Abstract
The variability and persistence of species are appropriate measures of stability for communities that have large fluctuations in composition, such as phytoplankton. Our hypothesis was that phytoplankton species richness and abundance depend on the environmental variability arising from different phases of hydrological cycles, since changes in the limnophase and potamophase promote environmental fluctuations in the floodplain. A sub-basin of the Upper Paraná River was assessed between 2000 and 2001, a prolonged limnophase period, and 2010–2011, a period of conspicuous potamophase and limnophase. The phytoplankton community differed between these two periods. The lowest species richness with elevated values of abundance was recorded in 2000–2001, when 18 taxa indicators were verified. Lower variability and greater persistence of phytoplankton were recorded in 2000–2001, when the environmental variability was low. Therefore, the prolonged limnophase had a negative influence on species richness and influenced the pattern of dominant species persistence, thus, corroborating our hypothesis, indicating greater phytoplankton persistence in irregular hydrological cycles. Hydrological cycles with limnophase and potamophase periods present greater variability and less persistence of phytoplankton species richness and abundance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept of stability has been widely discussed in theoretical ecology (Tilman, 1999; Ives & Carpenter, 2007; Mazancourt et al., 2013). Limnology has explored several characteristics of aquatic ecosystems that affect the stability of communities, including diversity, interactions among species, type of food webs and sensitivity of species to different environmental perturbations (Ptacnik et al., 2008; Corcoran & Boeing, 2012; Downing et al., 2014). Attempts have been made to understand the conditions that lead a community to persist in a given period of time (Townsend et al., 2010), since it is possible to define species persistence as the constancy in abundance, in the ranking of species or in their presence or absence over time (Rahel, 1990).
Stability can be measured in many different ways, commonly associated with different properties of the community, including variability, or inversely, species persistence (Pimm, 1984). Appropriate measures must be used, especially for communities that have large fluctuations in species composition (Ives et al., 2000), such as phytoplankton.
The phytoplankton community is temporally dynamic, and its variability is related to environmental characteristics and the temporal stability of the ecosystem. Several underlying processes may explain the increased community dissimilarity through time. It can be a result of the different responses of populations to environmental fluctuations, such as local extinction and colonisation processes (Schneck et al., 2010). The variability of phytoplankton structure, expressed here as changes in richness and abundance, is a potential indicator of environmental conditions, providing examples to understand how ecological systems respond to natural or anthropogenic disturbances.
Investigations of the community variability, as a response, have enabled the understanding of patterns and processes regarding a wide range of ecological systems, scales (spatial and/or temporal) and applications (Fraterrigo & Rusak, 2008). Furthermore, to understand the dynamics of ecological systems and the variability of organisms, it is necessary to provide information to management and monitoring programmes for biodiversity conservation (Palmer et al., 1997).
Alterations in both the limnophase (low water) and potamophase periods (high water; Neiff, 1990) promote environmental fluctuations in the floodplain. The hydrological (or hydrosedimentological, sensu Neiff, 1990) cycles of Paraná River are influenced by anthropogenic impacts, due to a large cascade of reservoirs that are located upstream of this area, and have generated hydrodynamic, physical, chemical and biological changes on the floodplain (Souza Filho et al., 2004; Roberto et al., 2009; Souza Filho, 2009; Bovo-Scomparim et al., 2013). In addition, climatic events such as El Niño and La Niña, which have caused rainfall anomalies in the region, have influenced the range of variation of the water levels in the main channel, with effects on phytoplankton (Train & Rodrigues, 2004; Bovo-Scomparim & Train, 2008; Borges & Train, 2009; Rodrigues et al., 2009).
The aim of this study was to assess the variability in richness and density of a phytoplankton community, and inversely, their persistence, in habitats of the sub-basin of the Upper Paraná River floodplain, as well as its relationship with the environmental variability generated during extreme hydrological cycles. Therefore, our hypothesis was that the variability and persistence of phytoplankton species richness and abundance depend on the environmental variability arising from different phases of the hydrological cycles. We predicted that years with irregular hydrological cycles, when no flood pulse occurs, there would be less variability and greater persistence of phytoplankton species richness and abundance. However, in hydrological cycles with conspicuous limnophase and potamophase, larger dispersion of the phytoplankton inoculum may occur during the potamophase, which may increase the variability and decrease the persistence of phytoplankton species richness and abundance.
Materials and methods
Study area
The Paraná River is the second largest river in South America and the tenth largest in the world regarding discharge water, completely inside the Brazilian territory, except for a stretch along the Itaipu Reservoir, bordering with Paraguay (Agostinho et al., 2008). The width:depth ratio is 100:1 and water velocity of approximately 0.56 m s−1. The Upper Paraná River floodplain occupies the entire channel of the Paraná River along the segment between Porto Primavera Dam (Primavera, SP) and the backwater of Itaipu Reservoir (Guaíra, PR). The river is about 230 km long and 20 km wide (Souza Filho & Stevaux, 2004).
The Baía River (BR) sub-basin is located on the right bank of the Paraná River. BR is an important lateral channel, located in the State of Mato Grosso do Sul (Fig. 1). The BR is separated from the Paraná River, on its left bank, by a wide floodplain, containing numerous channels and lakes. It has a depth:width ratio of 18:1 and considerable flow variations during the different phases of the hydrological cycle. During potamophase, the flow decreases, which can reverse the flow when water enters the Paraná River (Train & Rodrigues, 1998).
Guaraná Lake (CFL) is a floodplain lake with direct connection to the BR, with a rounded shape, presenting an average depth of 2.1 m, 386 m length, 1058 m perimeter and an area of 4.2 ha, beyond these margins, it is constituted of macrophytes, mainly in limnophase periods. Fechada Lake (IFL) is an isolated floodplain lake, without direct connection to the BR, with an elongated shape, presenting average depth of 2.4 m, 624 m length, 1376 m perimeter and an area of 7.5 ha.
Sampling and sample analysis
Water samples were taken quarterly in 2000–2001 (except in 2001, when only two samples were taken) and 2010–2011, indicating different hydrological cycles. Phytoplankton samples were taken on the subsurface (depth 20 cm) of the pelagic zone of each environment. Samples were directly collected with bottles. These samples were preserved with 1 % Lugol’s solution. We performed the counting randomly per field, using an inverted microscope, according to Utermöhl (1958) and Lund et al. (1958), estimating the phytoplankton density. Phytoplankton species richness considered the number of taxa present in each sample.
Water temperature (WT, °C), pH, electrical conductivity (Cond, μS cm−1) and dissolved oxygen (DO, mg l−1) were measured in situ using portable digital potentiometers. Water transparency (m) was measured with a Secchi disc. The euphotic zone (Z eu) was calculated as being 2.7-fold the depth of the Secchi disc (Cole, 1994). The maximum depth (Z max) was obtained in each habitat. Total phosphorus (TP), soluble reactive phosphorus (SRP), nitrate (NO3-N), nitrite (NO2-N) and ammonium (NH4-N) were measured using the methods described in Bovo-Scomparim et al. (2013). The dissolved inorganic nitrogen (DIN) was calculated as the sum of the NH4-N, NO2-N and NO3-N concentrations. The daily water levels of the Paraná River (WL) were provided by the Itaipu Binational, National Water Agency (ANA) and Limnology, Ichthyology and Aquaculture Research Center (Nupélia). Potamophase was considered when the water level of the Paraná River was ≥3.5 m, the beginning of the flooding process in the environments associated with the Upper Paraná River (Souza Filho et al., 2004). Limnophase was considered when the water level of the Paraná River was <3.5 m.
The variation in the hydrological cycle was considered by amplitude of limnophase (number of days with water level below reference level 3.5 m), amplitude of potamophase (number of days with water level above reference level 3.5 m) and connectivity index (ratio of the number of days under potamophase and limnophase) using PULSO software (Neiff & Neiff, 2003).
Data analysis
We performed a principal component analysis (PCA) to summarise environmental variability between different periods. In order to verify differences in phytoplankton richness and abundance between the two periods analysed (2000–2001 and 2010–2011) and the phases of the hydrological cycle (limnophase and potamophase), we performed a permutational multivariate analysis of variance (PERMANOVA; Anderson, 2001). Additionally, we identified species associated with each period in each habitat using the indicator species analysis (INDVAL; Dufrêne & Legendre, 1997). To test the variability and persistence of the phytoplankton species, we conducted a permutation test for homogeneity of multivariate dispersions among the sampling units of phytoplankton density collected in each period. The test was based on the distances from individual points (sampling unit) to their group centroid in the full dimensional space calculated through a principal coordinates analysis (PCoA). The analysis was based on a dissimilarity matrix obtained using Bray–Curtis index (Anderson, 2006). Significance was estimated through analysis of variance and permutation test. The data were log-transformed 10(x) + 1. To assess the relationship between phytoplankton abundance and environmental variables, we carried out a BioEnv test, which finds the best subset of environmental variables with the maximum Spearman’s rank correlation using the community dissimilarity matrix (Bray–Curtis index, log(x + 1) data; Clarke & Ainsworth, 1993). INDVAL analysis was performed using the PC-Ord 6.0 (McCune & Mefford, 1999). The remaining analyses were processed using R environment (R Development Core Team, 2012).
Results
Hydrological cycles and environmental variability
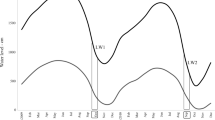
The variation in water level in the Paraná River was determined in two distinct periods. In the years of 2000 and 2001, the average water level was 2.7 ± 0.6 m (CV 21 %) with low intensity and amplitude of potamophase (11 and 3 days, respectively), the connectivity index ranged from 0.01 to 0.03 and limnophase predominated (355 and 362 days, respectively). The average water level of the Paraná River in the years of 2010 and 2011 was 3.3 ± 1.1 m (CV 32 %) with the occurrence of intense potamophase (102 and 81 days, respectively), and a connectivity index between 0.29 and 0.39. In these years, during the potamophase, the water levels of the Paraná River were >6 m (Fig. 2).
The first two axes generated through the PCA explained 60 % of the environmental variability in the environments of the BR sub-basin. The first axis had positive influence of the water level of the Paraná River (0.43), Z max (0.43) and Z eu (0.39) and negative influence of DO (−0.37), discriminated between the potamophase periods in 2010 and 2011, pointed out on the right of the diagram. The principal variables in the second axis were TP (0.48) and SRP (0.46) and pH (−0.41) (Fig. 3).
Dispersion of the scores of the first two axes of the principal component analysis performed for the abiotic variables of the Baía River sub-basin environments (CFL L: connected floodplain lake in limnophase, CFL P: connected floodplain lake in potamophase, IFL L: isolated floodplain lake in limnophase, IFL P: isolated floodplain lake in potamophase, BR L: Baía River in limnophase, BR P: Baía River in potamophase, WL: water level of the Paraná River, Zmax: maximum depth, Zeu: euphotic zone, DO: dissolved oxygen, pH: pH, TP: total phosphorus, SRP: soluble reactive phosphorus)
Phytoplankton community
Overall, 197 taxa were registered in BR, 235 in CFL and 162 in IFL. Chlorophyceae, Bacillariophyceae, Cyanobacteria and Euglenophyceae were the principal groups. Cryptophyceae, Chrysophyceae, Zygnemaphyceae, Xanthophyceae and Dinophyceae also revealed important contributions to these environments.
The phytoplankton species richness in BR ranged from 13 (May 2000) to 52 taxa (December 2010). In CFL, it ranged from 11 (August 2000) to 56 taxa (March 2010), while in IFL, the range was between 14 (February 2000) and 35 taxa (March 2010). The highest values for species richness were recorded in 2010–2011, for all of the analysed habitats (Fig. 4). Phytoplankton density ranged from 54 ind. ml−1 (December 2011) in BR to 9986 ind. ml−1 (February 2000) in CFL. The highest value of phytoplankton density was recorded during limnophase of 2000–2001 (Fig. 5). Significant differences in phytoplankton species richness and density were recorded in the different periods and phases of the hydrological cycle (Table 1).
The results of the INDVAL analysis suggest that only one taxon was indicator of the period 2010–2011, Monoraphidium convolutum (Corda) Komárková–Legnerová, in CFL, while in the period 2000–2001, 18 taxa with significant values were indicators (Table 2).
The PCoA indicated that along the period of 2000–2001 the sampling units in the three habitats were less dispersed than between 2010 and 2011 (Fig. 6a–c). The dispersion of the groups had significant differences in BR (P = 0.005), CFL (P = 0.03) and IFL (P = 0.01).
Principal coordinates analysis (PCoA) of phytoplankton density sampling units obtained in the three habitats in the Baía River sub-basin in periods 2000–2001 and 2010–2011: a river, b connected floodplain lake and c isolated floodplain lake. Polygons indicate the maximum dispersion of sampling units for each period. The solid circle and triangle in the centre of each polygon represent the respective group centroids
BioEnv indicated that the variables correlated with phytoplankton abundance in BR were pH, Z max and the water level of the Paraná River (Table 3). In CFL, the principal variables were electrical conductivity and Z eu (Table 4) and in IFL, the principal variables correlated were electrical conductivity, Z eu and SRP (Table 5).
Discussion
The variability of phytoplankton species richness and abundance in the environments of the BR sub-basin was strongly associated with the hydrological regime of the Paraná River. Lower temporal variability and greater phytoplankton persistence occurred in 2000–2001, when an extreme drought in the region resulted in anomalies in the hydrological cycles. The synergism of the La Niña events (McPhaden et al., 2006; CPTEC, 2012), which caused negative precipitation anomalies in the region, associated with Porto Primavera Reservoir filling, located upstream to the study area (Borges & Train, 2009; Rodrigues et al., 2009), was decisive to generate disturbances in the hydrological regime and extending the limnophase period.
Thus, the combination of intensive hydrological and climatic anomalies affected the dynamics of the phytoplankton community structure and caused greater persistence of abundance for some taxa. Devercelli (2006) also recorded high phytoplankton abundance, due to prolonged limnophase, associated with severe drought in the Middle Paraná River in 1999–2000. As attested by Pomati et al. (2011), the intensity of fluctuations of environmental variables has strong effect on the temporal stability of planktonic communities.
The change in the natural dynamics of potamophase periods may have influenced the local phytoplankton richness, since floods, along with habitat heterogeneity, are responsible for the maintenance of biodiversity in the floodplain (Neiff, 1990; Ward & Tockner, 2001). The low species richness and high density recorded in 2000–2001, provided the evidence, along with the presence of cyanobacteria during most of this period. In general, the continuous persistence of species is often greater in relatively constant environmental conditions (Collier, 2008).
The increase in phytoplankton species richness in periods of high water levels in Paraná River between 2010 and 2011 suggests the availability of ecological niches and inflows of inocula into the remaining habitats that were isolated during limnophase. Besides, the adjacent environments in the Upper Paraná River may constitute storage zones for algae, contributing significantly to phytoplankton species richness (Bovo-Scomparim, 2011). Therefore, it confirmed that a greater amplitude of potamophase favours phytoplankton diversity.
The phytoplankton composition of the BR and floodplain lakes presented remarkable differences between the two periods. According to INDVAL analysis, M. convolutum was the only significant indicator for CFL in 2010–2011, probably due to the decrease in competitive and dominant species during the potamophase, which favoured the increase of rare species (Train & Rodrigues, 2004). The lowest water retention time in the potamophase restricts phytoplankton growth, and our results suggest that this time is insufficient for the accumulation of phytoplankton, in other words, for the proliferation of dominant species (Schemel et al., 2004).
In the high water period, the positive precipitation anomalies were influenced by El Niño events according to McPhaden et al. (2006) and CPTEC (2012), which occurred concomitantly with the release of water from dams upstream, causing exceptional floods in the Upper Paraná River, with large amplitude of potamophase. In this phase of the hydrological cycle, occurred a marked reduction in the phytoplankton density, mainly in BR, that had influence of flooding caused by Paraná River.
According to Schemel et al. (2004), phytoplankton abundance is favoured in temperate floodplains with complex hydrological variability with several flooding periods. However, this was not a pattern evident in this sub-basin of the Upper Paraná River subtropical region. The highest value of phytoplankton abundance was recorded in 2000–2001, with the absence of potamophase. Grabowska et al. (2014) described significant increase in phytoplankton abundance at low water level condition and increased diversity of phytoplankton during high water level condition, the same pattern found in our study.
In 2000–2001, with the occurrence of lower variability and greater persistence in the phytoplankton abundance in the habitats, 18 taxa were selected as indicators. These taxa were favoured by environmental conditions imposed by limnophase, mainly at high concentrations of TP and low Z max. Among these taxa, a higher contribution of diatom Aulacoseira granulata (Ehrenberg) Simonsen var. granulata to density in BR in 2000–2001 pointed to the dependence of this taxon upon turbulence for suspension (Reynolds et al., 2002; Rodrigues et al., 2009), which was facilitated in the lower Z max period, when the winds promoted complete mixing in the water column. Aulacoseira herzogii (Lemmermann) Simonsen, an indicator species for CFL, was associated with high nutrient concentrations, such as TP and SRP. According to Padisák et al. (2009), the occurrence of these taxa is related to environmental conditions found in the shallow lakes, such as mesotrophic status and water column mixing, which were verified in this environment.
The dominance of cyanobacteria nitrogen fixers such as Dolichospermum circinalis (Rabenhorst ex Bornet & Flahault) P. Wacklin, L. Hoffmann & J. Komárek and Dolichospermum planctonicum (Brunnthaler) P. Wacklin, L. Hoffmann & J. Komárek has been attributed, among other factors, to their ability to survive in N-deficient systems (Reynolds et al., 2002). This feature combined with their high surface:volume ratio, confers a greater tolerance to nutrient-limiting conditions and results in the success of these taxa in low light, high-trophic status environments (Kruk & Segura, 2012). D. planctonicum in BR and CFL in February and August 2000 was associated with low Z eu and hypereutrophic conditions (TP >200 µg l−1). The cyanobacteria Aphanocapsa elachista West & G. S. West and Synechocystis aquatilis Sauvageau were favoured by the high concentrations of nutrients, mainly TP. Furthermore, A. elachista, with abundant mucilage, and S. aquatilis, with a high surface:volume ratio (Kruk et al., 2010), were favoured in limnophase, due to high water retention time, thereby enabling greater permanence in the water column. Thus, the synergism of these factors influenced the dominance of cyanobacteria in the phytoplankton community.
The chlorophyceans represented by Closteriopsis scolia A. Comas, Monoraphidium contortum (Thuret) Komárková–Legnerová and Monoraphidium griffithii (Berkeley) Komárková–Legnerová, with high requirements for nutrients (Padisák et al., 2009), contributed strongly to high phytoplankton density in BR and its connected lake in many periods. Euglena sp., Trachelomonas hispida var. hispida (Perty) Stein and Trachelomonas volvocinopsis Svirenko have commonly been described for meso–eutrophic shallow lakes rich in organic matter (Padisák et al., 2009; Kruk & Segura, 2012), and contributed to high densities only in CFL, in 2000–2001. The cryptomonads Chroomonas and Cryptomonas, selected as indicators for all environments, possess high surface area:volume ratios, rapid growth, rapid absorption of nutrients and tolerance to low light. These characteristics, including opportunistic life strategies, make them very adaptable to a wide range of habitats and environmental conditions, and enable their development in different habitats (Reynolds et al., 2002; Kruk et al., 2010). High values of phytoplankton density occurred in CFL as well as in the river in 2000 and 2001, when prolonged limnophase occurred. The low-flow conditions in BR favoured phytoplankton growth and the occurrence of cyanobacteria blooms (Train & Rodrigues, 1998) as well as promoted greater persistence of species.
The connectivity between the river and the lake facilitates the exchange of inocula, providing the lentic environment with hydrodynamic and nutritional conditions appropriate for the development and persistence of mostly A. granulata var. granulata and D. planctonicum in 2000–2001. Furthermore, the continuous fluctuations in water level of BR, connected to Guaraná Lake, influence the magnitude of environmental variation in this lake. Thus, the continuous exchange of water between BR and this CFL resulted in a greater homogeneity of environmental conditions, as well as of phytoplankton taxa, unlike those observed in IFL, which, with its particular dynamics, allowed the occurrence of distinct successional processes of the phytoplankton community, thereby establishing a distinct habitat and phytoplankton community from BR.
The variability of phytoplankton abundance followed a well-defined temporal pattern, with records of the highest variability or greatest dispersion of sampling units in time for 2010–2011, in the river and floodplain lakes. As indicated through the PCoA, potamophase increased temporal dissimilarity and modified the phytoplankton abundance. In this sense, the recurrence of floods maintains those environmental conditions that favour greater variability and lower persistence of the phytoplankton community. This indicates that the temporal variability of phytoplankton abundance was lowest in the years 2000 and 2001, when potamophase did not occur, providing the phytoplankton taxa with greater persistence. The opposite was reported in 2010 and 2011, with the occurrence of intensive potamophase, greater variability of phytoplankton abundance and lower taxa persistence.
The low diversity of planktonic algae may decrease the magnitude and stability of ecosystem processes, such as the use of resources and productivity (Cottingham et al., 2001). This pattern was observed in 2000–2001, when the prolonged limnophase altered the patterns of richness and influenced the dominance of some species. In the absence of floods, the autochthonous processes of productivity may have had greater contribution to energy input than the allochthonous processes, causing the accumulation of nutrients in the habitats, mainly TP. These conditions favour the dominance of species such as cyanobacteria. Effects of extreme variations in water level on other aquatic communities of the Upper Paraná River floodplain have been shown previously (Bonecker et al., 2009; Lansac-Tôha et al., 2009; Padial et al., 2009; Bonecker et al., 2013; Higuti et al., 2013; Petry et al., 2013).
The environmental variables selected by BioEnv, explain the dissimilarity of the phytoplankton in the river (pH, Z max and water level of Paraná River), in CFL (conductivity and Z eu) and IFL (conductivity, Z eu and SRP). The variables water level, Z eu and Z max showed a particularly strong variation between the two extreme periods; therefore, they appeared to be directly related to the temporal variation of phytoplankton abundance. These variables indicated that when there is low environmental variability, phytoplankton abundance is greater. According to Huszar et al. (2003), environmental constancy is required to promote and maintain phytoplankton persistence; however, little is known on the subject and its direct relationship with environmental stability (Schneck et al., 2010). Nevertheless, it is possible to conclude that the greater phytoplankton persistence was related to lower environmental variability.
Flooding is a regulatory factor and component of the ecosystem that benefits the maintenance of ecological processes and the biodiversity (Ward & Tockner, 2001). So, the limnophase extension interferes with the phytoplankton richness and influences the pattern of species dominance. A strong distinction of the phytoplankton richness and abundance was observed between the two periods analysed. Undoubtedly the regulation of the main channel of Paraná River by Porto Primavera Dam, along with the La Niña event, was the main drivers of alterations in the environmental conditions in 2000–2001, and influenced the extension of limnophase. Therefore, our hypothesis was confirmed that in irregular hydrological cycles without flood pulses reduce variability and increase abundance of the phytoplankton community, which is in contrast to the hydrological cycles with limnophase and potamophase when there is greater variability and lower persistence of phytoplankton richness and abundance.
References
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68(4, Suppl.): 1119–1132.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Anderson, M. J., 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62: 245–253.
Bonecker, C. C., A. S. M. Aoyagui & R. M. Santos, 2009. The impact of impoundment on the rotifer communities in two tropical floodplain environments: interannual pulse variations. Brazilian Journal of Biology 69(2 Suppl.): 529–537.
Bonecker, C. C., N. R. Simões, C. V. Minte-Vera, F. A. Lansac-Tôha, L. F. M. Velho & A. A. Agostinho, 2013. Temporal changes in zooplankton species diversity in response to environmental changes in an alluvial valley. Limnologica 43: 114–121.
Borges, P. A. F. & S. Train, 2009. Phytoplankton diversity in the Upper Paraná River floodplain during two years of drought (2000 and 2001). Brazilian Journal of Biology 69(2 Suppl.): 637–647.
Bovo-Scomparim, V. M., 2011. Variação temporal e espacial da comunidade fitoplanctônica do alto rio Paraná e ambientes associados, na área de influência dos reservatórios de Porto Primavera e Rosana. Universidade Estadual de Maringá, Tese de Doutorado.
Bovo-Scomparim, V. M. & S. Train, 2008. Long-term variability of the phytoplankton community in an isolated floodplain lake of the Ivinhema River State Park, Brazil. Hydrobiologia 610: 331–344.
Bovo-Scomparim, V. M., S. Train & L. C. Rodrigues, 2013. Influence of reservoirs to dispersion and seasonal variation of the phytoplankton community in the Upper Paraná River, Brazil. Hydrobiologia 702: 115–127.
Clarke, K. R. & M. Ainsworth, 1993. A method of linking multivariate community structure to environmental variables. Marine Ecology Progress Series 92: 205–219.
Cole, G. A., 1994. Textbook of Limnology. Waveland Press, Inc., Prospect Heights.
Collier, K. J., 2008. Temporal patterns in the stability, persistence and condition of stream macroinvertebrate communities: relationships with catchment land-use and regional climate. Freshwater Biology 53: 603–616.
Corcoran, A. A. & W. J. Boeing, 2012. Biodiversity increases the productivity and stability of phytoplankton communities. Plos One 7(11): 1–9.
Cottingham, K. L., B. L. Brown & J. T. Lennon, 2001. Biodiversity may regulate the temporal variability of ecological systems. Ecology Letters 4: 72–85.
CPTEC, 2012. Centro de Previsão do tempo e estudos climáticos. http://www.cptec.inpe.br/. Accessed 24 Nov 2012.
Devercelli, M., 2006. Phytoplankton of the Middle Paraná River during an anomalous hydrological period: a morphological and functional approach. Hydrobiologia 563: 465–478.
Downing, A. L., B. L. Brown & M. A. Leibold, 2014. Multiple diversity-stability mechanisms enhance population and community stability in aquatic food webs. Ecology 95(1): 173–184.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Fraterrigo, J. M. & J. A. Rusak, 2008. Disturbance-driven changes in the variability of ecological patterns and processes. Ecology Letters 11: 756–770.
Grabowska, M., K. Glińska-Lewczuk, K. Obolewski, P. Burandt, S. Kobus, J. Dunalska, R. Kujawa, A. Goździejewska & A. Skrzypczak, 2014. Effect of hydrological and physiochemical factors on phytoplankton communities in floodplain lakes. Polish Journal of Environmental Studies 23: 713–725.
Higuti, J., L. F. M. Velho, F. A. Lansac-Tôha & K. Martens, 2013. Plêuston communities are buffered from regional flood pulses: the example of ostracods in the Paraná River floodplain, Brazil. Freshwater Biology 52: 1930–1943.
Huszar, V., C. Kruk & N. Caraco, 2003. Steady-state assemblages of phytoplankton in four temperate lakes (NE U.S.A.). Hydrobiologia 502: 97–109.
Ives, A. R. & S. R. Carpenter, 2007. Stability and diversity of ecosystems. Science 317: 58–62.
Ives, A. R., J. L. Klug & K. Gross, 2000. Stability and species richness in complex communities. Ecology Letters 3: 399–411.
Kruk, C. & A. M. Segura, 2012. The habitat template of phytoplankton morphology-based functional groups. Hydrobiologia 698: 191–202.
Kruk, C., V. L. M. Huszar, E. H. M. Peeters, S. Bonilla, L. Costa, M. Lurling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Lansac-Tôha, F. A., C. C. Bonecker, L. F. M. Velho, N. R. Simões, J. D. Dias, G. M. Alves & E. M. Takahashi, 2009. Biodiversity of zooplankton communities in the Upper Paraná River floodplain: interannual variation from long-term studies. Brazilian Journal of Biology 69(2 Suppl.): 539–549.
Lund, J. W. G., C. Kipling & E. D. Lecren, 1958. The inverted microscope method of estimating algal number and the statistical basis of estimating by counting. Hydrobiologia 11: 980–985.
Mazancourt, C., I. Forest, A. Larocque, F. Berendse, E. Luca, J. B. Grace, B. Haegeman, H. W. Polley, C. Roscher, B. Schimid, D. Tilman, J. van Ruijven, A. Weigelt, B. J. Wilsey & M. Loreau, 2013. Predicting ecosystem stability from community composition and biodiversity. Ecology Letters 16: 617–625.
McCune, B. & M. J. Mefford, 1999. PC-ORD. Multivariate Analysis of Ecological Data, Version 4.0. MjM Software Design, Gleneden Beach.
McPhaden, M. J., S. E. Zebiak & M. H. Glantz, 2006. ENSO as an Integrating concept in earth science. Science 314: 1740–1745.
Neiff, J. J., 1990. Ideas para la interpretacion ecologica del Paraná. Interciência 15(6): 424–441.
Neiff, J. J. & M. Neiff, 2003. PULSO: software para análisis de fenómenos recurrentes. http://www.neiff.com.ar. Accessed 15 Feb 2012.
Padial, A. A., P. Carvalho, S. M. Thomaz, S. M. Boschilia, R. B. Rodrigues & J. T. Kobayashi, 2009. The role of an extreme flood disturbance on macrophyte assemblages in a neotropical floodplain. Aquatic Science 71: 389–398.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and m issue in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Palmer, M., C. Hakenkamp & K. Nelson-Baker, 1997. Ecological heterogeneity in streams: why variance matters. Journal of the North American Benthological Society 16: 189–202.
Petry, A. C., F. Abujanra, L. C. Gomes, H. F. Julio Jr. & A. A. Agostinho, 2013. Effects of the interannual variations in the flood pulse mediated by hypoxia tolerance: the case of the fish assemblages in the Upper Paraná River floodplain. Neotropical Ichthyology 11(2): 413–424.
Pimm, S. L., 1984. The complexity and stability of ecosystems. Nature 307: 321–326.
Pomati, F., B. Matthews, J. Jokela, A. Schildknecht & B. W. Ibelings, 2011. Effects of re-oligotrophication and climate warming on plankton richness and community stability in a deep mesotrophic lake. Oikos 121(8): 1317–1327.
Ptacnik, R., A. G. Solimini, T. Andersen, T. Tamminen, P. Brettum, L. Lepisto, E. Willén & S. Rekolainen, 2008. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proceedings of the National Academy of Science of USA 105(13): 5134–5138.
Rahel, F. J., 1990. The hierarchical nature of community persistence: a problem of scale. American Naturalist 136: 328–344.
R Development Core Team, 2012. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 18 Oct 2012.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Roberto, M. C., N. F. Santana & S. M. Thomaz, 2009. Limnology in the Upper Paraná River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Brazilian Journal of Biology 69(2 Suppl.): 717–725.
Rodrigues, L. C., S. Train, V. M. Bovo-Scomparin, S. Jati, C. C. J. Borsalli & E. Marengoni, 2009. Interannual variability of phytoplankton in the main rivers of the Upper Paraná River floodplain, Brazil: influence of upstream reservoirs. Brazilian Journal of Biology 69(2 Suppl.): 501–516.
Schemel, L. E., T. R. Sommer, A. B. Muller-Solger & W. C. Harrell, 2004. Hydrologic variability, water chemistry, and phytoplankton biomass in a large floodplain of the Sacramento River, CA, U.S.A. Hydrobiologia 513: 129–139.
Schneck, F., A. Schwarzbold, S. C. Rodrigues & A. S. Melo, 2010. Environmental variability drives phytoplankton assemblage persistence in a subtropical reservoir. Austral Ecology 36(7): 839–848.
Souza Filho, E. E., 2009. Evaluation of the Upper Paraná River discharge controlled by reservoirs. Brazilian Journal of Biology 69(2 Suppl.): 707–716.
Souza Filho, E. E. & J. C. Stevaux, 2004. Geology and geomorphology of the Baía–Curutuba–Ivinheima River complex. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 1–30.
Souza Filho, E. E., P. C. Rocha, E. Comunello & J. C. Stevaux, 2004. Effects of the Porto Primavera Dam on physical environment of the downstream floodplain. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 55–74.
Tilman, D., 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80: 1455–1474.
Townsend, S. E., D. T. Haydon & L. Matthews, 2010. On the generality of stability–complexity relationships in Lotka–Volterra ecosystems. Journal of Theoretical Biology 267: 243–251.
Train, S. & L. C. Rodrigues, 1998. Temporal fluctuations of the phytoplankton community of the Baia River, in the Upper Paraná River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 361: 125–134.
Train, S. & L. C. Rodrigues, 2004. Phytoplankton assemblage. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 103–124.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen phytoplankton-methodic. Mitt. d. internat. vereinig. f. limnol. 9: 1–39.
Ward, J. V. & K. Tockner, 2001. Biodiversity: towards a unifying theme for river ecology. Freshwater Biology 46: 807–819.
Acknowledgments
The authors are grateful to the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia) at Universidade Estadual de Maringá for logistic support; CNPq/PELD for financial support and CAPES for a scholarship granted to the first author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David Hamilton
Rights and permissions
About this article
Cite this article
Bortolini, J.C., Train, S. & Rodrigues, L.C. Extreme hydrological periods: effects on phytoplankton variability and persistence in a subtropical floodplain. Hydrobiologia 763, 223–236 (2016). https://doi.org/10.1007/s10750-015-2378-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2378-y