Abstract
Base on atmosphere CO2 concentration increases and Cd pollution stress, the response mechanism of rice to environmental change was studied. To explore the changes of endogenous hormones and organic acids in rice roots under high CO2 and Cd stress, which provide the theoretical basis for future rice production under the double environmental impacts of atmospheric CO2 changes and Cd stress. Rice seedlings (Oryza sativa L., “Beijing No. 2”) were treated from two-leaf stage, with two CO2 concentrations (400 ± 20 μmol/mol and 800 ± 20 μmol/mol, controlled by an artificial climate chamber) and/or three CdCl2 concentrations (0, 50, 150 μmol/L) for 7 days. The growth parameters of rice seedlings were measured. The root endogenous hormones and organic acids contents were determined by high-performance liquid chromatography (HPLC). Results:(1) Increased CO2 concentration promoted the accumulation of aboveground dry weight by 45.6%. The IAA (Indole-3-acetic acid), GA3 (Gibberellins A3) and ZT (Zeatin) contents increased by 15.7%, 1.6% and 26.7%. Citric and fumaric acid contents in roots increased11.7 and 19.8 fold, malic acid secreted from roots decreased by 23.4%. (2) The growth was inhibited under Cd stress alone, including the fresh weight and dry weight of the aboveground part decreased by 48.5% and 15.4%, respectively. The IAA, GA3, ZT, ABA (Abscisic acid), SA (Salicylic acid) and JA (Jasmonic acid) contents increased in roots. The large accumulation of malic acid, lactic acid and citric acid under Cd stress. Tartaric acid content increased 87.5% in roots. (3) Compared with Cd stress, under high CO2 and Cd stress, IAA, ZT and GA3 contents and endogenous hormones ratios significantly increased, and root length and biomass of rice increased (29.9%, 34.1% under high CO2 concentration and heavy Cd stress). The total organic acids secretions decreased. In conclusion, Cd stress inhibited the rice growth, the more produced (such as SA, JA and ABA) and the secreted (as Cd chelation agents) by roots were involved in the defense mechanisms and produced a detoxification mechanism; High CO2 promoted the root growth and resistance to Cd stress by changing hormones and organic acids contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentrations have reached an annual average of 410 μmol/mol from 2011 to 2019 and are projected to reach 570 μmol/mol by the middle of the twenty-first century (IPCC 2021; Uprety et al. 2010). As an essential raw material for photosynthesis, increasing CO2 concentration will promote plant photosynthesis and increase the contents of carbon, nitrogen, and starch in leaves (Lamichaney et al. 2021; Quirk et al. 2019), and root biomass can also be significantly increased (Madhu and Hatfield 2013). Elevated CO2 concentration also can significantly increase IAA (Indole-3-acetic acid) content in tomato roots, activate corresponding enzymes, and promote root growth, IAA may stimulate ET (Ethylene) synthesis, and the two can jointly regulate the elongation of root hair (Wang et al. 2009). The presence of organic acids, especially those intermediates of the tricarboxylic acid cycle, in all plants is involved in some functions in cellular metabolism (Medeiros et al. 2021). These results indicate that high CO2 concentration can promote crop growth and development by regulating root secretions and endogenous hormones.
Heavy metals pollution is a widely concerned environmental problem, which is mainly discharged into the air, soil and water through metals mining and fossil fuel burning. Cadmium (Cd) is a typical heavy metal pollutant. Cd toxicity may result in a disturbance of the uptake and translocation of mineral nutrients and plant metabolism and inhibit plant growth and development (Qin et al. 2020; Nahakpam and Shah 2011). Elevated rhizosphere Cd concentrations impede plant morphological, physiological, and biochemical processes and alter signal transduction pathways (such as antioxidant systems, photosynthesis, and hormonal systems) (Farhat et al. 2022). It was found that the low molecular weight secretions of roots in different rice varieties could affect Cd uptake (Liu et al. 2007). The secretion of organic acids can effectively alleviate Cd toxicity (Xie et al. 2013). Malic acid and citric acid, as the root exudates and the most reported organic ligands, play an important role in plant defense against metal contamination (Pinto et al. 2008). Plant endogenous hormones also play a key role in heavy metal stress response. It is suggested that plant hormones are involved in the early stress response and that Cd stress can induce ABA (Abscisic acid) accumulation in roots of Phragmites australis (Cav.), since the enzymatic activity is induced under stress conditions through an ABA mediated pathway (Fediu et al. 2005). Through the regulation of root secretions and endogenous hormones, plants can reduce the damage caused by heavy metal stress and enhance their adaptability to environmental stress, thus ensuring their growth and survival. An in-depth understanding of these mechanisms is important for improving plant tolerance to heavy metal pollution.
It is generally believed that high CO2 can affect the resistance mechanism of plants to heavy metal stress and enhance their resistance to heavy metal stress and adaptation ability. The previous studies have found that a high concentration of CO2 could significantly increase IAA, ZT (Zeatin) and GA3 (Gibberellins A3) contents, and reduce the oxalic acid and acetic malic acid contents (Qi et al. 2021), which can also increase the ratios of “promoting-hormone/ABA” in rice leaves under Pb stress and alleviate Pb harm in rice leaves (Wang et al. 2022). Membrane damage, antioxidant responses, and extensive metabolomic changes in rice leaves under high CO2 and Pb stress were also focused (Wang et al. 2023). All the previous studies focused on leaf tissue of rice. The growth of below-ground part or above-ground part, which is more sensitive to the complex environment (high CO2 concentration and/or Cd stress)? The changes of endogenous hormones and organic acids in rice roots have not been fully studied. This study hypothesizes that high CO2 concentrations can mitigate Cd damage by regulating root endogenous hormones and organic acids contents (root and root secretion), which regulate growth and defense. The response mechanism of rice seedling roots under increased CO2 concentration and/or Cd stress was discussed. The present study aims to provide a theoretical basis for rice growth and cultivation in future environments.
Materials and methods
Experimental design
High-quality rice seeds were selected and soaked in a beaker, and put in the artificial climate box to avoid light (28℃, 24 h). Then they were spread evenly on the wet filter paper in a tray to accelerate germination for 36 h. The seedlings were spread evenly and flatly on a yarn net which covered the plastic beakers (total of 18) filled with Hoagland nutrient solution (contains Ca(NO3)2, KNO3, MgSO4, KH2PO4, Na2-EDTA, FeSO4, H3BO3, MnSO4,CuSO4, ZnSO4, H2MoO4), and cultured in the artificial climate chamber (relative humidity 80%; light 16 h, darkness 8 h; light intensity 3000 lx; day temperature 28℃, night temperature 26℃), Hoagland nutrient solution was added regularly every day until double leaf stage of rice seedlings. Nine beakers of rice seedlings (under 400 ± 20 μmol/mol CO2 concentration treatment) were randomly selected as the first module, while the other nine beakers (under 800 ± 20 μmol/mol CO2concentration treatment) were selected as the second module. Beakers of each module were divided into three groups, random three beakers a group with three different CdCl2 concentrations (0, 50, 150 μmol/L) respectively. The total 6 treatments were named in Table 1. CO2 concentration was regulated and controlled by the artificial climate chamber. After7 days, the growth parameters of rice seedlings were measured, and the hormones contents in rice roots and the organic acids contents in and secreted from rice roots were determined by high-performance liquid chromatography (Fig. 1).
Measure of the growth parameters
Shoot length and Root length: The shoot length and taproot length of rice seedlings were accurately measured with a scale.
Fresh weight: The rice seedlings were divided into two parts from the base of the stem as the parts of above-ground and below-ground and weighted by electronic scale accurately.
Dry weight: The rice seedlings were placed in the oven for drying, and the temperature was set at 100℃ for 2 h and then 80℃ for 5 h and weighted by electronic scale accurately after drying.
Dry matter percentage: Total dry weight / total fresh weight × 100%.
Three plastic beakers were cultivated for each treatment, and 10 rice seedlings were randomly selected from each beaker. Total 30 samples of each treatment for the average value.
Determination of the contents of endogenous hormones
Firstly, 3 g fresh roots of rice seedlings were respectively weighed, cut into pieces, and added with 80% methanol. The roots were ground in an ice dish to homogenate in the dark, and the volume of 80% methanol was fixed to 8 mL. The roots were immersed in a refrigerator at 4℃ for 12 h and centrifuged for 20 min (10,000 r/min, 4℃), and the supernatant was taken. The residue was incubated to 5 mL with 80% methanol, and then placed in a refrigerator at 4℃ for 12 h, centrifuged for 20 min (10,000 r/min, 4℃), and the supernatant was taken. After that, the residue was continued to be filled with 80% methanol to 5 mL. The process was repeated for 3 times, and the supernatant was combined, and then transferred to the petri dish, placed in an incubator to avoid light, and blow-dried at 4℃. The low-dried samples were dissolved with 100% methanol and constant volume to 5 mL, centrifuged for 10 min (10000 r/min, 4℃), the obtained supernatant was filtered by disposable organic filters with pore sizes of 0.45 µm and 0.22 µm successively, and finally stored in the refrigerator at 4℃. The methodology was referred to the previous study (Wang et al. 2021).
The samples were determined by high-performance liquid chromatography (HPLC, Agilent 1200). The Chromatographic column is a Diamonsil C18 (2) column (250 × 4.6 mm). Methanol, glacial acetic acid and water were added in the ratio of 45: 0.8: 54.2, which was as the mobile phase (the ratio of JA was 65: 0.035: 34.965). The detection wavelength was set as 254 nm. The Column temperature was set as 35℃. The current speed was set as 0.8 ml/min. Three repeats of each treatment were calculated for the average value.
Determination of organic acids contents
Extraction of organic acids in roots: Firstly, 0.5 g fresh roots were cut into pieces, 3 mL distilled water was added, and grinded to homogenate. Then ultrasonic treatment was at 25℃ for 30 min, a water bath at 75℃ for 15 min, centrifugation of10000 r/min for 20 min at 4℃, and the supernatant was taken. Finally, they were filtered successively with the disposable water system filters with pore diameter of 0.45 µm and 0.22 µm and stored in a 4℃ refrigerator. The methodology was referred to the previous study (Qi et al. 2021).
Collection and determination of the organic acids secreted from roots: Firstly, rice seedling roots were cleaned several times to remove nutrient solution using deionized water, and the clean plastics sheet were put in the porcelain dish with wet filter paper, each rice root tip was clamped with two pre-treated chromatographic filter paper rounds, then onto a plastic sheet, and the other parts of roots were covered with a wet filter paper, the rice roots were covered with black shading paper finally. The organic acids secreted from roots were collected after 2 h under light. The 10 chromatographic filter paper rounds of the root tips were placed into a 1.5 mL centrifuge tube, 200 μL filtered water was added, and then shaken for 10 min, centrifuged for 5 min, then stored in the refrigerator at 4℃.
The samples were determined by high-performance liquid chromatography (HPLC, Agilent 1200). H2SO4 (0.01 mM) and methanol were added in the ratio of 96:4, which was the mobile phase. The detection wavelength was set as 210 nm. The other measurement conditions were same as those of endogenous hormones. The average value is obtained by 3 repeats.
Data analysis
The significant differences of parameters were using the method of two-way ANOVA followed by LSD’s multiple-range test for multiple comparisons. The data analysis was done with the SPSS statistical software (v26.0, SPSS, USA) and the figures were drawn by Origin (v2019b, Origin, USA) software.
Results
Changes of rice seedlings growth under high CO 2 and/or Cd stress
Compared with AC, EC increased the above-ground part dry weight by 45.6%; AC + L significantly (P < 0.05) increased the dry matter rate by 38.9%, reduced the shoot length, root length, above-ground parts fresh weight and above-ground parts dry weight by 42.5%, 30.2%, 48.5% and 15.4%; AC + H significantly increased the dry matter rate by 43.6%, other growth parameters decreased.
Compared with AC + L, EC + L significantly (P < 0.05) increased the shoot length, root length, and above-ground part dry weight by 15.3%, 25.1% and 32.7%; Compared with AC + H, EC + H significantly (P < 0.05) increased the root length and above-ground part dry weight by 29.9% and 34.1% respectively (Table 2).
Changes of rice roots endogenous hormones contents under high CO 2 and/or Cd stress
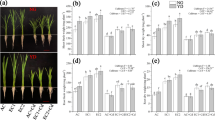
Compared with AC, EC significantly (P < 0.05) increased ZT, GA3 and IAA contents by 26.7%, 1.6% and 15.7%, while the ABA, JA and SA contents were decreased significantly by 2%, 22.7% and 6% (P < 0.05); AC + L and AC + H significantly (P > 0.05) increased ZT, ABA, IAA, GA3, SA and JA contents.
Compared with AC + L, EC + L increased IAA, ZT and GA3 contents by 190%, 41.27% and 1.8%, while decreased ABA, SA and JA contents significantly (P > 0.05) by 6.1%, 16.6% and 23.7%; Compared with AC + H, EC + H increased IAA, ZT and GA3 contents by 154.6%, 38.2%, 3.5%, while decreased ABA, SA and JA contents significantly (P > 0.05) by 4.7%, 12.5%, 8.3%. (Fig. 2 A–F).
Compared with AC, EC increased all ratios of endogenous hormones contents; AC + L increased IAA/ABA, ZT/ABA and decreased GA3/JA significantly (P > 0.05); AC + H increased IAA/ABA, ZT/ABA, GA3/ABA, IAA/JA and ZT/JA. Compared with Cd stress alone, EC + L and EC + H increased all ratios of endogenous hormones contents significantly (P > 0.05) (Fig. 3 A–F).
Changes of organic acids contents in rice roots under high CO 2 and/or Cd stress
Compared with AC, EC increased citric acid and fumaric acid contents in rice roots by 11.7 and 19.8 fold; AC + L increased malic acid, lactic acid, acetic acid, citric acid and the total organic acids contents in roots by 419.4%, 104.6%, 287.4% and 23.6%, while significantly (P < 0.05) decreased oxalic acid content by 3.3%; AC + H increased tartaric acid, malic acid, lactic acid, fumaric acid and the total organic acids contents by 87.5%, 790.7%, 142.7%, 202.9% and 24.6%.
Compared with AC + L, EC + L significantly (P < 0.05) decreased tartaric acid content in roots by 50%; Compared with AC + H, EC + H significantly (P < 0.05) decreased tartaric acid and lactic acid contents in roots while increased fumaric acid content by 74.8% (Fig. 4 A–H).
Changes of rice roots secreted organic acids contents under high CO 2 and/or Cd stress
Compared with AC, EC significantly (P < 0.05) decreased secreted malate acid and succinate acid contents by 23.4% and 41.1%; AC + L significantly (P < 0.05) increased secreted citric acid, acetic acid oxalic acid, malic acid, succinate acid and the total organic acids contents by 56.6%, 26.4%, 105.3%, 54.3%, 50%, 73.4%; AC + H increased secreted oxalic acid, malic acid, succinate acid and the total organic acids contents by 63.8%, 32.5%, 36% and 42.5%.
Compared with AC + L, EC + L decreased citric acid and succinic acid contents secreted by roots with 21.7% and 13.3% respectively. Compared with AC + H, EC + H significantly (P < 0.05) decreased oxalic acid, malic acid, succinic acid and total organic acids contents of roots secreted by 23.1%, 19%, 22.6% and 22% respectively (Fig. 5 A–F).
Discussion
Effects of high CO 2 concentration on rice roots growth, endogenous hormones and organic acids contents
Increased atmospheric CO2concentration has a positive effect on plant growth. As an important nutrient for plants, increased CO2 concentration can promote plant growth and development, including promoting plant height, root growth, biomass, fruit quality, and dry weight (Boretti and Florentine 2019; Huber et al. 2021; Uddin et al. 2018). The results of this study showed that compared with AC, EC significantly promoted dry weight accumulation in the above-ground part by 45.6%. This was indicated that high CO2 can promote rice root to absorb more nutrients and increase above-ground dry weight of rice seedlings. This trend was related to the positive effects of increasing CO2 concentration on plant biomass, plant height, root mass and lateral root number (Temme et al. 2015; Drag et al. 2020). The same results have been found in other studies (Roy et al. 2012; Wang et al. 2019).
Plant hormones promote or inhibit each other in plants and play an important role in regulating plant growth and development. High CO2 can change the balance of plant hormones, that leading to significant changes in plant growth and nutrient levels (Pinero et al. 2014). In this study, high CO2 increased IAA, GA3 and ZT contents in rice roots by 26.7%, 1.6% and 15.7%, while decreased ABA, JA, and SA contents by 2%, 22.7% and 6%. Studies have shown that increased CO2 concentration can increase the IAA content in plants and play an important role in inducing root hair development (Niu et al. 2011; Negishi et al. 2014). IAA and GA3 can promote plant growth by stimulating cell elongation and division (Teng et al. 2006). ZT has been shown to induce cell division in plants (Jameson 2023). These results indicate that high CO2 could regulate rice roots growth by increasing the accumulation of growth-promoting hormones (IAA, GA3, ZT), thus playing a positive role in rice growth.
The ratio of plant hormone contents can regulate plant growth and development under elevated CO2 concentration. In this study, the ratios of endogenous hormones in the root of rice seedlings showed an increasing trend under EC treatment, indicating the promotion effect of high CO2 on growth and metabolic activities of rice seedlings. The increases of IAA, GA3, and ZT contents in root was one of the reasons for the enhanced growth of rice in this study.
As the important metabolic regulators, organic acids play an important role in the metabolisms of secondary metabolites, amino acids and fatty acids (Nelson et al. 2008). This study shows that CO2 enrichment increased the citric acid and fumaric acid contents in roots by 11.7 and 19.8 fold, but significantly reduced the secreted malic acid and succinic acid by 23.4% and 41.1%. That maybe relate to the TCA cycle in rice root, the intrinsic physiological mechanism needs further study. Malic acid is an important node of plant carbon metabolism, and CO2 enrichment can reduce malic acid content (Sicher 2008). The production of citric acid may also act as a CO2 reservoir for some plants (López-Bucio et al. 2000). Malate import and citrate export are two of the most critical fluxes that mitochondria contribute to the cellular metabolic network in plants (Lee et al. 2021). In summary, high CO2 can enhance the metabolic capacity of rice roots, which can be demonstrated by the increased citric acid in roots and reduced malic acid secreted by roots.
Effects of Cd stress on rice roots growth, endogenous hormones and organic acids contents
Cd stress can inhibit rice growth, including root length, root area, root number, and stem length, meanwhile reduced seed germination, growth, yield, and quality of brown rice (Yu et al. 2006; Song et al. 2015; Rizwan et al. 2016). Under Cd stress, the plasma membrane of root epidermal cells is destroyed, cell activity is reduced, and Cd2+ enters root cells and inhibits root elongation (Dong et al. 2020). In this study, Cd stress significantly inhibited the growth of shoot length and root length of rice seedlings by 42.5% and 30.2%, and the greater the degree of stress, the more obvious the inhibition effect. Cd stress also had a significant effect on the dry matter percentage of rice seedlings. The study found that the above-ground part of rice seedlings was more sensitive to Cd stress than the below-ground part. When Cd was absorbed by root, the above-ground part was affected firstly.
When plants are stressed by heavy metals, phytohormones are involved in resistance to stress, and balance is disturbed (Guo et al. 2019). This study showed, AC + L increased ABA, SA and JA contents significantly by 6.1%, 16.6% and 23.7%, and AC + H increased ABA, SA and JA contents significantly by 4.7%, 12.5% and 8.3%.The enhancement of Cd enables plants to produce more endogenous hormones, such as SA, JA and ABA, which are involved in the signaling of plant stress responses to enhance their own defense mechanisms (Arin and Balci 2016; Khan et al. 2020; Matayoshi et al. 2022). Studies have shown that the expression levels and activities of stress-related hormones (under environmental stress), especially ABA, generally increase after stress, transcription factors from the MYC, MYB, and NAC protein families also enhance plant tolerance in an ABA-dependent manner (Asgher et al. 2015; Verma et al. 2016). The signaling pathways of ABA, SA and JA interact at various nodes, such as hormone-responsive transcription factors to regulate plant defense response. The crosstalk of ABA, SA and JA with the major growth-promoting hormones (IAA and GAs) plays an important role in mediating the stress responses. The signaling pathways of SA and JA are known to intersect at various points because SA and JA regulate biotic stress responses antagonistically (Bari and Jones 2009). This study showed that under AC + H, compared with AC + L, SA content increased but JA content decreased in roots. The changes of SA and JA contents showed the opposite results, indicated that under AC + L, the root system responds to Cd stress through JA, and SA plays an important role with the increase of Cd2+.
In the results of the ratios of endogenous hormones, the study found that Cd increased IAA/ABA; while GA3/JA decreased under AC + L, these results were caused by the accumulated ABA and JA in rice roots under Cd stress, which affected the root metabolic process and eventually led to the change of growth pattern to adapt to environmental stress. The different changes in hormone ratios in the results indicated that Cd stress disrupted the normal balance of hormone regulation in rice roots.
Organic acids are a class of extremely important metal ligands, which are mainly generated by TCA cycle in mitochondria and play a key role in the absorption, transportation, storage, and detoxification of heavy metals and other physiological metabolic processes. In this study, the lactic acid and tartaric acid increased 142.7% and 87.5%. Lactic acid is a metabolite produced by anaerobic metabolism and an important energy carrier; it is probably the most important energy source in the TCA cycle (Sun et al. 2020). Tartaric acid is efficient in complexing metals, The study showed that root tartaric acid content was positively correlated with Cd accumulation (Tao et al. 2020). The large accumulation of lactic acid in this study indicates that the TCA cycle of rice roots was sharply elevated to maintain roots energy metabolism under Cd stress. Tartaric acid plays an important role in detoxification. As the main channel for plants to absorb heavy metals, roots will quickly develop a coping mechanism when plants are damaged by an external adverse environment. This study showed that Cd stress increased malic acid, lactic acid, and citric acid contents in rice roots, TCA cycle-related metabolites were the main cause of rice root resistance to Cd stress, which was similar with the previous results (Wójcik et al. 2006). A large increase of malic acid content is beneficial to enhance restrict the Cd flow by forming strong bonds with Cd ions through metal chelation (Li et al. 2023).
Under environmental stress, roots rapidly synthesize and accumulate lots of organic acids to cope with the damage caused by the stress, and root secretions also significantly increase (López-Bucio et al. 2000; Hu et al. 2023). In this results, rice roots’ oxalic acid, citric acid, malic acid, acetic acid, and succinic acid secretion increased under a light Cd environment by 56.6%, 26.4%, 105.3%, 54.3%, 50%, those were consistent with previous report (Javed et al. 2017). These results indicate that Cd induced the secretion of organic acids from rice roots, and maybe produced a detoxification mechanism through the organic complexation of Cd ions, thus alleviation of Cd toxicity. Studies have also shown that citrate and malate are effective complexing agents of iron and can induce the dissolution of iron hydroxide (Joens 1998). Entry through Zn2+, Fe2+, and Ca2+ transporters is the molecular basis for the entry of Cd2+ into plant cells (Clemens 2006). Iron has been shown to inhibit the inflow of Cd (Thomas 2021). This indicates that there is a competitive relationship between Fe2+ and Cd2+ uptake by roots under Cd stress. The large increase in organic acids contents in roots of rice under Cd stress also indicates that the rice inhibits Cd uptake by absorbing more nutrients, such as iron, to achieve the purpose of reducing Cd toxicity.
Effects of high CO 2 and Cd on rice roots growth, endogenous hormones and organic acids contents
The increase of CO2 has a certain dilution effect on the accumulation of Cd in plants (Jia et al. 2010). In this study, it was found that high CO2 promoted the root growth of rice seedlings under Cd stress, and the dry weight and fresh weight of rice seedlings were effectively elevated. The effect was more obvious under EC + H treatment, and the above-ground part was more sensitive than the below-ground part. The research on Lolium perenne L seedlings showed that under Cd stress, when CO2 concentration increased, the Cd contents in roots and shoots significantly decreased, effectively alleviating Cd toxicity, promoting seedling growth, and increasing yield (Shi et al. 2021). Under Cd stress, increased CO2 can promote the accumulation of plant biomass, which is related to the dilution effect of CO2 (Jia et al. 2010). The above results indicate that the elevated CO2 concentration can alleviate the reduction of Cd treatment on the growth of rice seedlings to a certain extent.
The responses of plants to different environmental conditions are the result of the combined action of various factors. Plant hormones are necessary for stress adaptation and defense against abiotic stress such as metal toxicity (El Rasafi et al. 2020; Chu et al. 2020). This study showed that compared with Cd stress alone, EC + L and EC + H promoted the accumulation of IAA, ZT, and GA3 in rice seedlings roots, and decreased the contents of ABA, JA, and SA in roots. Some studies have shown that ABA, JA, SA and GAs played key roles in plant response to abiotic stress, and they interact by activating multiple proteins and transcription factors under stress (Verma et al. 2016; Vishwakarma et al. 2017). ABA, SA, and IAA also play a role in regulating plant response to heavy metal stresses (Yue et al. 2016; Shao et al. 2010). They are synergistic, and it has been reported that under Cd stress, plants mainly produce ABA and JA to regulate plant growth (Pérez et al. 2014). Down-regulation of auxin signaling is part of the plant-induced immune response, and auxin is also involved in the attenuation of the plant defense response (Bari and Jones 2009). The results indicate that high CO2 perhaps decreased the adverse effect of Cd stress, so more IAA was accumulated to promote the growth of rice. The decrease of ABA and JA contents indicates the same point. High CO2 can increase JA content in leaf under Pb stress in our prior study, while in this study, which decreased JA content in roots under Cd stress, may be due to different plant parts and heavy metal stresses (Wang et al. 2022). The hormone ratios in leaves under EC + L and EC + H treatment were higher than those under Cd stress alone, indicating that high CO2 increased the contents of growth-promoting hormones in rice roots under Cd stress, and reduced the inhibitory effect of Cd on rice growth ability. These findings provide a basis for future rice planting and breeding screening.
The research previously studied the response mechanism of rice seedling leaves under high concentrations of CO2 and Pb stress and found that CO2 can reduce the damage degree of leaf cell membrane under Pb stress and enhance the antioxidant capacity of cells, in which secondary metabolites play an important role (Wang et al. 2023). This study found that high CO2 also decreased tartaric acid content in root under Cd stress, indicated that CO2 had a detoxifying effect on Cd toxicity in roots and thus reduced tartaric acid accumulation (Chen et al. 2017; Riaz et al. 2018).In the combined of high CO2 and Cd stress, the increase of CO2 resulted in the decrease of oxalic acid, citrate acid and succinic acid secretion in rice roots, while under severe stress (H treatment), the total organic acid contents secreted by rice roots were decreased. Oxalic acid, as the most common dicarboxylic acid, has a strong ability to bind heavy metals, which can reduce the oxidative damage caused by abiotic stress, and then affect the accumulation of heavy metals in plants (Huang et al. 2023). Under Cd stress, high CO2 can reduce oxalic acid secretion and tartaric acid accumulation in roots, which may be related to the reduction of Cd in rice roots and the weakened Cd toxicity. The previous study found that the high CO2 decreased oxalic acid, citric acid, succinic acid, and lactic acid contents in rice leaves (Qi et al. 2021), which were similar with the present study on root. One reason may be that high CO2 inhibited the respiration of roots, resulting in reduced secretion of metabolites related to the TCA cycle. Another reason may be that CO2 could promote plant growth stress and alleviate root damage under Cd, thus reducing root organic acids secretion. It was also found that high CO2 promoted the organic acid content of wheat seedlings under Cd stress, which is different from the results of this study (Jia et al. 2014, 2016).
In summary, high CO2 can alleviate the damage of rice roots under Cd stress to a certain extent, and hormones play a major role, which is proved by the increase of IAA, GA3 and ZT contents. Interestingly, this study found that the root organic acids contents decreased under high CO2 and Cd, indicating that there was a complex change mechanism of rice root under the combined treatment.
Conclusion
The changes of endogenous hormones and organic acids were studied in rice seedling roots under high CO2 and Cd stress. The results showed:
-
(1)
High CO2 promoted the growth of rice seedlings by increasing the growth hormones (IAA, ZT and GA3) contents. High CO2 can enhance TCA cycle by increasing citric acid and fumaric acid contents in rice roots.
-
(2)
Under Cd stress, the growth of rice seedlings is strongly inhibited. ABA, SA and JA contents were changed to enhance Cd stress resistance. The total organic acids contents in and secreted from roots increased significantly. The secreted organic acids (especially tartaric acid) also showed positive metal complexation ability.
-
(3)
High CO2 effectively promoted the accumulation of IAA, ZT and GA3 in roots under Cd stress, but ABA, SA and JA content decreased. All the elevated hormone ratios indicated that CO2 promoted the growth of rice under Cd stress. High CO2 had a certain detoxification effect by decreasing tartaric acid and the secrete (by root) oxalic acid contents.
In summary, high CO2 can resist Cd stress by regulating the content of growth-promoting hormones, while organic acids play an important role. The above results verify the study hypothesis. It can provide data support for rice cultivation in future agriculture.
The molecular response mechanism of rice roots under high CO2 concentration and Cd stress is not clear. Those will be the future research directions. Cadmium ions (Cd2+) are similar in size and charge to some plant essential elements, these elements may compete with Cd. Under Cd stress, the interference of other ions in nutrient solution cannot be completely eliminated, so the method of this study has limitations. The stress treatment time in this study was 7 days. Future experiments will explore the mechanism of rice roots response to high CO2 and Cd stress during dynamic time.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arin L, Balci H (2016) The effect of some organic acid and plant-derived material treatments on the germination and emergence of lettuce. Org Agr 6:199–201. https://doi.org/10.1007/s13165-015-0128-0
Asgher M, Khan MI, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 252(2):399–413. https://doi.org/10.1007/s00709-014-0710-4
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69(4):473–488. https://doi.org/10.1007/s11103-008-9435-0
Boretti A, Florentine S (2019) Atmospheric CO2 concentration and other limiting factors in the growth of C3 and C4 plants. Plants (basel) 8(4):92. https://doi.org/10.3390/plants8040092
Chen YT, Wang Y, Yeh KC (2017) Role of root exudates in metal acquisition and tolerance. Curr Opin Plant Biol 39:66–72. https://doi.org/10.1016/j.pbi.2017.06.004
Chu Z, Munir S, Zhao GJ, Hou JD, Du WQ, Li N, Lu YE, Yu QH, Shabala S, Ouyang B (2020) Linking phytohormones with growth, transport activity and metabolic responses to cadmium in tomato. Plant Growth Regul 90:557–569. https://doi.org/10.1007/s10725-020-00580-w
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11):1707–1719. https://doi.org/10.1016/j.biochi.2006.07.003
Dong G, Nkoh JN, Hong ZN, Dong Y, Lu HL, Yang J, Pan XY, Xu RK (2020) Phytotoxicity of Cu2+ and Cd2+ to the roots of four different wheat cultivars as related to charge properties and chemical forms of the metals on whole plant roots. Ecotoxicol Environ Saf 196:110545. https://doi.org/10.1016/j.ecoenv.2020.110545
Drag DW, Slattery R, Siebers M, DeLucia EH, Ort DR, Bernacchi CJ (2020) Soybean photosynthetic and biomass responses to carbon dioxide concentrations ranging from pre-industrial to the distant future. J Exp Bot 71(12):3690–3700. https://doi.org/10.1093/jxb/eraa133
El Rasafi T, Oukarroum A, Haddioui A, Song H, Kwon EE, Bolan N, Rinklebe J (2020) Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol 52(5):675–726. https://doi.org/10.1080/10643389.2020.1835435
Farhat F, Arfan M, Wang X, Tariq A, Kamran M, Tabassum HN, Tariq I, Mora-Poblete F, Iqbal R, El-Sabrout AM, Elansary HO (2022) The impact of bio-Stimulants on Cd-Stressed wheat (Triticum aestivum L.): insights into growth, chlorophyll fluorescence, Cd accumulation, and Osmolyte regulation. Front Plant Sci 13:850567. https://doi.org/10.3389/fpls.2022.850567
Fediuc E, Lips SH, Erdei L (2005) O-acetylserine (thiol) lyase activity in phragmites and typha plants under cadmium and NaCl stress conditions and the involvement of ABA in the stress response. J Plant Physiol 162(8):865–872. https://doi.org/10.1016/j.jplph.2004.11.015
Guo J, Qin S, Rengel Z, Gao W, Nie Z, Liu H, Li C, Zhao P (2019) Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol Environ Saf 172:380–387. https://doi.org/10.1016/j.ecoenv.2019.01.069
Hu Y, Zhou XQ, Shi A, Yu YS, Rensing C, Zhang TX, Xing SH, Yang WH (2023) Exogenous silicon promotes cadmium (Cd) accumulation in sedum alfredii hance by enhancing Cd uptake and alleviating Cd toxicity. Front Plant Sci 14:1134370. https://doi.org/10.3389/fpls.2023.1134370
Huang HG, Lu RZ, Zhan J, He JS, Wang Y, Li TX (2023) Role of root exudates in cadmium accumulation of a low-cadmium-accumulating tobacco line (Nicotiana tabacum L.). Toxics 11(2):141. https://doi.org/10.3390/toxics11020141
Huber BM, Louws FJ, Hernández R (2021) Impact of different daily light integrals and carbon dioxide concentrations on the growth, morphology, and production efficiency of tomato seedlings. Front Plant Sci 12:615853. https://doi.org/10.3389/fpls.2021.615853
IPCC working group I (2021) Climate change: the physical science basis. Cambridge University Press, Cambridge (http://www.climate2021.org/spm)
Jameson PE (2023) Zeatin: The 60th anniversary of its identification. Plant Physiol 192(1):34–55. https://doi.org/10.1093/plphys/kiad094
Javed MT, Akram MS, Tanwir K, Javed Chaudhary H, Ali Q, Stoltz E, Lindberg S (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf 141:216–225. https://doi.org/10.1016/j.ecoenv.2017.03.027
Jia Y, Tang S, Wang R, Ju X, Ding Y, Tu S, Smith DL (2010) Effects of elevated CO2 on growth, photosynthesis, elemental composition, antioxidant level, and phytochelatin concentration in Lolium mutiforum and Lolium perenne under Cd stress. J Hazard Mater 180(1–3):384–394. https://doi.org/10.1016/j.jhazmat.2010.04.043
Jia X, Wang WK, Chen ZH, He YH, Liu JX (2014) Concentrations of secondary metabolites in tissues and root exudates of wheat seedlings changed under elevated atmospheric CO2 and cadmium-contaminated soils. Environ Exp Bot 107:134–143. https://doi.org/10.1016/j.envexpbot.2014.06.005
Jia X, Liu T, Zhao YH, He YH, Yang MY (2016) Elevated atmospheric CO2 affected photosynthetic products in wheat seedlings and biological activity in rhizosphere soil under cadmium stress. Environ Sci Pollut Res Int 23(1):514–526. https://doi.org/10.1007/s11356-015-5288-7
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44. https://doi.org/10.1023/A:1004356007312
Khan A, Bilal S, Khan AL, Imran M, Al-Harrasi A, Al-Rawahi A, Lee IJ (2020) Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol Environ Saf 188:109885. https://doi.org/10.1016/j.ecoenv.2019.109885
Lamichaney A, Tewari K, Basu PS, Katiyar PK, Singh NK (2021) Effect of elevated carbon-dioxide on plant growth, physiology, yield and seed quality of chickpea (Cicer arietinum L.) in Indo-Gangetic plains. Physiol Mol Biol Plants 27:251–263. https://doi.org/10.1007/s12298-021-00928-0
Lee CP, Elsässer M, Fuchs P, Fenske R, Schwarzländer M, Millar AH (2021) The versatility of plant organic acid metabolism in leaves is underpinned by mitochondrial malate-citrate exchange. Plant Cell 33(12):3700–3720. https://doi.org/10.1093/plcell/koab223
Li CQ, Fu YZ, Trotsenko V, Zhatova H (2023) Understanding the physiological and molecular mechanisms of grain cadmium accumulation conduces to produce low cadmium grain crops: a review. Plant Growth Regul. https://doi.org/10.1007/s10725-023-01105-x
Liu JG, Qian M, Cai GL, Zhu QS, Wong MH (2007) Variations between rice cultivars in root secretion of organic acids and the relationship with plant cadmium uptake. Environ Geochem Health 29(3):189–195. https://doi.org/10.1007/s10653-006-9063-z
López-Bucio J, Nieto-Jacobo MF, Ramírez-Rodríguez VV, Herrera-Estrella L (2000) Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci 160(1):1–13. https://doi.org/10.1016/s0168-9452(00)00347-2
Madhu M, Hatfield JL (2013) Dynamics of plant root growth under increased atmospheric carbon dioxide. Agro J 105(3):657–669. https://doi.org/10.2134/agronj2013.0018
Matayoshi CL, Pena LB, Arbona V, Gómez-Cadenas A, Gallego SM (2022) Biochemical and hormonal changes associated with root growth restriction under cadmium stress during maize (Zea mays L.) pre-emergence. Plant Growth Regul 96:269–281. https://doi.org/10.1007/s10725-021-00774-w
Medeiros DB, Aarabi F, Martinez Rivas FJ, Fernie AR (2021) The knowns and unknowns of intracellular partitioning of carbon and nitrogen, with focus on the organic acid-mediated interplay between mitochondrion and chloroplast. J Plant Physiol 266:153521. https://doi.org/10.1016/j.jplph.2021.153521
Nahakpam S, Shah K (2011) Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings. Plant Growth Regul 63(1):23–35. https://doi.org/10.1007/s10725-010-9508-3
Negishi N, Nakahama K, Urata N, Kojima M, Sakakibara H, Kawaoka A (2014) Hormone level analysis on adventitious root formation in eucalyptus globulus. New for 45(4):577–587. https://doi.org/10.1007/s11056-014-9420-1
Nelson DL, Lehninger AL, Cox MM (2008) Lehninger principles of biochemistry. Freeman Macmillan, New York
Niu Y, Jin C, Jin G, Zhou Q, Lin X, Tang C, Zhang Y (2011) Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ 34(8):1304–1317. https://doi.org/10.1111/j.1365-3040.2011.02330.x
Pérez Chaca MV, Vigliocco A, Reinoso H, Molina A, Abdala G, Zirulnik F, Pedranzani H (2014) Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol Plant 36:2815–2826. https://doi.org/10.1007/s11738-014-1656-z
Piñero MC, Houdusse F, Garcia-Mina JM, Garnica M, Del Amor FM (2014) Regulation of hormonal responses of sweet pepper as affected by salinity and elevated CO2 concentration. Physiol Plantarum 151(4):375–389. https://doi.org/10.1111/ppl.12119
Pinto AP, Simotildees I, Mota AM (2008) Cadmium impact on root exudates of sorghum and maize plants: a speciation study. J Plant Nutr 31(10):1746–1755. https://doi.org/10.1080/01904160802324829
Qi XF, Wang XH, Wang Q, Li M, Ma LJ, Li YY, Li XM, Wang LL (2021) Photosynthesis, stomatal conductance, endogenous hormones and organic acid synergistic regulation in leaves of rice (Oryza sativa L.) under elevated CO2. Appl Ecol Environ Res 19(5):3773–3787. https://doi.org/10.15666/aeer/1905_37733787
Qin SY, Liu HE, Nie ZJ, Rengel Z, Gao W, Li C, Zhao P (2020) Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2):168–180. https://doi.org/10.1016/S1002-0160(20)60002-9
Quirk J, Bellasio C, Johnson DA, Beerling DJ (2019) Response of photosynthesis, growth and water relations of a savannah-adapted tree and grass grown across high to low CO2. Ann Bot 124(1):77–90. https://doi.org/10.1093/aob/mcz048
Riaz M, Yan L, Wu X, Hussain S, Aziz O, Jiang C (2018) Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: a review. Ecotoxicol Environ Saf 165:25–35. https://doi.org/10.1016/j.ecoenv.2018.08.087
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-Ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879. https://doi.org/10.1007/s11356-016-6436-4
Roy KS, Bhattacharyya P, Neogi S, Rao KS, Adhya TK (2012) Combined effect of elevated CO2 and temperature on dry matter production, net assimilation rate, C and N allocations in tropical rice (Oryza sativa L.). Field Crops Res 139:71–79. https://doi.org/10.1016/j.fcr.2012.10.011
Shao HB, Chu LY, Ruan CJ, Li H, Guo DG, Li WX (2010) Understanding molecular mechanisms for improving phytoremediation of heavy metal-contaminated soils. Crit Rev Biotechnol 30(1):23–30. https://doi.org/10.3109/07388550903208057
Shi Y, Liu Y, Li H, Pei H, Xu Y, Ju X (2021) Phytochelatins formation kinetics and Cd-induced growth inhibition in Lolium perenne L at elevated CO2 level under Cd stress. Environ Sci Pollut Res Int 28(27):35751–35763. https://doi.org/10.1007/s11356-021-12883-0
Sicher RC (2008) Effects of CO2 enrichment on soluble amino acids and organic acids in barley primary leaves as a function of age, photoperiod and chlorosis. Plant Sci 174(6):576–582. https://doi.org/10.1016/j.plantsci.2008.03.001
Song W, Chen SB, Liu JF, Chen L, Song NN, Liu N, Liu B (2015) Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Integr Agric 14(9):1845–1854. https://doi.org/10.1016/S2095-3119(14)60926-6
Sun H, Wang X, Li H, Bi J, Yu J, Liu X, Rong Z (2020) Selenium modulates cadmium-induced ultrastructural and metabolic changes in cucumber seedlings. RSC Adv 10(30):17892–17905. https://doi.org/10.1039/d0ra02866e
Tao Q, Zhao J, Li J, Liu Y, Luo J, Yuan S, Li B, Li Q, Xu Q, Yu X, Huang H, Li T, Wang C (2020) Unique root exudate tartaric acid enhanced cadmium mobilization and uptake in Cd-hyperaccumulator Sedum alfredii. J Hazard Mater 383:121177. https://doi.org/10.1016/j.jhazmat.2019.121177
Temme AA, Liu JC, Cornwell WK, Cornelissen JH, Aerts R (2015) Winners always win: growth of a wide range of plant species from low to future high CO2. Ecol Evol 5(21):4949–4961. https://doi.org/10.1002/ece3.1687
Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J (2006) Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol 172(1):92–103. https://doi.org/10.1111/j.1469-8137.2006.01818.x
Thomas M (2021) A comparative study of the factors affecting uptake and distribution of Cd with Ni in barley. Plant Physiol Biochem 162:730–736. https://doi.org/10.1016/j.plaphy.2021.03.043
Uddin S, Löw M, Parvin S, Fitzgerald GJ, Tausz-Posch S, Armstrong R, O’Leary G, Tausz M (2018) Elevated [CO2] mitigates the effect of surface drought by stimulating root growth to access sub-soil water. PLoS ONE 13(6):e0198928. https://doi.org/10.1371/journal.pone.0198928
Uprety DC, Sen S, Dwivedi N (2010) Rising atmospheric carbon dioxide on grain quality in crop plants. Physiol Mol Biol Plants 16(3):215–227. https://doi.org/10.1007/s12298-010-0029-3
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86. https://doi.org/10.1186/s12870-016-0771-y
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:161. https://doi.org/10.3389/fpls.2017.00161
Wang Y, Du ST, Li LL, Huang LD, Fang P, Lin XY, Zhang YS, Wang HL (2009) Effect of CO2 elevation on root growth and its relationship with indole acetic acid and ethylene in tomato seedlings. Pedosphere 19(5):570–576. https://doi.org/10.1016/S1002-0160(09)60151-X
Wang WL, Cai C, He J, Gu JF, Zhu GL, Zhang WY, Zhu JG, Liu G (2019) Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res 248:107605. https://doi.org/10.1016/j.fcr.2019.107605
Wang XH, Zou JY, Qi XF, Li Q, Ma LJ, Li YY, Li XM, Wang LL (2022) High concentration of CO2 improve the Pb resistance of Oryza sativa L. seedlings by enhancing photosynthesis and regulating plant endogenous hormones. J Plant Growth Regul 41:3556–3567. https://doi.org/10.1007/s00344-021-10533-w
Wang LL, Yao YX, Wang JY, Cui JH, Wang XH, Li XM, Li YY, Ma LJ (2023) Metabolomics analysis reveal the molecular responses of high CO2 concentration improve resistance to Pb stress of Oryza sativa L. seedlings. Ecotoxicol Environ Saf 251:114515. https://doi.org/10.1016/j.ecoenv.2023.114515
Wójcik M, Skórzyńska-Polit E, Tukiendorf A (2006) Organic acids accumulation and antioxidant enzyme activities in thlaspicaerulescens under Zn and Cd stress. Plant Growth Regul 48:145–155. https://doi.org/10.1007/s10725-005-5816-4
Xie X, Weiss DJ, Weng B, Liu J, Lu H, Yan C (2013) The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandeliaobovata (S, L.) Yong) roots. Environ Sci Pollut Res Int 20(2):997–1008. https://doi.org/10.1007/s11356-012-1031-9
Yu H, Wang JL, Fang W, Yuan JG, Yang ZY (2006) Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ 370(2–3):302–309. https://doi.org/10.1016/j.scitotenv.2006.06.013
Yue R, Lu C, Qi J, Han X, Yan S, Guo S, Liu L, Fu X, Chen N, Yin H, Chi H, Tie S (2016) Transcriptome analysis of cadmium-treated roots in maize (Zea mays L.). Front Plant Sci 7:1298. https://doi.org/10.3389/fpls.2016.01298
Funding
This research was supported by the National Natural Science Foundation of China (31600314), the Department of Education of Liaoning Province, China (LJKZ0991), and the National College Student Innovation and Entrepreneurship Project (202310166026).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Cui Jinghui, OuYang Wanting, Song xiaohui, Wang xuhao, Luo zihan, Wang xiaoyu and Feng ziyuan, the experimental design was guided by Wang lanlan, Ma lianju, Li xuemei and Li yueying, the first draft of the manuscript was written by Cui jinghui and Wang lanlan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Conflict of interest on behalf of all authors, the corresponding author states that there is no conflict of interest.
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, J., OuYang, W., Wang, X. et al. Effects of high concentration of CO2 and/or Cd stress on endogenous hormones and organic acids contents in rice (Oryza sativaL.) seedling roots. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01162-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01162-w