Abstract
Lectins are non-immunological carbohydrate-binding proteins classified on the basis of their structure, origin, and sugar specificity. The binding specificity of such proteins with the surface glycan moiety determines their activity and clinical applications. Thus, lectins hold great potential as diagnostic and drug discovery agents and as novel biopharmaceutical products. In recent years, significant advancements have been made in understanding plant and microbial lectins as therapeutic agents against various viral diseases. Among them, mannose-specific lectins have being proven as promising antiviral agents against a variety of viruses, such as HIV, Influenza, Herpes, Ebola, Hepatitis, Severe Acute Respiratory Syndrome Coronavirus-1 (SARS-CoV-1), Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) and most recent Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). The binding of mannose-binding lectins (MBLs) from plants and microbes to high-mannose containing N-glycans (which may be simple or complex) of glycoproteins found on the surface of viruses has been found to be highly specific and mainly responsible for their antiviral activity. MBLs target various steps in the viral life cycle, including viral attachment, entry and replication. The present review discusses the brief classification and structure of lectins along with antiviral activity of various mannose-specific lectins from plants and microbial sources and their diagnostic and therapeutic applications against viral diseases.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several outbreaks of emerging and re-emerging pathogenic infections have plagued the global community in recent decades, posing severe concerns for health, immunity and the global economy. Due to urbanization, globalization, travel, international businesses, aging and climate change, newer viruses and their variants are emerging, spreading and being transmitted more frequently [1, 2]. As per the World Health Organization (WHO) report, RNA viruses such as HIV, Influenza virus, Dengue virus (DENV), Ebola virus (EBOV), Nipah virus, Zika virus (ZIKV), Hepatitis virus, SARS-CoV-1, MERS-CoV-1 and the current SARS-CoV-2 are the top global threats to mankind [3]. The global community is currently dealing with the SARS-CoV-2 pandemic. The overall scenario of pandemics has decimated the world due to the lack of sufficient vaccines and treatments accessible to the population. To combat severe pandemic situations, researchers are developing strategies for controlling the emergence and re-emergence of viruses with high epidemic potential. However, there is little understanding of the identity, epidemiology and pathophysiology of newly emerging or recurring viral diseases.
Antiviral drugs are known to limit viral infection by decreasing viral multiplication, targeting distinct viral proteins or host cellular components and neutralizing host receptors to inhibit virus uptake and prevent virus entrance. However, antiviral and antibiotic therapies are limited due to their non-specificity toward viral targets [4]. Viral infection or vaccination produces an adaptive immune response by neutralizing antibodies targeting specific surface viral antigens and interrupting the replication cycle before transcription and translation [5, 6]. However, early inhibition of viral penetration in target cells, selection of inhibitors and identification and characterization of the molecules to prevent viral entry are critical for treating fast-disseminating viral pandemics. Viral transmission and disease progression starts once the virus contacts glycoprotein-based host cell receptors; glycoproteins expressed on the surface of most viruses can be targeted to prevent virus entry [7, 8]. Lectins, the carbohydrate-binding proteins, bind to specific sugars of viral glycoproteins and have opened a wide range of possibilities to restrict viral entry to host cells by inhibiting the interaction between the viral envelope glycoproteins and host cell surface components [9]. Several plant lectins have antiviral activity against human-enveloped viruses like HIV [10], hepatitis C virus (HCV) [11], herpes simplex virus type 1 (HSV-I) [12], influenza virus [13], poxvirus [14], human cytomegalovirus (HCMV) [15], respiratory syncytial virus (RSV) [16], ebola virus (EBOV) [17] and coronavirus (SARS-CoV 2) [18].
Lectins are widely distributed proteins that reversibly and non-catalytically bind with high stereo-specificity to various sugars without modifying their carbohydrate moiety [19]. Lectins exist in animals, plants and microbes and possess high diversity in structure, amino acid composition, molecular weight and involvement of metal ions [20, 21]. Various lectins exhibit multiple biological and pharmacological activities such as anticancer, antiviral, insecticidal, antifungal, antiparasitic and immunomodulatory agents depending on their carbohydrate specificity and biochemical properties. Because of their significant immunomodulatory potential, plant lectins are also known to prevent microbial infections in several animal models [22].
On the basis of their carbohydrate-binding specificity, lectins can identify and bind distinct types of glycan structures present in the glycan shield of viruses. Antiviral lectins interact with high-mannose glycan structures of virus envelope proteins, added as post-translational modifications [23,24,25]. Mannose-binding lectins (MBLs) possess mannose-specific glycoconjugates with potent antiviral activity against human retroviruses [26]. They are widely found in all living species and have been isolated as well as identified most frequently in plants, algae, fungi and cyanobacteria [27]. Plant lectins are commonly found in storage tissues such as seeds, tubers, bulbs, corms, rhizomes, rootstocks and bark [28]. The envelope proteins have N-linked oligosaccharide attachment sites that help the virus evade the host immune system when glycosylated [29]. The envelope (Env) protein complex facilitates viral attachment and entry into target cells followed by activation of a cascade of conformational remodeling in the Env protein complex, leading to the viral envelope fusion with the host cellular membrane [30]. The lectin Griffithsin (GRFT) derived from red algae Griffithsia sp., is a mannose specific lectin known to bind and inhibit HIV. The HIV-1 Env spikes consist of a transmembrane gp41 trimer linked to an extracellular gp120 trimer having high-mannose glycans that help the virus to recognize and bind to CD4 cells [31]. The presence of high-mannose glycans in HCV has been reported to be recognized by mannose-specific lectin CVN (Cyanovirin) resulting in inhibition of HCV infection [11]. Moreover, Narcissus tazetta lectin (NTL) also a mannose-binding lectin, exhibits strong antiviral activity against influenza A (H1N1, H3N2, H5N1) and B viruses with IC50 values ranging from 0.20–1.33 μg/mL in a dose-dependent manner [16]. Furthermore, high mannose-type glycans have been identified on site N234 of spike glycoprotein of SARS-CoV-2 while complex-type N-glycans and high mannose type glycans have been identified at sites N165, N331 and N343 [32]. GRFT has also been found to inhibit spike protein-mediated SARS-CoV-2 cell-to-cell fusion with an IC50 of 323 nmol/L [33].
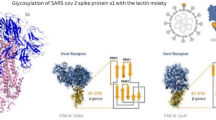
A pictorial representation of the interaction of different viruses with their human cell receptors and the blockade of this interaction in the presence of mannose-specific lectins is shown in Fig. 1. Each glycan site present on the viral surface (Fig. 1) has been modeled using the most prevalent sugar compositions, utilizing pre-existing Man5GlcNAc2 residues because compositional information was unavailable [34].
Enveloped viruses are highly adaptable pathogens that infect a wide range of hosts, including protozoans to mammals and exploit the molecular machinery of the host cell for reproduction. They have evolved together with their hosts and developed numerous strategies to undermine and evade the host's defense mechanisms. It has been found that viral envelope glycoproteins undergo spontaneous mutations causing the loss of oligosaccharide-attachment sites to evade recognition by exogenous (host-specific) lectins such as pattern recognition receptors (PRRs) and pathogen-associated molecular patterns (PAMPs) that are important components of innate immune system of the host. In this way, many viruses subvert and escape the host immune system surveillance leading to infection [35]. Depletion of the glycocalyx surrounding the envelope glycoproteins is detrimental to viral fitness [36]. In light of these facts, mannose-specific lectins can be used for developing antiviral medications by targeting the glycans of viral envelope proteins.
The present review provides an overview of the classification, structure and prospective diagnostic and prophylactic potential of mannose-specific lectins against viruses.
Classification of lectins
Lectins are also termed agglutinins or phytohemagglutinins and have the most extended scientific history of all plant proteins. The lectin terminology persisted in confusion until the most accepted definition came out. According to this, the presence of at least one noncatalytic domain that binds reversibly to a specific carbohydrate moiety is now considered the criterion for a protein to be called a lectin. Based on these considerations, plant lectins were recently defined as “all plant proteins” possessing at least one non-catalytic domain that binds reversibly to a specific mono- or oligosaccharide [37]. This definition, far less restrictive than all previous definitions, comprises a broad range of proteins with different agglutination and glycoconjugate precipitation properties [28]. Lectins have been classified on the basis of their carbohydrate-binding domain.
Over the years, there have been significant advancements in structural analysis techniques such as X-ray diffraction and NMR which are being utilized to investigate the molecular interactions between lectins and carbohydrates. These findings have resulted in the identification of an increasing number of high-resolution novel plant lectin structures [38]. The sequencing of amino acids of lectins has progressed more rapidly compared to other categories of plant proteins and the information obtained from lectin sequencing has facilitated search for lectin genes. The molecular study of these has genes provided essential insights into the fundamental structure of lectins and valuable information regarding their synthesis and post-translational modifications. Molecular cloning has confirmed the presence of extensive lectin gene families in certain plant species. Lastly, the genetic cloning of lectin genes from various plant species has shed light on the evolutionary connections between known plant lectins.
Lectin and glycan generally interact through hydrogen bonding and van der Waals forces. Most lectins are members of protein families with well-defined carbohydrate recognition domains (CRDs). The CRDs of different lectins within a family exhibit differential specificity and binding to N-glycans, O-glycans and glycolipids. Whereas some lectins have multiple CRDs that can participate in ligand binding, others have only a single CRD that relies on lectin clustering for high-affinity binding. CRD clustering allows stronger interactions with ligands and adds to the specificity of multivalent interactions. The lectin affinity for specific glycoconjugates depends on the structure, multivalency and density of glycans on molecules [39] (Fig. 2).
Latest classification of lectins based on carbohydrate recognition domain (CRD)
Lectins can be broadly divided into merolectins, hololectins, chimerolectins and superlectins. Merolectins consist of a single carbohydrate-binding domain. Being monovalent, they cannot precipitate glyco-conjugates or agglutinate cells. At present, only a few examples of merolectins, such as Hevein, a chitin-binding protein found in the latex of rubber trees (Hevea brasiliensis) [40] and the monomeric mannose-binding proteins from orchids [41] are well known. Hololectins have at least two identical and similar carbohydrate-binding domains. They are divalent and multivalent proteins that cause cell agglutination and glycoconjugate precipitation. The majority of plant lectins belong to this group. One example is Concanavalin A [42]. This tetrameric protein specifically binds to α-D-mannosyl and α-D-glucosyl residues (two hexoses differing only by the alcohol on carbon-2) [43]. Another example is peanut (Arachis hypogaea) agglutinin, a 110-kDa homotetrameric, non-glycosylated protein with high specificity towards tumor-associated T-antigen disaccharide Galβ1, 3GalNAc [44]. Chimerolectins are fusion proteins comprising one or more carbohydrate-binding domains arrayed in tandem with an unrelated domain. This unrelated domain has a well-defined biological activity and function independent of the carbohydrate-binding domain. Depending on the number of carbohydrate-binding domains, chimerolectins correspond to merolectins or hololectins. For example, ricin can be classified as lectin and type II ribosome-inactivating protein (RIP). Ricin protein is made up of A chain (with N-glycosidase activity/RIP activity) and a B chain (with hemagglutinating/lectin activity) capable of binding different carbohydrates such as β-D-glucose and β-D-galactose [45, 46]. Superlectins are a type of hololectins, classified as a unique group of chimerolectins. They have two distinct carbohydrate-binding domains that identify sugars with different structures [47]. TxLCI, for example, is a tulip bulb lectin with two distinct carbohydrate-binding domains that bind mannose and GalNAc sugar residues [28]. On the basis of their carbohydrate binding specificity, all known lectins are currently classified into twelve families [48].
Agaricus bisporus agglutinin (ABA)
A. bisporus agglutinin (ABA) was first isolated from the edible mushroom A. bisporus. Although fungi possess numerous homologs of this lectin, only a fewABA homologs have been found in lower plants, most notably the liverwort Marchantia polymorpha expresses at least four functional lectin homologs, which are dimeric proteins made up of subunits with 140–142 amino acid residues [48]. The occurrence of ABA homologs in lower plants most likely evolved as a result of horizontal transfer from a fungal ancestor, probably an endosymbiont [49]. ABA has been thoroughly investigated with respect to its biochemical properties, biological activities, and structure. Studies on specificity have revealed that ABA selectively interacts with the T antigen glycan (Gal β1,3GalNAc) and that the affinity of the lectin is strongly enhanced by the presence of high‐density polyvalent glycotope [50]. According to X-ray diffraction studies, the monomers of these fungal lectins are composed of a β‐sandwich of two bundles of β‐sheets joined by a helix-loop-helix motif comprised up of two short α helices. The structural analysis has additionally demonstrated that the ABA monomer has two distinct binding sites that are on the opposite side of the helix-loop-helix motif [51].
Amaranthin domain
The amaranthin family is named after Amaranthus caudatus seed lectin found in various amaranthus species. Amaranthin is a GalNAc-specific lectin with a high affinity towards T-antigen disaccharide Galβ(1,3) GalNAc [52]. This class of lectins has two identical subunits of around 33 kDa. The protomer has 300 amino acid residues and contains homologous domains N and C connected by a short α‐helical 310 segment. Each domain comprises six strands of antiparallel β-sheet with β-hairpin capping forming a β-barrel assembly [53]. A specific hydrogen-bonding pattern is required to bind carbohydrate residues to amaranthine on surface exposed hairpins and turns. Amaranthins naturally occur as hololectins, however, several chimerolectins have also been identified containing an amaranthin domain with an aerolysin toxin domain [54].
Class V chitinase-related agglutinin (CRA)
A novel class V chitinase-related lectin was initially reported from the bark of the legume tree Robinia pseudoacacia (black locust) in 2007 and shares nearly 50% sequence identity with plant class V chitinases but devoid of chitinase activity [55]. This CRA is a homodimer of 337 amino acid residue subunits. It interacts with the high mannose N-glycans that comprises the proximal pentasaccharide core structure with considerable affinity despite being a weak agglutinin. The crystal structure of CRA domain has revealed a TIM-barrel scaffold. This fold is composed of β‐sheet strands surrounded by an outer crown of α‐helices and an extra hairpin‐shaped loop consisting of three antiparallel strands of β ‐sheet that protrude from one edge of the TIM‐barrel structure [48].
Cyanovirin domain
The Cyanovirin family is named after a virucidal protein Cyanovirin‐N (CVN) that was initially purified from cyanobacterium (blue green alga) Nostoc ellipsosporum [56]. NMR and X-ray crystallography investigations have revealed that each CVN monomer has two binding sites which are positioned in deep clefts at the opposite extremities of the elongated structure made up of a triple-stranded β‐sheet and a β‐hairpin. It consists of a single polypeptide of 101 amino acid residue with an internal duplication [57]. Only a few other bacteria, except N. ellipsosporum, have genes that encode proteins like CVN. Similar proteins have recently been discovered in various kinds of fungi and the fern Ceratopteris richardii. A phylogenetic evaluation of the various subdomains of a number of bacterial, fungal, and plant CVN sequences revealed that the CVN domain evolved from a special ancient duplication that occurred in an associated ancestor [58]. Based on this distribution, it seems probable that the family originated through a horizontal transfer between bacteria and fungi as well as between fungi and Embryophyta. CVN irreversibly inactivates human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV). Due to the high affinity interactions of CVN with high‐mannose N‐glycans, it displays strong anti‐HIV activity for the viral surface envelope glycoprotein gp120 [59].
Euonymus europaeus lectin (EUL) domain
A lectin isolated and characterized from the arilli of spindle tree Euonymus europaeus (EUL), represents a novel family of plant lectins [60]. It is a homodimeric protein composed of 152 residue subunits that interacts with both blood group B oligosaccharides and high‐mannose N‐glycans [61]. In addition to being important in the classification of plant lectins, the uncovering of the EUL domain as a novel carbohydrate binding module also provides new insight into the potential roles of these lectins in plants. According to the genome and transcriptome databases, mosses, gymnosperms, bryophytes, filicophytes and angiosperms all contain this carbohydrate-binding domain. Additionally, all Poaceae members contain (many) genes that encode cytoplasmic EUL proteins with single and double domains. In contrast, dicotyledonous species only have one or two genes that encode a single domain EUL protein whereas several forms of single and double EUL domain proteins are synthesized by gymnosperms [48].
Galanthus nivalis agglutinin (GNA)
A lectin from snowdrop (Galanthus nivalis) bulbs has been isolated and characterized with exclusive specificity towards mannose and exhibits high affinity towards oligomannosides and high-mannose N-glycans [62]. It was initially called the ‘monocot mannose-binding lectin’, and as a result, after the first identified member, this group of lectins was termed 'GNA-related lectins' as they share a significant amount of sequence and structural similarity [62, 63]. Similar lectins have been found in plants other than the Liliopsida (monocots) family, including liverwort (Marchantia polymorpha), microbes and animals [64]. X-ray crystallography has shown that each β-prism fold subunit comprises of three antiparallel four-stranded β-sheets which form three mannose binding sites. GNA‐related lectins consist of 12–14 kDa subunits and exist either as monomers, dimers, or tetramers [48].
Hevein domain
The hevein domain was named after lectin isolated and purified from the latex of Hevea brasiliensis (rubber tree). Hevein is made up of a single 43 amino acid polypeptide that creates a distinctive structural motif in combination with two short α-helical segments and three antiparallel β-sheets. Four disulfide bridges (between eight exceptionally conserved Cys residues) stabilize the overall secondary structure of the backbone. Due to the availability of an expanded binding site, the hevein domain has a stronger affinity for longer GlcNAc oligomers [59]. The current distribution and domain architecture indicate that the hevein domain was vertically transmitted in plants and fungi, where it further evolved by domain duplication and/or fusion to unrelated domains and is thought to have originated in an early eukaryote (before the division of Viridiplantae and fungi/metazoa) [48].
Jacalin-related domain
All proteins with one or more domains structurally identical to jacalin, a Gal-binding lectin found in jack fruit (Artocarpus integrifolia) seeds, are classified as jacalin-related lectins [65]. These proteins have been further subdivided into two sub-families, each having its distinct specificity and molecular structure. First is Gal-specific jacalin-related proteins located in the vacuole, comprising of a small β (20 amino acid residues) and a large α (133 amino acid residues) subunit, which have a marked preference for Gal over Man residues [66]. Another is Man-specific jacalin-related lectins residing in the cytoplasm and nucleus, comprising ~ 150 amino acids each, with exclusive specificity towards Man. The Jacalin lectin family includes lectins from the family Moraceae (jacalin, MPA, artocarpin), Convolvulaceae (calsepa, conarva), Asteraceae (heltuba), Gramineae (barley and wheat lectins) and Musaceae (banana lectin) [67]. They can be found in seeds and vegetative tissues, have a low sequence similarity and differ in carbohydrate-binding specificity. The 3D structure of jacalin [68] is composed of a three-fold symmetric β-prism as revealed in X-ray diffraction analysis. Each β-prism further consists of three four-stranded β-sheets. Out of the twelve strands, eleven strands are constructed by α-chains, whereas β-chain forms the twelfth strand. Four protomers form a tetrameric structure linked to each other through noncovalent interaction. Each protomer contains a single carbohydrate-binding site with Gly, Tyr, Trp, Asp of the α-chains forming a network of hydrogen bonds with O3, O4, O5 and O6 of methyl α-D-Gal. Gal-specific subgroup shows a high affinity for Galβ(1,3)GalNAc [65, 69]. In contrast, the Man-specific jacalin-related lectins bind specifically to Man and maltose and show a high affinity for oligosaccharides [70, 71].
Legume lectin domain
Legume lectins represent the largest family of carbohydrate binding proteins and their physicochemical and biological properties have been broadly studied. Legume lectins have demonstrated promising antiviral activity against various pathogens, including human pathogenic microorganisms [72]. They have been identified as selective viral entry inhibitors, making them potential candidates for antiviral therapy [73]. Some legume lectins, such as those binding to mannose/glucose/N-acetylglucosamine, have been suggested to have inhibitory activity against SARS-CoV-2 [74]. The unique status of legume lectins as metalloproteins sets them apart from other plant lectins. They contain strongly bound Mn2+ and Ca2+ ions within their protomers, that are crucial for their carbohydrate-binding affinity [75]. Most legume lectins comprise two protomers that interact in a two-fold symmetric plane. This interaction forms a twelve-stranded β-sandwich structure with the two monomers at each end serving as the monosaccharide binding sites. The dome-shaped protomers also possess specific amino acid residues at their top, contributing to the monosaccharide binding sites.
LysM domain
Several enzymes involved in the breakdown of bacterial cell walls have the lysin domain (also known as the LysM domain). LysM domain-containing receptor-like kinases in plants that are involved in the perception of rhizobial signals were the first to be discovered. Previous studies have revealed that over 4000 proteins from prokaryotes and eukaryotes are reported to contain one or more LysM domains [48]. The secondary structure of the LysM domain is composed of two α-helices placed on the same side of an anti-parallel β-sheet [76].
Nicotiana tabacum agglutinin (Nictaba)
Nictaba, a jasmonate-inducible lectin, was initially identified in tobacco (Nicotiana tabacum) leaf samples. Nictaba is a homodimer comprising of two 19 kDa subunits having a high affinity for high‐mannose N‐glycans, and is synthesized in the cytoplasm, after which it is partially transported into the nucleus where it is thought to interact with a variety of glycoproteins [77]. According to molecular modeling, Nictaba has a β-sandwich structure comprising of two β-sheets linked by extented loops [78].
Ricin-B domain
The very first lectin to be identified, ricin from the castor bean (Ricinus communis) represents the ricin-B family, one of the most extensive families of naturally occurring carbohydrate-binding proteins. Ricin's structural analysis has revealed that the basic carbohydrate unit is made up of three tandemly arranged subdomains each containing around 40 amino acid residues. The term "ricin-B domain" is frequently used to signify this carbohydrate-binding domain. Type 2 RIPs are the most well-known plant lectins of the ricin-B family [79]. Type 2 RIPs are typical chimeric proteins having a functionally and structurally distinct A chain with a polynucleotide:adenosine glycosidase domain (PAG, formerly called RNA N‐glycosidase or RIP domain) and a B‐chain with a duplicated ricin‐B domain. The N-terminal A-chain (25–30 kDa) exhibits N-glycosidase activity, while the C-terminal B-chain (30–35 kDa) possesses two different carbohydrate-binding sites. RIP is a catalytic inhibitor of the eukaryotic ribosome by removing a highly conserved adenine residue from the sarcin/ ricin loop of the large ribosomal RNA. [80].The majority of plant lectins with ricin-B domains have been considered to be GalNAc or galactose-specific. RIPs are prevalent among angiosperms [59] and have anti-HIV, antiproliferative, anticancer, and immunosuppressive activities [81].
Scaffold organization of the mannose-binding lectins
Scaffold organization of the mannose-binding lectins has biomedical and biotechnological applications, such as characterization of glycans and as diagnostic and therapeutic agents for cancer cells [82, 83]. The possibility for lectin engineering and the emergence of cutting-edge therapeutic approaches is provided by the varied structural scaffolds of lectins [84]. They are divided into three structural scaffolds (Fig. 3) that produce more complicated oligomeric structures:
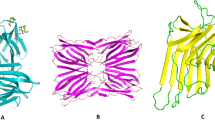
Different structures of mannose specific plant lectins. A β-sandwich structure- (i) Lentil lectin (PDB ID- 1LEM), (ii) Con- A (PDB ID-1CVN). B β-prism I fold- (i) Calsepa lectin (PDB ID- 1OUW), (ii) Artocarpin lectin (PDB ID- 1J4T), (iii) Parkia platycephala lectin (PDB ID- 1ZGR), (iv) Helianthus tuberosus lectin (PDB ID- 1OUW). C β-prism II fold- (i) Galanthus nivalis lectin (PDB ID- 1NIV), (ii) Narcissus pseudonarcissus lectin (PDB ID- 1NPL).[Source: RCSB protein data bank (www.rcsb.org)]
The β-Sandwich Fold
Legume lectins (Fabaceae) feature a jelly roll scaffold that consists of a single or two polypeptide chains. Traditionally, they are categorized as single-chain or two-chain lectins based on whether their protomers are cleaved. Single-chain lectins do not undergo protomeric chain cleavage, while two-chain lectins have their protomers separated into a light (α-chain) and a heavy (β-chain) through noncovalent linkages. The jelly roll scaffold of legume lectins is responsible for their carbohydrate-binding abilities. Tetravalent lectins with a single chain comprise four non-covalently associated identical protomers, each containing a carbohydrate recognition domain (CRD) that recognizes mannose and its derivatives. In two-chain lectins, the noncovalent binding of two identical protomers, each carrying a CRD specific for mannose and its derivatives, results in bivalent lectins (α2β2). Examples of single-chain and two-chain lectins are concanavalin A (Con A) from jack bean seeds (Canavalia ensiformis) and lentil lectin (LcA) from Lens culinaris, respectively (Fig. 3A) [85].
The β-Prism I Fold
The β-prism I scaffold is used to construct mannose-binding lectins in Moraceae seeds like artocarpin and a lectin found in the jackfruit seeds (Artocarpus integrifolia), which serves as a prototype for this group [86]. Their protomers are structured into three bundles of four antiparallel β-strands that form a β-prism structure along a longitudinal axis. As per the number of identical protomers connected by noncovalent bonds, mannose-specific jacalin-related lectins are bivalent, e.g., Calsepa from hedge bindweed Calystegia sepium [87]; tetravalent, e.g., Artocarpin from black mulberry Artocarpus integer [88]; hexavalent, e.g., PPL from the African locust bean Parkia platycephala [89]; or octavalent lectins, e.g., Heltuba from the Jerusalem artichoke Helianthus tuberosus [90] (Fig. 3B).
The β-Prism II Fold
GNA, a mannose-specific lectin isolated from the snowdrop bulbs (Galanthus nivalis), a monocot plant species in the Amaryllidaceae family, was the first lectin to be identified with the β-prism II scaffold [40]. In the protomer, three bundles of four antiparallel β-strands are organized into a flattened β-trefoil structure around a central pseudo axis. Each of the bundles of β-strands contains a CRD. Lectins from GNA-related category are formed by the noncovalent interaction of two protomers, with the exception of gastrodianin, which comprises a single protomer e.g., the hexavalent NPA from daffodil (Narcissus pseudonarcissus) bulbs [91]; or four protomers, e.g., the dodecavalent GNA from snowdrop (Galanthus nivalis) bulbs [40] (Fig. 3C).
Mannose-binding interactions
The sugar-binding sites of all MBLs are formed by noncovalent interactions between some hydroxyl groups of the sugar ring and a few polar amino acid residues, generating a shallow depression at the lectin surface. These polar amino acid residues interact non-covalently with mannose containing surface glycoproteins of viruses. This non-covalent interaction occurs through hydrogen bonding by polar but neutral (uncharged) amino acids (Asn, Gln), negatively charged polar amino acids (Asp, Glu), and non-polar amino acids (Gly) of mannose-specific lectins. Moreover, these hydrogen bond interactions are favored by the hydrophobic stacking interaction between the aromatic amino acid residues (Tyr and Trp) proximal to the mannose binding site of lectins and the pyranose ring of mannose residues of Env protein of the virus. Many carbohydrate-binding proteins contain aromatic amino acid residues in their binding sites. These residues interact with carbohydrates in a stacking geometry via CH/π interactions. These interactions can be found in carbohydrate-binding proteins, including lectins, enzymes and carbohydrate transporters. Besides this, many non-protein aromatic molecules (natural as well as artificial) can bind saccharides using these interactions [92]. Thus, stacking interaction is a common property of monosaccharide binding exhibited even by non-protein aromatic molecules. Additionally, both Asp and Glu play a crucial role in binding mannose residues of envelope proteins due to their capacity to create multiple H-bonds with the hydroxyl groups emerging from the pyranose ring [26]. For instance, Man binding sites of artocarpin lectin form a network of 9 H-bonds between four amino acid residues (Gly15, Asp138, Leu139, Asp141) located at the top of the β-prism protomer and the O1, O3, O4, O5, and O6 atoms of the sugar [86, 88]. Gln89, Asp91, Asn93, and Tyr97 residues of GNA, the Man-specific snowdrop (Galanthus nivalis) lectin, exhibits eight H-bonds with O2, O3, O4, and O6 atoms of Man glycoproteins [26].
Overview of glycosylation of viral proteins
Glycosylation of viral proteins is a widespread phenomenon observed in various viruses, including SARS-CoV-2, HIV-1, influenza, Lassa, Zika, dengue and Ebola. Glycosylation is crucial in viral life cycle, including nucleic acid replication, virus assembly, new virus particle transport, virus release and host-cell recognition. It promotes the formation of specific protein conformations, modulates viral protein-receptor interactions and affects viral replication and infectivity. Moreover, viral protein glycosylation allows viruses to exploit the host-cell machinery to modify their proteins. Understanding the glycosylation of viral proteins is essential for unraveling virus-host interactions, developing antiviral strategies and designing immunogens for vaccine development [93,94,95].
Glycosylation, the most prevalent co- and post-translational protein modification, significantly impacts protein structure (folding and stability) and function [96, 97]. It involves host organelles (ribosomes, endoplasmic reticulum and Golgi apparatus) and enzymes (glycotransferases and glycosidases) in viral protein synthesis, folding and glycosylation [96, 98]. The selective pressure on glycosylation sites arises from the involvement of glycans in viral adhesion and epitope shielding [94, 99], however, the current understanding of viral glycosylation patterns is somewhat limited [96, 100]. Different viral proteins follow distinct glycosylation routes compared to host glycoproteins [94].
They can be broadly categorized into O-linked and N-linked glycosylation. In O-linked glycosylation, the carbohydrate moiety is covalently bonded to hydroxyl oxygen, primarily in the Golgi apparatus, involving serine, threonine, tyrosine, 5-hydroxylysine, and 4-hydroxyproline [101]. Mucin-type O-glycosylation, also known as N-acetylgalactosamine (GalNAc)-type, is the most frequent in viral proteins [102].
In mammalian cells, N-glycosylation of viral proteins starts simultaneously with their synthesis. The glycan precursor, Glc3Man9GlcNAc2, is generated in the cytoplasm within [102]. It is then transported to the ER lumen and modified with monosaccharides [103]. An oligosaccharide transferase attaches the mature glycan structure to the Asn residue of an Asn-X-Ser/Thr motif ("X" representing any amino acid except proline) in the nascent protein chain [98].
Mucin-type O-glycosylation is more complex than N-glycosylation. In the Golgi apparatus, GalNAc monosaccharide is inserted into specific amino acid residues (Ser, Thr, or Tyr) by a group of GalNAc-transferases [96, 102]. The oligosaccharide repertoire remains limited in the ER and the early secretory pathway, but in the Golgi complex, glycans acquire diverse structures through terminal glycosylation, leading to significant heterogeneity. This variation differs among cell types, tissues and species, contributing to the diversification of glycan molecules classified as oligomannose, hybrid and complex-type N-glycan structures [94, 103].
N-linked glycosylation is the most prevalent form of protein modification and plays a crucial role in regulating many intracellular and extracellular functions. Several envelope glycoproteins have been the subject of viral glycosylation studies, including the envelope glycoprotein (Env) of human immunodeficiency virus-1 (HIV-1), hemagglutinin glycoprotein (HA) of influenza virus, spike glycoprotein (S) of coronaviruses, glycoprotein (GP) of Ebola virus, envelope (E) glycoprotein of dengue, Zika and other flaviviruses [104].
SARS-CoV-2
SARS-CoV-2 spike protein has 22 N-glycosylation sites with key glycosylation sites found at Asn-90 or Asn-322 positions of the S protein [32]. However, the detailed mechanisms for this site-specific glycosylation regulating the entry of SARS-CoV-2 have remained unclear. The glycosylation of viral spike protein is done by the host cellular glycosylation machinery through the secretory pathway. The mannose residues in these glycans are crucial moieties for interacting with cell surface attachment factors, like sialic acid and glycosaminoglycan (GAGs) containing oligosaccharides [105]. Glycans are sometimes referred to as the "glycan shield" because they sterically mask the underlying polypeptide epitopes from detection by feasible neutralizing antibodies [106]. Viruses utilize the host cell machinery for glycosylation and typically get decorated with host’s glycans (self glycans) as a strategy to escape the host immune response [107]. Recent characterization of the S-protein glycosylation profile in BTI-Tn-5B1-4 insect cells has revealed high-mannose N-glycans in all 22 expected sites, particularly covering the receptor-binding domain (RBD) areas [108].
HIV
Studies have revealed that N-linked glycans present on the surface of envelope glycoprotein (gp120) of HIV are essential for proper protein folding. gp120 has been found to be highly glycosylated, containing around 24 N-linked glycans. Among them, Asn260 glycosylation site defines correct expression of gp120 and gp41 [109]. Additionally, N-linked glycans may influence how HIV envelope proteins function and neutralizes antibodies [110].
HCV
There are 4 and 11 N-linked glycosylation sites present on the envelope glycoproteins E1 and E2 of HCV, respectively [111, 112]. Glycans at positions E2N1, E2N2, E2N4, E2N6 and E2N11 have been found to attenuate the accessibility of neutralizing antibodies to the epitopes of E2.Mutations at these glycosylation sites have been found to have a negative impact on virus particle assembly and/or secretion as well as virus entry. Additionally, these glycans may control CD81 and E2 binding, which may help to partially explain how HCV subverts the immune response [111]. Moreover, the glycans at the E2N2 and E2N4 positions are essential for facilitating HCV fusion and entry [104]. Hepatitis B virus (HBV) encodes three envelope glycoproteins, small (S), medium (M) and large (L) which share a potential N-glycosylation site at Asn-146 (N146) of their S domain [113]. Early N-glycan modification on the ER has been found to have a major impact on the production of infectious virus particles [114].
EBOV
The surface glycoprotein of Ebola virus (EBOV) viz. GP1 contains 15 N-glycosylation sites. Previous studies have been shown that the elimination of GP1 N-glycans enhances protease sensitivity of cathepsin B but does not have any effect on the binding of pseudoviral particles to the cell surface. Additionally, removal of N-linked glycans from GP1 can greatly increase the sensitivity of antibody neutralization, while adding N618D to the GP1 subunit (7Gm8G) could further increase the antibody neutralization sensitivity of the viral particles [104, 115].
HSV
HSV-1 encodes 12 envelope glycoproteins that play important roles in the viral replication. Glycoproteins gB, gC and gD have been implicated in the attachment of virus to host cell-receptors, while gB, gD and heterodimer gH/gL are essential for HSV attachment and penetration. HSV-1 gH contains 838 amino acids and has 7 consensus sites for N-linked oligosaccharides as well as 11 sites for O-linked glycosylation [116]. gL contains 224 amino acids and has one consensus site for N-linked oligosaccharides and 3 potential sites for O-linked glycosylation. gC-1 is heavily glycosylated which contains 9 consensus sites for N-linked glycosylation and numerous clustered O-linked glycans in a peptide stretch delimited by amino acids 30 and 124 [104].
Lectins as antiviral agents
The distinctiveness of mannose-specific plant lectins in recognizing high-mannose and complex N-glycans on the surface of viruses makes them potent inhibitors of viral infection [26, 117]. Mannose-specific plant lectins by binding to specific glycans on the viral surface interfere with the attachment of virus to receptors on host cells thus preventing viral fusion. [118,119,120,121]. Mannose recognition by mannose specific lectins is a complex process mediated by distinct receptors with unique specificity [122]. The mannose-binding lectin pathway is activated when mannose complexes bind to pathogens [123, 124]. Mannose-binding lectin is a key component of innate immunity and can recognize and bind to carbohydrates on microorganisms, leading to complement activation [125]. The binding of mannose-specific lectins to viral glycoproteins, such as gp120 in HIV, inhibits viral infectivity by blocking the interaction with host cell receptors [126, 127]. Additionally, mannose-specific lectins can disrupt viral replication by inhibiting the self-assembly of viruses [128]. Some virus strains have been found to develop an increased number of glycosylation sites to evade antibody pressures and change antigenicity [129].
Additionally, lectins from red algae, such as Grateloupia chiangii lectin (GCL), have been characterized for their antiviral activities [128]. Mannose-specific lectins have also been investigated for their potential applications in the prevention and control of viral infections, including COVID-19 [130]. Mannose-specific lectins from red algae, such as Grateloupia chiangii and Setcreasea purpurea, have been shown to possess antiviral activity.
While mannose-specific lectins hold promise as antiviral agents, there are several limitations and challenges that need to be addressed. One limitation is the potential safety concerns associated with lectin-based therapies, as lectins can interact with healthy human cells [131]. Another challenge is the need for further research to understand the exact mechanisms of action of mannose-specific lectins and their interactions with viral glycoproteins [132]. Additionally, the availability and production of mannose-specific lectins in large quantities may pose challenges for their widespread therapeutic use [128].
HIV-specific lectins
The surface of many enveloped viruses is covered with virally encoded glycoproteins. Among these, HIV-1 envelope glycoproteins gp120 and gp41 are extensively glycosylated with N-linked carbohydrates contributing up to 50% of total molecular weight of gp120 [133]. Gp120 contains numerous high mannose sugars and conserved glycosylation sites [134, 135]. The initial step in the invasion process of HIV is binding of envelope glycoprotein gp120 to the host cell receptor CD4. This binding is followed by a conformational modification that enables co-receptor binding (CCR5 or CXCR4), ultimately leading to the fusion of virus with host cell membrane [136]. Once gp120 interacts with CD4-positive host cells, it is delivered to T-lymphocytes and macrophages, resulting in the infection of these cells by HIV.
To block viral entry, anti-HIV lectins can bind to oligosaccharide epitopes on the surface glycoproteins of HIV. This binding either prevents the interaction between HIV and receptors on target cell membranes or affects the conformational modifications of the critical viral envelope and transmembrane glycoproteins [137].
HIV surface glycoproteins, namely HIV gp120 and HIV gp41 interact with CD4 receptors and CCR5/CXCR4 co-receptors on target cells facilitating the invasion of host cells. Initially expressed as gp160 precursors, these glycoproteins undergo proteolytic cleavage to form HIV gp120 and HIV gp41 [138]. In mature HIV viruses, HIV gp120 and HIV gp41 remain linked through noncovalent interactions. The association of gp120 with the CD4 receptor triggers the invasion of HIV into host cells. This interaction induces a conformational change in gp120, enhancing its affinity for chemokine receptors CXCR4 or CCR5 [139]. As a result, the transmembrane glycoprotein HIV gp41 undergoes another conformational alteration, leading to the fusion of viral and host cell membranes [140]. This fusion allows for the internalization of the HIV viral capsid, containing viral mRNA and crucial viral proteins, into the host cellcytoplasm [141, 142].
Studies by Mizuochi [143] have revealed the diverse oligosaccharide structures of HIV gp120. Oligomannoses, primarily composed of five to nine mannose residues, comprise approximately 33% of the total carbohydrate structures of HIV gp120 [143]. Complex-type glycan chains, including mono-, bi-, tri- and tetra-antennary structures, have also been found along with variations in N-acetyl lactosamine repeats and a core fucose residue [143, 144]. Recent advancements in glycoscience, genomics, proteomics and mass spectrometry have allowed for a more comprehensive characterization of HIV gp120 glycosylation. Mass spectrometry analyses have confirmed the prevalence of high mannose content in HIV gp120 across various viral clades and expression systems. The estimated number of N-glycosylation sites in HIV gp120 ranges from 20 to 35 [145,146,147,148].
Bonomelli et al. [31] have reported that the oligosaccharides of HIV gp120 derived from virus isolates are predominantly oligomannoses, while recombinant monomeric HIV gp exhibits lower oligomannose content. Additionally, a conserved mannose patch of Man5–9GlcNAc2 has been identified on HIV gp120 and is present across primary isolates and diverse HIV clades. The presence of this mannose patch on HIV gp120 is of significance in the development of an effective anti-HIV vaccine [133, 149,150,151]. HIV gp41, conversely, plays a crucial role in mediating membrane fusion, which is essential for HIV entrance into target cells, immune evasion and infectivity [152].
A mannose-specific plant lectin from Narcissus pseudonarcissus (NPL) has been proposed as a potential treatment for AIDS [119]. Lectins such as MAP30 from bitter melon, GAP31 from Gelonium multiflorum and jacalin have demonstrated inhibitory effects on viral proliferation, including HIV and cytomegalovirus [127]. Mannose-specific lectins have also been investigated for their potential in the treatment of other viral infections, including HIV and coronaviruses. HIV-1, for instance, has a high density of high-mannose glycans on its surface, making it a prime target for interaction with mannose-specific lectins [153]. Lectins such as GNA, HHA, concanavalin A and cyanovirin-N (CV-N) have been shown to bind to the HIV envelope glycoprotein (gp120) and inhibit HIV activity by preventing the virus from entering cells [154]. Similarly, lectins from other plant sources, including Polygonatum cyrtonema, Clematis montana, Ophiopogon japonicus and Narcissus pseudonarcissus have exhibited antiviral activity against HIV and HSV [155, 156]. Furthermore, lectins from cyanobacteria such as Nostoc ellipsosporum and Scytonema varium have shown antiviral activity against influenza and HIV [157, 158].
Recently, researchers have been exploring plant and microbial lectins, such as griffithsin (GRFT), actinohivin (AH), concanavalin-A (ConA), cyanovirin-N (CV-N), microvirin (MVN) and banana lectin (BanLec) for their antiretroviral properties [10]. These lectins possess multiple sugar-binding sites, allowing multivalent interactions with gp120 and neutralizing a broad range of HIV-1 and HIV-2 strains [9]. Table 1 provides examples of lectins that have been studied for their antiretroviral potential against HIV.
Cyanovirin-N (CVN)
The lectin cyanovirin-N (CVN) was first isolated from the cyanobacterium Nostoc ellipsosporum [159]. The CVN lectin is mainly composed of 101 amino acids and has a molecular weight of 11 kDa. It has two carbohydrate-binding sites specific for the terminal α1, 2-mannose sugars on high-mannose glycans [160]. CVN is mainly found as a monomer in solution; however, it forms a domain-swapped dimer in crystal. The identical (A) or similar (B) domains appear in the dimer, but they are made up of sequences originating from two different CVN monomers [161]. CVN binds large oligosaccharide structures seen in terminal branches and has a high affinity for Man α (1–2) substructures in D1 and D3 arms of Man-9 [160, 162]. As compared to complex N-linked oligosaccharides, CVN has the strongest affinity for α -Man-(1,2)- α -Man-(1,2)- α-Man and preferentially targets under processed glycans [121]. CVN effectively prevents the interaction of HIV gp120 with the CD4 T-cell receptor [163]. CD4 conformational changes and interaction with the relevant co-receptors CXCR4 and CCR5 are prevented by CVN binding to viral gp120 [164]. As a result, the virus cannot enter the cell, and transmission from an infected to a non-infected cell is inhibited. CVN specifically binds to the terminal Man α (1–2) Man unit of arms D1 and D3 on large high-mannose N-linked oligosaccharides (Man8GlcNAc2 (Man8) and Man9GlcNAc2 (Man9) of HIV gp120 [162]. In vitro deactivation of HIV has been reported at a nanomolar concentration of the lectin [165]. CVN has two binding domains that allow the protein to cross-link the branched oligomannosides and association with both domains is essential for the lectin's activity. On the other hand, domain B has a ten times stronger affinity for α (1–2) linked oligomannose than domain A. Resistance has been found to develope in CVN exposed HIV strains after the deletion of N-glycans in viral gp120 [166]. In addition to interaction with HIV gp120 and reduction of HIV (types 1 and 2) infection at a nanomolar level, CVN is active against other enveloped viruses like simian immunodeficiency virus (SIV) and the chimeric SIV/HIV-1 virus(SHIV89.6P) [167], feline immunodeficiency virus (FIV), human herpes virus 6 (HHV-6), measles virus (MeV) [168], Ebola virus [169], hepatitis virus [170] and influenza virus [171] all with the N-linked high mannose oligosaccharides as glycoprotein components.
CVN is a potent antiviral protein that inhibits the entry of HIV-1 by binding selectively and with high affinity to high-mannose oligosaccharides on the gp120 protein [172]. It has shown efficacy against various enveloped viruses, including Influenza A and B viruses, Herpes simplex virus type-1, and HIV [172]. The structure of CVN consists of an elongated, largely beta-sheet protein with internal two-fold pseudosymmetry [173]. CVN is a domain-swapped dimer in the crystalline state, while solution NMR reveals a monomeric form [174]. It has also been investigated as a potential component of microbicides for HIV prevention [175], and studies have demonstrated its protective effect against HIV-1 [176]. CVN exhibits strong antiviral properties and holds promise for therapeutic and preventive applications.
Microvirin (MVN)
Microvirin (MVN) is a lectin isolated from the cyanobacterium Microcystis aeruginosa consisting of 108 amino acids (13 kDa) [177]. In solution, MVN is a monomer with a single sugar-binding site that targets terminal α1, 2-mannose sugars and binds with HIV gp-120 [178]. The monomers are made up of 113 amino acid residues by forming two 50 percent identical domains, one is the N-terminal domain (SD1: residues 1–54), and the other is the C-terminal domain (SD2: residues 60–113) that are divided by a five-amino-acid linker [179]. Each monomer has two binding sites that have an affinity for N-linked oligomannosides with at least the Manα (1–6) Man β (1–4) GlcNAc β (1–4) GlcNAc tetrasaccharide core structure [180]. MVN has been found to exhibit anti-HIV-1 activity by inhibiting syncytium formation between HIV-1-infected HUT-78 cells and uninfected HUT-78 cells [181]. MVN also stimulates pro-infammatory cytokines secretion by peripheral blood mononuclear cells (PBMCs). The neutralizing anti-HIV mAb 2G12 antibody has shown affinity against high mannose carbohydrates of HIV gp-120. MVN has been shown to bind efficiently with gp-120, interfering with 2G12 mAb binding on HIV-1-infected cells [181].
Scytovirin (SVN)
Scytovirin is a 9.71 kDa lectin isolated from the cyanobacterium Scytonema varium [24, 182]. It is a single 95-amino-acid polypeptide chain with two highly symmetric structural domains, SD1 (residues 3–42) and SD2 (residues 51–90), that are 90% identical. Each domain constitutes three aromatic amino acids implicated in carbohydrate binding and two intra-domain disulfide linkages. SVN interacts with Manα (1–2), Manα (1–6), Manα (1–6) tetrasaccharides of the viral envelope glycoproteins, particularly gp120, gp160 and with gp41 less effectively [24]. Both domains (SD1 and SD2) bind oligosaccharides simultaneously. However, SD1 has a stronger affinity for oligosaccharides than SD2 [182]. Recombinant SVN encoded by the expression of the synthetic gene in E. coli has been found to yield 5–10 mg/L of lectin [183].
BanLec
BanLec, a lectin derived from banana (Musa paradisiacal) has been found to possess potent anti-HIV activity [184,185,186,187]. BanLec belongs to the family of jacalin-related lectins. The native lectin is a dimer comprising two identical 15 kDa subunits, each with 141 amino acids and two sugar-binding sites [188]. In vitro analysis of BanLec has been reported to prevent infection by a variety of HIV-1 isolates with distinct tropisms, with IC50 values in the low nanomolar range. BanLec, like the other carbohydrate-binding proteins, prevents HIV infection at the viral introductory stage by binding to high-mannose structures on densely glycosylated gp120 in a concentration-dependent manner, preventing virus interaction with the cell. According to Swanson et al. [189], BanLec is a potent mitogen for murine T-cells, although the effects depend on the mouse strain used. Remarkably, a mutation in BanLec's sugar-binding region impairs mitogenic activity without affecting HIV neutralization.
It has been shown to inhibit HIV fusion in cells with low-nanomolar IC50 values [185]. BanLec binds to specific viral glycans preventing viral attachment, entry and fusion [184]. In addition to HIV, BanLec has also demonstrated antiviral activity against other viruses, such as influenza [190], herpes simplex viruses [191] and coronaviruses, including SARS-CoV-2 [192]. These findings highlight the potential of BanLec as a candidate for developing antiviral therapies. Identifying BanLec and other lectins with varying binding, toxicity and anti-HIV activity has provided the basis for expansion of the possibilities for lectin-based microbicides [186].
Griffithsin (GRFT)
GRFT, derived from red algae Griffithsia sp., is a potent antiviral protein with an impressive track record against HIV and enveloped viruses and consists of 121 amino acids that form a stable domain-swapped homodimer with three monosaccharide-binding subunits [193]. Its excellent safety and efficacy profiles make it a HIV entry inhibitor [194]. This remarkable antiviral activity of the lectin stems from its ability to bind terminal mannoses on high-mannose oligosaccharides, effectively crosslinking these glycans on viral envelope glycoproteins [195]. With a broad spectrum of antiviral activity, GRFT has shown promise against various viruses like HSV-2, HCV and MERS-CoV [196, 197]. Its exceptional potency against HIV, even at low concentrations, makes it an attractive candidate for preventing sexual transmission of the virus [198]. Moreover, GRFT has displayed antiviral activity against the porcine epidemic diarrhea virus (PEDV) [199].
With its multifaceted antiviral activity and favorable safety profile, GRFT holds promise for developing novel therapies against viral infections. It is considered the most effective HIV entry inhibitor. GRFT has outperformed broadly neutralizing antibodies (bNAbs) like 2G12, which also target high-mannose-type glycans [200]. Notably, GRFT exhibits remarkable neutralization of HIV even at picomolar concentrations.
Efficacy of GRFT extends beyond specific viral clades, as it can inhibit both T-tropic and M-tropic viruses of clades A, B, and C. Furthermore, it has shown the ability to prevent HIV infection in human cervical explant tissues without triggering proinflammatory cytokine production [201]. As regards safety, GRFT has demonstrated a unique profile in rabbit vaginal irritancy models, exhibiting low toxicity when administered in single or chronic subcutaneous dosages to mice and guinea pigs [202].
HCV and HBV-specific lectins
HCV causes chronic blood-borne infection, leading to severe liver disease and further causing hepatocellular carcinoma [214]. The HCV envelope glycoproteins E1 and E2 are type I transmembrane proteins with an N-terminal ectodomain and a C-terminal hydrophobic anchor that act during the entry and fusion steps of the virus life cycle [215]. Sequence analyses of E1 and E2 have identified 5 and 11 potential N-glycosylation sites strongly conserved among different genotypes [216]. The glycan portion of the E1E2 heterodimer comprises up to one-third of its total molecular weight, effectively reducing the immunogenicity of envelope proteins [217]. It has been shown that several of these glycans play an essential role in HCV assembly and infectivity and can mask access of neutralizing antibodies to epitopes presented on the surface of the viral particle [111]. Thus, regions of gpE1E2 critical to the entry step of the HCV life cycle are highly conserved and can be an attractive target for antiviral drug therapy.
Carbohydrate-binding agents can be an effective strategy for determining the presence and function of carbohydrates on pathogens. In addition to their carbohydrate specificities, these lectins differ in their quaternary structures and valency. CVN has two carbohydrate-binding sites allowing for two-site binding with the branched glycans Man-8 and Man-9; MVL is a homodimeric protein with four carbohydrate-binding sites and GNA is a tetramer with 12 carbohydrate-binding sites. Similar to HIV, HCV also contains high-Man glycans. A similar approach has been employed to examine the inhibitory efficacy of the lectins CVN [170]., GNA [11], MVN [218], and GRFT [219] against HCV pseudoparticles (HCVpp) and HCV cell culture (HCVcc). As a result, lectins are known to inhibit HCV at millimolar to nanomolar concentrations and prevent HCV infection during the early stages. Among other lectins are Galectin-3-binding protein (Gal-3BP), which is suggested to function as a serum marker for the severity of hepatitis virus-induced liver fibrosis [220]. Pholiota squarrosa lectin (PhoSL), which specifically binds to core-fucose, has been found to inhibit HBV infection [221].
According to Kachko et al. [11], GNA has high specificity to HCV that recognizes glycans present in E1E2 region, which remains accessible for post-binding and can be susceptible to E1E2-directed inhibitors. MVL also binds oligomannosides containing the chitobiose core such as Man3GlcNAc2 and Man6GlcNAc2 with sub-micromolar affinities, while CVN binds to the Man1,2Man termini of Man8GlcNAc2 (Man-8) and Man9GlcNAc2 (Man-9) [11]. Moreover, GNA has a unique carbohydrate recognition profile, binding to Man termini in hybrid-type and complex-type N-glycans found at the termini of O-linked glycans [222]. Several plant lectins active against the hepatitis viruses are listed in Table 2.
Influenza virus-specific lectins
Influenza, an acute viral-borne respiratory disease, manifests with high fever, coryza, cough, headache, prostration, malaise and upper respiratory tract inflammation. The viruses causing influenza belong to the Orthomyxoviridae family and are enveloped negative-stranded RNA viruses, possessing seven to eight gene segments in their genomes and exhibiting viral surface envelope proteins of haemagglutinin and neuraminidase subtypes [227].
Types A and B classified under the same genus among the influenza viruses, while type C belongs to a distinct category, each differing in terms of host range and pathogenicity. Notably, types B and C predominantly affect humans [228]. Influenza B viruses have been found in seals and influenza C viruses have been isolated from pigs and dogs. However, history has witnessed devastating influenza pandemics, such as the one in 1918, which claimed approximately 40 million lives worldwide [229]. Similarly, the H1N1 pandemic in 2009 resulted in over 18,000 deaths within a year, as reported by the WHO. Furthermore, a new influenza virus, H7N9, emerged in eastern China, spreading to Vietnam and causing 13 deaths and 60 infections (WHO, April 14, 2013 report).
Lectins derived from the genus Pseudomonas and Malaysian banana have shown antiviral activity against influenza by blocking viral entry into host cells [230]. Viruses have a remarkable ability to mutate, posing challenges in developing effective treatments. Mutations that reduce antibody binding to haemagglutinin (HA) receptors decrease treatment efficacy [231]. Nonetheless, targeting virus particle components, such as post-translational glycosylations has shown promise as an antiviral approach against influenza. N-glycans attached to influenza surface glycoproteins play crucial roles in protein folding, trafficking, HA receptor binding and evasion of host antibody detection [232]. However, the potential toxicity of exogenous lectin treatment due to their ability to detect sugar moieties in host cells poses a significant obstacle.
Intranasal application of lectins such as CVN and H84T Banlec, which bind to high-mannose structures, has demonstrated protective effects against influenza virus challenges in mice [189]. Moreover, when administered intraperitoneally, H84T Banlec exhibited protective properties against influenza virus [233]. Lectins such as Nicotiana tabacum agglutinin (NTA) and Urtica dioica agglutinin (UDA), which bind to complex-type glycans, have also been found to suppress influenza virus [234]. Similarly, post-exposure treatment with the antiviral lectin CVN, which targets mannobiose substructures on oligomannose glycans, has shown significant survival benefit in a murine influenza model [202]. Additional plant lectins active against Inflenza viruses can be found in Table 3.
Studies involving three-dimensional modeling have revealed the structure of Narcissus tazetta lectin (NTL), a potent antiviral lectin. NTL consists of two identical 13-kDa subunits forming a homodimer and exhibits three subdomains, each containing a conserved mannose-binding site. This lectin shares significant homology with existing monocot mannose-binding lectins [235, 236]. NTL has potent antiviral activity against influenza A (H1N1, H3N2, H5N1) and influenza B viruses with IC50 values ranging from 0.20 to 1.33 µg/ml. Nonetheless, more research is needed to explore the therapeutic potential of lectins in combating influenza and other viral diseases caused by the remarkable mutability of viruses.
HSV-specific lectins
HSV is an enveloped DNA virus of types HSV-1 and 2 that are genetically similar but epidemiologically distinct. HSV-1 typically infects the mouth, leading to oral herpes and is primarily transmitted through oral-to-oral contact. On the other hand, HSV-2 is commonly associated with genital illness. These infections can have catastrophic consequences and even be fatal in patients with immature or weakened immune systems [242].
Researchers have been exploring the potential of lectins in combating HSV infections. For instance, the jackfruit lectin (JFL) isolated from Artocarpus heterophyllus has demonstrated inhibitory action against HSV-2 in vitro and has shown a cytopathogenic effect [243]. Another lectin with antiviral properties is derived from Typhonium divaricatum (L) Decne, a plant belonging to the family Araceae. This lectin has Man-binding capabilities and has been shown to inhibit HSV-2 [244]. Other Man-specific lectins have shown inhibitory efficacy against HSV infection and have been summarized in Table 4.
Recently, lectins NML-1 and -2, isolated from Nostoc muscorum have been tested for their resistance to HSV-1 infection using a plaque assay. NML-1 demonstrated early suppression of HSV-1 infection while NML-2 inhibited HSV-1 infection at a later stage. The findings suggest that Nostoc muscorum might serve as a novel source of antiviral lectins with diverse mechanisms of action, making it a promising candidate for antiviral therapeutic research [12]. Furthermore, BanLec has shown excellent antiviral activity against HSV-1 with a selectivity index (SI) of 10.8 and an effective concentration (EC50) of 16.0 µg/mL. However, its antiviral efficacy against HSV-2 is significantly less as evidenced by SI of 2.6 and an EC50 of 67.7 µg/mL [191].
EBOV-specific lectins
In December 2013, the WHO reported a new EBOV infection outbreak in the Republic of Guinea [249]. This outbreak extended to other West African nations, including Sierra Leone and Liberia. Despite efforts, the number of cases recorded in Guinea, Liberia and Sierra Leone from January to September 2014 showed little evidence that EBOV infection was declining [250].
EBOV belongs to the family Filoviridae and the order Mononegavirales. It is a negative-stranded, non-segmented RNA virus that causes a severe hemorrhagic illness. Alongside EBOV, the Marburg virus is the second genus in this family and causes a disease similar to EBOV infection. The third genus Cuevavirus [251] is only found in bats. Bats, particularly the fruit bat species Myonycteris torquata, appear to be the most plausible reservoir for EBOV infections, although they do not seem to get sick [252].
In contrast, humans experience symptoms after an incubation period of 1–25 days, including fever, headache, joint muscle and abdominal pain, diarrhea and vomiting. At this stage, EBOV infection can be easily mistaken for other tropical fevers, such as malaria or dengue. However, internal and subcutaneous bleeding during the hemorrhagic terminal phase distinguishes EBOV infection [253]. One potential treatment for EBOV infections involves the use of lectins. GRFT is a lectin that binds to the Asn(N)-linked Man5-9 GlcNAc2 structures on the envelopes of various viruses, including HIV-1, HIV-2, HCV, SARS coronavirus and EBOV. Therefore, GRFT and other lectins have shown potential in treating EBOV infections [202]. High-dose treatment with MBL has been shown to ameliorate EBOV infection and protect against Ebola virus disease (EVD) in vivo [17, 202]. Several lectins, such as Con A, syanovirin N and other Man-specific plant lectins listed in Table 5, have been considered as potential antiviral drugs.
Coronavirus-specific lectins
On January 12, 2020, the WHO confirmed the spread of a novel coronavirus (SARS-CoV-2) in Wuhan, China and termed the ensuing disease as "coronavirus disease 2019 (COVID-19)", an acute respiratory tract infection posing a severe threat to global health and economy [256]. Hundreds of millions of patients have been diagnosed as of February 2021 and millions more have died. The fatality rate has varied from 1 to 10% in different countries, and the disease is more contagious than SARS and MERS [257]. SARS-CoV, a coronavirus strain identified for its potential to cause severe acute respiratory syndrome (SARS), was discovered in China's Guangdong province in 2002 [258]. Another coronavirus variant, MERS-CoV, was isolated in Saudi Arabia in 2012. It was later confirmed to be clinically similar to SARS-CoV [259]. SARS, MERS and SARS-CoV-2 can cause life-threatening illnesses in humans. Coronaviruses are a vast family of RNA viruses (with a positive sense, single-stranded RNA molecule having size range of 27–32 kb) that belong to the Orthocoronavirinae subfamily of the Coronaviridae family in the order Nidovirales [260]. According to genome sequencing and phylogenetic analysis, SARS-CoV, MERS-CoV and SARS-CoV-2 are zoonotic diseases that originated from bat Coronaviruses and infected humans directly or indirectly through an intermediate host [261].
It has been reported that the lectin from Urtica dioica (UDA) interacts with GlcNAc-like residues on glycosylated envelope glycoprotein of SARS-CoV, limiting its binding to host cells [262]. MERS-CoV has been inhibited using lectin GNA, which can detect the disaccharide Man(1–3)Man near the end of the glycoprotein viral envelope [263]. SARS-CoV-2 spike protein is highly glycosylated with 23 potential N-glycosylation sites, 12 of which are adequately glycosylated [264]. As a result, lectins specific to the glycans found in the spike glycoprotein should be expected to limit SARS-CoV-2 infectivity.
The spike glycoproteins (S) of the SARS-CoV and SARS-CoV-2 bind to angiotensin-converting enzyme 2 (ACE2) allowing them to enter into host cells [265]. The difference in viral proteins between SARS-CoV and SARS-CoV-2 is only 25% [266]. Several N-glycosylated surface sites rich in Man, hybrid N-glycans and complex N-glycans are found on SARS-CoV spike protein (S) [267]. Furthermore, high-Man glycans are found at the top of the S-glycoprotein, whereas complex N-glycans are located at the bottom near the viral envelope surface [268]. S-glycoprotein can be the primary target for antiviral medications and is expected to prohibit coronavirus from adhering to these cells. Lectins such as Con A, and those from Lathyrus sativus (LEC), Lens culinaris (LCA), Onobrychis viciifolia (OVA), Pisum sativum (PSA), Vicia faba agglutinin (VFA) and Vicia cracca (VCA) can interact with S-glycoprotein [269].
SARS-CoV-2, like other RNA viruses, is prone to genetic evolution as it adapts to new human hosts. Over time, mutations occur, forming variants with distinct features from the original strains. Since then, additional SARS-CoV-2 variants have been identified, with a number categorized as variants of concern (VOCs) due to their public health concerns [270]. According to a recent WHO epidemiological bulletin, five SARS-CoV-2 VOCs have been diagnosed since the pandemic began in December, 2019. All five recognized VOCs have mutations in the RBD and NTD viz. Alpha (B.1.1.7); Beta (B.1.351); Gamma (P.1); Delta (B.1.617.2); and Omicron (B.1.1.529) [271], of which N501Y mutation located on the RBD is common to all variants except the Delta variant. As a result of the spike proteins' affinity for ACE2 receptors, viral attachment and subsequent invasion into host cells are enhanced [272].
The RBD is found in the S1 subunit of S-glycoprotein, which allows coronavirus to bind to the peptidase domain of ACE2. In contrast, the S2 subunit is majorly involved in membrane fusion during viral infection [93]. S-glycoprotein of SARS-CoV-2 has shown 22 N-glycan residues when the protein was expressed in human HEK-293 cells, indicating extensive glycosylation [273, 274]. 28% of the total molecular weight of the S protein comprises oligomannose-type glycans and the N234 glycoside beside the RBD exhibits Man5-GlcNAc2 oligomannose-type glycans [273].
GRFT has been reported to block the entry of the SARS-CoV-2 into Vero E6 cell lines and the type I interferon (IFN) receptor (IFNAR) knockout mouse models by attaching to the spike protein of the SARS-CoV-2 [275]. FRIL derived from Lablab purpureus is a Glc/Man-specific plant lectin, effective both in vivo and in vitro for neutralizing SARS-CoV-2 by binding to complex type N-glycans on viral glycoproteins [241]. In addition, a Man-specific plant lectin isolated from Lens culinaris has a significant SARS-CoV-2 inhibitory impact in the early stages of infections. This lectin obstructs the ACE2-S trimer by binding to oligomannose-type glycans and N-acetylglucosamine at glycosylation sites N165, N234 and N343 located around the receptor-binding domain and epidemic variants B.1.1.7, B.1.351 and P.1. Antiviral activity of lentil lectin has also been observed against both SARS-CoV and MERS-CoV [276].
According to molecular docking and MD simulation studies, BanLec targets N-glycans of S-glycoprotein to lower SARS-CoV-2 infectivity [277]. Some antiviral plant and microbial lectins are reportedly potent inhibitors of Coronaviruses as listed in Tables 6, 7 and 8. Withania somnifera (L.) Dunal, known as Indian ginseng has been reported as an antiviral medicinal herb with the potential to alleviate COVID-19 [278]. In silico docking and molecular dynamics studies have shown that the phytochemicals in W. somnifera (WS) can also stop the SARS-CoV-2 virus from infecting and replicating in host [279]. WS phytochemicals have displayed potent binding to host ACE2 receptor complex as well as the RBD of S protein of SARS-CoV-2 [280]. It is worth mentioning that a Man-specific lectin has been isolated from the leaves of WS W. somnifera having potential affinity for S protein of SARS-CoV-2 [281]. CVN has been found to have high binding energy scores with the spike protein, main protease (Mpro), and papain-like protease (PLpro) of SARS-CoV-2 [282].
Lectins as Adjuvants
The role of lectins as adjuvants in vaccines has been extensively studied and documented in the literature. Lectins are proteins that have the ability to bind to specific carbohydrates and they have been shown to enhance immune responses when used as adjuvants in vaccines [290]. In mammals, lectins have been widely used as adjuvants in vaccines against viral infections because they attract more cells to the site of inflammation and regulate the immune functions of the recruited cells. Lectins have a role in immunological recognition and host defense against pathogens [290]. For example, lectins are up-regulated in response to microorganismal/viral infection in fish cells inducing an antiviral state [290]. Lectins also display enhanced immune responses to intranasally co-administered HSV glycoprotein D2 [291].
Additionally, lectins have been explored as potential adjuvants for antiviral vaccines, including those against members of the Coronaviridae family [292]. The use of lectins as adjuvants for viral vaccines, particularly those against SARS-CoV-2, is an option that needs to be evaluated with COVID-19 vaccines [293]. Lectins can modulate dendritic cells resulting in the selective up-regulation of costimulatory molecules, making them strong mucosal adjuvants [291]. They have also been found to interact with glycosylated toll-like receptors (TLR) on macrophages and dendritic cells, fulfilling the role of vaccine adjuvants [294]. Furthermore, lectins have been investigated as potential adjuvants in development of coccidial vaccines [295].
Plant-derived lectins such as those from Abrus precatorius and Synadenium carinatum latex, have been shown to have immunostimulatory and adjuvant effects [296, 297]. Legume lectins, such as SBA, PNA, ConA and PHA induce Th1 response and are considered potential vaccine adjuvants [294]. Lectins from Xylaria hypoxylon and Pleurotus ostreatus have been reported to enhance immune responses in mice [298]. Moreover, lectins from mistletoe (Viscum album L.) have been used in adjuvant cancer therapy for more than 50 years [299]. The immunostimulatory activities of mistletoe lectins is exerted through lectin-carbohydrate interactions [299].
In conclusion, lectins have been extensively studied as adjuvants in vaccines and have shown promising results in enhancing immune responses. They have been used in mammalian systems, including humans as well as in aquaculture. Lectins have been found to modulate immune functions, induce antiviral states and enhance immune responses to specific antigens. They have also been explored as potential adjuvants for viral vaccines including those against SARS-CoV-2. Lectins from various sources, including plants and mistletoe have been investigated for their immunostimulatory and adjuvant effects. Using lectins as adjuvants offers the potential for developing highly effective vaccines and immunotherapies. Further research is needed to fully understand the mechanisms of action and optimize the use of lectins as adjuvants in different vaccine formulations.
Mannose-specific lectins as prophylactic, diagnostic and therapeutic agents
In Prophylaxis
Plant lectins have been investigated for their immunomodulatory properties and their potential to improve the host immune response against microbial infections [22]. Experimental studies have shown that plant lectins are used prophylactically to enhance the survival of mice under microbial challenge. Additionally, lectins have been used in combination with parasite antigens to induce more efficient immunization [22]. These findings suggest that immunomodulatory plant lectins have the potential to be used as prophylactic agents against microbial infections.
Vaccination is probably the most effective approach for assisting the immune system's activation of protective responses against illnesses and reducing morbidity and mortality, as proven by historical records. During public health emergencies, however, new and alternative vaccine design and development approaches are necessary for rapid and widespread immunization coverage in order to manage a disease outbreak and prevent the epidemic from spreading. Traditional vaccine approaches, such as attenuated or dead microorganisms, have failed to protect against numerous illnesses due to a variety of factors, including inability to trigger immune responses and low efficiency. Therefore, it is necessary to include a robust immunomodulatory adjuvant capable of enhancing and directing immune responses against specific disease antigens with vaccination [300]. The most challenging aspect of vaccination is induction of long-lasting immunity, which is typically lower using live or inactivated organisms [301, 302]. Plant lectins are being considered for exploration in adjuvant/carrier formulations for chemotherapeutic and preventive vaccine manufacturing due to their potential in medicinal applications [295]. Lectin-mediated vaccine formation has been found to enhance the potency of oral vaccines by targeting intestinal M-cells [303]. Certain plant-derived lectins such as SBA, PNA, Con A and PHA can act as TLR agonists by interacting with the glycosylated TLR receptor on macrophages and dendritic cells [295, 304].
Extensive studies have been conducted on the role of lectins as adjuvants in various fields including infectious diseases, cancer therapy and vaccine development. Lectins can serve as biopharmaceuticals, biomedical tools or design components of drugs. Lectins have demonstrated both promotive and suppressive roles in human cancers depending on their homology or heterology. Additionally, lectins have been investigated as potential therapeutics against viral infections including COVID-19, with advancements in protein engineering to enhance their efficacy. Furthermore, lectins have been explored as immunoadjuvants by promoting an immune response when co-administered with other antigens. They have also been identified as mediator molecules for bacterial adhesion to specific carbohydrate receptors on host tissues. Moreover, lectins can modulate the immune system depending on the context, acting as immunostimulants or immunoinhibitors. The fine structure of lectins and their binding affinity to carbohydrates play a crucial role in their adjuvant properties. Overall, lectins show promise as versatile and effective adjuvants in various applications, including vaccine development and cancer therapy [300,301,302,303,304].
In Disease Diagnosis
In addition to their antiviral activity, mannose-specific lectins have been widely used in viral diagnostics. Lectins can be immobilized on surfaces to trap viral particles and inhibit their spread and replication [119]. Concanavalin A (Con A), a mannose-specific lectin, has been used to separate coronaviruses from crude viral suspensions so that they can be used in molecular diagnostic methods [305]. Con A is the most commonly employed glucose- and mannose-selective lectin. In order to make glucose and hydrogen peroxide sensors, Con A is great for immobilizing enzymes like glucose oxidase (GOx) and horseradish peroxidase (HRP) to solid supports that are covered in intrinsic hydrocarbon chains. Con A biosensors that are sensitive to glucose, cancer cells and harmful microorganisms can be made using modified electrodes [306]. A Con A based zinc oxide nanobiosensor can identify mannose-glucose residues in viruses such as DENV2, Zika, Chikungunya and Yellow fever as well as discriminate between arboviral infections [307].
Another lectin utilized in biosensors for dengue detection is Cramoll, a glucose/mannose-binding lectin isolated from Cratylia mollis seeds [308]. A sensitive and selective dengue serotyping biosensor has been constructed using a gold nanoparticle polyaniline hybrid composite designed to immobilize Bauhinia monandra lectin (a galactose-specific lectin) [309]. Thus, mannose, glucose, galactose, N-acetyl-glucosamine and N-acetyl-galactosamine specific plant and microbial lectin-based biosensors have been designed to detect specific viruses in biological samples sensitively, including coronavirus [284, 310, 311].
Lectins are also used in clinical laboratory for the identification and taxonomic classification of microorganisms, including viruses [312]. The ability of lectins to bind to carbohydrates on the surface of cells and viral envelopes makes them valuable tools for the detection and characterization of viruses [9].
In Therapy
Apart from their diagnostic applications, mannose-specific lectins have been utilized in drug delivery systems. The tetrameric structure of dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN), a mannose-binding lectin, allows for great selectivity toward mannose- and/or fucose-containing glycopolymers [313]. This selectivity can be exploited to target drugs specifically to dendritic cells, which play a crucial role in the immune response to viral infections [313]. Mannose-specific lectins have also been investigated for their potential as carriers for the delivery of chemotherapeutic agents [314].
In recent years, several lectins from plants and microbes have been identified and studied including griffithsin (GRFT), actinohivin (AH), concanavalin-A (ConA), cyanovirin-N (CV-N), microvirin (MVN) and banana lectin (BanLec). Like GRFT, another lectin CV-N has been found to inhibit entry and cell-to-cell spread of HSV-1. Moreover, a mannose-binding lectin from Typhonium divaricatum (L.) Decne and Jackfruit lectin (JFL) from Artocarpus heterophyllus have shown potent antiviral activity against HSV-II. Furthermore, a GNA-related mannose-binding lectin from Polygonatum odoratum (POL) has been found to exert remarkable anti-HSV-II effects [10, 247]. Other investigations have shown that CV-N, Microcystis viridis lectin (MVL) and Galanthus nivalis agglutinin (GNA) inhibit HCV in vitro with IC50 values of 0.6 nM, 30.4 nM and 11.1 nM, respectively [11]
Lectins from red alga Kappaphycus alvarezii (KAA-2), green alga Boodlea coacta (BCA) and the mannose-specific Narcissus tazetta lectin (NTL) have been found to exert potent anti-influenza effects by directly binding hemagglutinin (HA) of multiple strains of influenza virus [10]. Additionally, by specific binding to the spike glycoprotein of coronaviruses, agglutinins like Hippeastrum hybrid agglutinin (HHA), GNA, Cymbidium agglutinin (CA), GRFT, and Urtica dioica agglutinin (UDA) have been found to exhibit antiviral activity against coronaviruses in vitro and in vivo [10].
Conclusion
The inadequacy of primary healthcare infrastructure in the underdeveloped countries of the world explains the disparities in health status and illness burden. As a result, research towards speedy, simple, cost-effective and uncomplicated diagnostics for infectious diseases is required to address the issue. Using millions of distinct tags of viruses and their serovars/serotypes, the science of glycoproteins holds immense potential. Many health-related issues can be addressed by using molecular biology and nanotechnology techniques at the point of-use to make quick diagnoses. As a result, we must revisit our current health-related research and practices to improve our diagnostic and healthcare delivery systems. Biosensors can become promising technologies for detecting viruses by using MBLs and other lectins as biolgical recognition elements in the biosensor. Biosensors are sensitive and analytical devices that translate biological responses into electrical, optical or mass signals [315]. They have grown in popularity over time due to benefits such as rapid real-time detection, portability, ease of use and multi-pathogen identification in field and laboratory tests [316].
These immunotherapeutic investigations have improved the understanding of the host-pathogen relationship, which may aid in the development of new therapeutic strategies. Plant lectins are known to have distinct biological targets yet their effects have not been studied clinically except in cancer therapy. Another disadvantage is the scarcity of suitable purification strategies for the synthesis of numerous lectins. Protein engineering and drug delivery technique improvements would enable production of large quantities of smaller lectin fragments with higher target specificity. Antiviral therapies can limit virus multiplication or prevent virus entry into host cells. Understanding host-pathogen interactions is central to developing modern treatments for infectious diseases.
Lectins, including cyanovirin-N, scytovirin and griffithsin, have shown antiviral activity against various enveloped and non-enveloped viruses. Investigations to understand in vivo efficacy of lectins as prophylactic, diagnostic and therapeutic agents, including their toxicological aspects, immunogenicity and large-scale production for clinical use are currently underway. The present review highlights recent improvements in understanding of antiviral potential of lectins and provides an update of the most recent research findings in this fiercely disputed area. Overall, more knowledge of "glycovirological" aspects, antiviral lectins and glyco-biotechnology would lead to the development of highly effective next-generation antiviral biotherapeutics and prophylactics against the currently raging SARS-CoV-2 pandemic and possibly other emerging viruses in the twenty-first century. The envelopes of many viruses are rich in mannose, hybrid and complex N-glycans. The envelope glycoprotein is crucial to the viral life cycle and is a primary target for antiviral drugs. The specificity of the viral envelope glycoproteins must be defined. Several attempts have been made in the context of developing potent antiviral compounds based on glycans to prevent many viral infections. Lectins have various applications as antimicrobial agents, absorbents, biological tools in nanosensors/ biosensors and diagnostics. Certain lectins are additionally utilized as adjuvants to formulate effective vaccines. Lectins can be promising R&D interventions against viruses and drug resistance issues, but their effectiveness as biopharmaceuticals depends on future advancements in research.
Future prospectives
Lectins, notably mannose-specific plant and microbial lectins, have shown potential as antiviral agents. They have been found to inhibit the replication of coronaviruses by interfering with multiple targets in the viral replication cycle [284]. Mannose-specific plant lectins derived from seaweed have demonstrated the ability to block the replication of various enveloped viruses including influenza virus, herpes virus and HIV-1 [268]. These lectins have also been shown to have anti-coronavirus properties, except garlic lectins [317]. Mannose-binding lectins have been suggested as potential antiviral agents due to their ability to interact with glycans involved in viral entry mechanisms [119]. Plant-derived mannose-specific lectins have been found to inhibit and neutralize SARS-CoV-2 infectivity in vitro, in vivo and in silico [18]. Additionally, antiviral mannose-binding plant lectins have potential applications in preventing and controlling viral infections [130]. Depending on their origin, mannose-specific lectins from plants, fungi, algae and bacteria have been found to recognize either complex-type N-glycans or high-mannose-type N-glycans [27]. Some authors consider mannose/glucose/N-Acetylglucosamine binding lectins potent antiviral agents against coronaviruses, including SARS-CoV-2 [74]. A high-mannose specific lectin derived from the green alga Halimeda renschii has exhibited potent anti-influenza virus activity through high-affinity binding to the viral hemagglutinin [318]. MBLs also play a crucial role in the lectin pathway of complement activation. [319]. MBL status in host can be either advantageous or disadvantageous when considered from the viewpoint of the severity of a particular illness. It is known that people having higher levels of MBL are better able to modulate inflammation in contrast to those deficient in MBL who are at risk of sepsis and systemic inflammatory response syndrome (SIRS). For these reasons, we believe that analyses of the relevance of MBLs should extend beyond their established role in infectious diseases and include clinical areas such as autoimmunity and inflammatory disorders.
References
Smith, K.M., Machalaba, C.C., Seifman, R., Feferholtz, Y., Karesh, W.B.: Infectious disease and economics: The case for considering multi-sectoral impacts. One Health. 7, 100080 (2019)
Bloom, D.E., Black, S., Rappuoli, R.: Emerging infectious diseases: A proactive approach. Proc. Natl. Acad. Sci. U.S.A. 114(16), 4055–4059 (2017)
Friedrich, M.J.: WHO’s top health threats for 2019. JAMA 321(11), 1041 (2019)
Kausar, S., Said Khan, F., Ishaq Mujeeb Ur Rehman, M., Akram, M., Riaz, M., Rasool, G., Hamid Khan, A., Saleem, I., Shamim, S., Malik, A.: A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 35, 20587384211002621 (2021)
Elfakharany, E., Gerges, M., Behery, E., Mohsen, A., Belald, F.: COVID-19 coronavirus: Pathogenesis, clinical features, diagnostics, epidemiology, prevention and control. Appl. Pharm. 12, 65–66 (2020)
Oroojalian, F., Haghbin, A., Baradaran, B., Hemmat, N., Shahbazi, M.A., Baghi, H.B., Mokhtarzadeh, A., Hamblin, M.R.: Novel insights into the treatment of SARS-CoV-2 infection: an overview of current clinical trials. Int. J. Biol. Macromol. 165, 18–43 (2020)
Maginnis, M.S.: Virus–receptor interactions: the key to cellular invasion. J. Mol. Biol. 430(17), 2590–2611 (2018)
Nassar, A., Ibrahim, I.M., Amin, F.G., Magdy, M., Elgharib, A.M., Azzam, E.B., Nasser, F., Yousry, K., Shamkh, I.M., Mahdy, S.M., Elfiky, A.A.: A review of human coronaviruses’ receptors: the host-cell targets for the crown bearing viruses. Molecules 26(21), 6455 (2021)
Nabi-Afjadi, M., Heydari, M., Zalpoor, H., Arman, I., Sadoughi, A., Sahami, P., Aghazadeh, S.: Lectins and lectibodies: potential promising antiviral agents. Cell. Mol. Biol. Lett. 27(1), 1–25 (2022)
Mazalovska, M., Kouokam, J.C.: Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. Biomed Res. Int. (2018)
Kachko, A., Loesgen, S., Shahzad-ul-Hussan, S., Tan, W., Zubkova, I., Takeda, K., Wells, F., Rubin, S., Bewley, C.A., Major, M.E.: Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin: mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. Mol. Pharm. 10(12), 4590–4602 (2013)
Saad, M.H., Sidkey, N.M., Khan, R.H., El-Fakharany, E.M.: Nostoc muscorum is a novel source of microalgal lectins with potent antiviral activity against herpes simplex type-1. Int. J. Biol. Macromol. 210, 415–429 (2022)
Thompson, A.J., Cao, L., Ma, Y., Wang, X., Diedrich, J.K., Kikuchi, C., Willis, S., Worth, C., McBride, R., Yates, J.R., III., Paulson, J.C.: Human influenza virus hemagglutinins contain conserved oligomannose N-linked glycans allowing potent neutralization by lectins. Cell Host Microbe 27(5), 725–735 (2020)
Kaur, A., Kamboj, S.S., Singh, J., Singh, R., Abrahams, M., Kotwal, G.J., Saxena, A.K.: Purification of 3 monomeric monocot mannose-binding lectins and their evaluation for antipoxviral activity: potential applications in multiple viral diseases caused by enveloped viruses. Biochem. Cell Biol. 85(1), 88–95 (2007)
Favacho, A.R., Cintra, E.A., Coelho, L.C., Linhares, M.I.: In vitro activity evaluation of Parkia pendula seed lectin against human cytomegalovirus and herpes virus 6. Biol. 35(3), 189–194 (2007)
Ooi, L.S., Ho, W.S., Ngai, K.L., Tian, L., Chan, P.K., Sun, S.S., Ooi, V.E.: Narcissus tazetta lectin shows strong inhibitory effects against respiratory syncytial virus, influenza A (H1N1, H3N2, H5N1) and B viruses. J. Biosci. 1, 95–103 (2010)
Covés-Datson, E.M., Dyall, J., DeWald, L.E., King, S.R., Dube, D., Legendre, M., Nelson, E., Drews, K.C., Gross, R., Gerhardt, D.M., Torzewski, L.: Inhibition of Ebola virus by a molecularly engineered banana lectin. PLoS Negl. Trop. Dis. 13(7), e0007595 (2019)
Ahmed, M.N., Jahan, R., Nissapatorn, V., Wilairatana, P., Rahmatullah, M.: Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 146, 112507 (2022)
Lagarda-Diaz, I., Guzman-Partida, A.M., Vazquez-Moreno, L.: Legume lectins: proteins with diverse applications. Int. J. Mol. Sci. 18(6), 1242 (2017)
Dias, R.D., Machado, L.D., Migliolo, L., Franco, O.L.: Insights into animal and plant lectins with antimicrobial activities. Molecules 20(1), 519–541 (2015)
Santos, A.F., Da Silva, M.D., Napoleão, T.H., Paiva, P.M., Correia, M.D., Coelho, L.C.: Lectins: Function, structure, biological properties andpotential applications. Curr. Top. Pept. Protein Res. (2014)
Jandú, J.J., Moraes Neto, R.N., Zagmignan, A., de Sousa, E.M., Brelaz-de-Castro, M.C., dos Santos Correia, M.T., da Silva, L.C.: Targeting the immune system with plant lectins to combat microbial infections. Front. Pharmacol. 8, 671 (2017)
O’Keefe, B.R., Shenoy, S.R., Xie, D., Zhang, W., Muschik, J.M., Currens, M.J., Chaiken, I., Boyd, M.R.: Analysis of the interaction between the HIV-inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol. Pharmacol. 58(5), 982–992 (2000)
Bokesch, H.R., O’Keefe, B.R., McKee, T.C., Pannell, L.K., Patterson, G.M., Gardella, R.S., Sowder, R.C., Turpin, J., Watson, K., Buckheit, R.W., Boyd, M.R.: A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochem. 42(9), 2578–2584 (2003)
Botos, I., Wlodawer, A.: Cyanovirin-N: a sugar-binding antiviral protein with a new twist. Cell. Mol. Life Sci. 60(2), 277–287 (2003)
Barre, A., Bourne, Y., Van Damme, E.J., Rougé, P.: Overview of the structure–function relationships of mannose-specific lectins from plants, algae and fungi. Int. J. Mol. Sci. 20(2), 254 (2019)
Barre, A., Van Damme, E.J., Simplicien, M., Le Poder, S., Klonjkowski, B., Benoist, H., Peyrade, D., Rougé, P.: Man-specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) coronaviruses: Biomedical perspectives. Cells 10(7), 1619 (2021)
Van Damme, E.J., Smeets, K., Peumans, W.J.: The mannose-binding monocot lectins and their genes. Lect. Biomed. pers. 27, 59–80 (1995)
Stewart-Jones, G.B., Soto, C., Lemmin, T., Chuang, G.Y., Druz, A., Kong, R., Thomas, P.V., Wagh, K., Zhou, T., Behrens, A.J., Bylund, T.: Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell 165(4), 813–826 (2016)
Davenport, Y.W., West, A.P., Jr., Bjorkman, P.J.: Structure of an HIV-2 gp120 in complex with CD4. Virol. J. 90(4), 2112–2118 (2016)
Bonomelli, C., Doores, K.J., Dunlop, D.C., Thaney, V., Dwek, R.A., Burton, D.R., Crispin, M., Scanlan, C.N.: The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS ONE 6(8), e23521 (2011)
Shajahan, A., Pepi, L.E., Rouhani, D.S., Heiss, C., Azadi, P.: Glycosylation of SARS-CoV-2: structural and functional insights. Anal. Bioanal. Chem. 413(29), 7179–7193 (2021)
Cai, Y., Xu, W., Gu, C., Cai, X., Qu, D., Lu, L., Xie, Y., Jiang, S.: Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting SARS-CoV-2 spike S2 subunit. Virol. Sin. 35(6), 857–860 (2020)
Watanabe, Y., Bowden, T.A., Wilson, I.A., Crispin, M.: Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta - Gen. Subj. 1863(10), 1480–1497 (2019)
Seal, S., Dharmarajan, G., Khan, I.: Evolution of pathogen tolerance and emerging infections: A missing experimental paradigm. Elife. 10, e68874 (2021)
Kolchinsky, P., Kiprilov, E., Sodroski, J.: Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. Virol. J. 75(5), 2041–2050 (2001)
Peumans, W.J., Van Damme, E.J.: Lectins as plant defense proteins. Plant Physiol. 109(2), 347 (1995)
Wright, C.S.: New folds of plant lectins. Curr. Opin. Struct. Biol. 7(5), 631–636 (1997)
Taylor, M., Drickamer, K., Imberty, A., van Kooyk, Y., Schnaar, R., Etzler, M., Varki, A.: Discovery and classification of glycan-binding proteins. Essentials of Glycobiology (2022)
Van Parijs, J., Broekaert, W.F., Goldstein, I.J., Peumans, W.J.: Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 183(2), 258–264 (1991)
Van Damme, E.J., Balzarini, J., Smeets, K., van Leuven, F., Peumans, W.J.: The monomeric and dimeric mannose-binding proteins from the Orchidaceae species Listera ovata and Epipactis helleborine: sequence homologies and differences in biological activities. Glycoconj. J. 4, 321–332 (1994)
Sumner, J.B.: The globulins of the jack bean, Canavalia ensiformis: preliminary paper. J. Biol. Chem. 37(1), 137–142 (1919)
Naismith, J.H., Emmerich, C., Habash, J., Harrop, S.J., Helliwell, J.R., Hunter, W.N., Raftery, J., Yariv, J.: Refined structure of concanavalin A complexed with methyl α-D-mannopyranoside at 2.0 Å resolution and comparison with the saccharide-free structure. Acta Crystallogr., Sect. D: Biol. Crystallogr. 50(6), 847–858 (1994)
Ravishankar, R., Thomas, C.J., Suguna, K., Surolia, A., Vijayan, M.: Crystal structures of the peanut lectin–lactose complex at acidic pH: Retention of unusual quaternary structure, empty and carbohydrate bound combining sites, molecular mimicry and crystal packing directed by interactions at the combining site. Proteins: Struct. Funct. Bioinfo. 43(3), 260–270 (2001)
Rutenber, E., Robertus, J.D.: Structure of ricin B‐chain at 2.5 Å resolution. Proteins: Struct. Funct. Bioinfo. 10(3), 260–269 (1991)
Bah, C.S., Fang, E.F., Ng, T.B.: Medicinal applications of plant lectins. Antitumor potential and other emerging medicinal properties of natural compounds. 55–74 (2013)
Mishra, A., Behura, A., Mawatwal, S., Kumar, A., Naik, L., Mohanty, S.S., Manna, D., Dokania, P., Mishra, A., Patra, S.K., Dhiman, R.: Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 134, 110827 (2019)
Van Damme, E.J., Lannoo, N., Peumans, W.J.: Plant lectins. In Advances in botanical research. Acad. Press. 48, 107–209 (2008)
Van Holle, S., Van Damme, E.J.: Messages from the past: New insights in plant lectin evolution. Front. Plant Sci. 10, 36 (2019)
Nakamura-Tsuruta, S., Kominami, J., Kuno, A., Hirabayashi, J.: Evidence that Agaricus bisporus agglutinin (ABA) has dual sugar-binding specificity. Biochem. Biophys. Res. Commun. 347(1), 215–220 (2006)
Carrizo, M.E., Capaldi, S., Perduca, M., Irazoqui, F.J., Nores, G.A., Monaco, H.L.: The anti neoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J. Biol. Chem. 280, 10614–10623 (2005)
Rinderle, S.J., Goldstein, I.J., Matta, K.L., Ratcliffe, R.M.: Isolation and characterization of amaranthin, a lectin present in the seeds of Amaranthus caudatus, that recognizes the T-(or cryptic T)-antigen. J. Biol. Chem. 264(27), 16123–16131 (1989)
Transue, T.R., Smith, A.K., Mo, H., Goldstein, I.J., Saper, M.A.: Structure of benzyl T-antigen disaccharide bound to Amaranthus caudatus agglutinin. Nat. Struct. Mol. Biol. 4(10), 779–783 (1997)
Dang, L., Rougé, P., Van Damme, E.J.M.: Amaranthin–like proteins with aerolysin domains in plants. Front. Plant Sci. 8, 1368 (2017)
Van Damme, E.J., Culerrier, R., Barre, A., Alvarez, R., Rougé, P., Peumans, W.J.: A novel family of lectins evolutionarily related to class V chitinases: an example of neofunctionalization in legumes. J. Plant Physiol. 144(2), 662–672 (2007)
Boyd, M.R., Gustafson, K.R., McMahon, J.B., Shoemaker, R.H., O’Keefe, B.R., Mori, T., Gulakowski, R.J., Wu, L., Rivera, M.I., Laurencot, C.M., Currens, M.J.: Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41(7), 1521–1530 (1997)
Gustafson, K.R., Sowder, R.C., II., Henderson, L.E., Cardellina, J.H., II., McMahon, J.B., Rajamani, U., Pannell, L.K., Boyd, M.R.: Isolation, primary sequence determination, and disulfide bond structure of cyanovirin-N, an anti-HIV (human immunodeficiency virus) protein from the CyanobacteriumNostoc ellipsosporum. Biochem. Biophys. Res. Commun. 238(1), 223–228 (1997)
Percudani, R., Montanini, B., Ottonello, S.: The anti‐HIV cyanovirin‐N domain is evolutionarily conserved and occurs as a protein module in eukaryotes. Proteins: Struct. Funct. Genet. 60(4), 670–678 (2005)
Tsaneva, M., Van Damme, E.J.: 130 years of plant lectin research. Glycoconj. J. 37, 533–551 (2020)
Pacak, F., Kocourek, J.: Studies on phytohemagglutinins: xxv. Isolation and characterization of hemagglutinins of the spindle tree seeds (Evonymus europaea L.). Biochim Biophys Acta Proteins Proteom. 400(2), 374–386 (1975)
Petryniak, J., Pereira, M.E., Kabat, E.A.: The lectin of Euonymus europeus: purification, characterization, and an immunochemical study of its combining site. Arch. Biochem. Biophys. 178(1), 118–134 (1977)
Van Damme, E.J., Allen, A.K., Peumans, W.J.: Isolation and characterization of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett. 215(1), 140–144 (1987)
Barre, A., Van Damme, E.J., Peumans, W.J., Rouge, P.: Structure-function relationship of monocot mannose-binding lectins. Plant Physiol. 112(4), 1531–1540 (1996)
Peumans, W.J., Barre, A., Bras, J., Rougé, P., Proost, P., Van Damme, E.J.: The liverwort contains a lectin that is structurally and evolutionary related to the monocot mannose-binding lectins. Plant physiol. 129(3), 1054–1065 (2002)
Sastry, M.V., Banarjee, P., Patanjali, S.R., Swamy, M.J., Swarnalatha, G.V., Surolia, A.: Analysis of saccharide binding to Artocarpus integrifolia lectin reveals specific recognition of T-antigen (beta-D-Gal (1–3) D-GalNAc). J. Biol. Chem. 261(25), 11726–11733 (1986)
Bourne, Y., Astoul, C.H., Zamboni, V., Peumans, W.J., Menu-Bouaouiche, L., Van Damme, E.J., Barre, A., Rougé, P.: Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem. J. 364(1), 173–180 (2002)
Bourne, Y., Zamboni, V., Barre, A., Peumans, W.J., Van Damme, E.J., Rougé, P.: Helianthus tuberosus lectin reveals a widespread scaffold for mannose-binding lectins. Struct. 7(12), 1473–1482 (1999)
Sankaranarayanan, R., Sekar, K., Banerjee, R., Sharma, V., Surolia, A., Vijayan, M.: A novel mode of carbohydrate recognition in jacalin, a Moraceae plant lectin with a β-prism fold. Struct. Mol. Biol. 3(7), 596–603 (1996)
Sarkar, M., Wu, A.M., Kabat, E.A.: Immunochemical studies on the carbohydrate specificity of Maclura pomifera lectin. Arch. Biochem. Biophys. 209(1), 204–218 (1981)
Van Damme, E.J., Barre, A., Mazard, A.M., Verhaert, P., Horman, A., Debray, H., Rouge, P., Peumans, W.J.: Characterization and molecular cloning of the lectin from Helianthus tuberosus. European J. Biochem. 259(1–2), 135–142 (1999)
Peumans, W.J., Winter, H.C., Bemer, V., Van Leuven, F., Goldstein, I.J., Truffa-Bachi, P., Van Damme, E.J.: Isolation of a novel plant lectin with an unusual specificity from Calystegia sepium. Glycoconj. J. 14(2), 259–265 (1997)
El-Araby, M.M., El-Shatoury, E.H., Soliman, M.M., Shaaban, H.F.: Characterization and antimicrobial activity of lectins purified from three Egyptian leguminous seeds. AMB Exp. 10, 1–4 (2020)
Mitchell, C.A., Ramessar, K., O’Keefe, B.R.: Antiviral lectins: Selective inhibitors of viral entry. Antiviral Res. 142, 37–54 (2017)
Costa, A., Malveira, E.A., Mendonça, L.P., Maia, M.E., Silva, R.R., Roma, R.R., Aguiar, T.K., Grangeiro, Y.A., Souza, P.F.: Plant Lectins: A Review on their Biotechnological Potential Toward Human Pathogens. Curr. Protein Pept. Sci. 23(12), 851–861 (2022)
Peumans, W.J., Van Damme, J.M., Barre, A., Rougé, P.: Classification of plant lectins in families of structurally and evolutionary related proteins. Mol. Immun. Compl. Carb. 2, 27–54 (2001)
Bateman, A., Bycroft, M.: The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299, 1113–1119 (2000)
Lannoo, N., Vandenborre, G., Miersch, O., Smagghe, G., Wasternack, C., Peumans, W.J., Van Damme, E.J.: The jasmonate-induced expression of the Nicotiana tabacum leaf lectin. Plant Cell Physiol. 48(8), 1207–1218 (2007)
Schouppe, D., Rougé, P., Lasanajak, Y., Barre, A., Smith, D.F., Proost, P., Van Damme, E.J.M.: Mutational analysis of the carbohydrate binding activity of the tobacco lectin. Glycoconj. J. 27, 613–623 (2010)
Stirpe, F., Battelli, M.G.: Ribosome-inactivating proteins: progress and problems. Cell. Mol. Life Sci. 63, 1850–1866 (2006)
Barbieri, L., Battelli, M.G., Stirpe, F.: Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta. Biomembr. Bba-Biomembr. 1154(3–4), 237–282 (1993)
Chan, W.Y., Ng, T.B.: Comparison of the Embryotoxic Effects of Saporin, Agrostin (Type 1 Ribosome-Inactivating Proteins) and Ricin (a Type 2 Ribosome-Inactivating Protein. J. Pharmacol. Toxicol. 88(6), 300–303 (2001)
Barre, A., Simplicien, M., Benoist, H., Van Damme, E.J., Rougé, P.: Mannose-specific lectins from marine algae: diverse structural scaffolds associated to common virucidal and anti-cancer properties. Mar. Drugs 17(8), 440 (2019)
Notova, S., Bonnardel, F., Lisacek, F., Varrot, A., Imberty, A.: Structure and engineering of tandem repeat lectins. Curr. Opin. Struct. Biol. 62, 39–47 (2020)
Hirabayashi, J., Arai, R.: Lectin engineering: the possible and the actual. J. R. Soc. Interface. Foc. 9(2), 20180068 (2019)
Agrawal, B.B., Goldstein, I.J.: Physical and chemical characterization of concanavalin A, the hemagglutinin from jack bean (Canavalia ensiformis). Biochim. Biophys. Acta 133(2), 376–379 (1967)
Pratap, J.V., Jeyaprakash, A.A., Rani, P.G., Sekar, K., Surolia, A., Vijayan, M.: Crystal structures of artocarpin, a Moraceae lectin with mannose specificity, and its complex with methyl-α-D-mannose: implications to the generation of carbohydrate specificity. J. Mol. Biol. 317(2), 237–247 (2002)
Baumann, C.M., Strosberg, A.D., Rüdiger, H.: Purification and Characterization of a Mannose/Glucose-Specific Lectin from Vicia cracca. European J. Biochem. 122(1), 105–110 (1982)
Chowdhury, S., Ahmed, H., Chatterjee, B.P.: Chemical modification studies of Artocarpus lakoocha lectin artocarpin. Biochimie 73(5), 563–571 (1991)
Mann, K., Farias, C.M., Del Sol, F.G., Santos, C.F., Grangeiro, T.B., Nagano, C.S., Cavada, B.S., Calvete, J.J.: The amino-acid sequence of the glucose/mannose-specific lectin isolated from Parkia platycephala seeds reveals three tandemly arranged jacalin-related domains. European J. Biochem. 268(16), 4414–4422 (2001)
Antonyuk, V.O.: Integrated use of the jerusalem artichoke (Helianthus tuberosus L.) tubers: purification of inulin, fructose and mannosespecific lectin. FARM ZH. (3), 50–60 (2014)
Kaku, H., Van Damme, E.J., Peumans, W.J., Goldstein, I.J.: Carbohydrate-binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch. Biochem. Biophys. 279(2), 298–304 (1990)
Spiwok, V.: CH/π interactions in carbohydrate recognition. Mol. 22(7), 1038 (2017)
Yan, R., Zhang, Y., Li, Y., Xia, L., Guo, Y., Zhou, Q.: Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367(6485), 1444–1448 (2020)
Watanabe, Y., Bowden, T.A., Wilson, I.A., Crispin, M.: Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta. Gen. Subj. BBA-Gen. Subjects. 1863(10), 1480–1497 (2019)
Zhou, D., Tian, X., Qi, R., Peng, C., Zhang, W.: Identification of 22 N-glycosites on spike glycoprotein of SARS-CoV-2 and accessible surface glycopeptide motifs: Implications for vaccination and antibody therapeutics. Glycobiol. J. 31(1), 69–80 (2021)
Cipollo, J.F., Parsons, L.M.: Glycomics and glycoproteomics of viruses: Mass spectrometry applications and insights toward structure–function relationships. Mass Spectrom. Rev. 39(4), 371–409 (2020)
Ohyama, Y., Nakajima, K., Renfrow, M.B., Novak, J., Takahashi, K.: Mass spectrometry for the identification and analysis of highly complex glycosylation of therapeutic or pathogenic proteins. Expert Rev. Proteomics 17(4), 275–296 (2020)
Carbaugh, D.L., Lazear, H.M.: Flavivirus envelope protein glycosylation: impacts on viral infection and pathogenesis. J. Virol. 94(11), e00104-e120 (2020)
Vankadari, N., Wilce, J.A.: Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microb. Infect. 9(1), 601–604 (2020)
Bagdonaite, I., Vakhrushev, S.Y., Joshi, H.J., Wandall, H.H.: Viral glycoproteomes: technologies for characterization and outlook for vaccine design. FEBS Lett. 592(23), 3898–3920 (2018)
Reily, C., Stewart, T.J., Renfrow, M.B., Novak, J.: Glycosylation in health and disease. Nat. Rev. Nephrol. 15(6), 346–366 (2019)
Hargett, A.A., Renfrow, M.B.: Glycosylation of viral surface proteins probed by mass spectrometry Curr. Opin. Virol. 36, 56–66 (2019)
Fung, T.S., Liu, D.X.: Post-translational modifications of coronavirus proteins: roles and function. Future Virol. 13(6), 405–430 (2018)
Feng, T., Zhang, J., Chen, Z., Pan, W., Chen, Z., Yan, Y., Dai, J.: Glycosylation of viral proteins: Implication in virus–host interaction and virulence. Virulence. 13(1), 670–683 (2022)
Tortorici, M.A., Walls, A.C., Lang, Y., Wang, C., Li, Z., Koerhuis, D., Boons, G.J., Bosch, B.J., Rey, F.A., de Groot, R.J., Veesler, D.: Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 26(6), 481–489 (2019)
Bagdonaite, I., Wandall, H.H.: Global aspects of viral glycosylation. Glycobiology 28(7), 443–467 (2018)
Wang, D.: Coronaviruses’ sugar shields as vaccine candidates. Trends Immunol. 21, 17 (2020)
Zhao, H., To, K.K., Sze, K.H., Yung, T.T., Bian, M., Lam, H., Yeung, M.L., Li, C., Chu, H., Yuen, K.Y.: A broad-spectrum virus-and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat. Commun. 11(1), 4252 (2020)
Mathys, L., François, K.O., Quandte, M., Braakman, I., Balzarini, J.: Deletion of the highly conserved N-glycan at Asn260 of HIV-1 gp120 affects folding and lysosomal degradation of gp120, and results in loss of viral infectivity. PLoS ONE 9(6), e101181 (2014)
Quiñones-Kochs, M.I., Buonocore, L., Rose, J.K.: Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 76(9), 4199–4211 (2002)
Helle, F., Vieyres, G., Elkrief, L., Popescu, C.I., Wychowski, C., Descamps, V., Castelain, S., Roingeard, P., Duverlie, G., Dubuisson, J.: Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J. Virol. 84(22), 11905–11915 (2010)
Goffard, A., Callens, N., Bartosch, B., Wychowski, C., Cosset, F.L., Montpellier, C., Dubuisson, J.: Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 79(13), 8400–8409 (2005)
Dobrica, M.O., Lazar, C., Branza-Nichita, N.: N-glycosylation and N-glycan processing in HBV biology and pathogenesis. Cells 9(6), 1404 (2020)
Lazar, C., Durantel, D., Macovei, A., Zitzmann, N., Zoulim, F., Dwek, R.A., Branza-Nichita, N.: Treatment of hepatitis B virus-infected cells with α-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antivir. Res. 76(1), 30–37 (2007)
Lennemann, N.J., Walkner, M., Berkebile, A.R., Patel, N., Maury, W.: The role of conserved N-linked glycans on Ebola virus glycoprotein 2. J. Infect. Dis. 204, 9 (2015)
Antoine, T.E., Park, P.J., Shukla, D.: Glycoprotein targeted therapeutics: a new era of anti-herpes simplex virus-1 therapeutics. Rev. Med. Virol. 23(3), 194–208 (2013)
Barre, A., Van Damme, E.J., Klonjkowski, B., Simplicien, M., Sudor, J., Benoist, H., Rougé, P.: Legume lectins with different specificities as potential glycan probes for pathogenic enveloped viruses. Cells 11(3), 339 (2022)
Martinez, D., Amaral, D., Markovitz, D., Pinto, L.: The use of lectins as tools to combat SARS-CoV-2. Curr. Pharm. Des. 27(41), 4212–4222 (2021)
Carneiro, D.C., Fernandez, L.G., Monteiro-Cunha, J.P., Benevides, R.G., Cunha Lima, S.T.: A patent review of the antimicrobial applications of lectins: Perspectives on therapy of infectious diseases. J. Appl. Microbiol. 132(2), 841–854 (2022)
Liu, Y., Liu, J., Pang, X., Liu, T., Ning, Z., Cheng, G.: The roles of direct recognition by animal lectins in antiviral immunity and viral pathogenesis. Molecul. 20(2), 2272–2295 (2015)
Fujimoto, Y.K., Green, D.F.: Carbohydrate recognition by the antiviral lectin cyanovirin-N. J. Am. Chem. Soc. 134(48), 19639–19651 (2012)
McGreal, E.P., Rosas, M., Brown, G.D., Zamze, S., Wong, S.Y., Gordon, S., Martinez-Pomares, L., Taylor, P.R.: The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiol. 16(5), 422–430 (2006)
Khan, H., Aziz, A.A., Sulahria, H., Khan, H., Ahmed, A., Choudhry, N., Narayanan, R., Danzig, C., Khanani, A.M.: Emerging treatment options for geographic atrophy (GA) secondary to age-related macular degeneration. Clin. Ophthalmol. 31, 321–327 (2023)
Lu, J., Zhao, Z., Li, Q., Pang, Y.: Review of the unique and dominant lectin pathway of complement activation in agnathans. Dev. Comp. Immunol. 140, 104593 (2023)
Riwes, M.M., Leather, H., Neal, D., Bennett, C., Sugrue, M., Cline, C., Stokes, J., Hiemenz, J., Hsu, J., Wingard, J.R.: Association of mannose-binding lectin levels and invasive fungal disease in hematologic malignancy patients receiving myelosuppressive chemotherapy or allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 51(9), 1228–1232 (2016)
Shan, L.H., Lee, P.L., Chen, H.W., Chen, L.K., Kao, C.L., King, C.C.: Analysis of the steps involved in dengue virus entry into host cells. Virol. 257(1), 156–167 (1999)
Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., Venkitanarayanan, K.: Combating pathogenic microorganisms using plant-derived antimicrobials: a minireview of the mechanistic basis. Biomed Res. Int. (2014)
Hwang, H.J., Han, J.W., Jeon, H., Cho, K., Kim, J.H., Lee, D.S., Han, J.W.: Characterization of a novel mannose-binding lectin with antiviral activities from red alga. Grateloupia chiangii. Biomolecul. 10(2), 333 (2020)
Sato, Y., Hirayama, M., Morimoto, K., Yamamoto, N., Okuyama, S., Hori, K.: High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 286(22), 19446–19458 (2011)
Gupta, A., Gupta, G.S.: Status of mannose-binding lectin (MBL) and complement system in COVID-19 patients and therapeutic applications of antiviral plant MBLs. Mol. Cell. Biochem. 476(8), 2917–2942 (2021)
Barton, C., Kouokam, J.C., Hurst, H., Palmer, K.E.: Pharmacokinetics of the Antiviral Lectin Griffithsin Administered by Different Routes Indicates Multiple Potential Uses. Viruses 8(12), 331 (2016)
Pengcheng, W., Bai, J., Liu, X., Wang, M., Wang, X., Jiang, P.: Tomatidine inhibits porcine epidemic diarrhea virus replication by targeting 3CL protease. Vet. Res. 51, 1–8 (2020)
Pritchard, L.K., Spencer, D.I., Royle, L., Bonomelli, C., Seabright, G.E., Behrens, A.J., Kulp, D.W., Menis, S., Krumm, S.A., Dunlop, D.C., Crispin, D.J.: Glycan clustering stabilizes the mannose patch of HIV-1 and preserves vulnerability to broadly neutralizing antibodies. Nat. Commun. 6(1), 1–1 (2015)
Ward, A.B., Wilson, I.A.: Insights into the trimeric HIV-1 envelope glycoprotein structure. Trends Biochem. Sci. 40(2), 101–107 (2015)
Wang, W., Nie, J., Prochnow, C., Truong, C., Jia, Z., Wang, S., Chen, X.S., Wang, Y.: A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirol. 10(1), 1–4 (2013)
Tilton, J.C., Doms, R.W.: Entry inhibitors in the treatment of HIV-1 infection. Antiviral Res. 85(1), 91–100 (2010)
Akkouh, O., Ng, T.B., Singh, S.S., Yin, C., Dan, X., Chan, Y.S., Pan, W., Cheung, R.C.: Lectins with anti-HIV activity: a review. Molecul. 20(1), 648–668 (2015)
Checkley, M.A., Luttge, B.G., Freed, E.O.: HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J. Mol. Biol. 410(4), 582–608 (2011)
Murugaiah, V., Yasmin, H., Pandit, H., Ganguly, K., Subedi, R., Al-Mozaini, M., Madan, T., Kishore, U.: Innate Immune Response Against HIV-1. Microb. Pathog. 23–58 (2021)
Xiao, T., Cai, Y., Chen, B.: HIV-1 entry and membrane fusion inhibitors. Viruses 13(5), 735 (2021)
Blumenthal, R., Durell, S., Viard, M.: HIV entry and envelope glycoprotein-mediated fusion. J. Biol. Chem. 287(49), 40841–40849 (2012)
Wilen, C.B., Tilton, J.C., Doms, R.W.: HIV: cell binding and entry. Cold Spring Harb. Perspect. Med. 2(8), a006866 (2012)
Mizuochi, T., Matthews, T.J., Kato, M., Hamako, J., Titani, K., Solomon, J., Feizi, T.: Diversity of oligosaccharide structures on the envelope glycoprotein gp 120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J. Biol. Chem. 265(15), 8519–8524 (1990)
Geyer, H., Holschbach, C., Hunsmann, G., Schneider, J.: Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J. Biol. Chem. 263(24), 11760–11767 (1998)
Go, E.P., Hewawasam, G., Liao, H.X., Chen, H., Ping, L.H., Anderson, J.A., Hua, D.C., Haynes, B.F., Desaire, H.: Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J. Virol. 85(16), 8270–8284 (2011)
Go, E.P., Herschhorn, A., Gu, C., Castillo-Menendez, L., Zhang, S., Mao, Y., Chen, H., Ding, H., Wakefield, J.K., Hua, D., Liao, H.X.: Comparative analysis of the glycosylation profiles of membrane-anchored HIV-1 envelope glycoprotein trimers and soluble gp140. J. Virol. 89(16), 8245–8257 (2015)
Raska, M., Takahashi, K., Czernekova, L., Zachova, K., Hall, S., Moldoveanu, Z., Elliott, M.C., Wilson, L., Brown, R., Jancova, D., Barnes, S.: Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J. Biol. Chem. 285(27), 20860–20869 (2010)
Zhu, X., Borchers, C., Bienstock, R.J., Tomer, K.B.: Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochem. 39(37), 11194–11204 (2000)
Behrens, A.J., Harvey, D.J., Milne, E., Cupo, A., Kumar, A., Zitzmann, N., Struwe, W.B., Moore, J.P., Crispin, M.: Molecular architecture of the cleavage-dependent mannose patch on a soluble HIV-1 envelope glycoprotein trimer. J. Virol. 91(2), e01894-e1916 (2017)
Sok, D., Doores, K.J., Briney, B., Le, K.M., Saye-Francisco, K.L., Ramos, A., Kulp, D.W., Julien, J.P., Menis, S., Wickramasinghe, L., Seaman, M.S.: Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci. Transl. Med. 6(236), 236ra63 (2014)
Coss, K.P., Vasiljevic, S., Pritchard, L.K., Krumm, S.A., Glaze, M., Madzorera, S., Moore, P.L., Crispin, M., Doores, K.J.: HIV-1 glycan density drives the persistence of the mannose patch within an infected individual. J. Virol. 90(24), 11132–11144 (2016)
Wang, L.X., Song, H., Liu, S., Lu, H., Jiang, S., Ni, J., Li, H.: Chemoenzymatic synthesis of HIV-1 gp41 glycopeptides: effects of glycosylation on the anti-HIV activity and α-helix bundle-forming ability of peptide C34. ChemBioChem 6(6), 1068–1074 (2005)
Ji, X., Chen, Y., Faro, J., Gewurz, H., Bremer, J., T Spear G.: Interaction of human immunodeficiency virus (HIV) glycans with lectins of the human immune system. Curr. Protein Pept. Sci. 7(4), 317–324 (2006)
Svarovsky, S.A., Joshi, L.: Biocombinatorial selection of carbohydrate binding agents of therapeutic significance. Curr. Drug Discov. Technol. 5(1), 20–28 (2008)
Jahan, R., Ahmed, M.N., Nissapatorn, V., Wilairatana, P., Rahmatullah, M.: Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 146, 112507 (2022)
Peng, H., Lv, H., Wang, Y., Liu, Y.H., Li, C.Y., Meng, L., Chen, F., Bao, J.K.: Clematis montana lectin, a novel mannose-binding lectin from traditional Chinese medicine with antiviral and apoptosis-inducing activities. Peptides 30(10), 1805–1815 (2009)
Van Damme, E.J., Rougé, P.: Lectins from plants, algae, fungi, bacteria and animal therapeutic tools for SARS-CoV-2 and other pathogenic enveloped viruses, in a “one-health” perspective. Front. Cell. Infect. Microbiol. 13, 49 (2023)
Jaakkonen, A., Volkmann, G., Iwaï, H.: An off-the-shelf approach for the production of fc fusion proteins by protein trans-splicing towards generating a lectibody In Vitro. Int. J. Mol. Sci. 21(11), 4011 (2020)
Boyd, M.R., Gustafson, K.R., McMahon, J.B., Shoemaker, R.H., O’Keefe, B.R., Mori, T., Gulakowski, R.J., Wu, L., Rivera, M.I., Laurencot, C.M., Currens, M.J.: Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1521, 30 (1997)
Bolmstedt, A.J., O’Keefe, B.R., Shenoy, S.R., McMahon, J.B., Boyd, M.R.: Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol. Pharmacol. 59(5), 949–954 (2001)
Barrientos, L.G., Gronenborn, A.M.: The highly specific carbohydrate-binding protein cyanovirin-N: structure, anti-HIV/Ebola activity and possibilities for therapy. Mini-Rev. Med. Chem. 5(1), 21–31 (2005)
Bewley, C.A., Otero-Quintero, S.: The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man8 D1D3 and Man9 with nanomolar affinity: implications for binding to the HIV envelope protein gp120. J. Am. Chem. Soc. 123(17), 3892–3902 (2001)
Liu, Y., Carroll, J.R., Holt, L.A., McMahon, J., Giomarelli, B., Ghirlanda, G.: Multivalent interactions with gp120 are required for the anti-HIV activity of Cyanovirin. Peptide Sci. 92(3), 194–200 (2009)
Nickoloff-Bybel, E.A., Festa, L., Meucci, O., Gaskill, P.J.: Co-receptor signaling in the pathogenesis of neuroHIV. Retrovirology 18(1), 24 (2021)
Keeffe, J.R., Gnanapragasam, P.N., Gillespie, S.K., Yong, J., Bjorkman, P.J., Mayo, S.L.: Designed oligomers of cyanovirin-N show enhanced HIV neutralization. Proc. Natl. Acad. Sci. 108(34), 14079–14084 (2011)
Balzarini, J., Van Laethem, K., Peumans, W.J., Van Damme, E.J., Bolmstedt, A., Gago, F., Schols, D.: Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J. Virol. 80(17), 8411–8421 (2006)
Tsai, C.C., Emau, P., Jiang, Y., Tian, B., Morton, W.R., Gustafson, K.R., Boyd, M.R.: Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89. 6P in macaques. AIDS Res. Hum. Retroviruses. 19(7), 535–541 (2003)
Dey, B., Lerner, D.L., Lusso, P., Boyd, M.R., Elder, J.H., Berger, E.A.: Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 74(10), 4562–4569 (2000)
Barrientos, L.G., O’Keefe, B.R., Bray, M., Sanchez, A., Gronenborn, A.M., Boyd, M.R.: Cyanovirin-N binds to the viral surface glycoprotein, GP1, 2 and inhibits infectivity of Ebola virus. Antiviral Res. 58(1), 47–56 (2003)
Helle, F., Wychowski, C., Vu-Dac, N., Gustafson, K.R., Voisset, C., Dubuisson, J.: Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 281(35), 25177–25183 (2006)
O’Keefe, B.R., Smee, D.F., Turpin, J.A., Saucedo, C.J., Gustafson, K.R., Mori, T., Blakeslee, D., Buckheit, R., Boyd, M.R.: Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 47(8), 2518–2525 (2003)
Wang, W., Cole, A.M., Hong, T., Waring, A.J., Lehrer, R.I.: Retrocyclin, an antiretroviral θ-defensin, is a lectin. J. Immunol. Res. 170(9), 4708–4716 (2003)
Bewley, C.A., Gustafson, K.R., Boyd, M.R., Covell, D.G., Bax, A., Clore, G.M., Gronenborn, A.M.: Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 5(7), 571–578 (1998)
Zweckstetter, M., Bax, A.: Prediction of sterically induced alignment in a dilute liquid crystalline phase: aid to protein structure determination by NMR. J.Am. Chem. Soc. 122(15), 3791–3792 (2000)
Vamvaka, E., Evans, A., Ramessar, K., Krumpe, L.R., Shattock, R.J., O’Keefe, B.R., Christou, P., Capell, T.: Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro. Plant Cell Rep. 35(6), 1309–1319 (2016)
Fischetti, L., Barry, S.M., Hope, T.J., Shattock, R.J.: HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS (London, England). 23(3), 319 (2009)
Kehr, J.C., Zilliges, Y., Springer, A., Disney, M.D., Ratner, D.D., Bouchier, C., Seeberger, P.H., De Marsac, N.T., Dittmann, E.: A mannan binding lectin is involved in cell–cell attachment in a toxic strain of Microcystis aeruginosa. Mol. Microbiol. 59(3), 893–906 (2006)
Shahzad-ul-Hussan, S., Gustchina, E., Ghirlando, R., Clore, G.M., Bewley, C.A.: Solution structure of the monovalent lectin microvirin in complex with Manα (1–2) Man provides a basis for anti-HIV activity with low toxicity. J. Biol. Chem. 286(23), 20788–20796 (2000)
Williams, D.C., Lee, J.Y., Cai, M., Bewley, C.A., Clore, G.M.: Crystal structures of the HIV-1 inhibitory cyanobacterial protein MVL free and bound to Man3GlcNAc2: structural basis for specificity and high-affinity binding to the core pentasaccharide from n-linked oligomannoside. J. Biol. Chem. 280(32), 29269–29276 (2005)
Bewley, C.A., Cai, M., Ray, S., Ghirlando, R., Yamaguchi, M., Muramoto, K.: New carbohydrate specificity and HIV-1 fusion blocking activity of the cyanobacterial protein MVL: NMR, ITC and sedimentation equilibrium studies. J. Mol. Biol. 339(4), 901–914 (2004)
Huskens, D., Férir, G., Vermeire, K., Kehr, J.C., Balzarini, J., Dittmann, E., Schols, D.: Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J. Biol. Chem. 285(32), 24845–24854 (2010)
Siqueira, A.S., Lima, A.R., de Souza, R.C., Santos, A.S., Vianez Júnior, J.L., Gonçalves, E.C.: In silico analysis of the cyanobacterial lectin scytovirin: new insights into binding properties. Mol. Biol. Rep. 44(4), 353–358 (2017)
Xiong, C., O’Keefe, B.R., Botos, I., Wlodawer, A., McMahon, J.B.: Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr. Purif. 46(2), 233–239 (2006)
Covés-Datson, E.M., King, S.R., Legendre, M., Swanson, M.D., Gupta, A., Claes, S., Meagher, J.L., Boonen, A., Zhang, L., Kalveram, B., Raglow, Z.: Targeted disruption of pi–pi stacking in Malaysian banana lectin reduces mitogenicity while preserving antiviral activity. Sci. Rep. 11(1), 656 (2021)
Hopper, J.T., Ambrose, S., Grant, O.C., Krumm, S.A., Allison, T.M., Degiacomi, M.T., Tully, M.D., Pritchard, L.K., Ozorowski, G., Ward, A.B., Crispin, M.: The tetrameric plant lectin BanLec neutralizes HIV through bidentate binding to specific viral glycans. Structure. 25(5), 773–782 (2017)
Singh, S.S., Devi, S.K., Ng, T.B.: Banana lectin: a brief review. Molecules 19(11), 18817–18827 (2014)
Mordi, R.C., Fadiaro, A.E., Owoeye, T.F., Olanrewaju, I.O., Uzoamaka, G.C., Olorunshola, S.J.: Identification by GC-MS of the components of oils of banana peels extract, phytochemical and antimicrobial analyses. Res. J. Phytochem. 10(1), 39–44 (2016)
Meagher, J.L., Winter, H.C., Ezell, P., Goldstein, I.J., Stuckey, J.A.: Crystal structure of banana lectin reveals a novel second sugar binding site. Glycobiology 15(10), 1033–1042 (2005)
Swanson, M.D., Boudreaux, D.M., Salmon, L., Chugh, J., Winter, H.C., Meagher, J.L., Andre, S., Murphy, P.V., Oscarson, S., Roy, R., King, S.: Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell 163(3), 746–758 (2015)
Lopandić, Z., Dragačević, L., Popović, D., Andjelković, U., Minić, R., Gavrović-Jankulović, M.: BanLec-eGFP chimera as a tool for evaluation of lectin binding to high-mannose glycans on microorganisms. Biomolecules 11(2), 180 (2021)
Subramaniam, G., Batcha, A.T., Wadhwani, A.: In vitro antiviral activity of BanLec against herpes simplex viruses type 1 and 2. Bangladesh J. Pharmacol. 15(1), 11–18 (2020)
Chan, J.F., Oh, Y.J., Yuan, S., Chu, H., Yeung, M.L., Canena, D., Chan, C.C., Poon, V.K., Chan, C.C., Zhang, A.J., Cai, J.P.: A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo. Cell Rep. 3(10), 100774 (2022)
Ziółkowska, N.E., Shenoy, S.R., O'Keefe, B.R., McMahon, J.B., Palmer, K.E., Dwek, R.A., Wormald, M.R., Wlodawer, A.: Crystallographic, thermodynamic, and molecular modeling studies of the mode of binding of oligosaccharides to the potent antiviral protein griffithsin. Proteins: Struct. Funct. 67(3), 661–670 (2007)
Derby, N., Lal, M., Aravantinou, M., Kizima, L., Barnable, P., Rodriguez, A., Lai, M., Wesenberg, A., Ugaonkar, S., Levendosky, K., Mizenina, O.: Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 9(1), 3881(2018)
Lusvarghi, S., Bewley, C.A.: Griffithsin: an antiviral lectin with outstanding therapeutic potential. Viruses 8(10), 296 (2016)
Alam, A., Jiang, L., Kittleson, G.A., Steadman, K.D., Nandi, S., Fuqua, J.L., Palmer, K.E., Tusé, D., McDonald, K.A.: Technoeconomic modeling of plant-based griffithsin manufacturing. Front. Bioeng. Biotechnol. 6, 102 (2018)
Millet, J.K., Séron, K., Labitt, R.N., Danneels, A., Palmer, K.E., Whittaker, G.R., Dubuisson, J., Belouzard, S.: Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 133, 1–8 (2016)
Hoelscher, M., Tiller, N., Teh, A.Y., Wu, G.Z., Ma, J.K., Bock, R.: High-level expression of the HIV entry inhibitor griffithsin from the plastid genome and retention of biological activity in dried tobacco leaves. Plant Mol. Biol. 97, 357–370 (2018)
Li, L., Yu, X., Zhang, H., Cheng, H., Hou, L., Zheng, Q., Hou, J.: In vitro antiviral activity of Griffithsin against porcine epidemic diarrhea virus. Virus Genes 55, 174–181 (2019)
Mori, T., O’Keefe, B.R., Sowder, R.C., Bringans, S., Gardella, R., Berg, S., Cochran, P., Turpin, J.A., Buckheit, R.W., McMahon, J.B., Boyd, M.R.: Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 280(10), 9345–9353 (2005)
O’Keefe, B.R., Vojdani, F., Buffa, V., Shattock, R.J., Montefiori, D.C., Bakke, J., Mirsalis, J., d’Andrea, A.L., Hume, S.D., Bratcher, B., Saucedo, C.J.: Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. 106(15), 6099–6104 (2009)
Barton, C., Kouokam, J.C., Lasnik, A.B., Foreman, O., Cambon, A., Brock, G., Montefiori, D.C., Vojdani, F., McCormick, A.A., O’Keefe, B.R., Palmer, K.E.: Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 58(1), 120–127 (2014)
Hoorelbeke, B., Huskens, D., Férir, G., François, K.O., Takahashi, A., Van Laethem, K., Schols, D., Tanaka, H., Balzarini, J.: Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope. Antimicrob. Agents Chemother. 54(8), 3287–3301 (2010)
Koharudin, L.M., Furey, W., Gronenborn, A.M.: Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J. Biol. Chem. 286(2), 1588–1597 (2011)
Whitley, M.J., Furey, W., Kollipara, S., Gronenborn, A.M.: B urkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: structures, carbohydrate binding and anti-HIV activity. FEBS J. 280(9), 2056–2067 (2013)
Férir, G., Huskens, D., Noppen, S., Koharudin, L.M., Gronenborn, A.M., Schols, D.: Broad anti-HIV activity of the Oscillatoria agardhii agglutinin homologue lectin family. J. Antimicrob. Chemother. 69(10), 2746–2758 (2014)
McFeeters, H., Gilbert, M.J., Wood, A.M., Haggenmaker, C.B., Jones, J., Kutsch, O., McFeeters, R.L.: Scytovirin engineering improves carbohydrate affinity and HIV-1 entry inhibition. Biochem. Physiol. S. 2(2) (2013)
López, S., Armand-Ugon, M., Bastida, J., Viladomat, F., Esté, J.A., Stewart, D., Codina, C.: Anti-human immunodeficiency virus type 1 (HIV-1) activity of lectins from Narcissus species. Planta Med. 69(2), 109–112 (2003)
Swanson, M.D., Winter, H.C., Goldstein, I.J., Markovitz, D.M.: A lectin isolated from bananas is a potent inhibitor of HIV replication. J. Biol. Chem. 285(12), 8646–8655 (2010)
Balzarini, J., Neyts, J., Schols, D., Hosoya, M., Van Damme, E., Peumans, W., De Clercq, E.: The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 18(2), 191–207 (1992)
Teixeira, C.S., Assreuy, A.M., da Silva Osterne, V.J., Amorim, R.M., Brizeno, L.A., Debray, H., Nagano, C.S., Delatorre, P., Sampaio, A.H., Rocha, B.A., Cavada, B.S.: Mannose-specific legume lectin from the seeds of Dolichos lablab (FRIL) stimulates inflammatory and hypernociceptive processes in mice. Process Biochem. 49(3), 529–534 (2014)
Jayaprakash, N.G., Singh, A., Vivek, R., Yadav, S., Pathak, S., Trivedi, J., Jayaraman, N., Nandi, D., Mitra, D., Surolia, A.: Correction: The barley lectin, horcolin, binds high-mannose glycans in a multivalent fashion, enabling high-affinity, specific inhibition of cellular HIV infection. J. Biol. Chem. 297(3) (2021)
Gondim, A.C., da Silva, S.R., Mathys, L., Noppen, S., Liekens, S., Sampaio, A.H., Nagano, C.S., Rocha, C.R., Nascimento, K.S., Cavada, B.S., Sadler, P.J.: Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. Med. Chem. Comm. 10(3), 390–398 (2019)
Ghany, M.G., Strader, D.B., Thomas, D.L., Seeff, L.B.: American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 49(4), 1335–1374(2009)
Moustafa, R.I., Dubuisson, J., Lavie, M.: Function of the HCV E1 envelope glycoprotein in viral entry and assembly. Future Virol. 171–184 (2019)
Lavie, M., Hanoulle, X., Dubuisson, J.: Glycan shielding and modulation of hepatitis C virus neutralizing antibodies. Front. Immunol. 9, 910 (2018)
Helle, F., Duverlie, G., Dubuisson, J.: The hepatitis C virus glycan shield and evasion of the humoral immune response. Viruses 3(10), 1909–1932 (2011)
Shahid, M., Qadir, A., Yang, J., Ahmad, I., Zahid, H., Mirza, S., Windisch, M.P., Shahzad-ul-Hussan, S.: An engineered microvirin variant with identical structural domains potently inhibits human immunodeficiency virus and hepatitis C virus cellular entry. Viruses 12(2), 199 (2020)
Meuleman, P., Albecka, A., Belouzard, S., Vercauteren, K., Verhoye, L., Wychowski, C., Leroux-Roels, G., Palmer, K.E., Dubuisson, J.: Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 55(11), 5159–5167 (2011)
Loimaranta, V., Hepojoki, J., Laaksoaho, O., Pulliainen, A.T.: Galectin-3-binding protein: A multitask glycoprotein with innate immunity functions in viral and bacterial infections. J. Leukoc. Biol. 104(4), 777–786 (2018)
Ouchida, T., Maeda, H., Akamatsu, Y., Maeda, M., Takamatsu, S., Kondo, J., Misaki, R., Kamada, Y., Ueda, M., Ueda, K., Miyoshi, E.: Pholiota squarrosa lectin (PhoSL), a lectin binding to core-fucose specifically, inhibits HBV infection. Res Sq. (2022)
Bertaux, C., Daelemans, D., Meertens, L., Cormier, E.G., Reinus, J.F., Peumans, W.J., Van Damme, E.J., Igarashi, Y., Oki, T., Schols, D., Dragic, T.: Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology 366(1), 40–50 (2007)
Jensen, S.M., Ruscetti, F.W., Rein, A., Bertolette, D.C., Saucedo, C.J., O’Keefe, B.R., Jones, K.S.: Differential inhibitory effects of cyanovirin-N, griffithsin, and scytovirin on entry mediated by envelopes of gammaretroviruses and deltaretroviruses. J. Virol. 88(4), 2327–2332 (2014)
Ko, S.M., Kwon, J., Vaidya, B., Choi, J.S., Lee, H.M., Oh, M.J., Bae, H.J., Cho, S.Y., Oh, K.S., Kim, D.: Development of lectin-linked immunomagnetic separation for the detection of hepatitis A virus. Viruses 6(3), 1037–1048 (2014)
Taghizadeh, S.F., Azizi, M., Asili, J., Madarshahi, F.S., Rakhshandeh, H., Fujii, Y.: Therapeutic peptides of Mucuna pruriens L.: Anti‐genotoxic molecules against human hepatocellular carcinoma and hepatitis C virus. Food Sci. Nutr. 9(6), 2908–14 (2021)
Al-Sohaimy, S.A., Hafez, E.E., Abdelwahab, A.E., El-Saadani, M.A.: Anti-HCV lectin from Egyptian Pisum sativum. Aust. J. Basic Appl. Sci. 1(3), 213–219 (2007)
Palese, P.: Orthomyxoviridae: the viruses and their replication. Fields virology. 1647–89 (2007)
Ran, Z., Shen, H., Lang, Y., Kolb, E.A., Turan, N., Zhu, L., Ma, J., Bawa, B., Liu, Q., Liu, H., Quast, M.: Domestic pigs are susceptible to infection with influenza B viruses. J. Virol. 89(9), 4818–4826 (2015)
Nickol, M.E., Kindrachuk, J.: A year of terror and a century of reflection: perspectives on the great influenza pandemic of 1918–1919. BMC Infect. Dis. 19(1), 1 (2019)
Morimoto, K., Sato, Y.: Anti-influenza virus activity of high-mannose binding lectins derived from genus Pseudomonas. Virus Res. 223, 64–72 (2016)
Wu, N.C., Young, A.P., Al-Mawsawi, L.Q., Olson, C.A., Feng, J., Qi, H., Chen, S.H., Lu, I., Lin, C.Y., Chin, R.G., Luan, H.H.: High-throughput profiling of influenza A virus hemagglutinin gene at single-nucleotide resolution. Sci. Rep. 4(1), 1–8 (2014)
Wu, C.Y., Lin, C.W., Tsai, T.I., Lee, C.C., Chuang, H.Y., Chen, J.B., Tsai, M.H., Chen, B.R., Lo, P.W., Liu, C.P., Shivatare, V.S.: Influenza A surface glycosylation and vaccine design. Proc. Natl. Acad. Sci. U S A. 114(2), 280–5 (2017)
Covés-Datson, E.M., King, S.R., Legendre, M., Gupta, A., Chan, S.M., Gitlin, E., Kulkarni, V.V., Pantaleón García, J., Smee, D.F., Lipka, E., Evans, S.E., Tarbet, E.B., Ono, A., Markovitz, D.M.: A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc Natl Acad Sci U S A. 117(4), 2122–2132 (2020)
Gordts, S.C., Renders, M., Férir, G., Huskens, D., Van Damme, E.J., Peumans, W., Balzarini, J., Schols, D.: NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J. Antimicrob. Chemother. 70(6), 1674–1685 (2015)
Ooi, L.S., Ng, T.B., Geng, Y., Ooi, V.E.: Lectins from bulbs of the Chinese daffodil Narcissus tazetta (family Amaryllidaceae). Biochem. Cell Biol. 78(4), 463–468 (2000)
Ooi, L.S., Sun, S.S., Ng, T.B., Ooi, V.E.: Molecular cloning and the cDNA-derived amino acid sequence of Narcissus tazetta isolectins. J. Protein Chem. 20(4), 305–310 (2001)
Sato, Y., Morimoto, K., Kubo, T., Sakaguchi, T., Nishizono, A., Hirayama, M., Hori, K.: Entry inhibition of influenza viruses with high mannose binding lectin ESA-2 from the red alga Eucheuma serra through the recognition of viral hemagglutinin. Mar. Drugs 13(6), 3454–3465 (2015)
Sato, Y., Morimoto, K., Hirayama, M., Hori, K.: High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem. Biophys. Res. Commun. 405(2), 291–296 (2011)
Vanderlinden, E., Van Winkel, N., Naesens, L., Van Damme, E.J., Persoons, L., Schols, D.: In vitro characterization of the carbohydrate-binding agents HHA, GNA, and UDA as inhibitors of influenza A and B virus replication. Antimicrob. Agents Chemother. 65(3), e01732-e1820 (2021)
Ooi, L.S., Sun, S.S., Ooi, V.E.: Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae). Int. J. Biochem. Cell Biol. 36(8), 1440–1446 (2004)
Liu, Y.M., Shahed-Al-Mahmud, M., Chen, X., Chen, T.H., Liao, K.S., Lo, J.M., Wu, Y.M., Ho, M.C., Wu, C.Y., Wong, C.H., Jan, J.T.: A carbohydrate-binding protein from the edible lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 32(6), 108016 (2020)
Kleinschmidt‐DeMasters, B.K., Keohane, C., Gray, F.: Herpes simplex virus infections of the CNS. Infections of the Central Nervous System: Pathology and Genetics. 43–54 (2020)
Lim, T.K.: Edible medicinal and non-medicinal plants. Dordrecht, The Netherlands: Springer. 1, 656–687 (2012)
Luo, Y., Xu, X., Liu, J., Li, J., Sun, Y., Liu, Z., Liu, J., Van Damme, E., Balzarini, J., Bao, J.: A novel mannose-binding tuber lectin from Typhonium divaricatum (L.) Decne (family Araceae) with antiviral activity against HSV-II and anti-proliferative effect on human cancer cell lines. J Biochem Mol Biol. 40(3), 358–67 (2007)
Tiwari, V., Shukla, S.Y., Shukla, D.: A sugar binding protein cyanovirin-N blocks herpes simplex virus type-1 entry and cell fusion. Antiviral Res. 84(1), 67–75 (2009)
Nixon, B., Stefanidou, M., Mesquita, P.M., Fakioglu, E., Segarra, T., Rohan, L., Halford, W., Palmer, K.E., Herold, B.C.: Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. Virol. J. 87(11), 6257–6269 (2013)
Yang, Y., Xu, H.L., Zhang, Z.T., Liu, J.J., Li, W.W., Ming, H., Bao, J.K.: Characterization, molecular cloning, and in silico analysis of a novel mannose-binding lectin from Polygonatum odoratum (Mill.) with anti-HSV-II and apoptosis-inducing activities. Phytomedicine. 18(8–9), 748–55 (2011)
Marchetti, M., Mastromarino, P., Rieti, S., Seganti, L., Orsi, N.: Inhibition of herpes simplex, rabies and rubella viruses by lectins with different specificities. Res. Virol. 146(3), 211–215 (1995)
Gatherer, D.: The 2014 Ebola virus disease outbreak in West Africa. J. Gen. Virol. 95(8), 1619–1624 (2014)
Briand, S., Bertherat, E., Cox, P., Formenty, P., Kieny, M.P.: The international Ebola emergency. N. Engl. J. 371(13), 1180–1183 (2014)
Negredo, A., Palacios, G., Vázquez-Morón, S., González, F., Dopazo, H., Molero, F., Juste, J., Quetglas, J., Savji, N., de la Cruz, M.M., Herrera, J.E.: Discovery of an ebolavirus-like filovirus in europe. PLoS Pathog. 7(10), e1002304 (2001)
Vogel, G.: Infectious disease. Are bats spreading Ebola across sub-Saharan Africa? Science. 344(6180), 140 (2014)
Paessler, S., Walker, D.H.: Pathogenesis of the viral hemorrhagic fevers. Annu. Rev. Pathol. 8(1), 411 (2013)
Maier, I., Schiestl, R.H., Kontaxis, G.: Cyanovirin-N binds viral envelope proteins at the low-affinity carbohydrate binding site without direct virus neutralization ability. Molecules. 26(12), 3621(2021)
Brudner, M., Karpel, M., Lear, C., Chen, L., Yantosca, L.M., Scully, C., Sarraju, A., Sokolovska, A., Zariffard, M.R., Eisen, D.P., Mungall, B.A.: Lectin-dependent enhancement of Ebola virus infection via soluble and transmembrane C-type lectin receptors. PLoS ONE 8(4), e60838 (2013)
Guo, Y.R., Cao, Q.D., Hong, Z.S., Tan, Y.Y., Chen, S.D., Jin, H.J., Tan, K.S., Wang, D.Y., Yan, Y.: The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil. Med. Res. 1, 1 (2020)
Baud, D., Qi, X., Nielsen-saines, K., Musso, D., Pomar, L., Favre, G.: Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. (2020)
El Zowalaty, M.E., Järhult, J.D.: From SARS to COVID-19: A previously unknown SARS-related coronavirus (SARS-CoV-2) of pandemic potential infecting humans–Call for a One Health approach. One Health. 9, 100124 (2020)
Shereen, M.A., Khan, S., Kazmi, A., Bashir, N., Siddique, R.: COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 24, 91–98 (2020)
Banerjee, A., Kulcsar, K., Misra, V., Frieman, M., Mossman, K.: Bats and coronaviruses. Viruses. 11(1), 41 (2019)
Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., Wang, W., Song, H., Huang, B., Zhu, N., Bi, Y.: Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet. 395(10224), 565–574 (2020)
Kumaki, Y., Wandersee, M.K., Smith, A.J., Zhou, Y., Simmons, G., Nelson, N.M., Bailey, K.W., Vest, Z.G., Li, J.K., Chan, P.K., Smee, D.F.: Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin. Urtica dioica agglutinin. Antiviral Res. 90(1), 22–32 (2011)
Koch, B., Schult-Dietrich, P., Büttner, S., Dilmaghani, B., Lohmann, D., Baer, P.C., Dietrich, U., Geiger, H.: Lectin affinity plasmapheresis for middle east respiratory syndrome-coronavirus and Marburg virus glycoprotein elimination. Blood Purif. 46(2), 126–133 (2018)
Tripathi, N., Goel, B., Bhardwaj, N., Vishwakarma, R.A., Jain, S.K.: Exploring the potential of chemical inhibitors for targeting post-translational glycosylation of coronavirus (SARS-CoV-2). ACS Omega 7(31), 27038–27051 (2022)
Sharifkashani, S., Bafrani, M.A., Khaboushan, A.S., Pirzadeh, M., Kheirandish, A., Yavarpour Bali, H., Hessami, A., Saghazadeh, A., Rezaei, N.: Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: potential therapeutic targeting. Eur. J. Pharmacol. 884, 173455 (2020)
Wu, F., Zhao, S., Yu, B., Chen, Y.M., Wang, W., Song, Z.G., Hu, Y., Tao, Z.W., Tian, J.H., Pei, Y.Y., Yuan, M.L.: A new coronavirus associated with human respiratory disease in China. Nature 579(7798), 265–269 (2020)
Ritchie, G., Harvey, D.J., Feldmann, F., Stroeher, U., Feldmann, H., Royle, L., Dwek, R.A., Rudd, P.M.: Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology 399(2), 257–269 (2010)
Barre, A., Damme, E.J., Simplicien, M., Benoist, H., Rougé, P.: Man-specific, GalNAc/T/Tn-specific and Neu5Ac-specific seaweed lectins as glycan probes for the SARS-CoV-2 (COVID-19) coronavirus. Marine drugs. 18(11), 543(2020)
Fouad, A.K.: Lectin therapy: A way to explore in order to inhibit the binding of COVID-19 to these host cells. Int J Innov Sci Res Technol. 5, 1280–1286 (2020)
Harvey, W.T., Carabelli, A.M., Jackson, B., Gupta, R.K., Thomson, E.C., Harrison, E.M., Ludden, C., Reeve, R., Rambaut, A., Peacock, S.J., Robertson, D.L.: SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19(7), 409–424 (2021)
Abdool Karim, S.S., de Oliveira, T.: New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N. Engl. J. Med. 384(19), 1866–1868 (2021)
Chi, X., Yan, R., Zhang, J., Zhang, G., Zhang, Y., Hao, M., Zhang, Z., Fan, P., Dong, Y., Yang, Y., Chen, Z.: A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 369(6504), 650–655 (2020)
Watanabe, Y., Berndsen, Z.T., Raghwani, J., Seabright, G.E., Allen, J.D., Pybus, O.G., McLellan, J.S., Wilson, I.A., Bowden, T.A., Ward, A.B., Crispin, M.: Vulnerabilities in coronavirus glycan shields despite extensive glycosylation. Nat. Commun. 11(1), 2688 (2020)
Shajahan, A., Supekar, N.T., Gleinich, A.S., Azadi, P.: Deducing the N-and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology 30(12), 981–988 (2020)
Ahan, R.E., Hanifehnezhad, A., Kehribar, E.S., Oguzoglu, T.C., Foldes, K., Özçelik, C.E., Filazi, N., Öztop, S., Palaz, F., Önder, S., Bozkurt, E.U.: A Highly Potent SARS-CoV-2 Blocking Lectin Protein. ACS Infect. Dis. 8(7), 1253–1264 (2021)
Wang, W., Li, Q., Wu, J., Hu, Y., Wu, G., Yu, C., Xu, K., Liu, X., Wang, Q., Huang, W., Wang, L., Wang, Y.: Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg Microbes Infect. 10(1), 1519–1529 (2021)
Lokhande, K.B., Apte, G.R., Shrivastava, A., Singh, A., Pal, J.K., Swamy, K.V., Gupta, R.K.: Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies. J. Biomol. Struct. Dyn. 40(9), 3880–3898 (2022)
Saggam, A., Limgaokar, K., Borse, S., Chavan-Gautam, P., Dixit, S., Tillu, G., Patwardhan, B.: Withania somnifera (L.) Dunal: opportunity for clinical repurposing in COVID-19 management. Front. pharmacol. 835 (2021)
Chikhale, R.V., Gurav, S.S., Patil, R.B., Sinha, S.K., Prasad, S.K., Shakya, A., Shrivastava, S.K., Gurav, N.S., Prasad, R.S.: Sars-cov-2 host entry and replication inhibitors from Indian ginseng: an in-silico approach. J. Biomol. Struct. Dyn. 39(12), 4510–4521 (2021)
Kumar, N., Shala, A.Y., Khurana, S.M.: Antiviral and immuno-boosting potential of Ashwagandha (Withania somnifera L.). Medicinal Plants-International Med. Plants - Int. J. Phytomed. 13(2), 237–44(2021)
George, B.S., Silambarasan, S., Senthil, K., Jacob, J.P., Ghosh, D.M.: Characterization of an Insecticidal Protein from Withania somnifera Against Lepidopteran and Hemipteran Pest. Mol. Biotechnol. 60(4), 290–301 (2018)
Naidoo, D., Kar, P., Roy, A., Mutanda, T., Bwapwa, J., Sen, A., Anandraj, A.: Structural insight into the binding of cyanovirin-n with the spike glycoprotein, mpro and PLpro of SARS-CoV-2: Protein–protein interactions, dynamics simulations and free energy calculations. Molecules 26(17), 5114 (2021)
O’Keefe, B.R., Giomarelli, B., Barnard, D.L., Shenoy, S.R., Chan, P.K., McMahon, J.B., Palmer, K.E., Barnett, B.W., Meyerholz, D.K., Wohlford-Lenane, C.L., McCray, P.B., Jr.: Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 84(5), 2511–2521 (2010)
Keyaerts, E., Vijgen, L., Pannecouque, C., Van Damme, E., Peumans, W., Egberink, H., Balzarini, J., Van Ranst, M.: Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 75(3), 179–187 (2007)
Jang, H., Lee, D.H., Kang, H.G., Lee, S.J.: Concanavalin A targeting N-linked glycans in spike proteins influence viral interactions. Dalton Trans. 49(39), 13538–13543 (2020)
Wang, D., Lu, J.: Glycan arrays lead to the discovery of autoimmunogenic activity of SARS-CoV.Physiol. Genom. 18(2), 245–248 (2004)
Alsaidi, S., Cornejal, N., Mahoney, O., Melo, C., Verma. N., Bonnaire, T., Chang, T., O'Keefe, B.R., Sailer, J., Zydowsky, T.M., Teleshova, N., Romero, J.A.F.: Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model. Mar. Drugs. 19(8), 418 (2021)
Gooldy, M., Roux, C.M., LaRosa, S.P., Spaulding, N., Fisher, C.J., Jr.: Removal of clinically relevant SARS-CoV-2 variants by an affinity resin containing Galanthus nivalis agglutinin. PLoS ONE 17(7), e0272377 (2022)
Idrees, M., Khan, S., Memon, N.H., Zhang, Z.: Effect of the Phytochemical Agents against the SARS-CoV and Some of them Selected for Application to COVID-19: A Mini-Review. Curr. Pharm. Biotechnol. 22(4), 444–450 (2021)
Wang, W., Sun, J., Liu, C., Xue, Z.: Application of immunostimulants in aquaculture: current knowledge and future perspectives. Aquac. Res. 48(1), 1–23 (2017)
Lavelle, E.C., Grant, G., Pusztai, A., Pfüller, U., Leavy, O., McNeela, E., Mills, K.H., O’Hagan, D.T.: Mistletoe lectins enhance immune responses to intranasally co-administered herpes simplex virus glycoprotein D2. Immunology 107(2), 268–274 (2002)
Nascimento da Silva, L.C., Mendonça, J.S., de Oliveira, W.F., Batista, K.L., Zagmignan, A., Viana, I.F., dos Santos, Correia. M.T.: Exploring lectin–glycan interactions to combat COVID-19: Lessons acquired from other enveloped viruses. Glycobiology. 31(4), 358–571(2021)
Kumar, A., Sharma, A., Tirpude, N.V., Padwad, Y., Hallan, V., Kumar, S.: Plant-derived immuno-adjuvants in vaccines formulation: a promising avenue for improving vaccines efficacy against SARS-CoV-2 virus. Pharmacol. Rep. 74(6), 1238–1254 (2022)
Katoch, R., Tripathi, A.: Research advances and prospects of legume lectins. J. Biosci. 46(4) (2021)
Sander, V.A., Corigliano, M.G., Clemente, M.: Promising plant-derived adjuvants in the development of coccidial vaccines. Front. Vet. Sci. 6, 20 (2019)
Tripathi, S., Maiti, T.K.: Efficiency of heat denatured lectins from Abrus precatorius as immunoadjuvants. Food Agric. Immunol. 15(3–4), 279–287 (2003)
Cardoso, M.R., Mota, C.M., Ribeiro, D.P., Noleto, P.G., Andrade, W.B., Souza, M.A., Silva, N.M., Mineo, T.W., Mineo, J.R., Silva, D.A.: Adjuvant and immunostimulatory effects of a D-galactose-binding lectin from Synadenium carinatum latex (ScLL) in the mouse model of vaccination against neosporosis. Vet. Res. 43, 1–3 (2012)
Kang, J., Zuo, Y., Guo, Q., Wang, H., Liu, Q., Liu, Q., Xia, G., Kang, Y.: Xylaria hypoxylon lectin as adjuvant elicited Tfh cell responses. Scand. J. Immunol. 82(5), 436–442 (2015)
Frantz, M., Jung, M.L., Ribereau-Gayon, G., Anton, R.: Modulation of mistletoe (Viscum album L.) lectins cytotoxicity by carbohydrates and serum glycoproteins. Arzneimittelforschung. 50(05), 471–478 (2000)
Moyle, P.M.: Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 35(3), 375–389 (2017)
Vetter, V., Denizer, G., Friedland, L.R., Krishnan, J., Shapiro, M.: Understanding modern-day vaccines: what you need to know. Ann. Med. 50(2), 110–120 (2018)
Shi, S., Zhu, H., Xia, X., Liang, Z., Ma, X., Sun, B.: Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine. 37(24), 3167–3178 (2019)
Azizi, A., Kumar, A., Diaz-Mitoma, F., Mestecky, J.: Enhancing oral vaccine potency by intestinal M cells. PLoS Pathog. 6(11), e1001147 (2010)
Unitt, J., Hornigold, D.: Plant lectins are novel Toll-like receptor agonists. Biochem. Pharmacol. 81(11), 1324–1328 (2011)
Montassier, H.J., Maria de Fatima, S.M., Piza, V.M., Okino, C.H., Brentano, L., Richtzenhain, L.J.: Development of a microplate lectin-capture RT-PCR (MLC-RT-PCR) for the detection of avian infectious bronchitis virus. (2013)
Wang, B., Anzai, J.I.: Recent progress in lectin-based biosensors. Materials. 8(12), 8590–8607 (2015)
Simão, E.P., Silva, D.B., Cordeiro, M.T., Gil, L.H., Andrade, C.A., Oliveira, M.D.: Nanostructured impedimetric lectin-based biosensor for arboviruses detection. Talanta 208, 120338 (2020)
Oliveira, M.D., Nogueira, M.L., Correia, M.T., Coelho, L.C., Andrade, C.A.: Detection of dengue virus serotypes on the surface of gold electrode based on Cratylia mollis lectin affinity. Sens. Actuators B Chem. 155(2), 789–795 (2011)
Andrade, C.A., Oliveira, M.D., De Melo, C.P., Coelho, L.C., Correia, M.T., Nogueira, M.L., Singh, P.R., Zeng, X.: Diagnosis of dengue infection using a modified gold electrode with hybrid organic–inorganic nanocomposite and Bauhinia monandra lectin. J. Colloid Interface Sci. 362(2), 517–523 (2011)
Silva, M.L.: Lectin-based biosensors as analytical tools for clinical oncology. Cancer Lett. 436, 63–74 (2018)
de Oliveira, W.F., dos Santos Silva, P.M., Coelho, L.C., dos Santos Correia, M.T.: Biomarkers, biosensors and biomedicine. Curr. Med. Chem. 27(21), 3519–3533 (2020)
Mislovičová, D., Gemeiner, P., Kozarova, A., Kožár, T., Lectinomics I.: Relevance of exogenous plant lectins in biomedical diagnostics. Biologia. 64(1), 1–9 (2009)
Beyer, V.P., Monaco, A., Napier, R., Yilmaz, G., Becer, C.R.: Bottlebrush glycopolymers from 2-oxazolines and acrylamides for targeting dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin and mannose-binding lectin. Biomacromol 21(6), 2298–2308 (2020)
Gupta, A., Gupta, G.S.: Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine. J. Nanopart. Res. 24(11), 228 (2022)
Azimzadeh, M., Nasirizadeh, N., Rahaie, M., Naderi-Manesh, H.: Early detection of Alzheimer’s disease using a biosensor based on electrochemically-reduced graphene oxide and gold nanowires for the quantification of serum microRNA-137. RSC Adv. 7(88), 55709–55719 (2017)
Velusamy, V., Arshak, K., Korostynska, O., Oliwa, K., Adley, C.: An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 28(2), 232–254 (2010)
Abbas, H.S., Kotakonda, M.: Lectins Are the Sparkle of Hope for Combating Coronaviruses and the Global COVID-19. Adv. Pharm. Bull. 12(2), 319–328 (2021)
Mu, J., Hirayama, M., Sato, Y., Morimoto, K., Hori, K.: A novel high-mannose specific lectin from the green alga Halimeda renschii exhibits a potent anti-influenza virus activity through high-affinity binding to the viral hemagglutinin. Mar. Drugs 15(8), 255 (2017)
Wallis, R., Dodd, R.B.: Interaction of mannose-binding protein with associated serine proteases: effects of naturally occurring mutations. J. Biol. Chem. 275(40), 30962–30969 (2000)
Funding
RA and KY are grateful to Indian Council of Medical Research (ICMR), New Delhi, India, for providing financial assistance in the form of approved ICMR Adhoc Project No. 2021-9508 (RFC No. ITR/Adhoc/2/2023-24 dated 05/09/2023; File No. 17X (3)/Adhoc/34/2022-ITR).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: KY, RA, AY and UND conceived the original idea and designed the contents of the manuscript; AG, KY, AY and DK drafted the manuscript. AG, AS and MAK prepared diagrams. RA, KY, AY, and UND critically edited and revised the final version of the manuscript. All authors provided critical feedback and helped shape the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, A., Yadav, K., Yadav, A. et al. Mannose-specific plant and microbial lectins as antiviral agents: A review. Glycoconj J 41, 1–33 (2024). https://doi.org/10.1007/s10719-023-10142-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-023-10142-7