Abstract
The biogeography and ecological preferences of Neotropical freshwater ostracods are poorly known, and more so the dynamics of populations and habitat selection of species living in pleustonic environments of temporary ponds. In the present survey we analyze the population changes of ostracods living in pleustonic environments of small freshwater bodies on Martín García Island (Río de la Plata, Argentina). Between June 2005 and June 2007, monthly samples of floating vegetation from eight different ponds on the island were collected, and limnological parameters were measured in situ. The results of multivariate logistic regression showed that the presence of ostracods was significantly related to high dissolved oxygen content and high water temperature. In addition, multivariate regression analysis indicated that, when ostracods were present, their total abundance was negatively related to floating vegetation dry weight. Four ostracod species were found: Strandesia bicuspis, Chlamydotheca incisa, Cypridopsis vidua, and Bradleytriebella trispinosa. The seasonal variation in abundances indicated that populations of the most common species, S. bicuspis and C. incisa, were denser during the summer and autumn months. The results of canonical correspondence analysis showed that individuals of S. bicuspis were more abundant at higher temperatures and lower conductivity than C. incisa. Further research is needed to clarify the observed negative correlation between floating vegetation dry weight and ostracod density and the possible differential thermal preference of the two species studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ecological characteristics of the environment influence the presence or absence of ostracod species in different ways, including, among others, water chemistry and hydroperiod (De Deckker, 1983; Geiger, 1998; Griffiths & Holmes, 2000; Smith & Horne, 2002; Laprida, 2006). The major advances in the study of ostracod ecology in relation to habitat traits have resulted mainly from works in the Northern Hemisphere and Australia, with little input from studies in Neotropical or Afrotropical regions. Indeed, there is an incongruence in the relatively low ostracod diversity of South America in comparison with Africa (Martens et al., 2008), probably derived from a lower research effort in the Neotropical region. Information relevant to Argentina is even scarcer, and reports that consider the current ostracod fauna of this area in relation to environmental parameters are particularly scarce (César et al., 2001; Schwalb et al., 2002; Laprida, 2006; César & Liberto, 2008). Recently, in her study of water bodies within the pampean plains, Laprida (2006) correlated the dominance of different species with the duration of water and the composition and variability of its ions.
According to Wiggins et al. (1980), temporary freshwaters represent a particular type of habitat among inland water bodies in that the dry period imposes such rigorous environmental conditions that only a small number of species can survive. Furthermore, in these water bodies, characteristic assemblages of ostracod species are usually found that could even be regarded as ecological indicators for such habitats (Laprida, 2006). Within the communities of these temporary environments, water volume is a prime regulating factor of the constituent population structure and the community dynamics. Within aquatic habitats, the pleuston constitutes a microcosm developed among the floating plants, which is a special type of habitat particularly common in South America (Esteves, 1998), considered as a full biochore by itself by Por & Rocha (1998), who called it the pleustal. Dioni (1967) defines hypopleuston as the community of organisms that inhabit aquatic rhizoids and the roots of floating macrophytes, and epipleuston as the fauna that live on the dry surfaces of floating leaves. These root systems can possess a rich biotic community (Poi de Neiff & Carignan, 1997; Por & Rocha, 1998; De Marco et al., 2001; Takeda et al., 2003). It is on these types of habitats that we have focused our study, since ostracod density is closely related to the presence or absence of aquatic vegetal communities along with their densities (Mourguiart, 1991), and recent research has shown that the ostracod community in hypopleustonic habitat can be rich and diverse (Higuti et al., 2007, 2009).
In general, the development and distribution of ostracod populations are closely linked to the cycle of aquatic vegetation, with this latter dynamic, in turn, being determined by the hydrologic development of the pond itself (Mourguiart, 1991). The plants present in these environments provide both food and refuge. Not much is known about the ostracod–plant association, but many species of these tiny crustaceans live in association with plants (Griffiths & Holmes, 2000), particularly in the pleustonic environment (e.g., Rocha & Por, 1998; Higuti et al., 2007).
The objectives of our investigations focused on determining the seasonal variation patterns of Ostracoda in pleustonic communities of water bodies on Martín García Island. These are small-scale ecosystems, mainly ponds with great variation in water level and environmental stress. We also aim to study the causes of ostracod population changes by measuring relevant parameters such as ostracod density and age structure (juveniles and adults) throughout the year, in relation to the environment in which the individuals live. We expected that, even taking into account that our studied water bodies are similar in size and close to each other, and most of them share a similar history, the ostracod populations’ structure and dynamics would differ according to subtle variations in habitat traits and plant communities composing the pleustonic habitat.
Materials and methods
Study area
The Multiple Use Reserve Island of Martín García is situated in the upper Río de la Plata (34°11′S, 58°15′W), has a surface area of 184 ha, and consists in an elevated and fractured block, part of the crystalline basement of Brasilia, subsequently covered by Pleistocene and Holocene deposits (Dalla Salda, 1981; Ravizza, 1982, 1984; González & Ravizza, 1987). Although the reserve’s current stable population does not exceed 100 inhabitants, the island does receive a sizable influx of tourists in autumn, spring, and summer.
The island’s climate is temperate and humid with annual precipitation of 980 mm and annual mean temperature of 17.6°C, with January being the warmest and July the coldest month. Frosts are rare, and the most humid months are June and July (with mean humidity of 81%). Different types of lentic small water bodies are present on the island; some ponds are of human origin and can be either (semi)permanent or temporary, formed from filling basaltic-rock quarries that were formerly exploited in some sectors around the plateau, whereas others consist in temporary ponds of natural origin formed in depressions in the ground within the bordering woods or near to inner sandy areas. The input of water into these bodies comes from increases in the water level and flooding from the Río de la Plata in some instances, from precipitation in others, or from both sources (César et al., 2001). In general, these types of ponds exhibit different degrees of filling and eutrophy, and within them carpets of floating vegetation develop (Lahitte & Hurrell, 1996) that contain diverse invertebrate fauna (Rumi et al., 1996; Damborenea et al., 1997; Armendáriz & César, 2001; César et al., 2001).
The sampling campaigns were performed at eight ponds (stations) of Martín García Island (Fig. 1) between June 2005 and June 2007. All sampling sites can be considered as being of human origin (abandoned small quarries), with the sole exception of station 6 (Laguna Arenalcito). Most of them are temporary, except sites 2 and 4, which never dried completely during the study period.
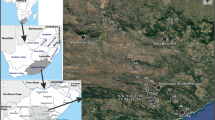
Location of the Island of Martín García in South America (A) and the estuary of Río de la Plata (B), and (C) general view of the island and location of sampling sites (1–8). C: modified from the INGEODAV webpage, accessed 2008 (http://ingeodav.fcen.uba.ar/Ingeodav/Escueladecampo/mgarcia/GUIADECAMPO.htm)
Sampling and physicochemical data
Three replicate samples per station were collected from floating vegetation (pleuston) with a 150 μm mesh size net supported by a 30 × 30 cm square frame. The sampling sites were selected in areas deeper than 50 cm to avoid bottom sediment influence. Three replicate samples were taken from different parts of each pond, trying to account for wider variability in ostracod communities that could be caused by heterogeneity in pleustonic vegetation structure. Although the replicated samples were later pooled together, they also increased the probability of finding ostracods with heterogeneous distributions when densities were very low. After identification and counting of ostracods, the three replicas were pooled together for further analysis. The collected material (floating plants and associated invertebrates) was fixed in situ in 10% (v/v) formalin. In the laboratory, the samples were washed on a sieve with 125 μm pore size, and ostracods were separated from plants, stained with erythrosin B, and preserved in 70% aqueous alcohol. The collected ostracods were observed by conventional microscopy for species identification (Ramírez, 1967; Moguilevsky & Whatley, 1995).
To estimate the dry weight of the vegetal carpet, each sample was washed with water and the macrophyte material (including both submersed and aerial parts) was removed for drying in an oven at 105°C and subsequent weighing using a precision balance (Dahus, Explorer).
The physicochemical variables of the water—water temperature, conductivity, total dissolved solids (TDS), dissolved oxygen, and pH—were measured at the site with a digital meter (Water Quality Meter Sper Sc. LTD), and the air temperature was also registered. Occasionally, when no data could be obtained for a particular variable because of probe failure, they were estimated by regression with related variables obtained at the same site; for example, water temperature could be estimated from air temperature measured in situ, and conductivity could be calculated from concentration of total dissolved solids.
Finally, in this study we considered the pluviometric data for this sampling period as corresponding to those provided throughout 2005–2007 by the National Meteorological Service Station located closest to the island (San Fernando, Buenos Aires, 34°27′S, 58°35′W).
Data analysis
To determine the relationship among the measured environmental parameters and to reveal patterns related to their spatial and seasonal variation, we applied the method of principal components analysis (PCA) using PAST version 1.82 software (Hammer et al., 2001). Variables that were mutually correlated were not considered; thus, the parameters introduced were water temperature, dissolved oxygen content, pH, conductivity, and dry weight of the vegetal carpet (air temperature, total dissolved solids, and oxygen saturation percentage were not used in this analysis). The conductivity and dry weight data were converted to their logarithms to base 10, since they contained a wide range of high values. The totality of the data was not used, only those values corresponding to samples where all five above-mentioned variables had been measured (or inferred by regression); thus, of the 112 samples collected, only data from 77 samples were included in this PCA.
The ostracod density (number of individuals m−2) was calculated for each environment sampled and for each species encountered, and the individuals counted were classified as either juveniles or adults.
Multivariate logistic regression models were built to determine if the occurrence of ostracods in the different locations examined could be related to the environmental variables registered. To do this we used the program SPSS version 15 (SPSS Inc., 2004).
In ostracod-containing samples, we carried out multiple linear regression with SPSS, introducing the variables of water temperature, dissolved oxygen, log10(conductivity), pH, and log10(dry weight) by means of a stepwise algorithm.
Finally, we carried out canonical correspondence analysis (CCA) to determine the distribution patterns for S. bicuspis and C. incisa in relation to the environmental variables. CCA is a technique of unimodal gradient analysis that, in this instance, was used to examine the relationship between the predictor environmental variables and the species’ responses (Ter Braak, 1986; Ramette, 2007). For this step we used the CANOCO program (Ter Braak, 1988) version 4.5 for Windows, selecting the option of manual “forward selection variables” (FSV). The variables initially introduced in the CCA were: the four ostracod species registered (with adults and juveniles as separate variables for the two more common species), water temperature, log10(conductivity), pH, log10(dry weight) of the vegetal carpet, dissolved oxygen, and the presence of eight species of aquatic macrophytes with unequal distributions among the ponds: Lemna gibba, Lemna minuta, Spirodela intermedia, Azolla filiculoides, Wolffia columbiana, Wolffiella oblonga, Hydrocotyle ranunculoides, and Limnobium spongia.
Results
During the study period the winters were dry (especially in 2006) and the greatest amount of rainfall occurred during the summers of 2006 (in January and March) and 2007 (in December and March) plus in April 2007 (Fig. 2). Major drought events in some of the studied ponds (see below) occurred during late spring–early summer in 2006, and mid to late summer 2007, when daily temperatures were high (between 15°C and 25°C approximately), particularly when daily precipitation was reduced (less than 20 mm) for more than 3 or 4 weeks.
Table 1 presents the physicochemical variables measured at the sampling sites on the island during the study (from June 2005 to June 2007), summarized per season. In general, the levels of dissolved oxygen were highly variable, pH was slightly acidic to neutral, and conductivity was variable; water temperature oscillated between 16°C and 20°C. In the PCA (Fig. 3), stations 6–8 were situated on the positive side of component 1, corresponding to sites with low conductivity and pH, while stations 2–5 were sites with higher values for both of these parameters. Station 1 was located near the origin, with intermediate values. Component 2 of the analysis reflects the variability in water temperature, oxygen levels, and biomass (dry weight) of the floating plants throughout the year. The samples distributed on the positive side of component 2 were for the most part collected during the warmer months (summer), while those oriented toward the negative portion of the component had higher oxygen content, greater vegetal coverage, and lower temperatures, corresponding to the colder months (winter).
Results of principal components analysis (PCA) of all samples that contained data on conductivity, pH, water temperature, oxygen concentration, and dry weight of aquatic plants. Codes indicate sampling site (first digit), sampling month (second and third digits), and year (last two digits). Site codes as in Table 1 and Fig. 1
During the sampling period of this study (2005–2007), four species of Ostracoda were present: Cypridopsis vidua (Müller, 1776), Chlamydotheca incisa (Claus, 1892), Strandesia bicuspis (Claus, 1892), and Bradleytriebella trispinosa (Pinto & Purper, 1965). It must be remarked that the last of these species (B. trispinosa) up until only recently was considered as belonging to the genus Strandesia and was transferred to the new genus Bradleytriebella by Savatenalinton & Martens (2009). The species B. trispinosa and C. vidua were found at low frequency and density. The former was present only at station 4 (“Tanque”) during March and April 2007 at respective densities of 14.8 and 7.4 ind m−2, while the latter was detected in December 2006 at station 1 (“Basural”) and in April 2007 at station 5 (“Boya 45”) at densities of 29.6 and 7.4 ind m−2, respectively. For Strandesia bicuspis and Chlamydotheca incisa, the total density of both species throughout the 2 years of sampling passed through two peaks: between February and April of both 2006 and 2007, i.e., between summer and autumn (see below for further details).
Figure 4 shows the main density changes through time (Fig. 4A) for the whole ostracod community (i.e., the mean ostracod density for all sites and raw values for each site, both at each sampling occasion), plus the mean and variability at each site for the whole sampling period (Fig. 4B). A trend is observed for highest densities occurring around summer–autumn in both years, and minimal densities in winter (Fig. 4A). The sites with highest ostracod densities were stations 6 and 7 (Fig. 4B).
Ostracods were found in only a little more than half of the samples (59.8%). The empirical statistical findings of stepwise multivariate logistic regression demonstrate that the presence of ostracods is related positively to relatively high oxygen content of the water, elevated temperature, and absence of Azolla filiculoides. Among these parameters the first introduced in the stepwise procedure was absence of Azolla filiculoides, the second was water temperature, and the last was dissolved oxygen content; no further variables were added to these, as the model was not significantly improved. With this model, tabulation of the categories indicated that a total of 74.5% of the data that we had classified as 0 (absence of ostracods, 40% of samples correctly classified) and as 1 (their presence, 88% of samples well classified) had been correctly assessed. The final model is significant at a level of P < 0.05, though with a very low percentage of variance explained (Cox and Snell R 2 = 0.262).
The results of the multiple stepwise linear regression indicated that the variable log10(dry weight) of floating vegetation best described the density of ostracods in samples where they were present, with the stepwise algorithm selecting this parameter alone (P < 0.05; R 2 = 0.397). The resulting regression model corresponded to a simple linear regression with the following equation (Fig. 5): log10(ostracod abundance) = 4.16 − 2.24 × log10(dry weight).
Variation of log10 of total ostracod abundance per sample in relation to log10 of the dry weight of aquatic plants. Sample codes as in Fig. 3. A regression line is included (see text for more details)
The variation in ostracod species abundance with time (Fig. 6) indicated that the populations of S. bicuspis and C. incisa both increased in density during the summer–autumn months. Strandesia bicuspis was found in all the aquatic environments studied (stations 1–8), but not in the same proportions. The stations where this species had greater representation were 1, 4, 6, and 7; in the latter two, the pattern of distribution over time during the sampling period was more clearly evident. S. bicuspis was registered during the summer months and persisted until autumn throughout the 2 years of monitoring (Fig. 6), and at station 6 both adults and juveniles were observed in February 2006. Noteworthy is the fact that this small lake had dried up shortly before, in December 2005. Chlamydotheca incisa was found at stations 1, 3, 5, 7, and 8, and its abundance was greater in autumn, though occasionally also in winter with both adults and juveniles. The populations of S. bicuspis became densely developed after periods of rainfall, even earlier than did C. incisa. The small variation between the two species at sites 3 and 8 shows that, when C. incisa dominated the ostracod community, it remained until the beginning of winter, when even a large proportion of juveniles of this species are observed, thus suggesting a slight preference or higher tolerance for lower temperatures in C. incisa over S. bicuspis, or a faster initial growth but reduced lifespan in the latter. A scattered occurrence of the two species was observed in the spring of both sampling years.
Change in abundance of the two ostracod species most commonly found at the study sites (stations 1–8) during the study period (June 2005 to June 2007). Dashed bars indicate sites that were dry at that time; pointed bars, almost dry sites. Dark grey bars: S. bicuspis, black bars: C. incisa. Filled bars: adults, empty bars: juveniles
The two first factors extracted by CCA (Fig. 7) accounted for 44.9% and 9.7% of the species data, respectively, and 70% and 15.1% of the relationships among the four ostracod species and the 12 environmental variables introduced. Through the stepwise forward selection of variables (FSV) procedure, the analysis showed that two of the variables were significantly (P < 0.05) related to the distribution of the species, namely water temperature and log10(conductivity). If we would use only conductivity and water temperature for the CCA, the percentage of explained variance of species data would be reduced to 28.8% for axis 1 and 3.7% for axis 2. However, testing separately (not sequentially) each variable using permutation tests, not only water temperature and log10(conductivity) were significant, but also the presence of plant species 8 (Limnobium spongia), pH, and log10(dry weight) of the floating vegetal mat were also significantly related to the distribution and abundance of ostracods. We finally used all these variables in the CCA ordering graph to appreciate in greater detail the distribution of the ostracod species among the samples in relation to the environmental gradients represented by the entire set of variables analyzed. Water temperature was associated with the negative sector of axis 1, while dissolved oxygen concentration along with log10(dry weight) of the vegetal carpet, conductivity, and pH were oriented in the positive sector. pH and log10(conductivity) were the most heavily weighted variables on the second axis, but in a negative relationship with it. With respect to the plant species building the floating vegetal carpet (pleuston), species 5 (Wolffia columbiana) was distinctive in that it became strongly associated with positive values on axis 1 (high conductivity and pH). The positioning of species 1 (Lemna gibba), 7 (Hydrocotyle ranunculoides), and 8 (Limnobium spongia), very close to each other in the upper left quadrant, indicated a relationship between their presence and low values of conductivity and pH, high temperature, and low levels of dissolved oxygen. The ordering diagram showed a quite clear separation among the dominant species of ostracods. C. incisa was located on the negative side of the water temperature gradient and on the positive side of the first axis, i.e., related to high dissolved oxygen concentration, pH, and log10(conductivity) but low water temperature, while S. bicuspis, in turn, was oriented on the negative side of axis 1 in association with higher water temperatures and lower quantities of dissolved oxygen, salts, and vegetation. The second axis separated only the samples situated on the positive side of axis 1, where C. incisa was predominant, and ordered the samples with more adults of this species on the negative portion of axis 2, with samples accounting for greater numbers of juveniles being on the positive side, in relation to reduced conductivity and pH values.
Results of canonical correspondence analysis (CCA) of samples where ostracods were found. Sample codes as in Fig. 3. Sp 1, Lemna gibba; Sp 2, Lemna minuta; Sp 3, Spirodela intermedia; Sp 4, Azolla filiculoides; Sp 5, Wolffia columbiana; Sp 6, Wolffiela oblonga; Sp 7, Hydrocotyle ranunculoides; Sp 8, Limnobium spongia
Discussion
During this study four species of Ostracoda were found on Martín García Island—Chlamydotheca incisa, Strandesia bicuspis, Bradleytriebella trispinosa, and Cypridopsis vidua—whose presence there had been documented by César et al. (2001) in samples obtained 10 years before the present survey. The species of the genera Chlamydotheca and Strandesia principally inhabit temporary aquatic environments, and therefore the appearance of these genera is determined by the duration of these aquatic environments. The previously reported occurrence of species of these genera on Martín García Island accordingly coincides with the spring and summer months (César et al., 2001). Recently, César & Liberto (2008) detected different spatial distributions for the two species in the island and called attention to an increase of tourist activities in Martín García Island that could affect some of the study sites through increased debris close to the water bodies. The number of ostracod species found in this survey is low, compared with other studies in Neotropical pleustonic environments (e.g., Rocha & Por, 1998; Higuti et al., 2007). Probably this low species richness is mostly caused by the recent origin of most of the pools, together with their isolation and temporary character.
The reduced content of salts, indicated by the low conductivity, and the pH values measured at the stations we monitored were consistent with the island’s granite bottom and with the precipitation pattern. The variability in the conductivity among the study sites was likely affected mainly by the influence of drainage from the Río de la Plata during the flooding of certain of those sites as well as the precipitation of salts and their accumulation on the rocky lakebed substrate in addition to contamination of anthropogenic origin. The conductivity of the internal Río de la Plata estuary (around Martín García Island) is about 70–80 μS cm−1 (Quirós & Senone, 1985; Jaime et al., 2001). Most of the study sites present much higher conductivity values for most of the year, with the exception of sites 6, 7 and 8, probably more influenced by rainwaters. The low values of dissolved oxygen in many samples were probably caused by the substrate’s high level of organic material at all of the sites and the covering of the surface of the water by the floating vegetation. Cantera Grande (site 2) exhibited the lowest values of dissolved oxygen and the maximum conductivity levels, followed by C. Gruta (site 3), while stations 6–8 exhibited the lowest conductivity and high dissolved oxygen content. Station 6 was the only natural small lake and located at the base of a sandbank, features which would account for its substrate of sand. The remaining sites were abandoned quarries whose substrate is rocky and covered by organic material.
Our findings indicated that ostracods were not present in a high proportion of the samples. Despite the low percentage of variation that was explained by our statistical model, the logistical regression results demonstrated that the probability of encountering ostracods was greater at high oxygen content and elevated water temperature along with the absence of Azolla filiculoides. The oxygen concentration in many samples was very low and sometimes much lower than that of the Río de la Plata surrounding waters (between 8 and 9 mg l−1 in September according to Quirós & Senone, 1985). Other authors (e.g., Mezquita et al., 2005) already recognized the negative effect of low oxygen content on the probability of finding freshwater ostracods, or discussed the scarcity of oxygen production by phytoplankton below floating vegetation, and its high consumption at night by their roots (Por & Rocha, 1998). However, the impact of particular aquatic plant species on ostracod occurrence has not been clearly acknowledged. Our results on the negative relationship between the presence of Azolla sp. and the probability of finding pleustonic ostracods need further attention. According to Costa et al. (1999), Azolla sp. is unique among the floating macrophytes in that it can grow in water lacking nitrogen compounds with sufficient phosphate present, owing to a symbiosis with the nitrogen-fixing cyanobacterium Anabaena azollae, a species that lives in the dorsal lobules of the macrophyte’s leaves. This characteristic enables the plant to maintain a high rate of growth in spite of the high algal biomass of cyanophytes. This overgrowth can exert a sequential impact on the structure of the various communities of biota present through the following domino effects: low planktonic diversity and a reduction of the herbivorous zooplankton, a lowering of the growth rates of other algae along with that of the floating and rooted macrophytes, with these effects producing a consequent drop in dissolved oxygen levels that would, in turn, result in the death of animals with low tolerance to hypoxia. Of relevance is the report of the production of neurotoxins by Nostoc in association with Azolla filiculoides (Usher et al., 2007), substances which could affect the presence of ostracods, though this possibility was not investigated here. While some herbivores can tolerate active or inactive toxins, species lacking this resistance can either remain in such a stressful environment or else migrate, a transition that would subject them to the risk of visibility to predators (Camacho, 2008). Mills & Wyatt (1974) observed that ostracod species exposed to blue–green algae exhibited generally low survival rates. In bialgal systems involving green plus blue–green algae, the ostracods would select one or the other for feeding, but they generally preferred strains of unicellular green algae. Nevertheless, certain chlorophytes (Zygnema, Hormidium, and Trentepohlia) were rejected in favor of blue–green algae such as Tolypothrix and Westiella, while erect and ramified strains (Fischerella, Westiella, and Tolypothrix) were chosen for consumption over Anabaena and Nostoc. These latter two genera were therefore less preferred by ostracods (Mills & Wyatt, 1974), which may be related to our results on the absence of ostracods when A. filiculoides is present, as this species can harbor these genera of cyanobacteria as symbionts. Future studies directed at investigation of the relationship between ostracods and this macrophyte species would be of interest.
From the results obtained in the multiple stepwise linear regression—performed to evaluate which environmental variables could be related to greater or lesser ostracod abundance—the only parameter selected by the model was seen to be log10(dry weight) for the vegetal carpet, where higher dry weight was associated with lower abundance (Fig. 5). This unexpected inverse relationship could have resulted from a reduction in the dissolved oxygen content (especially at night) through decomposition of algal organic material. In this sense, Marçal & Callil (2008) discussed that the reduced density of ostracods and other groups under low oxygen concentration could be related to the decay of floating macrophytes. An alternative explanation would be an increase of predation pressure over ostracods in more developed plant carpets, which take longer to grow. During the succession of invertebrate communities in temporary aquatic environments, bigger predator invertebrates potentially preying on ostracods need some time to develop, and therefore their trophic effects on ostracods and other smaller invertebrates are seen at later successional stages (Martins et al., 2009). However, according to results by Padial et al. (2009), high structural heterogeneity provided by roots of floating macrophytes can enhance survival of ostracods and other invertebrates to predators. However, these authors considered fishes as predators (not macroinvertebrate predators), which are seldom found in temporary ponds. Indeed, Poi de Neiff & Neiff (2006) discuss how this vegetation might serve as refuge for invertebrates from fishes, but actually the invertebrate community is usually rich in predatory invertebrates.
Strandesia bicuspis and Chlamydotheca incisa were the only two species found in the majority of the samples. S. bicuspis appeared to develop dense populations after periods of rainfall (and before C. incisa), as would correspond to a typical species of temporary environments. Mourguiart (1991) associated C. incisa with a supralittoral milieu (at 0 m), although this species was found in somewhat deeper regions too. In addition, Laprida (2006) located this species in oligohaline shallow temporary environments that were vegetated and contained sodium chloride but moderate levels of carbonates and bicarbonates, with pH between 7.5 and 9.0, dissolved oxygen levels between 4.1 and 6.5 mg l−1, and total dissolved solids between 680 and 2,070 mg l−1. In our study, C. incisa appeared at stations with lower pH and levels of total dissolved solids but similar concentrations of dissolved oxygen. Ramírez (1967) registered C. incisa in November, December, January, May, and July, at temperatures between 31°C in December and 13°C in July; in our survey, however, the water temperature never exceeded 27°C. We found no data in the literature regarding the ecological preferences of S. bicuspis, although Broodbakker (1984) reported that two other species of this genus—Strandesia longula Broodbakker, 1983 and Strandesia stocki Broodbakker, 1983—were found mostly in water with low chloride content, in accordance with our findings that S. bicuspis seems to be related to samples with lower ionic content than C. incisa. We found B. trispinosa (as adults) on only two occasions (in March and April) and at a single station (site 4) having an average temperature of 19°C. In the Pinto & Purper (1965) report, the authors mention that at, a few meters from where they registered B. trispinosa, a flooded pasture was located that contained a very different set of associated species: there Chlamydotheca and Strandesia were nearly the exclusive genera present, with only very few specimens from other genera being sighted. We ourselves observed dispersed or sporadic appearances of species from both of these genera (C. incisa and S. bicuspis) in the spring of 2005, but more consistently in 2006, probably as a result of the earlier and more abundant rainfall during the latter year. In this regard C. incisa seems to remain for longer, even persisting up to the end of autumn or the beginning of winter, perhaps because this species prefers prolonged hydroperiods more than does S. bicuspis, or tolerates lower temperatures as well. However, our results are not conclusive because this happened at only two sites.
Finally, CCA indicated that C. incisa seems to favor environments with lower temperatures, higher pH and conductivities, and greater content of dissolved oxygen in contrast to S. bicuspis, since the data from the latter became ordered on the negative side of axis 1 in samples with higher temperatures and lower pH, conductivities, and dissolved oxygen concentrations. The separation between the adults and juveniles of both species in the CCA ordination possibly results from the effects of the periods of rainfall, when the conductivity is reduced and more juveniles will hatch. In this regard, Ramírez (1967) found C. incisa in ponds in Buenos Aires Province during November, December, January, May, and July. In such environments the conditions vary in an accentuated manner, with the temperature oscillating between day and night, decreasing to the frost point in winter, and exceeding 30°C in summer. The author furthermore verified that this species tolerated freezing temperatures for as long as 10 h. In our results, besides some abiotic variables measured, the presence of plant species Limnobium spongia was significantly related to ostracod community ordination, perhaps related to the relatively large size of this plant in comparison with others. Results on invertebrate community variation in relation to floating vegetation composition are confusing; Dioni (1967) found high similarity in the pleustonic invertebrate composition on different species of plants, but Poi de Neiff & Neiff (2006) found an important effect of plant structure on invertebrate community composition.
In this investigation, we have shown how, in certain relatively simple aquatic environments such as those of temporary ponds, a differential response to the existing conditions can even be observed for the few ostracod species that have managed to colonize there. To date, the relationship between the various species and their environment has only been sparsely studied in many species of the Neotropical region. More ecological research of this type should be carried out in the future to determine with greater precision the niches of the various species within the region, so as to utilize, at the greatest level of precision, the preferred niches of the regional species and thereby enable the ostracods’ enormous potential as indicators of environmental conditions.
References
Armendáriz, L. C. & I. I. César, 2001. The distribution and ecology of littoral Oligochaeta and Aphanoneura (Annelida) of the Natural and Historical Reserve of Isla Martín García, Río de la Plata River, Argentina. Hydrobiologia 463: 207–216.
Broodbakker, N. W., 1984. The distribution and zoogeography of freshwater Ostracoda (Crustacea) in the West Indies, with emphasis on species inhabiting wells. Bijdragen tot de Dierkunde 54: 25–50.
Camacho, F. A., 2008. Macroalgal and cyanobacterial chemical defenses in freshwater communities. In Charles, D. A. (ed.), Algal Chemical Ecology. Springer, Berlin: 105–116.
César, I. I. & R. Liberto, 2008. Ostracoda. ¿Posibles indicadores de deterioro ambiental de la Reserva de Usos Múltiples Isla Martín García? Ciencia 3: 99–109.
César, I. I., L. C. Armendáriz & M. C. Damborenea, 2001. Ostrácodos (Crustacea) de la Isla Martín García, Río de la Plata, Argentina. Natura Neotropicalis 32: 147–151.
Costa, M. L., M. C. Santos & F. Carrapiço, 1999. Biomass characterization of Azolla filiculoides grown in natural ecosystems and wastewater. Hydrobiologia 415: 323–327.
Dalla Salda, L., 1981. El basamento de Martín García. Revista Asociación Geológica Argentina 36: 9–43.
Damborenea, M. C., I. I. César & L. C. Armendáriz, 1997. Especies de Temnocephala (Platyhelminthes, Themnocephalidae) de la Isla Martín García, Buenos Aires, Argentina. Neotropica 43: 123–124.
De Deckker, P., 1983. Notes on the ecology and distribution of non-marine ostracods in Australia. Hydrobiologia 106: 223–234.
De Marco, P. Jr., M. A. R. Araujo, M. K. Barcelos & M. B. L. Santos, 2001. Aquatic invertebrates associated with the water-hyacinth (Eichhornia crassipes) in an eutrophic reservoir in tropical Brazil. Studies on Neotropical Fauna and Environment 36: 73–80.
Dioni, W., 1967. Investigación preliminar de la estructura básica de las asociaciones de la micro y meso fauna de las raíces de las plantas flotantes. Acta Zoologica Lilloana 23: 111–137.
Esteves, F. A., 1998. Fundamentos em Limnologia, 2nd ed. Interciência-finep, Rio de Janeiro.
Geiger, W., 1998. Populations dynamics, life histories and reproductive modes. In Martens, K. (ed.), Sex and Parthenogenesis: Evolutionary Ecology of Reproductive Modes in Non-Marine Ostracods. Backhuys, Leiden, The Netherlands.
González, M. A. & G. B. Ravizza, 1987. Sedimentos estuáricos del Pleistoceno tardío y Holoceno en la Isla Martín García. Revista Asociación Geológica Argentina 42: 223–243.
Griffiths, H. I. & J. A. Holmes, 2000. Non-Marine Ostracods and Quaternary Palaeoenvironments. Technical Guide No. 8. Quaternary Research Association, London.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4(1): 9 pp.
Higuti, J., L. F. M. Velho, F. A. Lansac-Tôha & K. Martens, 2007. Pleuston communities are buffered from regional flood pulses: the example of ostracods in the Paraná River floodplain, Brazil. Freshwater Biology 52: 1930–1943.
Higuti, J., F. A. Lansac-Tôha, L. F. M. Velho & K. Martens, 2009. Biodiversity of non-marine ostracods (Crustacea, Ostracoda) in the alluvial valley of the upper Paraná River, Brazil. Brazilian Journal of Biology 69: 661–668.
Jaime, P., A. N. Menéndez & O. E. Natale, 2001. Balance y dinámica de nutrientes principales en el Río de la Plata Interior, Proyecto INA 10.4-01. Unpublished report.
Lahitte, H. B. & J. A. Hurrell, 1996. Plantas hidrófilas de la Isla Martín García (Buenos Aires, República Argentina). Comisión de Investigaciones Científicas (CIC), La Plata.
Laprida, C., 2006. Ostrácodos recientes de la llanura pampeana, Buenos Aires, Argentina: ecología e implicancias paleolimnológicas. Ameghiniana 43: 181–204.
Marçal, S. F. & C. T. Callil, 2008. Structure of invertebrates community associated with Eichhornia crassipes Mart. (Solms-Laubach) after the introduction of Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) in the Upper Paraguay River, MT, Brazil. Acta Limnologica Brasiliensia 20(4): 359–371.
Martins, M. J. F., J. Vandekerkhove, F. Mezquita, O. Schmit, J. Rueda, G. Rossetti & T. Namiotko, 2009. Dynamics of sexual and parthenogenetic populations of Eucypris virens (Crustacea: Ostracoda) in three temporary ponds. Hydrobiologia 636(1): 219–232.
Martens, K., I. Schön, C. Meisch & D. J. Horne, 2008. Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. Hydrobiologia 595: 185–193.
Mezquita, F., J. R. Roca, J. M. Reed & G. Wansard, 2005. Quantifying species–environment relationships in non-marine Ostracoda: examples using Iberian data. Palaeogeography, Palaeoclimatology, Palaeoecology 225: 93–117.
Mills, D. H. & J. T. Wyatt, 1974. Ostracod reactions to non-toxic and toxic algae. Oecologia (Berlin) 17: 171–177.
Moguilevsky, A. & R Whatley, 1995. Ostrácodos. Metodología para su estudio. In: Lopretto, E. C. & G. Tell (Dir.), Ecosistemas de Aguas Continentales, La Plata: 973–999.
Mourguiart, P., 1991. Los ostrácodos. In: La Paz. Institut français de recherche scientifique pour le dévelopment en coopération. ORSTOM-Instituto de Historia Social Boliviana (HISBOL) (Ed.), El lago Titicaca: síntesis del conocimiento limnológico actual, La Paz: 345–352.
Padial, A. A., S. M. Thomaz & A. A. Agostinho, 2009. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia 624: 161–170.
Pinto, I. D. & I. Purper, 1965. A new fresh-water ostracode Cyprinotus trispinosus Pinto et Purper, sp. nov., from southern Brazil, its ontogenetic carapace variation and seasonal distribution. Escola Geologia Porto Alegre 7: 1–53.
Poi de Neiff, A. & R. Carignan, 1997. Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Paraná River floodplain. Hydrobiologia 345: 185–196.
Poi de Neiff, A. & J. J. Neiff, 2006. Riqueza de especies y similaridad entre invertebrados que viven en macrófitas de la planicie de inundación del río Paraná. Interciencia 31(3): 220–225.
Por, F. D. & C. E. F. Rocha, 1998. The Pleustal, a third limnic biochore and its neotropical centre. Verhandlungen der Internationale Vereinigung fuer Theoretische und Angewandte Limnologie 26: 1876–1881.
Quirós, R. & H. Senone, 1985. Niveles de nutrientes y pigmentos fotosintéticos en el Río de la Plata interior (55°–59°W 34°–36°S). INIDEP Informes Técnicos del Departamento de Aguas Continentales, 1: 37.
Ramette, A., 2007. Multivariate analyses in microbial ecology. FEMS Microbial Ecology 62: 142–160.
Ramírez, F. C., 1967. Ostrácodos de lagunas de la provincia de Buenos Aires. Revista del Museo de La Plata (nueva serie) Sección Zoología 10: 5–54.
Ravizza, G. B., 1982, Geología del Pleistoceno-Holoceno de la Isla Martín García. Río de La Plata Superior. Trabajo Final de Licenciatura Facultad Ciencias Exactas y Naturales Universidad Buenos Aires.
Ravizza, G. B., 1984. Principales aspectos geológicos del Cuaternario en la Isla Martín García. Río de la Plata Superior. Revista de la Asociación Geológica Argentina 39: 125–130.
Rocha, C. E. & F. D. Por, 1998. Preliminary comparative data on the fauna of the pleuston in the southern Pantanal, Brazil, with emphasis on the microcrustaceans. Verhandlungen der Internationale Vereinigung fuer Theoretische und Angewandte Limnologie 26: 2137–2140.
Rumi, A., S. M. Martín, M. P. Tassara & G. Darrigran, 1996. Moluscos de agua dulce de la Reserva Natural e Histórica Isla Martín García, Río de la Plata, Argentina. Comunicaciones de la Sociedad Malacológica de Uruguay 7: 7–12.
Savatenalinton, S. & Martens, 2009. Generic revision of Cypricercinae McKenzie, 1971 (Crustacea, Ostracoda), with the description of three new genera and one new species and a phylogenetic analysis of the subfamily. Hydrobiologia 632: 1–48.
Schwalb, A., S. J. Burns, G. Cusminsky, K. Kelts & V. Margraff, 2002. Assemblage diversity and isotopic signals of modern ostracodes and host waters from Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology 187: 323–339.
Smith, A. & D. J. Horne, 2002. Ecology of marine, marginal-marine and non-marine ostracods. In Chivas, A. R. & A. Holmes (eds), The Ostracoda: Applications in Quaternary Research. Washington DC: 37–64.
SPSS Inc., 2004. SPSS ® 13.0 Guía Breve. SPSS Inc, Chicago. 241.
Takeda, A. M., G. M. Souza-Franco, S. M. Melo & A. Monkolski, 2003. Invertebrados associados as macrófitas aquáticas da planície de inundacão do alto rio Paraná (Brasil). In Thomaz, S. M. & L. M. Bini (eds.), Ecologia e manejo de macrófitas aquáticas. EDUEM, Maringá: 243–260.
Ter Braak, C. J. F., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
Ter Braak, C. J. F., 1988. CANOCO—a FORTRAN program for canonical community ordination. Microcomputer Power, Ithaca, NY.
Usher, K. M., B. Bergman & J. A. Raven, 2007. Exploring cyanobacterial mutualisms. Annual Review of Ecology, Evolution, and Systematics 38: 255–273.
Wiggins, G. B., R. J. Mackay & I. M. Smith, 1980. Evolutionary and ecological strategies of animals in annual temporary ponds. Archiv für Hydrobiologie Supplement 58: 97–206.
Acknowledgments
The authors wish to thank Dr. Donald Francis Haggerty, a retired career investigator and native English speaker, for translating the final version of the manuscript into English. F.M.-J. acknowledges help from the Spanish Ministry of Science and Innovation (Project CGL2008-01296/BOS) for funding to participate in the 16th ISO, and from the Valencian Government—Conselleria d’Educació de la Generalitat Valenciana for funding for a short stay in La Plata. Financial support for this work was provided by an institutional Grant from Comisión de Investigaciones Científicas de la Provincia de Buenos Aires. The authors wish to thank the staff members of the Prefectura Naval Argentina Isla Martín García and Mr. José D. Maciel. Liberto, R., Becaria CONICET and César, I., Investigador CIC. We are very grateful to two anonymous referees for their thorough review and detailed comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: D. A. Dermeval, R. L. Pinto & K. Martens / Ostracoda – Biostratigraphy and Applied Ecology
Rights and permissions
About this article
Cite this article
Liberto, R., Mesquita-Joanes, F. & César, I. Dynamics of pleustonic ostracod populations in small ponds on the Island of Martín García (Río de la Plata, Argentina). Hydrobiologia 688, 47–61 (2012). https://doi.org/10.1007/s10750-011-0600-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0600-0