Abstract
Knowledge of the environmental correlates of species’ distributions is essential for understanding population dynamics, responses to environmental changes, biodiversity patterns, and the impacts of conservation plans. Here we examine how environment controls the distribution of the neotropical genus Montrichardia at regional and local spatial scales using species distribution models (SDMs) and logistic regression, respectively. Montrichardia is a genus of aquatic macrophytes with two species, Montrichardia linifera and Montrichardia arborescens, and is often an important component of flooded habitats. We find that for each species, altitude, precipitation and temperature of the driest month figure in the best performing SDMs as the most important factors controlling large-scale distributions, suggesting that the range limits of both species are climatically constrained by plant water-energy balance and cold intolerance. At small spatial scales, logistic regression models indicate the species partition types of aquatic habitat along local gradients of water pH, conductivity, and water transparency. In summary, a hierarchy of factors may control Montrichardia distribution from large to small spatial scales. While at large spatial scales, evolutionarily conserved climatic niches may control the range limits of the genus, at small spatial scales niche differentiation allows individual species to grow in environmentally distinct aquatic habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Observed species distribution is biologically rooted in local demographic processes—survival, growth, reproduction and dispersal—that can vary widely on an environmentally heterogeneous landscape (Merow et al., 2014). As such, an understanding of the factors that control species distributions from local to regional spatial scales is of key importance in predicting species response to global climate change, local habitat restoration, and conservation programs (Chambers et al., 2008; Lopes et al., 2015, 2016). At regional scales, the spatial distribution of plants is often understood to be limited by climatic factors and geographical barriers that prevent migration (Sculthorpe, 1985; Santamaría, 2002). At local spatial scales, both abiotic (e.g., environmental constraints) and biotic processes (e.g., competition) have been shown to determine species distribution (Silvertown et al., 1999; Ferreira et al., 2015). In general, however, a mechanistic understanding of species distributions across scales is still lacking for most species (Wiens, 2011).
Improved understanding of the factors controlling the spatial distribution of freshwater aquatic macrophytes may be particularly enigmatic, largely because of their restriction to wetlands. For example, because of the patchy distribution of wetlands, the effects of dispersal limitation on the distribution of aquatic macrophytes might be expected to be greater relative to terrestrial plants, leading to more restricted distributions (Lopes et al., 2016). On the other hand, wetland habitats may facilitate dispersal, by both serving as migration corridors and buffering species from adverse climates by generating more favorable microclimates at local scales (Meave et al., 1991; Householder et al., 2015). In light of this, some researchers have tended to emphasize large range sizes and wide environmental tolerance of aquatic macrophytes (e.g., Candolle, 1855; Darwin, 1859; Good, 1953; Santamaría, 2002; Lopes & Piedade, 2014), while others have focused on patterns of rarity, endemicity, and ecological specificity (e.g., Weddell, 1872; Guppy, 1906; Chambers et al., 2008; Figueroa et al., 2013). The need to more precisely understand the environmental drivers of macrophyte distribution across spatial scales has been increasingly recognized, especially in light of potentially large economic and ecological repercussions of changes in macrophyte distribution, either as a result of climate change or human-mediated introduction (Piedade et al., 2010; Lopes et al., 2015).
The distribution and growth of aquatic vegetation have traditionally been understood in terms of plant ecophysiological response to local environmental conditions (such as light, temperature, nutrient availability, pH, salinity, water velocity, and water-level variation) (Barendregt & Bio, 2003; Neiff & Poi de Neiff, 2003; Piedade et al., 2010; Figueroa et al., 2013). While such studies have increased our understanding of ecological function and physiology of aquatic plants, a local-scale perspective is often not adequate to understand distribution pattern on regional scales. Consequently, species distribution modeling is increasingly employed to investigate the factors that control the broader range limits of aquatic macrophytes, especially with regard to regional scale applications, such as in the prediction of the invasive expansion of aquatic plants (Lehtonen, 2009; Loo et al., 2009). Because different factors may control species distribution at different spatial scales, a combined approach aimed at elucidating environmental determinants of species distribution from local to regional spatial scales can arguably lead to novel insight.
In this study, we apply distribution models—using both species distribution modeling and logistic regression—to identify the environmental factors controlling the spatial distribution of the genus Montrichardia (Araceae) at regional and local spatial scales, respectively. The genus Montrichardia occurs exclusively in the Neotropics (Mayo et al., 1997) and contains two species of emergent aquatic macrophytes, M. linifera and M. arborescens, both known popularly as Aroid Marsh or as “Aninga” in Brazil. The distributions of the two Montrichardia species overlap in the Amazon Basin, where they often form monospecific stands along floodplain lakes and rivers. In this study, we aim to determine (1) what environmental factors constrain the range limits of Montrichardia species and how these environmental associations compare among species and (2) what environmental factors are associated with the local distribution patterns of the genus Montrichardia and how these associations compare among species. We expect that while climatic factors similarly control the range limits of Montrichardia species at continental scales, at local scales, individual species distribution may be strongly differentiated by non-climatic factors.
Materials and methods
Focal region
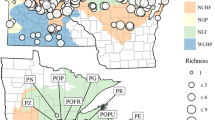
Our field sampling was restricted to the Amazon basin, where the distributions of both Montrichardia species overlap. The Amazon provides a potentially interesting focal region because of the high environmental diversity of wetland habitat types (Junk et al., 2011). Indeed, some aquatic organisms in the Amazon Basin have their distribution restricted to specific wetland habitat types (Piedade et al., 2010; Lopes et al., 2011, 2014). The most important of these include black-, white-, and clear-water habitats, differentiated according to the geology of the drainage basin (Sioli, 1968). This simple categorization of water types is possible, because water color reflects physical and chemical water characteristics (Sioli, 1968; Furch, 2000; Junk et al, 2011). White-water rivers are rich in dissolved minerals and are characterized by intense erosional and depositional processes resulting in high loads of suspended matter and muddy-colored water (várzea) (Furch & Junk, 1997). In contrast, clear and black-water rivers (igapó) drain geologically old formations of the Brazilian and Guiana Shields and carry little suspended sediment (Furch & Junk, 1997). Black-water rivers are further characterized by high levels of dissolved humic substances and low pH (acidic waters) (Junk et al., 2015). Clear-water rivers are characterized by intermediate pH and a high phytoplankton production, comparable to várzea lakes (Richey et al., 1990; Junk, 1997). Both white- and clear-water wetlands show higher abundance of aquatic plants and floating meadows than black-water wetlands (Piedade et al., 2010). Relatively few species of aquatic macrophytes occur in black-water floodplains, mostly belonging to the families Cyperaceae, Poaceae, Maranthaceae, and Araceae (Piedade et al., 2010; Lopes et al., 2014). Because differences among Amazonian wetland habitat types covary, relatively few and easily measured variables can be used as broad surrogates of environmental variation in Amazonian wetland habitats, including pH, conductivity, water transparency, and even botanical criteria (Junk et al., 2011, 2012, 2015).
Field sampling and analysis
To examine the distributions of species along local physiochemical water gradients field sampling in 45 sites distributed over an area of approximately 3.8 million km2 within the Amazon Basin was undertaken during the period of 2009–2012 in the Brazilian states of Roraima, Amazonas, Rondônia, Pará, and Amapá (Fig. 1). The study sites included the three major Amazonian water types, white, black, and clear waters (Table 1). Field sampling was performed in local populations of isolated Montrichardia stands that were located at least 500 meters from a neighboring Montrichardia population. Each population was georeferenced with aid of a GPS Garmin using UTM coordinates. Water pH (WTW, model pH 315i, Germany), conductivity (WTW, model cond 315i, Germany), Secchi disk depth (water transparency), and water column depth were measured with standard portable devices. Conductivity, water transparency, and pH are strong indicators of different Amazonian water types (Sioli, 1968). The degree of water-level fluctuation experienced by each population was estimated by measuring the height above ground level of the most recent annual high-water event, as determined by watermarks on woody trees near the sampling area (see more details in Wittmann et al., 2004; Schöngart et al., 2005).
We used logistical regression to examine how pH, conductivity, water transparency, and water column depth influence species occurrence at local spatial scales. Statistical analyses were performed using R 3.0.1 software. In addition, direct ordination of presence/absence data along environmental gradients was analyzed using the software Comunidata 1.6 (Dias, 2009).
Distribution modeling
To examine the neotropical spatial distributions of Montrichardia species, we gathered georeferenced data available in online herbaria (Appendix 1 in Supplementary Material; Fig. 2), totaling 284 records for Montrichardia arborescens and 114 records for M. linifera. We used Maximum Entropy Method (MAXENT version 3.3.3 k) to model the potential distribution of Montrichardia species within the neotropics. MaxEnt has been identified as one of the most accurate methods for species niche modeling for geographically sparse occurrence records (Elith et al., 2006; Pearson et al. 2007). The method combines biological data of species occurrence (presence-only data) with environmental grid data to estimate the probability of distribution, subjected to the set of constraints provided by environmental characteristics of grid cells where the species has been recorded (Phillips et al., 2006). For each species, plant records were split into a 70% “training set” and a 30% model “test set,” for model validation. Duplicate records were excluded. All other settings were set to default values.
Fifteen environmental variables were used (aspect, elevation, annual accumulation flux, direct flux, inclination, digital soil map, wetlands, global vegetation index-EVI, temperature of hottest month, temperature of coldest month, temperature of driest month, average annual temperature, total annual rainfall, rainfall of hottest, and coolest months) with a resolution of 1 km2, extracted from public data bases (Appendix 2 in Supplementary Material). Variables were used individually and in several combinations in the search for the best MAXENT model with the lowest number of variables for each species. All models were performed using 1000 permutations. Model performance was assessed using two methods: (i) the area under the curve (AUC) of the receiver-operating characteristic (ROC) and (ii) the jackknife validation method. The AUC of ROC is obtained by plotting sensitivity (proportion of correct prediction true positive, or absence of omission) and 1-specificity (proportion of false predicted presence—false-positive or commission error) for all possible thresholds of probability (threshold independent evaluation). In presence-only models, the AUC value represents the probability that the model scores a presence site (test locality) higher than a random background site (Phillips et al., 2006). The value ranges from 0.5 to 1 − a/2, where a is the fraction of pixels covered by the species’ distribution that remains unknown (Phillips et al., 2006). An AUC value closer to 1 indicates that the model predicts better than a random model, while a value of 0.5 indicates that the prediction is worse than random (Phillips et al., 2006). AUC values below 0.8 indicates poor model performance, 0.8–0.9 moderate model performance, 0.90–0.95 good model performance, and above 0.95 excellent model performance (Guisan & Thuiller, 2005).
Jackknife tests were used to estimate which of the variables contributed more to the model (Efron, 1981). The percent contribution of each variable was calculated on the basis of how much the variable contributed to an increase in the regularized model gain as averaged over each model run. Individual variable contribution is determined by randomly permuting the values of that variable among the training points (both presence and background) and measuring the resulting decrease in training AUC. A large decrease indicates that the model depends heavily on that variable. Values are normalized to give percentages (MaxEnt Tutorial; http://www.cs.princeton.edu/∼schapire/MaxEnt/).
Results
Logistic regressions indicate that physiochemical properties of water are important determinants of the local distribution of both species. The occurrence of M. arborescens is significantly associated with low pH (AUC = 0.87; P < 0.0001), low conductivity (AUC = 0.87; P < 0.0001), and high water transparency (AUC = 0.77; P = 0.003). In contrast, the occurrence of M. linifera is significantly associated with higher pH values (AUC = 0.87; P < 0.0001), high conductivity (AUC = 0.80; P < 0.0001), and low water transparency (AUC = 0.70; P = 0.04) (Fig. 3). Occurrence of neither species was related to water column depth or maximum flood level (P > 0.05, Fig. 3d).
MAXENT models demonstrated low rates of omissions and high statistical significance (Table 2). The same combination of factors resulted in the highest values of AUC in both species (Table 2, model 1; presented in Fig. 4). The Jackknife test for model 1 revealed that the variables altitude (alt) and total precipitation (rain_tot) were the most important variables for modeling the distribution of the two species (Table 2; Fig. 5). While altitude was the most important variable influencing the distribution of M. arborescens, total precipitation was the most important variable influencing the distribution of M. linifera (Fig. 5). The variables “soil” and “veg 2002” (compost by EVI = Global vegetation index) commonly used to predict the distribution of species (Brown, 1994) had only little importance in model performance (Fig. 5) but remained in the final models to refine the forecast area of species occurrence.
Actual distribution by herbarium data (black dots) and potential distribution (colored area) calculated by average of Model 1 (Table 2) of: a Montrichardia arborescens and b Montrichardia linifera
Jackknife analyses of individual predictor variables important in the development of the full model for Montrichardia spp. in relation to the overall model quality or the “regularized training gain.” Black bars indicate the gain achieved when including only that variable and excluding the remaining variables; gray bars show how much the gain is diminished without the given predictor variable. Alt altitude, rain_tot rain total, soil map of soil, veg2002 global vegetation index, 2002. a Montrichardia arborescens; b Montrichardia linifera
Generated distribution maps based on the averages of the MAXENT models showed that M. arborescens has a Neotropical distribution, with the central part of the Amazon and the North of Amazonas State being the areas with highest probability of species occurrence (Fig. 4a). On the other hand, M. linifera has a fairly wide distribution in Central and South America and along the coastal region of Brazil. The Maxent models demonstrated a good (AUC > 0.9) and excellent (AUC > 0.95) forecast of species occurrence, confirmed by observations made in the field (Fig. 6a, b).
Model 1 results (Table 2) overlaid with field data of a Montrichardia arborescens and b Montrichardia linifera
Discussion
At a regional spatial scale, both Montrichardia species were restricted to neotropical lowlands, with preference for hot and per humid tropical climates. Altitude and precipitation were important variables influencing the distribution of both species in all ten best models, while temperature of the driest months was an important variable for the distribution of both species in six of the ten best models. Temperature, and its strong effect on plant water-energy balance, is known to be one of the most important climate factors affecting the distribution range of many aquatic and wetland plant species (Sculthorpe, 1985; Santamaría, 2002; Bornette & Puijalon, 2011). It affects plant physiology, including germination and the periodicity and rate of seasonal growth (Short & Neckles, 1999). In addition, both Montrichardia species were detected to occur in regions where high annual precipitation (usually >1800 mm/year) is coupled with the absence of a distinct dry season. This becomes especially evident in extra-Amazonian regions, such as in Northeastern Brazil and the Gulf of Mexico, where the genus Montrichardia is restricted to per humid climates of coastal regions and absent in adjacent semi-arid or arid continental climates toward the interior (Fig. 4).
At local scales, our results are consistent with the idea that aquatic macrophytes are, in general, sensitive to the physical and chemical attributes of water (Ferreira et al., 2015). In the Amazon, Montrichardia species demonstrate the ability to colonize environments with distinct water characteristics, including wetlands with low amounts of nutrients, such as clear- and black-water river floodplains, as well as wetlands with high nutrient levels, such as white-water river floodplains. The occurrence of M. arborescens in the Brazilian part of the Amazon seems to be related to black- and clear-water rivers, although some records for white-water rivers are available in herbaria. M. linifera does not occur along black-water rivers and is likely to be limited to environments with high nutrient availability and neutral pH. M. linifera individuals are located in open, non-shaded areas along rivers and lakes and were more aggregated than those of M. arborescens, the latter being more sparsely distributed on the floodplain (Lopes et al., 2016). M. linifera can be observed on mineral substrates of floodplains as well as on organic substrates of floating islands, called “Matupás” in the várzeas of the Amazon basin (Junk & Piedade, 1997; Freitas et al., 2015). In sum, our results are consistent with the notion that local habitat variation along strong environmental gradients can lead to phenotypic differentiation and diversification in the Amazon basin and thus ultimately could lead to niche differentiation among closely related, co-existing species (i.e., Gentry, 1988; Fine et al., 2005).
Although the model predicted a potential overlap in the distribution of both Montrichardia species, both field observations and field data indicated that local habitat variation strongly segregates Montrichardia species along physical and chemical water gradients in the Amazon basin. While species segregation by physical and chemical water characteristics within the Amazon basin is evident, the species distribution model was not able to predict the segregation of local niche differences adequately. For example, soil variables that are commonly of high importance for the prediction of species distributions (Brown, 1994) had only little importance for the prediction of the distribution of both Montrichardia species. While it might be argued that in aquatic habitats plants might not be expected to respond strongly to soil attributes, the physical and chemical attributes of their aquatic habitat often reflect basin-wide soil properties, especially in the Amazon region. Also, Montrichardia is firmly rooted in the soil. Likely, the resolution of the soil layer in the present model is not sufficient to detect the contrasting differences in nutrient conditions between nutrient-rich and nutrient-poor alluvial substrates. We thus call attention to the fact that variations in physical and chemical soil variables may influence species distributions in the Amazon basin at very small spatial scales (<1 km), which are yet not detectable with the available resolution. Very likely, this is also the case for the global vegetation index (EVI), which had only little influence in our models.
IPCC (2013) predicts a 1.5–5.5°C increase of mean annual temperatures in most parts of the Amazon basin by 2100, coupled with a continuous increase in atmospheric CO2 concentrations. In our models, both Montrichardia species showed potentially wider spatial distribution under moderately increased temperature scenarios (average 33–35°C). However, contracting distributions would be indicated in the case of reduced annual precipitation, as predicted by IPCC (2013) for most regions of Northeastern South America for the dry season (April-September). In another approach, a recent experimental study by Lopes et al. (2015) subjected M. arborescens to elevated temperature and atmospheric CO2 concentrations in microcosms. Results indicated that primary productivity of M. arborescens was negatively affected when temperature and CO2 surpassed 33°C 800 ppm respectively, indicating the presence of physiological stress and the sensitivity of this species to climate change (Lopes et al., 2015). Such findings, and how they translate to determine species distributions at large scales are not clear, but they do indicate that a stronger mechanistic basis for understanding species distributions is essential if we are to accurately model the future ranges of species under different climate scenarios.
As inventories of aquatic macrophytes are still poor and sparse in the Amazon Basin (Piedade et al., 2010), the possibility of species niche modeling with few occurrence data opens a large potential for the interpretation of biogeographic patterns. Moreover, with just three or four independent variables, it was possible to develop reliable distribution models, which may be of advantage in remote areas such as the Amazon basin, where environmental data are still scarce. For example, one possible use of potential species distribution maps could be their use in sustainable management plans, in order to discover new populations and to select priority areas for conservation (Kumar & Stohlgren, 2009; Adams et al., 2015).
Conclusions
Our results suggest that a hierarchy of environmental factors may control Montrichardia species distribution from large to small spatial scales. While at large spatial scales climatic factors may similarly control the range limits of the genus, at small spatial scales individual species may colonize very different aquatic habitats. Both species of Montrichardia prefer environments with tropical climates with comparatively high temperature and elevated precipitation. Water chemistry influences the distribution of Montrichardia species at local scales where species distributions overlap in the Amazon basin. While M. linifera occurs mostly in white-water rivers, M. arborescens preferentially occurs in black-water rivers and upland streams. These findings indicate that in Montrichardia, an evolutionarily conserved climatic niche co-occurs with a strong capacity for niche differentiation among types of wetland habitats.
Distribution models were able to predict the large-scale distributions of species and their probable climatic determinants. However, they were not able to predict the segregation of the two species across different types of aquatic habitat types in the Amazon. We thus call attention to the need of environmental data at small scale resolution in vast areas such as the Amazon basin.
References
Adams, M. P., M. I. Saunders, P. S. Maxwell, D. Tuazon, C. M. Roelfsema, D. P. Callaghan, J. Leon, R. G. Alistair & K. R. O’Brien, 2015. Prioritizing localized management actions for seagrass conservation and restoration using a species distribution model. Marine and Freshwater Ecosystems, Aquatic Conservation. doi:10.1002/aqc.2573.
Barendregt, A. & A. M. F. Bio, 2003. Relevant variables to predict macrophytes communities in running waters. Ecological Modelling 160: 205–217.
Bornette, G. & S. Puijalon, 2011. Response of aquatic plants to abiotic factors: a review. Aquatic Sciences 73(1): 1–14.
Brown, D. G., 1994. Predicting vegetation types at tree line using topography and biophysical disturbance variables. Journal of Vegetation Science 5: 641–656.
Candolle, A., 1855. Géographie botanique. Paris.
Chambers, P. A., P. Lacoul, K. J. Murphy & S. M. Thomaz, 2008. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595: 9–26.
Darwin, C., 1859. The Origin of Species, by Means of Natural Selection. Murray, London.
Dias, R. L., 2009. Softwear Comunidata 1.6.
Efron, B., 1981. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika 68(3): 589–599.
Elith, J., C. H. Graham, R. P. Anderson, M. Dudik, S. Ferrier, A. Guisan, R. Hijmans, F. R. Huettmann, J. Leathwick, A. Lehmann, J. G. Li, L. A. Lohmann, B. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J. M. M. Overton, A. J. Townsend Peterson, S. Phillips, K. Richardson, R. E. Scachetti-Pereira, R. Schapire, J. Soberón, S. S. Williams, E. Wisz & N. Zimmermann, 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151.
Ferreira, F. A. R. P., G. Mormul, A. Pott Catian & G. Pedralli, 2015. Distribution pattern of neotropical aquatic macrophytes in permanent lakes at a Ramsar site. Brazilian Journal of Botany 38(1): 131–139.
Figueroa, J. M. T., M. J. López-Rodríguez, S. Fenoglio, P. Sánchez-Castillo & R. Fochetti, 2013. Freshwater biodiversity in the rivers of the Mediterranean Basin. Hydrobiologia 719(1): 137–186.
Fine, P., D. C. Daly, G. Villa Muñoz, I. Mesones & K. M. Cameron, 2005. The contribution of edaphic heterogeneity to the evolution and diversity of Burseraceae trees in the Western Amazon. Evolution 59(7): 1464–1478.
Freitas, C. T., G. H. Shepard & M. T. F. Piedade, 2015. The floating forest: traditional knowledge and use of matupá vegetation islands by riverine peoples of the Central Amazon. Plos One 10(4): e0122542.
Furch, K., 2000. Chemistry and bioelement inventory of contrasting Amazonian forest soils. In Junk, W. J., J. J. Ohly, M. T. F. Piedade & M. G. M. Soares (eds), The Central Amazon floodplain: actual use and options for a sustainable management. Backhuys Publishers, Leiden: 109–128.
Furch, K. & W. J. Junk, 1997. Physicochemical conditions in the floodplains. Ecological Studies 126: 69–108.
Gentry, A. H., 1988. Changes in plant community diversity and floristic composition on environmental and geographic gradients. Annals of the Missouri Botanical Garden 75(1): 1–34.
Good, R., 1953. The geography of the flowering plants. Longmans Green, London.
Guisan, A. & W. Thuiller, 2005. Predicting species distribution: offering more than simple habitat models. Ecological Letters 8: 993–1009.
Guppy, H., 1906. Observations of a naturalist in the Pacific between 1826 and 1899. II Plant dispersal. Macmillan Publishers Ltd, London.
Householder, E., F. Wittmann, M. Tobler & J. Janovec, 2015. Montane bias in lowland Amazonian peatlands: plant assembly on heterogenous landscapes and potential significance to palynological inference. Palaeogeography, Palaeoclimatology, Palaeoecology 423: 138–148.
IPCC, 2013. Climate Change 2013: the physical science basis. In Stocker, T. F., D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley (eds), Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK. http://www.climatechange2013.org/images/report/WG1AR5_TS_FINAL.pdf.
Junk, W. J., 1997. The Central Amazon Floodplain: Ecology of a Pulsing System. Ecological Studies, Vol. 126. Springer-Verlag, Berlin: 525.
Junk, W. J. & M. T. F. Piedade, 1997. Plant life in the floodplain with special reference to herbaceous plants. In Junk, W. J. (ed.), The Central Amazon Floodplain, Vol. 126. Springer-Verlag, New York: 147–181.
Junk, W. J., M. T. F. Piedade, J. Schöngart, M. Cohn-Haft, J. M. Adeney & F. Wittmann, 2011. A classification of major naturally-occurring Amazonian lowland wetlands. Wetlands 31: 623–640.
Junk, W. J., M. T. F. Piedade, J. Schöngart & F. Wittmann, 2012. A classification of major natural habitats of Amazonian white water river floodplains (várzeas). Wetlands Ecology and Management 20(6): 461–475.
Junk, W. J., F. Wittmann, J. Schöngart & M. T. F. Piedade, 2015. A classification of the major habitats of Amazonian black-water river floodplains and a comparison with their white-water counterparts. Wetlands Ecology and Management 23(4): 677–693.
Kumar, S. & T. J. Stohlgren, 2009. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. Journal of Ecology and The Natural Environment 1(4): 094–098.
Lehtonen, S., 2009. On the origin of Echinodorus grandiflorus (Alismataceae) in Florida (“E. floridanus”), and its estimated potential as an invasive species. Hydrobiologia 635(1): 107–112.
Loo, S. E., R. Mac Nally, D. J. O’Dowd, J. R. Thomson & P. S. Lake, 2009. Multiple scale analysis of factors influencing the distribution of an invasive aquatic grass. Biological invasions 11(8): 1903–1912.
Lopes, A. & M. T. F. Piedade, 2014. Experimental study on the survival of the water hyacinth (Eichhornia crassipes (Mart.) Solms-Pontederiaceae) under different oil doses and times of exposure. Environmental Science and Pollution Research 21(23): 13503–13511.
Lopes, A., J. D. A. Paula, S. F. Mardegan, N. Hamada & M. T. F. Piedade, 2011. Influência do hábitat na estrutura da comunidade de macroinvertebrados aquáticos associados às raízes de Eichhornia crassipes na região do Lago Catalão, Amazonas, Brasil. Acta Amazonica 41: 493–502.
Lopes, A., F. Wittmann, J. Schöngart & M. T. F. Piedade, 2014. Herbáceas aquáticas em seis igapós na Amazônia Central: composição e diversidade de gêneros. Revista Geográfica Academica 8(1): 5–17.
Lopes, A., A. B. Ferreira, P. O. Pantoja, P. Parolin & M. T. F. Piedade, 2015. Combined effect of elevated CO2 level and temperature on germination and initial growth of Montrichardia arborescens (L.) Schott (Araceae): a microcosm experiment. Hydrobiologia. doi:10.1007/s10750-015-2598-1.
Lopes, A., P. Parolin & M. T. F. Piedade, 2016. Morphological and physiological traits of aquatic macrophytes respond to water chemistry in the Amazon Basin: an example of the genus Montrichardia Crueg (Araceae). Hydrobiologia 766(1): 1–15.
Mayo, S. J., J. Bogner & P. C. Boyce, 1997. The Genera of Araceae. RBGKew Press, London.
Meave, J., M. Kellman, A. MacDougall & J. Rosales, 1991. Riparian habitats as tropical forest refugia. Global Ecology and Biogeography Letters 1: 69–76.
Merow, C., A. M. Latimer, A. M. Wilson, S. M. McMahon, A. G. Rebelo & J. A. Silander, 2014. On using integral projection models to generate demographically driven predictions of species’ distributions: development and validation using sparse data. Ecography 37(12): 1167–1183.
Neiff, J. J. & A. S. G. Poi de Neiff, 2003. Connectivity processes as a basis for the management of aquatic plants. In Thomaz, S. & L. M. Bini (eds), Ecologia e Manejo de Macrófitas Aquáticas. Nupélia – Maringá. Eduem, Maringá: 39–58.
Pearson, R. G., C. J. Raxworthy, M. Nakamura & A. T. Peterson, 2007. Predicting species’ distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography 34: 102–117.
Phillips, S. J., R. P. Anderson & R. E. Schapire, 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259.
Piedade, M. T. F., W. J. Junk, S. A. D’Ângelo, F. Wittmann, J. Schöngart, K. M. D. N. Barbosa & A. Lopes, 2010. Aquatic herbaceous plants of the Amazon floodplains: state of the art and research needed. Acta Limnologica Brasiliensia 22(2): 165–178.
Richey, J. E. E., J. I. Hedges, A. H. Devol, P. D. Quay, R. Victoria, L. Martinelli & B. R. Forsberg, 1990. Biogeochemistry of carbon in the Amazon River. Limnol. Oceanogr 35(2): 352–371.
Santamaría, L., 2002. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica 23(3): 137–154.
Schöngart, J., M. T. F. Piedade, F. Wittmann, W. J. Junk & M. Worbes, 2005. Wood growth patterns of Macrolobium acaciifolium (Benth.) Benth. (Fabaceae) in Amazonian black-water and white-water floodplain forests. Oecologia 145: 454–461.
Sculthorpe, C. D., 1985. The Biology of Aquatic Vascular Plants. Edward Arnold, London: 610.
Short, F. T. & H. A. Neckles, 1999. The effects of global climate change on seagrasses. Aquatic Botany 63(3): 169–196.
Silvertown, J., M. Dodd, D. Gowing & O. Mountford, 1999. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400: 61–63.
Sioli, H., 1968. Hydrochemistry and geology in the Brazilian Amazon region. Amazoniana 3: 267–277.
Weddell, H., 1872. Sur les Podostémacées en général, et leur distribution géographique en particulier. Bulletin de la Société botanique de France 19: 50–57.
Wiens, J. J., 2011. The niche, biogeography and species interactions. Philosophical Transactions of the Royal Society of London B: Biological Sciences 366(1576): 2336–2350.
Wittmann, F., W. J. Junk & M. T. Piedade, 2004. The várzea forests in Amazonia: flooding and the highly dynamic geomorphology interact with natural forest succession. Forest Ecology and Management 196: 199–212.
Acknowledgments
This work is support by INCT ADAPTA—Brazilian Ministry of Science, Technology and Innovation (Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq/Fundação de Amparo à Pesquisa do Estado do Amazonas—FAPEAM), and the Universal CNPq (14/2009; 14/2011), PRONEX “Áreas alagáveis” (CNPq/FAPEAM), PELD MAUA (CNPq/FAPEAM) and FAPEAM EDITAL N. 017/2014—FIXAM/AM Nº Processo: 062.01174/2015 to Dr. Aline Lopes. We thank Marcelo Santos Junior and Marina Anciães for their help with MAXENT Software, and Conceição Lucia Costa, Celso R. Costa, and Valdeney de A. Azevedo for their efforts in collecting field data. Aline Lopes thanks CNPq for her Doctoral Grant and the MAUA Research Group at INPA for logistical and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Adalberto L. Val, Gudrun De Boeck & Sidinei M. Thomaz / Adaptation of Aquatic Biota of the Amazon
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes, A., Wittmann, F., Schöngart, J. et al. Modeling of regional- and local-scale distribution of the genus Montrichardia Crueg. (Araceae). Hydrobiologia 789, 45–57 (2017). https://doi.org/10.1007/s10750-016-2721-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2721-y