Abstract
Pain represents an unpleasant sensation linked to actual or potential tissue damage. In the early phase, the sensation of pain is caused due to direct stimulation of the sensory nerve fibers. On the other hand, the pain in the late phase is attributed to inflammatory mediators. Current medicines used to treat inflammation and pain are effective; however, they cause severe side effects, such as ulcer, anemia, osteoporosis, and endocrine disruption. Increased attention is recently being focused on the examination of the analgesic potential of phytoconstituents, such as glycosides of traditional medicinal plants, because they often have suitable biological activities with fewer side effects as compared to synthetic drugs. The purpose of this article is to review for the first time the current state of knowledge on the use of glycosides from medicinal plants to induce analgesia and anti-inflammatory effect. Various databases and search engines, including PubMed, ScienceDirect, Scopus, Web of Science and Google Scholar, were used to search and collect relevant studies on glycosides with antinociceptive activities. The results led to the identification of several glycosides that exhibited marked inhibition of various pain mediators based on different well-established assays. Additionally, these glycosides were found to induce most of the analgesic effects through cyclooxygenase and lipoxygenase pathways. These findings can be useful to identify new candidates which can be clinically developed as analgesics with better bioavailability and reduced side effects.

Analgesic mechanisms of plant glycosides
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain represents an unpleasant sensation linked to actual or potential tissue damage which results in the activation of specific nerve fibers, transmitting the signal to the brain where its conscious perception may be modified by various factors [1]. The experienced discomfort sensation signals possible injuries to the body [1]. Various causes, mostly associated with injury, can induce pain that ultimately results in noxious stimulation of the sensory nerve endings [1,2,3]. As reported by the International Association of the Study of Pain, three different types of pain have been identified [4], namely acute pain, chronic pain, and cancer pain [5,6,7].
Acute pain, also known as nociceptive pain, is characterized by sharp, throbbing, or abrupt sensation [8, 9], which mostly occurs as a physiological response to tissues damage or trauma [10, 11]. This type of pain usually lasts for a short period (up to 6 months) and terminates when the damage is healed.
Chronic pain, also known as neuropathic pain [12, 13], lasts for a long time (more than 6 months) and is the result of damage and incorrect processing of the input signals by the nervous system [14, 15]. The causes and mechanisms of chronic pain are often unknown or complex, and dramatically influences the patient’s quality of life by causing negative psychological consequences, such as anxiety, anger, and depression [16,17,18].
Cancer pain is a specific type of severe chronic pain which is caused by a particular malignancy (e.g., pancreatic, head and neck cancer) or as an iatrogenic effect of chemotherapy and radiotherapy (i.e., cisplatin-induced peripheral neuropathy) [19, 20]. The pain in the early stage is the result of a direct stimulation of the sensory nerve fibers. On the other hand, the pain in the late phase is mediated by various inflammatory regulators, such as serotonin, histamine, prostaglandins (PGs), and bradykinins [21, 22].
Despite substantial progress in the field of treatment of chronic pain and inflammatory diseases for the past decades, including the validation of new druggable targets (e.G. sigma and serotonin receptors) [23,24,25,26,27], these ailments still represent one of the significant health burdens of the world. Inflammation is linked to a complex array of responses, including release of mediators, enzyme activation, extravasation and cell migration, tissue breakdown, and repair. Current medicines used to treat inflammation and induce analgesia possess severe adverse effects, including gastric lesions caused by non-steroidal anti-inflammatory drugs, or tolerance and dependence caused by opiates [20]. Moreover, these drugs are not always effective in the management of inflammation and induction of analgesia; therefore, new analgesic drugs are urgently needed [28]. As stated in a published report from the World Health Organization, around 80% of the world population still depend primarily on herbal remedies [29,30,31,32]. Following this trend, researchers focused their attention on the efficacy of constituents extracted from plants used in the various traditional medicinal systems which might exhibit useful bioactivities [33,34,35,36,37,38] and fewer undesirable adverse effects as compared to synthetic drugs. The scope of this review is to critically analyze available literature on various medicinal plant-derived glycosides with potential antinociceptive effects that could be candidates in the discovery and development of effective analgesic drugs in the future.
Overview of glycosides
Glycosides are organic molecules which can be isolated from plant or animal sources. The enzymatic or acid hydrolysis of the glycosidic bond of glycosides leads to release of one or more sugar moieties together with non-sugar entities [39, 40]. The sugar moiety is called glycone, while the non-sugar portion is referred to as aglycone or genin. Regarding O-glycosides, glycosidic linkages are formed by the interaction of hemiacetal (or hemiketal) group of the glycone and the hydroxyl group of the aglycone. Similarly, glycosides can be linked by N-, S-, or C-glycosidic bond to give glycosylamines, thioglycosides, and C-glycosides, respectively. This classification takes into account the type of glycosidic bond. Alternatively, based on the aglycone portion, different types of glycosides have been classified, for example, triterpene glycosides, β-sistosterol, flavonoid glycosides, iridoid, phenylpropanoid, anthraquinon glycosides, kaempferol glycosides, and saponine [39, 40]. For saponins, the aglycone moiety is called a sapogenin, whereas the glycone part is commonly known as oligosaccharide. The linkage of oligosaccharides to the sapogenin through one or two glycosylation sites leads to the corresponding monodesmosidic or bidesmosidic saponins. Similarly, the attachment of the glycone to three sites give tridesmosidic saponins; however, these are rare [41, 42]. Emerging evidence suggests that various glycosides have great therapeutic potential, including antifungal, anticancer, antiplatelet activities [43,44,45,46,47].
Glycosides as analgesic agents
A large number of structurally different glycosides, isolated from different medicinal plants, showed in vivo analgesic effects and/or in vitro biological activities (Table 1). Quercetin-3-O-(6-feruloyl)-β-D-galactopyranoside, a flavonoid glycoside, has been isolated from the aerial part of Polygounm viscosum (Polygonaceae) [48]. The intraperitoneal (i.p.) administration of this flavonoid glycoside showed statistically significant analgesic activity in pain models as well as anti-inflammatory effects in carrageenan-induced rat paw edema model. At a dose of 50 mg/kg, quercetin-3-O-(6-feruloyl)-β-D-galactopyranoside exhibited moderate analgesic activity as compared to conventional drug morphine. On the other hand, in a murine model of acetic acid-induced writhing, quercetin-3-O-(6-feruloyl)-β-D-galactopyranoside at a dose of 50 mg/kg exhibited 23.3% effect, while the standard drug acetylsalicylic acid displayed 64% effect [48]. It has been reported that a triterpene glycoside-rich extract from the stem bark of Cussonia paniculata showed significant analgesic effects as evidenced by the reduction in the number of writhes compared with standard drugs, such as indomethacin and cyproheptadine, when administered through i.p. route [49]. Treatment with the aqueous extract of C. paniculate at 50, 100 or 200 mg/kg and indomethacin at 10 mg/kg i.p. caused a significant decrease in licking time as well as frequency of licking of the formalin-injected paw of rats. The extract at 50 mg/kg dose (i.p.) showed the highest analgesic activity [49]. Similarly, rutin and quercetin were isolated from Achillea millefolium extract and evaluated for their analgesic activities [50]. In this study, rutin- and quercetin-rich extracts were enaluated using the hot plate, writhing, formalin as well as intestinal transit tests in order to justify their folk uses as an analgesic, anti-inflammatory, and antispasmodic agents. As a result, the crude extract of A. millefolium at a dose of 500 or 1000 mg/kg significantly inhibited abdominal contortions by 65% and 23%, respectively [50]. Furthermore, hydro-alcoholic extract of aerial part of Artemisia vulgaris (Asteraceae) containing rutin exhibited significant analgesic activity in writhing test in mice by significantly reducing the number of contortions. A. vulgaris extract at a dose of 500 or 1000 mg/kg inhibited pain and inflammation by 48% and 59%, respectively. However, these extracts did not affect the intestinal transit and the response time in the hot plate test as well as the immediate or late responses in the formalin test [50].
Backhouse et al. [51] reported the anti-inflammatory (in paw- and ear-induced edema), analgesic (in writhing test) and antioxidant property (based on inhibition of lipid peroxidation, superoxide anion and xanthine oxidase) of Buddleja globose extract which contained apigenin-7-O-glucoside, luteolin-7-O-glucoside and verbascoside. Individually, β-sitosterol glucoside (0.1%), verbascoside (1.8%), apigenin-7-O-glucoside (0.03%) and luteolin-7-O-glucoside (1.1%) were isolated from the methanolic extract which exerted analgesic and anti-inflammatory effects (38.5% and 61.4%, respectively), and was found to be active as a topical anti-inflammatory agent in 12-O-tetradecanoylphorbol-13- (TPA)-induced edema (56.7%) [51].
Rodriguez et al. [52] isolated pseudopterosin and seco-pseudopterosin, two diterpene glycosides, from Pseudopterogorgia elisabethae. Pseudopterosin exhibited significant analgesic and anti-inflammatory effects by blocking the release of eicosanoids through inhibition of thromboxane B2 (50% inhibitory concentration, IC50 = 4.7 μM) and superoxide anion (IC50 = 11.2 μM) [52].
Another group of researchers isolated ilwensisaponin A, ilwensisaponin C, ajugol, and picroside IV from the methanolic extract of the flowers of Verbascum pterocalycinum [53]. Ilwensisaponin A and C are saponin glycosides while ajugol and picroside IV represent iridoid glycosides. Ilwensisaponine A and C significantly inhibited abdominal contraction caused by benzoquinone, without causing any toxicity or acute gastric injury. Ilwensisaponin C had profound inhibition of TPA-induced ear edema. In prostaglandin E1-induced hind paw edema in mice, intravenous (i.v.) administration of picroside at a dose of 100 mg/kg caused significant swelling thickness inhibition during various assessment times. In mice with carrageenan-induced hind paw edema, ilwensisaponine C at a dose of 100 mg/kg showed higher activity as compared to 200 mg/kg. Ilwensisaponine C, at a dose of 100 mg/kg, markedly attenuated the carrageenan-induced paw edema at various application durations [53].
It has been found that harpagoside was the main iridoid glycoside in aqueous extract of Harpagophytum procumbens (Pedaliaceae) [54]. Harpagoside displayed significant analgesic activity at 10 mg/kg in acetic acid-induced writhing test [54].
Kaemferol and kaempferol-3-O-glucoside were isolated from the whole plant of Thesium chinense Turcz [55]. These glycosides displayed considerable analgesic activity in acetic acid-induced abdominal constriction test and also exhibited significant anti-inflammatory activity in carrageenan-induced hind paw edema and xylene-induced ear edema in experimental animals. Kaempferol at a dose of 50 and 100 mg/kg showed 40.9% and 64.3% inhibition of writhing, respectively, against acetic acid-induced nociception in mice. Kaempferol-3-O-glucoside at a dose of 50 and 100 mg/kg caused writhing inhibition of 30.50% and 20.50%, respectively, while aspirin (positive control) at a dose of 100 mg/kg showed 87% inhibition [55].
Kaempferol-3-O-[α-L-rhamnopyranosyl(1 → 6)-β-D-glucopyranoside] and kaempferol-3-O-[β-D-glucopyranoside] were extracted from the leaves of Hedyosmu bonplandianum (Chloranthaceae). The n-butanol extract, containing these glycosides, at a dose of 100 and 140 mg/kg, exhibited substantial analgesic activities in mice by reducing the number of stretching and writing after injection of an acetic acid solution [56]. Moreover, morphine-like analgesic activity was determined using the hot plate test.
Researchers have isolated haypolaetin-8-glucoside from Sideritis mugronensis, which possessed significant analgesic activity based on pressure pain threshold test in rats [57]. Haypolaetin-8-glucoside also displayed considerable anti-inflammatory activity when tested in the acute phase of carrageenan-induced inflammation model. This flavonoid glycoside was found to be superior to phenylbutazone in blunting the acute phase of carrageenan-induced inflammation, but was less effective during the prolonged inflammatory stage. According to the results, hypolaetin-8-glycoside possesses a combination of anti-inflammatory and antiulcer properties, suggesting that it or its derivative may be useful therapeutic agent, such as aspirin, for the treatment of inflammatory disorders [57].
Choi et al. [58] isolated syringin using activity-guided fractionation of the ethyl acetate extracts of the stem bark of Magnolia sieboldii. Syringin showed significant analgesic activity in acetic acid-induced writhing test and hot plate test. Moreover, sinapyl alcohol, the hydrolysate of syringin, at a dose of 20 or 30 mg/kg/day showed significant anti-inflammatory activity by inhibiting the increase of vascular permeability by acetic acid in mice and reducing acute paw edema induced by carrageenan in rats. Therefore, sinapyl alcohol resulted more potent analgesic activity than syringin. Thus, the authors speculated that the higher anti-inflammatory and antinociceptive effects were due to the in vivo transformation of syringin into sinapyl alcohol [58].
Kaempferol-3,7-O-α-dirhamnoside and quercetin-3,7-O-α-dirhamnoside, isolated from the leaves of Tilia argentea (Tiliaceae), showed analgesic activity based on p-benzoquinone-induced writhing test, and anti-inflammatory effect in the carrageenan-induced hind paw edema in mice. These two compounds displayed significant antinociceptive and anti-inflammatory properties at a dose of 50 mg/kg, and these effects were not associated with any acute toxicity or gastric damage [59].
When pectolinarin (isolated from Cirsium subcoriaceum) and linarin (extracted from Buddleia cordata) were tested for antinociceptive effect in the acetic acid-induced writhing test in mice, the compounds caused a marked dose-dependent pain-relieving effect with 50% effective dose (ED50) of 28.44 and 89.0 mg/kg, respectively [60].
The antinociceptive effect of carumbelloside–I, a pregnane glycoside isolated from Caralluma umbellate, was evaluated [61]. In vivo studies in mice showed that carumbelloside–I significantly reduced abdominal constriction inflicted by acetic acid in a dose-related fashion, with maximum protection of 55.7% at a dose of 10 mg/kg when administered orally [61].
Several known glycosides isolated from Sedum dendroideum have showed varying degrees of antinociceptive effect when tested at 10 mg/ml (per os, p.o.) for inhibition of the abdominal acetic acid-induced writhing in mice. The effects of oral administration of flavonoids were as follow: kaempferitrin displaying 47.3% inhibition, kaempferol 3-O-β-glucopyranoside-7-O-α-rhamnopyranoside showing 25.7% inhibition, kaempferol 3-O-neohesperidoside-7-O-α-rhamnopyranoside registering 60.2% inhibition, and kaempferol 7-O-α-rhamnopyranoside inducing 58% inhibition [62].
Zhang et al. [63] investigated the analgesic and anti-inflammatory properties of a salicylate derivative fraction (SDF) isolated from Gaultheria yunnanensis. Methyl salicylate 2-O-β-D-xylopyranosyl (1 → 6) β-D-glucopyranoside (gaultherin) was identified as the major constituent of SDF. The SDF exhibited a significant inhibition of carrageenan-induced hind paw edema in rats (at 200 or 400 mg/kg, p.o.) and croton oil-induced ear swelling in mice (200, 400 or 800 mg/kg, p.o.). Additionally, SDF (400 or 800 mg/kg, p.o.) inhibited only the second phase (inflammatory) in the formalin test and exhibited minimal effect in the hot-plate test in mice. The same research group also evaluated the analgesic and anti-inflammatory potential of gaultherin [64]. The results indicated that gaultherin (200 mg/kg) significantly suppressed the abdominal contractions in the acetic acid-induced writhing test in mice. Additionally, the anti-inflammatory effect of gaultherin was demonstrated in the croton oil-induced ear edema model in mice. These results also revealed that gaultherin produced comparable effects to that of aspirin [64].
In another study, a fraction from the leaves of Rhododendron aureum was extracted and led to the isolation of a glycoside named rhododendrin. Subsequently, in vivo studies showed that rhododendrin caused a significant analgesic effect on the acetic acid-induced writhing test in mice at various doses after oral administration [65].
More recently, Wang et al. [66] isolated two glycosides, namely sacroroside A and B, from the ethyl acetate fraction Artemisa sacrorum. These two flavone C-glycosides elicited marked analgesic effects at various test doses (30 and 40 mg/kg, p.o.) in rats [66].
Analgesic mechanisms of plant glycosides
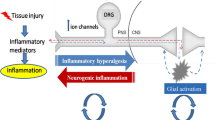
The analgesic effects of plant glycosides have been demonstrated with several proposed mechanisms of action. For instance, rutin, quercetin, luteolin, and triterpenoid glycosides exhibited anti-inflammatory activities through inhibition of cyclooxygenase (COX) and lipoxygenase (LOX) pathways which are involved in the production of inflammation-mediating agent, namely prostaglandin E2 (PGE2), from arachidonic acid (Fig. 1) [49, 67]. The enzyme COX is known to convert arachidonic acid to prostaglandin H2 which is subsequently converted to various active metabolites. For instance, thromboxane A2 is a potent vasoconstrictor which stimulates platelet aggregation. Likewise, prostaglandin I2 (PGI2), also known as prostacyclin, is expressed in the vascular endothelium and suppresses platelet aggregation. Both PGE2 and PGI2 are potent vasodilators which cause elevated blood flow in inflamed tissues to stimulate pain and inflammation in the localized area. Furthermore, verbascoside and luteolin-7-O-glucoside inhibited oxidative stress mediators, such as superoxide anion and lipoperoxides. In particular, the in vitro anti-inflammatory effect of luteolin-7-O-glucoside was achieved by the inhibitory effect on activated human neutrophils. Similarly, luteolin-7-O-glucoside also showed a vital inhibitory effect on matrix metalloproteinase-2 (MMP-2) and MMP-9 and this effect may contribute to its modulating influence on the extracellular matrix degradation and remodelling. Moreover, luteolin exhibited a decrease of lipopolysaccharide-induced COX-2 expression and completely suppressed the formation of PGE2 [51]. Indeed, luteolin glucoside displayed a significant suppression of the synthesis of leukotriene as well as thromboxane and consequently produced anti-inflammatory activity. Verbascoside was found to exert inhibitory influence on histamine- and bradykinin-induced contractions with resultant anti-inflammatory activity. The results also revealed an inhibition of nitric oxide release in lipopolysaccharide-exposed cells in parallel with suppression of inducible nitric oxide synthase and anti-inflammatory properties [18, 51].
Quercetin glycoside showed a potent anti-inflammatory action on exudative and proliferative phases of inflammation [68]. Leukotriene B4, a potent chemotactic mediator for the polimorphonuclear leukocytes, is produced by lipoxygenase, an enzyme which is very sensible to steroidal anti-inflammatory drugs, such as dexamethasone. Finally, several glycosides inhibited the synthesis of leukotriene, showing the potential to function as anti-inflammatory drugs [69].
The mediators of COX cascade as well as the function of prostaglandins in the inflammatory response and body homeostasis have been studied widely [70]. Besides, leukotrienes, the second major family of arachidonic acid derivatives, are synthesized by the action of 5-LOX that plays a significant role in the inflammatory pathway [70]. It is also observed that constitutive expression of COX-1 is implicated in homeostatic processes, whereas its isoform COX-2 plays a vital role in the inflammatory process and associated pain sensation; however, the precise contributions of two COX isoforms towards various pathophysiological processes is still not clearly understood. COX-2 is known to be induced during the resolution of an inflammatory response with subsequently production of anti-inflammatory and not pro-inflammatory PGs [71]. In fact, cellular infiltration and edema were noticed for longer periods in COX-2-deficient mice than in wild-type mice, suggesting that COX-2 may have an important role during the resolution phase of inflammation [72].
Leukotrienes (LTs) are synthesized via the action of 5-LOX which plays a significant role in the inflammatory process [73]; however, this enzyme needs the presence of 5-LOX activating-protein in intact cells [74]. Likewise, although analgesic glycosides inhibit 5-LOX activity in intact human leucocytes, these agents were unsuccessful in inhibiting the enzyme activity in broken cells [74]. This result might be attributed to the ability of glycosides to block surface-associated LOX. The final and biologically active metabolites of the 5-LOX cascade are LTB4 and cysteinyl LTs (e.g., LTC4, LTD4, and LTE4). The cysteinyl LTs are recognized as slow-reacting substances associated with anaphylaxis and originated from the unstable intermediate LTA4 [75]. LTB4 is also known to be a potent stimulator of leucocyte activation, and it has been shown that adhesion of cells to vascular endothelium leads to elicit chemokinetic as well as chemotactic effects [76].
During short-term exposure to LTB4, polymorphonuclear leukocytes are largely recruited, whereas during protracted exposure, their presence is located in tissues and exudates [77]. Furthermore, LTB4 is implicated in the pathogenesis of numerous inflammatory disorders as well. Experimental evidences support that LTs stimulate the synthesis and release of proinflammatory cytokines from macrophages and lymphocytes [78, 79].
Pain accompanies tissue disruption in a normal healthy individual, and the intensity of such pain varies depending on the nature of trauma, the healing process and various other host immune factors. Pain mediators or analgesics are those compounds which mediate the decrease in sensation of pain. Thus, various pain mediators may have promoting effects on wound healing in the short term [80, 81]. Treatment of pain involves addressing various inflammatory mechanisms involving the direct participation of cytokines and other immune molecules. These molecules generate inflammation which in turn mediated formation of the wound and localized pain [81, 82]. Glycosides discussed in the present review have potential analgesic and anti-inflammatory effect; thus, these compounds may act as pain mediators. It can be summarized that COX inhibition may act through a shunt pathway of the arachidonic acid metabolism in the direction of the leukotriene pathway to normalize inflammation and sensation of pain.
Bioavailability of flavonoid glycosides
The potential beneficial impact of flavonoid glycosides on human health has been the focus of various medicinal interests. Based on prior studies, the disposition of these glycosides may be dependent on the sugar moiety of the glycoside or the plant matrix. In the case of quercetin, two isolated quercetin glycosides were administered to 12 healthy volunteers in a four-way crossover study. Interestingly, only quercetin glucuronides, but no free quercetin, were detected in human plasma. A notable variation in the bioavailability and pharmacokinetic parameters among quercetin glucosides was observed. Peak plasma concentrations of 2.3 ± 1.5 μg/mL and 2.1 ± 1.6 μg/mL (mean ± SD) were noted after 0.7 ± 0.2 h and 0.7 ± 0.3 h of administration, respectively. However, following administration of rutin, peak plasma levels were higher despite the higher dose. Peak plasma concentrations were reached at 7.0 ± 2.9 h after administration of rutin with an elimination half-life of 11 h. From the data mentioned above, it can be stated that the disposition of quercetin in humans primarily depends on sugar moiety [83,84,85,86,87].
Other investigators have evaluated the bioavailability of luteolin in Caco-2 cells as well as rats using HPLC and LC-MS. Following oral administration of 22.8 and 58.3 μmol/kg of luteolin and luteolin-7-O-glucoside, respectively, to rats, luteolin and luteolin monoglucuronide were detected in the plasma. The plasma concentration was highest (0.76 ± 0.27 μM) after 1 h administration [85,86,87].
Another research group has investigated the pharmacokinetic parameters of harpagoside in an open, single-dose, two-treatment, two-period, randomized cross-over study. Six horses received a single dose (5 mg/kg) of harpagoside and following 7 days washout period, a second dose (10 mg/kg) of harpagoside was administered through nasogastric tube. Subsequently, plasma samples at various time intervals (before and 24 h following administration) were collected, and it was observed that harpagoside could be detected up to 9 h following administration. Moreover, Cmax was found to be 25.59 and 55.46 ng/ml, T1/2 at 2.53 and 2.32 h, respectively, and Tmax at 1 h in both studies. The area under the curve (AUC) was found to be 70.46 and 117.85 ng-h/ml, respectively. Additionally, distribution was 259.04 and 283.83 L/kg and clearance was 70.96 and 84.86 L/h/kg, respectively. Based upon the experimental results, a proportional relationship among dose, Cmax and AUC was established. Interestingly, no adverse effects were observed in horses with harpagophytum extract treatment [88, 89].
In an attempt to determine the bioavailability of kaempferol in rodents, hepatic and small intestinal microsomes fortified with either NADPH or uridine diphosphoglucuronic acid (UDPGA) were incubated with various concentrations of kaempferol for a period up to 120 min. Based on the values of the kinetic constants (Km and Vmax), the propensity for UDPGA-dependent conjugation as compared to NADPH-dependent oxidative metabolism was found to be greater for both hepatic and small intestinal microsomes. In another experiment, rats were treated with kaempferol via i.v. (10, or 25 mg/kg) or oral (100 or 250 mg/kg) route. The investigators observed gastro-intestinal first pass effect based on analysis of portal blood collected following oral administration of 100 mg/kg kaempferol. In case of i.v. route, the plasma concentration-time profiles for 10 and 25 mg/kg doses were in line with a high clearance (~ 3 L/h/kg) and large volumes of distribution (8–12 L/kg). Moreover, the disposition was represented by a terminal half-life value of 3–4 h. Following oral administration, the plasma concentration-time profiles exhibited rapid absorption (tmax ~1–2 h). Interestingly, the AUC values following i.v. and oral administrations elevated proportionally to the dose. Another observation was poor bioavailability at ~2%. Analysis of portal plasma following oral administration indicated low to moderate absorption. Generally, the low bioavailability of kaempferol was ascribed, at least in part, to substantial first-pass metabolism via glucuronidation and other metabolic pathways in the gastro-intestinal tract and the liver [90,91,92,93].
In the case of syringin at a dose of 100 g/kg, the maximal plasma glucose lowering activity in Wistar rats received treatment was about 22.62 ± 2.13% after 60 min. It has been observed that syringin failed to modulate inflammation in normal rats 60 min following an i.v. injection; however, an anti-inflammatory effect could be achieved after 90 min [94,95,96].
All these preliminary bioavailability data support that glycosides can reach plasma level slowly, and they can execute their pharmacological effects gradually.
Future perspective
One of the major goals in the development of analgesic drugs, other than efficacy and potency, is to avoid undesired effects on the gastrointestinal tract [97] due to the high risk of inducing gastric ulceration associated with the chronic use of certain analgesics. Thus, the development of alternative synthetic analgesics with lower ulcer-inducing effects is a significant challenge [98, 99]. On the other hand, the search for new active plant-based compounds with a superior safety profile is an area of intense research worldwide [100, 101]. Indeed, in recent times, the computational techniques applied to the drug discovery processes have further supported the characterization of ulcer-free analgesic agents from medicinal plants [102]. Additionally, different type of novel pharmaceutical approaches and formulations are also used to find solutions of these problems [15, 103]; however, often these strategies are not successful in resolving the side effects related to clinically used analgesics.
Among the compounds reviewed in this work, the flavonoid glycosides, such as kaempferol-3,7-O-α-dirhamnoside and quercetin-3,7-O-α-dirhamnoside from Tilia argentea, exhibited considerable analgesic effect without acute toxicity and adverse gastric effects at the tested doses [59]. Unfortunately, as in the case of other natural polyphenols, the overall low bioavailability and the rapid metabolism of these derivatives might limit their use in vivo. For these reasons, the incorporation of plant glycosides into various delivery systems, such as liposomes, polymeric and lipid nanoparticles as well as the conversion into prodrugs to improve their physicochemical properties, might represent a valuable approach [104, 105]. Further studies on these and other active molecules are needed to ascertain their potential applications. Besides, the acute and chronic safety profiles for most of these compounds should be further evaluated in order to facilitate the potential clinical uses of such compounds.
Conclusion
Based on studies presented here, a broad range of glycosides extracted from medicinal plants showed potential anti-inflammatory and antinociceptive effects towards different in vitro and in vivo models, respectively. Although a large number of pieces of evidence supporting the folkloric use of plant extracts to treat pain or associated disorder are available, we need to explore their full characterization, the toxicological profile and the drug-drug interaction. Moreover, the lack of studies regarding metabolic stability and the pharmacokinetic profile for various natural products might limit their further clinical development. On the other hand, the identification and characterization of new natural agents with an interesting pharmacological profile is still an attractive and successful approach in modern drug discovery. In this context, phytochemicals and plant extracts are attracting more and more attention for their potential uses as analgesic agents due to their ability to modulate specific biochemical pathways involved in the inflammatory and analgesic response (e.g., COX and LOX pathways). As reported in this review, triterpene glycosides, β-sitosterol glycosides, flavonoid glycosides, saponin glycosides, iridoid glycosides, pregnane glycosides, phenylpropanoid glycosides, and kaempferol glycosides, displayed promising biological activity. We may conclude that glycosides from medicinal plants might represent valuable pharmacological tools to develop new analgesic drug candidates.
Abbreviations
- AUC:
-
area under the curve
- COX:
-
cyclooxygenase
- ED50 :
-
50% effective dose
- IC50 :
-
50% inhibitory concentration
- i.p.:
-
intraperitoneal
- i.v.:
-
intravenous
- LOX:
-
lipoxygenase
- LTs:
-
leukotrienes
- MMP:
-
metalloproteinase
- PGs:
-
prostaglandins
- PGE2 :
-
prostaglandin E2
- PGI2 :
-
prostaglandin I2
- p.o.:
-
per os
- SDF:
-
salicylate derivative fraction
- TPA:
-
12-O-tetradecanoylphorbol-13-acetate
- UDPGA:
-
uridine diphosphoglucuronic acid
References
Andersen LPH, Gogenur I, Fenger AQ, Petersen MC, Rosenberg J, Werner MU. Analgesic and antihyperalgesic effects of melatonin in a human inflammatory pain model: a randomized, double-blind, placebo-controlled, three-arm crossover study. Pain. 2015;156(11):2286–94. https://doi.org/10.1097/j.pain.0000000000000284.
Adedapo AA, Sofidiya MO, Maphosa V, Moyo B, Masika PJ, Afolayan AJ. Anti-inflammatory and analgesic activities of the aqueous extract of Cussonia paniculata stem bark. Rec Nat Prod. 2008;2(2):46–53.
Rosenberg NL, Lovejoy B. CHAPTER 11 - work-related low Back pain. In: Rosenberg NL, editor. Occupational and Environmental Neurology: Butterworth-Heinemann. 1995:279–308.
Khan MA, Raza F, Khan IA. Pain: history. Culture and Philosophy Acta Med-Hist Adriat. 2015;13(1):113–30.
Afzal M, Gupta G, Kazmi I, Rahman M, Afzal O, Alam J et al. Anti-inflammatory and analgesic potential of a novel steroidal derivative from Bryophyllum pinnatum. Fitoterapia. 2012;83(5):853–8. doi:https://doi.org/10.1016/j.fitote.2012.03.013.
Li C, Chen M, Li X, Yang M, Wang Y, Yang X. Purification and function of two analgesic and anti-inflammatory peptides from coelomic fluid of the earthworm, Eisenia foetida. Peptides. 2017;89:71–81. doi:https://doi.org/10.1016/j.peptides.2017.01.016.
Muhammad N, Lal Shrestha R, Adhikari A, Wadood A, Khan H, Khan AZ, et al. First evidence of the analgesic activity of govaniadine, an alkaloid isolated from Corydalis govaniana wall. Nat Prod Res. 2014;29(5):430–7. https://doi.org/10.1080/14786419.2014.951933.
Tedore T, Weinberg R, Witkin L, Giambrone GP, Faggiani SL, Fleischut PM. Acute pain management/regional anesthesia. Anesthesiol Clin. 2015;33(4):739–51. https://doi.org/10.1016/j.anclin.2015.07.005.
Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477–87. https://doi.org/10.1111/papr.12108.
Odoma S, Umar Zezi A, Mohammed Danjuma N, Ahmed A, Garba Magaji M. Elucidation of the possible mechanism of analgesic actions of butanol leaf fraction of Olax subscorpioidea Oliv. Journal of Ethnopharmacology. 2017;199:323–7. doi:https://doi.org/10.1016/j.jep.2016.12.052.
Rauf A, Ali J, Khan H S, Mubarak M, Patel S. Emerging CAM Ziziphus nummularia with in vivo sedative-hypnotic, antipyretic and analgesic attributes. 3Biotech. 2016;6:11–20.
Crofford LJ. Chronic pain: where the body meets the brain. Trans Am Clin Climatol Assoc. 2015;126:167–83.
Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119–30. https://doi.org/10.1037/a0035514.
Rauf A, Khan R, Raza M, Khan H, Pervez S, De Feo V et al. Suppression of inflammatory response by chrysin, a flavone isolated from Potentilla evestita Th. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia. 2015;103(0):129–35. doi:https://doi.org/10.1016/j.fitote.2015.03.019.
Shchegol'kov EV, Shchur IV, Burgart YV, Saloutin VI, Trefilova AN, Ljushina GA et al. Polyfluorinated salicylic acid derivatives as analogs of known drugs: Synthesis, molecular docking and biological evaluation. Bioorganic and Medicinal Chemistry. 2017;25(1):91–9. doi:https://doi.org/10.1016/j.bmc.2016.10.014.
Qadir MI, Abbas K, Hamayun R, Ali M. Analgesic, anti-inflammatory and anti-pyretic activities of aqueous ethanolic extract of Tamarix aphylla L.(Saltcedar) in mice. Pak J Pharm Sci. 2014;27(6):1985–8.
Ali M, Rauf A, Ben Hadda T, Bawazeer S, Abu-Izneid T, Khan H, et al. Mechanisms underlying anti-hyperalgesic properties of Kaempferol-3, 7-di-O-α-L-rhamnopyranoside isolated from Dryopteris cycadina. Curr Top Med Chem. 2017;17:383–90.
Isacchi B, Iacopi R, Bergonzi MC, Ghelardini C, Galeotti N, Norcini M, et al. Antihyperalgesic activity of verbascoside in two models of neuropathic pain. J Pharm Pharmacol. 2011;63(4):594–601.
Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. International journal of molecular sciences. 2019;20(6). doi:https://doi.org/10.3390/ijms20061451.
Reis-Pina P, Lawlor PG, Barbosa A. Cancer-related pain management and the optimal use of opioids. Acta Medica Port. 2015;28(3):376–81.
Rauf A, Uddin G, Khan H, Siddiqui BS, Arfan M. Anti-hyperalgesic activity of crude extract and 7-methyljuglone of Diospyros lotus roots. Nat Prod Res. 2015;29(23):2226–9. https://doi.org/10.1080/14786419.2014.1003297.
Rauf A, Uddin G, Siddiqui BS, Khan H, Shah SUA, Hadda TB et al. Antinociceptive and anti-inflammatory activities of flavonoids isolated from Pistacia integerrima galls. Complementary Therapies in Medicine. 2016;25:132–8. doi:https://doi.org/10.1016/j.ctim.2016.02.002.
Li JX, Zhang Y. Emerging drug targets for pain treatment. Eur J Pharmacol. 2012;681(1–3):1–5. https://doi.org/10.1016/j.ejphar.2012.01.017.
Yan YY, Li CY, Zhou L, Ao LY, Fang WR, Li YM. Research progress of mechanisms and drug therapy for neuropathic pain. Life Sci. 2017;190:68–77. https://doi.org/10.1016/j.lfs.2017.09.033.
Cirino TJ, Eans SO, Medina JM, Wilson LL, Mottinelli M, Intagliata S, et al. Characterization of sigma 1 receptor antagonist CM-304 and its analog, AZ-66: novel therapeutics against Allodynia and induced pain. Front Pharmacol. 2019;10:678. https://doi.org/10.3389/fphar.2019.00678.
Romeo G, Prezzavento O, Intagliata S, Pittala V, Modica MN, Marrazzo A, et al. Synthesis, in vitro and in vivo characterization of new benzoxazole and benzothiazole-based sigma receptor ligands. Eur J Med Chem. 2019;174:226–35. https://doi.org/10.1016/j.ejmech.2019.04.056.
Modica MN, Lacivita E, Intagliata S, Salerno L, Romeo G, Pittala V, et al. Structure-activity relationships and therapeutic potentials of 5-HT7 receptor ligands: an update. J Med Chem. 2018;61(19):8475–503. https://doi.org/10.1021/acs.jmedchem.7b01898.
Raziq N, Saeed M, Shahid M, Muhammad N, Khan H, Gul F. Pharmacological basis for the use of Hypericum oblongifolium as a medicinal plant in the management of pain, inflammation and pyrexia. BMC Alternative Complimentary Medicine. 2016;16:41–7.
Ullah H, Khan H. Anti-Parkinson Potential of Silymarin: Mechanistic Insight and Therapeutic Standing. Frontiers in Pharmacology. 2018;9(422). doi:https://doi.org/10.3389/fphar.2018.00422.
Zafar M, Khan H, Rauf A, Khan A, Lodhi MA. In silico study of alkaloids as α-glucosidase inhibitors: Hope for the discovery of effective lead compounds. Frontiers in Endocrinology. 2016;7(153). doi:https://doi.org/10.3389/fendo.2016.00153.
Amin S, Khan H. Revival of natural products: utilization of modern technologies. Current Bioactive Compounds. 2016;12(2):103–6.
Semwal K, Badoni Semwal R, Semwal R, Jacob V, Singh G. Analgesic and antipyretic activities of Gindarudine, a morphine alkaloid from Stephania glabra. Current Bioactive Compounds. 2011;7(3):214–7.
Fazel Nabavi S, Braidy N, Habtemariam S, Sureda A, Manayi A, Mohammad NS. Neuroprotective effects of fisetin in alzheimer's and parkinson's diseases: from chemistry to medicine. Curr Top Med Chem. 2016;16(17):1910–5.
Rehman S, Khan H. Advances in antioxidant potential of natural alkaloids. Current Bioactive Compounds. 2017;13(2):101–8.
Khan H, Amin S, Patel S. Targeting BDNF modulation by plant glycosides as a novel therapeutic strategy in the treatment of depression. Life Sci. 2018;196:18–27. https://doi.org/10.1016/j.lfs.2018.01.013.
Khan H. Medicinal plants in light of history recognized therapeutic modality. Journal of Evidence-based Complementary and Alternative Medicine. 2014;19(3):216–9.
Khan H, Nabavi SM, Sureda A, Mehterov N, Gulei D, Berindan-Neagoe I, et al. Therapeutic potential of songorine, a diterpenoid alkaloid of the genus aconitum. Eur J Med Chem. 2018;153(10):29–33. https://doi.org/10.1016/j.ejmech.2017.10.065.
Khan H, Rengasamy KRR, Pervaiz A, Nabavi SM, Atanasov AG, Kamal MA. Plant-derived mPGES-1 inhibitors or suppressors: a new emerging trend in the search for small molecules to combat inflammation. Eur J Med Chem. 2018;153:2–28. https://doi.org/10.1016/j.ejmech.2017.12.059.
Bartnik M, Facey PC. Chapter 8 - glycosides A2 - Badal, Simone. In: Delgoda R, editor. Pharmacognosy. Boston: Academic Press; 2017. p. 101–61.
Deshpande PO, Mohan V, Pore MP, Gumaste S, Thakurdesai PA. Prenatal developmental toxicity study of glycosides-based standardized fenugreek seed extract in rats. Pharmacogn Mag. 2017;13(Suppl 1):S135–41.
Fan B-Y, Li Z-R, Ma T, Gu Y-C, Zhao H-J, Luo J-G et al. Further screening of the resin glycosides in the edible water spinach and characterisation on their mechanism of anticancer potential. Journal of Functional Foods. 2015;19, Part A:141–54. doi:https://doi.org/10.1016/j.jff.2015.09.027.
Kallemeijn WW, Witte MD, Wennekes T, Aerts JMFG. Chapter 4 - Mechanism-Based Inhibitors of Glycosidases: Design and Applications. In: Derek H, editor. Advances in Carbohydrate Chemistry and Biochemistry. Academic Press; 2014. p. 297–338.
Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Acute and repeated doses (28 days) oral toxicity study of glycosides based standardized fenugreek seed extract in laboratory mice. Regul Toxicol Pharmacol. 2015;72(2):323–34. https://doi.org/10.1016/j.yrtph.2015.05.003.
Kang L-P, Zhang J, Cong Y, Li B, Cheng-qi X, et al. Steroidal glycosides from the rhizomes of Anemarrhena asphodeloides and their antiplatelet aggregation activity. Planta Med. 2012;78:611–6.
Khan H, Khan Z, Amin S, Mabkhot YN, Mubarak MS, Hadda TB, et al. Plant bioactive molecules bearing glycosides as lead compounds for the treatment of fungal infection: a review. Biomed Pharmacother. 2017;93:498–509. https://doi.org/10.1016/j.biopha.2017.06.077.
Khan H, Saeedi M, Nabavi S, Mubarak M, Bishayee A. Glycosides from medicinal plants as potential anticancer agents: Emerging trends towards future drugs. Current Medicinal Chemistry. 2018;DOI : https://doi.org/10.2174/0929867325666180403145137.
Khan H, Pervaiz A, Kamal MA, Patel S. Antiplatelet potential of plant-derived glycosides as possible lead compounds. Curr Drug Metab. 2018;19:856–62.
Datta B, Datta S, Chowdhury M, Khan T, Kundu J, Rashid M, et al. Analgesic, antiinflammatory and CNS depressant activities of sesquiterpenes and a flavonoid glycoside from Polygonum viscosum. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2004;59(3):222–5.
Adedapo AA, Sofidiya MO, Maphosa V, Moyo B, Masika PJ, Afolayan AJ. Anti-inflammatory and analgesic activities of the aqueous extract of Cussonia paniculata stem bark. Record of Natural Products. 2008;2(2):46–53.
Pires JM, Mendes FR, Negri G, Duarte-Almeida JM, Carlini EA. Antinociceptive peripheral effect of Achillea millefolium L. and Artemisia vulgaris L.: both plants known popularly by brand names of analgesic drugs. Phytother Res. 2009;23(2):212–9.
Backhouse N, Rosales L, Apablaza C, Goïty L, Erazo S, Negrete R, et al. Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa. Buddlejaceae Journal of Ethnopharmacology. 2008;116(2):263–9.
Rodríguez II, Shi Y-P, García OJ, Rodríguez AD, Mayer AM, Sánchez JA, et al. New pseudopterosin and s eco-pseudopterosin diterpene glycosides from two colombian isolates of pseudopterogorgia e lisabethae and their diverse biological activities. J Nat Prod. 2004;67(10):1672–80.
Akkol EK, Tatli II, Akdemir ZS. Antinociceptive and anti-inflammatory effects of saponin and iridoid glycosides from Verbascum pterocalycinum var. mutense hub.-Mor. Zeitschrift für Naturforschung C. 2007;62(11–12):813–20.
Lanher M-C, Fleurentin J, Mortier F, Vinche A, Younos C. Anti-inflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med. 2007;58:117–23.
Parveen Z, Deng Y, Saeed MK, Dai R, Ahamad W, Yu YH. Antiinflammatory and analgesic activities of Thesium chinense Turcz extracts and its major flavonoids, kaempferol and kaempferol-3-O-glucoside. Yakugaku Zasshi. 2007;127(8):1275–9.
Cárdenas LC, Rodríguez J, Villaverde MC, Riguera R, Cadena R, Otero JA. The analgesic activity of Hedyosmum bonplandianum: flavonoid glycosides. Planta Med. 1993;59(1):26–7.
Villar A, Gasco M, Alcaraz M. Anti-inflammatory and anti-ulcer properties of hypolaetin-8-glucoside, a novel plant flavonoid. J Pharm Pharmacol. 1984;36(12):820–3.
Choi J, Shin K-M, Park H-J, Jung H-J, Kim HJ, Lee YS, et al. Anti-inflammatory and antinociceptive effects of sinapyl alcohol and its glucoside syringin. Planta Med. 2004;70(11):1027–32.
Toker G, Küpeli E, Memisoğlu M, Yesilada E. Flavonoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden). J Ethnopharmacol. 2004;95(2–3):393–7.
Martinez V, Ramirez T, Lastra A. A comparative study of the analgesic and anti-inflammatory activities of pectolinarin isolated from Cirsium subcoriaceum and linarin isolated from Buddleia cordata. Planta Med. 1998;64:134–7.
Ramesh M, Rao YN, Kumar MR, Rao AVNA, Prabhakar M, Reddy BM. Antinociceptive and anti-inflammatory activity of carumbelloside-I isolated from Caralluma umbellata. J Ethnopharmacol. 1999;68(1):349–52.
De Melo GO. Malvar DdC, Vanderlinde FA, Rocha FF, Pires PA, Costa EA et al. Antinociceptive and anti-inflammatory kaempferol glycosides from Sedum dendroideum. J Ethnopharmacol. 2009;124(2):228–32. https://doi.org/10.1016/j.jep.2009.04.024.
Zhang B, Li JB, Zhang DM, Ding Y, Du GH. Analgesic and anti-inflammatory activities of a fraction rich in gaultherin isolated from Gaultheria yunnanensis (FRANCH.) REHDER. Biol Pharm Bull. 2007;30(3):465–9. https://doi.org/10.1248/bpb.30.465.
Zhang B. He XL, Ding Y. Du GH. Gaultherin, a natural salicylate derivative from Gaultheria yunnanensis: towards a better nonsteroidal anti-inflammatory drug. Eur J Pharmacol. 2006;530(1–2):166–71. https://doi.org/10.1016/j.ejphar.2005.11.030.
Kim M-H, Nugroho A, Choi J, Park JH, Park H-J. Rhododendrin, an analgesic/anti-inflammatory arylbutanoid glycoside, from the leaves of Rhododendron aureum. Arch Pharm Res. 2011;34(6):971–8.
Wang Q-H, Han N-R-C-K-T, Dai N-Y-T WR-J, Wu J-S. Analgesic effects and structural elucidation of two new flavone C-glycosides from Artemisa sacrorum. Chin J Nat Med. 2015;13(10):786–90. https://doi.org/10.1016/S1875-5364(15)30080-7.
Norregaard R, Kwon T-H, Frokiær J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Research and Clinical Practice. 2015;34(4):194–200. https://doi.org/10.1016/j.krcp.2015.10.004.
Ghannadi A, Hajhashemi V, Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 2005;8(4):488–93.
Neto A, Costa J, Belati C, Vinholis A, Possebom L, Da Silva FA, et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J Ethnopharmacol. 2005;96(1):87–91.
Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. https://doi.org/10.1038/9550.
Ballou LR BC, Raghow R. Elucidation of the pathophysiological functions of prostaglandins using cyclooxygenase gene deficient mice. In: Vane JR, Botting RM, eds. Therapeutic roles of selective COX2 inhibitors. London, UK: William Harvey Press 2001:128–67.
Sala A, Zarini S, Bolla M. Leukotrienes: lipid bioeffectors of inflammatory reactions. Biochemistry (Mosc). 1998;63(1):84–92.
Radmark OP. The molecular biology and regulation of 5-lipoxygenase. Am J Respir Crit Care Med. 2000;161(2 Pt 2):S11–5. https://doi.org/10.1164/ajrccm.161.supplement_1.ltta-3.
Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343(6255):282–4. https://doi.org/10.1038/343282a0.
Penrose JF, Austen KF, Lam BK. Leukotrienes: biosynthetic pathways, release and receptor-mediated actions with relevance to disease states. In: Gallin JL, Snyderman R, editors. Inflammation basic principles and clinical correlates. Philadelphia: Lippicort Williams & Wilkins; 1999. p. 361–71.
Bray MA, Ford-Hutchinson AW, Smith MJ. Leukotriene B4: an inflammatory mediator in vivo. Prostaglandins. 1981;22(2):213–22. https://doi.org/10.1016/0090-6980(81)90036-8.
Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323(10):645–55. https://doi.org/10.1056/NEJM199009063231006.
Rainsford KD, Ying C, Smith F. Effects of 5-lipoxygenase inhibitors on interleukin production by human synovial tissues in organ culture: comparison with interleukin-1-synthesis inhibitors. J Pharm Pharmacol. 1996;48(1):46–52. https://doi.org/10.1111/j.2042-7158.1996.tb05875.x.
He W, Pelletier JP, Martel-Pelletier J, Laufer S, Di Battista JA. Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J Rheumatol. 2002;29(3):546–53.
Ronchetti S, Migliorati G, Delfino DV. Association of inflammatory mediators with pain perception. Biomed Pharmacother. 2017;96:1445–52. https://doi.org/10.1016/j.biopha.2017.12.001.
McHugh JM, McHugh WB. Pain: neuroanatomy, chemical mediators, and clinical implications. AACN Clin Issues. 2000;11(2):168–78.
Widgerow AD, Kalaria S. Pain mediators and wound healing--establishing the connection. Burns. 2012;38(7):951–9. https://doi.org/10.1016/j.burns.2012.05.024.
Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharmacol. 1975;9(2–3):229–34. https://doi.org/10.1007/bf00614022.
Graefe EU, Derendorf H, Veit M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int J Clin Pharmacol Ther. 1999;37(5):219–33.
Gee JM, DuPont MS, Day AJ, Plumb GW, Williamson G, Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr. 2000;130(11):2765–71. https://doi.org/10.1093/jn/130.11.2765.
Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C. Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. J Nutr. 2001;131(8):2109–14. https://doi.org/10.1093/jn/131.8.2109.
O'Leary KA, Day AJ, Needs PW, Mellon FA, O'Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–91. https://doi.org/10.1016/s0006-2952(02)01510-1.
Jang MH, Lim S, Han SM, Park HJ, Shin I, Kim JW, et al. Harpagophytum procumbens suppresses lipopolysaccharide-stimulated expressions of cyclooxygenase-2 and inducible nitric oxide synthase in fibroblast cell line L929. J Pharmacol Sci. 2003;93(3):367–71. https://doi.org/10.1254/jphs.93.367.
Inaba K, Murata K, Naruto S, Matsuda H. Inhibitory effects of devil's claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J Nat Med. 2010;64(2):219–22. https://doi.org/10.1007/s11418-010-0395-8.
Nielsen SE, Breinholt V, Justesen U, Cornett C, Dragsted LO. In vitro biotransformation of flavonoids by rat liver microsomes. Xenobiotica. 1998;28(4):389–401. https://doi.org/10.1080/004982598239498.
Chen Y, Xie S, Chen S, Zeng S. Glucuronidation of flavonoids by recombinant UGT1A3 and UGT1A9. Biochem Pharmacol. 2008;76(3):416–25. https://doi.org/10.1016/j.bcp.2008.05.007.
Yodogawa S, Arakawa T, Sugihara N, Furuno K. Glucurono- and sulfo-conjugation of kaempferol in rat liver subcellular preparations and cultured hepatocytes. Biol Pharm Bull. 2003;26(8):1120–4. https://doi.org/10.1248/bpb.26.1120.
Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304(3):1228–35. https://doi.org/10.1124/jpet.102.046409.
Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55(2):159–69. https://doi.org/10.1007/s00280-004-0842-x.
Aquilonius SM, Hartvig P. Clinical pharmacokinetics of cholinesterase inhibitors. Clin Pharmacokinet. 1986;11(3):236–49. https://doi.org/10.2165/00003088-198611030-00005.
Cho JY, Nam KH, Kim AR, Park J, Yoo ES, Baik KU, et al. In-vitro and in-vivo immunomodulatory effects of syringin. J Pharm Pharmacol. 2001;53(9):1287–94. https://doi.org/10.1211/0022357011776577.
Nolan AM. Chapter 3 - pharmacology of analgesic drugs A2 - Flecknell, Paul a. In: Waterman-Pearson A, editor. Pain Management in Animals. Oxford: W.B. Saunders; 2000. p. 21–52.
Hegazy GH, Ali HI. Design, synthesis, biological evaluation, and comparative Cox1 and Cox2 docking of p-substituted benzylidenamino phenyl esters of ibuprofenic and mefenamic acids. Bioorganic and Medicinal Chemistry. 2012;20(3):1259–70. doi:https://doi.org/10.1016/j.bmc.2011.12.030.
Husain A, Ahmad A, Khan SA, Asif M, Bhutani R, Al-Abbasi FA. Synthesis, molecular properties, toxicity and biological evaluation of some new substituted imidazolidine derivatives in search of potent anti-inflammatory agents. Saudi Pharmaceutical Journal. 2016;24(1):104–14. doi:https://doi.org/10.1016/j.jsps.2015.02.008.
Gülçin I, Küfrevioǧlu Öİ, Oktay M, Büyükokuroǧlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90(2–3):205–15.
Adeoye AT, Adedapo AA, Abatan MO. Study on acute ulcerous pain in rats treated with aqueous root extract of Lonchocarpus cyanescens. Journal of Acute Disease. 2016;5(6):454–7. https://doi.org/10.1016/j.joad.2016.09.002.
Umre R, Ganeshpurkar A, Ganeshpurkar A, Pandey S, Pandey V, Shrivastava A, et al. In vitro, in vivo and in silico antiulcer activity of ferulic acid. Future Journal of Pharmaceutical Sciences. 2018. https://doi.org/10.1016/j.fjps.2018.08.001.
Tomić M, Micov A, Pecikoza U, Stepanović-Petrović R. Chapter 1 - Clinical Uses of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Potential Benefits of NSAIDs Modified-Release Preparations A2 - Čalija, Bojan. Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs. Boston: Academic Press; 2017. p. 1–29.
Biasutto L, Zoratti M. Prodrugs of quercetin and resveratrol: a strategy under development. Curr Drug Metab. 2014;15(1):77–95.
Intagliata S, Modica MN, Santagati LM, Montenegro L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants (Basel, Switzerland). 2019;8(8). doi:https://doi.org/10.3390/antiox8080244.
Acknowledgements
AGA acknowledges the support from the Polish KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal-Safe Food” of Ministry of Science and Higher Education (05-1/KNOW2/2015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, H., Pervaiz, A., Intagliata, S. et al. The analgesic potential of glycosides derived from medicinal plants. DARU J Pharm Sci 28, 387–401 (2020). https://doi.org/10.1007/s40199-019-00319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00319-7