Abstract
In the last decades, petroleum activities have increased in the Brazilian Amazon where there is oil exploration on the Urucu River, a tributary of the Amazon River, about 600 km from the city of Manaus. Particularly, transportation via the Amazon River to reach the oil refinery in Manaus may compromise the integrity of the large floodplains that flank hundreds of kilometers of this major river. In the Amazon floodplains, plant growth and nutrient cycling are related to the flood pulse. When oil spills occur, floating oil on the water surface is dispersed through wind and wave action in the littoral region, thus affecting the vegetation of terrestrial and aquatic environments. If pollutants enter the system, they are absorbed by plants and distributed in the food chain via plant consumption, mortality, and decomposition. The effect of oil on the growth and survival of vegetation in these environments is virtually unknown. The water hyacinth [Eichhornia crassipes (Mart.) Solms] has a pantropical distribution but is native to the Amazon, often growing in high-density populations in the floodplains where it plays an important role as shelter and food source for aquatic and terrestrial biota. The species is well known for its high capacity to absorb and tolerate high levels of heavy metal ions. To study the survival and response of water hyacinth under six different oil doses, ranging from 0 to 150 ml l−1, and five exposure times (1, 5, 10, 15, and 20 days), young individuals distributed in a completely randomized design experiment composed of vessels with a single individual each were followed over a 50-day period (30-day acclimatization, 20 days under oil treatments). Growth parameters, biomass, visual changes in the plants, and pH were recorded at 1, 5, 10, 15, and 20 days. Increasing the time of oil exposure caused a decrease in biomass, ratio of live/dead biomass and length of leaves, and an increase in the number of dead leaves. Dose of oil and time of exposure are the most important factors controlling the effects of petroleum hydrocarbons on E. crassipes. Although the species is able to survive exposure to a moderate dose of oil, below 75 ml l−1 for only 5 days, severe alterations in plant growth and high mortality were observed. Therefore, we conclude that Urucu oil heavily affects E. crassipes despite its known resistance to many pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, petroleum and derivatives are the most common forms of energy used in the world. The growing demand for petroleum has justified new prospecting in areas of difficult access in the Amazon region, leading to the discovery of large reserves of oil near the Urucu River, a tributary of the Amazon River (Val and Almeida-Val 1999; ANP - Agência Nacional do Petróleo, Gás Natural e Biocombustíveis 2008). These areas of petroleum exploration constitute a mosaic of different environments (i.e., nonflooded land and aquatic habitats such as floodplains along rivers and lakes), with high levels of species diversity. The extraction, storage, and especially the transport of oil are activities that represent potential dangers and can cause irreparable damage to the environment (Val and Almeida-Val 1999; Keramitsoglou et al. 2003).
Petroleum produced in Urucu is a light crude oil with low density, low viscosity, low specific gravity, and high API (American Petroleum Institute) gravity due to the presence of a high proportion of light hydrocarbon fractions (ANP - Agência Nacional do Petróleo, Gás Natural e Biocombustíveis 2008). This oil type acts at the cellular level, changing membrane permeability or interrupting the metabolic processes of various plants (Pezeshki et al. 2000). The specificity of action of petroleum hydrocarbons in plants at the chemical and physical levels (Pezeshki et al. 2000) may result in changes in species composition, diversity, and structure of plant communities in a given area (Burk 1977). However, studies of oil toxicity in freshwater aquatic plants are limited, and most investigations have focused mainly on brackish and salt marsh species (Lin and Mendelssohn 1996). Studies dealing with the effects of contamination on Amazonian freshwater aquatic plant species are scarce (Lopes and Piedade 2009; Lopes and Piedade 2011). Nevertheless, Amazon floodplains are colonized by a large number of aquatic macrophytes with important ecological functions, such as shelter and food sources for aquatic and terrestrial fauna (Piedade et al. 2010).
Oil spills seriously compromise the integrity of floodplain areas throughout the world. In the tropics, the floodplains associated with large rivers are environments where nutrient cycling depends on the variation in the water level related to the flood pulse (Junk et al. 1989), which favors the replenishment of nutrients during each annual hydrologic cycle (Furch and Junk 1997). If pollutants are released into the river system, they will be replenished as well (Lopes et al. 2009). With the entrance of petroleum into the aquatic-terrestrial system, a series of alterations will take place, mainly related to the substantial increase in organic material as a result of the increased death and decomposition of plants (Akinluyi and Odeyemi 1987), alterations in soil pH, and the introduction of heavy metals such as nickel and mercury (Ekundayo and Obuekwe 2000). Additionally, contamination can spread throughout the food chain, enhanced by the flood pulse that widely distributes contaminants to both terrestrial and aquatic systems (Piedade et al. 2010).
In the Amazon region, aquatic macrophytes are very productive, contributing 65 % of the primary production of water bodies (Melack and Forsberg 2001). Eichhornia crassipes, for example, may double its weight in 12–13 days, reaching a productivity of 150 t ha−1 year−1 and a biomass of 988 g m−2 (Junk and Howard-Williams 1984; Westlake 1963). Other productive aquatic macrophytes in the Amazon are Echinochloa polystachya (6,880 g m−2) (Piedade et al. 1991), Paspalum repens (2,210 g m−2), Oryza perenis (1,720 g m−2), and Hymenachne amplexicaulis (2,270 g m−2) (Junk and Howard-Williams 1984).
Due to their large biomass per meter squared, biogeochemical cycles in floodplain areas are influenced by aquatic macrophytes (Furch and Junk 1997). In a white water floodplain (várzea) near Manaus, Junk and Piedade (1993) identified 388 species of herbaceous plants. Among the most abundant species are the aquatic plants Pistia stratiotes, Scirpus cubensis, E. crassipes, and Salvinia auriculata. The variety of herbaceous plants in the várzea floodplains provides many options in the detection of plants with useful characteristics for phytoremediation or bioindication of environmental contamination. Therefore, the study of the effect of crude oil on aquatic macrophytes can contribute to the establishment of techniques for the early detection as well as for the recovery of affected areas after major contamination events.
The water hyacinth [E. crassipes (Mart.) Solms] is a perennial aquatic herbaceous plant that belongs to the pickerel weed family (Pontederiaceae). It is native to tropical America (Gichuki et al. 2012) and has been classified as one of the worst aquatic weeds in the world (Holm et al. 1991). The plant has spread to many parts of the world due to its popularity as an ornamental plant for ponds (Penfound and Earle 1948). The leaves of E. crassipes contain 11 to 15 % protein (Junk 1979) and provide food for various species of animals, including manatees and species of turtles (Piedade et al. 1992).
E. crassipes is a good model to study the effects of crude oil because the species is highly effective in removing excess nutrients (Desougi 1984; Vora and Rao 1988; Oke and Elmo 1990), heavy metals (Harley 1990; Vesk and Allaway 1997; Soltan and Rashed 2003), toxic metals (Kelley et al. 1999, 2000), minerals and organic chemicals, and herbicides (Boyd 1967; Reay 1972) from polluted water. Accumulation of ions in the roots and leaves of water hyacinth has also been shown in field studies using this species as a biological monitor of water pollution (Ajmal et al. 1987; Zaranyika and Ndapwadza 1995). Laboratory studies of the water hyacinth have demonstrated the potential use of this species in removing petroleum from contaminated environments (Casabianca et al. 1995; Crema et al. 2012; Prado and Rubim 2003; Lopes et al. 2009).
Considering that the contamination of freshwater aquatic plants is expected to increase in the Amazon due to the growth of petroleum extraction (Lopes and Piedade 2010), the assessment of its impacts on aquatic macrophytes is essential. Therefore, this study evaluated the effect of Urucu crude oil on the survival and growth of E. crassipes to answer two main questions: (1) Does the morphology and biomass production of the species respond to different doses of oil? (2) Does increasing time of exposure affect biomass production and mortality of the plant?
Methods
Plant collection and experimental design
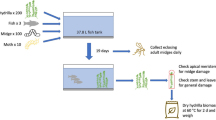
Individuals of E. crassipes were collected from stands of the species in lakes on the Central Amazon Marchantaria Island (03° 15′ S, 060° 00′ W, Fig. 1a). The experiment was conducted for 50 days in the INPA/Max-Planck greenhouse (Manaus, Brazil; Fig. 1b) with natural sunlight and temperature (25.5–35.5 °C). The water used was provided by an artesian well located by the greenhouse. The artesian water was used to provide constant nutrient conditions of the water over the experimental period because preliminary tests showed that individuals of E. crassipes survived for periods longer than 3 months in artesian water (Lopes et al. 2009). The crude oil was obtained from the operations base “Geólogo Pedro de Moura, Petrobras” near the Urucu River, Amazonas, Brazil. This oil is considered light, with 45.8 API gravity (ANP - Agência Nacional do Petróleo, Gás Natural e Biocombustíveis 2008). Details of the oil composition can be found in Lopes et al. (2009).
The sample units consisted of 300 individual plants in vessels distributed in a completely randomized 6 × 5 (oil doses × harvest times) factorial design with ten replicates. Young healthy individuals of E. crassipes with three leaves of approximately 4 × 7 cm were collected in the field and transported to the laboratory in Manaus, where they were washed in tap water to the remove sediments from roots. Subsequently, the plants were placed in vessels (20 cm in diameter and 18.5 cm in height) with 2 l of water where they remained for a 30-day acclimatization period (Fig. 1b). Whenever necessary, the water level was adjusted. Subsequently, crude oil was placed on the surface of the water without mixing with the substratum and avoiding direct contact with leaves. The oil doses, established after determination of the LD50 (median lethal dose) (see Lopes et al. 2009), were 0, 3, 12.5, 25, 75, and 150 ml l−1of water (0, 0.32, 1.32, 2.64, 7.95, and 15.89 l m−2). At days 1, 5, 10, 15, and 20, the plants were measured and harvested for biomass; pH of the water was measured at the same intervals. The dose-response test was performed according to the OECD toxicity protocol 227 (Organization for Economic Cooperation and Development OECD 2006).
Parameters measured after oil addition
The mortality rate in each treatment was used to indicate the sensitivity of the species to crude oil; the number of leaves indicated the investment of the plants in maintaining leaf production. In addition, the longest leaf and longest root were measured, variables indicating the plant’s growth during the experimental period. Aerial biomass was harvested, separated into dead and live components, and dried at 65 °C until constant weight. Root biomass was determined by collecting the roots and washing them and drying them in an oven at 65 °C to constant weight, without separating live and dead material. Total biomass was calculated using the following equation: TB = lab + dab + rb, where TB = total biomass, lab = live aerial biomass, dab = dead aerial biomass, and rb = root biomass. The ratio of live/dead biomass was calculated as an early indicator of oil impact on the plants, and the ratio of root/shoot biomass was calculated to represent the cumulative effect of the oil.
Statistical analysis
Statistical analysis was performed using Systat 10.2 software (Systat Inc 2002). Plant survival was evaluated using the nonparametric Kaplan-Meier estimator. This method estimates the probability of occurrence of events throughout a short period of days or months with intervals not necessarily equally spaced. The effect of the treatments was analyzed using a general linear model. Two-way analysis of variance was used to analyze the effect of the dose and time of exposure. For this analysis, the dead plants and those exposed to the 150-ml l−1 dose were excluded due to the death of all plants in the treatment of 20 days (Table 1). Tukey’s post hoc test was performed when there was a significant difference between the treatments (α < 0.05). For the analysis of dose effect, dead plants were excluded as well as the ones exposed to 150 ml l−1 in treatments of 15 and of 20 days due to high mortality. Significant differences were recorded at the 0.05 probability level, unless otherwise stated. Biomass data were normalized using log (g) to follow the assumptions of the methods applied.
Results
The survival of E. crassipes decreased significantly with increasing oil level during the time of exposure (t (294) = 3.282, p = 0.001; Table 1). After 20 days of oil exposure, the plants showed visual changes due to the petroleum contamination. While the control individuals did not die, appearing healthy, plants exposed to 150 ml l−1 oil were heavily affected, losing leaves and showing morphological modifications in the remaining ones. Factorial analysis showed that increasing the dose from 3 to 150 ml l−1 resulted in a 60 % increase in plant mortality, and increasing the time of exposure from 5 to 20 days resulted in a 40 % increase in plant mortality. Additionally, the interaction of these two factors resulted in a 10 % increase in plant mortality.
The number of leaves was affected by the oil dose together with time of exposure (Table 2). After 1 day of treatment, the control plants showed a 9.0 % increase in the median number of leaves; the oil caused a decrease in the number of leaves by 8.5 % in the treatments of 25 ml l−1 and 9.0 % with a dose of 150 ml l−1 (F (5,54) = 2.768, p = 0.027). After 5 days, there was an increase of 13.8 % in the number of leaves in the control treatment and a reduction of 15.8 % with the 75-ml l−1 dose and 47.3 % with the 150-ml l−1 dose (F (5,52) = 5.921, p < 0.001). After 10 days, the control plants showed a 13.8 % increase in the number of leaves, while there was a reduction of 53.6 % in the number of leaves in the treatment of 75 ml l−1 and 79.5 % with 150 ml l−1 oil (F (5,44) = 5.650, p < 0.001). At 15 and 20 days of exposure, this parameter could not be analyzed since dead plants were excluded.
The length of the longest leave among treatments was significantly reduced in response to oil dose together with exposure time (Table 2). This reduction was less intense in the control (from 7.4 ± 0.9 to 5.0 ± 1.0 cm after 20 days) and in the oil treatment of 3 ml l−1 (from 7.7 ± 1.1 to 4.9 ± 1.9 cm after 20 days) than in treatments of 12.5, 25, and 75 ml l−1 (from 8.0 ± 0.8 to 3.7 ± 0.7 cm after 20 days). Root length was not affected by the oil dose or by the time of exposure (Table 2) with a mean value of 13.5 ± 4.3 cm.
The total biomass (log) of E. crassipes did not change significantly between treatments with different oil doses (Table 2, Fig. 2), although live biomass trended to decrease and dead biomass tended to augment with oil dose. Oil dose and time of exposure caused a decrease in the aerial biomass of E. crassipes (Table 2, Fig. 2). After 10 days of exposure, there was a significant reduction in the 150-ml l−1 treatment in relation to control (F (5,43) = 3.008, p = 0.002), and after 20 days, with the exclusion of the treatment of 150 ml l−1, there was a tendency of a reduction in aerial biomass (F (4,38) = 2.536, p = 0.056). Oil dose together with time of exposure affected the accumulation of dead aerial biomass of the species (Table 2, Fig. 2). The oil caused an increase in the accumulation of dead biomass after 5 days of exposure in the oil treatment of 150 ml l−1 (F (5,23) = 3.667, p = 0.014). Ten days after oil application, there was an increase in dead biomass in the 75- and 150-ml l−1 treatments (F (5,36) = 3.996, p = 0.006). After 15 days of exposure, there was a significant increase in dead biomass with treatments of over 12.5 ml l−1 (F (4,32) = 8.934, p < 0.0001), and the same pattern was observed after 20 days of exposure (F (4,37) = 7.255, p < 0.0001). Changes in the root biomass of E. crassipes did not show a significant effect of time and oil dose (Table 2, Fig. 2).
The decrease in the ratio of live/dead aerial biomass was a response to the effect of both dose and time of exposure (Table 2, Fig. 3). Furthermore, there was a significant decrease in the ratio after 5 days of exposure to 150 ml l−1 oil in relation to control (F (5,52) = 3.806, p = 0.005). After 10 days of exposure, the ratio of live/dead biomass decreased in treatments of 75 and 150 ml l−1 (F (5,43) = 5.608, p < 0.0001), while after 15 days, the reduction was significant for the 12.5- and 25-ml l−1 doses (F (4,36) = 5.291, p = 0.002). After 20 days of treatment, a significant reduction in the ratio live/dead biomass was observed in the treatment with oil doses over 12.5 ml l−1 (F (4, 38) = 6.949, p < 0.0001; Fig. 3).
The ratio of root/shoot biomass was significantly increased in response to oil dose and time of exposure (Table 2, Fig. 4). When analyzing each time of exposure, the ratio of root/shoot biomass increased with oil dose only after 20 days (F (4,38) = 3.772, p = 0.011; Fig. 4). In the treatment of 25 ml l−1, an increase in the ratio of root/shoot biomass was observed between 5 and 20 days (Fig. 4).
The pH changed due to oil application and time of exposure (Tables 2 and 3). At the beginning of the experiment, pH was neutral becoming acid in the first day after the oil addition [mean pH value of 4.87 (±0.57) in 150-ml l−1 treatment and 6.64 (±0.61) in the control]; this difference continued until the end of the experiment, when the pH was 5.73 ± 0.24 in the 150-ml l−1 treatment and 7.53 ± 0.83 in the control.
Discussion
Although the exposure of E. crassipes to Urucu crude oil had a limited impact on the growth parameters, the interaction of oil dose and time of exposure increased the oil’s effect causing great mortality in the plants. The impact of 20 days of exposure to oil led to a reduction in the relative growth rate of leaves in all oil treatments and reductions in the ratio of live/dead biomass due to the increase in dead biomass. According to Lin et al. (2002), dose and oil type are two of the most important variables that determine the degree of oil damage in plants. Since Urucu oil is light, and therefore more damaging to the metabolism of plants (Lin and Mendelssohn 1996; Pezeshki et al. 2000), this may explain the increased mortality observed especially at the higher oil doses. In fact, anatomical modifications and strong alterations in cell organization were observed in leaves of E. crassipes when the plant was subjected to high concentrations of Urucu oil (Lopes et al. 2009). These alterations, together with decrease in biomass and increase in plant death, indicate that the negative effects of this oil are comparable to those of other oils evaluated elsewhere (Mendelssohn and McKee 1988; Lin and Mendelssohn 1996; Lin et al. 2002).
Besides the type of oil, the intensity of its impact depends on a number of biotic and abiotic factors including the amount of oil, the plant species, and the season of the spill, and the weather conditions at the time of the spill (Mendelssohn and Mckee 1988; Lin and Mendelssohn 1996; Pezeshki et al. 2000; Lopes and Piedade 2010). Different combinations of these factors have resulted in conflicting results, even for the same species. For instance, in the first days after applying doses of up to 3 l m−2 of Urucu oil in E. crassipes, Crema et al. (2012) observed that growth was not affected, but that after 45 days of exposure, there was dead biomass. In another study with the same species and oil at low doses of 1 ml l−1, Prado and Rubim (2003) found that limitation in plant development occurred only after 90 days of exposure. In the present study, the effects of oil were evident at oil doses over 12.5 ml l−1 after only 15 days of exposure. This difference in plant response may reflect the experimental conditions. While in the present study, each experimental unit contained only one individual, Prado and Rubim (2003) used six individuals in each experimental unit, and when grouping plants together, important biotic effects related to density, such as competition, may play an important role, masking the individual results. Crema et al. (2012) conducted their study in Rio Claro, São Paulo (Southeast Brazil), where the prevailing temperatures are lower than those of the Amazon region. This may have increased the time of survival of plants. A temperature just a few degrees higher may amplify the toxic effects of petroleum, as demonstrated by Lopes and Piedade (2010) for the aquatic macrophyte species E. polystachya.
In experimental conditions, it is hardly possible to reproduce the complexity of the Amazon floodplains and therefore population and community analyses. Thus, the use of individual plants in controlled experiments with conditions as close as possible to those prevailing in the field is more reliable to identify the effects of petroleum on biomass and metabolism. To expand the analysis from individuals to the population and community levels, the ideal combination whenever possible would be the combination of laboratory experiments and fieldwork.
The oil dose did not affect the maximum length of the roots of live plants and its biomass in the 20 days of exposure in the present study. However, studying E. crassipes in a period of 35 days, comparing the initial and final length of roots of all plant material (live and dead), showed that Urucu oil causes a significant reduction in the length of the roots (Lopes et al. 2009), which indicates that this parameter may be affected with time of exposure. Investigations with other species have indicated the interruption in growth as an initial effect of oil penetration of the root; over time, the chemical effects will take place especially at elevated doses, causing higher mortality of the plants (Pezeshki et al. 2000; Lin et al. 2002).
The root system of water hyacinths has pH-dependent charge sites, which are responsible for the absorption and accumulation of large amounts of cations (Yahya 1990). pH levels generally decrease with increasing metal concentrations [Urucu crude oil has 5 ppm nickel, 5 ppm vanadium, and 0.05 % (w/v) sulfur (Petrobras 1997; ANP - Agência Nacional do Petróleo, Gás Natural e Biocombustíveis 2008)] and over time in the growth solution. This observation, which was supported by our results, may result from the ion-exchange capability of water hyacinths and the marked proton secretion from this plant during metal accumulation. The toxicity of heavy metal ions is chiefly related to their interference with the electron transport in respiration and photosynthesis processes and the inactivation of vital enzymes resulting in decreased growth owing to the lower energy status and uptake of mineral nutrients (Salt et al. 1995; Larcher 2003). Oil may affect other water parameters over time, reducing dissolved oxygen, increasing electrical conductivity, and increasing concentrations of total phosphorus (Crema et al. 2012), leading to further reductions in growth.
In this study, at doses of up to 150 ml l−1, Urucu oil did not cause a reduction in total biomass of the plant, but a great decrease in the ratio of live/dead biomass was observed. These reductions demonstrated the unhealthy state of the plants, leading to further reduction in their time of survival. An oil spill may cause total mortality in some macrophyte species, resulting in a change of species diversity in the impacted region. For instance, E. polystachya was extremely affected in a previous study, since all plants died after 4-day exposure to 75, 100, and 150 ml l−1 oil (Lopes et al. 2009). The exposure of P. stratiotes for 98 days to 0.3 l m−2 oil resulted in the mortality of all individuals (Silva 2005). Victorio et al. (2006) observed an increase in total biomass after exposing Egeria densa to doses of 0.06 and 0.12 l m−2 oil (Urucu oil), over a 20-day period. Although E. densa appears to be more resistant than E. crassipes to Urucu crude oil, this species does not occur in the Amazon floodplain.
The rate of E. crassipes mortality was directly proportional to the oil dose and increased considerably with time of exposure. Although this species is very tolerant, surviving in environments contaminated by different kinds of wastes, such as from sugar cane, domestic sewage, agroindustry, and fishing (Harley 1990; Esteves 1992), Urucu crude oil, with a relatively high toxicity, reduced the growth and survival of water hyacinth at doses over 12.5 ml l−1. This may limit the use of E. crassipes for phytoremediation of environments contaminated only with low oil doses.
The root/shoot ratio may reflect the cumulative response of vegetation to biotic and abiotic factors (Mokany et al. 2006). The biomass of roots provides a large number of key functions, such as structural support, storage, transport, and absorption (Schulze 1983), while aerial biomass corresponds to organs performing a variety of functions, including support, transport, storage, photosynthesis, and reproduction (Litton et al. 2003). Since E. crassipes adjusts the pattern of root/shoot partitioning in response to petroleum dose and time of exposure, and since these two factors act synergistically in enhancing plant mortality, oil spills in the Amazon floodplain are expected to cause major impacts.
Besides the aesthetic appeal of E. crassipes, the species produces shadows on the surface of the water, preventing the explosive growth of algae (Beyruth 1992). Moreover, the leaves provide food for various species of animals, including manatees and some species of turtles (Piedade et al. 1992). Therefore, limiting the normally large amount of biomass produced by E. crassipes as a result of oil contamination in the Amazon floodplain can be very hazardous for this species and, owing to its key importance, for the entire ecosystem.
Conclusion
Dose and time of exposure are two of the most important factors controlling the effects of Urucu petroleum hydrocarbons on E. crassipes. While relatively low doses of oil may cause a reduction in the number of leaves and plant biomass, high doses can even cause death of the plants. The lower dose of Urucu oil tested, 3 ml l−1, did not cause significant alteration in morphology and biomass of the plants. Moderate oil doses, 12.5 and 25 ml l−1, caused an increase in dead biomass and a decrease in the ratio of live/dead biomass and number of leaves. When exposed to high doses, 75 and 150 ml l−1, the decline in biomass and increase in plant mortality were intensified. Considering that the higher dose tested is well below the values currently cited in literature, we may conclude that E. crassipes is highly affected by Urucu oil. The time of exposure and oil dose had a synergistic effect characterized by the intensification of the alterations in morphology, decrease in biomass production, and increase in dead biomass, from the first 5 days of exposure up to the end of the experiment.
References
Ajmal M, Kahn R, Kahn AU (1987) Heavy metals in water, sediments, fish and plants of river Hind, UP. India Hydrobiol 148:151–157
Akinluyi TO, Odeyemi O (1987) Decreasing fish productivity in the Niger delta of Nigeria. A microbiological perspective. Niger J Appl Sci 5:97–105
Beyruth Z (1992) Aquatic macrophytes from a marginal pond at Embu-mirim river, S. Paulo, Brazil. Rev. Saúde Pública 26:272–282
Boyd C (1967) Some aspects of aquatic plant ecology. Proceedings of the Reservoir Fishery Resources Symposium. Southern Division, American Fisheries Society, pp. 114–129
Burk CJ (1977) A four year analysis of vegetation following an oil spill in a freshwater marsh. J Appl Ecol 14:515–522
Casabianca ML, Laugier T, Posada F (1995) Petroliferous wastewaters treatment with water hyacinths (Raffinerie de Provence, France): experimental statement. Waste Manag 15(8):651–655
Crema LC, Biudes JFV, Camargo AFM (2012) Effect of Urucu oil (Brazilian Amazon) on the biomass of the aquatic macrophyte Eichhornia crassipes (Mart.) Solms (Pontederiaceae). Acta Limnol Bras 23(4):406–411, Online
Desougi LA (1984) Mineral nutrient demands of the water hyacinth (Eichhornia crassipes (Mart.) Solms) in the White Nile. Hydrobiologia 110:99–108
Ekundayo EO, Obuekwe O (2000) Effects of an oil spill on soil physico-chemical properties of a spill site in a typic udipsamment of the Niger delta basin of Nigeria. Environ Monit Assess 60:235–249
Esteves FA (1992) Biomass and analysis of the major inorganic components of floating aquatic macrophyte (Eichhornia crassipes (Mart.) Solms) in sex reservoirs of São Paulo State (Brazil). Cienc Cult 34(9):1197–1200
Furch K, Junk WJ (1997) The chemical composition, food value, and decomposition of herbaceous plants, leaves, and litter of floodplain florets. In: Junk WJ (ed) The Central Amazon Floodplain, vol 126. Springer-Verlag, New York, pp 187–205
Gichuki J, Omondi R, Boera P, Okorut T, Matano AS, Jembe T, Ofulla A (2012) Water Hyacinth Eichhornia crassipes (Mart.) Solms-Laubach dynamics and succession in the Nyanza Gulf of Lake Victoria (East Africa): implications for water quality and biodiversity conservation. Sci World J 2012:1–10
Harley KLS (1990) The role of biological control in the management of water hyacinth. Biocontrol News Inf 11:11–22
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1991) The world’s worst weeds, distribution and biology. Krieger Publishing Co., Malabar
Junk WJ (1979) Macrófitas aquáticas nas várzeas da Amazônia e possibilidades do seu uso da agropecuária. Imprensa Oficial do Estado do Amazonas, 24p
Junk WJ, Howard-Williams C (1984) Ecology of aquatic macrophytes in Amazonia. In: Siolo H (ed) The Amazon: limnology and landscape ecology of a mighty tropical river and its basin. W. Junk Publishers, Dordrecht, pp 269–293
Junk WJ, Piedade MTF (1993) Herbaceus plants of the Amazon floodplain near Manaus: species diversity and adaptations to the flood pulse. Amazoniana 12(3/4):467–484
Junk WJ, Bayley PB, Sparks RE (1989) The floodpulse concept in the river-floodplain systems. Can Spec Publ Fish Aquat Sci 106:110–127
Kelley C, Mielke RE, Dimaquabo D, Curtis AJ, Dewitt JG (1999) Adsorption of Eu(III) onto roots of water hyacinth. Environ Sci Technol 33:1439–1443
Kelley C, Curtis AJ, Uno JK, Berman CL (2000) Spectroscopic studies of the interaction of Eu(III) with the roots of water hyacinth. Water Air Soil Pollut 119:171–176
Keramitsoglou I, Certalis C, Kassomenos P (2003) Decision support system for managing oil spill events. Environ Manag 32(2):290–298
Larcher W (2003) Physiological plant ecology. Plants under stress. Springer, Austria, 513 p
Lin Q, Mendelssohn IA (1996) A comparative investigation of the effects of Louisiana crude oil on the vegetation of fresh, brackish, and salt marsh. Mar Pollut Bull 32(2):202–209
Lin Q, Mendelssohn IA, Suidam MT, Lee K, Venosa AD (2002) The dose-response relationship between No. 2 fuel oil and the growth of the salt marsh grass, Spartina alterniflora. Mar Pollut Bull 44:897–902
Litton CM, Ryan MG, Tinker DB, Knight DH (2003) Belowground and aboveground biomass in young postfire lodge pole pine forests of contrasting tree density. Can J For Res 33:351–363
Lopes A, Piedade MTF (2009) Estabelecimento de Echinochloa polystachya (H.B.K.) Hitchcock (Poaceae) em solo de várzea contaminado com petróleo de Urucu. Acta Amazon 39:583–590
Lopes A, Piedade MTF (2010) O período da contaminação com petróleo influencia a rebrota de Echinochloa polystachya (H.B.K.) Hitchcock em solo de várzea da Amazônia Central? Biota Neotropica 10(4):143–148
Lopes A, Piedade MTF (2011) Time of exposure and oil dosage affecting biomass production of the Amazonian semi-aquatic grass Echinochloa polystachya. Rodriguesia 62:901–908
Lopes A, Rosa-Osman SM, Piedade MTF (2009) Effects of crude oil on survival, morphology, and anatomy of two aquatic macrophytes from the Amazon floodplains. Hydrobiology 636(1):295–305
Melack JM, Forsberg BR (2001) Biochemistry of Amazon floodplain lakes and associates wetlands. In: McClain ME, Victoria RL, Richey JE (eds) The Biochemistry of the Amazon Basin. Oxford University Press, Oxford, pp 235–274
Mendelssohn IA, McKee KL (1988) Spartina alterniflora dieback in Louisiana: time-course investigation of soil waterlogging effects. J Ecol 76:509–521
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root : shoot ratios in terrestrial biomes. Glob Chang Biol 12:84–96
Oke OA, Elmo BO (1990) Elemental analysis of water hyacinth growing in the Badagry lagoon of Lagos state, Nigeria. Niger J Weed Sci 3:67–70
Organization for Economic Cooperation and Development (OECD) (2006) Test No. 227: Terrestrial Plant Test: Vegetative Vigour Test. OECD guidelines for the testing of chemicals [cited 8 Feb 2014; available on internet at http://browse.oecdbookshop.org/oecd/pdfs/browseit/9722701E.PDF]
Penfound WT, Earle TT (1948) The biology of water hyacinth. Ecol Monogr 18:447–472
Petrobras (1997) Características do petróleo Urucu. CNPES:SEPESQ:DIQUIM:SETAV Anexo V
ANP - Agência Nacional do Petróleo, Gás Natural e Biocombustíveis (2008) Despacho do Diretor-Geral nº 683, Anexo II: relação de tipos de petróleo nacional, pp.13. [Internet]. [cited 2014 Fev 18]. Available from: http://www.anp.gov.br/doc/participacoes_governamentais/2008/Revisao_2008.pdf
Pezeshki SR, Hester MW, Lin Q, Nyman JA (2000) The effect of oil spill and cleanup on dominant US Gulf coast marsh macrophytes: a review. Environ Pollut 180:129–139
Piedade MTF, Junk WJ, Long SP (1991) The productivity of the C4 grass Echinochloa polystachya on the Amazon floodplain. Ecology 72(4):1456–1463
Piedade MTF, Junk WJ, Mello JASN (1992) A floodplain grassland of the Central Amazon. In: Long SP, Jones MB, Roberts JM (eds) Productivity of grass ecosystems of the Tropics and Sub-Tropics. Chapman, Hall/UNEP, London, pp 127–158
Piedade MTF, Junk W, D'Ângelo SA, Wittmann F, Schöngart J, Barbosa KMN, Lopes A (2010) Aquatic herbaceous plants of the Amazon floodplains: state of the art and research needed. Acta Limnol Bras 22(2):165–178, Online
Prado KLL, Rubim MAL (2003) Efeito de petróleo sobre a macrófita aquática Eichhornia crassipes em experimento de laboratório. Anais do VI Congresso de Ecologia do Brasil 83-85
Reay P (1972) The accumulation of arsenic from arsenic-rich natural waters by aquatic plants. J Appl Ecol 9:577–583
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley D, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Schulze ED (1983) Root–shoot interactions and plant life forms. Neth J Agric Sci 4:291–303
Silva RSde (2005) Efeito do Petróleo sobre o crescimento da macrófita aquática Pistia stratiotes. Trabalho de Conclusão de Curso, Instituto de Biociências, Universidade Estadual Paulista, 20p
Soltan ME, Rashed MN (2003) Behaviour of water hyacinth in river Nile water (Egypt). In: Proceedings of the Vth conference on Engineering in the Chemical Industry, Hungarian Chemical Society, Budapest 228–235
Systat Inc. (2002) SYSTAT 10.2 SYSTAT Software Inc
Val AL, Almeida-Val VM, Val AL (1999) Effects of crude oil on respiratory aspects of some fish species of the Amazon. In: Almeida-Val VMF (ed) Biology of Tropical Fishes, 22nd edn. INPA, Manaus, pp 277–291
Vesk PA, Allaway WG (1997) Spatial variation of copper and lead concentrations of water hyacinth plants in a wetland receiving urban run-off. Aquat Bot 59:33–44
Victorio SS, Camargo AFM, Henry-Silva GG (2006) Influência de diferentes concentrações de petróleo sobre macrófita aquática submersa enraizada Egeria densa. Livro de Resumos da Reunião Anual de Avaliação PRH-ANP 2006. 117-120
Vora AB, Rao V (1988) Water hyacinth as a scavenger of heavy metals from polluted waters of the river Sabarmati Ahmedabad. Adv Plant Sci 1:1–6
Westlake DF (1963) Comparisons of plant productivity. Biol Rev 38:385–425
Yahya MN (1990) The absorption of metal ions by Eichhornia crassipes. Chem Speciat Bioavailab 2:82–91
Zaranyika MF, Ndapwadza T (1995) Uptake of Ni, Zn, Fe, Co, Cr, Pb, Cu and Cd by water hyacinth in Mukuvisi and Manyame rivers, Zimbabwe. J Environ Sci Health A 30:157–169
Acknowledgments
This work is part of the INCT ADAPTA, Brazilian Ministry of Science and Technology, MCT. INPA/Max-Planck Project, and PPI 1090-5 provided financial support to this research. Petrobras Oil Company provided oil through the PIATAM project. The authors thank Conselho Nacional de Pesquisas (CNPq) for the Master’s Grant awarded to Aline Lopes. Funding and logistical and technical support were provided by INPA/Max-Planck Project and Laboratório de Ecofisiologia e Evolução (LEEM). We deeply thank Mr. Ethan Householder for the valuable and critical comments on earlier versions of the manuscript as well as for the English correction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Lopes, A., Piedade, M.T.F. Experimental study on the survival of the water hyacinth (Eichhornia crassipes (Mart.) Solms—Pontederiaceae) under different oil doses and times of exposure. Environ Sci Pollut Res 21, 13503–13511 (2014). https://doi.org/10.1007/s11356-014-3307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3307-8