Abstract
Since the Industrial Revolution, crops have been exposed to various changes in the environment, including elevated atmospheric carbon dioxide (CO2) concentration and cadmium (Cd) pollution in soil. However, information about how combined changes affect crop is limited. Here, we have investigated the changes of japonica and indica rice subspecies seedlings under elevated CO2 level (1200 ppm) and Cd exposure (5 μM Cd) conditions compared with ambient CO2 level (400 ppm) and without Cd exposure in CO2 growth chambers with hydroponic experiment. The results showed that elevated CO2 levels significantly promoted seedling growth and rescued the growth inhibition under Cd stress. However, the elevated CO2 levels led to a significant increase in the shoot Cd accumulation of the two rice subspecies. Especially, the increase of shoot Cd accumulation in indica rice was more than 50% compared with control. Further investigation revealed that the decreases in the photosynthetic pigments and photosynthetic rates caused by Cd were attenuated by the elevated CO2 levels. In addition, elevated CO2 levels increased the non-enzymatic antioxidants and significantly enhanced the ascorbate peroxidase (APX) and glutathione reductase (GR) activities, alleviating the lipid peroxidation and reactive oxygen species (ROS) accumulation induced by Cd. Overall, the research revealed how rice responded to the elevated CO2 levels and Cd exposure, which can help modify agricultural practices to ensure food security and food safety in a future high-CO2 world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The atmospheric carbon dioxide (CO2) concentration has sharply increased from 280 ppm before industrialization to the current levels of approximately 415 ppm, primarily because of soaring human activities (e.g., excessive fossil fuel consumption and deforestation) (AbdElgawad et al. 2021; Yu and Chen 2019). Moreover, the atmospheric CO2 concentration will rise to over 800 ppm by the end of this century (Bouain et al. 2022). Many researches have showed that elevated atmospheric CO2 concentrations will significantly impact on plant growth, productivity, and species composition in agricultural and ecosystems (Li et al. 2023).
With the functions of photosynthetic substrates and signal transduction, CO2 levels in plants would deeply affect the plants’ photosynthesis and carbon metabolism (Loladze 2014; Sage and Khoshravesh 2016). As a matter of fact, the current CO2 concentration does not meet the demand of the maximal photosynthetic efficiency for the C3 species (Thompson et al. 2017). With the increase in CO2 concentration, the CO2 capture of the C3 species would be significantly enhanced, leading to an improvement in biomass, which is called “CO2 fertilization” (AbdElgawad et al. 2021; Seibert et al. 2021). Thirty years of free-air carbon dioxide enrichment (FACE) research has shown that a 35% yield increase was recorded in the rice cultivars of the highest yield potential at elevated CO2 concentrations (Ainsworth and Long 2021).
The “CO2 fertilization” generated by elevated CO2 concentrations would obviously relieve the future problem of food shortages, but some negative effects have also been firmly recognized recently. For example, elevated CO2 concentrations incur the reductions in most of the mineral and nutrient concentrations in plant tissues. Research has shown that the beneficial influence of elevated CO2 concentrations on cotton, as relayed by P supplementation, might be an adverse impact in response to severe P deficiencies (Singh and Reddy 2014). With a FACE platform, a field experiment showed that elevated CO2 reduced the prolamin, albumin, glutelin, and total protein concentrations in rice (Oryza sativa L.) grain (Wei et al. 2021). In wheat (Triticum aestivum L.) grain, the S, Zn, Fe, and protein concentrations were decreased with elevated CO2 concentrations (Dier et al. 2020). At the Giessen FACE site, the Ca, Mg, N, P, and K concentrations of many grasses were significantly reduced (Seibert et al. 2021), and elevated CO2 concentrations decreased the concentrations of N, P, K, S, and Mg in the edible parts of lettuce (Giri et al. 2016). Many mineral element concentrations (Mn, Fe, Zn, Mg, Mo, K, and Ca) in wheat grain were significantly decreased under increasing atmospheric CO2 concentrations (Beleggia et al. 2018). Previously, these negative effects have been regarded as the results of the “dilution effect” because of the increase in C capture and biomass (Taub and Wang 2008). However, the “dilution effect” was not an effectively hypothesis. For instance, the changes in the different nutrient element concentrations are very different (Myers et al. 2014). The decreased efficiency in nutrient assimilation and/or acquisition appears to be the main issue of the negative effects of elevated CO2 concentrations on plants’ mineral elements (Gojon et al. 2022). Collectively, with the atmospheric CO2 concentration increased, the yield increase but loss of mineral elements (also called “hidden hunger”) would be a prominent problem in the future.
Rice, as a C3 cereal crop species, is a staple food for over half of the world’s population (Zhao and Chang 2022). Elevated CO2 concentrations also could lead to the gain of crop yield and loss of nutrients for rice. Elevated CO2 concentrations contributed to a significant increase in rice grain yield, and this is partly regulated by qPE9-1, a G protein γ subunit which is associated with N uptake in response to elevated CO2 concentrations (Wang et al. 2022). Elevated CO2 would also reduce the accumulation of P, and PHT4;3 is a P transporter that regulates the loss of P in chloroplasts in the face of elevated CO2 to support plant growth in Arabidopsis thaliana, and this mechanism is conserved in rice (Bouain et al. 2022). Besides the potential loss of nutrients under elevated CO2 concentrations, rice also faces a risk of Cd pollution. Because of the Cd pollution of paddy field caused by the industrial activities (e.g., non-ferrous metal manufacturing, energy production and mining) and application of phosphate fertilizer, livestock manure, and sewage sludge, the rice Cd exposure has become a serious public problem for plant growth and human health in many countries, and at present, many researchers have shown that rice is the major source of human Cd intake (McGrath 2022). Rice shares over 55.8% of the Cd intake among the Chinese population (Song et al. 2017). Although many researchers have studied the influence of elevated CO2 concentrations or Cd exposure on rice, a clear assessment is necessary when rice simultaneously faces elevated CO2 concentrations and Cd pollution. Here, we have mainly analyzed the biomasses, Cd concentrations, gas exchange parameters, and antioxidant-related parameters of rice seedlings to illustrate the impacts of Cd exposure and elevated CO2 concentrations on rice using hydroponics experiments in CO2 growth chambers.
Materials and methods
Plant materials and culture conditions
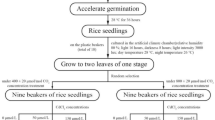
Two rice cultivars, Yangdao No. 6 (YD) and Ninggeng No. 7 (NG), were used in this study. Yd belongs to the indica rice subspecies, and NG belongs to the japonica rice subspecies. Rice seeds were soaked in distilled water that was kept in a 28 °C incubator in the dark. After 2 days, the germinated seeds were transferred to home-made hydroponic grow trays containing 28 °C distilled water, where they remained for 2 days. Then, the uniform seedlings of approximately 1.5 cm in height were transferred to a hydroponic box containing 1/8 Kimura B solution for 3 days, and then 1/4 Kimura B solution was added, and they were kept for an additional 3 days. Finally, 1/2 Kimura B solution (180 μM Ca(NO3)2, 90 μM KNO3, 90 μM KH2PO4, 270 μM MgSO4, 180 μM (NH4)2SO4, 3 μM H3BO3, 0.5 μM MnCl2, 1 μM (NH4)6Mo7O24, 0.4 μM ZnSO4, 0.2 μM CuSO4, and 20 μM Fe(II)-EDTA, at a pH of 5.6–5.7) was added, and this solution was renewed in the hydroponic box every 3 days. Each hydroponic box contained 6 seedlings as one biological replicate. The rice seedlings were grown in CO2 growth chambers (RGL-500C-CO2, Hefei Dascate Biotechnology Co., Ltd, Hefei, China) under a daily cycle of 28 °C and 14 h day, and 26 °C and 10 h night, with 65% humidity and a light intensity of approximately 350 μmol m−2 s−1. The CO2 concentrations of the two CO2 growth chambers used in this study were 400 ppm (i.e., ambient CO2) and 1200 ppm (i.e., elevated CO2), respectively.
Cd and CO2 treatments
For the Cd concentration gradient experiments, 10-day-old seedlings were treated with 0, 1, 2, 5, or 10 μM of Cd. Each treatment was applied to four biological replicates.
Three CO2 conditions were set as follows: (1) 4-day-old seedlings that had always been grown at an ambient CO2 concentration (AC) as the control; (2) 4-day-old seedlings that had been grown in a growth chamber with ambient CO2 for 6 days and then were transferred to an elevated CO2 concentration, named elevated CO2 condition 1 (EC1); and (3) seedlings that had always been grown in a growth chamber with elevated CO2, named elevated CO2 condition 2 (EC2). To investigate the effects of Cd stress at different CO2 concentrations, 10-day-old seedlings grown in the CO2 conditions described above were treated with 0 or 5 μM of Cd. Each treatment was applied to four biological replicates.
Measurements of the shoots, root lengths, and weights
After the rice seedlings grown at different CO2 conditions had been treated with Cd for 9 days, the shoot and root lengths of the seedlings were measured. The rice seedling roots were then washed with 5 mM of CaCl2 twice to remove the nutrient elements and Cd adhering to the root surfaces, and they were further cleaned with distilled water twice to remove any CaCl2 that remained attached to the root surfaces. The seedlings were then separated into shoots and roots, and their fresh weights were weighed and totally dried at 70 °C. The dry shoot and root samples were used to measure the dry weights and Cd concentrations.
Cd concentration assay
The dry samples were sheared and put into mixed acid (HClO4: HNO3 = 15:85, v/v) overnight and then digested with a heating block (DigiBlock ED54-iTouch, LabTech, Beijing, China) that was applied using the following procedure: initial heating to 80 °C, where it was held for 1 h and then heating to 100 °C for 1 h, 120 °C for 1 h, 160 °C for 1 h, and 180 °C until the solution was clarified and further crystallized. The solution was then mixed with 10 ml of 2.5% HNO3 and put into a water bath at 60 °C to dissolve the samples before taking the Cd concentration measurements (Fang et al. 2020).
The Cd concentrations were determined using an ICP-OES (inductively coupled plasma optical emission spectrometer, Optima 2100DV, Perkin Elmer, Gaithersburg, MD, USA). The Cd translocation factor (TF) was the ratio of the Cd concentrations in the shoots to the Cd concentrations in the roots (Wang et al. 2017). For quality control, reagent blanks and orange leaves which was a certified reference plant material (GBW10020, National Research Center for Standards, China) were applied in the assay. The Cd recovery rates of the reference materials were 95–101%.
Photosynthetic system-related assay
The contents of the photosynthetic pigments (total chlorophyll (total Chl), chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid (Car)) were measured as follows: approximately, 100 mg of fresh leaves was cut up and placed in 10 ml tubes containing 5 ml of 95% ethanol. The tubes containing the samples were kept in the dark until the leaves were completely decolorized. The absorbance levels of the extracted solutions at 665 nm, 649 nm, and 470 nm were measured, respectively, with a spectrophotometer (SHIMADZU UV-2450, Kyoto, Japan). The contents of the photosynthetic pigments were then calculated as follows (Huang et al. 2019):
and
The contents of photosynthetic pigment (mg/g) = C × V × N/W.where C was the content of the photosynthetic pigment, V was the volume of extracting solution (mL), N was the dilution ratio, and W was the fresh weight (g).
The leaf gas exchange parameters (Pn, net photosynthetic rate; Tr, transpiration rate; Ci, intercellular CO2 concentration; and Gs, stomatal conductance) were recorded with a Li-6400 photosynthesis system with an LED light source (Li-Cor Biosciences, Lincoln, NB, USA) on a flag leaf from 10:00 am to 12:00 pm (Ulfat et al. 2021). The light intensity of the leaf chamber was set to 1500 μmol m−2 s−1 with a flow rate of 500 μmol s−1, the leaf temperatures were set at 28 °C, and leaf chamber conditions were set at 400 or 1200 ppm CO2.
Analysis of the MDA contents, H2O2 contents, O2 - contents, and plasma membrane integrity
To measure the MDA contents, 0.2 mg of fresh sample was triturated with liquid nitrogen and mixed with 5 ml of 5% TCA (trichloroacetic acid). After being centrifuged at 4 °C for 10 min at 8000 × g, 2 ml of the supernatant was transferred to a new tube and mixed with 5 ml of 6% TBA (thiobarbituric acid) dissolved with 10% TCA. Then, the mixture was boiled for 30 min and immediately cooled using running water (Nie et al. 2016). The absorbance levels of the mixture at 450 nm, 532 nm, and 600 nm were measured, and the MDA contents were calculated according to the following formula:
The superoxide anion (O2−) contents were measured as follows: A quantity of 0.2 g of fresh sample was triturated with liquid nitrogen and homogenized with 1 mL of 50 mM phosphate buffer (pH of 7.8). After being centrifuged at 4 °C for 10 min at 12000 × g, 0.5 ml of the supernatant was moved to a new tube and mixed with 0.5 ml of 50 mM phosphate buffer (pH of 7.8) and 0.1 ml of hydroxylamine hydrochloride (10 mM). The mixture was incubated at 25 °C for 1 hand further mixed with 1 ml of 17 mM P-aminobenzene sulphonic acid anhydrous and 1 ml of 7 mM a-naphthylamine. Afterward, the samples were incubated for 20 min at 25 °C, and the absorbance at 390 nm was measured. NaNO2 was used to establish the standard curve (Huang et al. 2008). The histochemical stain of O2− of the rice roots under the Cd and CO2 treatments was applied with NBT (nitro-blue tetrazolium) and imaged with a stereoscopic microscope (Model Stemi 2000-C; Carl Zeiss, Jena, Germany) (Huang et al. 2019).
The H2O2 contents were measured as follows: A quantity of 0.2 g of fresh sample was triturated with liquid nitrogen and homogenized with 5 mL of 5% TCA (trichloroacetic acid). After being centrifuged at 4 °C for 15 min at 12000 × g, 0.5 ml of the supernatant was moved to a new tube and mixed with 0.5 ml of phosphate buffer (10 mM, pH of 7.0) and 1 ml KI (1 M). The samples were then used to measure the absorbance at 390 nm to obtain the H2O2 content. H2O2 was used to establish the standard curve (Shah et al. 2020). The histochemical stain of H2O2 of the rice roots under the Cd and CO2 treatments was applied with 3, 3′-diaminobenzisine (DAB) and imaged with a stereoscopic microscope (Model Stemi 2000-C; Carl Zeiss, Jena, Germany) (Huang et al. 2019).
The plasma membrane integrity was analyzed with Evans blue (Martins et al. 2013). The roots were stained with 0.25% (w/v) Evans blue solution for 10 min at room temperature, then washed with distilled water, and imaged with a stereoscopic microscope (Model Stemi 2000-C; Carl Zeiss, Jena, Germany), the equivalent root tips were sampled and soaked in N,N-dimethylformamide to release the stain, and the concentration was determined by measuring absorbance at 600 nm.
Determination of the NPT and total phenolic contents
The non-protein thiol (NPT) content was measured with Ellman’s reagent (Tian et al. 2011) as follows: A quantity of 0.2 g of fresh sample was triturated with liquid nitrogen and mixed with 3 mL of sulfosalicylic acid (5%, w/v). Then, the homogenate was centrifuged at 4 °C for 30 min at 10000 × g, and 0.3 ml of the supernatant was added to a new tube and mixed with 1.2 ml of 100 mM phosphate buffer (pH of 7.6) and 25 μl of 6 mM DTNB (5,5’-dithiobis-2-nitrobenzoic acid) solution (the DNTB was dissolved with 100 mM phosphate buffer (pH of 7.6) with 5 mM EDTA). The mixed solution was incubated for 20 min at room temperature, and then the absorbance at 412 nm was measured. Glutathione was applied to establish the standard curve.
The total phenolic content was measured using Folin–Ciocalteu reagent (Li et al. 2012) as follows: A quantity of 0.2 g of fresh sample was triturated with liquid nitrogen and mixed with 10 ml of 50% ethanol, and then the mixture was ultrasound treated for 1.5 h. After being centrifuged for 15 min at 4000 × g, 1 ml of the supernatant was homogenized with 1 ml of Folin–Ciocalteu reagent and 2 ml of 15% Na2CO3 (w/v). After incubation at room temperature in the dark for 50 min, the absorbance at 765 nm was detected. Pyrogallol was used to obtain the standard curve.
Determination of the ascorbate and glutathione contents
The ascorbate (ASA) and glutathione (GSH) contents were measured as follows: A quantity of 0.2 g of fresh sample was triturated with liquid nitrogen and mixed with 3 ml of chilled extraction buffer (5% meta-phosphoric acid dissolved with 1 mM EDTA solution). After centrifugation at 4 °C for 20 min at 12000 g, the supernatants were moved to new tubes and used for detecting the ascorbate and glutathione contents (Hasanuzzaman et al. 2011).
For ASA the content measurements, 0.3 ml of the supernatant was mixed with 750 μl of 0.15 M phosphate buffer (pH of 7.4, with 5 mM EDTA), 0.6 ml of TCA (10%), 0.6 ml of H3PO4 (44%), 0.6 ml of 2,2′-dipyridyl (4%, dissolved in 70% ethanol), and 0.15 ml of FeCl3 (0.3%) before being incubated for 30 min at 40 °C. The absorbance at 525 nm was recorded. For the GSH content measurements, 2.6 ml of 150 mM phosphate buffer (pH of 7.7) and 0.15 ml of DTNB were mixed with 0.25 ml of supernatant. After incubation for 5 min at 30 °C, the absorbance at 412 nm was recorded. ASA and GSH were used to establish the respective standard curves.
Determination of APX and GR activity
0.2 g of fresh sample was triturated with liquid nitrogen and mixed it with 2 ml of chilled phosphate buffer (100 mM, pH of 7.0, containing 20 mM EDTA-Na2 and 10% polyvinyl pyrrolidone) before centrifuging it at 4 °C for 15 min at 12000 × g. The supernatant (i.e., the enzyme extract) was then used to analyze the activities of ascorbate peroxidase (APX) and glutathione reductase (GR) (Hasanuzzaman et al. 2017).
To measure the APX activity, the supernatant was reacted with 100 mM phosphate buffer (pH of 7.0), 0.1 mM EDTA, 0.1 mM H2O2, and 0.5 mM AsA, and the changes in the absorbance at 412 nm were recorded.
To measure the GR activity, enzyme extract was reacted with 100 mM phosphate buffer (pH of 7.0), 20 mM EDTA-Na2, 5 mM GSSG, and 1.5 mM NADPH, and then it was used to measure the changes in the absorbance at 340 nm.
Statistical analysis
The data were analyzed by two-way analysis of variance by Duncan’s multiple comparison test (P < 0.05) using with SPSS 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Elevated CO2 attenuated the growth inhibition of the rice caused by Cd
Here, the effects on the rice of different Cd concentrations were analyzed, and their growth was inhibited when treated with 1 μM Cd, and the relative shoot elongation and relative root elongation were inhibited approximately 50% in two cultivars (Fig. S1). The Cd concentrations in the roots and shoots of both cultivars showed that YD had the stronger ability to transport Cd from the roots to the shoots, although the Cd concentrations in the roots of the YD rice were significantly higher than those of the NG rice (Fig. S2). With the changes of relative root elongation under different Cd concentrations together, we selected 5 μM of Cd to proceed follow-up experiments.
To identify the combined effects of the elevated CO2 and Cd exposure on the rice, we used Yangdao No. 6 (YD) and Ninggeng No. 7 (NG), which belong to the indica and japonica rice subspecies, respectively. The seedlings were grown at three different CO2 conditions as follows: (1) grown at ambient CO2 levels (AC); (2) grown at ambient CO2 levels for 6 days and then at elevated CO2 levels, which we named elevate CO2 condition 1 (EC1); and (3) grown at elevated CO2 levels, which we named elevate CO2 condition 2 (EC2).
The fresh weights and dry weights of the shoots of two cultivars were significantly increased by approximately 20% for both EC1 and EC2 compared to the AC rice (P < 0.05) (Fig. 1 a–c). The different elevated CO2 conditions (EC1 and EC2) did not result in significant differences in the fresh weights and dry weights of the shoots of the two rice cultivars. A similar trend was observed for the root weights, but the increases in root weights enhanced for the EC1 and EC2 samples were not as big as the increases in the shoot weights (Fig. 1d–e). Elevated CO2 conditions (EC1 and EC2) attenuated the inhibition caused by the Cd treatment (Fig. 1a, b, and d). In the indica rice, NG, under Cd stress, the elevated CO2 treatment restored the fresh weights of both the shoots and roots to the levels of the rice grown at ambient CO2 without Cd treatment (Fig. 1b and d). These results suggested that elevated CO2 attenuated the growth inhibition of the rice caused by Cd treatment.
Phenotypes and biomasses of the rice under the Cd and CO2 treatments: a phenotypes, b fresh weights of the shoots, c dry weights of the shoots, d fresh weights of the roots, and e dry weights of the roots. NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC1, growth in the chamber of ambient CO2 concentration and then transferred to elevated CO2 concentration chamber treated with Cd; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above the bars indicate significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
Elevated CO2 levels promoted Cd transportation from the roots to the shoots
The Cd concentrations in the shoots and roots of the rice exposed to Cd were analyzed (Fig. 2). The Cd concentrations in the NG shoots were significantly lower than those of the YD shoots, regardless of the CO2 condition used (Fig. 2a). The Cd concentrations in the shoots of the NG rice grown at elevated CO2 conditions (EC1 and EC2) were significantly increased compared with the NG rice grown at an ambient CO2 condition (AC). Interestingly, the Cd concentrations in the NG roots grown at an ambient CO2 condition (AC) were higher than those of the YD rice grown at elevated CO2 conditions (EC1 or EC2) (Fig. 2b), resulting in the lowest Cd translocation factor (the Cd concentration ratio between the shoots and roots) at AC (Fig. 2c). Although a similar trend was observed for the Cd concentrations in the YD rice roots, the Cd concentrations in the YD shoots did not show significant differences at either CO2 condition, leading to similar Cd translocation factors for the YD rice for all the three CO2 conditions. In addition, the Cd translocation factors for the YD rice at all three CO2 conditions were significantly higher than those of the NG rice (Fig. 2c). The Cd accumulations in the shoots (Fig. 2d), roots (Fig. 2e), and whole seedling (Fig. 2f) significantly increased at the elevated CO2 concentrations (EC1 and EC2). This revealed that elevated CO2 conditions enhanced Cd accumulation and promoted Cd translocation from the roots to the shoots of the rice.
Cd concentrations, translocation factors, and accumulations of rice under the Cd and CO2 treatments. a Cd concentrations in the shoots, b Cd concentrations in the roots, c Cd translocation factors, d Cd accumulations in the shoots, e Cd accumulations in the roots, and f total Cd accumulations. NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC1, growth in the chamber of ambient CO2 concentration and then transferred to elevated CO2 concentration chamber treated with Cd; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above the bars indicate the significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
Elevated CO2 concentrations increased the contents of the photosynthetic pigments
Compared with the AC treatment, the elevated CO2 concentrations (EC1 and EC2) significantly increased the contents of the photosynthetic pigments in the two cultivars, and the contents of the photosynthetic pigments in the two cultivars were significantly reduced in response to Cd stress (AC + Cd), while the elevated CO2 concentration significantly boosted the contents of the photosynthetic pigments of the two cultivars under Cd stress (Fig. 3). In particular, the carotenoid contents of the two cultivars did not show any significant differences for the AC, EC1, or EC2 treatments, but when the seedlings were stressed by the Cd treatment, the carotenoid contents of the NG rice were significantly higher than those of the YD rice for each of the AC + Cd, EC1 + Cd, and EC2 + Cd treatments (Fig. 3c). The two cultivars showed similar patterns.
Contents of the photosynthetic pigments of the rice under the Cd and CO2 treatments: a chlorophyll a (Chl a), b chlorophyll b (Chl b), c carotenoid (Car), and d total chlorophyll (total Chl). NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC1, growth in the chamber of ambient CO2 concentration and then transferred to elevated CO2 concentration chamber treated with Cd; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above each bar indicate the significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
Elevated CO2 concentrations changed the gas exchange parameters of the rice leaves
The net photosynthetic rates (Pn) (Fig. 4a) and intercellular CO2 concentrations (Ci) (Fig. 4b) of the rice leaves were significantly increased under the elevated CO2 concentrations (EC1 and EC2) compared with the AC treatment (Fig. 4a). While the Pn of the AC treatment was significantly greater than the Pn of the AC + Cd treatment and the elevated CO2 concentrations (EC1 + Cd and EC2 + Cd) significantly contributed to the Pn but could not rescue the concentrations of only the elevated CO2 treatments (EC1 and EC2), the Pn values of the elevated CO2 concentrations (EC1 + Cd and EC2 + Cd) remained significantly lower compared to those without elevated CO2 concentrations and without Cd exposure (EC1 + Cd and EC2 + Cd) (Fig. 4a). The intercellular CO2 concentration (Ci) met the concentration of the CO2 treatment (Fig. 4b). Only the elevated CO2 concentration, the stomatal conductance (gs) (Fig. 4c), and transpiration rate (Tr) (Fig. 4d) were significantly reduced, and we found the same results for the Cd exposure cases (AC + Cd compared with the EC1 + Cd or EC2 + Cd). The gs of the AC + Cd treatment was significantly lower compared with that of the control (AC), but the elevated CO2 concentrations did not significantly affect the gs under Cd stress (EC1 compared with EC1 + Cd and EC2 compared with EC2 + Cd) (Fig. 4c). The Tr showed a similarly tendency, but Cd stress did not significantly inhibit the Tr (AC compared with AC + Cd) (Fig. 4d). The two cultivars showed similar patterns.
The gas exchange parameters of rice leaf under Cd and CO2 treatment. a Pn (net photosynthetic rate), b Ci (intercellular CO2 concentration), c gs (stomatal conductance), d Tr (transpiration rate). NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC1, growth in the chamber of ambient CO2 concentration and then transferred to elevated CO2 concentration chamber treated with Cd; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above each bar indicated significant differences at Duncan’s test (P < 0.05). Values are means ± SD (n = 4)
Elevated CO2 concentrations reduced the MDA, O2 −, and H2O2 accumulations in the rice under Cd stress
Cd stress caused MDA, O2−, and H2O2 to accumulate in the rice (Fig. 5). In detail, compared with the AC treatment, only the EC2 elevated CO2 concentration did not significantly influence the MDA, O2−, and H2O2 accumulations in the rice shoots and roots. However, when the seedlings were exposed to Cd, the elevated CO2 concentrations significantly reduced the MDA, O2−, and H2O2 accumulations in the rice shoots and roots, except the root O2− contents of the NG rice, which were lower than those of the AC + Cd treatment. The two cultivars showed similar patterns, whereas when the seedlings were treated with Cd, the MDA, O2−, and H2O2 accumulations in the NG rice were significantly lower than those of the YD rice, except for the shoots’ MDA and H2O2 contents under the EC2 + Cd treatment. Also, the roots of rice seedlings were stained by DAB, NBT, and Evans blue to analyze the O2−, and H2O2 accumulations and plasma membrane integrity. The images of histochemical staining roots were shallower treated with EC2 compared with control (AC), but darker treated with Cd (AC+Cd) compared with control (AC), and further treated with elevated CO2 (EC2+Cd) rescued the darker tendence, and the two cultivars showed similar patterns (Fig. S3).
The MDA, H2O2, and O2− contents of the rice under the Cd and CO2 treatments: a MDA contents of the shoots, b H2O2 contents of the shoots, c O2− contents of the shoots; d MDA contents of the roots, e H2O2 contents of the roots, and f O2− contents of the roots. NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above each bar indicate significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
Elevated CO2 concentrations increased the NPT and total phenolic contents
The NPT contents of the shoots were significantly increased in the rice treated with elevated CO2 concentrations compared with the AC treatment. Moreover, under Cd stress, the NPT contents of the shoots (AC + Cd) were further significant increased compared with the AC and EC2 treatments, and the NPT contents of the EC2 + Cd treatment were significantly higher than those of the AC + Cd treatment (Fig. 6a). For the rice treated with elevated CO2 concentrations, the NPT contents of the roots showed consistent tendencies compared to those of the shoots, but under Cd stress, at either of the elevated CO2 concentrations, the NPT contents of the roots did not show any significant differences between the AC + Cd and EC2 + Cd treatments, and the NPT contents of the roots of the YD rice were significantly lower than those of the NG rice (Fig. 6c).
NPT and total phenolic contents of the rice under the Cd and CO2 treatments: a NPT contents of the shoots, b total phenolic contents of the shoots, c NPT contents of the roots, and d total phenolic contents of the roots. NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above each bar indicate significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
The total phenolic contents in the shoots of the rice under the EC2 treatment were significantly higher than those of the AC treatment for both two cultivars, and under Cd stress, the elevated CO2 concentrations still significantly increased the total phenolic contents (Fig. 6b). The total phenolic contents in the roots of the NG rice showed similar patterns to those of the shoots, but regardless of the treatment, the total phenolic contents in the roots of the NG rice were significantly higher than those of the YD rice (Fig. 6d).
Elevated CO2 concentrations increased the ASA and GSH contents of the rice
The ASA contents in the roots and shoots of both cultivars treated with elevated CO2 concentrations were significantly increased compared with the control (AC), and under Cd stress, the elevated CO2 concentration (EC2 + Cd) significantly increased the ASA contents in the roots and shoots compared with the Cd treatment (AC + Cd) (Fig. 7a and c). In addition, Cd exposure affected the ASA contents, and regardless of whether the AC or elevated CO2 concentration treatments were applied, the ASA contents in the roots and shoots of the YD rice were significantly reduced (Fig. 7a and c).
ASA and GSH contents of the rice under the Cd and CO2 treatments: a ASA contents in the shoots, b GSH contents in the shoots, c ASA contents in the roots, and d GSH contents in the roots. NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above each bar indicate significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
The GSH contents in the shoots and roots of the two rice cultivars were significantly increased when treated with Cd, and the elevated CO2 concentrations increased the GSH contents in the two cultivars (Fig. 7b and d). The GSH contents in the roots of the two cultivars treated with EC2 were significantly increased compared to those of the AC treatment (Fig. 7d), but they did not show significant increases in the shoots (Fig. 7b).
Elevated CO2 concentrations enhanced the APX and GR activities
The APX activity in the shoots of the NG rice did not show significantly differences among the AC, EC2, and AC + Cd treatments, but the EC2 + Cd treatment significantly promoted APX activities in the shoots, and only the elevated CO2 concentrations significantly enhanced the APX activities in the shoots of the YD rice. Cd exposure affected APX activity, but the APX activity in the shoots of the YD rice treated with EC2 + Cd was significantly higher than the other treatments of the YD rice (Fig. 8a). There were no significant differences between the APX activities of the AC and AC + Cd treatments of the NG rice, and the EC2 and EC2+Cd treatments of the NG rice showed the same patterns. Elevated CO2 concentrations (EC2) significantly increased the APX activity in the roots of the two rice cultivars, but the APX activity in the roots of the YD rice was significantly inhibited under Cd stress (AC + Cd), and when further treated with elevated CO2 concentrations (EC2 + Cd), the APX activity significantly increased compared with the AC + Cd treatment, though this increase was not significantly higher than that of the control (AC) (Fig. 8c).
The APX and GR activities of the rice under the Cd and CO2 treatments: a APX activity in the shoots, b GR activity in the shoots, c APX activity in the roots, and d GR activity in the roots. NG, Ninggeng No. 7, indica rice; YD, Yangdao No. 6, japonica rice; AC, ambient concentration; EC2, growth in the chamber of elevated CO2 concentration from beginning to end. The different letters above each bar indicate significant differences with Duncan’s test (P < 0.05). The values shown are the means ± SDs (n = 4)
Overall, elevated CO2 concentrations increased GR activity in the two cultivars in both the shoots and roots, and Cd exposure also increased GR activity. The two factors worked together and further enhanced GR activity (Fig. 8b and d). In detail, the GR activity in the shoots of the NG rice between the AC and EC2 treatments did not show significant differences, but the GR activity was significantly increased under Cd stress (AC + Cd). In addition, when treated with elevated CO2 concentrations and Cd, the GR activity was significantly enhanced (Fig. 8b). For the YD cultivar, the GR activity was significantly increased step by step when consecutively treated with EC2, Cd, and EC2 + Cd (Fig. 8b). The GR activity in the roots of the two cultivars showed a similar pattern to the GR activity in the shoots, whereas the GR activity in the roots of the YD rice under the AC + Cd treatment was not significantly higher than that of the EC treatment (Fig. 8d).
Discussion
In this study, we selected an indica cultivar (YD) and a japonica cultivar (NG). Both two cultivars are widely planted in the Middle and Lower Yangtze River areas of China. We analyzed the effects on the two rice cultivars under different Cd concentrations. The two cultivars showed significant differences in shoot elongation, root elongation, shoot biomass, root biomass, shoot Cd concentration, root Cd concentration, and Cd translocation factor. The Cd concentrations in the roots and shoots of the two cultivars also indicated that the indica rice could accumulate more Cd in its grain than that of the japonica rice (Yan et al. 2019). The roots were firstly faced with Cd exposure (Song et al. 2018). We based on treatment volumes on root lengths and chose 5 μM Cd as the Cd stress treatment for the follow-up experiments.
To analyze the rice growth differences between the elevated CO2 concentrations, combined with the elevated CO2 concentration and the Cd stress treatments, we applied two elevated CO2 concentration approaches (EC1 and EC2). According to the biomass analysis, the elevated CO2 concentrations both promoted plant growth, and the “CO2 fertilization” has been widely proven, with previous studies including Eucalyptus trees (Wujeska-Klause et al. 2019), grass (Andresen et al. 2018), and so on. We then detected the Cd concentrations, which showed that elevated CO2 concentrations reduced Cd concentrations in the roots but promoted Cd translocation from the roots to the shoots. Although plants could block largely Cd in root tissues by the mechanisms of cell-wall binding, complexation, and vacuolar sequestration (Tang et al. 2023), the Cd TFs in this study were quite low, but still some Cd could be transfer to shoot and reached to the toxic level to rice. In addition, the elevated CO2 concentrations increased the biomasses of shoot and root. As a result, elevated CO2 concentrations led to the significantly increases of the Cd accumulations both in the shoots and roots of the rice, and total Cd accumulations of whole plant were also significantly increased compared with control. Up to here, we had a simple but serious problem that elevated CO2 concentrations would enhance the ability of rice to absorb Cd and transport Cd to shoot. Also, previous studies found the similar results. For Lolium perenne, researchers found that applying CO2 enhanced the Cd uptake (Song et al. 2015) and enhanced the phytoremediation efficiency of ryegrass in Cd-polluted soils (Guo et al. 2014). But here, we used hydroponics experiments; the results cannot be clearly explained by the changes of the bioavailability of Cd, soil pH, and microbial biomasses as previous studies. And another toxic metal, arsenic (As), its change of bioavailability was opposite with Cd in response to the change of soil pH; the As concentration of rice grain would be also increased by elevated CO2 (Muehe et al. 2019). In addition, the “dilution effect” could not answered the results yet, because the correlation of different element concentrations depended ever more on plant species and cultivars (Yi et al. 2023). So, it was thought that CO2 maybe provides a signal which motivated the Cd uptake by root and Cd transport within the plants, or CO2 induced signal molecule (e.g. NO) accumulation to answer this effect (Wang et al. 2013). Because of the serious toxicity of Cd, many researches have tried to reduce the Cd accumulation in edible parts of crops to protect people from Cd, especially for rice (McGrath 2022). But Cd pollution of paddy field was not easily scavenged; the Cd accumulated in edible parts of crops can only try to reduce. With the atmosphere CO2 concentrations increased in the future, the Cd pollution of crops should threat human health. Thus, Cd pollution should receive attention in future studies on elevated CO2 concentrations.
The photosynthetic pigments and leaf gas exchange parameters showed that the elevated CO2 concentrations caused increased photosynthesis in the plants (Thompson et al. 2017). The patterns for the changes of photosynthetic pigment contents were consistent with the changes of Pn values. Moreover, because of the treatments of elevated CO2 concentrations, Ci and its related attributes, gs and Tr, showed main variances between control and Cd stress; furthermore, the tendency of Pn, gs, and Tr indicated the decrease of water loss and enhancement of the water use efficiency. Elevated CO2 concentrations and Cd altered the carbon gain and water loss abilities of plants. Overall, elevated CO2 concentrations would improve the photosynthesis efficiency, but Cd exposure weakened the benefits of elevated CO2.
The approaches of two elevated CO2 concentrations showed similar results, and elevated CO2 concentration in advance showed greater risks than the treatments with simultaneous Cd exposure; in addition, the culture condition of elevated CO2 concentration in advance was more aligned with the future environment. Therefore, we treated the seedlings with the elevated CO2 concentrations when the seedlings were transferred to hydroponic boxes for the experiments. Cd toxicity causes the overproduction of ROS which would cause plants suffer from ROS stress (Huang et al. 2019). We measured the MDA contents and ROS accumulations. Also, we analyzed plasma membrane integrity of roots by Evans blue. The MDA measurements were an index of lipid peroxidation (Ali et al. 2014). Cd stress significantly induced an over-accumulation of O2− and H2O2 and an increase in the MDA contents in the roots and shoots of the plants and caused the cell death of plasma membrane. To deal with the ROS stress, plants evolved the antioxidant systems to scavenging ROS. The plant’s antioxidant systems include non-enzymatic antioxidants and antioxidant enzymes (Nahar et al. 2016). Many studies have reported that antioxidant enzymes play important roles in Cd detoxification (Naliwajski and Sklodowska 2021). The presence of antioxidant enzymes suggested that the plants’ growth was affected when they were treated by elevated CO2 concentrations (Gillespie et al. 2011). Cd-induced oxidative stress has been shown to be modulated by the AsA-GSH pathway (Hasanuzzaman et al. 2017). NPTs including several acid-soluble sulfhydryl components such as GSH, PC (phytochelatins), and cysteine, have been shown to play an important role in plant detoxification (in response to toxic metal exposure) (Shi et al. 2022). Here, we found that the NTPs mainly functioned in rice seedling Cd detoxification, and elevated CO2 concentrations had litter effect on NPT content. Both the Cd stress and the elevated CO2 concentrations increased phenolic accumulation in this study. Yet, there were evidences suggested that the phenolic accumulation was likely to happen during the rice maturity stage (Goufo et al. 2014). As for the litter attention on non-enzymatic antioxidants, we analyzed the non-enzymatic antioxidants and the APX and GR activities in the AsA-GSH cycle. The ASA and GSH contents showed that the elevated CO2 concentrations mainly affected the ASA contents and the GSH contents were mainly affected by the Cd exposure. The APX and the GR activities also indicated that the elevated CO2 concentrations mainly enhanced the plants’ non-enzymatic antioxidant systems. Past research has also indicated that plants have reduced the negative effects of drought mainly by maintaining their total antioxidants (Ulfat et al. 2021). Taken together, elevated CO2 concentrations could enhance the antioxidant capacity ability of rice to promote plant growth, Cd would cause rice suffer ROS stress, and elevated CO2 concentrations alleviated the stress by scavenging ROS.
Conclusions
Although many previous studies have indicated that elevated CO2 concentrations have a strong positive impact on crop yields due to the “CO2 fertilization” effect, a growing number of studies have shown the challenge of the negative impacts on crop quality where the crop could not cope with the demand for nutrients under elevated CO2 concentrations. In addition, through large-scale and long-term experiments, the seasonal precipitation (Hovenden et al. 2019) and intensifying drought cycles (Gray et al. 2016) may eliminate growth in crop yields, and predicting the carbon feedback to plants, even with the best-supported short-term models, might not effectively predict long-term results (Reich et al. 2018).
In our study, elevated CO2 concentrations enhanced the photosynthetic capacity to promote plant growth, and the ASA–GSH cycle enhanced the activity of antioxidants induced by elevated CO2 concentrations which contributed to Cd detoxification. In addition, elevated CO2 concentrations affected Cd translocation, especially in the indica rice. We suggested that CO2 played a role of a signal molecule which regulated the ion transportation and distribution mechanisms in rice. In conclusion, a promising strategy for identifying the mechanisms of Cd uptake and transfer is still needed for a future high-CO2 world to maintain food security and food safety.
Data availability
The data that support the findings of this study are available from the first author on reasonable request.
References
AbdElgawad H, Schoenaers S, Zinta G, Hassan YM, Abdel-Mawgoud M, Alkhalifah DHM, Hozzein WN, Asard H, Abuelsoud W (2021) Soil arsenic toxicity differentially impacts C3 (barley) and C4 (maize) crops under future climate atmospheric CO2. J Hazard Mater 414. https://doi.org/10.1016/j.jhazmat.2021.125331
Ainsworth EA, Long SP (2021) 30 years of free-air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Glob Chang Biol 27:27–49. https://doi.org/10.1111/gcb.15375
Ali E, Maodzeka A, Hussain N, Shamsi IH, Jiang L (2014) The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul 75:641–655. https://doi.org/10.1007/s10725-014-9966-0
Andresen LC, Yuan N, Seibert R, Moser G, Kammann CI, Luterbacher J, Erbs M, Müller C (2018) Biomass responses in a temperate European grassland through 17 years of elevated CO2. Glob Chang Biol 24:3875–3885. https://doi.org/10.1111/gcb.13705
Beleggia R, Fragasso M, Miglietta F, Cattivelli L, Menga V, Nigro F, Pecchioni N, Fares C (2018) Mineral composition of durum wheat grain and pasta under increasing atmospheric CO2 concentrations. Food Chem 242:53–61. https://doi.org/10.1016/j.foodchem.2017.09.012
Bouain N, Cho H, Sandhu J, Tuiwong P, Prom UTC, Zheng L, Shahzad Z, Rouached H (2022) Plant growth stimulation by high CO2 depends on phosphorus homeostasis in chloroplasts. Curr Biol 32(4493-4500):e4494. https://doi.org/10.1016/j.cub.2022.08.032
Dier M, Huther L, Schulze WX, Erbs M, Kohler P, Weigel HJ, Manderscheid R, Zorb C (2020) Elevated atmospheric CO2 concentration has limited effect on wheat grain quality regardless of nitrogen supply. J Agric Food Chem 68:3711–3721. https://doi.org/10.1021/acs.jafc.9b07817
Fang Z, Hu Z, Yin X, Song G, Cai Q (2020) Exogenous glutathione alleviation of Cd toxicity in Italian Ryegrass (Lolium multiflorum) by modulation of the Cd absorption, subcellular distribution, and chemical form. Int J Environ Res Public Health 17:8143. https://doi.org/10.3390/ijerph17218143
Gillespie KM, Rogers A, Ainsworth EA (2011) Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J Exp Bot 62:2667–2678. https://doi.org/10.1093/jxb/erq435
Giri A, Armstrong B, Rajashekar CB (2016) Elevated carbon dioxide level suppresses nutritional quality of lettuce and spinach. Am J Plant Sci 07:246–258. https://doi.org/10.4236/ajps.2016.71024
Gojon A, Cassan O, Bach L, Lejay L, Martin A (2022) The decline of plant mineral nutrition under rising CO2: physiological and molecular aspects of a bad deal. Trends Plant Sci 28:185–198. https://doi.org/10.1016/j.tplants.2022.09.002
Goufo P, Pereira J, Moutinho-Pereira J, Correia CM, Figueiredo N, Carranca C, Rosa EAS, Trindade H (2014) Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ Exp Bot 99:28–37. https://doi.org/10.1016/j.envexpbot.2013.10.021
Gray SB, Dermody O, Klein SP, Locke AM, McGrath JM, Paul RE, Rosenthal DM, Ruiz-Vera UM, Siebers MH, Strellner R, Ainsworth EA, Bernacchi CJ, Long SP, Ort DR, Leakey AD (2016) Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat Plants 2:16132. https://doi.org/10.1038/nplants.2016.132
Guo J, Feng R, Ding Y, Wang R (2014) Applying carbon dioxide, plant growth-promoting rhizobacterium and EDTA can enhance the phytoremediation efficiency of ryegrass in a soil polluted with zinc, arsenic, cadmium and lead. J Environ Manage 141:1–8. https://doi.org/10.1016/j.jenvman.2013.12.039
Hasanuzzaman M, Hossain MA, Fujita M (2011) Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep 5:353–365. https://doi.org/10.1007/s11816-011-0189-9
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front. Plant Sci 8:1061. https://doi.org/10.3389/fpls.2017.01061
Hovenden MJ, Leuzinger S, Newton PCD, Fletcher A, Fatichi S, Luscher A, Reich PB, Andresen LC, Beier C, Blumenthal DM, Chiariello NR, Dukes JS, Kellner J, Hofmockel K, Niklaus PA, Song J, Wan S, Classen AT, Langley JA (2019) Globally consistent influences of seasonal precipitation limit grassland biomass response to elevated CO2. Nat Plants 5:167–173. https://doi.org/10.1038/s41477-018-0356-x
Huang H, Li T, Tian S, Gupta DK, Zhang X, Yang X (2008) Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Biores Technol 99:6088–6096. https://doi.org/10.1016/j.biortech.2007.12.056
Huang Y, Zhu Z, Wu X, Liu Z, Zou J, Chen Y, Su N, Cui J (2019) Lower cadmium accumulation and higher antioxidative capacity in edible parts of Brassica campestris L. seedlings applied with glutathione under cadmium toxicity. Environ Sci Pollut Res 26:13235–13245. https://doi.org/10.1007/s11356-019-04745-7
Li F, Xiao J, Chen J, Ballantyne A, Jin K, Li B, Abraha M, John R (2023) Global water use efficiency saturation due to increased vapor pressure deficit. Science 381:672–677. https://doi.org/10.1126/science.adf5041
Li X, Lin J, Gao Y, Han W, Chen D (2012) Antioxidant activity and mechanism of Rhizoma cimicifugae. Chem Cent J 6:140. https://doi.org/10.1186/1752-153X-6-140
Loladze I (2014) Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife 3:e02245. https://doi.org/10.7554/eLife.02245
Martins N, Gonçalves S, Romano A (2013) Aluminum inhibits root growth and induces hydrogen peroxide accumulation in Plantago algarbiensis and P. almogravensis seedlings. Protoplasma 250:1295–1302. https://doi.org/10.1007/s00709-013-0511-1
McGrath SP (2022) Keeping toxic cadmium out of the food chain. Nat Food 3:569–570. https://doi.org/10.1038/s43016-022-00579-8
Muehe EM, Wang T, Kerl CF, Planer-Friedrich B, Fendorf S (2019) Rice production threatened by coupled stresses of climate and soil arsenic. Nat Commun 10:4985. https://doi.org/10.1038/s41467-019-12946-4
Myers SS et al (2014) Increasing CO2 threatens human nutrition. Nature 510:139–142. https://doi.org/10.1038/plants13179
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255. https://doi.org/10.1016/j.ecoenv.2015.12.026
Naliwajski M, Sklodowska M (2021) The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells 10:609. https://doi.org/10.3390/cells10030609
Nie J, Liu Y, Zeng G, Zheng B, Tan X, Liu H, Xie J, Gan C, Liu W (2016) Cadmium accumulation and tolerance of Macleaya cordata: a newly potential plant for sustainable phytoremediation in Cd-contaminated soil. Environ Sci Pollut Res 23:10189–10199. https://doi.org/10.1007/s11356-016-6263-7
Reich PB, Hobbie SE, Lee TD, Pastore MA (2018) Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 360:317–320. https://doi.org/10.1126/science.aas9313
Sage RF, Khoshravesh R (2016) Passive CO2 concentration in higher plants. Curr Opin Plant Biol 31:58–65. https://doi.org/10.1016/j.pbi.2016.03.016
Seibert R, Andresen LC, Jarosch KA, Moser G, Kammann CI, Yuan N, Luterbacher J, Laughlin RJ, Watson CJ, Erbs M, Müller C (2021) Plant functional types differ in their long-term nutrient response to eCO2 in an extensive grassland. Ecosystems 25:1084–1095. https://doi.org/10.1007/s10021-021-00703-y
Shah AA, Ahmed S, Ali A, Yasin NA (2020) 2-Hydroxymelatonin mitigates cadmium stress in Cucumis sativus seedlings: modulation of antioxidant enzymes and polyamines. Chemosphere 243:125308. https://doi.org/10.1016/j.chemosphere.2019.125308
Shi G, Liu H, Zhou D, Zhou H, Fan G, Chen W, Li J, Lou L, Gao Y (2022) Sulfur reduces the root-to-shoot translocation of arsenic and cadmium by regulating their vacuolar sequestration in wheat (Triticum aestivum L.). Front. Plant Sci 13:1032681. https://doi.org/10.3389/fpls.2022.1032681
Singh SK, Reddy VR (2014) Combined effects of phosphorus nutrition and elevated carbon dioxide concentration on chlorophyll fluorescence, photosynthesis, and nutrient efficiency of cotton. J Plant Nutr Soil Sci 177:892–902. https://doi.org/10.1002/jpln.201400117
Song G, Yuan S, Wen X, Xie Z, Lou L, Hu B, Cai Q, Xu B (2018) Transcriptome analysis of Cd-treated switchgrass root revealed novel transcripts and the importance of HSF/HSP network in switchgrass Cd tolerance. Plant Cell Rep 37:1485–1497. https://doi.org/10.1007/s00299-018-2318-1
Song N, Ma Y, Zhao Y, Tang S (2015) Elevated ambient carbon dioxide and Trichoderma inoculum could enhance cadmium uptake of Lolium perenne explained by changes of soil pH, cadmium availability and microbial biomass. Appl Soil Ecol 85:56–64. https://doi.org/10.1016/j.apsoil.2014.09.007
Song Y, Wang Y, Mao W, Sui H, Yong L, Yang D, Jiang D, Zhang L, Gong Y (2017) Dietary cadmium exposure assessment among the Chinese population. PloS ONE 12:e0177978. https://doi.org/10.1371/journal.pone.0177978
Tang Z, Wang HQ, Chen J, Chang JD, Zhao FJ (2023) Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J Integr Plant Biol 65:570–593. https://doi.org/10.1111/jipb.13440
Taub DR, Wang X (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50:1365–1374. https://doi.org/10.1111/j.1744-7909.2008.00754.x
Thompson M, Gamage D, Hirotsu N, Martin A, Seneweera S (2017) Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: a perspective on root sugar sensing and hormonal crosstalk. Front Physiol 8:578. https://doi.org/10.3389/fphys.2017.00578
Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84:63–69. https://doi.org/10.1016/j.chemosphere.2011.02.054
Ulfat A, Shokat S, Li X, Fang L, Großkinsky DK, Majid SA, Roitsch T, Liu F (2021) Elevated carbon dioxide alleviates the negative impact of drought on wheat by modulating plant metabolism and physiology. Agr Water Manage 250. https://doi.org/10.1016/j.agwat.2021.106804
Wang H, Xiao W, Niu Y, Jin C, Chai R, Tang C, Zhang Y (2013) Nitric oxide enhances development of lateral roots in tomato (Solanum lycopersicum L.) under elevated carbon dioxide. Planta 237:137–144. https://doi.org/10.1007/s00425-012-1763-2
Wang J, Yu N, Mu G, Shinwari KI, Shen Z, Zheng L (2017) Screening for Cd-safe cultivars of Chinese cabbage and a preliminary study on the mechanisms of cd accumulation. Int J Environ Res Public Health 14. https://doi.org/10.3390/ijerph14040395
Wang K, Xu F, Yuan W, Feng Z, Sun L, Xu W (2022) The G protein γ subunit is important for nitrogen uptake and grain yield in rice under elevated CO2. Plant Soil 482:543–551. https://doi.org/10.1007/s11104-022-05707-8
Wei L, Wang W, Zhu J, Wang Z, Wang J, Li C, Zeng Q, Ziska LH (2021) Responses of rice qualitative characteristics to elevated carbon dioxide and higher temperature: implications for global nutrition. J Sci Food Agric 101:3854–3861. https://doi.org/10.1002/jsfa.11021
Wujeska-Klause A, Crous KY, Ghannoum O, Ellsworth DS (2019) Lower photorespiration in elevated CO2 reduces leaf N concentrations in mature Eucalyptus trees in the field. Glob Chang Biol 25:1282–1295. https://doi.org/10.1111/gcb.14555
Yan H et al (2019) Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat Commun 10:2562. https://doi.org/10.1038/s41467-019-10544-y
Yi Q, Wang Y, Yi C, Li L, Chen Y, Zhou H, Tong F, Liu L, Gao Y, Shi G (2023) Agronomic and ionomics indicators of high-yield, mineral-dense, and low-Cd grains of wheat (Triticum aestivum L.) cultivars. Ecotoxicol Environ Saf 261:115120. https://doi.org/10.1016/j.ecoenv.2023.115120
Yu T, Chen Y (2019) Effects of elevated carbon dioxide on environmental microbes and its mechanisms: a review. Sci Total Environ 655:865–879. https://doi.org/10.1016/j.scitotenv.2018.11.301
Zhao FJ, Chang JD (2022) A weak allele of OsNRAMP5 for safer rice. J Exp Bot 73:6009–6012. https://doi.org/10.1093/jxb/erac323
Funding
This research was funded by the Jiangsu Science and Technology Support Program for Social Development, grant number BE2014709, and the Science and Technology Project of Jiangxi Provincial Department of Education, grant number GJJ200464.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Z.H., Y.Z., and Y.W. The first draft of the manuscript was written by Z.H., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2237 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Z., Zou, Y., Wang, Y. et al. Elevated carbon dioxide concentrations increase the risk of Cd exposure in rice. Environ Sci Pollut Res 30, 120300–120314 (2023). https://doi.org/10.1007/s11356-023-30646-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30646-x