Abstract
Effect of Cd2+ toxicity and heat stress in sensitive rice cv. DR-92 and tolerant rice cv. Bh-1 grown in North East region of India were studied in sand cultures. Increasing levels of 0–500 μM Cd2+ alone and/or heat stress showed increased activities of superoxide dismutase, guaiacol peroxidase, ascorbate peroxidase and glutathione reductase enzymes which were associated with induced oxidative stress and altered enzyme activities. The values for SOD and POD activities were always more in cv. DR-92 whereas CAT and GR activities were higher in cv. Bh-1 in roots and shoots under Cd2+ or heat stress alone in sensitive cv. DR-92. Upon imposition of a combination of Cd2+ + heat the activities of SOD and POD decreased significantly in root/shoot of both the sensitive and tolerant rice varieties. A nine fold increase in GR activity under combination of heat + 100 μM Cd2+ stress in shoots of cv. Bh-1 at day 15 was noted when compared to controls. The dual stress combination of Cd2+ + heat did not alter catalase activity in vivo in both the rice varieties. Results suggest a time-specific and varietal distribution of the antioxidant enzymes in rice plants subjected to Cd2+ and/or heat stress. Tolerant cv. Bh-1 has better survival to combined stressors like Cd2+ and heat than sensitive rice cv. DR-92 and heat stress when given in combination with Cd2+ toxicity seem to mitigate the effect of Cd2+ stress alone in rice. The study indicates individual Cd2+ toxicity and heat stress and a combination of the two stresses to have separate implications on antioxidative defense mechanism in rice plants. Among enzymes of the defense apparatus ascorbate peroxidase and glutathione reductase appear to serve as an important component for better survival of rice plants under combination of Cd2+ + heat stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are continually under threat due to adverse environmental conditions. In nature, plants encounter a number of biotic and abiotic stress factors simultaneously that may include drought, heat shock and heavy metals (both from air and water) (Kochhar and Kochhar 2005). The altered geochemical and biochemical balance of heavy metals due to human activities presently makes metal pollution a major environmental concern (Lei et al. 2007). Heavy metals can bind to functionally important domains of biomolecules and thereby inactivate them or render oxidative stress by direct electron transfer or inhibition of normal metabolic reactions (Hall 2002). Heat stress affects plant growth throughout its ontogeny, though heat threshold level varies considerably at different developmental stages (Wahid et al. 2007). Heat stress has been shown to alter the plant metabolism either through synthesis of heat-shock proteins (HSPs) (Vierling 1991) or by altered antioxidant enzyme activities due to enhanced production of reactive oxygen species (ROS) (Kochhar and Kochhar 2005). Thus increasing contamination and consequential accumulation of heavy metals in the soil as well as increasing temperature have become serious problems to crop yield and productivity in agricultural terms (Shah et al. 2001; Sharma and Dubey 2005; Howarth 2005).
In general, heavy metals and heat stress result in oxidative damage to plants. ROS are always formed by the inevitable leakage of electrons onto molecular oxygen from the electron transport activities of chloroplast, mitochondria and plasma membrane or as a byproduct of various metabolic pathways localized in different cellular compartments (Xiaozhong and Huang 2000; Polle 2001). ROS may cause lipid peroxidation and subsequent membrane injury as well as protein and nucleic acid damage (Gao et al. 2008). The production of ROS under normal growth conditions in cells is low (240 μMs−1 O ·−2 ) and in a steady state (0.5 μM H2O2) in chloroplasts, however, an enhanced production of ROS under various stressful conditions of the environment is reported to result in altered cellular homeostasis (upto 720 μMs−1 O ·−2 ) and a steady state of 5–15 μM H2O2 in chloroplast (Polle 2001).
Plant cells contain a range of protective and repair systems, which under normal circumstances, minimize the occurrence of oxidative damage. There are systems which either react with reactive forms of oxygen and keep them at a low level or are antioxidant enzymes as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidases (POD), ascorbate peroxidase (APX), glutathione reductase (GR) that quench ROS supported by antioxidants like glutathione, ascorbic acid, ά-tocopherol and carotenoids (Sairam et al. 2000; Shah et al. 2001) or systems that regenerate oxidized antioxidants (glutathione, mono- and dehydroascorbate) (Markovska et al. 2009).
The non-specific enzyme guaiacol peroxidases so named as they can metabolize guaiacol as substrate and can function as effective quencher of ROS and peroxy radical induced by stressful conditions in the cell (Van Assche and Clijsters 1990). Usually an altered peroxidase activity is reported to be associated with the response of higher plants to an uptake of toxic amounts of heavy metals (Van Assche and Clijsters 1990; Shah et al. 2001) or to heat injury (Kochhar and Kochhar 2005). Very often therefore, peroxidases are known as stress ameliorating enzymes which actively responds to damages in plant metabolism and may act as early and sensitive indicators of heavy metal toxicity (Shah et al. 2001). In coordination with enzyme superoxide dismutases, catalase and ascorbate peroxidase, the peroxidases play an essential protective role in the scavenging process. Once SOD converts superoxide radical to H2O2, it is reduced to water and oxygen either by APX in ascorbate–glutathione cycle or by POD and CAT in cytoplasm and in other cellular compartments (Howarth 2005). An enhanced or altered expression of these key antioxidant enzymes in response to biotic and abiotic stresses is known (Bowler et al. 1992; Rizhsky et al. 2004; Sharma and Dubey 2005).
Rice can be grown under various climatic conditions at latitudes ranging from 53ºN to 40ºS by long period induction and domestication (Lu and Chang 1980). Generally, several latitude-dependent indica rice cultivars are grown in the North-East Region of India. Of these two rice cv. DR-92 and cv. Bh-1 were studied for their sensitivity to cadmium toxicity and heat stress. The present study was undertaken with the objective to examine the effect of low and high cadmium toxicity in combination with heat stress on the antioxidant enzymes in sensitive rice cv. DR-92 and tolerant rice cultivar Bh-1 grown at low and high altitudes, respectively in North East India. Attempts are also made to test the hypothesis that whether there occurs a cross-talk between the various antioxidant enzymes and that cadmium and heat stress either individual or combination have different oxidative response in rice seedlings. If either of the two stresses when given in combination help in better survival.
Materials and methods
Plant material and stress conditions
Seeds of two rice (Oryza sativa) cvs. DR-92 (sensitive) and Bh-1 (tolerant) and grown at low and high altitudes in North-East region of India were surface sterilized with 0.1% sodium hypochlorite solution and imbibed in water for 24 h. Seeds were germinated in petriplates for 5 days and seedlings were raised for 20 days in sand cultures, saturated either with Hoagland nutrient solution (Hoagland and Arnon 1938) that served as control or nutrient solution supplemented with 10, 50, 100 and 500 μM Cd(NO3)2 as treatments (Shah and Dubey 1998). Cd2+ levels were ascertained as low toxic (10, 50 μM) and high toxic (100, 500 μM) concentrations with full viability of seeds. Pots were maintained at field saturation capacity at pH 7.0 and irrigation done when required. Seedlings were maintained in the growth chamber for 20 day at 28 ± 1°C, 80% relative humidity and 12-h light (irradiance 40–50 μmol m−2 s−1) followed by a dark period. Seedlings were uprooted at 5 day intervals, roots and shoots were separated which served as Cd2+ treated plant samples. For heat treatments the control as well as Cd-treated seedlings were uprooted and kept at 40°C for 2 h. The roots and shoots were separated and used for experimental studies. All the estimations were carried out in triplicate. Cadmium was estimated in plant parts according to the method of Shah and Dubey (1995) using Atomic Abbsorption Spectrophotometer (AAS) fitted with Perkin-Elmer-2380.
Assay of guaiacol peroxidase (POD) activity
Guaiacol peroxidase (POD) (EC1.11.1.7) activity assay was performed spectrophotometrically according to the method of Egley et al. (1983). POD was extracted by homogenizing about 200 mg of root and shoot samples in 5 ml of 60 mM phosphate buffer (pH 6.0) using a chilled mortar and pestle at 4°C. The homogenates were centrifuged at 22,000×g for 10 min and supernatant were used for enzyme assay. Assay mixture in a final volume of 2 ml contained 50 μl enzyme, 200 μl guaiacol and 50 μl H2O2 in 1.7 ml of buffer. The increase in absorbance was measured at 470 nm (extinction coefficient 26.6 mM−1 cm−1) using a Beckman DU- 530 (Germany) spectrophotometer. Enzyme specific activity is expressed as μmol H2O2 reduced mg−1 protein min−1.
Assay of superoxide dismutase (SOD) activity
The superoxide dismutase (SOD) (EC 1.15.1.1) was assayed according to the method of Mishra and Fridovich (1972). For extraction of the enzyme, about 200 mg fresh root and shoot samples were homogenized in 5 ml of 100 mM potassium-phosphate buffer (pH 7.5), containing 1.0 mM EDTA, 0.1% (v/v) Triton X-100 and 2% (w/v) of soluble polyvinyl pyrrolidone (PVP) using prechilled mortar and pestle. After centrifugation at 22,000×g for 10 min at 4°C, the supernatant was dialyzed in cellophane membrane tubings against the cold extraction buffer for 4 h with 3–4 changes of the buffer. After dialysis the SOD activity was assayed in the supernatant. The assay mixture contained 50 mM sodium carbonate-bicarbonate buffer (pH 9.8), containing 0.1 mM EDTA, 0.6 mM epinephrine and 0.1 ml enzyme in a total volume of 3 ml. Epinephrine was the last component to be added. The adrenochrome formation during the next 4 min was recorded at 470 nm in a UV–Vis spectrophotometer (ELICO, SL-159, India). One unit of SOD activity is expressed as the amount of enzyme required to cause 50% inhibition of epinephrine oxidation under the experimental conditions.
Assay of ascorbate peroxidase (APX) activity
Ascorbate peroxidase (APX) (EC1.11.1.11) was assayed as given by Nakano and Asada (1981). About 200 mg of fresh roots and shoots were homogenized in 5 ml of 50 mM potassium-phosphate buffer (pH 7.8) containing 1% PVP, 1 mM EDTA and 1 mM ascorbic acid (added just before use) in a chilled mortar and pestle. The homogenate was centrifuged at 22,000×g at 4°C for 10–15 min and the supernatant was used for enzyme assay. Reaction mixture in a total volume of 3 ml contained 50 mM potassium-phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 0.5 mM ascorbic acid, 0.1 mM H2O2 and 0.1 ml dialyzed enzyme extract. H2O2 was the last component to be added and the absorbance was read at 290 nm (extinction coefficient 2.8 mM−1 cm−1). Enzyme specific activity is expressed as μmol ascorbate oxidized mg−1 protein min−1.
Assay of catalase (CAT) activity
Catalase (CAT) (EC 1.11.1.6) was assayed as given by Beers and Sizers (1952). The enzyme was extracted from 200 mg fresh root and shoot samples by homogenization in 5 ml of 50 mM Tris-NaOH buffer (pH 8.0) containing 0.5 mM EDTA, 2% (w/v) PVP and 0.5% (v/v) Triton X-100 using a chilled mortar and pestle. The homogenate was centrifuged at 22,000×g for 10 min at 4°C and after dialysis supernatant was used for enzyme assay. Assay mixture in a total volume of 1.5 ml contained 1 ml of 100 mM potassium-phosphate buffer (pH 7.0), 400 μl of 200 mM H2O2 and 100 μl dialyzed enzyme extract. The rate of H2O2 decomposition was monitored at 240 nm (extinction coefficient 0.036 mM−1 cm−1). Enzyme specific activity is expressed as μmol of H2O2 oxidized mg−1 protein min−1.
Assay of glutathione reductase (GR) activity
Glutathione reductase (GR) (EC 1.6.4.2) was assayed based on the method of Carlberg and Mannervik (1985) with minor modification (Dalton et al. 1986). About 200 mg of fresh root and shoot tissues were homogenized in 5 ml of 0.1 M potassium phosphate buffer (pH 7.0) using chilled mortar and pestle. The homogenate was centrifuged at 22,000×g for 10 min at 4°C and the supernatant so obtained was used for determination of enzyme activity. The reaction mixture contained 0.25 mM GSSG, 0.125 mM NADPH, 50 mM tricine (pH 7.8), 0.5 mM EDTA and 50 μl of extract in a final volume of 2 ml. The decrease in absorbance due to NADPH oxidation was recorded at 340 nm (extinction coefficient of 6.22 mM cm−1) and expressed in terms of nmol NADPH oxidized mg−1 protein min−1.
In all the enzymatic preparations protein was determined by the Lowry’s method (1951) using bovine serum albumin (BSA, Sigma) as standard.
Statistical analyses
The seedlings were distributed over a completely randomized samples. All the experiments were performed in triplicate. Values in the figures indicate mean values ± SD. based on three independent experiments and were significantly different as assessed by the analysis of variance (ANOVA) test.
Results
Effect of Cd2+ toxicity and heat on guaiacol peroxidase (POD) activity
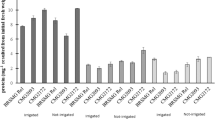
Increasing levels of Cd2+ led to a concomitant increase in guaiacol peroxidase activity in both roots and shoots of sensitive rice cv. DR-92 whereas as expected an opposite trend was observed for POD activity in tolerant cv. Bh-1 (Table 1, Fig. 1). The POD activity in cv. DR-92 were always higher in Cd2+ treatments than that in controls. Under low Cd2+ levels a decline in POD activity at day 15 followed by a significant increase at 20 day of growth period were observed in the roots of cv. DR-92. A high toxic (100 and 500 μM) level of Cd2+ however, resulted in a dip in POD activity much earlier i.e. at day 10 followed by a gradual increase till 20 day of growth. Almost two fold increase in the POD activity in shoots of cv. DR-92 were noted under 50 μM Cd2+ treatments at day 10 and a threefold increase under 500 μM Cd2+ treatments at day 5 of the growth period. Seedlings grown under 500 μM Cd2+ for 20 days showed about 50 to 80% increase in POD activity in roots whereas a 30 to 72% increase in POD activity in shoots were observed at day 15 when compared to control grown seedlings of rice cv. DR-92. Heat stress alone led to a significant elevation in POD activity in sensitive cv. DR-92. A combination of Cd2+ treatments + heat resulted in a significant decline in POD activity at 20 d of growth in cv. DR-92, the values being lower than those under heat stress alone.
Effect of 10 and 50 μM and 100 and 500 μM Cd2+ toxicity and heat stress on the activity of guaiacol peroxidase in the roots and shoots of rice cvs. DR-92 and Bh-1 grown in sand cultures. The data are mean of three replicates ± SD. ANOVA significant at P ≤ 0.01. Values with different letters are significantly different at P < 0.05
The levels of POD activity were always higher in roots and shoots of controls and all treatments in cv. Bh-1 than that in cv. DR-92, and declined gradually in former throughout the 5–20 day growth period and under all treatments. Cd and heat stress alone resulted in a lowered POD activity in tolerant cv. Bh-1 except at day 10 where 50 μM Cd2+ concentration caused a slight elevation (5–15%) in enzyme activity. The combined effect of 500 μM Cd2+ and heat stress resulted in decrease in the POD activity in roots at 10 day in cv. Bh-1 which declined thereafter. In all Cd2+ + heat treated seedlings a significant decrease in the activity of POD is noted beyond 10 day in the rice cv. Bh-1 (Fig. 1, Table 1).
Effect of Cd2+ toxicity and heat on superoxide dismutase (SOD) activity
Table 1 and Fig. 2 show the activity levels of superoxide dismutase in control, Cd2+ stressed, heat stress as well as Cd2+ and heat stressed seedlings in roots and shoots of the rice sensitive cv. DR-92 and tolerant rice cv. Bh-1. SOD activity was higher in cv. DR-92 than in cv. Bh-1 in both roots and shoots. An increase in SOD activity during 5–15 day growth period with 30–50% higher activity in roots and shoots of 10 μM Cd2+ and 50 μM Cd2+ treatments than that in controls were noted in cv. DR-92 followed by a decline thereafter. Under 500 μM Cd2+ toxicity a significant increase of ~1.8 fold in SOD activity at 15 day in shoots and a similar increase under 100 μM Cd2+ in roots of cv. DR-92 were recorded. In tolerant cv. Bh-1 however, a 500 μM Cd2+ with heat stress revealed ~2 fold increase in SOD activity at day 15 in both roots and shoots as compared to controls. Heat treatments alone led to a decline in SOD activity in roots of cv. Bh-1 when compared to that in Cd treatments alone, whereas in sensitive cv. DR-92, heat stress led to a significant increase in enzyme activity in shoots. The combined effect of 100 and 500 μM Cd2+ + heat stress caused moderate amplification in SOD activity in roots and shoots cv. DR-92 during 10–15 day (Table 1), whereas the enzyme activity either remain unchanged or decreased in both roots and shoots of tolerant cv. Bh-1, suggesting a protective effect of heat over Cd stress.
Effect of 10 and 50 μM and 100 and 500 μM Cd2+ toxicity and heat stress on the activity of superoxide dismutase in the roots and shoots of rice cvs. DR-92 and Bh-1 grown in sand cultures. The data are mean of three replicates ± SD. ANOVA significant at P ≤ 0.01. Values with different letters are significantly different at P < 0.05
Effect of Cd2+ toxicity and heat on ascorbate peroxidase (APX) activity
The specific activity of APX under Cd2+ treatments and combination of Cd2+ and heat stress is shown as Table 1, Fig. 3. A 10 and 50 μM Cd2+ treatments alone caused a significant increase in APX activity during 10–15 days of growth period in sensitive cv. DR-92, when compared with controls that further enhanced under higher Cd levels. The values for activity of APX remained lower in tolerant cv. Bh-1 than that in sensitive cv. DR-92. Heat stress led to ~2.0 fold increase in APX activity in roots of cv. Bh-1 and ~2.5 fold elevation in roots of cv. DR-92 at day 10 which gradually declined during 5–20 days of growth period in cv. Bh-1 but continued to increase in cv. DR-92. Cd2+ treatments in combination with heat stress caused an elevation in APX activity during 10–15 days in both roots and shoots of cv. DR-92, however a gradual decline was noted for cv. Bh-1 under similar conditions.
Effect of 10 and 50 μM and 100 and 500 μM Cd2+ toxicity and heat stress on the activity of ascorbate peroxidase in the roots and shoots of rice cvs. DR-92 and Bh-1 grown in sand cultures. The data are mean of three replicates ± SD. ANOVA significant at P ≤ 0.01. Values with different letters are significantly different at P < 0.05
Effect of Cd2+ toxicity and heat on catalase (CAT) activity
The activity of CAT under low/high Cd2+ levels, heat stress alone and Cd2+ + heat stress is shown as Fig. 4. Activity of CAT was always higher under 50 μM Cd2+ stress at day 10, in both the cultivars, with a further increase at day 15 under combined effect of 50 μM Cd2+ + heat stress in cv. DR-92 in roots. In cv. Bh-1 also a similar trend was noted but at rather a lower Cd2+ treatments of 10 μM Cd2+ alone. Seedlings grown under high Cd2+ level of 100 μM always exhibited a high catalase activity at 15 day in the two cultivars which declined sharply thereafter. Heat stress caused a slight rise in CAT activity in shoots of tolerant cv. Bh-1 as well as sensitive cv. DR-92 at day 10 with a decline thereafter. The Cd2+ + heat treatments did not exhibit any significant variation in the CAT activities and remained almost unchanged except on day 10 where cv. Bh-1 had a 1.5 to 2.0 fold elevation in the CAT activity in roots than that in cv. DR-92. A 100 μM Cd2+ + heat stress together caused 1.6 fold elevation in CAT activity in shoots of cv. DR-92 at day 15. A similar increase was noted in cv. Bh-1 in corresponding plant samples. A higher Cd2+ levels of 100 and 500 μM in combination with heat caused a 30% inhibition in catalase activity in roots of cv. Bh-1 at 15 day of growth.
Effect of 10 and 50 μM and 100 and 500 μM Cd2+ toxicity and heat stress on the activity of catalase in the roots and shoots of rice cvs. DR-92 and Bh-1 grown in sand cultures. The data are mean of three replicates ± SD. ANOVA significant at P ≤ 0.01. Values with different letters are significantly different at P < 0.05
Effect of Cd2+ toxicity and heat on glutathione reductase (GR) activity
Figure 5 show the specific activity of glutathione reductase subjected to Cd2+ and/or heat stress during 5–20 days of growth period in the roots and shoots of rice cvs. DR-92 and Bh-1. Under Cd2+ alone and stress combination of Cd2+ and heat a slight increase in GR activity were observed in cv. DR-92 at day 15 followed by a gradual decline thereafter. A marked change in GR activity could be observed in roots and shoots of tolerant cv. Bh-1, where a 10 and 50 μM Cd-treatments led to a ~2.5 to 3.0 fold increase in GR activity at day 10 in roots that further enhanced by ~3.3 to 4.0 folds under combined effects of Cd2+ + heat stress. Maximum GR activity were noted in 100 μM Cd-treated roots and shoots of cv. Bh-1 at day 10 of growth, where a 2.0 fold increase in GR activity in roots of cv. Bh-1 followed by a sharp decline thereafter were noticed. Under combined effects of 100 μM Cd2+ and heat, a nearly ninefold increase in GR activity were observed in shoots of cv. Bh-1 at 15 day of growth, when compared with controls. Shoots maintained higher GR activity than roots in both the rice cultivars and under all stress treatments. Heat alone increased GR activity in roots and shoots of tolerant cv. Bh-1 during 10 days and declined thereafter, however in sensitive cv. DR-92 the activity of GR did not exhibit any significant change throughout the growth period. Unlike the combined effect of Cd2+ and heat stress where activity was high at day 10, a 100 μM and 500 μM Cd2+ treatments alone led to a significant increase in GR activity in shoots later at day 15 of the growth period. The activity of glutathione reductase declined beyond 15 day of growth in all test samples in both roots and shoots of the two cultivars.
Effect of 10 and 50 μM and 100 and 500 μM Cd2+ toxicity and heat stress on the activity of glutathione reductase in the roots and shoots of rice cvs. DR-92 and Bh-1 grown in sand cultures. The data are mean of three replicates ± SD. ANOVA significant at P ≤ 0.01. Values with different letters are significantly different at P < 0.05
Discussion
Cadmium toxicity and elevated temperature are the two major risks in crop plants. There is an increasing evidence suggesting that oxidative stress is a key damaging factor in plants exposed to a variety of stressful conditions including metal toxicity and that the plants resist damage due to oxidative stress by inducing the activities of antioxidative enzymes (Bowler et al. 1992). Therefore, the present study has been carried out to develop an understanding towards a combination of Cd2+ toxicity and heat stress, induced oxidative stress and the antioxidative defense system in growing rice plants. Results indicate induction of a high degree of oxidative stress paralleled with increasing Cd2+ levels and heat stress alone with altered activities of key antioxidative enzymes. Combinatorial effects of Cd + heat stress indicate a protective role of heat on Cd induced oxidative stress in rice seedlings.
An elevated activity of enzyme guaiacol peroxidase under increasing concentration of Cd2+ stress alone has been observed earlier in rice (Shah et al. 2001). In bean roots Cd2+ although caused oxidative stress it did not change the activity of POD. In contrast, in pea roots Cd2+ strongly stimulated POD activity mainly at higher Cd2+ concentration (Chaoui et al. 1997, 2004). When the root/shoot samples from rice cv. DR-92 were subjected to a combination of Cd2+ and heat stress a significant elevation in the activity of POD was noted suggesting a synergistic effect of the two stresses leading to an increased guaiacol peroxidase activity in this cultivar. Similar reports in different plants subjected to various abiotic stresses like drought, heat, cold, pathogen attack etc. are available in literature (Rizhsky et al. 2004). Most of these studies show that peroxidase activity and oxidative stress are induced by different physical, chemical or biological agents. An expressive enhancement of POD is also observed in soybean plants exposed to 150 μM Al (Shamsi et al. 2008). Enhancement in POD activity in Cd2+ stressed rice plants suggest the role of POD in removal of excess H2O2 produced under Cd2+ toxicity and/or heat stress. A decrease in POD activity as observed under stress conditions in roots and shoots of rice cv. Bh-1 could be due to varietal differences and better adaptability of cv. Bh-1 towards elevated ROS levels.
The result show a concomitant increase in SOD activity in the two cvs. DR-92 and Bh-1, the effect being more profound in roots than in shoots. This could be due to enhanced oxidative damage involved in roots of plants. With increase in Cd levels an enhanced uptake in roots were observed with less translocation to shoots (results submitted for publication elsewhere). An elevated SOD activity in leaves and roots under Cd2+ stress were also reported in Phragmites australis (Iannelli et al. 2002) and also in soybean plants exposed to low pH, Al alone and under combined effects of Al with Cd2+ (Shamsi et al. 2008). The decline in SOD activity beyond 15 days of growth is also reported in penncross grass at 28 days of growth (Xiaozhong and Huang 2000). Increase in SOD activity in response to stresses has also been attributed to the de-novo synthesis of enzymatic protein (Shah and Dubey 1998). Activities of Cu/Zn SOD, Fe-SOD and Mn-SOD have been shown to increase in plant cells under stressful conditions, which appear to be a part of defense mechanism under oxidative stress generated in cytosol, mitochondria and chloroplasts (Ushimaru et al. 1995). However, a Cu/Zn-SOD precursor was found to be down-regulated by heat stress in rice leaves (Lee et al. 2007). Sato et al. (2001) reported that SOD activity was not altered in rice seedlings exposed to heat (42°C), suggesting that SOD might respond to thermal stress in a complex manner or different isoforms could be differentially regulated.
Increased SOD activity during short-term heat stress may provide protection from oxidative stress, however prolonged periods of heat stress could be related to the reduction in SOD activity, which cause accumulation of O ·−2 especially in chloroplasts and mitochondria (Xiaozhong and Huang 2000). It is quite pertinent that the overall change in the ratio of O ·−2 scavenging enzyme (SOD) to H2O2 scavenging enzyme (CAT and POD) activity rather than individual changes in enzyme activities would result in the net oxidative stress in roots/shoots of rice seedlings (Kanazawa et al. 2000; Shah et al. 2001).
An increase in the activity of enzyme APX under increasing Cd2+ levels in sensitive cv. DR-92 as observed in our experiments suggest that cadmium inhibits plant growth and causes alterations in plant metabolism due to its interference with activities of enzymes and growth processes (Sharma and Dubey 2005). Cd stress alone did not lead to an increase in APX activity in tolerant cv. Bh-1 however under heat stress an increase in APX in tolerant cv. Bh-1 was noted suggesting thereby that oxidative stress is induced under heat stress and that cv. Bh-1 is tolerant to Cd alone. A combination of Cd + heat caused a decrease in APX activity suggesting that heat counteracts the effect of Cd in tolerant rice cultivar by lowering of APX in plants parts. High temperature stress can on the other hand account for significant decline in enzyme activities (Anderson 1997; Shah and Dubey 2005). However it is the oxidative stress which is the major damaging factor in plants exposed to abiotic and biotic stresses, albeit the magnitude of stress varies (Alcazar et al. 1995). Furthermore an increase in APX activity under increasing levels of Cd2+ suggest an important role of APX in scavenging H2O2 under stressful conditions. Among H2O2 decomposing enzymes, APX has higher affinity for H2O2 than CAT and POD (Wang et al. 1999). Since ascorbate is the primary antioxidant and H2O2 is the major stable oxidant, the ratio of these redox components is indicative of the redox balance within the tissues (Markovska et al. 2009).
As observed in this study, an increase in the activity of enzyme catalase is also reported in some plant species exposed to toxic concentrations of heavy metals Cu, Pb, Zn (Prasad et al. 1999) but in other environmental stresses like drought (Zhang and Kirkham 1994; Sairam et al. 2000), senescence (Kanazawa et al. 2000), chilling (Anderson 1997), salinity (Singh et al. 2007) and highlight (Anderson 1997), an overall decline in CAT activity is reported. In the leaves of Brassica juncea (Markovska et al. 2009) decrease in activities of both APX and CAT was observed under Cd2+ stress suggesting an involvement in competition to remove H2O2. However, under Al3+ toxicity, CAT was reported to be less efficient scavenger of H2O2 as compared with APX enzyme (Sharma and Dubey 2005). It is also reported that Cd2+ metal may inhibit enzymes in H2O2 removal (CAT and APX) causing H2O2 accumulation in plants (Hatata and Abdel-Aal 2008).
In both roots and shoots of sensitive cv. DR-92 almost a constant activity of GR throughout the growth period was observed. A low activity of GR in A. maritimum is also reported under Cd stress (Schickler and Caspi 1999). Recently Zhou et al. (2008) showed that GR activities in leaves of alfalfa were not affected by Hg2+ exposure at low (1–10 μM) concentrations as compared to controls, but raising the Hg2+ concentrations (20–40 μM) remarkably increased the GR activities. It is known that GSH is oxidized during removal of the accumulating H2O2 under abiotic stress conditions (Szalai et al. 2009). Stress induced changes in level of H2O2 affects GSH/GSSG ratio, that affects the signaling causing thereby, changes in GR levels. With imposition of Cd2+ and heat stress, an increased GR activity in cv. Bh-1 observed herein could possibly be a result of changes in the expression of gene, regulating its synthesis. Miller and Mittler (2006) reported that the heat shock, transcription factors (Hsfs) that control the rapid induction of heat shock proteins (HsPs) in response to heat shock also function as molecular sensors and directly sense reactive oxygen species (ROS) and control the expression of oxidative stress response genes during oxidative stress. In the present study this oxidative stress was caused due to Cd toxicity. A recent report from Chao et al. (2009) suggest that accumulation of H2O2 during heat shock signals the increase in GSH levels thereby protecting the plant from oxidative damage caused by other abiotic stressors. Cd stress caused increase in HsfA4a expression, together with the metallothionein gene (MT) in roots of wheat and rice (Shim et al. 2009). These authors suggested that this HsfA4a confers Cd tolerance by upregulating MT gene expression in planta. The indirect regulation of protein by H2O2 and GSH may occur due to a cross-talk between the two species that affects thiol-disulfide transitions reported specially for GR (Dixon et al. 2005) playing thereby a role in signaling and responses to abiotic stress (Rausch et al. 2007). An interaction between the H2O2/GSH/GR redox system in combating a combination of stresses has also been suggested by Riechheld et al. (2007). Enhanced activities of enzymes of ascorbate–glutathione cycle (glutathione reductase) observed in Cd2+ stressed rice seedlings appear to be due to the need to maintain a favourable redox status, by maintaining sufficient level of reduced ascorbate and reduced glutathione and to overcome the possible problems of oxidation (Ranieri et al. 1996). Higher GR values in shoots than in roots suggest a tissue specific distribution of the enzyme in rice seedlings. The tolerant rice cv. Bh-1 possibly has enhanced formation of PCs resulting from more utilization of GSH and elevated GR activity, the effect being enhanced significantly in Cd treatments at day 10 in both roots/shoots than under Cd + heat treatments or heat stress alone. Results therefore also suggest better performance of GR under Cd stress alone than under heat or Cd + heat.
In conclusion results suggest that the Cd2+ toxicity + heat combinations should be regarded as a new state of abiotic stress in rice plants that requires a new defense or acclimation response perhaps in addition to the existing one. The potential effects of Cd2+ or/and heat stress combinations could vary depending on the relative level of each of the different stresses taken together and varietal differences. An involvement of cellular antioxidant defense systems in the adaptation of Oryza sativa to Cd2+ toxicity and/or heat stress, is indicated and rice cv. Bh-1 seems to be better adapted to combat Cd2+ metal concentrations and heat stress than cv. DR-92. The effect of Cd2+ concentrations and heat stress on growth and metabolism of rice seedlings when given individually is different than that observed in stress combination. In all there seems to be no linear relationship in antioxidant enzymes and stress combination in rice cvs. DR-92 and Bh-1, as both up and down regulations of antioxidant enzymes were observed that perhaps occur at both transcriptional and translational levels and also vary with the type and duration of stress and the plant variety. Heat stress alone causes enhanced oxidative stress but when given in combination with Cd largely seem to mitigate the effect of latter, in rice seedlings in cv. DR-92. APX activity play an important role in conferring better survival of rice seedlings of cv. DR-92 under combination of Cd + heat stresses whereas GR confers better survival towards Cd stress in tolerant cv. Bh-1.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione reductase
- HSP:
-
Heat shock protein
- MDAR:
-
Monodehydroascorbate reductase
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Alcazar MD, Egea C, Espin A, Candela EM (1995) Peroxidase isoenzymes in the defense response of Capsicum annuum to Phytophthora capsici. Physiol Plant 94:736–742
Anderson A (1997) Heavy metals in commercial fertilizers, manure and lime, cadmium balance for cultivated soils. Reports of Agricultural College of Sweden. Series A Nr 283
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Current Sci 10:1227–1238
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. J Biol Chem 195:133–139
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–91
Carlberg I, Mannervik B (1985) Glutathione reductase. Method Enzymol 113:488–495
Chao YY, Hsu YT, Kao CH (2009) Involvement of glutathione in heat shock-and hydrogen peroxide-induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 318:37–45
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani EL (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chaoui A, Jarrar B, El Ferjani E (2004) Effects of cadmium and copper on peroxidase, NADH oxidase and IAA oxidase activities in cell wall, soluble and microsomal membrane fractions of pea roots. J Plant Physiol 161:1225–1234
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reaction of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci (USA) 38:3811–3815
Dixon DP, Skipsay M, Grundy NM, Edwards R (2005) Stress induced protein S- glutathionylation in Arabidopsis. Plant Physiol 138:2233–2244
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxide in the development of water impermeable seed coats in Sida spinosa L. Planta 157:224–232
Gao JG, Xiao Q, Ding LP, Chen MJ, Yin L, Li JZ, Zhou SY, He GY (2008) Differential responses of lipid peroxidation and antioxidants in Alternanthera philoxeroids and Oryza sativa subjected to drought stress. Plant Growth Regul 56:89–95
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Expt Bot 53:1–11
Hatata MM, Abdel-Aal EA (2008) Oxidative stress and antioxidant defense mechanisms in response to cadmium treatments. American-Eurasian J Agric Environ Sci 4(6):655–669
Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. Cal Agri Exp Sta Cir 3:346–347
Howarth CJ (2005) Genetic improvements of tolerance to high temperature. In: Ashraf M, Harris PJC (eds) Abiotic Stresses: Plant Resistance Through Breeding and Molecular Approaches. Howarth Press Inc, New York
Iannelli MA, Pietrini F, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites australis plants. Plant Physiol Biochem 40:977–982
Kanazawa S, Sano S, Koshiba T, Ushimaru T (2000) Changes in antioxidative enzymes in cucumber cotyledons during natural senescence: comparison with those during dark-induced senescence. Physiol Plant 109:211–216
Kochhar S, Kochhar VK (2005) Expression of antioxidant enzymes and heat shock proteins in relation to combined stress of cadmium and heat in Vigna mungo seedlings. Plant Sci 168:921–929
Lee U, Rioflorido I, Hong SW, Larkindale J, Waters ER, Vierling E (2007) The Arabidopsis ClpB/Hsp 100 family of proteins: chaperones for stress and chloroplast development. Plant J 49:115–127
Lei Y, Chen K, Tian X, Korpelainen H, Li C (2007) Effect of Mn toxicity morphological and physiological changes in two Populus cathayana populations originating from different habitats. Trees 21:569–580
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent J Biol Chem 193:265–275
Lu JJ, Chang TT (1980) Rice in its temporal and spatial perspective. In: Luh BS (ed) Rice, production and utilization. AVI Publishing Co., Westport, CT, pp 1–74
Markovska YK, Gorinova NI, Nedkovska MP, Miteva KM (2009) Cadmium-induced oxidative damage and antioxidant responses in Brassica juncea plants. Biol Plant 53(1):151–154
Miller G, Mittler R (2006) Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Annals Bot 98:279–288
Mishra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxides in spinach chloroplast. Plant Cell Physiol 22:867–880
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Prasad KVSK, Pardha Saradhi P, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot 42:1–10
Ranieri A, D’Urso G, Nali C, Lorenzini G, Soldaniti GF (1996) Ozone stimulates apoplastic antioxidant systems in pumpkin leaves. Physiol Plant 97:381–387
Rausch T, Gromes R, Liedschulle V, Muller I, Bogs J, Galovic V, Wachter A (2007) Novel insight into the regulation of GSH triosynthesis in higher plants. Plant Biol 9:565–572
Riechheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y (2007) Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 19:1851–1865
Rizhsky L, Hongjian L, Joel S, Vladimir S, Sholpan D, Mittler R (2004) When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Sairam RK, Srivastava GC, Saxena DC (2000) Increased antioxidant activity under elevated temperature–a mechanism of heat stress tolerance in wheat genotypes. Biol Plant 43:245–251
Sato Y, Murakami T, Funatsuki H, Matsuba S, Saruyama H, Tanida M (2001) Heat Shock- mediated APX gene expression and protection against chilling injury in rice seedlings. J Expt Bot 52:145–151
Schickler H, Caspi H (1999) Response of antioxidative enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum. Physiol Plant 105:39–44
Shah K, Dubey RS (1995) Effect of cadmium on RNA level as well as activity and molecular forms of ribonuclease in growing rice seedlings. Plant Physiol Biochem 33:577–584
Shah K, Dubey RS (1998) Cadmium elevates level of protein, amino acids and alters activity of proteolytic enzymes in germinating rice seeds. Acta Physiol Plant 20:189–196
Shah K, Dubey RS (2005) Plant metabolism under temperature stress. In: Dwivedi P, Dwivedi RS (eds) Physiology of Abiotic Stress in Plants. Agrobios (India), Jodhpur, pp 243–274
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shamsi IH, Wei K, Zhang GP, Jilani GH, Hassan MJ (2008) Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biol Plant 52(1):165–169
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Shim D, Hwang JU, Lee J, Lee S, Choi Y, An G, Martinoia E, Lee Y (2009) Orthologs of the class A4 Heat Shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. The Plant Cell online acc no. 10.1105/tpc.109.066902
Singh MP, Singh DK, Rai M (2007) Assessment of growth, physiological and biochemical parameters and activities of antioxidative enzymes in salinity tolerant and sensitive Basmati rice varieties. J Agron Crop Sci 193:398–412
Szalai G, Kellos T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80
Ushimaru T, Ogawa K, Ishida N, Shibasaka M, Kanematsu S, Asada K, Tsuji H (1995) Changes in organelle superoxide dismutase isoenzymes during air adaptation of submerged rice seedlings: differential behaviour of isoenzymes in plastids and mitochondria. Planta 196:606–613
Van Assche F, Clijster H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Vierling E (1991) The role of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42:579
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang J, Zhang H, Allen RD (1999) Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol 40:725–732
Xiaozhong L, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
Zhang J, Kirkham MB (1994) Drought-stress induced changes in activities in superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhou ZS, Wang SJ, Yang Z-M (2008) Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 70:1500–1509
Acknowledgments
Financial support for the work by Department of Science and Technology, Govt. of India, New Delhi in the form of a project (SR/FT/L-75/2003) is gratefully acknowledged. Banaras Hindu University for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Nahakpam and K. Shah both contributed equally to the present work.
Rights and permissions
About this article
Cite this article
Nahakpam, S., Shah, K. Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings. Plant Growth Regul 63, 23–35 (2011). https://doi.org/10.1007/s10725-010-9508-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9508-3