Abstract
Elevated CO2 levels may alleviate toxicities induced by environmental stresses in plants, such as heavy metals. To assess this possibility, seedlings of Lolium perenne L. were exposed to different Cd stress and CO2 levels during hydroponic culture. The kinetics of growth, Cd accumulation, and thiol formation were investigated. Elevated CO2 levels increased the growth rate from 30 to 75%, and decreased the Cd accumulation rate from 36 to 42%, leading to a decrease of Cd content in root and shoot. However, an increase in Cd transport from root to shoot was observed at elevated CO2 under Cd stress. The production of phytochelatins (PCs) occurred earlier at elevated CO2 level than at ambient CO2 level after exposure to Cd stress. The mean SH/Cd ratio was relatively higher at elevated CO2 level, but elevated CO2 level significantly decreased thiol contents. The reduction of Cd contents, earlier production of PCs, and relatively higher SH/Cd ratio at the elevated CO2 level alleviated Cd toxicity in root and shoot to some extent, causing significant yield increase of L. perenne after exposure to Cd stress. This study could provide an important data support and theoretical basis in understanding the effects of elevated CO2 on plant growth, heavy metal accumulation, and thiol formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination, caused by mining, manufacturing, usage of synthetic products, and land application of industrial or domestic sludge, has becomes a serious environmental problem especially in some developing countries like China (Li et al. 2010; Luo et al. 2010). Cadmium (Cd) causes higher toxicity in plants than other heavy metals due to its solubility, mobility, absorption, and incorporation into tissues (Shi et al. 2009; Lou et al. 2013). However, plants have developed various ways to combat Cd-induced adverse effects, such as chelating by low molecular weight thiol such as cysteine (Cys), glutathione (GSH), and phytochelatins (PCs) which play crucial roles in detoxifying mechanisms (Yadav 2010; Sytar et al. 2013; Wu et al. 2013; Negrin et al. 2017). In addition, Cys and GSH were involved in PCs syntheses (Pal and Rai 2020; Wu et al. 2013). The syntheses of PCs are catalyzed by phytochelatin synthase (PCS) using GSH as substrate and metal ion as activator (Wang and Wang 2009; Branco et al. 2010; Etelvina and Victoria 2014), and dependent upon plant species, the toxic degree of metal ions and interactions among metals (Etelvina and Victoria 2014). It was reported that PCs production occurred earlier than any other physiological parameter and could be used as biochemical indicators/markers to assess metal toxicity to biota (Wang and Wang 2009; Branco et al. 2010; Ju et al. 2011). Morelli et al. (2009) found that the determination of PCs was a better approach than chemical analyses of metals.
The CO2 level in the atmosphere has been continuously rising due to the ongoing combustion of fossil fuels, resulting in the well-known consequences of the greenhouse effect and global climate change (Li et al. 2010; Jia et al. 2010). Plants adapt to elevated CO2 levels through alterations of physiological and physicochemical processes, and it was summarized that elevated CO2 level increased net photosynthetic rate and carbon assimilation, but decreased photorespiration and oxidative stress (Jia et al. 2010). The effects of elevated CO2 level on plant growth, development, and element uptake under Cu, Cd, or Cs stress have been investigated, and results generally demonstrated that elevated CO2 level alleviated the toxicities of heavy metals in plants to some extent (Li et al. 2010; Jia et al. 2010). However, very little information is available regarding the effects of elevated CO2 level on phytochelatins formation kinetics and Cd-induced growth inhibition in L. perenne.
On the basis of previous studies, it is hypothesized that elevated CO2 could alleviate Cd toxicity through increasing growth rate, reducing Cd accumulation rate and favoring thiol formation in plants. Therefore, the object of the present study was to investigate the kinetics of growth, Cd accumulation, and thiol formation in L. perenne exposed to Cd stress under ambient and elevated CO2 levels. The knowledge gained might constitute an important advancement in the understanding of the effects of elevated CO2 on plant growth, heavy metals accumulation, and formation of thiol especially PCs, and the mechanisms of elevated CO2 in terms of alleviation toxicities of heavy metals in plants, possibly providing evidence for potential phytoremediation practices.
Materials and methods
Plant growth and Cd exposure
Seeds of L. perenne were obtained from the Plant Protection Institute, Chinese Academy of Agricultural Sciences (CAAS), P.R. China. Seeds were soaked in 1% (w/v) sodium hypochlorite (NaClO) solution for 15 min, washed with deionized water several times, sown into moist mixture of vermiculite and perlite (v:v, 1:1), and kept in growth chamber at 25 °C, with 16/8 h photoperiod at 105 μmol·m-2 s-1 and a relative humidity of 60%.
One week after planting, the containers were filled with full Hoagland’s solution, and the culture medium was renewed once a week. The Hoagland’s solution consisted of 4 mmol·L-1 Ca (NO3)2·4H2O, 6 mmol·L-1 KNO3, 2 mmol·L-1 MgSO4·7H2O, 1 mmol·L-1 NH4H2PO4, 15 μmol·L-1 H3BO3, 1 μmol·L-1 MnSO4·4H2O, 0.5 μmol·L-1 ZnSO4·7H2O, 0.2 μmol·L-1 CuSO4·5H2O, 0.01 μmol·L-1 (NH4)6Mo7O24, and 100 μmol·L-1 Fe-EDTA. Solution pH was adjusted to about 6.5 by 0.1 mol·L-1 NaOH solution.
After growth for 2 weeks, healthy and uniform-sized seedlings were randomly transplanted into plastic containers (length 45 cm × width 15 cm × height 15 cm) with 10 L aerated hydroponic culture of half-strength Hoagland’s solution at a density of 10 seedlings per container, and kept in a controlled environmental room under the same condition as described above.
After growth for 3 weeks in full Hoagland’s solution, the seedlings were randomly sorted into two groups, and each set was randomly divided into three subsets, in which seedlings were exposed to 0, 20, or 80 μmol·L-1 of Cd. A solution Cd2+ was supplied with 2CdCl2·5H2O. Four independent repeats were performed with each pot.
During the experimental period, the two sets of seedlings were grown in two controlled environment rooms under the same conditions as described above with the exception of CO2 level. The carbon dioxide concentration in one chamber was maintained at 760 ± 28 μL·L-1 as elevated CO2, and in the other, maintained at 380 ± 12 μL·L-1 as ambient CO2, using an automatically controlled CO2 release facility connected to CO2 cylinders.
Sample collection
One of the ten seedlings in each container was randomly collected and separated into the root and shoot after 0, 1, 3, 6, 12, 24, 48, 72, 144, and 216 h of exposure to the Cd solution and CO2 level. The intensive monitoring of many time points was set to observe clearer change of dry weight of root and shoot of Lolium perenne L. seedlings. Root and shoot, respectively, were each cut into small pieces (less than 0.5 cm in length) with stainless steel scissors and randomly divided into several subsamples. One subsample (approximately 0.20 g) was wrapped with tin foil, frozen in liquid N2, and stored in dark at − 80 °C for thiol analyses. The remaining subsample was dried in an oven at 75 °C for 72 h, weighed, and ground into a fine, homogeneous powder using a stainless steel cutter-blender (IKA T2500, Germany) for Cd assay.
Cadmium quantification
Aliquots (approximately 0.30 g) of fine homogeneous powder were digested with 8:1 (v/v) HNO3/H2O2 mixture using a microwave digestion system (CEM MARS 240/50, USA), and analyzed for Cd content by an Atomic Absorption Spectrometer (ZEEnit 700, Analytik Jena, Germany). Calibration curves were established using a commercial standard solution at a concentration of 1000 mg·L−1 purchased from Sigma Chemical. The reliability of the digestion and analytical procedure was assessed using blanks and standards as QA/QC samples.
Extraction procedure and derivatization of thiol
The samples (approximately 0.20 g), previously stored in the dark at − 80 °C, were homogenized in liquid N2 using mortar and pestle, and 1.8 mL of extraction buffer containing 0.1% (v/v) TFA and 5 mmol·L-1 DTPA were added. The homogenates were transferred to a 2-mL centrifuge tube, vigorously mixed, and centrifuged at 12,000g for 10 min at 4 °C. The resulting supernatants were immediately put into derivatization of thiol.
The supernatant (250 μL) was transferred and mixed with 650 μL of 200 mmol·L-1 HEPES buffer (pH about 9.0) in 5 mmol·L-1 DTPA and 25 μL of 20 mmol·L-1 TCEP in HEPES buffer. This reaction mix was pre-incubated at room temperature (25 °C) for 5 min and the derivatization was performed by incubating the mix in dark for 30 min at room temperature (25 °C) after adding 20 μL of 50 mmol·L-1 mBBr in ACN. The reaction was terminated by adding 100 μL of 1 mol·L-1 MSA. The derivatized samples were filtered with 0.20-μm nylon syringe filters (Millipore Corp. Bedford, MA, USA) for HPLC analysis. The whole protocol was conducted in darkness and speed.
HPLC analysis of thiol
The separation of derivatized thiol was performed using an Agilent Technologies 1200 series HPLC system (Agilent Technologies Inc., Hambruecker Landstrasse, Waghaeusel-Wiesental, Germany) consisting of a quaternary pump with degasser, thermostat for ALS/FC/Spotter, thermostatted column compartment, diode array detector, fluorescence detector, and autosampler fitted with a 100-μL loop. The column was an Agilent Zorbax Eclipse XDB-C18 column (4.6 × 30 mm, 1.8 μm; Agilent Technologies Inc., Princeton, MN, USA). The temperatures of the sample capsule and the column oven were maintained at 4 °C and 25 °C, respectively. The excitation and emission wavelengths were set at 380 and 470 nm, respectively. Data were integrated using ChemStation software (Agilent Technologies Inc., Version B.03.02).
Derivatized samples (20, 50, or 100 μL) were run with linear gradient elution. Solvent A was 0.1% (v/v) TFA in water and solvent B was ACN. The gradient profile was described as 0–20 min, 8–26% B; 20–22 min, 26–100% B; 22–24 min, isocratic 100% B; 24–28 min, 100–8% B; 28–30 min, isocratic 8% B, and total analysis time was 30 min. The flow rate was 0.8 mL·min-1. All solvents were filtered with a 0.2-μm nylon filter (Nylaflo; Pall Corp., Ann Arbor, MI, USA) and degassed before use. The identification was carried out by comparing the traces obtained from blank (extract buffer), sample extract and standards of Cys, GSH (Fluka, Milwaukee, WI, USA), and PC2-6 (AnaSpec, San Jose, CA, USA), and the quantification was performed by external standard method.
Mathematical and statistical analysis
The root/shoot ratio is one measure to assess the overall health of plants and is obtained by comparing root dry weight to shoot dry weight. The tolerance index (Ti) (Jia et al. 2010) represents the ability of plants to survive exposed to Cd stress, and it is calculated according to the equation: Ti = average of root or shoot biomass exposed to Cd stress /average of root or shoot biomass in control. The translocation accumulation factor (TAF) represents the distribution of plant growth and heavy metal absorption in above ground and underground organs, as an important index to evaluate the remediation capacity of plants (Nie 2005), and TAF = Cd content in shoot × shoot biomass)/Cd content in root × root biomass (Lin et al. 2014). The SH/Cd ratio represents the chelating ability of SH in root or shoot and is calculated as total SH content in the root or shoot × fresh weight of root or shoot/ (Cd content in the root or shoot × dry weight of root or shoot).
All experiments were conducted with four replicates, and results are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using PASW Statistics (SPSS Inc., USA, and release 18.0.0) and the figure was drawn using Origin Pro (Origin Lab Corp., USA, v8.0724). Difference was significant at the 5% level.
Results
Plant growth
The growth of L. perenne seedlings was promoted under elevated CO2 and was inhibited after exposure to Cd stress (Fig. 1 and Table 1). Regardless of CO2 level and Cd concentration, the dry weight of L. perenne seedlings increased with time. During the time course, the average dry weight at elevated CO2 respectively increased by 5.22%, 8.73%, and 9.31% in roots at Cd concentrations of 0, 20, and 80 mol·L-1 compared to the ambient CO2 control. Meanwhile, the average dry weight at elevated CO2 respectively increased by 7.89%, 10.88%, and 15.05% in shoots at Cd concentrations of 0, 20, and 80 mol·L-1 compared to the ambient CO2 control.
Dry weight of root and shoot of Lolium perenne L. seedlings. Six-week-old seedlings were exposed to Cd at concentration of 0 (■), 20 (●), and 80 μmol·L-1 (▲) for 216 h at CO2 level of 380 μL·L-1 (solid line) and 760 μL·L-1(dash line), respectively. Points, means of four replicates. Vertical lines exceeding the symbols, SD
Previous studies have shown that root growth of both L. perenne and T. repens increased under elevated CO2 conditions (Jongen et al. 1995; Blagodatskaya et al. 2010). Shoot biomass at elevated CO2 significantly increased after 48 h for the same Cd concentration treatment (p < 0.05). Meanwhile, after treatment with the same Cd concentration and exposure time, the increase rate (%) in shoot stimulated by elevated CO2 was relatively greater than its in root (p < 0.05).
However, the growth inhibition of root and shoot was aggravated by longer exposure duration in each process, and more severity of growth inhibition was observed in root than its in shoot at different CO2 concentrations and Cd concentrations, the growth inhibition of root and shoot increased with the prolongation of exposure time, and more severity of growth inhibition was observed in roots than its in shoots (Fig. 2). That may be related to higher concentrations of Cd in the roots than in the shoots (Jia et al. 2011). The growth inhibition both in root and shoot became significant after 48 h of Cd exposure at ambient CO2, and 72 h at elevated CO2, respectively (Fig. 1). At elevated CO2, root biomass increased significantly after 72 h of exposure to 20 μmol·L-1 and 80 μmol·L-1 Cd stress compared to that at ambient CO2 (p < 0.05), and Cd control treatment did not increase. The biomass of shoot had significantly increased after 48 h at elevated CO2 of exposure to Cd stress and Cd control treatment. During the time course, Ti in roots generally began to decrease below 1 after 48 h at ambient CO2, and decreased with increasing of Cd concentration and treatment time (Table S1). At elevated CO2, the Ti in shoot generally started to be less than 1 after 72 h, and decreased with increasing of Cd concentration and treatment time (Table S1). Ti in roots and shoots at elevated CO2 was relatively greater than at ambient CO2 after exposure to the same Cd concentration and treatment time (Table S1). Therefore, elevated CO2 delayed the growth inhibition of L. perenne and increased the biomass of L. perenne.
Cd contents in root and shoot of L. perenne seedlings. Six-week-old seedlings were exposed to Cd at concentration of 20 (●) and 80 μmol·L-1 (▲) for 216 h at CO2 level of 380 μL·L-1 (solid line) and 760 μL·L-1(dash line), respectively. Points, means of four replicates. Vertical lines exceeding the symbols, SD
Accumulation of Cd
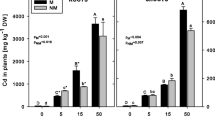
The accumulation of Cd in root and shoot was dependent upon CO2 level, Cd stress concentration, and exposure duration (Fig. 2 and Table 1). The Cd contents in root and shoot were lower at elevated CO2 than the ambient CO2 control for the same Cd concentration treatment, and the Cd contents in root were much higher than those in shoot at the same CO2 level. During the first 6 h, there was no significant difference in the Cd content in shoot treated both with 20 and 80 μmol·L-1 Cd (p > 0.05) (Fig. 2a). By contrast, the Cd contents in root exposed to 80 μmol·L-1 Cd were higher than those exposed to 20 μmol·L-1 Cd (Fig. 2b). It should be noted that after 24 h of Cd exposure, there was a significant reduction in the Cd contents in root and shoot at elevated CO2, compared to the ambient CO2 control (p < 0.05). Additionally, the reduction of Cd contents could alleviate the Cd-induced growth inhibition. After exposure to 20 μmol·L-1 and 80 μmol·L-1 Cd stress, the accumulation of Cd in root and shoot significantly increased with the exposure duration irrespective of the CO2 level. Furthermore, the effects of CO2 level, Cd concentration, and exposure time on TAFs were analyzed in the present study (Table 1), and it was found that the alteration of TAFs has a positive correlation with CO2 level (r = 0.179, p < 0.05), which meant that TAFs increased at elevated CO2 under Cd stress.
Syntheses of thiol
At the start of treatment, the content of GSH in shoot was (287.9 ± 33.6) nmol/g shoot fresh weight (mean ± SD, n = 24), and the GSH took account of total thiols in shoot about 90%. Meanwhile, the content of GSH in root was (34.0 ± 1.0) nmol/g root fresh weight (mean ± SD, n = 24), and the GSH took account of total thiols in root about 40%. After treatment with Cd, the proportion of GSH in root and shoot significantly reduced; however, the proportion in root was nearly 2.0% for 216 h, and always more than 50% in shoot. The proportion of PC2 in root firstly increased and then decreased, in shoot continually increased. In general, the proportion of PC3 in root and shoot presented increasing trend under Cd stress, especially after treatment of 12 h, the proportion of PC3 markedly increased, and that maintained about 70% of total thiols in root and 10% in shoot after treatment of 48 h. The proportion of PC4 and PC5 presented the same trend with PC3 in roots and shoots. The proportion of PC6 in roots firstly reduced and then slightly increased, and that altered slightly in shoots.
In most cases, the CO2 concentration had a negative effect on thiol content (Figs. 3, 4, 5, 6, 7, 8, and Figs. S3 and S4, and Table 1). During the first 24 h, the total contents of SH (the SH is functional group which is composed of sulfur atom and a hydrogen atom) in root increased first and then reduced irrespective of CO2 concentration with the exception of 80 μmol·L-1 Cd treatment at ambient CO2. After 24 h of Cd exposure, the contents of total SH in root rapidly increased (Fig. 3a). Regardless of Cd concentration in solution, no significant changes were found in the total SH contents in shoot during the first 12 h and 24 h at ambient and elevated CO2 level, and then continually increased (Fig. 3b).
SH contents in root and shoot of Lolium perenne L. seedlings. Six-week-old seedlings were exposed to Cd at concentration of 20 (●) and 80 μmol·L-1 (▲) for 216 h at CO2 level of 380 μL·L-1 (solid line) and 760 μL·L-1(dash line), respectively. Points, means of four replicates. Vertical lines exceeding the symbols, SD. In addition, it is hard to see the trend of the line from 0 to 12 h; thus, there is a magnified graph in the top left corner
Cys contents in root and shoot of Lolium perenne L. seedlings. Six-week-old seedlings were exposed to Cd at concentration of 20 (●) and 80 μmol·L-1 (▲) for 216 h at CO2 level of 380 μL·L-1 (solid line) and 760 μL·L-1(dash line), respectively. Points, means of four replicates. Vertical lines exceeding the symbols, SD
GSH contents in root and shoot of Lolium perenne L. seedlings. Six-week-old seedlings were exposed to Cd at concentration of 20 (●) and 80 μmol·L-1 (▲) for 216 h at CO2 level of 380 μL·L-1 (solid line) and 760 μL·L-1(dash line), respectively. Points, means of four replicates. Vertical lines exceeding the symbols, SD
PC2 contents in root and shoot of Lolium perenne L. seedlings. Six-week-old seedlings were exposed to Cd at concentration of 20 (●) and 80 μmol·L-1 (▲) for 216 h at CO2 level of 380 μL·L-1 (solid line) and 760 μL·L-1(dash line), respectively. Points, means of four replicates. Vertical lines exceeding the symbols, SD
Regardless of Cd concentration, the contents of Cys in root exhibited a reduction trend during the first 12 h and 24 h at the ambient and elevated CO2 level, respectively, then dramatically increased and reached a maximum at 72 h before decreasing rapidly (Fig. 4a). At the ambient CO2 level, the maximum content of Cys in root was observed at 72 h exposure of Cd at three different Cd concentrations, whereas at elevated CO2 level, it occurred after 72 h, 48 h, and 144 h exposure to 0 μmol·L-1, 20 μmol·L-1, and 80 μmol·L-1 Cd, respectively. The contents of Cys in shoot varied without significance during the first 12 h and 24 h at the ambient and elevated CO2 level, respectively, and then increased to reach its peak, and finally decreased over time (Fig. 4b). Regardless of Cd concentrations, the peak contents were observed at 72 h exposure at the ambient CO2 level, whereas it occurred at 48 h in Cd control and 144 h of Cd exposure at the elevated CO2 level.
The GSH content exhibited a response pattern similar to the Cys content in most cases. At ambient CO2 level, the root GSH content decreased during the first 12 h after Cd exposure, whereas at elevated CO2 level, this trend appeared much less pronounced (Fig. 5a). At ambient CO2 level, the GSH content dramatically increased after 24 h of exposure to 20 μmol·L-1 and 80 μmol·L-1 Cd stress, reached its maximum at 72 h, and then slightly decreased. However, the dramatic increase in the GSH content at elevated CO2 level occurred after 12 h of exposure to Cd stress and the peak content was observed at 144 h. At ambient CO2 level, the shoot GSH content was generally lower under Cd stress than the Cd control during the first 48 h; however, the variation trend became the opposite after 48 h exposure. By contrast, the shoot GSH content at elevated CO2 level varied a little during the first 72 h, and afterward, it was generally higher under Cd stress than the Cd control (Fig. 5b).
There was little variation in root and shoot PC2 content in the Cd control treatment at two CO2 levels. At ambient CO2 and under Cd stress, the root PC2 content kept increasing until 72 h, subsequently, slightly decreased, whereas at elevated CO2, it continuously increased during the time course (Fig. 6a, b). The shoot PC2 content kept increasing with the time course irrespective of CO2 levels and Cd concentrations.
An increase in root PC3-6 contents was observed under 20 μmol·L-1 and 80 μmol·L-1 Cd stress regardless of CO2 levels while in the Cd control treatment, very little variation was observed (Fig. S1a, S2a, S3a, and S4a). After exposure to 20 μmol·L-1 and 80 μmol·L-1 Cd stress, PC3-5 contents in shoots tended to increase irrespective of Cd concentrations and CO2 levels (Fig. S1b, S2b, and S3b), and PC6 contents had no obvious regularity with time (Fig. S4b).
The proportion of thiol was significantly altered after exposure to Cd stress at the ambient and elevated CO2 level, and it was noted that after treatment with Cd, the thiol in root were mainly PC3 and PC4, while the thiols in shoots were primarily GSH and PC3. However, PC production occurred when the Cd concentration was 0 μmol·L -1 due to the presence of other metal ions other than Cd (Figs. 7 and 8). During the time course, the proportion of Cys in roots dramatically decreased at 20 μmol·L-1 and 80 μmol·L-1 Cd stress and altered slightly in shoots after exposed to Cd stress.
Relationship of thiol and Cd
The binding ability of thiol including Cys, GSH, and PC2-6 was evaluated by determining the molar ratio of thiol to Cd during the time course study. The SH/Cd ratio in roots decreased firstly and increase with ranging from 3.07 to 0.22, and in shoots, dramatically decreased during the time course from 69.64 to 4.57 (Table S1). During the time course, the overall mean of SH/Cd ratio in roots and shoots reduced with increasing Cd concentration at the same CO2 level and was relatively higher at elevated CO2 than at ambient CO2 in roots, and no significant alteration was observed in shoots (p > 0.05).
The Cd contents and total SH contents in roots and shoots are plotted against each other in Fig. S5 exposure to Cd stress. The correlation between Cd and total SH contents in roots and shoots was calculated to estimate the importance of SH in the detoxification mechanism after exposure to Cd. Generally, the correlations of content of total SH and Cd were expressed as C total SH in fresh root = − 126.279 + 130.272C Cd in dry root (n = 160, r2 = 0.894, p<0.01) and C total SH in fresh shoot = 348.845 + 448.351C Cd in dry shoot (n = 160, r2 = 0.781, p<0.01) in roots and shoots, respectively. There was a strong correlation between total SH content and Cd content in both root and shoot (p<0.01), indicating that the synthesis of thiols was closely related to Cd uptake.
Discussion
Increasing attention has been paid to single and/or interactive effects on plants of elevated CO2 and salinity (Shimono et al. 2012; Zaghdoud et al. 2013), ozone (Clausen et al. 2011; Gillespie et al. 2011), cold (Tyagi et al. 2014), drought (Hebbar et al. 2013; Wang et al. 2017), organic pollutants (Hagedorn and Machwitz 2017), heavy metals (Li et al. 2010; Jia et al. 2010), or heat (Darbah et al. 2011; Pendall et al. 2016). Based on previous studies, it was found that elevated CO2 could ameliorate the toxicity and damages induced by these environmental stresses to some extent, and the same result was obtained in present study.
Elevated CO2 stimulates plant growth
Cd toxicity is generally characterized by the inhibition of root and shoot biomass production, leaf chlorosis, and a loss of photosynthetic activity, as well as formation of free radicals (Jia et al. 2010; Dias et al. 2013; SALEHI 2014; Muradoglu et al. 2015). The effects of elevated CO2 on plants were summarized in terms of increasing photosynthetic rate and carbon assimilation (Martínez-Lüscher et al. 2015), as well as reduction of photorespiration and oxidative stress (Rogers et al. 2004). The toxicity and damage induced by Cd in plants were ameliorated under the elevated CO2 conditions, resulting in greater biomass production with Cd-induced damage (Li et al. 2010; Jia et al. 2010). This study showed that the growth of L. perenne was significantly inhibited after exposure to Cd at both ambient and elevated CO2 levels. Inhibition of growth caused by Cd was greater in root than that in shoot, and the improvement in shoot was more than that in root at elevated CO2, causing a reduction of root/shoot ratio with the increase of exposure duration (Table S1). On the contrary, the growth of L. perenne seedlings was improved at elevated CO2 compared with those at ambient CO2 when exposed to the same concentration of Cd (Table S1). It was found that the growth inhibition occurred earlier in root after exposure to Cd stress at both CO2 levels, and the growth inhibition occurred later at elevated CO2 levels after exposure to the same Cd concentration, which indicated that elevated CO2 may increase tolerance of L. perenne to Cd stress.
Elevated CO2 helps Cd transportation
Cd contents in roots and shoots were significantly reduced at elevated CO2 level in comparison with those at ambient CO2, which may be due to an increase in growth rate and a reduction of Cd concentration in L. perenne. (Table S1). The similar phenomenon has also been observed by Li et al. (2010) and Jia et al. (2010, 2011), which might be formed due to the dilution effect of elevated CO2 (Loladze 2002). Elevated CO2 could decrease essential microelements in plants (Yang et al. 2007; Zheng et al. 2008; Rajashekar 2018; Senghor et al. 2017). The reduction of Cd contents could ameliorate the Cd toxicity to plant, enhance the tolerance ability, and reduce the food safety risk to some extent (Jia et al. 2010). In present study, it was also found that translocation accumulation factor (TAF) increased at elevated CO2 under Cd stress, and it was previously found that transport index (Ti) was higher under elevated CO2 than under ambient CO2, regardless of Cd concentrations and exposure times (Jia et al. 2011). Therefore, the very meaningful results were obtained that elevated CO2 could increase Cd translocation accumulation from root to shoot, indicating that L. perenne has potential for use in phytoremediation under elevated CO2 levels, according to according to Nie (2005).
Elevated CO2 favors the synthesis of thiol especially PCs
There are many ways for plants to resist Cd stress (Pal and Rai 2020). Thiol including Cys, GSH, and PCs play important roles in detoxification under Cd stress (Sytar et al. 2013) by chelation of Cd and compartmentalization of thiol-Cd compounds into the vacuoles (Clemens and Peršoh 2009; Guo et al. 2012), whose syntheses are related to Cd concentration in plant tissues (Choppala et al. 2014). This study found that the contents and proportion of thiol, especially PC2-6, increased after treatment with Cd in root and shoot at both CO2 levels, which occurred earlier than growth inhibition occurred. In present study, the content of GSH in roots and shoots significantly reduced after treatment of Cd, but the proportion of GSH accounted for above 50% of total thiols in shoots. The content of PC2 firstly increased and then decreased in roots under Cd stress, and continually increased in shoots. The content of PC3, PC4, and PC5 in roots and shoots increased under Cd stress, especially after treatment of 12 h, which occurred earlier than growth inhibition occurred. The content of PC6 in roots firstly reduced and then slightly increased after treatment of 72 h, and that altered slightly in shoots. The different alterations of GSH and PC2-6 in roots and shoots indicated that different sulfhydryl compounds played different roles in detoxification in roots and shoots. In roots, the thiols of PC2, PC3, and PC4 mainly performed the roles of detoxification after treatment of Cd, yet, GSH, PC2, and PC3 in shoots.
It has been proved that thiols especially PCs participate in Cd transportation from shoot to root and from root to shoot, and the transported PCs are possible to bind and aid in long-distance Cd transport (Gong et al. 2003; Chen et al. 2006). Under the elevated CO2, higher molecular PCs (PC4, PC5, PC6) were observed in both shoots and roots (Jia et al. 2011), which is possible to explain that the enhanced PCs products in roots under elevated CO2 helped the transport of Cd from roots to above-ground parts. In present study, thiol content was generally reduced at elevated CO2, which may be caused by the dilution effect and lower Cd contents in plants. However, the production of PCs occurred earlier at elevated CO2 than that at ambient CO2, which was involved in the improvement of plant growth and earlier Cd transport from root to shoot.
It was found that Cd stress significantly altered the SH/ Cd ratio in both roots and shoots (p < 0.01) (Table 1), and elevated CO2 significantly altered the SH/ Cd ratio in roots (p < 0.01), but no alteration was obtained in shoots (p > 0.05). In previous study, the PC-Cd ratios were not affected by elevated CO2 in roots and shoots of L. mutiforum and L. perenne after growth for 58 days in Cd-contaminated soil (Jia et al. 2010). In shoot, the SH/Cd ratio was higher than 2.0, indicating that enough thiol were produced against Cd stress and elevated CO2 scarcely altered the SH/Cd ratio to counter Cd toxicity in shoot. However, thiol in root could not effectively combat Cd stress, and additional ways such as cysteine and glutathione act, therefore, were involved in detoxification in L. perenne exposed to Cd at ambient and elevated CO2 (Jia et al. 2010). The overall mean of the SH/Cd ratio in root was relatively higher at elevated CO2 than at ambient CO2, which may provide higher Cd binding ability of thiol in root and help Cd transport from root to shoot.
The PCs have been expected to be useful as biomarkers of environmental metal pollution, since they were identified (Grill et al. 1985), for the reason that the relationship between the metal uptake and the synthesis of PCs has a significant implication regarding metal toxicity. The similar results were obtained in present study that the synthesis of thiols was closely related to Cd uptake. However, in order to better understand the role of PCs in detoxification and utilize PCs as biomarkers, the PC kinetics-metal uptake-metal sensitivity relationship must be directly tested using quantitative experiments (Wu et al. 2016).
Conclusions
Elevated CO2 resulted in a significant reduction of Cd content in root and shoot, and an increase of Cd translocation accumulation from root to shoot. The production of PCs occurred earlier than growth inhibition, which similarly occurred earlier at elevated CO2 level than at ambient CO2 level after exposure to Cd stress. Additionally, the average value of the SH/Cd ratio increased as the CO2 concentration increased, and the reduction of Cd contents, earlier production of PCs, and relatively higher SH/Cd ratio due to elevated CO2 level alleviated Cd toxicity in root and shoot to some extent. In summary, at elevated CO2 level, not only Cd toxicity was alleviated in L. perenne to some extent, Cd translocation accumulation from root to shoot were obtained after exposure to Cd stress, but also the significant increases of yields of L. perenne. Those were shown that L. perenne has great potential for phytoremediation of Cd-contaminated environment combining with elevated CO2 level.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Blagodatskaya E, Blagodatsky S, Dorodnikov M, Kuzyakov Y (2010) Elevated atmospheric CO2 increases microbial growth rates in soil: results of three CO2 enrichment experiments. Glob Chang Biol 16:836–848
Branco D, Lima A, Almeida SF, Figueira E (2010) Sensitivity of biochemical markers to evaluate cadmium stress in the freshwater diatom Nitzschia palea (Kützing) W. Smith. Aquat Toxicol 99:109–117
Chen A, Komives EA, Schroeder JI (2006) An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol 141(1):108–120
Choppala G, Saifullah BN, Bibi S, Iqbal M, Rengel Z, Kunhikrishnan A, Ashwath N, Ok YS (2014) Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci 33:374–391
Clausen SK, Frenck G, Linden L, Mikkelsen TN, Lunde C, Jørgensen RB (2011) Effects of single and multifactor treatments with elevated temperature, CO2 and ozone on oilseed rape and barley. J Agron Crop Sci 197:442–453
Clemens S, Peršoh D (2009) Multi-tasking phytochelatin synthases. Plant Sci 177:266–271
Darbah JN, Sharkey TD, Calfapietra C, Karnosky DF (2011) Differential response of aspen and birch trees to heat stress under elevated carbon dioxide. Environ Pollut 158:1008–1014
Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Gonçalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Actaphysiol Plant 35:1281–1289
Etelvina G, Victoria S (2014) Spatiotemporal evolution of Leucophyllum pringlei and allies (Scrophulariaceae): A group endemic to North American xeric regions. Mol Phylogenet Evol 76:93–101
Gillespie KM, Rogers A, Ainsworth EA (2011) Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J Exp Bot 62:2667–2678
Gong JM, Lee DA, Schroeder JI (2003) Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci U S A 100(17):10118–10123
Grill E, Winnacker EL, Zenk MH (1985) Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230:674–676
Guo J, Xu W, Ma M (2012) The assembly of metals chelation by thiols and vacuolar compartmentalization conferred increased tolerance to and accumulation of cadmium and arsenic in transgenic Arabidopsis thaliana. J Hazard Mater 199:309–313
Hagedorn F, Machwitz M (2017) Controls on dissolved organic matter leaching from forest litter grown under elevated atmospheric CO2. Soil Biol Biochem 39:1759–1769
Hebbar K, Sheena T, Kumari KS, Padmanabhan S, Balasimha D, Kumar M, Thomas GV (2013) Response of coconut seedlings to elevated CO2 and high temperature in drought and high nutrient conditions. J Ornam Plants 2251–6441
Jia Y, Tang S, Wang R, Ju X, Ding Y, Tu S, Smith DL (2010) Effects of elevated CO2 on growth, photosynthesis, elemental composition, antioxidant level, and phytochelatin concentration in Lolium mutiforum and Lolium perenne under Cd stress. J Hazard Mater 180:384–394
Jia Y, Ju X, Liao S, Song Z, Li Z (2011) Phytochelatin synthesis in response to elevated CO2 under cadmium stress in Lolium perenne L. J Plant Physiol 168(2011):1723–1728
Jongen M, Jones MB, Hebeisen T, Blum H, Hendrey G (1995) The effects of elevated CO2 concentrations on the root growth of Lolium perenne and Trifolium repens grown in a FACE system. Glob Chang Biol 1:361–371
Ju XH, Tang S, Jia Y, Guo J, Ding Y, Song Z, Zhao Y (2011) Determination and characterization of cysteine, glutathione and phytochelatins (PC2–6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. J Chromatogr B 879:1717–1724
Li Z, Tang S, Deng X, Wang R, Song Z (2010) Contrasting effects of elevated CO2 on Cu and Cd uptake by different rice varieties grown on contaminated soils with two levels of metals: implication for phytoextraction and food safety. J Hazard Mater 177:352–361
Lin L, Liao M, Ren Y, Luo L, Zhang X, Yang D, He J (2014) Effects of mulching tolerant plant straw on soil surface on growth and cadmium accumulation of Galinsoga parviflora. PLoS One 9:e114957. https://doi.org/10.1371/journal.pone.0114957
Loladze I (2002) Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol Evol 17:457–461
Lou YH, Luo H, Hu T, Li H, Fu J (2013) Toxic effects, uptake, and translocation of Cd and Pb in perennial ryegrass. Ecotoxicology 22:207–214
Luo, Y, Mao, DQ, Rysz, M, Zhou, Q, Zhang, H, Xu, L, Alvarez, PJJ (2010) Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ. Sci Technol 44:7220–7225
Martínez-Lüscher J, Morales F, Sánchez-Díaz M, Delrot S, Aguirreolea J, Gomès E, Pascual I (2015) Climate change conditions (elevated CO2 and temperature) and UV-B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates. Plant Sci 236:168–176
Morelli E, Marangi ML, Fantozzi L (2009) A phytochelatin-based bioassay in marine diatoms useful for the assessment of bioavailability of heavy metals released by polluted sediments. Environ Int 35:532–538
Muradoglu F, Gundogdu M, Ercisli S, Encu T, Balta F, Jaafar HZ, Zia-Ul-Haq M (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res 48:1–7
Negrin VL, Teixeira B, Godinho RM, Mendes R, Vale C (2017) Phytochelatins and monothiols in salt marsh plants and their relation with metal tolerance. Mar Pollut Bull 121:78–84
Nie FH (2005) New comprehensions of hyperaccumulator. Ecol Environ 14(1):136–138
Pal R, Rai J (2020) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160:945–963
Pendall E, Blumenthal D, Carrillo Y, Dijkstra F, Mueller K, Nelson L, Nie M, Ogle K, Ryan E, Samuels-Crow K (2016) Interactive effects of experimental warming and elevated CO2 on belowground allocation and soil organic matter decomposition at the prairie heating and CO2 enrichment experiment. In AGU Fall Meeting Abstracts
Rajashekar C (2018) Elevated CO2 levels affect phytochemicals and nutritional quality of food crops. Am J Plant Sci 9:150–162
Rogers A, Allen D, Davey P, Morgan P, Ainsworth E, Bernacchi C, Cornic G, Dermody O, Dohleman F, Heaton E (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant Cell Environ 27:449–458
SALEHI ES (2014) Cadmium toxicity: the investigation of Cd toxic level in different organs of cherry tomato plant and the effect of Cd accumulation. J Plant Crop 41(2):118–122
Senghor A, Dioh R, Müller C, Youm I (2017) Cereal crops for biogas production: a review of possible impact of elevated CO2. Renew. Sust Energ Rev 71:548–554
Shi G, Cai Q, Liu Q, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31:969–977
Shimono H, Konno T, Sakai H, Sameshima R (2012) Interactive effects of elevated atmospheric CO2 and waterlogging on vegetative growth of soybean (Glycine max (L.) Merr.). Plant Prod Sci 15:238–245
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad M (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Actaphysiol Plant 35:985–999
Tyagi S, Singh R, Javeria S (2014) Effect of climate change on plant-microbe interaction: an overview. Eur J Mol Biotechnol 5:149–156
Wang MJ, Wang WX (2009) Cadmium in three marine phytoplankton: accumulation, subcellular fate and thiol induction. Aquat Toxicol 95:99–107
Wang YH, Yan DH, Wang JF, Ding Y, Song XSH (2017) Effects of elevated CO2 and drought on plant physiology, soil carbon and soil enzyme activities. Pedosphere 27:846–855
Wu Z, Zhang C, Yan J, Ge Y (2013) Separation and quantification of cysteine, glutathione and phytochelatins in rice (Oryza sativa L.) upon cadmium exposure using reverse phase ultra performance liquid chromatography (RP-UPLC) with fluorescence detection. Anal Methods-UK 5:6147–6152
Wu Y, Guo Z, Zhang W, Tan Q, Zhang L, Ge X, Chen M (2016) Quantitative relationship between cadmium uptake and the kinetics of phytochelatin induction by cadmium in a marine diatom. Sci Rep 6:65–71
Yadav S (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yang L, Wang Y, Dong G, Gu H, Huang J, Zhu J, Yang H, Liu G, Han Y (2007) The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crop Res 102:128–140
Zaghdoud C, Mota-Cadenas C, Carvajal M, Muries B, Ferchichi A, del Carmen Martínez-Ballesta M (2013) Elevated CO2 alleviates negative effects of salinity on broccoli (Brassica oleracea L. var Italica) plants by modulating water balance through aquaporins abundance. Environ Exp Bot 95:15–24
Zheng J, Wang H, Li Z, Tang S, Chen Z (2008) Using elevated carbon dioxide to enhance copper accumulation in Pteridium revolutum, a copper-tolerant plant, under experimental conditions. Int J Phytoremediat 10:161–172
Funding
This work was financially supported by the Central Public Research Institute Basic Funds for Research and Development (Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, P.R. China).
Author information
Authors and Affiliations
Contributions
YS—drafting of the manuscript, manuscript preparation
YQL—carrying out the experiment
HYL—chemical analysis and interpretation
HPP—statistical calculations
YX and XHJ—manuscript preparation and overall corrections
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 560 kb)
Rights and permissions
About this article
Cite this article
Shi, Y., Liu, Y., Li, H. et al. Phytochelatins formation kinetics and Cd-induced growth inhibition in Lolium perenne L. at elevated CO2 level under Cd stress. Environ Sci Pollut Res 28, 35751–35763 (2021). https://doi.org/10.1007/s11356-021-12883-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12883-0