Abstract
Coastal areas are urbanized and industrialized environments, affected by dredging operations, discharges of untreated municipal wastewaters, and farming. Developing countries are in continuous growth and will deal, in a close future, with the highest rate of coastal transformation, posing serious risks for the ecological and environmental value of ecosystem assets. This research aims to study the dissolved and particulate Cr, Ni, Pb and Zn values within an argentinean estuarine environment which is currently under human-induced pressures. Concentrations of all the metals under analyses showed seasonal variability of both dissolved and particulate metals. An important outcome of this study was that dissolved Cr, Pb and Zn attained maximum values and overall increased concentrations with respect to previous records from the same area. Indeed, the highest concentrations were found during the dredging operations or in association with increases in the metal levels from wastewater discharges. The results also indicated that human activities contributed the least to the dissolved Ni concentrations. The particulate fraction of Cr, Ni and Zn showed an upward trend in the concentrations, particularly during the last two sampling dates, being also positively correlated between each other. Regarding their respective environmental quality standards, many samples achieved dissolved Cr and Zn concentrations above the maximum values recommended by international guidelines. Thus, this study highlights the possibility of stressors like dredging activities and municipal wastewaters to cause increases in the water column pollution levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal areas are urbanized and industrialized environments, and human-induced pressures, referred to as stressors, modify these areas in several ways (Verdonschot et al. 2013). Among coastal environments, estuaries play a key role as carriers of inorganic elements, like metals, from terrestrial environments to the open sea. Metals are elements of interest due to their non-biodegradability and biogeochemical recycling (De Souza Machado et al. 2016). They originate from natural sources, mainly the weathering of soil and rocks, erosion, forest fires, and volcanic eruptions, and from a huge variety of human activities (Yao et al. 2016). Anthropogenic metal sources ranged from agricultural and industrial to domestic effluents (Bhattacharya et al. 2015). Then, due to atmospheric depositions and during storms, maritime traffic, and dredging, metals might be mobilized and redistributed within the aquatic environment and they might also be incorporated by biota (e.g., Guerra et al. 2009; Hedge et al. 2009; Liang et al. 2016; Tornero and Hanke 2016).
Both dissolved and particulate fractions in seawater are important for metals dynamics within estuaries. Metals remain only for a short time in the dissolved fraction (Botté et al. 2013) and then, from this fraction, metals can be incorporated into the particulate fraction, sediments, or biota (Alquezar et al. 2007; Doig and Liber 2007). During dredging operations, the resuspension of bottom sediments usually lead to an increase in the water turbidity, and if the sediments act as a sink of contaminants, they could be remobilized during the oxidation of the anoxic sediments. These environmental alterations are specific for each ecosystem due to the degree of the sediments contamination levels (Vale et al. 1998). Then, the dissolved metals found in waters might not only belong to the current metal incomes but also to remobilization operations.
Metal behavior in estuaries throughout the globe experience substantial differences since each estuary is affected to a greater or lesser degree by anthropogenic activities and specific physical forces that take place in the area of influence (Diop et al. 2014). These systems are influenced by land, fresh water, and ocean inputs, resulting in unique and dynamic marine environments. The Bahía Blanca estuary (BBE), located in Argentina, South America, is a coastal system influenced by urban and industrial activities. It is affected by the continuous maintenance and dredging operations as a consequence of the maritime traffic (Grecco et al. 2011) that arise from being the most important deep water port system of the country.
The current study reports the concentration of the metals Cr, Ni, Pb and Zn in the dissolved and particulate seawater fractions of the BBE. The goal was to evaluate the anthropogenic influence (with emphasis on stressors like dredging and untreated wastewaters) on the estuarine environment by means of the analysis of Cr, Ni, Pb and Zn. These four metals have been previously identified as priority contaminants by the European Community Water Framework Directive (2000/60/EC; Anonymous 2000) and are widely used in environmental quality criteria (Burton 2002; Gao et al. 2015). A comprehensive investigation into the temporal-spatial metal distribution over a 3-year period (2011–2014) was conducted. It was expected to achieve variations in the metal concentrations throughout the sampling period of analysis as a result of human and industrial developments all along, mainly considering a major dredging operation that was carried out in the BBE during part of the sampling period.
This article is part of a series of studies dealing with the effects of urban and industrial impact on the accumulation pattern of metals on biotic and abiotic substrates (i.e., La Colla et al. 2015, 2017, 2018).
Materials and methods
Study area
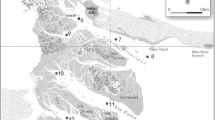
The study was conducted in the BBE (Fig. 1), located in the southeast coast of Argentina. This mesotidal system, the second largest estuary of the country, has an elongated shape with a NW–SE orientation (Piccolo and Perillo 1990) and a main navigation channel of 60 km long (Perillo and Piccolo 1999). The estuary is formed by a series of tidal channels separated by extensive tidal flats and saltmarsh patches that cover an area of approximately 2300 km2 (Piccolo et al. 2008). The estuary has been regarded as turbid (Andrade et al. 2000) with an annual mean of particulate suspended matter toward the inner zone of 78 mg/L (Guinder et al. 2009). The BBE is characterized by high salinities that usually vary between 33 and 40 PSU, even though values as low as 10 PSU have been reported (Botté et al. 2007; La Colla et al. 2015).
On the north coast the estuary receives freshwater inflows (mean discharge of 241,000 m3/day), mainly from two contributors: the Sauce Chico river and the Naposta Grande stream (Limbozzi and Leitào 2008). Other tributaries comprise small volumes of water, whereas some channels discharge water only during periods of rainfall and behave as tidal channels during the rest of the time. The BBE also receives groundwater contributions, with an overall estimated inflow of 2000 m3/day (CTE 2003). The BBE receives wastewater discharges from Bahía Blanca, the main city located adjacent to the estuary which supports approximately 300,000 inhabitants (INDEC 2010). The mean discharge is 70,000 m3/day, according to studies performed during the period 2001–2010 (Cifuentes et al. 2011). Punta Alta, another city close to the BBE, with 61,000 inhabitants (INDEC 2010), also produces wastewater discharges of around 19,000 m3/day (CTE 2003). Wastes generated during industrial operations on the north shoreline also flow into the estuary. Discharges from the petrochemical center reach the estuary with different degree of treatment and with a mean volume of 106,000 m3/day, according to studies performed during the period 2001–2012 (Cifuentes et al. 2014).
The cities and industries enclosing the BBE are in continuous development (expansion and production). By 2002, the industrial area surrounding the petrochemical center embraced only 9 industries while in 2012, it included more than 135 (Sznaiberg 2012). The harbor area modifies the coastal environment with the maintenance dredging activities, the infill material from the dredged areas, and the modification of the coastlines. Altogether, anthropogenic stressors result in the deterioration of the Bahía Blanca estuarine environment. One of the major dredging operations was carried out in the BBE during part of the sampling period (May 2013–November 2013) and included the removal of approximately 5,700,000 m3 of sediment from the inner estuary to go deep into the navigation routes. These dredging operations allowed ships with depths of up to 14 m to reach commercial and recreational ports, docks, and harbors within the estuary (Schnegelberger 2014). These operations directly influenced sampling sites 5 to 9 from this research.
The assessment of the environmental condition of the BBE has previous records regarding metal determinations in sediments, saltmarshes, microbial mats, and tissues of aquatic organisms (e.g., Botté et al. 2010; Fernandez Severini et al. 2011; La Colla et al. 2017, 2018; Negrin et al. 2016; Serra et al. 2017; Simonetti et al. 2013, 2017, among others).
Sampling sites
The sampling was carried out between November 2011 and May 2014 along the BBE on a trimonthly basis (Fig. 1). The sampling procedures involved the inner and the middle-outer estuary. All sampling sites are under human impact and the metal inputs are mainly due to untreated municipal wastewater, farming, industrial activities, dredging activities, and maritime traffic. The sampling sites and dates, together with the fraction analyzed (dissolved and/or particulate) and the description of the areas under study, are detailed in Table 1.
Cleaning procedures
All material used was cleaned according to internationally recommended protocols (APHA 1998). The conditioning procedure includes washing the material with non-ionic detergent, tap water, and then deionized water. After that, the material is soaked for 24 h in an acid nitric solution (5.0% HNO3) and finally washed with deionized water. The suspended particulate material (SPM) filters are also conditioned in 0.70% HNO3 for 72 h, rinsed with deionized water, dried to constant weight, and then re-weighed.
Sample collection and laboratory analyses
Water samples were obtained using 1500 ml polyethylene-tereftalate (PET) acid pre-cleaned bottles. Before water collection, bottles were rinsed three times with estuarine water. Then the bottles were filled by moving it upward through the water column (typical depth of 20–50 cm). Physicochemical parameters (salinity, pH, turbidity, and temperature) were measured in situ with a multisensor Horiba U-10.
The water samples were transported to the laboratory in refrigerated boxes and filtered, during the next hours, through 0.45 μm mesh cellulose acetate Millipore HA filters in order to obtain the dissolved and particulate fractions for metals determination. Filtered fractions were kept in bottles, acidified with concentrated analytical quality HCl up to pH 2, and stored in darkness at 4 °C. The SPM retained in the filters was dried in a stove at 50 ± 5 °C until constant weight, then weighted in an analytical balance (OHAUS, Adventurer) and stored in dry conditions. The content of SPM (mg) from each sample was calculated as follows: (weight of the filter with SPM) − (weight of the same filter before filtration).
A preconcentration procedure and organic extraction was applied for the dissolved metals determination (Botté et al. 2007; La Colla et al. 2015). Samples of 500 ml (by duplicate) were put in separatory funnels, its pH adjusted to 4.0 ± 0.020, and then 10 ml of APDC 1.0% (ammonium pyrrolidine dithiocarbamate) and 20 ml of MIBK (methyl isobutyl ketone) were added as chelating agent and as organic surfactant, respectively. After that, samples were shaken for 30 min, then the upper organic phase was separated in a beaker and the volume was reduced. Metals contained in the final extract were diluted up to 10 ml with saturated MIBK and were measured using an AAS (Perkin-Elmer AA-2380) with air/acetylene flame.
Particulate metals analyses were performed based on Botté et al. (2010) and La Colla et al. (2015). The filters (by duplicate) were subjected to an acid pre-digestion with 5.0 ml of HNO3 (65%) for at least 3 h. Then, 1.0 ml of HClO4 was added and the samples were put in a glycerin bath at 110 ± 10 °C for at least 72 h. The acidic extracts were transferred to centrifuge tubes and brought to 10 ml using 0.70% HNO3. Metals were determined using an inductively coupled plasma-optical emission spectrometer (ICP OES, Perkin Elmer Optima 2100 DV) with axial view. All reagents used were always Merck or similar analytical quality.
Data processing and statistical analyses
The analytical method detection limit (MDL) for each dissolved metal (μg/L) was 0.89 for Cr; 0.81 for Ni; 0.38 for Pb; 1.6 for Zn. The MDL for each particulate metal (μg/g) was 2.2 for Cr; 6.7 for Ni; 9.3 for Pb; 4.0 for Zn. Metal concentrations reported as below MDL were substituted by one-half the MDL for statistical analyses (Jones and Clarke 2005). Because of the small sampling size, no statistical analyses were performed when 50% or more of the metal concentrations under evaluation were below the MDL (EPA 2000).
The analytical quality was tested against reference materials (pond sediment flour R.M.No. 82- and estuarine water R.M.No. 39-) provided by the National Institute for Environmental Studies (NIES) from Tsukuba University (Japan). The recovery percentages were within 85–115% of the certified values for the four measured elements. Blanks of reagents were used simultaneously in each batch of analysis to corroborate the analytical quality. All analyses were done in duplicate, and the uncertainty based on one relative standard deviation of replicates was < 15%.
Normality and homogeneity of variance were tested with Shapiro-Wilk and Levene tests, respectively. Nonparametric tests were chosen as the data analyzed did not meet the assumptions of the parametric statistics and there were no possible transformations. The differences in the physicochemical parameters and the metal concentrations between sampling sites and sampling dates were evaluated with the nonparametric test Kruskal-Wallis ANOVA. The significant results were analyzed a posteriori with the Conover scores after a Kruskal-Wallis test (Conover 1999). Error values, either in tables or in the text, represent standard deviation. Metals might be originated from similar sources and/or have similar reactivity toward physicochemical parameters. Therefore, significant relationships were evaluated with the Spearman correlation tests. All statistical analyses were carried out using STATISTICA 7.0 (StatSoft, Inc.), following Zar (1996). The acceptable level of statistical significance used throughout the study was p < 0.05. The graphics were performed using software R in version 3.2.0 from the R-project (Wickham 2009).
Results and discussion
Analyses of the physicochemical variables
All the physicochemical parameters under study showed no significant differences between sampling sites (p > 0.30). However, some interesting differences between sampling dates were found.
Mean salinity was 36 ± 2.6 PSU, ranging from 29 to 42 PSU (Fig. 2). The distribution pattern of salinity fluctuated seasonally, with February 2014 (summer) achieving the statistically highest value compared to November 2011 (spring), August 2012 (winter), December 2013 (spring), and May 2014 (autumn) (p < 0.05). Salinity is directly related to the rainfall rates and the freshwater incomes, and the BBE normally has high salinity values with low variability due to the reduced river water that flows towards the estuary (Guinder et al. 2012 and references therein). During the present research, summer samplings reached to the highest salinities together with the highest temperatures (Fig. 2) and the values were similar to those previously registered in the BBE (Fernandez Severini et al. 2011; Guinder et al. 2012).
Temperature varied between 6 and 26 °C with a mean of 16 ± 5.6 °C (Fig. 2). Temperature values showed the regular seasonal cycle, recording the highest values in November 2012 (spring) and the summer seasons of February 2012, 2013, and 2014 compared to August 2013 (winter). Also, February 2014 (summer) reported higher values than August 2012 (winter) (p < 0.05). The values achieved were also in agreement with data from other previous studies from the BBE (Botté et al. 2007; Ferrer et al. 2000; Marcovecchio et al. 2010; Perillo et al. 2001).
Mean pH was 8.1 ± 0.31, with values between 7.4 and 9.1 (Fig. 2). There were significant differences between sampling dates (p = 0.030), with values in August 2013 being higher than in October 2012, February 2013, and February 2014. The lowest values were achieved in February 2013. The pH range was within the typical scope already described for the BBE (Freije et al. 2008; Guinder et al. 2012).
Mean turbidity was 110 ± 100 NTU, fluctuating from 10 to 490 NTU (Fig. 2). May 2014 exhibited the lowest mean concentrations, even though no significant differences between sampling dates were recorded (p > 0.50). Turbidity concentrations in this study were as high as expected, since the BBE is highly affected by winds and tides (Piccolo 2008), becoming a turbid estuary due to the resuspension of fine sediments (silt and clay) (Cuadrado et al. 2005). Moreover, the regular dredging of the main navigation channel contributes to the resuspension of fine sediments (Marcovecchio and Freije 2004).
The concentration and variation of these physicochemical parameters probably influenced the distribution of metals in both the dissolved and particulate seawater fraction, since physical and chemical processes which happen in these estuarine areas throughout the year influence the geochemistry of metallic elements, changing the behavior of metals in the water (Milazzo et al. 2014).
Evaluation of water quality by means of dissolved metals
Dissolved Cr, Ni, Pb and Zn concentrations were measured in the selected sites of the BBE (Fig. 1, Table 1) throughout the sampling period 2011–2014. No significant differences were found in the metal values between the different sampling sites (p > 0.35).
Dissolved Cr values obtained during the sampling period ranged from below the MDL to 57 μg/L (Fig. 3). Cr concentrations exhibited an upward trend in time, with the highest concentrations found in October 2012 (45 μg/L), February 2013 (57 μg/L), and August 2013 (48 μg/L). Both sampling dates, October and February, showed mean values that were significantly higher than the ones found in August 2012 (p < 0.0090). August 2013 also achieved high mean but with no statistical differences against the other dates (p > 0.50). As for the sampling sites, it is important to note that the highest mean dissolved value was achieved at site 5 (mean of 23 μg/L), which is located at the beginning of the zone where the dredging operations were carried out.
Cr values from this study were compared with those registered directly from the industrial effluent discharge sites during the period 2011–2014, and provided by the municipal government of the city (CTE 2011, 2012, 2013, 2014). The recorded dissolved Cr values were as high as 70 μg/L, with mean values of 17 μg/L during the sampling period. So, increases in the Cr values found in the present research could be partly explained by the industrial effluent discharges. Also, the high mean concentrations found in August 2013 might be influenced by the increased resuspension of the bottom sediment due to the major dredging operations (May 2013–November 2013). This is in concordance with the metal rises registered by other authors in anthropogenic impacted environments directly influenced by dredging procedures (e.g., Cabrita 2014; Caetano et al. 2003).
In this study, all dissolved Ni concentrations were below the MDL. These values were in agreement with the Ni values recorded directly from within industrial effluent discharge sites during the studied period (CTE 2011, 2012, 2013, 2014), which were also below the MDL.
Pb values found in the dissolved seawater fraction ranged from below the MDL to 15 μg/L (Fig. 3). The highest value was achieved in February 2014, compared to November 2011, August 2012, and October 2012 (p = 0.048). The rest of the values were generally close to the MDL. Metal values recorded directly from industrial effluent discharge sites during the period 2011–2014 (CTE 2011, 2012, 2013, 2014), registered values mostly below the MDL, and were in agreement with the dissolved Pb concentrations obtained during the present research. Indeed, the increase observed in the Pb values in February 2014 took place after an increase registered by the CTE in January 2014 (32 μg/L). Hence, industrial discharges might have influenced, to some extent, the maximum mean Pb values achieved in February 2014. As regards the sampling sites, site 6 showed the highest mean value during the sampling period.
Dissolved Zn concentrations showed wide ranges, with values from below the MDL to 55 μg/L in the sampling site 2 (August 2013) (Fig. 3). The mean maximum concentration was registered in December 2013 (23 μg/L). More than 50% of the data were below the MDL and thus, no statistical analyses were performed. All the maximum concentrations were achieved while the dredging activities were being conducted. Nevertheless, the values from the study were lower than the ones registered directly from the industrial effluent discharge sites (CTE 2011, 2012, 2013, 2014), as these discharges reached to concentrations as high as 580 μg/L, with mean values of 140 μg/L. The dissimilarities in the concentrations highlight the processes, like tides, that might be affecting the distribution, dissolution, and/or precipitation of Zn from the effluent discharges.
Concerning the hazardous properties of metals, dissolved Cr concentrations were, in 30% of the samples, higher than the maximum recommended of 15 μg/L in account to the Environmental Quality Standard (EQS, for estuarine waters) (Cole et al. 1999). During the period 2011–2014, the dissolved Cr concentrations were also higher than those recorded in previous studies from the BBE (Table 2). In this research, Cr reached maximum values of 57 μg/L, whereas Cr concentrations in previous studies registered maximum values of only 5.8 μg/L (Botté et al. 2007). Regarding dissolved Cr values in impacted estuaries from around the world, Cr values found in the BBE were usually similar than those found in other coastal environments, with the exception of the values found in the Tagus estuary in Portugal that were considerably higher (Table 2).
Ni values from the study period, being all below the MDL, would indicate that human activities contributed the least to the levels of this dissolved metal. Ni values were also lower compared to preceding concentrations from the BBE (maximum of 3.5 μg/L in Botté et al. 2007) and to data from other anthropogenically impacted coastal systems like the Tagus Estuary, world-known as a sink of historically accumulated metals, with values up to 350 μg/L (Duarte et al. 2014) (Table 2).
Dissolved Pb levels found in this research were always below the maximum of 25 μg/L recommended by the EQS (Cole et al. 1999). Nevertheless, these Pb concentrations were higher than those recorded from previous studies in the BBE (Table 2). Preceding values indicated maximums of 7.5 μg/L (Botté et al. 2007). The concentrations registered for Pb could be indicative of the slow decrease in the use of leaded gasoline and/or a consequence of the role that cities and industries surrounding the BBE have, since they are in continuous development. After making comparisons with other anthropogenically impacted estuaries worldwide (Table 2), the Tagus estuary (Duarte et al. 2014 and references therein) was the only system with higher values compared to the BBE, whereas the rest of the coastal systems used for comparison displayed similar or lower mean Pb values.
Regarding dissolved Zn values, 6.3% of the samples were above the maximum recommended value of 40 μg/L, set by the EQS (Cole et al. 1999). Also showed higher maximum concentrations compared to previous studies from the BBE like Andrade (2001), with a maximum of 25 μg/L (Table 2). Compared to estuaries from around the world, the present research showed, in general, higher or similar maximum values, with the exception of the ones found in the Tagus estuary (Table 2).
Relevance of the suspended particulate fraction in metal distribution
SPM includes, among others, the finest grain sediment, usually clay and silt; consequently, these types of particles, with a large surface:volume relationship, present great ability to trap substances or elements through sorption processes (Pasternack and Brown 2006). Moreover, SPM concentrations are the result of bioturbation processes, physical disturbances such as winds and tides, and urban impacts such as dredging operations, among others.
In this study, the concentrations of the metals Cr, Ni, Pb and Zn associated to the SPM fraction were measured in all sites throughout the sampling period 2011–2013 (Fig. 1, Table 1). Due to economic constraints, particulate samples from the period between August 2013 and May 2014 could not be used in this study. All metals evaluated showed a uniform distribution between the different sampling sites, thus, no significant differences were achieved (p > 0.68).
Particulate Cr concentrations ranged from below the MDL to 35 μg/g (Fig. 4). An upward trend in metal levels was reported toward the end of the sampling period, showing significant higher mean values in October 2012 (31 μg/g) in comparison to all the previous sampling dates (p = 0.042). Moreover, in February 2013, Cr values also reached to higher mean values (26 μg/g), although compared, exclusively, to November 2011 (p = 0.030). The highest concentrations found in October 2012 and February 2013 could be related to the trend of also highest dissolved Cr values during the same sampling dates, since dissolved metals could rapidly become part of the particulate fraction.
As for particulate Ni concentrations, they ranged from below the MDL to 32 μg/g (Fig. 4). An upward trend in the concentrations was observed by the end of the period, just like for particulate Cr. Indeed, Ni values in October 2012 were significantly higher than those found in November 2011 (p = 0.012). The presence of Ni exclusively in the particulate fraction might be linked to the fact that metals usually remain in the dissolved fraction for a short time and then become part of the particulate fraction by the adsorption to the fine grain sediment with great contents of organic matter.
For particulate Pb, concentrations ranged from below the MDL to 21 μg/g (Fig. 4), with no statistical differences between the sampling dates (p = 0.15). The particulate Zn concentrations found during the sampling period varied between 54 and 470 μg/g (Fig. 4). Zn levels remained approximately the same throughout most of the sampling period, with the exception of an upward trend found toward the last two sampling dates, like for Cr and Ni (October 2012 and February 2013). Moreover, an extremely high maximum value (470 μg/g) was found at the sampling site 9 in October 2012. These achieved concentrations might be related to the dissolved values, as high as 570 μg/, registered in the industrial effluent discharge sites during the period 2011–2013 (CTE 2011, 2012, 2013). These dissolved metal concentrations might have influenced over the suspended particulate metal values found during this research through adsorption processes.
In comparison to previous metal values from the same study area, particulate Cr values were slightly higher than those concentrations previously recorded by Andrade (2001) and reaching up to 28 μg/g. Zn values from this study were similar to those previously recorded in the BBE (Table 2) whereas particulate Ni and Pb values were below those maximum concentrations registered in the BBE (Table 2).
Cr and Ni values were similar than those found in other anthropogenically impacted estuaries (Table 2). Meanwhile, current Pb and Zn values from this study were below those registered in estuaries from around the world (Table 2).
Relationships among the studied metals and between metals and the physicochemical parameters
The correlation analyses between the physicochemical parameters and the concentrations of dissolved and particulate metals were, in most cases, not significant (p > 0.50) (Table 3). Among the statistical significant correlations, those with values of correlation higher than 60% were taken into account. Positive significant correlations were found, in the particulate fraction, between Cr and Ni (r2:0.97), Cr and Zn (r2:0.87), and Ni and Zn (r2:0.86). Dissolved Cr also correlated positively with particulate Cr (r2:0.66) and particulate Ni (r2:0.71). Moreover, high negative significant correlations were found between pH and the particulate fraction of Cr (r2: − 0.65) and Ni (r2: − 0.62).
The closer inter-element correlations observed for the elements (Cr, Ni and Zn) in seawater might indicate possible similar biogeochemical transformations, and could provide interesting information for characterizing the sources. These correlations might be the result of similar sources or be a function of bottom-sediment remobilization and re-release of previously sorbed contaminants. Studies performed in other coastal systems (e.g., Beltrame et al. 2009) have mentioned positive correlations between Cr, Ni and Zn. Specifically for Cr and Ni, it is important to highlight that the high correlations might be related to the increased of both metals in the particulate fraction by the end of the sampling period.
As regards the negative correlation between pH and some metals in the SPM fraction, the highest Cr and Ni levels achieved in October 2012 and February 2013 could be explained, at least in part, by decreases in the pH. In the case of Cr, a small pH decrease can favor an increase of the metal in the particulate fraction (Sadiq 1992). In the case of Ni, as a consequence of natural processes, like the decrease in pH, Ni is usually released into the water column, especially in turbid environments (Angel et al. 2010).
Conclusion
An important outcome of this study was that dissolved Cr, Pb and Zn attained maximum concentrations and increased values with respect to previous records from the same area. The results were linked to the role that cities and industries surrounding the BBE have, since they are in continuous development. Also, the increases could be affected by the effluent discharges of the industrial and urban activities and the increased resuspension of the bottom sediment due to the major dredging operations. Dissolved concentrations of Cr and Zn were found above their respective environmental quality standards according to international guidelines. The exception was Ni, with the dissolved values being all below the limit of detection of the method. These results would indicate that human activities contributed less to the levels of this metal than to the levels of the rest of the evaluated elements.
The particulate concentrations of Cr, Ni and Zn showed an upward trend toward the last two sampling dates with the concentrations of Cr, Ni and Zn in close association. The results could be mainly linked to the wastewater discharges and the changes in the physicochemical parameters in the case of Cr and Ni.
References
Alquezar, R., Markich, J. S., & Twining, J. R. (2007). Uptake and loss of dissolved 109Cd and 75Se in estuarine macroinvertebrates. Chemosphere, 67, 1202–1210.
Andrade, S. (2001). Metales pesados en el agua de la zona interna de Bahía Blanca, y su toxicidad sobre algunas especies fitoplanctónicas. PhD Thesis, Universidad Nacional del Sur, Argentina, 244 pp.
Andrade, S., Pucci, A., & Marcovecchio, J. E. (2000). Cadmium concentrations in the Bahía Blanca estuary (Argentina). Potential effects of dissolved cadmium on the diatom Thalassiosira curviseriata. Oceanologia, 42, 505–520.
Angel, B. M., Hales, L. T., Simpson, S. L., Apte, S. C., Chariton, A. A., Shearer, D. A., & Jolley, D. F. (2010). Spatial variability of cadmium, copper, manganese, nickel and zinc in the Port Curtis estuary, Queensland, Australia. Marine Freshwater Research, 61(2), 170–183.
Anonymous. (2000). Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000, establishing a framework for community action in the field of water policy (water framework directive). Official Journal of European Community, L327, 1–72.
APHA-AWWA-WPCF. (1998). Standard methods for the examination of water and wastewater. En: Clesceri, L.S., Greenberg, A.E., Eaton, A.D. (Eds,), 20th ed. American Public Health Association, Washington.
Beltrame, M. O., De Marco, S. G., & Marcovecchio, J. E. (2009). Dissolved and particulate heavy metals distribution in coastal lagoons. A case study from Mar Chiquita Lagoon, Argentina. Estuarine, Coastals and Shelf Science, 85(1), 45–56.
Bhattacharya, B. D., Nayak, D. C., Sarkar, S. K., Biswas, S. N., Rakshit, D., & Ahmed, M. K. (2015). Distribution of dissolved trace metals in coastal regions of Indian Sundarban mangrove wetland: a multivariate approach. Journal of Clean Production, 96, 233–243.
Birch, G., & O’Hea, L. (2007). The chemistry of suspended particulate material in a highly contaminated embayment of Port Jackson (Australia) under quiescent, high-wind and heavy-rainfall conditions. Environmental Geology, 53(3), 501–516.
Botté, S. E., Freije, R. H., & Marcovecchio, J. E. (2007). Dissolved heavy metal (Cd, Pb, Cr, Ni) concentrations in surface water and porewater from Bahía Blanca estuary tidal flats. Bulletin of Environmental Contamination and Toxicology, 79, 415–421.
Botté, S. E., Freije, R. H., & Marcovecchio, J. E. (2010). Distribution of several heavy metals in tidal flats sediments within Bahía Blanca estuary (Argentina). Water Air and Soil Pollution, 210, 371–388.
Botté, S. E., Marcovecchio, J. E., Fernández-Severini, M. D., Negrin, V. L., Panebianco, V., Simonetti, P., Buzzi, N. S., & Delucchi, F. (2013). Ciclo de metales (pp. 227–258). In: Procesos químicos en estuarios, Universidad Tecnológica Nacional.
Burton, G. A. (2002). Sediment quality criteria in use around the world. Limnology, 3(2), 65–76.
Cabrita, M. T. (2014). Phytoplankton community indicators of changes associated with dredging in the Tagus estuary (Portugal). Environmental Pollution, 191, 17–24.
Caetano, M., Madureira, M. J., & Vale, C. (2003). Metal remobilisation during resuspension of anoxic contaminated sediment: short-term laboratory study. Water Air and Soil Pollution, 143, 23–40.
Cifuentes, O., Escudero, D., Medus, S., Bohn, A., & Dosso, A. (2011). Estudio de la dinámica (espacial y temporal) de los efluentes líquidos industriales y urbanos en la zona del Polo Petroquímico y área portuaria de Bahía Blanca. Contaminación atmosférica e hídrica en Argentina. Puliefito (Ed). Universidad Tecnológica Nacional, 539–545.
Cifuentes, O., Escudero, D., Bohn, A., & Dosso, A. (2014). Estudio de la dinámica (espacial y temporal) de los efluentes líquidos industriales y urbanos en la zona del Polo Petroquímico y área portuaria de Bahía Blanca. Universidad Tecnológica Nacional, 2011, 165 pp.
Cole, S., Codling, I. D., Parr, W., & Zabel, T. (1999). Guidelines for managing water quality impacts within UK European marine sites. En: Hailey, N. and Burn, (Eds.), UK Marine SAC Project, 449 pp.
Conover, W. J. (1999). Practical nonparametric statistics (3rd ed.pp. 250–257). New York: Wiley.
CTE (Comite Tecnico Ejecutivo). (2003). Programa integral de monitoreo Bahía Blanca. http://www.bahiablanca.gov.ar/areas-de-gobierno/medioambiente/comite-tecnico-ejecutivo/
CTE (Comite Tecnico Ejecutivo). (2011). Programa integral de monitoreo Bahía Blanca. http://www.bahiablanca.gov.ar/areas-de-gobierno/medioambiente/comite-tecnico-ejecutivo/
CTE (Comite Tecnico Ejecutivo). (2012). Programa integral de monitoreo Bahía Blanca. http://www.bahiablanca.gov.ar/areas-de-gobierno/medioambiente/comite-tecnico-ejecutivo/
CTE (Comite Tecnico Ejecutivo). (2013). Programa integral de monitoreo Bahía Blanca. http://www.bahiablanca.gov.ar/areas-de-gobierno/medioambiente/comite-tecnico-ejecutivo/
CTE (Comite Tecnico Ejecutivo). (2014). Programa integral de monitoreo Bahía Blanca. http://www.bahiablanca.gov.ar/areas-de-gobierno/medioambiente/comite-tecnico-ejecutivo/
Cuadrado, D. G., Gomez, E. A., & Ginsberg, S. S. (2005). Tidal and longshore sediment transport associated to a coastal estructure. Estuarine, Coastal and Shelf Science, 62, 291–300.
De Souza Machado, A. A., Spencer, K., Kloas, W., Toffolon, M., & Zarfl, C. (2016). Metal fate and effects in estuaries: a review and conceptual model for better understanding of toxicity. Science of the Total Environment, 541, 268–281.
Diop, C., Dewaelé, D., Diop, M., Touré, A., Cabral, M., Cazier, F., Fall, M., Diouf, A., & Ouddane, B. (2014). Assessment of contamination, distribution and chemical speciation of trace metals in water column in the Dakar coast and the Saint Louis estuary from Senegal, West Africa. Marine Pollution Bulletin, 86(1), 539–546.
Doig, L. E., & Liber, K. (2007). Nickel speciation in the presence of different sources and fractions of dissolved organic matter. Ecotoxicology and Environmental Safety, 66, 169–177.
Duarte, B., Silva, G., Costa, J. L., Medeiros, J. P., Azeda, C., Sá, E., Metelo, I., Costa, M. J., & Caçador, I. (2014). Heavy metal distribution and partitioning in the vicinity of the discharge areas of Lisbon drainage basins (Tagus estuary, Portugal). Journal of Sea Research, 93, 101–111.
EPA, U. S. (2000). Guidance for data quality assessment. Practical methods for data analysis. Office of Environmental Information. EPA QA/G-9, QA00 Version Washington. In Dc.
Fernandez Severini, M. D., Botté, S. E., Hoffmeyer, M. S., & Marcovecchio, J. (2011). Lead concentrations in zooplankton, water, and particulate matter of a southwestern Atlantic temperate estuary (Argentina). Archives of Environmental Contamination and Toxicology, 61(2), 243–260.
Ferrer, L. D., Contardi, E., Andrade, S., Asteasuain, R., Pucci, A. E., & Marcovecchio, J. E. (2000). Environmental cadmium and lead concentrations in the Bahía Blanca estuary (Argentina): potential toxic effects of Cd and Pb on crab larvae. Oceanologia, 43, 493–504.
Ferrer, L. D., Andrade, S., Contardi, E., Asteasuain, R. O., & Marcovecchio, J. E. (2003). Copper and zinc concentrations in Bahía Blanca estuary (Argentina), and their acute lethal effects on larvae of the crab Chasmagnathus granulata. Chemical Speciation and Bioavailability, 15(1), 7–14.
Freije, R. H., Spetter, C. V., Marcovecchio, J., Popovich, C. A., Botté, S. E., Negrín, V. L., Arias, A., Delucchi, F., & Asteasuain, R. O. (2008). Water chemistry and nutrients of the Bahía Blanca estuary. In J. Baretta & M. Mateus (Eds.), Neves, R (pp. 241–254). Lisboa: Perspectives on integrated coastal zone management in South America.
Gao, X., Zhou, F., Chen, C. T. A., & Xing, Q. (2015). Trace metals in the suspended particulate matter of the Yellow River (Huanghe) estuary: concentrations, potential mobility, contamination assessment and the fluxes into the Bohai Sea. Continental Shelf Research, 104, 25–36.
Grecco, L. E., Gómez, E. A., Botté, S. E., Marcos, Á. O., Marcovecchio, J. E., & Cuadrado, D. G. (2011). Natural and anthropogenic heavy metals in estuarine cohesive sediments: geochemistry and bioavailability. Ocean Dynamics, 61(2), 285–293.
Guerra, R., Pasteris, A., & Ponti, M. (2009). Impacts of maintenance channel dredging in a northern Adriatic coastal lagoon. I: Effects on sediment properties, contamination and toxicity. Estuarine, Coastal and Shelf Science, 85, 134–142.
Guinder, V. A., Popovich, C. A., & Perillo, G. M. E. (2009). Particulate suspended matter concentrations in the Bahía Blanca estuary, Argentina: implication for the development of phytoplankton blooms. Estuarine, Coastal and Shelf Science, 85(1), 157–165.
Guinder, V. A., Popovich, C. A., & Perillo, G. M. E. (2012). Phytoplankton and physicochemical analysis on the water system of the temperate estuary in South America: Bahía Blanca estuary, Argentina. International Journal of Environmental Research, 6(2), 547–556.
Hedge, L., Knott, A., & Johnston, E. (2009). Dredging related metal bioaccumulation in oysters. Marine Pollution Bulletin, 58, 832–840.
INDEC. (2010). Instituto Nacional de Estadística y Censos, Argentina. http://www.indec.gov.ar.
Jonas, P. J. C., & Millward, G. E. (2010). Metals and nutrients in the Severn estuary and Bristol Channel: contemporary inputs and distributions. Marine Pollution Bulletin, 61(1), 52–67.
Jones, R. P., & Clarke, J. U. (2005). Analytical chemistry detection limits and the evaluation of dredged sediment, ERDC/TN EEDP-04-36, U.S. Vicksburg, MS: Army Engineer Research and Development Center.
La Colla, N. S., Negrin, V. L., Marcovecchio, J. E., & Botté, S. E. (2015). Dissolved and particulate metals dynamics in a human impacted estuary from the SW Atlantic. Estuarine, Coastal and Shelf Science, 166, 45–55.
La Colla, N. S., Botté, S. E., Oliva, A. L., & Marcovecchio, J. E. (2017). Tracing Cr, Pb, Fe and Mn occurrence in the Bahía Blanca estuary through commercial fish species. Chemosphere, 175, 286–293.
La Colla, N. S., Botté, S. E., & Marcovecchio, J. E. (2018). Metals in coastal zones impacted with urban and industrial wastes: Insights on the metal accumulation pattern in fish species. Journal of Marine Systems, 181, 53–62.
Liang, J., Fang, L., Wu, T., & Zhang, X. (2016). Characterization, distribution, and source analysis of metals and polycyclic aromatic hydrocarbons (PAHs) of atmospheric bulk deposition in shanghai, China. Water Air and Soil Pollution, 227, 1–14.
Limbozzi, F., & Leitào, T. E. (2008). Characterization of Bahía Blanca main existing pressures and their effects on the state indicators for surface and groundwater quality. En: Neves, R., Baretta, J., Mateus, M. (Eds.), Perspectives on integrated coastal zone management in South America, Lisboa, 315–331.
Marcovecchio, J. E., & Freije, R. H. (2004). Efectos de la intervención antrópica sobre sistemas marinos costeros: El estuario de Bahía Blanca. Anales de la Academia Nacional de Ciencias Exactas. Físicas Nat., 56, 115–132.
Marcovecchio, J., Botté, S., Fernandez Severini, M. D., & Delucchi, F. (2010). Geochemical control of heavy metal concentrations and distribution within Bahia Blanca estuary (Argentina). Aquatic Geochemica, 16(2), 251–266.
Mendiguchía, C., Moreno, C., & García-Vargas, M. (2007). Evaluation of natural and anthropogenic influences on the Guadalquivir River (Spain) by dissolved heavy metals and nutrients. Chemosphere, 69(10), 1509–1517.
Milazzo, A. D. D., Silva, A. C. M., De Oliveira, D. A. F., & Da Cruz, M. J. M. (2014). The influence of seasonality (dry and rainy) on the bioavailability and bioconcentration of metals in an estuarine zone. Estuarine, Coastal and Shelf Science, 149, 143–150.
Negrin, V. L., Botté, S. E., Pratolongo, P. D., Trilla, G. G., & Marcovecchio, J. E. (2016). Ecological processes and biogeochemical cycling in salt marshes: synthesis of studies in the Bahía Blanca estuary (Argentina). Hydrobiologia, 774(1), 217–235.
Pasternack, G. B., & Brown, K. J. (2006). Natural and anthropogenic geochemical signatures of floodplain and deltaic sedimentary strata, Sacramento-San Joaquin Delta, California, USA. Environmental Pollution, 141, 295–309.
Perillo, G. M. E., & Piccolo, M. C. (1999). Geomorphological and physical characteristics of the Bahía Blanca estuary, Argentina. En: Perillo, G.M.E., Piccolo, M.C., Pino-Quiriva, M. (Eds.), Estuaries of South America. Their geomorphology and dynamics, Berlin, 195–216.
Perillo, G. M., Pierini, J. O., Pérez, D. E., & Gómez, E. A. (2001). Suspended sediment circulation in semi-enclosed docks, Puerto Galván, Argentina. Terra et Aqua, 13–20.
Piccolo, M. C. (2008). Climatological features of the Bahía Blanca estuary. In: Neves, R., Baretta, J., Mateus, M. (Eds.), Perspectives on Integrated Coastal Zone Management in South America, Lisboa, 231–239.
Piccolo, M. C., & Perillo, G. M. (1990). Physical characteristics of the Bahía Blanca estuary (Argentina). Estuarine, Coastal and Shelf Science, 31(3), 303–317.
Piccolo, M. C., Perillo, G. M. E., & Melo, W. D. (2008). The Bahía Blanca estuary: an integrated overview of its geomorphology and dynamics. En: Neves, R., Baretta, J., Mateus, M. (Eds.), Perspectives on integrated coastal zone management in South America, Lisboa, 219–229.
Sadiq, M. (1992). Toxic metal chemistry in marine environments. New Yor: Marcel Dekker 390 pp.
Schnegelberger, M. A. (2014). Dragado de profundización del canal interior y antepuerto de los puertos Ingeniero White y Galván y ensanchamiento de su canal de vinculación. Buenos aires, Argentina: En: Resúmenes del VIII Congreso argentino de ingeniería portuaria.
Serra, A. V., Botté, S. E., Cuadrado, D. G., La Colla, N. S., & Negrin, V. L. (2017). Metals in tidal flats colonized by microbial mats within a south-American estuary (Argentina). Environmental Earth Science, 76(6), 254.
Simonetti, P., Botté, S. E., Fiori, S. M., & Marcovecchio, J. E. (2013). Burrowing crab (Neohelice granulata) as a potential bioindicator of heavy metals in the Bahía Blanca estuary, Argentina. Archives of Environmental Contamination and Toxicology, 64(1), 110–118.
Simonetti, P., Botté, S. E., & Marcovecchio, J. E. (2017). Occurrence and spatial distribution of metals in intertidal sediments of a temperate estuarine system (Bahía Blanca, Argentina). Environmental Earth Science, 76(18), 636.
Sznaiberg, L. (2012). Parques Industriales: Luz verde para producir futuro. Revista Informe Industrial N_ 233. http://www.informeindustrial.com.ar/.
Tornero, V., & Hanke, G. (2016). Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Marine Pollution Bulletin, 112(1–2), 17–38.
Vale, C., Ferreira, M., Micaelo, C., Caetano, M., Pereira, E., Madureira, M. J., & Ramalhosa, E. (1998). Mobility of contaminants in relation to dredging operations in a mesotidal estuary (Tagus estuary, Portugal). Water Science and Technology, 37(6–7), 25–31.
Van Ael, E., Blust, R., & Bervoets, L. (2017). Metals in the Scheldt estuary: from environmental concentrations to bioaccumulation. Environmental Pollution, 228, 82–91.
Verdonschot, P. F. M., Spears, B. M., Feld, C. K., Brucet, S., Keizer-Vlek, H., Borja, A., Elliott, M., Kernan, M., & Johnson, R. K. (2013). A comparative review of recovery processes in rivers, lakes, estuarine and coastal waters. Hydrobiologia, 704(1), 453–474.
Wickham, H. (2009). ggplot2: Elegant graphics for data analysis. Berlin: Springer Science & Business Media.
Yao, Q., Wang, X., Jian, H., Chen, H., & Yu, Z. (2016). Behavior of suspended particles in the Changjiang estuary: size distribution and trace metal contamination. Marine Pollution Bulletin, 103(1), 159–167.
Yin, S., Feng, C., Li, Y., Yin, L., & Shen, Z. (2015). Heavy metal pollution in the surface water of the Yangtze estuary: A 5-year follow-up study. Chemosphere, 138, 718–725.
Zar, J. H. (1996). Biostatistical analysis (third ed.). New Jersey, USA: Prentice Hall.
Acknowledgements
The authors are greatly indebted to F.E. Garcia and M.N. Chiarello for their help in sample collection and analyses. Authors are very grateful to Dr. C. Domini for her valuable collaboration in improving the preconcentration procedure. The assistance of G.S. Gutierrez in sample collection was also appreciated. N. S. La Colla has received a doctoral grant funded by the National Council of Scientific and Technological Research (CONICET-Argentina).
Funding
This study was funded through research grants by CONICET (PIP D-738 2011) and by Universidad Nacional del Sur (Secyt-UNS, PGI 24/ZB59).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
La Colla, N.S., Botté, S.E., Negrin, V.L. et al. Influence of human-induced pressures on dissolved and particulate metal concentrations in a South American estuary. Environ Monit Assess 190, 532 (2018). https://doi.org/10.1007/s10661-018-6930-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6930-x