of E. najas on the ostracod communities. Thereafter, the variability of the ostracod species composition remains relatively high, when compared to cycle 5. The roots of free-floating macrophytes, mainly of E. crassipes, offer a substrate of high complexity that allows the occurrence of several species in high abundances (Higuti et al., 2007; Matsuda et al., 2015), allowing immigrant individuals to cohabit with the others.
In addition to the invasion of E. najas, the connecting channel between the lake and the river started silting up since c 2011, so that even during the regular floods of cycles 6 and 7, less connectivity occurred during these potamophases.
Indicator species
The potamophase periods allow the addition of new individuals by immigration through the water connections formed by the flood, as proposed by Thomaz et al. (2007), as well as the creation of new opportunities for temporal niches (Chesson & Huntly, 1997). Some species were more abundant during the potamophase periods, as evidenced by the test of indicator species, most of which belong to the family Cyprididae (see Tables S1, 3). Ostracod species of this family can produce dry-resistant eggs (Meisch, 2000) that can hatch irregularly over different generations (Smith et al., 2015). The floods might thus have contributed to the hatching of eggs that were not inundated in the limnophase, for example because they were attached to dried macrophytes, remnants of the previous potamophase, hanging several meters high in the trees.
Alicenula serricaudata, V. pagliolii, and P. brasiliensis (family Darwinulidae) do not produce dry-resistance eggs, but may benefit from the increased potential for colonization during potamophase periods. In addition, high temperatures (which coincide with the flooding period in summer) might increase the metabolic rate of organisms, resulting in shorter life cycles, as proposed for Darwinulidae by Higuti et al. (2009). However, this remains to be tested in controlled experiments.
Conclusions
Our hypothesis that during regular flood pulses the ostracod communities were stable and persistent, while in the extreme and long duration floods there was markedly more variability, is here corroborated. It should be stressed that the difference between regular and extreme floods could only be detected in the course of a long term, multi-annual monitoring program. Most ecological studies span one to a few years only, because funding for research projects is mostly for 2–4 years. The present results show the importance of long term ecological monitoring programs for the detection of the effects of extreme and rare events.
Our results are relevant for understanding the dynamics of the ostracod community structure, and as a model for pleuston communities in general. Such understanding is important for decision-making by natural resources managers, because large-scale environmental changes are expected as a result of global climate change and, consequently, significant changes in community structure might be expected. For rivers with many man-made dams, such as the Upper Paraná River, the effects of extreme floods resulting from unpredictably increased precipitation could at least partly be moderated by regulating water release from these dams. This would require that the authorities in charge of these dams take ecosystem health of downstream floodplains into account when deciding on their water release schedules.
References
Agostinho, A. A., S. M. Thomaz, C. V. Minte-Veral & K. O. Winemiller, 2000. Biodiversity in the High Parana River Floodplain. In Gopal, B., W. J. Junk & J. A. Davis (eds), Biodiversity in Wetlands: Assessment, Junction and Conservation. Backhuys Publishers, Leiden: 89–118.
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9: 683–693.
Bêche, L. A. & V. H. Resh, 2007. Short-term climatic trends affect the temporal variability of macroinvertebrates in California “Mediterranean” streams. Freshwater Biology 52: 2317–2339.
Bengtsson, J., S. R. Baillie & J. Lawton, 1997. Community variability increases with time. Oikos 78: 249–256.
Bortolini, J. C., S. Train & L. C. Rodrigues, 2016. Extreme hydrological periods: effects on phytoplankton variability and persistence in a subtropical floodplain. Hydrobiologia 763: 223–236.
Bozelli, R. L., S. M. Thomaz, A. A. Padial, P. M. Lopes & L. M. Bini, 2015. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 753: 233–241.
Brown, L. E., A. M. Milner & D. M. Hannah, 2006. Stability and persistence of alpine stream macroinvertebrate communities and the role of physicochemical habitat variables. Hydrobiologia 560: 159–173.
Bunn, S. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Campos, R., E. O. Conceição, M. B. O. Pinto, A. P. S. Bertocin, J. Higuti & K. Martens, 2017. Evaluation of quantitative sampling methods in pleuston: an example from ostracod communities. Limnologica 63: 36–41.
Cera, J. C. & S. E. T. Ferraz, 2015. Variações climáticas na precipitação no sul do Brasil no clima presente e futuro. Revista Brasileira de Meteorologia 30: 81–88.
Chesson, P. & N. Huntly, 1997. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. The American Naturalist 150: 519–553.
Collier, K. J., 2008. Temporal patterns in the stability, persistence and condition of stream macroinvertebrate communities: relationships with catchment land-use and regional climate. Freshwater Biology 53: 603–616.
CPTEC, 2016. Centro de Previsão do tempo e estudos climáticos [available on internet at http://www.cptec.inpe.br/]. Accessed 24 Nov 2016.
Dufrene, M. & P. Legendre, 1997. Species assemblages and indicator species – the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Edvard, E. C., A. C. Petry, M. R. Russo, A. M. Santos, R. R. A. Rocha & R. A. Leimig, 2000. A planície de inundação do alto rio Paraná: Site 6 PELD/CNPq - Relatório anual 2000. Descrição dos Locais de Amostragem [available on internet at http://www.peld.uem.br/Relat2000/2_2_CompBioticoDesLocAmost.PDF]. Accessed 17 Oct 2015.
Grimm, A. M. & R. G. Tedeschi, 2009. ENSO and extreme rainfall events in South America. Journal of Climate 22: 1589–1609.
Higuti, J. & K. Martens, 2012a. On a new cypridopsine genus (Crustacea, Ostracoda, Cyprididae) from the Upper Paraná River Floodplain (Brazil). Zootaxa 38: 23–38.
Higuti, J. & K. Martens, 2012b. Description of a new genus and species of Candonopsini (Crustacea, Ostracoda, Candoninae) from the alluvial valley of the Upper Paraná River (Brazil, South America). European Journal of Taxonomy 33: 1–31.
Higuti, J. & K. Martens, 2014. Five new species of Candoninae (Crustacea, Ostracoda) from the alluvial valley of the Upper Paraná River (Brazil, South America). European Journal of Taxonomy 106: 1–36.
Higuti, J. & K. Martens, 2016. Invasive South American floating plants are a successful substrate for native Central African pleuston. Biological Invasions 18: 1191–1201.
Higuti, J. L. F. M., F. M. Velho, F. A. Lansac-Tôha & K. Martens, 2007. Pleuston communities are buffered from regional flood river pulses: the example of ostracods in the Paraná River Floodplain, Brazil. Freshwater Biology 52: 1930–1943.
Higuti, J., F. A. Lansac-Tôha, L. F. M. Velho, R. L. Pinto, L. C. G. Vieira & K. Martens, 2009. Composition and distribution of Darwinulidae (Crustacea, Ostracoda) in the alluvial valley of the Upper Paraná River, Brazil. Brazilian Journal of Biology 69: 253–262.
Higuti, J. I., I. Schön, L. Audenaert & K. Martens, 2013. On the Strandesia obtusata/elliptica lineage (Ostracoda, Cyprididae) in the alluvial valley of the Upper Paraná River (Brazil), with the description of three new species. Crustaceana 86: 182–211.
Junk, W. J. & R. E. Sparks, 1989. The flood pulse concept in river–floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Junk, W. J. & K. M. Wantzen, 2007. Flood pulsing and the development and maintenance of biodiversity in floodplains. In Batzer, D. (ed.), Ecology of Freshwater and Estuarine Wetlands. University of California Press, Berkeley: 407–435.
Kim, W., S. W. Yeh, J. H. Kim, J. S. Kug & M. Kwon, 2011. The unique 2009–2010 El Niño event: a fast phase transition of warm pool El Niño to la Niña. Geophysical Research Letters 38: 1–5.
Lee, T., W. R. Hobbs, J. K. Willis, D. Halkides, I. Fukumori, E. M. Armstrong, A. K. Hayashi, W. T. Liu, W. Patzert & O. Wang, 2010. Record warming in the South Pacific and western Antarctica associated with the strong central-Pacific El Niño in 2009–10. Geophysical Research Letters 37: 1–6.
Lourenço, L. S., I. M. Fernandes, J. Penha & L. A. F. Mateus, 2012. Persistence and stability of cichlid assemblages in neotropical floodplain lagoons. Environmental Biology of Fishes 93: 427–437.
Martens, K. & F. Behen, 1994. A checklist of the non-marine ostracods (Crustacea, Ostracoda) from the inland waters of South America and adjacent islands. Travaux Scientifiques du Musée National d’Histoire Naturelle de Luxembourg, Luxembourg 22: 1–81.
Matsuda, J. T., F. A. Lansac-Tôha, K. Martens, L. F. M. Velho, R. P. Mormul & J. Higuti, 2015. Association of body size and behaviour of freshwater ostracods (Crustacea, Ostracoda) with aquatic macrophytes. Aquatic Ecology 49: 321–331.
McCune, B. & M. J. Mefford, 1999. PC-ORD for Windows – Multivariate Analysis of Ecological Data. MjM Software, Gleneden Beach.
Meisch, C., 2000. Freshwater Ostracoda of Western and Central Europe. In Schwoerbel, J. & P. Zwick (eds), Süßwasserfauna von Mitteleuropa 8/3. Spektrum Akademischer Verlag, Berlin.
Mormul, R. P., S. M. Thomaz, J. Higuti & K. Martens, 2010. Ostracod (Crustacea) colonization of a native and a non-native macrophyte species of Hydrocharitaceae in the Upper Paraná Floodplain (Brazil): an experimental evaluation. Hydrobiologia 644: 185–193.
Napiórkowski, P. & T. Napiórkowska, 2017. Limnophase versus potamophase: how hydrological connectivity affects the zooplankton community in an oxbow lake (Vistula River, Poland). Annales de Limnologie - International Journal of Limnology 53: 143–151.
Neiff, J. J., 1990. Ideas para la interpretacion ecologica del Paraná. Interciência 15: 424–441.
Neiff, J. J. & M. Neiff, 2003. PULSO: software para análisis de fenómenos recurrentes [available on internet at http://www.neiff.com.ar]. Accessed 15 Feb 2016.
Oberdorff, T., B. Hugueny & T. Vigneron, 2001. Is assemblage variability related to environmental variability? An answer for riverine fish. Oikos 93: 419–428.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2016. Vegan: Community Ecology Package. R Package Version 2.4-1 [available on internet at https://cran.r-project.org/package=vegan]. Accessed 10 Feb 2016.
R Development Core Team, 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [available on internet at http://www.r-project.org/]. Accessed 10 Feb 2016.
Räsänen, T. A. & M. Kummu, 2013. Spatiotemporal influences of ENSO on precipitation and flood pulse in the Mekong River Basin. Journal of Hydrology 476: 154–168.
Ratnam, J. V., S. K. Behera, Y. Masumoto, K. Takahashi & T. Yamagata, 2012. Anomalous climatic conditions associated with the El Niño Modoki during boreal winter of 2009. Climate Dynamics 39: 227–238.
Robinson, C. T. & U. Uehlinger, 2006. Experimental floods cause ecosystem regime shift. Ecological Applications 18: 511–526.
Rossetti, G. & K. Martens, 1998. Taxonomic revision of the Recent and Holocene representatives of the family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie 68: 55–110.
Sarkar, D., 2008. Lattice: Multivariate Data Visualization with R. Springer, New York.
Scarsbrook, M. R., 2002. Persistence and stability of lotic invertebrate communities in New Zealand. Freshwater Biology 47: 417–431.
Schneck, F., A. Schwarzbold, S. C. Rodrigues & A. S. Melo, 2011. Environmental variability drives phytoplankton assemblage persistence in a subtropical reservoir. Austral Ecology 36: 839–848.
Seager, R., Y. Kushnir, J. Nakamura, M. Ting & N. Naik, 2010. Northern Hemisphere winter snow anomalies: ENSO, NAO and the winter of 2009/10. Geophysical Research Letters 37: 1–6.
Simões, N. R., F. A. Lansac-Tôha & C. C. Bonecker, 2013. Drought disturbances increase temporal variability of zooplankton community structure in floodplains. International Review of Hydrobiology 98: 24–33.
Simpson, G. L., 2016. permute: Functions for Generating Restricted Permutations of Data. R package version 0.9-4 [available on internet at https://cran.r-project.org/package=permute]. Accessed 10 Feb 2016.
Smith, A. J., D. J. Horne, K. Martens & I. Schön, 2015. Class Ostracoda. In Thorpe & Covich’s Freshwater Invertebrates. Elsevier, New York: 757–780.
Souza Filho, E. E., 2009. Evaluation of the Upper Paraná River discharge controlled by reservoirs. Brazilian Journal of Biology 69: 707–716.
StatSoft, Inc., 2005. STATISTICA (Data Analysis Software System), Version 7.1. www.Statsoft.com.
Thomaz, S. M., L. M. Bini & T. A. Pagioro, 2003. Macrófitas aquáticas em Itaipu: ecologia e perspectivas para o manejo. In Thomaz, S. M. & L. M. Bini (eds), Ecologia e manejo de macrófitas aquáticas. EDUEM, Maringá: 319–341.
Thomaz, S. M., T. A. Pagioro, L. M. Bini, M. C. Roberto & R. R. A. Rocha, 2004. Limnological characterization of the aquatic environments and the influence of hydrometric levels. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and Its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 75–102.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river–floodplain systems. Hydrobiologia 579: 1–13.
Ward, J. V., K. Tockner & F. Schiemer, 1999. Biodiversity of floodplain river ecosystems: ecotones and connectivity. Regulated Rivers: Research and Management 15: 125–139.
Ward, P. J., W. Beets, L. M. Bouwer, J. C. J. H. Aerts & H. Renssen, 2010. Sensitivity of river discharge to ENSO. Geophysical Research Letters 37: 1–6.
Ward, P. J., B. Jongman, M. Kummu, M. D. Dettinger, F. C. Sperna Weiland & H. C. Winsemius, 2014. Strong influence of El Nino Southern Oscillation on flood risk around the world. Proceedings of the National Academy of Sciences of USA 111: 15659–15664.
Yu, J. Y. & S. T. Kim, 2013. Identifying the types of major El Niño events since 1870. International Journal of Climatology 33: 2105–2112.
Acknowledgements
We would like to thank Dr. Roger Paulo Mormul and Dr. Luiz Carlos Gomes for assistance with the statistical analyses and Jaime Luiz Lopes Pereira for help with the production of the map. We thank the Nucleus of Research in Limnology, Ichthyology and Aquaculture (Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Nupélia) and the Post-graduate Program in the Ecology of Continental Aquatic Environments (Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais, PEA) of the State University of Maringá (Universidade Estadual de Maringá, UEM) for the logistic support. This Research forms part of Projects supported by National Council for Scientific and Technological Development (CNPq Nr 472434/03-9), through the Long-Term Ecological Research (LTER) Program, and the SETI/Fundação Araucária/MCT/CNPq (Nr 232/10). EOC and RC would like to thank CNPq and the Coordination of Improvement of Higher Education Personnel (CAPES) for granting their Master’s Scholarships, respectively. Dr. Tadeusz Namiotko (Gdansk, Poland) and Dr. Thijs Van der Meeren (Ghent, Belgium) suggested important improvements. The Universidade Estadual de Maringá (UEM, Maringá) and the Royal Belgian Institute of natural Sciences (RBINS, Brussels) have a bilateral Memorandum of Understanding regarding collaborative Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Conceição, E.O., Higuti, J., Campos, R. et al. Effects of flood pulses on persistence and variability of pleuston communities in a tropical floodplain lake. Hydrobiologia 807, 175–188 (2018). https://doi.org/10.1007/s10750-017-3392-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3392-z