Abstract
The European Water Framework Directive aims to improve ecological status within river basins. This requires knowledge of responses of aquatic assemblages to recovery processes that occur after measures have been taken to reduce major stressors. A systematic literature review comparatively assesses recovery measures across the four major water categories. The main drivers of degradation stem primarily from human population growth and increases in land use and water use changes. These drivers and pressures are the same in all four water categories: rivers, lakes, transitional and coastal waters. Few studies provide evidence of how ecological knowledge might enhance restoration success. Other major bottlenecks are the lack of data, effects mostly occur only in short-term and at local scale, the organism group(s) selected to assess recovery does not always provide the most appropriate response, the time lags of recovery are highly variable, and most restoration projects incorporate restoration of abiotic conditions and do not include abiotic extremes and biological processes. Restoration ecology is just emerging as a field in aquatic ecology and is a site, time and organism group-specific activity. It is therefore difficult to generalise. Despite the many studies only few provide evidence of how ecological knowledge might enhance restoration success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human population growth in Europe has led to industrialisation, agricultural intensification and urbanisation, which in turn have produced a large number of environmental pressures that impact the aquatic environment. Human-induced pressures (often also referred to as stressors) typically alter the environment in many ways. For example, ‘urbanisation’ affects water quantity and quality, discharge and thermal regimes, habitat availability and degradation, longitudinal connectivity, dispersal and establishment of invasive species (Paul & Meyer, 2001; Allan, 2004; Lotze, 2010; Stelzenmüller et al., 2010; Atkins et al., 2011). Furthermore, pressures from several sources often coincide and impose multiple stresses on aquatic ecosystems (Adams, 2005; Crain et al., 2008; Ormerod et al., 2010). As such, it is of value to separate the stressors into exogenic unmanaged pressures and endogenic managed pressures (Elliott, 2011). The former are those pressures over which local management has no control over their causes but which must respond to their consequences, such as climate change and isostatic rebound. Endogenic managed pressures are those operating inside the system to be managed and over which management can address the causes and consequences. The latter in turn can be separated into those which put materials into the system, for example waste water discharges, sediment erosion, alien and introduced species, infrastructure such as weirs and bridges and those which remove materials such as fishing, sediment, space and water.
In a natural state, aquatic ecosystems are largely controlled by geomorphic and physiographic factors such as geology, geomorphology and hydrology. Centuries of land use has, however, altered the geomorphology and hydrology of many aquatic systems (Solimini et al., 2006). These processes have resulted in altered connectivity, such as upstream–downstream, stream-riparian zone and land-coastal interactions (Boyes & Allen, 2007; Zaldivar et al., 2008; Dallas & Barnard, 2011). The processes affecting aquatic ecosystems operate at spatial scales, ranging from the regional (landscape) to the local (site) scale. Disentangling scale-related effects on aquatic ecosystems has proven difficult, although a number of variance partitioning studies have tried to elucidate local versus regional effects (e.g. Johnson et al., 2004; Kernan et al., 2009), and, indeed, understanding the scale-related interactions within entire catchments is fundamental for sound water management (Frissell et al., 1986; Allan & Johnson, 1997; Johnson et al., 2007).
There is a growing awareness that aquatic ecosystems nested in terrestrial environments are intrinsically connected with catchment land use/cover; nevertheless, restoration studies have largely ignored the impacts of pressures operating beyond the local scale and have focused mainly on the quantification and management of single pressures. Moreover, although there is consensus on the importance of restoration (e.g. Ormerod, 2003; Palmer, 2009), the effectiveness of management measures and understanding of factors confounding organism responses are largely lacking as a result of the traditional focus of restoration studies.
The Water Framework Directive (WFD; European Commission, 2000), Directive 2000/60/EC, was adopted in 2000 as a single piece of legislation covering groundwater and the four surface water categories: rivers, lakes, transitional (estuarine) and coastal waters. The WFD introduced several concepts to unify water management all over Europe. The WFD recognised the use of typology for partitioning natural variability and for establishing type-specific biological reference conditions. Ecological status represents the quality of the structure and functioning of aquatic ecosystems, according to normative definitions of the biological community associated with the High, Good, Moderate, Poor and Bad status classes. Member States are required to implement Programmes of Measures (POMs) in order that all surface water bodies achieve at least Good Ecological Status (GES), by 2015 or within a prescribed time table. The status of the biological community is measured in metric values for Biological Quality Elements (BQEs) that are compared to the values for the reference condition. The values are standardised and termed Ecological Quality Ratio (EQR). The division of the value range of an EQR into classes provides the mechanism for categorising the ecological status (Furse et al., 2006).
The WFD aims to expand the traditional approach to environmental management by combining a catchment-scale understanding across a range of aquatic ecosystems to improve ecological status within entire river basins (Hering et al., 2010). This requires an assessment of the ecological responses of aquatic assemblages to major (multiple) stressors, such as eutrophication, hydromorphological change and acidification, and their interactions within and across lakes, rivers and transitional (e.g. estuaries and lagoons) and coastal waters (EU, 2000).
The European WISER project (Water bodies in Europe: Integrative Systems to assess Ecological status and Recovery: www.wiser.eu; see also Hering et al., 2012) aimed at gathering the knowledge required to support catchment-wide integrated river basin management (RBM). Understanding important cause–effect relationships, i.e. the relationships between the causes of environmental status change and their effect (impact) on the aquatic environment and biota is an important part of RBM planning. Furthermore, this requirement applies to both cause–effect relationships of degradation as well as recovery after restoration. This requires knowledge on driver–pressure–state–impact–response–recovery chains (i.e. the DPSIR framework according to European Environment Agency (1995) and Smeets and Weterings (1999)); expanded by a Recovery element in WISER to the DPSIRR framework by Feld et al. (2011) across rivers, lakes and estuarine and coastal waters and their different taxa groups. Feld et al. (2011) differentiated between Response as the response of the abiotic conditions to the Impact (often restoration measures) and Recovery as the success of the indicators to the Impact and environmental Response.
The DPSIRR framework illustrates the conceptual chain of causal links, starting with ‘driving forces’ (e.g. economic growth, human demand for food) through ‘pressures’ (emissions, waste disposal) to ‘states’ (physical habitat, chemical status) and ‘impacts’ on ecosystems, human health and functions, eliciting political and societal ‘responses’ (waste and waste water treatment, environmental regulations) that will, it is hoped, result in biological ‘recovery’ (improvement of ecological functions and processes, recolonisation). More recently, the DPSIR philosophy described above has been modified such that S implies State change (changes in the natural system) and the Impact refers only to those consequences for the human system (Atkins et al., 2011). It is of note that in a recent EU project, KNOWSEAS, the I has been replaced by W for welfare thus emphasising the human dimension (Laurence Mee, pers. comm.). Although a few case studies exist for which the DPSIRR chain can be described for specific ecosystems and pressures, to date, no comprehensive assessment across rivers, lakes, estuarine and coastal waters and considering all organism groups included in the WFD (fish, macrophytes, microalgae, and macroinvertebrates) has been conducted. Instead, the DPSIRR chain is commonly reported for the driver-pressure-state-impact (the ‘degradation chain’) or the response-recovery (‘restoration chain’) relationships. Although the ‘degradation chain’ is well described for major pressures, such as eutrophication (Zaldivar et al., 2008; Nixon, 2009), acidification (Schindler, 1988; Driscoll et al., 2001), hydromorphological degradation (CIS, 2006) and oxygen depletion (Diaz & Rosenberg, 2008; Rabalais et al., 2010; Zhang et al., 2010), empirical analysis of the different parts of the ‘recovery’-chain is rarely achieved, despite consensus of its importance (e.g. Ormerod, 2003; Palmer et al., 2005; Elliott et al., 2007; Borja et al., 2010; Feld et al., 2011).
We conducted a systematic literature review to comparatively assess recovery measures, effectiveness, successes and failures, underlying processes, confounding factors and organism group responses across rivers, lakes, estuaries and coastal waters. Meta-data were extracted from available publications to: (1) identify the main pressure scenarios, management measures, recovery time scales and knowledge gaps associated with each ecosystem; (2) identify common pressures resulting in shifting ecological baselines and thresholds and (3) identify processes and interactions that may confound or delay the recovery process.
Methods
For the comparison of restoration and recovery among and across water categories and organism groups, a number of parameters were extracted from the literature using ISI Web of Knowledge, SCOPUS and Google Scholar. Key items in the literature searches were: water category (river, lake, transitional/estuarine and coastal water), stressor (eutrophication, acidification, hydromorphological change, global/climate/land use change), recovery (restoration, rehabilitation, habitat or trajectory improvement, long-term effect), organism group (algae/phytoplankton/diatoms, macrophyte, zooplankton, macroinvertebrate, fish) and method (before/after, control/impact, space-for-time substitution, time series).
For each water category, a general review of all publications was combined with a more targeted review of a subset of publications. Meta-data were extracted from the subsets and used to prepare comparable data sets based on the extraction scheme in Table 1. For rivers, 370 papers were reviewed, and 168 papers were analysed in more detail by Feld et al. (2011). For eutrophication management in lakes, 364 peer reviewed publications representing 743 lake-equivalent recovery case studies (LECs) were reviewed (Spears et al., 2011) with a subset of 43 LECs from 30 peer reviewed publications being assessed in more detail. For recovery from acidification, 81 papers were reviewed including studies focusing on lakes, streams and datasets combining both. A more detailed review was undertaken on 30 of these focusing specifically on biological recovery. For estuarine and coastal waters the review of 51 studies by Borja et al. (2010) was the major information source. No distinction was made between overall and detailed selection of publications because of the large variety in pressures and studies. The information from the reviews was collated and used to provide meta-assessments of the different restoration measures studied (Fig. 1; Table 2), evaluation techniques used (Fig. 2), targeted responses (Fig. 3), organism groups examined (Fig. 4) and recovery periods observed. The results section summarises the main outcomes of the reviews structured according to the key concepts underpinning this assessment of ecosystem restoration and recovery across different water body types:
-
(i)
conceptual restoration frameworks,
-
(ii)
comparison of degradation chains,
-
(iii)
comparison of restoration measures,
-
(iv)
data availability and analytical methods,
-
(v)
time lines of recovery,
-
(vi)
recovery of different organism groups,
-
(vii)
recovery time,
-
(viii)
recovery failure or delay,
-
(ix)
shifting baselines,
-
(x)
effect of biological interactions and
-
(xi)
impacts of global change on recovery.
Results
Conceptual models on ‘restoration chains’ across water categories
Feld et al. (2011) applied the DPSIRR chain as a conceptual framework for their analysis of the effects of restoration on biological recovery of river systems as reported in the literature. Although these authors did not find empirical studies covering all parts of the DPSIRR chain, they argued that the framework allowed for a structured review and comparison of the findings reported in the literature. For instance, well-documented relationships between single components of the DPSIR framework may help inform practitioners about expected effects of restoration on recovery. Likewise, poorly documented linkages in the framework clearly point at knowledge gaps and may help identify important research needs. Similarly, some applications of the DPSIR approach to marine waters have been undertaken recently (e.g. Elliott, 2002; Borja et al., 2006; Atkins et al., 2011). For example, DPSIR analysis has been demonstrated as a useful approach for assessing the risk of failing to achieve WFD objectives in a case study in the Basque Country (Borja et al., 2006). For lakes some provisional conceptual models on recovery were available with chain fragments mainly focussing on eutrophication. Elliott et al. (2007) indicated the response chain by describing the use of habitat creation schemes to allow recovery from physical modification of estuaries, for example by port expansion, in itself the reversal of hydrophysical modifications carried out in estuaries since 1700 (see also Lotze, 2010).
Comparison of ‘degradation chains’ across water categories
Globally, the magnitude and spatial extent of human alteration of land cover continues to increase (Turner et al., 1990, Lambin et al., 1999; Lambin & Geist, 2006). However, the control of primary (e.g. population growth) and secondary (e.g. agricultural practices, industrial, urban and rural development) drivers is often not the focus of specific water management programmes, although these programmes have to respond to those drivers even if they have no control over them. This is most likely due to socioeconomic interests at the national and catchment scales and due to the practical difficulties in assessing the impacts of regional and global scale drivers on local scale pressures and ecosystem impacts. More commonly, the literature suggests that the impacts of specific pressures were more often reported, such as atmospheric deposition, pollutant run-off, point source discharges, physical alteration, biomanipulation and the ingress of non-native invasive species (Scott & Helfman, 2001; Pirrone et al., 2005; Palmer et al., 2010).
Aquatic ecosystems are often simultaneously affected by multiple pressures (Allan, 2004, Withers & Haygarth, 2007). For example, in freshwaters a decrease in pH and an increase in ammonium concentrations are associated with acid deposition, phosphorus and nitrogen commonly both increase as a result of fertiliser run-off (Hart et al., 2003) and the reduction of stream velocity coupled with an increase in siltation rate are associated with river canalisation (Meyer et al., 1999). However, pressures are often water category-specific. In general, rivers integrate the adverse effects of various human activities and associated pressures within a catchment, with hydromorphological degradation predominating (Feld, 2004), lake ecosystems are mainly affected by eutrophication and shoreline modification (at the global scale) and acidification (at the regional scale) (Søndergaard et al., 2007), while estuaries and coastal waters comprise the ultimate sink for nutrients, contaminants and other sources of pollution originating from entire river basins (Cloern, 2001; Diaz & Rosenberg, 2008) and are being physically modified (e.g. Pollard & Hannan, 1994; van der Wal et al., 2002). By contrast, the driver–pressures–stressor relations are comparable between different water categories (Fig. 5).
A notable finding of our study was the common hierarchy for all four water categories regarding scale of degradation from global and supra-regional scale of primary and secondary drivers from population growth and climate change, respectively. For example, at the catchment level, pressures such as land use, urbanisation and industrial development and run-off, alterations in riparian zones, longitudinal profiles and substance flows were often the main drivers again for all four water categories. Likewise, at the local scale (e.g. a single lake or river stretch), pressures and processes were similar to those found at the catchment scale, but organism composition differed. Commonalities among water categories imply that legislative decisions and management covering broad spatial scales will affect a wide range of water categories. Such measures tackle the problems at their core source. At the intermediate (catchment) level both source and effects-oriented measures, such as regional legislation and the creation of large buffer strips can be effective. Measures at this intermediate scale mostly deal with external pressures impacting the aquatic ecosystem. At the finest (local) scale effects-oriented measures are the most effective. Such measures are taken within the aquatic environment and are as such labelled internal measures, hence the importance of distinctions between exogenic unmanaged and endogenic managed pressures (Elliott, 2011).
Comparison of restoration measures across water categories
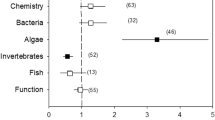
Applying the conceptual framework to lakes revealed a major focus on the reduction of and recovery from eutrophication (e.g. Andersson, 1979; Schindler, 2006). About 83% of the lake studies reviewed were concerned with this pressure and reported nutrient reduction as the main measure (Gulati & Van Donk, 2002; Jeppesen et al., 2005; Ibelings et al., 2007; Søndergaard et al., 2007). Only 46 equivalent lake studies reported additional secondary measures (Fig. 2). These 46 studies often related to biomanipulation (Gulati et al., 1990) and to the reduction of external and internal nutrient loads (Gulati et al., 2008). For acidification, liming the lake water directly or increasing the buffering capacity of surrounding soils along with international agreements on decreasing emission of acidic N and S compounds are the most common forms of restoration (Henrikson & Brodin, 1995; Brouwer et al., 2002). Most of the acidification recovery studies focusing on lakes were concerned with ‘natural’ recovery following reduced acid deposition (66%) (e.g. Gunn & Keller, 1990; Monteith et al., 2005) rather than liming (e.g. Gunn, et al., 1990; Blomqvist et al., 1995).
In contrast to lakes, river studies mainly focussed on three different kinds of restoration measures: habitat improvement, riparian buffer creation and weir removal. However, this may be an artefact of the study design in that the focus was on hydromorphology. Nevertheless, physical habitat-related improvements in rivers were most commonly reported, with explicit measures to enhance habitat (e.g. additions of wood and boulders) comprising nearly half of the reviewed studies (Roni & Quinn, 2001; Kail et al., 2007; Baillie et al., 2008; Feld et al., 2011). Improvement of water quality using riparian buffer strips was primarily aimed at mitigating the adverse impacts of intensive agricultural land use adjacent to streams and rivers (Castelle et al., 1994; Parkyn et al., 2005; Davies-Colley et al., 2009). Restoring the connectivity of large rivers was mainly used to improve migration potential between systems (Gregory et al., 2002; Doyle et al., 2005). Finally, recovery was also, and in more detail, studied after natural catastrophic events like high flows (e.g. Fisher et al., 1982; Lamberti et al., 1991).
For marine environments, measures rarely addressed direct effects on estuarine and coastal waters, but more often related to legislation on nutrient reduction in the major river basins (Borja et al., 2010). Stressors in the marine and estuarine environment are many and include hydromorphological and sediment barriers (e.g. dams), toxic chemical pollutants, excess nutrient inputs, hypoxia, turbidity, suspended sediments, introduced and alien species and over fishing (e.g. Russell et al., 1983; Hawkins et al., 1999; McLusky & Elliott, 2004; Crain et al., 2008). Physical change, elevated nutrient levels and organic load are the most common pressures currently addressed in the reviewed literature, with restoration measures focusing on the removal of barriers, the restoration of water flow and salinity balance, the creation of habitats and the reduction of nutrient load (Borja et al., 2010).
All observations on measures implemented to date show that elevated nutrient levels are the main drivers affecting the biodiversity of lakes, and (in the short-term) estuarine and coastal systems, while hydromorphological stress, affecting habitat availability, is more important in rivers and, in the long-term, estuarine waters (notably through land-claim and associated hydromorphological change). Regionally, the consequences of acidification of rivers and lakes in areas of poorly buffered soils and geology remain a concern. Another surprising outcome of the literature review was the paucity of information available on the importance of other stressors, despite a general consensus that secondary stressors can easily hamper full recovery. Furthermore, it became clear that land use strongly affected rivers, and as rivers transport nutrients to lakes, estuaries and coastal waters there is a direct link between all water categories. For example, the nutrient reduction in a river over 20 years led to an increase of water transparency and overall improvement of water quality in the lower part of the river, its estuary and coastal lagoons (Ibanez et al., 2008). That nutrient loads are not the main focus of many river or lake restoration schemes underlines the importance of multiple stressors and highlights a gap in best management practices, the need to address the connectivity and interactions among water categories situated at lower elevations (Spears et al., 2007, 2011).
Similarly, the essence of estuarine functioning lies in the maintenance of connectivity and the satisfactory ecological functioning of the adjacent freshwater and marine areas across notable ecotones (Elliott & Whitfield, 2011; Whitfield et al., 2012; Basset et al., in press). Hence, the maintenance and protection of connectivity is the dominant protection measure in estuaries and the restoration of systems from habitat loss becomes paramount. Restoration of permanent (e.g. land-claim) and temporary (e.g. water quality barriers) habitat loss therefore is detailed in many studies (e.g. Russell et al., 1983; Hawkins et al., 1999; Elliott et al., 2007). The uses of habitat creation schemes, such as managed realignment/depolderisation, to compensate for the previous loss of wetlands due to industrialisation and agricultural use, and of water purification schemes, to allow recovery from hypoxia caused by organic enrichment, are thus proving successful in restoring damaged estuarine ecosystems (Elliott et al., 2007; Luisetti et al., 2011).
Assessing data availability and statistical approaches across water categories and organism groups
Substantially more monitoring data are available for rivers and lakes (e.g. Bernhardt et al., 2005) than estuarine and coastal waters (Borja et al., 2006). However, despite the wealth of monitoring programmes focused on rehabilitating lotic systems, most studies are designed to address local conditions and single pressures. For example, Bernhardt et al. (2005) stated that of 37,000 river restoration projects in the United States, only 10% included some form of monitoring, and the authors argued that the information was often inadequate to evaluate successes and failures. Similarly, an overview of 16 European papers on river restoration by Reitberger et al. (2010) showed that none of the studies used time series analysis to monitor restoration. This emphasises the need for high quality monitoring data to properly evaluate the efficacy of restoration effort and to make generalisations and improvements which might increase the frequency of successes. Poor availability of data can be due to several reasons. Firstly, an overwhelming majority of restoration measures have not included monitoring, probably because there is no legal requirement. Secondly, when restoration measures are monitored, the methods and time scales applied are often inadequate considering knowledge of recovery time lags. Thirdly, most water authorities do not focus on long-term or whole-system ecological processes, but strive for rapid results, with little or no interest in properly evaluating the outcome. For example, in estuarine restoration studies, often as compensation schemes for port developments or coastal squeeze due to sea-level rise or isostatic rebound, it has been questioned whether the schemes are ‘good for the ecology or good for the ecologist’, i.e. while they have produced benefits for the ecology, for human safety (by allowing storage under storm surges) and for the economy (by reducing the need for elevated dykes), they have often been carried out ‘where they can be rather than where they should be’ (Elliott et al., 2007). Fourthly, studying recovery processes seems to have been given low priority in science.
A Before–After Control–Impact Paired Series (BACI-PS) monitoring design is considered the best approach for monitoring recovery (Smith et al., 1993; Gray & Elliott, 2009), as only this approach is capable of distinguishing the effects of restoration and separate them from other factors, such as seasonal or annual variability. Surprisingly, the BACI design is primarily applied to experimental studies (Fig. 3), while restoration monitoring usually, at best, follows a Before–After sampling design (Skilleter et al., 2006; Whomersley et al., 2007; Gee et al., 2010). Long-term time series data, commonly available for lakes (e.g. Søndergaard et al., 2007; Johnson & Angeler, 2010; May & Spears, 2012a) are usually lacking for rivers and even more so for marine systems (Stein & Cadien, 2009; Borja et al., 2010). For lakes, monitoring programmes typically do not encompass the pre-impact period, both for eutrophication and acidification; although there are a few notable exceptions (e.g. Jeppesen et al., 2005; Maberly & Elliott, 2012; May & Spears, 2012a). One way of assessing ecological degradation and recovery for lake ecosystems that have not been comprehensively monitored is through palaeoecology (Bennion et al., 2011). Here, analysis of sub-fossil organisms remains in the sediment record can track species composition for various organism groups (e.g. diatoms, cladocera, macrophytes) over time identifying changes in assemblages before impact, during the degradation phase, and, following restoration efforts, during the recovery trajectory, the start of which often predates monitoring in many studies (Salgado et al., 2010; Dong et al., in press). Only from monitoring of biological and environmental changes after restoration can new knowledge on recovery processes be gained and implemented. Indeed, this information provides the opportunity for practitioners and scientists to evaluate the success and efficacy of the restoration measures (May & Spears, 2012b). Restoration monitoring requires a tailor-made sampling design (preferably a BACI design) that allows sound statistical analysis according to state-of-the-art methods. Furthermore, given that the science of monitoring change is now well-established and the criteria for successful and effective monitoring, such as the 18 features indicated by Elliott (2011), and the detection of signal-to-noise relationships are well understood, then effective monitoring schemes can be devised and implemented.
Comparing time lines of recovery across water categories
In rivers, environmental improvement (a positive response) was reported in 33% of the restoration projects evaluated, with a positive biological response was reported in 50% of the projects. By contrast, for lakes restoration successes for both eutrophication and acidification were higher. For eutrophication, in total 66% of the restoration projects showed positive responses (reduction) for phosphorus and/or nitrogen and related environmental parameters, and 64% reported positive biological responses. Similarly, lake liming has been shown to be effective in restoring water chemistry to pre-disturbed conditions following acidification, and positive biological responses are frequently observed (Henrikson & Brodin, 1995). However, some studies have shown that community changes following liming are not stable and that liming is suitable for partial remediation of acidification impacts rather than producing long-term ecosystem recovery (Angeler & Goedkoop, 2010). Most studies reported some biological improvements following reductions in acid deposition but this still lags behind chemical recovery (Schindler, 2001; Stendera & Johnson, 2008a, b) and none suggest a return to assemblages found prior to the onset of acidification, where these can be inferred from the palaeolimnological record or using analogues (e.g. Kernan et al., 2010a). For estuarine and coastal waters, most of the studies included in Borja et al. (2010) showed some sign of recovery. However, pre-disturbance data were often lacking, confounding evaluation of success. Some estuarine and coastal ecosystems did not fulfil the technical definition of being restored, instead reaching an alternative state (Duarte et al., 2009, Borja et al., 2010). For example, Duarte et al. (2009) did not observe recovery of simple biological variables (such as chlorophyll a concentration) following the assumed reduction of nutrient loads in four well-studied coastal ecosystems over two decades.
Successful restoration is hard to define as ‘end points’ and goals are often vaguely described and usually not defined in quantitative terms before the restoration commences (Elliott et al., 2007). Despite this, for each of the water categories, studies were available that showed one or more indication(s) of restoration successes (Fig. 4), although it is apparent from these studies is that ecological recovery takes time, can be delayed or even can fail (e.g. Lake, 2001; Bond & Lake, 2003; Spears et al., 2011).
Recovery of different organism groups
Most restoration studies in rivers and in estuarine and coastal waters have focused on benthic invertebrates (Fig. 5) (Matthews et al., 2010; Feld et al., 2011; Borja et al., 2010). This is expected given the value of using a sedentary component and also because of the basic understanding of the structure and dynamics of this taxonomic assemblage. In rivers, fish are also often used as indicators (Karr, 1981; Karr & Chu, 1999) and in estuaries, for example, perhaps the best known (and of highest public awareness) restoration case is the recovery of the fish community in the Thames Estuary (McLusky & Elliott, 2004). In lakes, phytoplankton has been the focus of most eutrophication studies (Spears et al., 2011), whereas zooplankton assemblages are often the focus of acidification research (Yan et al., 2003). The highly dynamic nature and transient nature of estuarine plankton populations, together with the effects of natural physical characteristics such as turbidity, make them a less useful restoration focus (e.g. Gunn & Keller, 1990; Locke & Sprules, 1994). In essence, their high variability confounds the signal-to-noise relationship in estuarine restoration studies. Differences in the selection of indicator group(s) used to track recovery are generally linked to putative pressures. In lakes, eutrophication is the most important pressure, and phytoplankton assemblages have been shown to reflect changes in nutrient status. In acid-sensitive regions diatoms are highly sensitive to changes in pH and have been used to highlight and monitor change in acidified and recovering lakes (Battarbee et al., 2008). In rivers, most degradation is associated with hydromorphological change and in estuarine and coastal waters macroinvertebrates and fish respond strongly to these types of changes (e.g. Borja et al., 2009; Uriarte & Borja, 2009). The confounding factor in estuarine and coastal waters for phytoplankton is water movement, which reduces its indicative value.
Recovery time
Restoration and recovery ecology has often focussed on the meanings of the relevant terms and has produced relevant and applicable conceptual models giving the trajectories both of degradation and of recovery (e.g. see Elliott et al. (2007) for both the definitions and the models for estuarine and coastal waters). The term recovery implies that a system will return to a previous condition after being in a degraded or disrupted one, which is often interpreted as being in poor ecological health. This condition can be evaluated and communicated in different terms, depending upon the questions being asked. The studies can examine fundamental ecological processes; they can seek to examine community function, possibly in response to human activities or they can seek to inform questions on how various ecosystem services are affected by human and ecosystem interactions. Long-term studies of recovery in rivers, lakes and estuarine and coastal waters are scarce (Fig. 6). One important question before comparing timespans of recovery between water categories is the definition of ‘full recovery’. ‘Full recovery’ refers to an optimal functioning of the aquatic ecosystem under the given environmental circumstances that are not or only slightly changed by human activity. Literature for both riverine and marine systems addresses this issue, while for many lakes in lowland areas focus is more on a shift from turbid to clear water states. Monitoring for a large proportion of studies was <5–10 years (Fig. 5), and only a few studies (one each) in rivers and estuarine and coastal waters extended >20 years.
Large discrepancies exist between the length of monitoring programmes and the time needed for the ecosystem to reach ‘full recovery’ and although most studies do not address ‘full recovery’, some estimates are available (Table 1). Bednarek (2001) suggested recovery after weir removal may take as long as 80 years. Recovery after riparian buffer instalment may take at least 30–40 years (Jowett et al., 2009). In lakes, time for recovery from eutrophication varies from 10 to 20 years for macroinvertebrates, 2 to >40 years for macrophytes and 2 to >10 years for fish. Natural recovery from acidification takes much longer compared to recovery after liming, and like eutrophication, biological recovery is taxon-specific and often decades are needed to achieve pre-disturbed conditions. Estuarine and coastal waters have long periods of recovery (>10 years) (Borja et al., 2010), although macroinvertebrates have the potential to recover within months to <5 years (Bolam et al., 2006; Borja et al., 2009) though mostly take >6 years (Hiddink et al., 2006; Diaz et al., 2008; Stein & Cadien, 2009). Fish recover within 1–3 years (Able et al., 2008; Uriarte & Borja, 2009), depending on the type and intensity of pressure. In general, after intense and large pressures, periods of 15–25 years for attainment of the original biotic composition, diversity and complete functioning may be needed in all four water categories (Jowett et al., 2009; Borja et al., 2010; Spears et al., 2011).
In both rivers and lakes the success rate of restoration measures appears to be much higher for the abiotic conditions than for the biotic indicators and this is particularly so for hydromorphological restoration and liming. Since eutrophication is also considered to be an important pressure in rivers and lakes, this might be a major factor hampering recovery. In lakes internal nutrient loading often delays recovery. For rivers the evidence for macroinvertebrate recovery following hydromorphological restoration is equivocal; some studies have shown recovery while other studies do not, possibly due to the nutrient levels remaining high (Bond & Lake, 2003). Restoration of lakes by biomanipulation (in particular fish removal) and liming also showed only short-term recovery (e.g. Søndergaard et al., 2007). Fish removal in shallow eutrophic lakes, has been shown to have short-term effects on lake water quality, transparency and chlorophyll a in many lakes in the Netherlands and Denmark. By contrast, long-term effects (>8–10 years) are less obvious and a return to turbid conditions often occurs unless fish removal is repeated (Søndergaard et al., 2007). The same constraints apply to the mitigation of acidification by liming (Henrikson & Brodin, 1995).
Recovery failure or delay
In most restoration projects measures are taken to reduce the primary stressor, but secondary stressors often confound recovery. Confounding factors such as water quality, with particular emphasis on nutrient enrichment (e.g. Pretty et al., 2003), large scale hydrological change such as floods and droughts (e.g. Beechie et al., 2010) and catchment management/land use practices (e.g. Larson et al., 2001; Levell & Chang, 2008) and multiple pressures (Schindler, 2006; Borja et al., 2006), presence/absence of neighbouring source populations and dispersal barriers (e.g. Shields et al., 1995, 2006) and project size (Feld et al., 2011) cause delays or failures in aquatic system recovery (Lake, 2001; Walsh et al., 2005; Jähnig et al., 2010). Recovery has not necessarily failed, but the presence of secondary pressures may have pushed response times beyond those over which monitoring is typically performed (Bond & Lake, 2003). Acidification (Blomqvist et al., 1993), fisheries management (Carpenter & Kitchell, 1996), industrial pollution (Borja et al., 2006), non-native species (e.g. Manchester & Bullock, 2000; Matsuzaki et al., 2009) and climate change (Kernan et al., 2010a, b) were the main secondary pressures impacting de-eutrophication projects in aquatic systems (May & Carvalho, 2010). In particular, internal P loading impedes recovery in many eutrophic lakes (Søndergaard et al., 2007). Schindler (2006) reviewed a range of factors known to confound the recovery of lakes from eutrophication and stressed the need for better understanding of multiple pressures and identified the following secondary pressures as being of particular importance: (1) the aggravation of eutrophication by climate warming, (2) the overexploitation of piscivorous fishes and (3) changes in silica supply from the catchment as a result of climate change. A review of 81 papers from the peer reviewed literature included an examination of the factors hindering or preventing recovery from acidification. Most papers reported that chemical recovery had taken place following deposition reductions although there were exceptions. A lack of chemical recovery was ascribed to insufficient reduction of sulphur deposition, the effects of nitrogen deposition, soil acidification and increases dissolved organic carbon (one paper in each case) with two papers each highlighting the acid episodes and failure of liming measures. In many more cases limited or no biological recovery was reported. Abiotic constraints included the effects of nitrogen, acid episodes, toxic metals, UV, site characteristics, increases in DOC, climate change and calcium response. Biotic constraints were also highlighted including community closure, recolonisation, decoupled food-webs, functional shifts, within-species adaptation, absence of fish predation, stable simplified food-webs and competitive resistance. In estuarine and coastal waters recovery is often confounded by contaminants that can be released back into solution when they come in contact with toxic water (after reducing eutrophication or organic pollution), causing toxic effects in the biota (Calmano et al., 1993; Trannum et al., 2004; Borja et al., 2006). Furthermore, ecosystem characteristics account for differences in the response of chlorophyll a to changing nitrogen conditions and can determine the outcome of restoration efforts in estuarine and coastal waters (Carstensen et al., 2011).
Shifting baselines in recovery
Shifting baselines imply that the present state of the system is not an adequate reference with which to evaluate the effectiveness of restoration effort, as the future status of the ecosystem would differ from that at the present under a ‘do nothing’ scenario (Andersen et al., 2009). In our literature review, we found few references to shifting baselines or (quantified) thresholds (e.g. Ibelings et al., 2007). Even in lakes, where these concepts originated, few studies specifically addressed response trajectories. One exception is the suggestion of Carstensen et al. (2011) that shifting baselines, result from global change, may explain the reported failure to restore eutrophic coastal ecosystems to their previous state following reduction of nutrient inputs as reported by Duarte et al. (2009). Johnson & Angeler (2010) tracked recovery from acidification and included among-year variability of reference sites, while recent data from the UK Acid Waters Monitoring Network show how the recovery trajectories in some lakes are not tracking back towards the species communities found at the equivalent stage of the degradation phase but towards new assemblages not previously identified in the sediment record (Kernan et al., 2010a).
In addition to the influence and confounding effects of moving baselines through climate change, there is the need to acknowledge that in any event the trajectories of degradation and recovery are seldom similar. These pathways may differ thus giving rise to the concept of hysteresis, as a type of memory in the system, in which the status reached after recovery from the removal of a stressor may differ from the original (pre-stressor) situation (Scheffer, 2001; Elliott et al., 2007; Borja et al., 2010). The ability to recover from that stressor thus being termed resilience in the system and again the fundamental properties of recovery potential and resilience will depend on the nature of the communities available for recolonisation. For example, the ability of estuarine communities to withstand and recover from a stressor is greatly influenced by their high ability to withstand the natural stressors and high variability in transitional waters (Elliott & Whitfield, 2011).
Effects of biological interactions on recovery
Although restoring the appropriate abiotic (physicochemical) habitat is still the main focus of many restoration efforts, there is a growing awareness that biological factors should be considered in freshwater restoration (Bond & Lake, 2003; Jansson et al., 2007). Indeed, several, more or less connected, issues are often emphasised, such as incorporating:
-
(1)
the spatial and temporal scale (i.e. maximum and minimum) of the habitat and the connectivity between the various habitat patches, including both abiotic and biotic components (e.g. Wiens, 2002; Lake et al., 2007; Palmer, 2009; Elliott & Whitfield, 2011);
-
(2)
knowledge of source populations and dispersal ability or constraints in predicting restoration outcome (Havel & Medley, 2006; Lotze et al., 2011). However, few studies attempt to match this ecological background with empirical data, as was done by Blakely et al. (2006).
-
(3)
mitigating measures to prevent non-native species to colonise and set priority effects (e.g. Schreiber et al., 2002; D’Antonio & Meyerson, 2002; van Riel et al., 2006).
Impacts of climate/global change on recovery
Observed climate change over the last century is influencing aquatic ecosystems in many ways (e.g. Arnell, 1999; Räisänen et al., 2003), affecting ecosystems directly as well as indirectly through societal and economic systems, such as agricultural practices and land use (EEA, 2004; Solomon et al., 2007). In many cases, climate change is an additional stress factor (e.g. Straile et al., 2003). The direct effects of climate change on ecosystems impact the performance of individuals at various stages in their life history cycle via changes in physiology, morphology and behaviour. Climate impacts also occur at the population level via changes in transport processes that influence dispersal and recruitment. Community-level effects are mediated by interacting species (e.g. predators, competitors), and include climate-driven changes in both the abundance and the per capita interaction strength of these species. The proximate ecological effects of climate change thus include shifts in the performance of individuals, the dynamics of populations and the structure of communities. Combined, these proximate effects lead to emergent patterns, such as changes in species distributions, biodiversity, productivity and microevolutionary processes (Harley et al., 2006; Thackeray et al., 2010). Changes in species distributions and massive invasions by non-indigenous species and pathogens introduce an extra problem for recovery (e.g. priority effects, competitive displacement, food web changes or disease epidemics; Mack et al., 2000).
A range of management practices and extreme weather events (Gasith & Resh, 1999; Havens et al., 2001; Noges et al., 2010) were identified as key factors responsible for slowing down or counteracting recovery processes (Fisher et al., 1982; Jeppesen et al., 2005). In contrast, the loss of dissolved nitrogen (N) through denitrification and biological uptake, leading to a switch from P- to N-limitation of primary production in summer/autumn, was identified as a potential recovery enhancing process due to warming in lakes (van Donk et al., 1993; Weyhenmeyer et al., 2008). Alterations in nutrient concentrations and biogeochemical cycling at the sediment–water interface, following nutrient management, can influence the magnitude and timing of nutrient delivery to downstream ecosystems (Spears et al., 2011). This phenomenon is likely to be highly sensitive to changes in local weather conditions associated with climate change (Spears et al., 2012). As stable populations and intact communities appear to be more resilient to climatic disturbances, such protective measures may help to minimise the risk of population collapses, community disruption and biodiversity loss (Hughes et al., 2003).
Concluding Comments and Summary
The main drivers of eutrophication, acidification and hydromorphological degradation in rivers, lakes, estuarine and coastal waters stem primarily from human population growth and increases in urbanisation (changes in flows of water run-off and of nutrients and other substances), industrialisation (air pollution/acidification and flows of substances), land use (agricultural intensification affecting flows of water, landscape morphology and run-off of substances) and water use changes (e.g. drinking water, recreation). There is a common agreement that drivers and pressures in general are the same in lakes, rivers, estuarine and coastal waters. From the reviews here, it is, however, clear that eutrophication and acidification have received the most attention in lake studies, hydromorphological changes were the focus of river studies and recovery studies in estuarine and coastal marine waters were limited and diverse in drivers and pressures studied. A comparison of concepts and models used in both degradation and restoration studies clearly revealed that processes following restoration do not mirror those during degradation, i.e. the trajectories of degradation and recovery differ, will indicate hysteresis in the system and may result in a different overall status for a water body.
Although many studies provide theoretical frameworks, guidelines, research needs and issues that are important for aquatic ecosystem restoration, few provide evidence of how this ecological knowledge might enhance restoration success. Goals of restoration projects typically encompass many objectives (species groups, ecological, cultural and landscape values) and measures. Thus, at first, the evaluation of the response of a single factor to a single measure tends to be difficult (Roni et al., 2008). Surprisingly, little information on secondary stressors is available, despite knowledge that other pressures can easily prevent or delay full recovery. Furthermore, knowledge on recovery processes with respect to changes in ecological processes, especially changes in food web relationships, competition, predator–prey relationships and so on, is lacking.
A second point and major bottleneck is the lack of sufficient data (Palmer et al., 2005), preventing learning from both successful and unsuccessful restoration projects (Jansson et al., 2007; Elliott et al., 2007; Palmer, 2009). Restoration measures are often costly although the costs are much less than if systems are left to degrade even more; however in increasingly open systems such as estuarine and coastal areas in contrast to more closed river and lake systems, active restoration is difficult and passive restoration is used, i.e. to remove the stressor and allow the system to recover with little further intervention (Elliott et al., 2007, and references therein). Therefore, the basic requirements of future restoration endeavours and monitoring should include: (1) well-designed BACI-PS monitoring schemes, (2) monitoring over sufficient timescales, (3) quantified knowledge on thresholds and (4) well-defined objectives set at the outset for the restored condition. Furthermore, only a small fraction of the investment would be initially required to test the hypotheses defined initially and thereby, to establish a sound scientific and applicable basis for future restoration.
A third problem is related to effects that occur only in the short-term and at the local (site) scale; this raises the question of what scale is appropriate when designing restoration. Although empirical evidence is often lacking, several reviews supported the notion that the local scale is inappropriate to achieve long-term measurable improvements (e.g. Feld et al., 2011). As indicated here, a whole-system approach is required rather than an individual, site-specific approach to restoration. This is particularly important in estuaries which are totally dependent for successful function on connectivity with adjacent systems (Elliott & Whitfield, 2011).
A fourth point is that attention should focus on the indicative value of the organism group(s) selected to assess the different aspects of recovery. To date, most restoration studies in rivers and in estuarine and coastal ecosystems have focused on benthic invertebrate assemblages. In rivers, fish are also important indicators. In lakes phytoplankton is studied most extensively. The differences in indicator groups used, relates both to the primary causes of degradation and to their ability to reflect change in more or less-dynamic systems. The use of multiple indicator groups often result in multiple lines of evidence, thereby improving diagnostics and the ability to detect changes associated with multiple stressors (e.g. Stendera & Johnson, 2008a, b; Johnson & Hering, 2009).
Fifth, the time lags of recovery after removal of the stressor(s) are highly variable in all four water categories, from months to many decades. Recovery depends on the type and magnitude of the stressor(s), especially if some are still present, and on the organism group(s) used to assess recovery. Delays in recovery can be attributed to several factors, and different water types are exposed to different combinations of stressors resulting in differences in response times. Furthermore, there needs to be agreement upon the restoration goals for the system and also what criteria will be used to determine attainment of the desired or targeted system (Simenstad et al., 2006). For example, from the outset it should be stated whether a system is being restored merely for its abiotic features, its structural elements, i.e. the appropriate species or full functioning. We emphasise here that the attainment of a functioning ecosystem (e.g. self-sustaining population and communities undergoing all required interactions) is more important and more relevant to the definitions of recovery than merely achieving the presence of structural features (e.g. species presence).
A sixth point is that in freshwaters often restoration projects incorporate restoration of abiotic conditions to a fixed end-point, which either is a developmental stage or an ideal average condition. However, communities tend to be shaped by abiotic extremes and restoration planning should be shaped accordingly. Re-colonisation of a species is only likely when the entire scope, i.e. the maximum and minimum spatial extent and temporal duration of habitat use, is restored. Furthermore, extreme events (such as acidic episodes in streams) underlie the importance of refugia. Together with habitat enhancement, restoring refugia is one way of enhancing resistance to and resilience from both natural and anthropogenic disturbances, and may be critical to the survival and colonisation of target populations. Furthermore, a restoration outcome often comprises communities that need to develop over time, indicating the importance of incorporating the role of biological interactions in restoration planning.
It is difficult to judge the importance of shifting baselines as empirical data are largely lacking (but see palaeoecological studies of lakes). Even in the estuarine and coastal examples it is questionable whether the observed responses are due to alternative states or to other (overlooked or external) stressors. Often in many lake examples the latter is the case. However, there is little doubt that climate and global change will continue to affect recovery of aquatic ecosystems, the extent will depend on local factors, catchment properties and in particular, economic and societal developments.
This study emphasises a number of research priorities, including the need for:
-
statistical understanding of ecological responses;
-
more comprehensive and long-term monitoring to underpin quantitative assessment of management measures;
-
quantitative assessments of cause–effect relationships during the recovery process;
-
case studies relevant to WFD targets;
-
specific knowledge on certain BQEs in certain water categories;
-
knowledge on maintenance, and recurring management;
-
knowledge on the most important factor(s) for recovery and their interactions;
-
knowledge on shifting baselines and thresholds.
In general, restoration ecology requires (based on amongst others, Shields et al., 2003; Jeppesen et al., 2005; Elliott et al., 2007; Miller et al., 2010; Palmer et al., 2010; Reitberger et al., 2010; Feld et al., 2011):
-
agreement across habitats of the degradation and recovery trajectories and the case studies indicating the time scale for these under the action of different stressors;
-
definition of clear goals for restoration at the site and catchment scale that are based on recent biological and physico-chemical monitoring results and the distribution of targeted species or communities;
-
identification of best-practice restoration measures to address the specific pressures and the acknowledgement of the relative merits of active and passive recovery;
-
balancing all measures within a catchment in order to reach the best possible synergy effects of single component measures, and ultimately to achieve recovery of the entire catchment;
-
knowledge of indicators that can be monitored at the relevant (often large) scale and be relevant for the measure taken;
-
a monitoring design extracted from an experimental design that addresses the goals defined for restoration and that is likely to be successful at the large scale and in the long-term;
-
pre-restoration monitoring as a basis for monitoring of progress, and ultimately of success;
-
indication of the time span for each restoration measure to become successful;
-
monitoring of the post-restoration (abiotic) hydromorphological and biological developments based on before–after control–impact paired series surveys;
-
analysis of monitoring data according to state-of-the-art statistical techniques to identify potential shortcomings and to help to develop new indicators that also cover restoration effects on processes and community functions;
-
development of predictive models to support the design of future restoration projects and to assess their potential to become successful.
Comparison of recovery between aquatic and terrestrial ecosystems is difficult due to fundamental differences in their physical environments (i.e. the relative prevalence of water and air) and current patterns of human impacts (Carr et al., 2003). For example, some of the most profound differences are the large rate and extent of dispersal of materials, nutrients, organisms and their reproduction propagules in the relative ‘openness’ (longitudinal in rivers and within lakes or coastal waters) of aquatic systems. Nevertheless, some comparisons are possible. For instance, Prach et al. (2001) reached similar conclusions for spontaneous vegetation succession in terrestrial ecosystem restoration: (1) a plea for long-term research to better understand mechanisms, (2) studies of ecosystem function and key ecological processes, (3) extrapolating results in a landscape framework, (4) linking regional, local and community species pools and site conditions, (5) dispersal, (6) upscaling results to large geographical areas and (7) development of knowledge and GIS based expert systems towards the level of predictability of patterns and processes. Furthermore, they advocate setting clear aims, describing expected developmental processes and functioning, communicate with researchers, practitioners and authorities, including public awareness and last but not least monitoring.
In summary, restoration ecology is an emerging field, albeit with a longer history in terrestrial systems. The extensive body of aquatic literature reviewed here showed that restoration is a site, time and organism group-specific activity and that, as a consequence, generalisations on recovery processes are challenging. Despite the many studies in rivers, lakes and estuarine and coastal waters that provided theoretical frameworks, guidelines, research needs and issues that are important for restoration, only few studies provide evidence of how this ecological knowledge might enhance restoration success.
References
Able, K. W., T. M. Grothues, S. M. Hagan, M. E. Kimball, D. M. Nemerson & G. L. Taghon, 2008. Long-term response of fishes and other fauna to restoration of former salt hay farms: Multiple measures of restoration success. Reviews in Fish Biology and Fisheries 18(1): 65–97.
Adams, S. M., 2005. Assessing cause and effect of multiple stressors on marine systems. Marine Pollution Bulletin 51: 649–657.
Allan, J. D., 2004. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annual Review of Ecology Evolution and Systematics 35: 257–284.
Allan, J. D. & L. B. Johnson, 1997. Catchment-scale analysis of aquatic ecosystems. Freshwater Biology 37: 107–111.
Andersen, T., J. Carstensen, E. Hernández-García & C. M. Duarte, 2009. Ecological thresholds and regime shifts: Approaches to identification. Trends in Ecology & Evolution 24: 49–57.
Andersson, G., 1979. Internal loading of phosphorus and how to reduce it. Some examples from Lake Trummen. In Bjork, S. (ed.), Lake Management, Studies and Results at the Institute of Limnology in Lund. Arch. Hydrobiol. Beih. Ergebn. Limnol. 13: 43–48.
Angeler, D. & W. Goedkoop, 2010. Biological responses to liming in boreal lakes: An assessment using plankton, macroinvertebrates, and fish communities. Journal of Applied Ecology 47: 478–486.
Arnell, N. W., 1999. The effect of climate on hydrological regimes in Europe: A continental perspective. Global Environmental Change 9: 5–23.
Atkins, J. P., D. Burdon, M. Elliott & A. J. Gregory, 2011. Management of the marine environment: Integrating ecosystem services and societal benefits with the DPSIR framework in a systems approach. Marine Pollution Bulletin 62: 215–226.
Baillie, B. R., L. G. Garrett & A. W. Evanson, 2008. Spatial distribution and influence of large woody debris in an old-growth forest river system, New Zealand. Forest Ecology and Management 256: 20–27.
Basset, A., E. Barbone, M. Elliott, B.-L. Li, S. E. Jorgensen, P. Lucena-Moya, I. Pardo & D. Mouillot, in press. A unifying approach to understanding transitional waters: Fundamental properties emerging from ecotone ecosystems. Estuarine, Coastal & Shelf Science. Available online 9 June 2012.
Battarbee, R. W., D. T. Monteith, S. Juggins, G. L. Simpson, E. M. Shilland, R. J. Flower & A. M. Kreiser, 2008. Assessing the accuracy of diatom-based transfer functions in defining reference pH conditions for acidified lakes in the United Kingdom. The Holocene 8: 57–67.
Bednarek, A. T., 2001. Undamming rivers: A review of the ecological impacts of dam removal. Environmental Management 27(6): 803–814.
Beechie, T. J., D. A. Sear, J. D. Olden, G. R. Pess, J. M. Buffington, H. Moir, P. Roni & M. M. Pollock, 2010. Process-based principles for restoring river ecosystems. BioScience 60: 209–222.
Bennion, H., R. W. Battarbee, C. D. Sayer, G. L. Simpson & T. A. Davidson, 2011. Defining reference conditions and restoration targets for lake ecosystems using palaeolimnology: A synthesis. Journal of Paleolimnology 45: 533–544.
Bernhardt, E. S., M. A. Palmer, J. D. Allan, G. Alexander, K. Barnas, S. Brooks, J. Carr, S. Clayton, C. Dahm, J. Follstad-Shah, D. Galat, S. Gloss, P. Goodwin, D. Hart, B. Hassett, R. Jenkinson, S. Katz, G. M. Kondolf, P. S. Lake, R. Lave, J. L. Meyer, T. K. O’Donnell, L. Pagano, B. Powell & E. Sudduth, 2005. Synthesizing U.S. River restoration efforts. Science 308: 636–637.
Blakely, T. J., J. S. Harding, A. R. McIntosh & M. J. Winterbourn, 2006. Barriers to recovery of aquatic insect communities in an urban stream. Freshwater Biology 51: 1634–1645.
Blomqvist, P., R. T. Bell, H. Olofsson, U. Stensdotter & K. Vrede, 1993. Pelagic ecosystem responses to nutrient additions in acidified and limed lakes in Sweden. Ambio 22: 283–289.
Blomqvist, P., R. T. Bell, H. Olofsson, U. Stensdotter & K. Vrede, 1995. Plankton and water chemistry in Lake Njupfatet before and after liming. Canadian Journal of Fish and Aquatic Science 52: 551–565.
Bolam, S. G., M. Schratzberger & P. Whomersley, 2006. Macro- and meiofaunal recolonisation of dredged material used for habitat enhancement: Temporal patterns in community development. Marine Pollution Bulletin 52: 1746–1755.
Bond, N. R. & P. S. Lake, 2003. Local habitat restoration in streams: constraints on the effectiveness of restoration for stream biota. Ecological Management & Restoration 4: 193–198.
Borja, A., I. Muxika & J. Franco, 2006. Long-term recovery of soft-bottom benthos following urban and industrial sewage treatment in the Nervión estuary (southern Bay of Biscay). Marine Ecology Progress Series 313: 43–55.
Borja, A., I. Muxika & J. G. Rodríguez, 2009. Paradigmatic responses of marine benthic communities to different anthropogenic pressures, using M-AMBI, within the European Water Framework Directive. Marine Ecology 30: 214–227.
Borja, A., D. M. Dauer, M. Elliott & C. A. Simenstad, 2010. Medium- and long-term recovery of estuarine and coastal ecosystems: patterns, rates and restoration effectiveness. Estuaries and Coasts 33: 1249–1260.
Boyes, S. J. & J. H. Allen, 2007. Topographic monitoring of a middle estuary mudflat, Humber estuary, UK—Anthropogenic impacts and natural variation. Marine Pollution Bulletin 55: 543–554.
Brouwer, E., R. Bobbink & J. G. M. Roelofs, 2002. Restoration of aquatic macrophyte vegetation in acidified and eutrophied softwater lakes: An overview. Aquatic Botany 73: 405–431.
Calmano, W., J. Hong & U. Förstner, 1993. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Science & Technology 28: 223–235.
Carpenter, S. R. & J. F. Kitchell, 1996. The Trophic Cascade in Lakes. Cambridge University Press, Cambridge: 385 pp.
Carr, M. H., J. E. Neigel, J. A. Estes, S. Andelman, R. R. Warner & J. L. Largier, 2003. Comparing marine and terrestrial ecosystems: Implications for the design of coastal marine reserves. Ecological Applications 13: 90–107.
Carstensen, J., M. Sánchez-Camacho, C. M. Duarte, D. Krause-Jensen & N. Marbà, 2011. Connecting the dots: Responses of coastal ecosystems to changing nutrient concentrations. Environmental Science and Technology 45(21): 9122–9132.
Castelle, A. J., A. W. Johnson & C. Conolly, 1994. Wetland and stream buffer size requirements—A review. Journal of Environmental Quality 23: 878–882.
CIS, 2006. WFD and hydromorphological pressures. Good practice in managing the ecological impacts of hydropower schemes; flood protection works; and works designed to facilitate navigation under the Water Framework Directive. Water Framework Directive Technical Report, European Communities: 68.
Cloern, J. E., 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Crain, C. M., K. Kroeker & B. S. Halpern, 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11: 1304–1315.
Dallas, K. L. & P. L. Barnard, 2011. Anthropogenic influences on shoreline and nearshore evolution in the San Francisco Bay coastal system. Estuarine, Coastal and Shelf Science 92: 195–204.
D’Antonio, C. & L. A. Meyerson, 2002. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Restoration Ecology 10: 703–713.
Davies-Colley, R. J., M. A. Meleason, G. M. Hall & J. C. Rutherford, 2009. Modelling the time course of shade, temperature, and wood recovery in streams with riparian forest restoration. New Zealand Journal of Marine and Freshwater Research 43: 673–688.
Diaz, R. & R. Rosenberg, 2008. Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929.
Diaz, R., D. B. Rhoads, J. R. Kropp & K. Keay, 2008. Long-term trends of benthic habitats related to reduction in wastewater discharge to Boston Harbor. Estuaries and Coasts 31: 1184–1197.
Dong, X., H. Bennion, S. C. Maberly, C. D. Sayer, G. L. Simpson & R. W. Battarbree, in press. Nutrients provide a stronger control than climate on diatom communities in Esthwaite Water. Freshwater Biology. doi:10.1111/j.1365-2427.2011.02670.x.
Doyle, M. W., E. H. Stanley, C. H. Orr, A. R. Selle, S. A. Sethi & J. M. Harbor, 2005. Stream ecosystem response to small dam removal: Lessons from the Heartland. Geomorphology 71: 227–244.
Driscoll, C. T., G. B. Lawrence, A. J. Bulger, T. J. Butler, C. S. Cronan, C. Eagar, K. F. Lambert, G. E. Likens, J. L. Stoddard & K. C. Weathers, 2001. Acidic deposition in the northeastern United States: Sources and inputs, ecosystem effects, and management strategies. Bioscience 51: 180–198.
Duarte, C. M., D. J. Conley, J. Carstensen & M. Sánchez-Camacho, 2009. Return to Neverland: Shifting baselines affect eutrophication restoration targets. Estuaries and Coasts 32: 29–36.
EEA, 2004. Impacts of Europe’s changing climate: An indicator-based assessment. EEA report no. 2/2004. European Environment Agency, Copenhagen.
Elliott, M., 2002. The role of the DPSIR approach and conceptual models in marine environmental management: An example for offshore wind power. Marine Pollution Bulletin 44: iii–vii.
Elliott, M., 2011. Marine science and management means tackling exogenic unmanaged pressures and endogenic managed pressures—A numbered guide. Marine Pollution Bulletin 62: 651–655.
Elliott, M. & A. Whitfield, 2011. Challenging paradigms in estuarine ecology and management. Estuarine, Coastal & Shelf Science 94: 306–314.
Elliott, M. D., K. Burdon, L. Hemingway & S. Apitz, 2007. Estuarine, coastal and marine ecosystem restoration: Confusing management and science—A revision of concepts. Estuarine, Coastal & Shelf Science 74: 349–366.
EU, 2000. Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities L327: 1–72.
European Environment Agency (EEA), 1995. A General Strategy for Integrated Environmental Assessment at EEA. Internal Report European Environment Agency, Copenhagen: 20 pp.
Feld, C. K., 2004. Identification and measure of hydromorphological degradation in Central European lowland streams. Hydrobiologia 516: 69–90.
Feld, C. K., S. Birk, D. C. Bradley, D. Hering, J. Kail, A. Marzin, A. Melcher, D. Nemitz, M. L. Petersen, F. Pletterbauer, D. Pont, P. F. M. Verdonschot & N. Friberg, 2011. From natural to degraded rivers and back again: A test of restoration ecology theory and practice. Advances in Ecological Research 44: 119–209.
Fisher, S. G., L. J. Gray, N. B. Grimm & D. E. Busch, 1982. Temporal succession in a desert stream ecosystem following flash flooding. Ecological Monographs 52(1): 93–110.
Frissell, C. A., W. J. Liss, C. E. Warren & M. D. Hurley, 1986. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environmental Management 10(2): 199–214.
Furse, M., D. Hering, O. Moog, P. Verdonschot, R. K. Johnson, K. Brabec, K. Gritzalis, A. Buffagni, P. Pinto, N. Friberg, J. Murray-Bligh, J. Kokes, R. Alber, P. Usseglio-Polatera, P. Haase, R. Sweeting, B. Bis, K. Szoszkiewicz, H. Soszka, G. Springe, F. Sporka, I. Krno, 2006. The STAR project: Context, objectives and approaches. Hydrobiologia 566: 3–29.
Gasith, A. & V. H. Resh, 1999. Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. Annual Review of Ecology and Systematics 30: 51–81.
Gee, A., K. Wasson, S. Shaw & J. Haskins, 2010. Signatures of restoration and management changes in the water quality of a Central California Estuary. Estuaries and Coasts 33: 1004–1024.
Gray, J. S. & M. Elliott, 2009. Ecology of Marine Sediments: Science to Management. Oxford University Press, Oxford: 260 pp.
Gregory, S., H. Li & J. Li, 2002. The conceptual basis for ecological responses to dam removal. BioScience 52: 713–723.
Gulati, R. D. & E. Van Donk, 2002. Lakes in the Netherlands, their origin, eutrophication and restoration: State-of-the-art review. Hydrobiologia 478: 73–106.
Gulati, R. D., E. H. R. R. Lammens, M.-L. Meijer & E. Van Donk, 1990. Biomanipulation—Tool for Water Management. Developments in Hydrobiology 61. Kluwer Academic Publishers, Dordrecht: 628 pp (Reprinted from Hydrobiologia 200/201).
Gulati, R. D., L. M. D. Pires & E. Van Donk, 2008. Lake restoration studies: Failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38: 233–247.
Gunn, J. M. & W. Keller, 1990. Biological recovery of an acid lake following reductions in industrial emissions of sulphur. Nature 345: 431–433.
Gunn, J. M., J. G. Hamilton, G. M. Booth, C. D. Wren, G. L. Beggs, H. J. Rietveld & J. R. Munro, 1990. Survival, growth and reproduction of lake trout (Salvelinus namaycush) and yellow perch (Perca flavescens) after neutralization of an acid lake near Sudbury, Ontario. Canadian Journal of Fish and Aquatic Science 47: 446–453.
Harley, C. D. G., A. R. Hughes, K. M. Hultgren, B. G. Miner, C. B. J. Sorte, C. S. Thornber, L. F. Rodriguez, L. Tomanek & S. L. Williams, 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9(2): 228–241.
Hart, M. R., B. F. Quin & M. L. Nguyenc, 2003. Phosphorus runoff from agricultural land and direct fertilizer effects. A review. Journal of Environmental Quality 33: 1954–1972.
Havel, J. & K. Medley, 2006. Biological invasions across spatial scales: Intercontinental, regional, and local dispersal of Cladoceran zooplankton. Biological Invasions 8: 459–473.
Havens, K. E., K. R. Jin, A. J. Rodusky, B. Sharfstein, M. A. Brady, T. L. East, N. Iricanin, R. T. James, M. C. Harwell & A. D. Steinman, 2001. Hurricane effects on a shallow lake ecosystem and its response to a controlled manipulation of water level. Scientific World Journal 1: 44–70.
Hawkins, S. J., J. R. Allen, N. J. Fielding, S. B. Wilkinson & I. D. Wallace, 1999. Liverpool Bay and the estuaries: Human impact, recent recovery and restoration. In: Greenwood, E. F. (ed.), Ecology and Landscape Development: A History of the Mersey Basin. Conference Proceedings: 155–165.
Henrikson, L. & Y. W. Brodin, 1995. Liming of acidified surface waters. A Swedish synthesis. Springer, Berlin.
Hering, D., A. Borja, J. Carstensen, L. Carvalho, M. Elliott, C. K. Feld, A. S. Heiskanen, R. K. Johnson, J. Moe, D. Pont, A. L. Solheim & W. Van de Bund, 2010. The European Water Framework Directive at the age of 10: A critical review of the achievements with recommendations for the future. Science of the Total Environment 408: 4007–4019.
Hering, D., A. Borja, L. Carvalho & C. K. Feld, 2012. Assessment and recovery of European water bodies: key messages from the WISER project. Hydrobiologia: this issue.
Hiddink, J. G., S. Jennings & M. J. Kaiser, 2006. Indicators of the ecological impact of bottom-trawl disturbance on seabed communities. Ecosystems 9: 1190–1199.
Hughes, T. P., A. H. Baird, D. R. Bellwood, M. Card, S. R. Connolly & C. Folke, 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933.
Ibanez, C., N. Prat, C. Duran, M. Pardos, A. Munne, R. Andreu, N. Caiola, N. Cid, H. Hampel, R. Sanchez & R. Trobajo, 2008. Changes in dissolved nutrients in the lower Ebro river: Causes and consequences. Limnetica 27: 131–142.
Ibelings, B. W., R. Portielje, E. H. R. R. Lammens, R. Noordhuis, M. S. van den Berg, W. Joosse & M. L. Meijer, 2007. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10(1): 4–16.
Jähnig, S. C., K. Brabec, A. Buffagni, S. Erba, A. W. Lorenz, T. Ofenböck, P. F. M. Verdonschot & D. Hering, 2010. A comparative analysis of restoration measures and their effects on hydromorphology and benthic invertebrates in 26 central and southern European rivers. Journal of Applied Ecology 47: 671–680.
Jansson, R., C. Nilsson & B. Malmqvist, 2007. Restoring freshwater ecosystems in riverine landscapes: The roles of connectivity and recovery processes. Freshwater Biology 52: 589–596.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Kohler, E. Lammens, T. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Noges, G. Persson, G. Phillips, R. Portielje, C. L. Schelske, D. Straile, I. Tatrai, E. Willen & M. Winder, 2005. Lake responses to reduced nutrient loading—An analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Johnson, R. K. & D. G. Angeler, 2010. Tracing recovery under changing climate: Response of phytoplankton and invertebrate assemblages to decreased acidification. Journal of North American Benthological Society 29: 1472–1490.
Johnson, R. K. & D. Hering, 2009. Response of taxonomic groups in streams to gradients in resource and habitat characteristics. Journal of Applied Ecology 46: 175–186.
Johnson, R. K., W. Goedkoop & L. Sandin, 2004. Spatial scale and ecological relationships between the macroinvertebrate communities of stony habitats of streams and lakes. Freshwater Biology 49: 1179–1194.
Johnson, R. K., M. T. Furse, D. Hering & L. Sandin, 2007. Ecological relationships between stream communities and spatial scale: implications for designing catchment-level monitoring programmes. Freshwater Biology 52: 939–958.
Jowett, I. G., J. Richardson & J. A. T. Boubée, 2009. Effects of riparian manipulation on stream communities in small streams: Two case studies. New Zealand Journal of Marine and Freshwater Research 43(3): 763–774.
Kail, J., D. Hering, S. Muhar, M. Gerhard & S. Preis, 2007. The use of large wood in stream restoration: Experiences from 50 projects in Germany and Austria. J. Appl. Ecol. 44: 1145–1155.
Karr, J. R., 1981. Assessment of biotic integrity using fish communities. Fisheries 6(6): 21–27.
Karr, J. R. & E. W. Chu, 1999. Restoring Life in Running Waters: Better Biological Monitoring. Island Press, Washington, DC.
Kernan, M., M. Ventura, P. Bitusik, A. Brancel, G. Clarke, G. Velle, G. Raddum, E. Stuchlik & J. Catalan, 2009. Regionalisation of remote European mountain lake ecosystems according to their biota: Environmental vs. geographical patterns. Freshwater Biology 54: 2470–2493.
Kernan, M., R. W. Battarbee, C. J. Curtis, D. T. Monteith & E. W. Shilland (eds), 2010a. Recovery of lakes and streams in the UK from acid rain. The United Kingdom Acid Waters Monitoring Network 20 year interpretative report. Report to the Department for Environment, Food and Rural Affairs (Contract EPG 1/3/160).
Kernan, M., R. W. Battarbee & B. Moss, 2010b. Climate Change Impacts on Freshwater Ecosystems. Wiley-Blackwell, Chischester.
Lake, P. S., 2001. Restoring streams: Re-building and reconnecting. In: Rutherfurd, I., F. Sheldon, G. Brierley & C. Kenyon (eds) Third Australian Stream Management Conference, Cooperative Research Centre for Catchment Hydrology, Brisbane, Canberra: 369–371.
Lake, P. S., N. Bond & P. Reich, 2007. Linking ecological theory with stream restoration. Freshwater Biology 52: 597–615.
Lamberti, G. A., S. V. Gregory, L. R. Ashkenas, R. C. Wildman & K. M. S. Moore, 1991. Stream ecosystem recovery following a catastrophic debris flow. Canadian Journal of Fisheries and Aquatic Sciences 48(2): 196–208.
Lambin, E. F. & H. J. Geist, 2006. Land Use and Land Cover Change. Local Processes and Global Impacts. Springer, Berlin.
Lambin, E. F., X. Baulies, N. Bockstael, G. Fischer, T. Krug, R. Leemans, E. F. Moran, R. R. Rindfuss, Y. Sato, D. Skole, B. L. Turner II & C. Vogel, 1999. Land-use and land-cover change (LUCC): Implementation strategy. IGBP Report No. 48, IHDP Report No. 10, Stockholm, Bonn.
Larson, M. G., D. B. Booth & S. A. Morley, 2001. Effectiveness of large woody debris in stream rehabilitation projects in urban basins. Ecological Engineering 18: 211–226.
Levell, A. P. & H. Chang, 2008. Monitoring the channel process of a stream restoration project in an urbanizing watershed: A case study of Kelley Creek, Oregon, USA. River Research and Applications 182: 169–182.
Locke, A. & W. G. Sprules, 1994. Effects of lake acidification and recovery on the stability of zooplankton food webs. Ecology 75: 498–506.
Lotze, H. K., 2010. Historical reconstruction of human-induced changes in U.S. estuaries. Oceanography and Marine Biology: An Annual Review 48: 267–338.
Lotze, H. K., M. Coll, A. M. Magera, C. Ward-Paige & L. Airoldi, 2011. Recovery of marine animal populations and ecosystems. Trends in Ecology and Evolution 26: 595–605.
Luisetti, T., R. K. Turner, I. J. Bateman, S. Morse-Jones, C. Adams & L. Fonseca, 2011. Coastal and marine ecosystem services valuation for policy and management: Managed realignment case studies in England. Ocean & Coastal Management 54: 212–224.
Maberly, S. C. & J. A. Elliott, 2012. Insights from long-term studies in the Windermere catchment: External stressors, internal interactions and the structure and function of lake ecosystems. Freshwater Biology 57: 233–243.
Mack, R. N., D. Simberloff, W. M. Lonsdale, H. Evans, M. Clout & F. A. Bazzaz, 2000. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecological Applications 10(3): 689–710.
Manchester, S. J. & J. M. Bullock, 2000. The impacts of non-native species on UK biodiversity and the effectiveness of control. Journal of Applied Ecology 37: 845–864.
Matsuzaki, S., N. Usio, N. Takamura & I. Washitani, 2009. Contrasting impacts of invasive engineers on freshwater ecosystems: An experiment and meta-analysis. Oecologia 158: 673–686.
Matthews, J., B. Reeze, C. K. Feld & A. J. Hendriks, 2010. Lessons from practice: Assessing early progress and success in river rehabilitation. Hydrobiologia 655: 1–14.
May, L. & L. Carvalho, 2010. Maximum growing depth of macrophytes in Loch Leven, Scotland, United Kingdom, in relation to historical changes in estimated phosphorus loading. Hydrobiologia 646: 123–131.
May, L. & B. M. Spears, 2012a. Loch Leven: 40 years of scientific research. Understanding the links between pollution, climate change and ecological response. Developments in Hydrobiology 218: 130.
May, L. & B. M. Spears, 2012b. Managing ecosystem services at Loch Leven, Scotland, UK: Actions, impacts and unintended consequences. Hydrobiologia 681: 117–130.
McLusky, D. S. & M. Elliott, 2004. The Estuarine Ecosystem: Ecology, Threats and Management, 3rd edn. Oxford University Press, Oxford: 216 pp.
Meyer, J. L., M. J. Sale, P. J. Mulholland & N. L. Poff, 1999. Impacts of climate change on aquatic ecosystem functioning and health. Journal of the American Water Resources Association 35: 1373–1386.
Miller, S. W., P. Budy & J. C. Schmidt, 2010. Quantifying macroinvertebrate responses to in-stream habitat restoration: Applications of meta-analysis to river restoration. Restoration Ecology 18: 8–19.
Monteith, D., A. G. Hildrew, R. J. Flower, P. J. Raven, W. R. B. Beaumont, P. Collen, A. M. Kreiser, E. M. Shilland & J. H. Winterbottom, 2005. Biological responses to the chemical recovery of acidified fresh waters in the UK. Environmental Pollution 137: 83–101.
Nixon, S., 2009. Eutrophication and the macroscope. Hydrobiologia 629: 5–19.
Noges, P., U. Mischke, R. Laugaste & A. G. Solimini, 2010. Analysis of changes over 44 years in the phytoplankton of Lake Vrtsjarv (Estonia): The effect of nutrients, climate and the investigator on phytoplankton-based water quality indices. Hydrobiologia 646: 33–48.
Ormerod, S. J., 2003. Restoration in applied ecology: Editor’s introduction. Journal of Applied Ecology 40: 44–50.
Ormerod, S. J., M. Dobson, A. G. Hildrew & C. R. Townsend, 2010. Multiple stressors in freshwater ecosystems. Freshwater Biology 55: 1–4.
Palmer, M. A., 2009. Invited Odum essay: Reforming watershed restoration: Science in need of application and applications in need of science. Estuaries and Coasts 32: 1–17.
Palmer, M. A., E. S. Bernhardt, J. D. Allan, P. S. Lake, G. Alexander, S. Brooks, J. Carr, S. Clayton, C. N. Dahm, J. Follstad Shah, D. L. Galat, S. G. Loss, P. Goodwin, D. D. Hart, B. Hassett, R. Jenkinson, F. M. Kondolf, R. Lave, J. L. Meyer, T. K. O’Donnel, L. Pagano & E. Sudduth, 2005. Standards for ecologically successful river restoration. Journal of Applied Ecology 42: 208–217.
Palmer, M. A., H. L. Menninger & E. S. Bernhardt, 2010. River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshwater Biology 55: 1–18.
Parkyn, S., R. Davies-Colley, A. Cooper & M. Stroud, 2005. Predictions of stream nutrient and sediment yield changes following restoration of forested riparian buffers. Ecological Engineering 24: 551–558.
Paul, M. J. & J. Meyer, 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics 32: 333–365.
Pirrone, N., G. Trombino, S. Cinnirella, A. Algieri, G. Bendoricchio & L. Palmeri, 2005. The Driver–Pressure–State–Impact–Response (DPSIR) approach for integrated catchment-coastal zone management: Preliminary application to the Po catchment-Adriatic Sea coastal zone system. Regional Environmental Change 5: 111–137.
Pollard, D. A. & J. C. Hannan, 1994. The ecological effects of structural flood mitigation works on fish habitats and fish communities in the lower Clarence River system of south-eastern Australia. Estuaries 17: 427–461.
Prach, K., S. Bartha, C. B. Joyce, P. Pyšek, R. van Diggelen & G. Wiegleb, 2001. The role of spontaneous vegetation succession in ecosystem restoration: A perspective. Applied Vegetation Science 4(1): 111–114.
Pretty, J. L., S. S. C. Harrison, D. J. Shepherd, C. Smith, A. G. Hildrew & R. D. Hey, 2003. River rehabilitation and fish populations: Assessing the benefit of instream structures. Journal of Applied Ecology 40(2): 251–265.
Rabalais, N. N., R. J. Díaz, L. A. Levin, R. E. Turner, D. Gilbert & J. Zhang, 2010. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7: 585–619.
Räisänen, J., U. Hansson, A. Ullerstig, R. Döscher, L. P. Graham, C. Jones, M. Meier, P. Samuelsson & U. Willén, 2003. GCM driven simulations of recent and future climate with the Rossby Centre coupled atmosphere—Baltic Sea regional climate model RCAO. SWECLIM programmeme and EU PRUDENCE project, report number: RMK No. 101, January 2003: 61 pp.
Reitberger, B., J. Matthews, C. K. Feld, W. Davis & M. A. Palmer, 2010. River monitoring and indication of restoration success: Comparison of EU and BCG frameworks. University of Duisburg-Essen, Essen: 154 pp.
Roni, P. & T. P. Quinn, 2001. Effects of wood placement on movements of trout and juvenile Coho Salmon in natural and artificial stream channels. Transactions of the American Fisheries Society 130: 675–685.
Roni, P., K. Hanson & T. Beechie, 2008. Global review of the physical and biological effectiveness of stream habitat rehabilitation techniques. North American Journal of Fisheries Management 28: 856–890.
Russell, G., S. J. Hawkins, L. C. Evans, H. D. Jones & G. D. Holmes, 1983. Restoration of a disused dock basin as a habitat for marine benthos and fish. Journal of Applied Ecology 20: 43–58.
Salgado, J., C. Sayer, L. Carvalho, T. Davidson & I. Gunn, 2010. Assessing aquatic macrophytes community change through integration of palaeolimnological and historical data at Loch Leven, Scotland. Journal of Paleolimnology 43: 191–204.
Scheffer, M., 2001. Ecology of Shallow Lakes. Kluwer Academic Press, London.
Schindler, D. W., 1988. Effects of acid rain on freshwater ecosystems. Science 239: 149–157.
Schindler, D. W., 2001. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Canadian Journal of Fisheries and Aquatic Sciences 58: 18–29.
Schindler, D. W., 2006. Recent advances in the understanding and management of eutrophication. Limnology and Oceanography 51: 356–363.
Schreiber, E. S. G., P. S. Lake & G. P. Quinn, 2002. Facilitation of native stream fauna by an invading species? Experimental investigations of the interaction of the snail, Potamopyrgus antipodarum (Hydrobiidae) with native benthic fauna. Biological Invasions 4: 317–325.
Scott, M. C. & G. S. Helfman, 2001. Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries 26: 6–15.
Shields Jr. F. D., S. S. Knight & C. M. Cooper, 1995. Use of the index of biotic integrity to assess physical habitat degradation in warmwater streams. Hydrobiologia 312(3): 191–208.
Shields Jr. F. D., R. R. Copeland, P. C. Klingeman, M. W. Doyle & A. Simon, 2003. Design for stream restoration. Journal of Hydraulic Engineering 129: 575–584.
Shields, F. D., S. S. Knight & J. M. Stofleth, 2006. Large wood addition for aquatic habitat rehabilitation in an incised, sand-bed stream, Little Topashaw Creek, Mississippi. River Research and Applications 22: 803–817.
Simenstad, C., D. Reed & M. Ford, 2006. When is restoration not? Incorporating landscape-scale processes to restore self-sustaining ecosystems in coastal wetland restoration. Ecological Engineering 26: 27–39.
Skilleter, G. A., A. Pryor, S. Miller & B. Cameron, 2006. Detecting the effects of physical disturbance on benthic assemblages in a subtropical estuary: A beyond BACI approach. Journal of Experimental Marine Biology and Ecology 338: 271–287.
Smeets, E. & R. Weterings, 1999. Environmental indicators: Typology and overview. Technical report No. 25, European Environment Agency, Copenhagen: 19 pp.
Smith, E. P., D. R. Orvos & J. Cairns, 1993. Impact assessment using the before–after-control–impact model: Concerns and comments. Canadian Journal of Fisheries and Aquatic Sciences 50: 627–637.
Solimini, A. G., A. C. Cardoso & A. S. Heiskanen, 2006. Indicators and methods for the ecological status assessment under the Water Framework Directive. Linkages between chemical and biological quality of surface waters. Institute for Environment and Sustainability, Joint Research Center, European Communities: 262.
Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor & H. L. Miller, 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: Successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105.
Spears, B. M., L. Carvalho & D. Paterson, 2007. Phosphorus partitioning in a shallow lake: Implications for water quality management. The Water and Environment Journal 21(47): 53.
Spears, B., I. Gunn, S. Meis & L. May, 2011. Analysis of cause–effect–recovery chains for lakes recovering from eutrophication. CEH-report. Contribution to Deliverable D6: 2–4.
Spears, B. M., L. Carvalho, R. Perkins & D. M. Paterson, 2012. Long-term variation and regulation of internal loading in Loch Leven. Hydrobiologia 681: 23–33.
Stein, E. D. & D. B. Cadien, 2009. Ecosystem response to regulatory and management actions: The southern California experience in long-term monitoring. Marine Pollution Bulletin 59: 91–100.
Stelzenmüller, V., J. Lee, A. South & S. I. Rogers, 2010. Quantifying cumulative impacts of human pressures on the marine environment: A geospatial modelling framework. Marine Ecology Progress Series 398: 19–32.
Stendera, S. & R. K. Johnson, 2008a. Tracking recovery trends of boreal lakes: Use of multiple indicators and habitats. Journal of North American Benthological Society 27: 529–540.
Stendera, S. & R. K. Johnson, 2008b. Tracking recovery trends of boreal lakes: Use of multiple indicators and habitats. Journal of the North American Benthological Society 27: 529–540.
Straile, D., D. M. Livingstone, G. A. Weyhenmeyer & D. G. George, 2003. The response of freshwater ecosystems to climate variability associated with the North Atlantic Oscillation. Geophysical Monographs 134: 263–279.
Thackeray, S. J., T. H. Sparks, M. Frederiksen, S. Burthes, P. J. Bacon, J. R. Bell, M. S. Botham, T. M. Brereton, P. W. Bright, L. C. Carvalho, T. Clutton-Brock, A. Dawson, M. Edwards, J. M. Elliott, R. Harrington, D. Johns, I. D. Jones, J. T. Jones, D. I. Leech, D. B. Roy, W. A. Scott, M. Smith, R. J. Smithers, I. J. Winfield & S. Wanless, 2010. Trophic level asynchrony in rates of phonological change for marine, freshwater and terrestrial environments. Global Change Biology 16: 3304–3313.
Trannum, H. C., F. Olsgard, J. M. Skei, J. Indrehus, S. Overas & J. Eriksen, 2004. Effects of copper, cadmium and contaminated harbour sediments on recolonisation of soft-bottom communities. Journal of Experimental Marine Biology and Ecology 310: 87–114.
Turner II, B. L., W. C. Clark, R. W. Kates, J. F. Richards, J. T. Mathews & W. B. Meyer, 1990. The Earth as transformed by human action: Global and regional changes in the biosphere over the past 300 years. Cambridge University Press, Cambridge.
Uriarte, A. & A. Borja, 2009. Assessing fish quality status in transitional waters, within the European Water Framework Directive: Setting boundary classes and responding to anthropogenic pressures. Estuarine, Coastal and Shelf Science 82: 214–224.
Van der Wal, D., K. Pye & A. Neal, 2002. Long-term morphological change in the Ribble Estuary, northwest England. Marine Geology 189(3–4): 249–266.
Van Donk, E., R. D. Gulati, A. Iedema & J. T. Meulemans, 1993. Macrophyte-related shifts in the nitrogen and phosphorus contents of the different trophic levels in a biomanipulated shallow lake. Hydrobiologia 251: 19–26.
Van Riel, M. C., G. Van der Velde, S. Rajagopal, S. Marguillier, F. Dehairs & A. Bij de Vaate, 2006. Trophic relationships in the Rhine food web during invasion and after stablishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565: 39–58.
Walsh, C. J., T. D. Fletcher & A. R. Ladson, 2005. Stream restoration in urban catchments through redesigning stormwater systems: Looking to the catchment to save the stream. Journal of the American Benthological Society 24: 690–705.
Weyhenmeyer, G. A., A. K. Westoo & E. Willen, 2008. Increasingly ice-free winters and their effects on water quality in Sweden’s largest lakes. Hydrobiologia 599: 111–118.
Whitfield, A. K., M. Elliott, A. Basset, S. J. M. Blaber & R. J. West, 2012. Paradigms in estuarine ecology—the Remane diagram with a suggested revised model for estuaries: A review. Estuarine, Coastal & Shelf Science 97: 78–90.
Whomersley, P., M. Schratzberger, M. Huxham, H. Bates & H. Rees, 2007. The use of time-series data in the assessment of macrobenthic community change after the cessation of sewage-sludge disposal in Liverpool Bay (UK). Marine Pollution Bulletin 54: 32–41.
Wiens, J. A., 2002. Riverine landscapes: Taking landscape ecology into the water. Freshwater Biology 47: 501–515.
Withers, P. J. A. & P. M. Haygarth, 2007. Agriculture, phosphorus and eutrophication: A European perspective. Soil Use and Management 23: 1–4.
Yan, N. D., B. Leung, B. Keller, S. E. Arnott, J. M. Gunn & G. G. Raddum, 2003. Developing conceptual frameworks for the recovery of aquatic biota from acidification. Ambio 32: 165–169.
Zaldivar, J. M., A. C. Cardoso, P. Viaroli, A. Newton, R. de Wit, C. Ibanez, S. Reizopoulou, F. Somma, A. Razinkovas, A. Basset, M. Holmer & N. Murray, 2008. Eutrophication in transitional waters: An overview. Transitional Waters Monographs 1: 1–78.
Zhang, J., D. Gilbert, A. J. Gooday, L. Levin, S. W. A. Naqvi, J. J. Middelburg, M. Scranton, W. Ekau, A. Peña, B. Dewitte, T. Oguz, P. M. S. Monteiro, E. Urban, N. N. Rabalais, V. Ittekkot, W. M. Kemp, O. Ulloa, R. Elmgren, E. Escobar-Briones & A. K. Van der Plas, 2010. Natural and human-induced hypoxia and consequences for coastal areas: Synthesis and future development. Biogeosciences 7: 1443–1467.
Acknowledgments
This paper is part of the WISER project (Water bodies in Europe: Integrative Systems to assess Ecological status and Recovery) funded by the European Union under the 7th Framework Programme, Theme 6 (Environment including Climate Change) (contract No. 226273), www.wiser.eu.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: C. K. Feld, A. Borja, L. Carvalho & D. Hering / Water bodies in Europe: integrative systems to assess ecological status and recovery
Rights and permissions
About this article
Cite this article
Verdonschot, P.F.M., Spears, B.M., Feld, C.K. et al. A comparative review of recovery processes in rivers, lakes, estuarine and coastal waters. Hydrobiologia 704, 453–474 (2013). https://doi.org/10.1007/s10750-012-1294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1294-7