Abstract
Lectins are non-immune carbohydrate-binding proteins/glycoproteins that are found everywhere in nature, from bacteria to human cells. They have also been a valuable biological tool for the purification and subsequent characterisation of glycoproteins due to their carbohydrate binding recognition capacity. Antinociceptive, antiulcer, anti-inflammatory activities and immune modulatory properties have been discovered in several plant lectins, with these qualities varying depending on the lectin carbohydrate-binding site. The Coronavirus of 2019 (COVID-19) is a respiratory disease that has swept the globe, killing millions and infecting millions more. Despite the availability of COVID-19 vaccinations and the vaccination of a huge portion of the world's population, viral infection rates continue to rise, causing major concern. Part of the reason for the vaccine's ineffectiveness has been attributed to repeated mutations in the virus's epitope determinant elements. The surface of the Coronavirus envelope is heavily glycosylated, with approximately sixty N-linked oligomannose, composite, and hybrid glycans covering the core of Man3GlcNAc2Asn. Some O–linked glycans have also been discovered. Many of these glyco-chains have also been subjected to multiple mutations, with only a few remaining conserved. As a result, numerous plant lectins with specificity for these viral envelope sugars have been discovered to interact preferentially with them and are being investigated as a potential future tool to combat coronaviruses such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by preventing viral attachment to the host. The review will discuss the possible applications of plant lectins as anti-coronaviruses including SARS-CoV-2, antinociceptive, anti-inflammation and its immune modulating effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell surface glycoconjugates coat are important in a variety of biological pathways, including cell–cell adhesion, inflammatory translocation, host–pathogen interactions, immune response initiation, and cancer metastasis [1,2,3,4]. Carbohydrate-binding proteins that traverse the surface of opposing cells are involved in all of these activities [5]. Plant parts such as leaves, barks, seeds, and roots have been utilised by human groups in the prevention and treatment of diseases, as well as for healthcare, since ancient times [6]. Over 90% of folk medicine treatments in Africa and Asia are made up of plants, and the bulk of these medicines are employed by peasants or nomads. These plant products have long been used to treat a variety of illnesses, including infectious disorders caused by bacteria, fungus, and viruses, as well as non-communicable diseases like heart disease, cancer, diabetes, and chronic lung disease [7, 8]. The current analgesia-inducing pharmaceuticals, such as opioids and non-steroidal anti-inflammatory drugs (NSAIDs), are widely believed to be unsuitable for many patients because to their side effects and low efficacy [9]. As a result, new drug development becomes necessary. The accidental discovery in 1888 by German doctoral student Peter Hermann Stillmark that the castor bean (Ricinus communis) extract can agglutinate erythrocytes, a mechanism later confirmed to occur through erythrocyte surface glycoconjugates, had marked a watershed moment in our current understanding the plant carbohydrate-binding proteins in particular, as well as their animal/microbial equivalents in general [10]. These glycan-interacting proteins were given the name lectin, which comes from the Latin word "legere," which meaning "to choose or take up." In the years afterwards, research has proven the presence of such proteins not just in plant and animal cells, but also in bacteria, viruses, yeast, and parasites, where they may help these microorganisms bind to glycoproteins and glycolipids and permeate the host cell surface [11]. These proteins have been characterised as any multivalent protein/glycoprotein that has at least one non-catalytic domain that can bind reversibly with sugars or carbohydrates and hence produces glycan interaction [12, 13]. Lectins are assigned to conduct several biological functions such as endocytosis, act as intracellular transport vehicles for glycoproteins, and regulate the protein composition in the blood due to their specific carbohydrate-binding site(s) [14]. Animal lectin was identified in 1872 before plant lectin, however it was not recognised as a glycan-binding protein [15]. Although there are no obvious structural similarities between animal and plant lectins, they both have the ability to interact with and recognise certain glycan receptors, underscoring the importance of these proteins in molecular recognition [16]. Plant lectins are found in practically every area of the body plant like seeds, leaves, bark, stem, flower, roots, etc., but animal and microbial lectins are found in much less quantities [17, 18].
Plant lectin can account for up to 10% of the total soluble protein in the seeds [19, 20]. The abundance of plant lectin [21], their ease of isolation [17], and the rapid advancement in affinity chromatography preparation that facilitated the purification of plant lectin in a single or two steps [22, 23], have all aided in conducting in-depth studies to resolve the ambiguity of their structures, possible biological effects, and clinical applications. Despite the fact that many plant lectins have similar primary and secondary structures, they have distinct biological effects, which are most likely due to their various glycan recognition specificities. Furthermore, scientific evidence is growing that several plant lectins have antinociceptive, anti-inflammatory, antioxidant, and gastroprotective activities. Others are known to suppress a wide range of microorganisms, including viruses, parasites, nematodes, and bacteria [24,25,26,27,28]. Coronavirus is the principal cause of COVID-19, an acute respiratory illness that originated in Wuhan, China and produced a worldwide outbreak that killed millions and sickened millions [29]. To combat the disease's high mortality and morbidity rates, many vaccinations have been developed and licenced around the world. The efficacy of these vaccines, however, is questioned due to the increased frequency of virus spike protein changes [30,31,32]. However, because the various N-linked glycosylation points of coronavirus-2 protein, which play a key role in viral virulence, are largely conserved, the possibility of using carbohydrate-binding agents like lectins to target the virus' glycans and thus interfere with its initial binding stage to the host cell surface receptors has been explored [33,34,35]. In this review, we will go through the possible usefulness of plant lectins in combating coronavirus illnesses, as well as their antiulcer, anti-inflammatory, and antinociceptive properties.
The glycosylation of spike protein
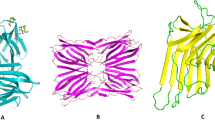
Spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins are the four primary structural proteins of the coronavirus. SARSCoV-2's principal structural protein antigen, spike protein (S), mediates coronavirus fusion into susceptible human cells through interactions with the angiotensin-converting enzyme-2 (ACE-2) receptor [36]. Unlike other SARS-CoV-2 viral proteins, however, it is responsible for inducing the host immune response, and antibodies directed against the S protein can provide protection against future infections [37]. The S protein (180–200 kDa) is a homotrimer made up of two subunits, S1 and S2, that are connected by a membrane-embedded serine 2 protease. The receptor-binding site is found in S1, while the viral fusion is found in S 2. Cryo-EM and mass spectrometry structural studies of the Spike protein revealed that it is heavily glycosylated, with as many as 66 N-glycosylation points (22 per monomer), covering the protein's surface and helping to mediate the pathogen's virulence while also shielding the vulnerable viral receptor-binding domain (RBD) from neutralising human antibodies [38, 39] Fig. 1. The glycosylation sites N165 and N234 (mannose-rich glycans), which are positioned near the ACE-2 RBD and have been discovered to have a role in the ACE2-S protein interaction by all-atom molecular dynamic modelling (MD), are of particular interest. The receptor glycan interaction was effectively reduced, but not completely eliminated, by point mutations of N165A and N234A, which caused in glycosylation depletion at these locations. Glycoproteins on the envelope of SARS CoV-2 point to potential uses of lectins as a therapeutic method [40]. When a virus is budding, the host cell creates a two-layer envelope, which makes the virus's components reliant on the cell membrane of origin [41]. In the layers of the SARS-CoV-2 envelope, host enzymes glycosylate a few of the proteins. These glycoproteins assist the virus' adherence, invasion, and entrance as well as the development and control of immune responses. Examples of glycoproteins on the envelope of SARS-CoV-2 that play crucial roles in its pathogenesis include the spike and membrane proteins known as S-protein and M-protein, respectively. The S-protein forms trimers and interacts with the angiotensin-converting enzyme 2 (ACE2) to mediate the adhesion between SARS-CoV-2 and the host cell [42]. There are three potential O-glycosylation sites and 22 potential N-glycosylation sites located in subunits S1 and S2, respectively. Regarding this, the S1 glycoprotein of SARS-CoV-2 reveals ligands for a number of innate immunological receptors, especially C-type lectin receptors (CLRs), which are known to bind certain glycans primarily in a way dependent on C-type lectin. Immune system cells like macrophages, dendritic cells (DCs), and monocytes commonly express CLRs, such as macrophage mannose receptor (MMR), macrophage galactose-type lectin (MGL), dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), lymph node-specific intercellular adhesion molecule-3-grabbing integrin (L-SIGN), and Dectin-2. All SARS-CoV-2 variants classified as variants of interest (B.1.427/epsilon, B.1.429/epsilon, B.1.525/eta, B.1.526/iota, B.1.617.1/kappa, B.1.617.3, and P2/zeta) and variants of concern (B.1.1.7/alpha, P.1/gamma, B1.351/beta, B.1.617. Data indicate that with the S-614G variation, half of the N-glycosylation sequences altered their glycan distribution. These findings highlighted the potential importance of these glycosylation sites in appropriately orienting the virus's receptor binding domain conformation [43] and references mentioned therein). Several researchers have reported recurring mutations at N-glycans utilising various expression vectors [39]. Despite this, 19 glycosylation sites were discovered to be preserved. However, only a little amount of O-7 glycosylation has been discovered [31]. The SARS-CoV-2 genome was expressed on the human cell line HEK-293, which resulted in a variety of complicated glycosylation patterns, most of which were of the mannose-rich type glycan.
The spike protein is broken down by host proteases into two functional subunits, S1 (blue) and S2 (red) (shown in pink). S1, which forms the trimer's apex, is crucial for lectin attachment and glycosylation. S2 is in charge of fusing with the membrane. Lectin glycosylated with the S1 spike protein of SARS corona virus
Targeting SARS-CoV spike glycoprotein with lectin
Drug repurposing has already begun in the search for an appropriate and effective treatment for SARS-CoV-2 infection. The inclusion of antimalarial medications like chloroquine and hydroxychloroquine in the COVID-19 therapy regimen sparked a lot of criticism before they were discontinued after being found to be ineffective [44, 45]. Anticancer medicines have also been postulated as potential inhibitors of viral replication; however, the increased toxicity associated with their administration has been challenged [46]. As previously noted, the extensively glycosylated surface of the SARS-CoV-2 S protein made it an appealing choice for lectins that bind to glycans, particularly those that have a plausible interaction with these glycans.
It is widely assumed that they will prevent the virus from attaching to host cells by generating a conformational shift that favours exposing the virus's epitope recognition site, so neutralising the virulent effect of the provoked immune response (Fig. 1). The presence of two glycosylation sequences N165 and N234 near the RBD of the spike protein, which are mostly made up of Man3 GlcNAc core, could be an attractive target for plant lectins with complex-type biantennary oligo-mannosyl saccharides. Greig and Bouillant discovered substantial binding of Concanavalin A (ConA), a lectin from the Canavalia ensiformis, to encephalomyelitis virus, a Coronavirus, over four decades ago. Snake venom phospholipase removes viral surface glycans. ConA-virus interactions were no longer possible highlighting the significance of the sugar chains in viral attachment [47].
Urtica dioica agglutinin (UDA), on the other hand, is a tiny 8.7 kDa lectin isolated from the Nettle (Urtica dioica L.), with a specificity for N, N′, N′′- tri-acetyl chitotriose (polymer of acetylated acetylglucosamines). This peptide lectin has a hydrophobic binding region adjacent to its sugar-binding site in addition to its sugar-binding site [48]. It stops SARS-CoV from replicating by interfering with viral attachment to the host cell, most likely by binding to N-acetylglucosamine (GlcNAc) units in the spike protein [49, 50]. The effect of the mannose-binding lectin griffithsin on MERS-CoV infection was investigated, and it was discovered that griffithsin, despite having no apparent cytotoxicity, had a strong inhibitory effect on MERS-CoV infection by binding to the mannose-rich viral surface protein [51].
In several other articles, the same lectin was shown to have a broad antiviral spectrum. The N-linked glycosylation sites on HIV-1 gp-120 have been confirmed to be recognised by griffithsin; lectin binding causes a change in the structure of gp-120, exposing the virus CD4 binding site [52, 53]. Similar results were achieved for the lectin from the banana (Musa acuminata), which inhibited HIV-1 by recognising the mannose-rich gp-120 viral outer layer glycoprotein in a range of picomole levels and thereby interfering with viral adherence to human cells [54]. A collection of 33 plant lectins with various specificities was tested, and the strongest antiviral action was found to be confined to lectins with the highest mannose binding specificity [55]. Using Vero B4 cells, antiviral activity of Wheat Germ Agglutinin (WGA) purified from Triticum vulgaris was discovered not only against the initially emerged SARSCoV-2, but also against its recent major two variants Alpha and Beta, with an IC50 of 10 ng/mL at both the pre-incubation period with the virus and during the viral infection. Surprisingly, this lectin exhibited a tight specificity for coronaviruses, as it had no effect on non-coronaviruses that cause respiratory distress [56].
Barre and his colleagues looked at the viral envelope shielding glycans of several viruses, including Ebola, herpes simplex, human cytomegalovirus, human immunodeficiency virus, influenza, chikungunya, Lassa, MERS-CoV, SARS-CoV, SARS-CoV-2, and Zika, all of which had high coat glycan heterogeneities. They proposed homodimeric mannose-specific legume lectins with a high affinity for the 1,6 fucosylated Man3 3GlcNAc2 core based on this. They came to the conclusion that lectins from Pisum sativum, Lens culinaris, Lathyrus ochrus, Canavalia ensiformis, Pterocarpus angolensis, and Vicia faba could be classed as having the best capability for binding SARS-CoV-2 spike envelopes [34, 57]. They did not consider these lectins to be coronavirus replication inhibitors because they only bind to the mannose-rich glycan receptors on the viral envelope surface and do not interfere with the inside viral genome, but they could be used in the future to prevent viral attachment to host cells and thus block the early stages of virulence processes (Fig. 2).
The N – linked glycosylation types – complex, hybrid and high mannose; O linked glycosylation (www.cryst.bbk.ac.uk)
In N-glycans (GlcNAc), N-acetylglucosamine is frequently the first sugar residue discovered. It is linked to the nitrogen of the Asn amide in the protein. Asn—X—Ser/Thr is the target sequence for N-glycosylation; X can be any amino acid residue other than Pro or Asp. Pro's side chain would cause steric hindrance, whereas Asp's negatively charged side chain would result in unfavourable interactions with negatively charged sugar residues. In several bacterial glycoproteins, an Asn residue has been linked to Glc, GalNAc, and L-Rha. There are three families of N-glycans: complex type, elevated mannose variety, and hybrid kind. They share a Penta saccharide core and are all derived from the same precursor oligosaccharide.
N-acetyl galactosamine (GalNAc) is usually the first sugar residue to remain when oligosaccharides in O-glycans are connected to a hydroxyl group of either serine or threonine. Less frequently, galactose, mannose, or xylose form O-glycosidic connections with Ser or Thr. The Ser or Thr hydroxyl group is linked to a single N-acetylglucosamine residue in the majority of nuclear and cytosolic proteins that are glycosylated. These exceptions include a number of transcription factors and proteins that are part of the nuclear pore complex. One Gal residue or glucosyl galactose disaccharide is added to hydroxylysine (Hyl) to glycosylate it (Table 1).
Potential applications of plant lectin as antinociceptive and anti-inflammatory agents
Apart from the current surge in demand for effective and strong analgesics, which has been followed by careful progress in pharmaceutical biotechnology and drug discovery, the demand for effective and powerful analgesics remains constant [72]. Morphine, thebaine, and the recently identified and commercialised serratiopeptidase are just a few of the regularly prescribed medications that were first taken from medicinal plants [73, 74]. Local populations across the globe, particularly in Africa and Asia, have long used various plant parts for pain treatment and inflammation reduction. Traditional medicine is believed to be used by 65 percent of Indians and 90 percent of Sudanese people, respectively [75, 76]. Treating experimental animals with clove aqueous extract considerably increased the latency period upon thermal stimulation (hotplate test), demonstrating the plant's analgesic properties. Clove (Syzygium aromaticum) buds are commonly used by indigenous to cure toothache [77]. Peppermint leaves, a fragrant herb, are used to calm an upset stomach on a regular basis [78]. The anti-inflammatory and antinociceptive activities of mint oil derived from three species, Mentha piperita L. var. pallescens, Mentha spicata L. subsp. Crispata, and Mentha suaveolens Ehrh, were investigated [79]. While various plant crude aqueous extracts have been evaluated for antinociceptive qualities and found to be effective, only a few studies have been published on the subject. Variable approaches such as abdominal writhing, formalin, and the hotplate tests are often used to investigate lectin anti-nociceptive effects in mouse and rat models. Anti-inflammatory responses are frequently tested by inducing paw oedema in animals using carrageenan, dextran, or serotonin. The migration of neutrophils and leukocytes into the peritoneal cavity is monitored in order to confirm the anti-inflammatory effect of lectins [80]. When mice were confronted with 1% carrageenan-induced inflammation and 0.8 percent acetic acid-induced abdominal writhing, an affinity-purified galactose-specific lectin isolated from the leaves of Bauhinia monandra demonstrated antinociceptive and anti-inflammatory effects in a dose-dependent manner. There was a 60 percent reduction in inflammation at a dosage of 60 mg lectin/kg mice. In the case of acetic acid pain induction, the anguish decrease was 71.3 percent. The presence of lectin in this plant, according to the authors, is responsible for the plant's widespread usage in traditional medicine as an anti-inflammatory and analgesic agent [81]. The anti-inflammatory activity of a heterodimer lectin-like protein isolated from the seeds of Clitoria fairchildiana was 64 percent attenuation in the mouse paws oedema caused by carrageenan administration. The lectin also stopped neutrophils from migrating. When mice were given acetic acid to generate pain, this lectin showed a 72 percent reduction in belly writhing, indicating that it had both anti-inflammatory and antinociceptive properties [82]. Several algal lectins have been found to have analgesic and anti-inflammatory properties, such as Caulerpa cupressoides lectin, which can reduce the effect of acetic acid-induced writhing by up to 86 percent. However, it was unable to produce significant antinociceptive effects in the hot plate experiment, indicating that the peripheral rather than central acting mechanism is involved [28]. In many cases, the antinociceptive properties of lectins have been attributed to the possible inhibition of inflammation-producing molecules such as bradykinin, prostaglandins, substance P, and some cytokines, such as IL-1 and TNF, which will lead to activation of chemo sensitive nociceptors and thus induction of pain [28]. Purification of a lectin from C. fairchildiana revealed that it is a glycoprotein with an electrophoretic pattern consisting of two bands with molecular weights of 100 and 116 kDa. The ability of the lectin to bind native rabbit erythrocytes has been confirmed. This lectin has antinociceptive and anti-inflammatory properties, which are linked to a neutrophil migration inhibition mechanism. These findings highlight the importance of further research into the use of C. fairchildiana lectin as a prototype in the development of new anti-inflammatory medicines [82].
Antiulcer properties of plant lectins
A gastric ulcer, caused by acid secretion or pepsin, is a disorder in which the layer protecting the stomach lining breaks down as a result of stomach acid. Carica papaya seeds flour is commonly used in Nigeria to cure peptic ulcers [83], whereas Acacia senegal and Aerva javanica are well-known in Sudan for their peptic ulcer-healing properties [75]. Many gastric-lesion inducers, including ethanol, Indomethacin, and Aspirin, have been used in the experimental animals. In mice, a homotetrameric galactose-binding lectin purified from Artocarpus incise and known as frutalin was successful in providing significant protection against both ethanol and indomethacin gastric injury, but in a dose unrelated manner, with a lectin at a concentration as low as 500 g/kg able to provide potent protection. However, pre-treatment with the 2- receptor antagonist Yohimbine had no effect on frutalin protection against ethanol lesions, implying that the 2- receptor was not involved in the lectin's caused action. Simultaneous administration of glibenclamide, a K + ATP channel inhibitor, resulted in a partial but considerable reduction of frutalin action, illustrating the role of the K + ATP channel in protecting the stomach lining against external mucosal attackers [84]. The preventive effect of a rabbit erythrocyte-specific seeds lectin isolated from the Brazilian plant Mucuna pruriens (L.) DC (MpLec) on ethanol-induced gastro-damage in mice was also investigated. However, pre-treatment with Yohimbine removed the MpLec protective effect, emphasising the importance of 2 adrenoceptors in the achieved defensive mechanism [27]. Another intriguing GlcNAc specific seeds lectin (Vicia cracca) that only agglutinates the human A-blood group was capable of decreasing ethanol damage by up to 63 percent [85]. The lectin was given at three different doses: 10, 100, and 1000 g/kg, and while all of them had a substantial effect, the 1000 g/kg dose provided the most protection.
Plant lectin induce the immune modulatory effect
Lectins are non-immune-derived carbohydrate-binding proteins. Cell–cell recognition, cell proliferation, cell migration, cell adherence to the extracellular matrix, and host parasite interactions are only a few of the biological processes in which they play a role [86, 87]. Because their interactions with receptor-link glycans on cell surfaces can induce cell signalling and physiological reactions, plant lectins have been widely exploited as valuable tools in biomedical research since the 1960s. Several plant lectins have immunomodulatory properties that are triggered by interactions with glycan moieties on immune cell surfaces. This contact may cause signal transduction, resulting in the production of certain cytokines and the induction of effective immune responses against malignancies or microbial infections. As a result, immunomodulatory lectins could have pharmacological applications or could aid in the identification of sugar targets for new therapeutic techniques. The most well-known plant lectin with immunomodulatory and anticancer properties is found in European mistletoe (Viscum album). Mistletoe lectins (ML) type I, II, and III are glycosylated, 56–64 kDa cytotoxic proteins that are type-2 ribosome-inactivating proteins and consist of two non-covalently connected pairs of disulfide-linked A-B dimers (RIP). The B-chain binds to galactosides preferentially [88], whereas the A-chain catalyses hydrolysis of the N-glycosidic bond at adenine4324 in eukaryotic 28S ribosomal RNA, blocking the protein biosynthesis elongation step [89]. The active component of the Viscum album extract, ML-I, has been isolated and is used as a supplemental treatment for cancer patients [90]. The ML-I B-chain attaches to glycans on cancer cells' surfaces, allowing the A-chain to enter the cytoplasm, where it is enzymatically active and highly cytotoxic. In vitro and in vivo studies have revealed that ML-antitumor I's effects are not only cytotoxic, but also immunomodulatory [91], an activity that is critical to the antitumor qualities [92]. The immunostimulatory action of ML-I is primarily exhibited by enhanced IL-12 production and cytokine-induced Natural Killer Cell activation according to the cloning of the mistletoe lectin gene and separate heterologous expression of the single chains [93]. Apart from European mistletoe, extracts from Korean and Chinese mistletoes (Viscum album coloratum and Viscum articulatum, respectively) include type-2 RIPs that bind D galactose and are structurally similar to ML [94]. In vitro and in vivo studies have shown that they, like ML-I, possess immunomodulatory characteristics. The immunomodulatory and anticancer properties of the Korean mistletoe lectin (KML) are due to the B-chain. The KML B-chain stimulates NK cell activation as well as macrophage production of cytokines and inflammatory mediators [95, 96]. Macrophage activation and cytokine production are triggered by KML interactions with TLR-4 molecules. TLR4 expression and TNF- production are increased when macrophages are stimulated by KLM, which can be inhibited by using anti-TLR4 antibodies or testing macrophages from different environments. Immunomodulatory plant lectins trigger Th1 immunity. The Th1 immune response, which is characterised by high levels of IFN ˠ, is usually mediated by an IL-12-dependent mechanism. Six plant lectins cause the production of IL-12 and IFN- ˠ: in vitro experiments with 12 distinct plant lectins revealed that six of the lectins induce the production of IL-12 and IFN- ˠ: PSA from Pisum sativum binds to N-glycans containing O-linked mannose with a fucose residue linked to N-acetylchitobiose; PHA-E and PHA-L from Phaseolus vulgaris, which respectively bind to bisected bi- and tri-antennary complex N-glycans and highly branched non-bisected complex N-glycans; and WGA from Triticum. Plant lectins like ArtinM from Artocarpus heterophyllus [97], the Korean mistletoe lectin from Viscum album coloratum [98], Cramoll from Cratylia mollis [99], BanLec from Musa paradisiaca [98], and garlic lectin from Alium sativum [100] promote the generation of Th1 cytokines. Th2 immunity is regulated by plant lectins. ScLL, a lectin from Synadenium carinatum that binds galactosides-containing glycans, reduced leukocyte trafficking and Th2 cytokine production in mice [101]. In animal models of the chronic inflammatory illness asthma, ScLL also reduced the pathological consequences [102]. The lectin Bchain of type-2 RIP from Ricinus communis, which binds to Dgalactose-containing glycans, including several glycoproteins produced on the surface of enterocytes, can trigger Th2 immunity. This characteristic prompted researchers to attach the ricin B-chain to the proinsulin gene's coding area, resulting in a fusion protein expressed in E. coli [101] or Solanum tuberosum [103]. Systemic tolerance to the fused autoantigen is favoured by lectin interaction with glycans on the surface of enterocytes. Fusion proteins (immunomodulatory lectin/autoantigen) expressed in edible plant tissues could be used to boost Th2 immunity and reduce autoimmunity. Plant lectins that recognise glycans on the surface of M cells may favour mucosal immunity against antigens that are given orally [104]. M cells have a specific glycosylation pattern on their surface, including lipid link fucose containing glycans [105] and transport a variety of materials from the intestinal lumen to the underlying lymphoid tissue of the mucosae, where a local and systemic potent immune response is initiated. Ulex europaeus agglutinin (UEA-1) binds to the surface of murine M cells and detects lipid linked fucose. This feature explains why UEA-1 is the most researched lectin when it comes to improving the potency of oral or nasal particle vaccinations. The apical surface of M cells from mice inoculated with these particles was able to attach to UEA-1-poly-L-lysine coated microparticles expressing HIV-1 genes [106].
Immunomodulatory effect of Artocarpus heterophyllus mannose binding lectin
The trisaccharide Man1-3 [Man1-6] Man core of N-glycans is recognised by ArtinM, also known as Artocarpin or KM + [107]. Innate immune cells, such as neutrophils, mast cells, dendritic cells, and macrophages, are activated when ArtinM interacts with some N-glycans on the cell surface. ArtinM treatment protects against Leishmania spp. and Paracoccidioides brasiliensis infection in the laboratory. ArtinM confers resistance by recognising N-glycans in the ectodomain of Toll-like receptors (TLR) expressed on the surface of innate immune cells, which leads to the production of interleukin 12 (IL-12) and the development of the Th1 adaptive immune response (Table 2). ArtinM's immunomodulatory effect and the mechanisms that underpin it are discussed in this article. We describe infection models in mice and discuss how they could be used to treat patients.
The homotetrameric ArtinM is made up of 13-kDa subunits. The main structure of ArtinM is a 149-amino-acid polypeptide chain with 52 percent similarity to the Jacalin sequence. The absence of internal post-translational cleavage in ArtinM, which keeps a short glycine-rich linker sequence holding the sections comparable to the Jacalin—and -chains together is credited with the distinctions between Jacalin and ArtinM [124]. These noncovalently linked Jacalin chains are made up of 133 and 20 residues, respectively [125], and are derived from a 17 kDa precursor that is not cleaved in the ArtinM molecule [126]. Each monomer's three-dimensional structure is a -barrel with a -prism folding. Each unit has a Mannose-binding carbohydrate-recognition domain (CRD). ArtinM is thus a four-CRD tetramer. The ligand mannotriose was discovered in the structure of ArtinM complexes. Three peptide loops (residues 14–17, 137–141, and 88–95) constitute a deep-seated binding site in the lectin. The primary and secondary sites make up this binding site. Hydrogen bonds dominate interactions at the principal site, which corresponds to two of the loops (residues 14–17 and 137–141). The third loop (residues 88–95) forms the secondary site, which establishes mostly van der Waals' interactions. Molecular modelling and crystallisation studies have revealed that structural differences between ArtinM and Jacalin are responsible for their different carbohydrate-binding specificities particularly in ArtinM's recognition of Dmannose but not D-galactose. ArtinM has a 1633-fold greater affinity for the glycoprotein horseradish peroxidase (HRP) than D-mannose. ArtinM binds to the mannosyl end of the branched oligosaccharide, which reinforces the trimannoside core of the HRP N-glycan. The xylose residue and lectin loops 86–95 is severely sterically clashed because to the mannosyl end's superposition with the trisaccharide in the complex. Eight hydrogen bonds are formed as a result, and binding energy is enhanced. GlcNAc or Fuc are the saccharides that can be attached to the core of mammalian N-glycans, and both can form numerous van der Waals interactions with ArtinM loop residues 87–93 [127]. Glycoarray investigation of ArtinM specificity indicated that the lectin recognises subsets of complex-type bi-antennary N-glycans having Man1-3(Man1-6) Man1-4GlcNAc1- 4GlcNAc. ArtinM recognition is aided by the branch linked to Man1-6, but Man1-3 elongation lowers lectin binding. Prior research of ArtinM specificity found that 1-6Man-extended mono-antennary glycans were better recognised than 1-3Man-extended mono-antennary glycans [128]. The selectivity of ArtinM binding to certain N-glycans, such as those associated to some protein cell receptors, is due to this peculiar binding mechanism. ArtinM's biological features, such as neutrophil chemotaxis and mast cell degranulation, are reproduced by rArtinM (recombinant ArtinM). When murine macrophages are treated with natural or recombinant versions of ArtinM, IL-12 is produced [129]. In addition, the recombinant form can trigger the same level of release of additional inflammatory products including TNF- and NO as the native form. In addition to the in vitro study, the immunomodulatory activity of ArtinM was replicated by rArtinM in a systemic fungal illness model induced by Paracoccidioides brasiliensis. ArtinM or rArtinM administration to mice prior to or after fungal inoculation enhanced Th1 immunity, as evidenced by high TNF- and IL-12 levels and low IL-4 levels. ArtinM's potential to increase IL-12 production by murine macrophages was the first indication that it has immunomodulatory properties. The ability to block IL-12 synthesis is dependent on lectin concentration and CRD, and D-mannose inhibits it selectively ArtinM stimulates a protective Th1 response against intracellular pathogens by promoting the production of IL-12. The importance of IL-12 in ArtinM-induced resistance was revealed by the reversal of its positive effect in IL-12 genetically defective animals. The interaction of cell-surface TLR with pathogen-associated molecular patterns is thought to trigger IL-12 synthesis by phagocytes (PAMPs). In animals, toll-like receptors are important for the start of innate immune responses against pathogens. They also recognise PAMPs from bacteria, viruses, and fungi [41]. TLRs have been identified in over a dozen distinct ways so far. TLRs 1–9 is conserved between humans and mice, TLR10 is expressed exclusively in humans, while TLR11 is active in mice (West et al. 2006). Transmembrane proteins of type I are known as TLRs. PAMP recognition is mediated by their ectodomains, which include leucine-rich repeats. Their intracellular Toll–IL-1 receptor (TIR) domains are needed for downstream signalling. Each TLR has a particular function in terms of PAMP detection and immune response induction, according to studies on mice lacking different TLRs [130]. As evidenced by the use of a TLR agonist, this discovery opens new doors in the development of treatment techniques. TLR2 recognition by ArtinM also activates dendritic cells to generate IL-12 (unpublished data). Indeed, ArtinM causes bone marrow-derived dendritic cells (BMDC) to develop, as seen by increased expression of MHC class II, CD80, and CD86 markers, which define a mature DC profile capable of priming T cells (Table 3). We have demonstrated that ArtinM administration elicits IFN-γ secretion by murine spleen cells. ArtinM was able to control the infection of L. amazonensis by acting on the early immune response. The immunomodulatory effect of ArtinM toward a Th1 profile is well supported by the results of Leishmania infection in mice. They also support the in vitro findings presented as “ArtinM targets TLR2 N-glycans to promote IL-12 production," which show that elevated IL-12 production is important for ArtinM's protection against Leishmania spp. ArtinM's pleiotropic effects are due to additional interactions with immune cells. Pleiotropism refers to a mediator's ability to function on different cell types, such as cytokines. It's a crucial trait that all cytokines possess, and it explains how they can affect both innate and adaptive immunity. The pleiotropic activities of cytokines are demonstrated by a variety of examples. In innate immunity, IL-12 increases NK cell cytotoxicity, while in adaptive immunity, it drives Th1 cell differentiation. In turn, IFN- stimulates macrophages in both the innate and adaptive cell-mediated immune responses. Furthermore, it boosts MHC molecule expression as well as antigen processing and presentation. Furthermore, it boosts MHC molecule expression as well as antigen processing and presentation. IL-10 inhibits activated macrophages and dendritic cells, reduces inflammation by suppressing Th1 cells, and inhibits macrophage IL-12 release. It is produced by macrophages, certain T helper cells, and mast cells. Despite the fact that pleiotropism permits cytokines to mediate a wide range of actions, their therapeutic utility is limited due to a number of unpleasant side effects. Pleiotropism is shown by ArtinM's biological characterisation.
Conclusion
Plant lectins have a high effectiveness against microorganisms such as bacteria, viruses, parasites, and fungus, as well as their reported function as antioxidants, antinociceptives, antitumor, antiulcer and immune modulation. Clinical investigations on their potential applicability as a drug shuttle for cancer treatment are currently underway, thanks to their unique sugar recognition site, which matches the recognised tumour cellular glycosylation alterations in minute details. Some of their disadvantages, such as their huge molecular weight, which will almost certainly cause immunogenicity and toxicity, may limit their widespread use in medicinal applications. As a result, the notion of using small molecular weight lectins seems encouraging and could pave the way to address the problem. The good news is that stronger variations of these helpful proteins, like lectibodies can be made by applying computational techniques or gene modification to boost their stability and durability and remove their mitogenic potential. In this regard, lectibodies are a novel and promising approach that, with great specificity and cheap cost, targets and neutralises carbohydrates on the surface of the viral envelope to treat viral infections. Lectibodies can prevent the virus or infected cells from entering the host cell by blocking specific cell surface receptors, but they can also trigger the immune system's CDC, ADCC, and ADCP to clear the virus or impacted cells from the body. It is possible that virus envelope glycans can use their inherent defence mechanisms to reduce the capacity of antiviral antibodies to restore normal host defences. We established the structural basis for ArtinM's sugar recognition and used our understanding of lectin specificity to explain how it interacts with glycosylate receptors on the cell surface. While there is still much to learn about the in-vivo and in-vitro biological effects of plant lectins, current research on modified lectins that have less unwanted activity without causing large structural changes may give hope for future plant lectin drug production and uses. The COVID-19 pandemic has led to an increase in viral infection rates, causing concern. The virus's surface is heavily glycosylated, with approximately sixty N-linked oligomannose, composite, and hybrid glycans covering the core of Man3GlcNAc2Asn. Many glyco-chains have been subjected to multiple mutations, with only a few remaining conserved. Consequently, plant lectins with specificity for viral envelope sugars have been discovered to interact preferentially with them. These plant lectins are being investigated as potential future tools to combat coronaviruses like SARS-CoV-2 by preventing viral attachment to the host. Furthermore, our laboratory's ongoing study on several lectins from tropical medicinal plants with extreme thermal and chemical stability has a lot of promise for exciting discoveries in the near future.
Data availability
The authors declares that the manuscript lacks any information necessary to access the dataset and other supporting files.
References
Colgan, S.P., et al.: Receptors involved in carbohydrate binding modulate intestinal epithelialneutrophil interactions. J. Biol. Chem. 270(18), 10531–10539 (1995). https://doi.org/10.1074/jbc.270.18.1053121
Gorelik, E., Galili, U., Raz, A.: On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 20(3–4), 245–277 (2001). https://doi.org/10.1023/a:1015535427597
Hevey, R.: Strategies for the development of Glycomimetic drug candidates. Pharmaceuticals 12(2), 55 (2019). https://doi.org/10.3390/ph12020055
Nardy, A.F.F.R., Freire-de-Lima, L., Freire-de-Lima, C.G., Morrot, A.: The sweet side of immune evasion: Role of glycans in the mechanisms of cancer progression. Front. Oncol. 6, 54 (2016). https://doi.org/10.3389/fonc.2016.00054
Brandley, B.K., Schnaar, R.L.: Cell-surface carbohydrates in cell recognition and response. J. Leukoc. Biol. 40(1), 97–111 (1986). https://doi.org/10.1002/jlb.40.1.97
Jones, M.B., Kansiime, F., Saunders, M.J.: The potential use of papyrus (Cyperus papyrus L.) wetlands as a source of biomass energy for sub-Saharan Africa. GCB Bioenergy 10(1), 4–11 (2018). https://doi.org/10.1111/gcbb.12392
Sofowora, A., et al.: The role and place of medicinal plants in the strategies for disease prevention. J. Altern. Complement. Med. 10(5), 210–229 (2013). https://doi.org/10.4314/ajtcam.v10i5.2
Spilatro, S.R., et al.: Characterization of a new lectin of soy bean vegetative tissues. Plant Physiol 110(3), 825–834 (1996). https://doi.org/10.1104/pp.110.3.825
Ahmadiani, A., Fereidoni, M., Semnanian, S., Kamalinejad, M., Saremi, S.: Antinociceptive and anti-inflammatory effects of Sambucus ebulus rhizome extract in rats. J. Ethnopharmacol. 61(3), 229–235 (1998). https://doi.org/10.1016/s0378-8741(98)00043-9
Sharon, N., Lis, H.: History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 14(11), 53R-62R (2004). https://doi.org/10.1093/glycob/cwh122
Sharon, N., Lis, H.: Microbial lectins and their glycoprotein receptors. New Compr. Biochem. 29, 475–506 (1997). https://doi.org/10.1016/S0167-7306(08)60626-2
Goldstein, I.J., et al.: What should be called a lectin? Nature 285(5760), 66–66 (1980)
Gomes, F.S., et al.: Antimicrobial lectin from S Chinus terebinthifolius leaf. J Appl Microbiol 114(3), 372–379 (2013)
Dias, R.O., Machado, L.S., Migliolo, L., Franco, O.L.: Insights into animal and plant lectins with antimicrobial activities. Molecules 20(1), 519–541 (2015). https://doi.org/10.3390/molecules20010519
Kilpatrick, D.C., Pusztai, A., Grant, G., Graham, C., Ewen, S.W.B.: Tomato lectin resists digestion in the mammalian alimentary canal and binds to intestinal villi without deleterious effects. FEBS Lett. 185, 299–305 (1985)
Reyes-Montaño, E. A., & Vega-Castro, N. (2018). Plant lectins with insecticidal and insectistatic activities. Insecticides Agriculture and Toxicology, Ghousia Begum, IntechOpen. https://www.intechopen.com/chapters/60115. https://doi.org/10.5772/intechopen.74962
Mishra, A., et al.: Structure-function and application of plant lectins in disease biology and immunity. Food Chem Toxicol 134, 110827 (2019). https://doi.org/10.1016/j.fct.2019.110827
Mo, H., et al.: Purification and characterization of Dolichos lablab lectin. Glycobiology. 9(2), 173–179 (1999). https://doi.org/10.1093/glycob/9.2.173
Roopashree, S., Singh, S.A., Gowda, L.R., Rao, A.G.: Dual-function protein in plant defence: Seed lectin from Dolichos biflorus (horse gram) exhibits lipoxygenase activity. Biochemical Journal 395(3), 629–639 (2006). https://doi.org/10.1042/BJ20051889
Sathe, S. K., & Deshpande, S. S. (2003). Beans. In B. Caballero (Ed.), Encyclopedia of Food Science and Nutrition (2nd ed) (pp. 403–412)
Spilatro, S.R., Cochran, G.R., Walker, R.E., Cablish, K.L., Bittner, C.C.: Characterization of a new lectin of soybean vegetative tissues. Plant Physiol. 110(3), 825–834 (1996)
Freeze, H.H.: Lectin Affinity Chromatography. Current Protocols in Protein Science (1995). https://doi.org/10.1002/0471140864.ps0901s00
O’Connor, B.F., et al.: Lectin Affinity Chromatography (LAC). Methods Mol. Biol. 1485, 411–420 (2017). https://doi.org/10.1007/978-1-4939-6412-3_23
Coelho, B.B., L. C., et al.: Lectins as antimicrobial agents. J. Appl. Microbiol. 125(5), 1238–1252 (2018). https://doi.org/10.1111/jam.14055
Gaofu, Q., et al.: In vitro assessment of plant lectins with anti-pinwood nematode activity. J. Appl. Microbiol. 98(1), 40–45 (2008). https://doi.org/10.1016/j.jip.2007.11.00422
Lusvarghi, S., Bewley, C.A.: Griffithsin: An antiviral lectin with outstanding therapeutic potential. Viruses (2016). https://doi.org/10.3390/v8100296
Pinto, I.R., Chaves, H.V., Vasconcelos, A.S., de Sousa, F.C.F., Santi-Gadelha, T., de Lacerda, J.T.J.G., Ribeiro, K.A., Freitas, R.S., Maciel, L.M., Filho, S.M.P., Viana, A.F.S.C., de Almeida Gadelha, C.A., Filho, G.C., de Paulo Teixeira Pinto, V., Pereira, K. M. A., Rodrigues e Silva, A. A., & Bezerra, M. M.: Antiulcer and Antioxidant Activity of a Lectin from Mucuna pruriens Seeds on ethanol- induced gastropathy: Involvement of Alpha-2 adrenoceptors and prostaglandins. Curr. Pharm. Des. 25(12), 1430–1439 (2019). https://doi.org/10.2174/1381612825666190524081433
VanderLei, E.S., Patoilo, K.K., Lima, N.A., Lima, A.P., Rodrigues, J.A., Silva, L.M., Lima, M.E., Lima, V., Benevides, N.M.: Antinociceptive and anti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int. Immunopharmacol. 10(9), 1113–1118 (2010). https://doi.org/10.1016/j.intimp.2010.06.014
Hu, B., Guo, H., Zhou, P., Shi, Z.L.: Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19(3), 141–154 (2021). https://doi.org/10.1038/s41579-020-00459-7
Baraniuk, C.: Covid-19: How effective are vaccines against the delta variant? BMJ 374, n1960 (2021). https://doi.org/10.1136/bmj.n1960
Hayawi, K., Shahriar, S., Serhani, M.A., Alashwal, H., Masud, M.M.: Vaccine versus Variants (3Vs): Are the COVID-19 vaccines Effective against the Variants? A Systematic Review. Vaccines 9(11), 1305 (2021). https://doi.org/10.3390/vaccines9111305
Khan, A., Khan, T., Ali, S., Aftab, S., Wang, Y., Qiankun, W., Khan, M., Suleman, M., Ali, S., Heng, W., Ali, S.S., Wei, D.Q., Mohammad, A.: SARS-CoV-2 new variants: Characteristic features and impact on the efficacy of different vaccines. Biomed. Pharmacother. 143, 112176 (2021). https://doi.org/10.1016/j.biopha.2021.112176
Ahmed, M.N., Jahan, R., Nissapatorn, V., Wilairatana, P., Rahmatullah, M.: Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 146, 112507 (2022). https://doi.org/10.1016/j.biopha.2021.112507
Barre, A., Van Damme, E.J.M., Simplicien, M., Le Poder, S., Klonjkowski, B., Benoist, H., Peyrade, D., Rougé, P.: Man-specific lectins from plants, fungi, algae and Cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) coronaviruses: Biomedical perspectives. Cells (2021). https://doi.org/10.3390/cells10071619
Martinez, D., Amaral, D., Markovitz, D., Pinto, L.: The use of lectins as tools to combat SARS-CoV-2. Curr. Pharm. Des. 27(41), 4212–4222 (2021). https://doi.org/10.2174/1381612827666210830094743
Hsieh, P.K., Chang, S.C., Huang, C.C., Lee, T.T., Hsiao, C.W., Kou, Y.H., Chen, I.Y., Chang, C.K., Huang, T.H., Chang, M.F.: Assembly of severe acute respiratory syndrome coronavirus RNA packaging signal into virus-like particles is nucleocapsid dependent. J. Virol. 79(22), 13848–13855 (2005). https://doi.org/10.1128/JVI.79.22.13848-13855.2005
Boechat, J.L., Chora, I., Morais, A., Delgado, L.: The immune response to SARS-CoV-2 and COVID-19 immunopathology—Current perspectives. Pulmonology 27(5), 423–437 (2021). https://doi.org/10.1016/j.pulmoe.2021.03.008
Huang, H.C., Lai, Y.J., Liao, C.C., Yang, W.F., Huang, K.B., Lee, I.J., Chou, W.C., Wang, S.H., Wang, L.H., Hsu, J.M., Sun, C.P., Kuo, C.T., Wang, J., Hsiao, T.C., Yang, P.J., Lee, T.A., Huang, W., Li, F.A., Shen, C.Y., Li, C.W.: Targeting conserved N-glycosylation blocks SARS-CoV-2 variant infection in vitro. EBioMedicine 74, 103712 (2021). https://doi.org/10.1016/j.ebiom.2021.103712
Shajahan, A., Pepi, L.E., Rouhani, D.S., Heiss, C., Azadi, P.: Glycosylation of SARS-CoV-2: Structural and functional insights. Anal. Bioanal. Chem. 413(29), 7179–7193 (2021). https://doi.org/10.1007/s00216-021-03499-x
Coltri, K.C., Oliveira, L.L., Pinzan, C.F., Vendruscolo, P.E., Martinez, R., Goldman, M.H., Panunto-Castelo, A., Roque-Barreira, M.C.: Therapeutic administration of KM+ lectin protects mice against Paracoccidioides brasiliensis infection via interleukin-12 production in a toll-like receptor 2-dependent mechanism. Am. J. Pathol. 173(2), 423–432 (2008). https://doi.org/10.2353/ajpath.2008.080126
Takeda, K., Akira, S.: Toll receptors and pathogen resistance. Cell. Microbiol. 5(3), 143–153 (2003). https://doi.org/10.1046/j.1462-5822.2003.00264.x
Zalpoor, H., Akbari, A., & Ephrin, N.-A. M. (2022). (Eph) receptor and downstream signaling pathways: A promising potential targeted therapy for COVID‑19 and associated cancers and diseases. Human Cell, 1–14
Zhao, X., Chen, H., Wang, H.: Glycans of SARS-CoV-2 spike protein in virus infection and antibody production. Frontiers in Molecular Biosciences (2021). https://doi.org/10.3389/fmolb.2021.629873
Altulea, D., et al.: What makes (hydroxy) chloroquine ineffective against COVID-19: Insights from cell biology. J. Mol. Cell Biol. 13(3), 175–184 (2021). https://doi.org/10.1093/jmcb/mjab016 JournalofMolecularCellBiology
Ferner, R.E., Aronson, J.K.: Chloroquine and hydroxychloroquine in covid-19. BMJ 369, m1432 (2020). https://doi.org/10.1136/bmj.m1432
El Bairi, K., Trapani, D., Petrillo, A., Le Page, C., Zbakh, H., Daniele, B., Belbaraka, R., Curigliano, G., Afqir, S.: Repurposing anticancer drugs for the management of COVID-19. Eur. J. Cancer 141, 40–61 (2020). https://doi.org/10.1016/j.ejca.2020.09.014
Greig, A.S., Bouillant, A.M.: Binding effects of concanavalin A on a coronavirus. Canadian Journal of Comparative Medicine: Revue Canadienne de Medecine Comparee 41(1), 122–126 (1977)
Shibuya, N., Goldstein, I.J., Shafer, J.A., Peumans, W.J., Broekaert, W.F.: Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch. Biochem. Biophys. 249(1), 215–224 (1986). https://doi.org/10.1016/0003-9861(86)90577-1
Kumaki, Y., Wandersee, M.K., Smith, A.J., Zhou, Y., Simmons, G., Nelson, N.M., Bailey, K.W., Vest, Z.G., Li, J.K., Chan, P.K., Smee, D.F., Barnard, D.L.: Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin. Urtica dioica agglutinin. Antiviral Research 90(1), 22–32 (2011). https://doi.org/10.1016/j.antiviral.2011.02.003
Saul, F.A., Rovira, P., Boulot, G., Van Damme, E.J., Peumans, W.J., Truffa-Bachi, P., Bentley, G.A.: Crystal structure of Urtica dioica agglutinin, a superantigen presented by MHC molecules of class I and class II. Structure. Academic Press 8(6), 593–603 (2000). https://doi.org/10.1016/S0969-2126(00)00142-8
Millet, J.K., Séron, K., Labitt, R.N., Danneels, A., Palmer, K.E., Whittaker, G.R., Dubuisson, J., Belouzard, S.: Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 133, 1–8 (2016). https://doi.org/10.1016/j.antiviral.2016.07.011
Alexandre, K.B., Gray, E.S., Pantophlet, R., Moore, P.L., McMahon, J.B., Chakauya, E., O’Keefe, B.R., Chikwamba, R., Morris, L.: Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site. J. Virol. 85(17), 9039–9050 (2011). https://doi.org/10.1128/JVI.02675-10
Fischer, K., Nguyen, K., LiWang, P.J.: Griffithsin retains anti-HIV-1 potency with changes in gp120 glycosylation and complements broadly neutralizing antibodies PGT121 and PGT126. Antimicrob. Agents Chemother. 64(1), e01084-e1119 (2019). https://doi.org/10.1128/AAC.01084-19
Swanson, M.D., et al.: A lectin isolated from bananas is a potent inhibitor of HIV replication. Int. J. Biol. Chem. 285(12), 8646–8655 (2010). https://doi.org/10.1074/jbc.M109.034926
Keyaerts, E., Vijgen, L., Pannecouque, C., Van Damme, E., Peumans, W., Egberink, H., Balzarini, J., Van Ranst, M.: Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 75(3), 179–187 (2007). https://doi.org/10.1016/j.antiviral.2007.03.003
Auth, J., Fröba, M., Große, M., Rauch, P., Ruetalo, N., Schindler, M., Morokutti-Kurz, M., Graf, P., Dolischka, A., Prieschl-Grassauer, E., Setz, C., Schubert, U.: Lectin from Triticum vulgaris (WGA) inhibits infection with SARS-CoV-2 and its variants of concern alpha and beta. Int. J. Mol. Sci. 22(19), 10205 (2021). https://doi.org/10.3390/ijms221910205
Garcia-Pino, A., Buts, L., Wyns, L., Imberty, A., Loris, R.: How a plant lectin recognizes high mannose oligosaccharides. Plant Physiol. 144(4), 1733–1741 (2007). https://doi.org/10.1104/pp.107.100867
Charan, R.D., Munro, M.H., O’Keefe, B.R., Rcii, S., McKee, T.C., Currens, M.J., Pannell, L.K., Boyd, M.R.: Isolation and characterization of myrianthus holstii lectin, a potent HIV-1 inhibitory protein from the plant myrianthus holstii (1). J. Nat. Prod. 63(8), 1170–1174 (2000). https://doi.org/10.1021/np000039h
David, M., et al.: A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARSCoV-2 and MERS-CoV infection in vivo. Cell Rep Med (2022). https://doi.org/10.1016/j.xcrm.2022.100774
Koshte, V.L., van Dijk, W., van der Stelt, M.E., Aalberse, R.C.: Isolation and characterization of BanLec-I, a mannoside-binding lectin from Musa paradisiac (banana). Biochemical Journal 272(3), 721–726 (1990). https://doi.org/10.1042/bj2720721
Chan, Y.S., Yu, H., Xia, L., Ng, T.B.: Lectin from green speckled lentil seeds (Lens culinaris) triggered apoptosis in nasopharyngeal carcinoma cell lines. Chinese Medicine 10(1), 25 (2015). https://doi.org/10.1186/s13020-015-0057-6
Wang, W., Li, Q., Wu, J., Hu, Y., Wu, G., Yu, C., Xu, K., Liu, X., Wang, Q., Huang, W., Wang, L., Wang, Y.: Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerging Microbes and Infections 10(1), 1519–1529 (2021). https://doi.org/10.1080/22221751.2021.1957720
LeVine, D., Kaplan, M.J., Greenaway, P.J.: The purification and characterization of wheat-germ agglutinin. Biochemical Journal 129(4), 847–856 (1972). https://doi.org/10.1042/bj1290847
Sheehan, S.A., Hamilton, K.L., Retzbach, E.P., Balachandran, P., Krishnan, H., Leone, P., Goldberg, G.S.: Evidence that Maackia amurensis seed lectin (MASL) exerts pleiotropic actions on oral squamous cells to inhibit SARS-CoV-2 infection and COVID-19 disease progression. Research Square. (2020). https://doi.org/10.21203/rs.3.rs-93851/v1
Van Damme, E.J.M., Van Leuven, F., Peumans, W.J.: Isolation, characterization and molecular cloning of the bark lectins from Maackia amurensis. Glycoconj. J. 14(4), 449–456 (1997). https://doi.org/10.1023/A:1018595300863
Gordts, S.C., Renders, M., Férir, G., Huskens, D., Van Damme, E.J., Peumans, W., Balzarini, J., Schols, D.: NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J. Antimicrob. Chemother. 70(6), 1674–1685 (2015). https://doi.org/10.1093/jac/dkv034
Kaur, R., Neetu, M., R., Jose, J., Kumar, P., & Tomar, S.: Glycan-dependent chikungunya viral infection divulged by antiviral activity of NAG specific chi-like lectin. Virology 526, 91–98 (2019). https://doi.org/10.1016/j.virol.2018.10.009
Balzarini, J., Hatse, S., Vermeire, K., Princen, K., Aquaro, S., Perno, C.F., De Clercq, E., Egberink, H., Vanden Mooter, G., Peumans, W., Van Damme, E., Schols, D.: Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 48(10), 3858–3870 (2004). https://doi.org/10.1128/AAC.48.10.3858-3870.2004
Hwang, H.J., Han, J.W., Jeon, H., Cho, K., Kim, J.H., Lee, D.S., Han, J.W.: Characterization of a Novel mannose-binding Lectin with antiviral Activities from Red Alga. Grateloupia chiangii. Biomolecules 10(2), 333 (2020). https://doi.org/10.3390/biom10020333
Sharma, A. et al. (2009). Purification and characterization of a lectin from Phaseolus vulgaris cv. (Anasazi Beans). Biomed. biotechnol. PubMed: 929568 https://doi.org/10.1155/2009/929568.
Yang, Y., Xu, H.L., Zhang, Z.T., Liu, J.J., Li, W.W., Ming, H., Bao, J.K.: Characterization, molecular cloning, and in silico analysis of a novel mannose-binding lectin from Polygonatum odoratum (Mill.) with anti-HSV-II and apoptosis-inducing activities. Phytomedicine 18(8–9), 748–755 (2011). https://doi.org/10.1016/j.phymed.2010.11.001
Khan, H., et al.: The analgesic potential of glycosides derived from medicinal plants Daru. DARU: J Pharm Sci 28(1), 387–401 (2020). https://doi.org/10.1007/s40199-019-00319-7
Bhagat, S., Agarwal, M., Roy, V.: Serratiopeptidase: A systematic review of the existing evidence. Int. J. Surg. 11(3), 209–217 (2013). https://doi.org/10.1016/j.ijsu.2013.01.010
Jehan, A. R. N. et al. (2017). Analgesic potential of extracts and derived natural products from medicinal plants, pain relief. In C. Maldonado (Ed.). https://www.intechopen.com/chapters/54987, Analgesics to Alternative Therapies vol. https://doi.org/10.5772/intechopen.68631. IntechOpen.
Karar, M.G.E., Kuhnert, N.: Herbal drugs from Sudan: Traditional uses and phytoconstituents. Pharmacogn. Rev. 11(22), 83–103 (2017). https://doi.org/10.4103/phrev.phrev_15_15
Prashantkumar, P., & Vidyasagar, G. M. (2008). Traditional knowledge on medicinal plants used for the treatment of skin diseases in Bidar district. http://hdl.handle.net/123456789/1588. (CRIS). Karnataka Council of Scientific & Industrial Research (CRIS). vol. India (pp. 273–276).
Kamkar Asl, M., et al.: Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna Journal of Phytomedicine 3(2), 186–192 (2013)
Uritu, C.M., Mihai, C.T., Stanciu, G.D., Dodi, G., Alexa-Stratulat, T., Luca, A., Leon-Constantin, M.M., Stefanescu, R., Bild, V., Melnic, S., Tamba, B.I.: Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manage. 2018, 7801543 (2018). https://doi.org/10.1155/2018/7801543
Mogosan, C., Vostinaru, O., Oprean, R., Heghes, C., Filip, L., Balica, G., Moldovan, R.I.: A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of mentha cultivated in Romania. Molecules 22(2), 263 (2017). https://doi.org/10.3390/molecules22020263
Nunes, B.S., Rensonnet, N.S., Dal-Secco, D., Vieira, S.M., Cavada, B.S., Teixeira, E.H., Moura, T.R., Teixeira, C.S., Clemente-Napimoga, J.T., Cunha, F.Q., Napimoga, M.H.: Lectin extracted from Canavalia grandiflora seeds presents potential antiinflammatory and analgesic effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 379(6), 609–616 (2009). https://doi.org/10.1007/s00210-009-0397-9
Campos, J.K.L., et al.: Anti-inflammatory and antinociceptive activities of Bauhinia monandra leaf lectin. Biochim Open 2, 62–68 (2016). https://doi.org/10.1016/j.biopen.2016.03.001
Leite, J.F., Assreuy, A.M., Mota, M.R., Bringel, P.H., Lacerda, R.R., Gomes, V.M., Cajazeiras, J.B., Nascimento, K.S., Pessôa, H.L., Gadelha, C.A., Delatorre, P., Cavada, B.S., Santi-Gadelha, T.: Antinociceptive and anti-inflammatory effects of a lectin-like substance from Clitoria fairchildiana R. Howard seeds. Molecules 17(3), 3277–3290 (2012). https://doi.org/10.3390/molecules17033277
Sverdén, E., et al.: Peptic ulcer disease. BMJ 367, l5495 (2019). https://doi.org/10.1136/bmj.l5495 BMJ
De Vasconcellos Abdon, A.P., Coelho de Souza, G., Coelho, N., de Souza, L., Prado Vasconcelos, R., Araújo Castro, C., Moreira Guedes, M., Pereira Lima, R.C., de Azevedo Moreira, R., de Oliveira Monteiro-Moreira, A.C., Rolim Campos, A.: Gastroprotective potential of frutalin, a d-galactose binding lectin, against ethanol-induced gastric lesions. Fitoterapia 83(3), 604–608 (2012). https://doi.org/10.1016/j.fitote.2012.01.005
Gorakshakar, A.C., Ghosh, K.: Use of lectins in immunohematology. Asian J Transfus Sci 10(1), 12–21 (2016). https://doi.org/10.4103/0973-6247.172180. (PMID: 27011665; PMCID: PMC4782487)
Van Damme, E.J.M., Peumans, W.J., Barre, A., Rougé, P.: Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 17(6), 575–692 (1998)
Van Damme, E.J.M., Lannoo, N., Peumans, W.J.: Plant lectins. Adv. Bot. Res. 48, 107–209 (2008). https://doi.org/10.1016/S0065-2296(08)00403-5
Olsnes, S., Stirpe, F., Sandvig, K., Pihl, A.: Isolation and characterization of viscumin, a toxic lectin from Viscum album L. (mistletoe). J. Biol. Chem. 257(22), 13263–13270 (1982). https://doi.org/10.1016/S0021-9258(18)33440-9
Endo, Y., Tsurugi, K., Franz, H.: The site of action of the A-chain of mistletoe lectin I on eukaryotic ribosomes. The RNA Nglycosidase activity of the protein. FEBS Letters 231(2), 378–380 (1988). https://doi.org/10.1016/0014-5793(88)80853-6
Klopp, R., Schmidt, W., Werner, E., Werner, M., Niemer, W., Beuth, J.: Influence of complementary Viscum album (iscador) administration on microcirculation and immune system of ear, nose and throat carcinoma patients treated with radiation and chemotherapy. Anticancer Res 25(1B), 601–610 (2005)
Hajto, T., Hostanska, K., Gabius, H.J.: Modulatory potency of the beta-galactoside-specific lectin from mistletoe extract (iscador) on the host defense system in vivo in rabbits and patients. Can. Res. 49(17), 4803–4808 (1989)
Bocci, V.: Mistletoe (Viscum album) lectins as cytokine inducers and immunoadjuvant in tumor therapy. A review: Journal of Biological Regulators and Homeostatic Agents 7(1), 1–6 (1993)
Eck, J., Langer, M., Möckel, B., Baur, A., Rothe, M., Zinke, H., Lentzen, H.: Cloning of the mistletoe lectin gene and characterization of the recombinant A-chain. Eur. J. Biochem. 264(3), 775–784 (1999). https://doi.org/10.1046/j.1432-1327.1999.00638.x
Yoon, T.J., Yoo, Y.C., Kang, T.B., Shimazaki, K., Song, S.K., Lee, K.H., Kim, S.H., Park, C.H., Azuma, I., Kim, J.B.: Lectins isolated from Korean mistletoe (Viscum album coloratum) induce apoptosis in tumor cells. Cancer Lett. 136(1), 33–40 (1999). https://doi.org/10.1016/s0304-3835(98)00300-0
Kang, T.B., Yoo, Y.C., Lee, K.H., Yoon, H.S., Her, E., Kim, J.B., Song, S.K.: Korean mistletoe lectin (KML-IIU) and its subchains induce nitric oxide (NO) production in murine macrophage cells. J. Biomed. Sci. 15(2), 197–204 (2008). https://doi.org/10.1007/s11373-007-9210-2
Park, H.J., Hong, J.H., Kwon, H.J., Kim, Y., Lee, K.H., Kim, J.B., Song, S.K.: TLR4-mediated activation of mouse macrophages by Koreanmistletoe lectin-C (KML-C). Biochem. Biophys. Res. Commun. 396(3), 721–725 (2010). https://doi.org/10.1016/j.bbrc.2010.04.169
Panunto-Castelo, A., Souza, M.A., Roque-Barreira, M.C., Silva, J.S.: KM (+), a lectin from Artocarpus integrifolia, induces IL-12 p40 production by macrophages and switches from type 2 to type 1 cell-mediated immunity against Leishmania major antigens, resulting in BALB/c mice resistance to infection. Glycobiology 11(12), 1035–1042 (2001). https://doi.org/10.1093/glycob/11.12.1035
Yoon, T.J., Yoo, Y.C., Kang, T.B., Her, E., Kim, S.H., Kim, K., Azuma, I., Kim, J.B.: Cellular and humoral adjuvant activity of lectins isolated from Korean mistletoe (Viscum album colaratum). Int. Immunopharmacol. 1(5), 881–889 (2001). https://doi.org/10.1016/s1567-5769(01)00024-8
De Melo, C.M., de Castro, M.C., de Oliveira, A.P., Gomes, F.O., Pereira, V.R., Correia, M.T., Coelho, L.C., Paiva, P.M.: Immunomodulatory response of Cramoll 1,4 lectin on experimental lymphocytes. Phytother. Res. 24(11), 1631–1636 (2010). https://doi.org/10.1002/ptr.3156
Dong, Q., Sugiura, T., Toyohira, Y., Yoshida, Y., Yanagihara, N., Karasaki, Y.: Stimulation of IFN-gamma production by garlic lectin in mouse spleen cells: Involvement of IL-12 via activation of p38 MAPK and ERK in macrophages. Phytomedicine 18(4), 309–316 (2011). https://doi.org/10.1016/j.phymed.2010.06.008
Carter, J.E., Yu, J., Choi, N.W., Hough, J., Henderson, D., He, D., Langridge, W.H.: Bacterial and plant enterotoxin B subunit-autoantigen fusion proteins suppress diabetes insulitis. Mol. Biotechnol. 32(1), 1–15 (2006). https://doi.org/10.1385/MB:32:1:001
Rogerio, A.P., Cardoso, C.R., Fontanari, C., Souza, M.A., Afonso-Cardoso, S.R., Silva, E.V., Koyama, N.S., Basei, F.L., Soares, E.G., Calixto, J.B., Stowell, S.R., Dias-Baruffi, M., Faccioli, L.H.: Anti-asthmatic potential of a D-galactose-binding lectin from Synadenium carinatum latex. Glycobiology 17(8), 795–804 (2007). https://doi.org/10.1093/glycob/cwm053
Carter, J.E., 3rd., Odumosu, O., Langridge, W.H.: Expression of a ricin toxin B subunit: Insulin fusion protein in edible plant tissues. Mol. Biotechnol. 44(2), 90–100 (2010). https://doi.org/10.1007/s12033-009-9217-1
Azizi, A., Kumar, A., Diaz-Mitoma, F., Mestecky, J.: Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 6(11), e1001147 (2010). https://doi.org/10.1371/journal.ppat.1001147
Jang, M.H., Kweon, M.N., Iwatani, K., Yamamoto, M., Terahara, K., Sasakawa, C., Suzuki, T., Nochi, T., Yokota, Y., Rennert, P.D., Hiroi, T., Tamagawa, H., Iijima, H., Kunisawa, J., Yuki, Y., Kiyono, H.: Intestinal villous M cells: An antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. U.S.A. 101(16), 6110–6115 (2004). https://doi.org/10.1073/pnas.0400969101
Manocha, M., Pal, P.C., Chitralekha, K.T., Thomas, B.E., Tripathi, V., Gupta, S.D., Paranjape, R., Kulkarni, S., Rao, D.N.: Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex europaeus-I lectin as M cell target. Vaccine 23(48–49), 5599–5617 (2005). https://doi.org/10.1016/j.vaccine.2005.06.031
Pereira-da-Silva, G., Roque-Barreira, M.C., Van Damme, E.J.: Artin M: A rational substitution for the names artocarpin and KM+. Immunol. Lett. 119(1–2), 114–115 (2008). https://doi.org/10.1016/j.imlet.2008.06.002
Fontenelle, T.P.C., Lima, G.C., Mesquita, J.X., Lopes, J.L.S., de Brito, T.V., Vieira Júnior, F.D.C., Sales, A.B., Aragão, K.S., Souza, M.H.L.P., Barbosa, A.L.D.R., Freitas, A.L.P.: Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 112, 1122–1130 (2018). https://doi.org/10.1016/j.ijbiomac.2018.02.058
Bitencourt, F.S., Figueiredo, J.G., Mota, M.R., Bezerra, C.C., Silvestre, P.P., Vale, M.R., Nascimento, K.S., Sampaio, A.H., Nagano, C.S., Saker-Sampaio, S., Farias, W.R., Cavada, B.S., Assreuy, A.M., de Alencar, N.M.: Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red marine alga Hypnea cervicornis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 377(2), 139–148 (2008). https://doi.org/10.1007/s00210-008-0262-2
Abreu, T.M., Ribeiro, N.A., Chaves, H.V., Jorge, R.J., Bezerra, M.M., Monteiro, H.S., Vasconcelos, I.M., Mota, É.F., Benevides, N.M.: Antinociceptive and anti-inflammatory activities of the lectin from marine red alga Solieria filiformis. Planta Med. 82(7), 596–605 (2016). https://doi.org/10.1055/s-0042-101762
Araújo, T.S., Teixeira, C.S., Falcão, M.A., Junior, V.R., Santiago, M.Q., Benevides, R.G., Delatorre, P., Martins, J.L., Alexandre-Moreira, M.S., Cavada, B.S., Campesatto, E.A., Rocha, B.A.: Anti-inflammatory and antinociceptive activity of chitin-binding lectin from Canna limbata Seeds. Applied Biochemistry and Biotechnology 171(8), 1944–1955 (2013). https://doi.org/10.1007/s12010-013-0470-1
Silva, H.C., Bari, A.U., Rocha, B.A., Nascimento, K.S., Ponte, E.L., Pires, A.F., Delatorre, P., Teixeira, E.H., Debray, H., Assreuy, A.M., Nagano, C.S., Cavada, B.S.: Purification and primary structure of a mannose/glucose-binding lectin from Parkia biglobosa Jacq. seeds with antinociceptive and anti-inflammatory properties. Journal of Molecular Recognition 26(10), 470–478 (2013). https://doi.org/10.1002/jmr.2289
de Oliveira Leite, G., Santos, S.A.A.R., Bezerra, F.M.D.H., Silva, S.E., F. E., de Castro Ribeiro, A. D., Roma, R. R., Silva, R. R. S., Santos, M. H. C., Santos, A. L. E., Teixeira, C. S., & Campos, A. R.: Is the orofacial antinociceptive effect of lectins intrinsically related to their specificity to monosaccharides? International Journal of Biological Macromolecules. MHC. CS, and AR 161, 1079–1085 (2020). https://doi.org/10.1016/j.ijbiomac.2020.06.132
Araújo, R.M.S., Vaz, A.F.M., Aguiar, J.S., Coelho, L.C.B.B., Paiva, P.M.G., Melo, A.M.M., Silva, T.G., Correia, M.T.S.: Lectin from Crataeva tapia bark exerts antitumor, anti-inflammtory and analgesic activities. Natural Products and Bioprospecting 1(2), 97–100 (2011). https://doi.org/10.1007/s13659-011-0014-8
Araújo, R.M., Ferreira, R.S., Napoleão, T.H., Carneiro-da-Cunha, Md., Coelho, L.C., Correia, M.T., Oliva, M.L., Paiva, P.M., Araújo, M.R.MSd., et al.: Crataeva tapia bark lectin is an affinity adsorbent and insecticidal agent. Plant Sci. 183, 20–26 (2012). https://doi.org/10.1016/j.plantsci.2011.10.018
Assreuy, A.M., Shibuya, M.D., Martins, G.J., De Souza, M.L., Cavada, B.S., Moreira, R.A., Oliveira, J.T., Ribeiro, R.A., Flores, C.A.: Anti-inflammatory effect of glucose-mannose binding lectins isolated from Brazilian beans. Mediators Inflamm. 6(3), 201–210 (1997). https://doi.org/10.1080/09629359791695
Moreira, R.A., Barros, A.C., Stewart, J.C., Pusztai, A.: Isolation and characterization of a lectin from the seeds of Dioclea grandiflora (Mart.). Planta 158(1), 63–69 (1983). https://doi.org/10.1007/BF00395404
Márcio, V.R., et al.: Purification and partial characterization of a lectin from Dioclea virgata Benth seeds. Fascículos Revista Brasileira de Fisiologia Vegetal. 8, 37–42 (1996)
Holanda, F.R., Coelho-de-Sousa, A.N., Assreuy, A.M., Leal-Cardoso, J.H., Pires, A.F., do Nascimento, K. S., Teixeira, C. S., Cavada, B. S., & Santos, C. F.: Antinociceptive activity of lectins from Diocleinae seeds on acetic acid-induced writhing test in mice. Protein Pept. Lett. 16(9), 1088–1092 (2009). https://doi.org/10.2174/092986609789055304
Renato, D.A.M., et al.: isolation and partial characterization of a lectin from seeds of Dioclea violacea. Braz. J. Plant. Physiol. 8(1), 23–29 (1996)
Do Nascimento, F.L.F., et al.: The anti-inflammatory effect of Andira Anthelmia lectin in rats involves inhibition of the prostanoid pathway. TNF-α and lectin domain. Research Square. (2021). https://doi.org/10.21203/rs.3.rs-718940/v1
De Freitas Pires, A., Bezerra, M.M., Amorim, R.M.F., do Nascimento, F. L. F., Marinho, M. M., Moura, R. M., Silva, M. T. L., Correia, J. L. A., Cavada, B. S., Assreuy, A. M. S., & Nascimento, K. S.: Lectin purified from Lonchocarpus campestris seeds inhibits inflammatory nociception. Int. J. Biol. Macromol. 125, 53–60 (2019). https://doi.org/10.1016/j.ijbiomac.2018.11.233
Oladokun, et al.: Anti-nociceptive and anti-inflammatory activities of Tetracarpidium conophorum seed lectin. Sci. Afr 3, e00073 (2019)
Pratap, J.V., Jeyaprakash, A.A., Rani, P.G., Sekar, K., Surolia, A., Vijayan, M.: Crystal structures of artocarpin, a Moraceae lectin with mannose specificity, and its complex with methyl-alpha-Dmannose: Implications to the generation of carbohydrate specificity. J. Mol. Biol. 317(2), 237–247 (2002). https://doi.org/10.1006/jmbi.2001.5432
Young, N.M., Johnston, R.A., Watson, D.C.: The amino acid sequences of jacalin and the Maclura pomifera agglutinin. FEBS Lett. 282(2), 382–384 (1991). https://doi.org/10.1016/0014-5793(91)80518-8
Ngoc, L.D., Brillard, M., Hoebeke, J.: The alpha- and betasubunits of the jacalins are cleavage products from a 17-kDa precursor. Biochem. Biophys. Acta. 1156(2), 219–222 (1993). https://doi.org/10.1016/0304-4165(93)90139-y
Lerouge, P., Cabanes-Macheteau, M., Rayon, C., Fischette-Lainé, A.C., Gomord, V., Faye, L.: N-glycoprotein biosynthesis in plants: Recent developments and future trends. Plant Mol. Biol. 38(1–2), 31–48 (1998). https://doi.org/10.1023/A:1006012005654
Nakamura-Tsuruta, S., Uchiyama, N., Peumans, W.J., Van Damme, E.J., Totani, K., Ito, Y., Hirabayashi, J.: Analysis of the sugar-binding specificity of mannose-binding-type Jacalinrelated lectins by frontal affinity chromatography—An approach to functional classification. FEBS J. 275(6), 1227–1239 (2008). https://doi.org/10.1111/j.1742-4658.2008.06282.x
Pranchevicius, M.C., Oliveira, L.L., Rosa, J.C., Avanci, N.C., Quiapim, A.C., Roque-Barreira, M.C., Goldman, M.H.: Characterization and optimization of ArtinM lectin expression in Escherichia coli. BMC Biotechnol. 12, 44 (2012). https://doi.org/10.1186/1472-6750-12-44
Akira, S., Uematsu, S., Takeuchi, O.: Pathogen recognition and innate immunity. Cell 124(4), 783–801 (2006). https://doi.org/10.1016/j.cell.2006.02.015
Santos, A.L.E., Leite, G.O., Carneiro, R.F., Roma, R.R., Santos, V.F., Santos, M.H.C., Pereira, R.O., Silva, R.C., Nagano, C.S., Sampaio, A.H., Rocha, B.A.M., Delatorre, P., Campos, A.R., Teixeira, C.S.: Purification and biophysical characterization of a mannose/N-acetyl-dglucosamine-specific lectin from Machaerium acutifolium and its effect on inhibition of orofacial pain via TRPV1 receptor. Arch. Biochem. Biophys. 664, 149–156 (2019). https://doi.org/10.1016/j.abb.2019.02.009
Nolte, S., de Castro, D., Damasio, A.C., Baréa, J.G., Magalhães, A., Mello Zischler, L.F.C., Stuelp-Campelo, P.M., Elífio-Esposito, S.L., Roque-Barreira, M.C., Reis, C.A., Moreno-Amaral, Andréa Novais.: BJcuL, a lectin purified from Bothrops jararacussu venom, induces apoptosis in human gastric carcinoma cells accompanied by inhibition of cell adhesion and actin cytoskeleton disassembly. Toxicon 59(1), 81–85 (2012). https://doi.org/10.1016/j.toxicon.2011.10.012. (ISSN 0041-0101)
Teixeira, C.R., Cavassani, K.A., Gomes, R.B., Teixeira, M.J., Roque-Barreira, M.C., Cavada, B.S., da Silva, J.S., Barral, A., Barral-Netto, M.: Potential of KM+lectin in immunization against Leishmania amazonensis infection. Vaccine 24(15), 3001–3008 (2006). https://doi.org/10.1016/j.vaccine.2005.11.067
Trinchieri, G., Wysocka, M., D’Andrea, A., Rengaraju, M., Aste-Amezaga, M., Kubin, M., Valiante, N.M., Chehimi, J.: Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog. Growth Factor Res. 4(4), 355–368 (1992). https://doi.org/10.1016/0955-2235(92)90016-b
Funding
This study received no financial support.
Author information
Authors and Affiliations
Contributions
All the authors are equally contributed until the completion of final drafting of the manuscript. OSA, RK and BNS helped in searching the review of literature, designing the tables. HJS, MAD and SSS did the concepts designed, drafting and preparation of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
For this paper, the authors say they have no competing interests.
Ethical approval
The authors declares that ethical approval was not required for this study or not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, O.S., Singh, S.S., Kamei, R. et al. Glycosylated SARs Cov 2 interaction with plant lectins. Glycoconj J 41, 185–199 (2024). https://doi.org/10.1007/s10719-024-10154-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-024-10154-x