Abstract
In order to assess the geochemical mechanism liable for fluoride contamination in groundwater and its health effects on the people of the Shanmuganadhi River basin, Tamil Nadu, India, 61 groundwater samples were collected during post- and pre-monsoon seasons from the wells used for drinking purposes. Collected samples were analysed for various physico-chemical parameters. The parameters estimated in the present study are hydrogen ion concentration (pH), total dissolved solids, electrical conductivity, calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (HCO3−), chloride (Cl−), sulphate (SO42−), nitrate (NO3−), phosphate (PO43−) and fluoride (F−). The fluoride ion concentration in the groundwater samples of this region varied from 0.01 to 2.50 mg/l and 0.01 to 3.30 mg/l during post- and pre-monsoon seasons, respectively. Out of 61 groundwater samples, 14 samples of post-monsoon season and 16 samples of pre-monsoon season represented high, very high and extremely high classes of fluoride, which cause dental fluorosis in this region. The fluoride-bearing minerals in the granitic and gneissic rocks such as apatite, hornblende, muscovite, biotite and amphiboles are the major sources for fluoride contamination in this area. In addition to the geogenic sources, applications of synthetic fertilizers in the agricultural fields also contribute significant amount of fluoride ions to groundwater. The spatial distribution of fluoride in different geological formations clearly indicate that the wells located in charnockite terrain were possessing very low fluoride concentration when compare with the wells located in the hornblende–biotite gneiss formation. Therefore, dental fluorosis risks are mostly associated with rock types in this region. People living over the basement rock comprising of hornblende–biotite gneiss are prone for fluorosis. Fluoride exhibited good positive correlation with bicarbonate in groundwater. As fluoridated endemic regions normally acquire lot of bicarbonate in groundwater samples, Shanmuganadhi basin falls under fluoride endemic category. The present study identified 26 villages in Shanmuganadhi basin as probable fluorosis risk areas where attention should be given to treat the fluoride-rich groundwater before drinking water supply. The groundwater level fluctuation study also designates that rise in water level reduces the concentration of fluoride due to dilution mechanism. Therefore, recharge of groundwater by artificial methods will definitely improve the present scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is the most valuable resource in the world. Human health completely depends on this source. Hence, we have to both ensure and upgrade water for well-being. Groundwater quality is mainly evaluated by chemical and physical parameters, and it depends on dissolved inorganic contaminants, sediments, toxic materials, natural organic compounds and bacteria accumulated over a period of time (Bhargava and Killender 1988; Prasad 1984). Fluoride is a vital component for healthy bones and teeth (Choi et al. 2012). High concentration of fluoride causes negative impacts on human system including the brain (Choi et al. 2012). The preliminary level of dental fluorosis creates the causes of white horizontal striations and opaque patches (Ahada and Suthar 2017; Susheela 2003; Rao 2003). In 1930s, fluorosis study was carried out by the USA (Dean and Elvove 1937). After that many studies on fluoride contamination in groundwater were carried out in different parts of the world (Ghosh et al. 2013; Gao et al. 2012). The World Health Organization has also reported that many countries such as South America, China, Central Africa and India have more fluoride concentration in groundwater (WHO 2011). Nowadays, researchers concentrate more on fluoride impact on human health (Vikas et al. 2013; Keshavarzi et al. 2010; Li et al. 2009; Kim and Jeong 2005; Karthikeyan and Shanmugasundarraj 2000; Rao 1997). In India also few studies on human health risk evaluation based on fluoride contamination in groundwater were carried out (Karunanidhi et al. 2019; Adimalla et al. 2018; Narsimha and Rajitha 2018; Raju 2016; Sudhakar and Narsimha 2013).
Fluoride contamination in groundwater is serious threat, while it exceeds the permissible limit of WHO standards (> 1.5 mg/l). The countries for example Yemen (Aqeel et al. 2017), Kenya (Francisca et al. 2017), India (Raj and Shaji 2017; Salve et al. 2008; Shaji et al. 2007), Iran (Aghapour et al. 2018; KheradPisheh et al. 2016), Thailand (Chuah et al. 2016), Tanzania (Bhattacharya et al. 2016), South Africa (Kut et al. 2016), Pakistan (Rasool et al. 2015), China (Li et al. 2018; Gao et al. 2013), Libya (Tejaswi et al. 2013), Saudi Arabia (Zabin et al. 2008) and Turkey (Oruc 2008) have excess fluoride in groundwater. In India, Tamil Nadu, Telangana, Rajasthan, Jharkhand, West Bengal, Gujarat, Andhra Pradesh and Uttar Pradesh are the states which possess high fluoride in groundwater leading dental fluorosis (Batabyal 2018; Sahu et al. 2018; Sakram et al. 2018; Prajapati et al. 2017; Patolia and Sinha 2017; Mondal et al. 2016; Ali et al. 2016; Thivya et al. 2015; Subba Rao 2011). Many researchers have carried out geochemical evaluation of fluoride contamination in groundwater in different districts of Tamil Nadu state such as Sajil Kumar (2017) in Vellore, Gowrisankar et al. (2017) in Krishnagiri, Panneer et al. (2017) and Satheeshkumar et al. (2017) in Dharmapuri, Chidambaram et al. (2013) in Dindigul, Manikandan et al. (2012) in Krishnagiri, Gopalakrishnan et al. (2012) in Tirunelveli, Srinivasamoorthy et al. (2012) and Karunanidhi et al. (2013) in Salem, Dar et al. (2011) in Kancheepuram, Anandakumar et al. (2009) and Karthikeyan et al. (2010) in Erode, Vennila et al. (2008) in Coimbatore, Jayaprakash et al. (2008) in Neyveli, Giridharan et al. (2008) in Chennai and Periakali et al. (2001) in Namakkal.
Fluoride ion originates in all sets of water of low absorptions to high absorptions, and it happens into the rocks, air, soil, water, floras and faunas. The groundwater, maximum which is formed from precipitation or shallow water bodies, gains minerals through its passage through the earth’s crust (Patel et al. 2016; Singh et al. 2015). Usually, the igneous rocks, the formation of sedimentary rocks and mineralized strains are linked with fluoride accessory minerals (Edmunds and Smedley 2001). Its occurrence in light coloured rocks is higher than dark coloured and meta-sedimentary rocks (Ali et al. 2016; Young et al. 2011). Weathering of fluoride-bearing minerals is the most important natural cause of fluoride content in water. The important sources of fluoride comprise fluoride-bearing minerals for example apatite [Ca5(Cl,F,OH)(PO4)3], cryolite (Na3AlF6), micas [AB2–3(X, Si)4O10(O, F, OH)2], amphiboles [A0–1B2C5T8O22(OH,F,Cl)], sellaite (MgF2) and fluorite (CaF2) (Jadhav et al. 2015; Datta et al. 1996; Hem 1985). The F− concentration in groundwater is elevated for the reason that it is impacted in rocks and minerals (Wasana et al. 2017; Chuah et al. 2016; Irigoyen-Camacho et al. 2016). The high concentration of bicarbonate, sodium and pH available in groundwater falls under the domination of the above rock–water interactions.
The present investigation is an attempt to evaluate the physico-chemical parameters so as to comprehend the physico-chemical process on the chemistry of the groundwater and to classify the fluoride influencing factors in groundwater such as anthropogenic and geochemical processes. The study also aims to identify the fluorosis risk zones in Shanmuganadhi River basin, Tamil Nadu, India, due to daily consumption of fluoride-rich groundwater.
Study location and geology
The present study was conducted in hard rock terrain situated between 10°25′–10°64′N latitudes and 77°39′–78°56′E longitudes. The total area covered under the study is 807.56 km2, where the plain area occupies about 634.66 km2 and the reserved forest covers 172.90 km2. Geologically, the river basin lies over the Archaean crystalline formation chiefly consists of hornblende–biotite gneiss, charnockite and granite (Fig. 1). Groundwater in this basin exists under phreatic condition in the weathered formation and in semi-confined nature in the fractured zones. Shanmuganadhi is one of the major streams of Amaravathi River, which originates from Kodaikanal hills in the Western Ghats and runs from south to north in the basin. It is a non-perennial river, which mainly carries water during monsoon seasons. The basin area falls under subtropical climate with moderate humidity. The average yearly rainfall of this area varies between 760 and 910 mm. Palani hills located in the southern part of the basin is the pilgrimage tourist spot. Paddy, coconut and sugarcane are the major wet crops in this region.
Materials and methods
The Survey of India (SOI) topographic maps available on 1:50,000 scale (58F/06, 58F/07, 58F/08, 58F/10, 58F/11 and 58F/12) were digitized to prepare the base map including the important features such as major streams, roads and settlements. The sampling locations were also included along with geological formations in the study area map using ArcGIS software (Fig. 1). Sampling wells were randomly identified in every 3 km using Global Positioning System (GPS). Totally 61 observation wells spread over the basin were chosen for sampling. Groundwater samples were collected from these wells during post-monsoon (January 2018) and pre-monsoon (May 2018) seasons. Depth of water level with respect to below ground level (bgl) was measured in all the wells. In addition to the location of wells (latitude and longitude), height with respect to mean sea level (MSL) was also noted using GPS. Collected groundwater samples were poured in 250-ml pre-cleaned Teflon bottles without any air bubbles. Before filling the bottles, they were rinsed with respective groundwater samples. The sample bottles were properly sealed to prevent interaction with atmosphere and transported to the laboratory for storing at 4 °C in the refrigerator. Using the portable water quality field kit, physical parameters like electrical conductivity (EC), total dissolved solids (TDS) and hydrogen ion concentration (pH) were measured immediately after sampling. Flame photometer was used to estimate sodium (Na+) and potassium (K+) in the laboratory. Bicarbonate (HCO3−), chloride (Cl−), calcium (Ca2+) and magnesium (Mg2+) were measured by using titration method based on the standard procedures stated in the American Public Health Association (APHA 2005). Spectrophotometric method was used to determine the concentrations of fluoride (F−), sulphate (SO42−), nitrate (NO3−) and phosphate (PO43−).
Groundwater samples were grouped into six categories based on the fluoride concentration to assess the level of contamination and its implication on human health (Narsimha and Sudarshan 2016). World Health Organization (WHO) has prescribed 1.5 mg/l as upper permissible limit for fluoride in drinking water. Regular consumption of fluoride-rich groundwater can create dental and skeletal fluorosis (WHO 2011). Geographical Information System (GIS) is capable of creating information on spatial and temporal domain, which is extremely vital for fruitful investigation, prediction and validation of geochemical data (Kumar et al. 2008; Saraf and Choudhury 1998; Duraisamy et al. 2018). Therefore, Inverse Distance Weighted (IDW) interpolation method was used to create various thematic layers and isopleth maps (Hema et al. 2010). The fluoride concentration values were weighted based on the average of the surrounding sample points by interpolation technique and spatial variation maps were generated. The correlation coefficient is used to express the elevated levels of negative and positive relationship between various elements. The relationship between two variables of geochemical parameters plotted as X, Y scatter diagram can be designated as positive and negative. Gibbs diagram was prepared to identify the major processes controlling groundwater chemistry such as precipitation dominance, rock dominance and evaporation dominance. Finally, human health survey was also carried out in each sampling site to assess the implication of fluoride-rich groundwater on human system. Methodology adopted in the present study is shown in Fig. 2.
Results and discussion
Based on the concentration of fluoride, groundwater samples of the basin were grouped into six categories. They were Class I (Very Low): concentration of fluoride in groundwater was less than 0.49 mg/l, Class II (Low): concentration of fluoride varied from 0.50 to 0.99 mg/l, Class III (Moderate): concentration of fluoride varied from 1.00 to 1.49 mg/l, Class IV (High): concentration of fluoride varied from 1.50 to 1.99 mg/l, Class V (Very High): concentration of fluoride varied from 2.00 to 2.49 mg/l and Class VI (Extremely High): concentration of fluoride in groundwater was more than 2.49 mg/l (Table 1). Accordingly, all other geochemical parameters were also grouped into six categories to understand the relationship among fluoride and other geochemical parameters during post- and pre-monsoon seasons (Tables 2 and 3). The minimum, maximum, mean, median and standard deviation of statistical parameters were calculated for all the six sample groups, which are presented in Tables 4 and 5, respectively, for post- and pre-monsoon seasons. In the present study, attempt was also made to understand the association of fluoride classes with different geological formations.
Hydrogeochemistry
Acidity and alkalinity
The groundwater samples were grouped into six classes based on fluoride ion concentrations during post- and pre-monsoon seasons. Hydrogen ion concentration (pH) is an important parameter in groundwater because it identifies whether the fluid is acid or alkaline in nature. The pH variations in all six groups of samples during post-monsoon season were, respectively, 6.34–8.28, 5.90–8.05, 7.23–7.86, 7.03–7.9, 6.83–8.24 and 7.29–7.29. During pre-monsoon season, its variations were 7.12–7.91, 7.14–7.94, 7.22–7.91, 7.18–7.61, 7.16–7.79 and 7.11–7.95 in the sample groups I–VI, respectively. According to the WHO (2011) standards, the pH values are to be in the range from 6.5 to 8.5 for the portable condition. It is observed that the pH values ranged from 6.5 to 8.5 during pre- and post-monsoon seasons. This shows moderately acidic to alkaline nature of groundwater exists in the study area.
Salinity
The electrical conductivity is a good indicator of TDS, which decides the taste of drinking water (Hanipha and Hussain 2013; Jain 1998; Subramani et al. 2005). However, post-monsoon season, the electrical conductivity of groundwater samples were in the range of 635.00–3175.00, 1008.57–3170.00, 1142.86–3540.00, 985.71–3270.00, 1100.00–3900.00 and 3757.14–3757.14 μS/cm in the groups I–VI, respectively. During the pre-monsoon season, its variations were 260.00–1312.00, 364.00–2560.00, 996.00–3430.00, 405.00–2084.00, 1079.00–1942.00 and 1200.00–1807.00 μS/cm in the sample groups I–VI, respectively.
The TDS concentration was in the range of 310.00–2784.00, 615.00–2630.00, 715.00–3170.00, 690.00–2410.00, 760.00–3170.00 and 2630.00–2630.00 mg/l in the sample groups I–VI correspondingly during post-monsoon season. Its variations during pre-monsoon were 182.00–918.40, 254.80–1792.00, 697.20–2401.00, 283.50–1458.80, 755.30–1359.40 and 840–1264.90 mg/l in the sample groups I–VI, respectively. The most desirable limit of TDS for drinking purpose is 500 mg/l, and the maximum allowable limit is 1500 mg/l (WHO 2011). The higher concentration of total dissolved solids is the major cause for gastro-intestinal irritation to the people, and the prolonged consumption of water with higher TDS causes kidney stones and heart complaints (Ali and Ali 2018; Garg et al. 2009).
Major cations
High concentration of calcium in groundwater may cause stomach ache, and it is unpleasant for drinking uses. Variation of calcium concentration in the post-monsoon sample groups of I–VI were, respectively, 8.00–132.00, 8.00–304.00, 69.97–256.00, 63.94–232.0, 27.74–312.00 and 83.39–83.39 mg/l. During pre-monsoon season, its variations were 16.00–67.20, 22.40–147.20, 67.20–193.60, 30.40–124.80, 65.60–92.80 and 46.40–116.80 mg/l in the sample groups of I–VI, respectively. The upper limit of calcium in the drinking water should be 200 mg/l as per the World Health Organization (WHO 2011) standards. Variation of magnesium ion concentration in the six sample groups was, respectively, 2.40–49.70, 0.00–76.64, 14.08–127.20, 12.92–72.00, 10.60–74.40 and 55.20–55.20 mg/l during post-monsoon season. In the pre-monsoon sample groups of I–VI, its variations were 4.80–34.56, 5.76–75.84, 23.04–96.00, 11.52–55.68, 20.16–40.32 and 20.16–50.88 mg/l, respectively. High magnesium content in the drinking groundwater can cause cathartic and diuretic problems, whereas it can cause laxative effect if its concentration is huge (Marghade et al. 2011). The major source for magnesium ions in groundwater is rocks and minerals. Weathering of common rock forming minerals like mica, feldspar, calcite, dolomite and hornblende can contribute magnesium as well as calcium ions into groundwater (Nag and Suchetana 2016; Subramani et al. 2010). The higher concentration of sodium ions in drinking groundwater may cause nervous sickness and high blood pressure in human beings (Aref and Roosta 2016). Its variation in the six sample groups (I–VI) of post-monsoon season samples was 0.90–73.00, 1.10–141.10, 11.00–283.00, 12.00–104.00, 6.9.0–110.00 and 99.90–99.90 mg/l, respectively. In the pre-monsoon sample groups of I–VI, sodium ion variations were, respectively, 22.00–148.00, 40.00–300.00, 100.00–350.00, 22.00–250.00, 112.00–240.00 and 140.00–250.00 mg/l. Potassium concentration will be generally low in the groundwater samples when compare with the other cations. However, its higher concentration may lead to muscle weakness and increase blood pressure (Nag and Suchetana 2016). Potassium values were 0.15–20.40, 0.40–11.40, 0.45–16.20, 0.62–30.0, 2.00–9.00 and 18.80–18.80 mg/l, respectively, in the six groups of samples collected during post-monsoon season. Its variation in the pre-monsoon sample groups of I–VI were 5.00–30.00, 8.00–60.00, 12.00–50.00, 5.00–50.00, 34.00–60.00 and 20.00–60.00 mg/l, respectively.
Major anions
Bicarbonate levels were 20.79–624.76, 47.64–428.26, 48.94–587.33, 183.54–452.73, 48.94–489.44 and 489.44–489.44 mg/l, respectively, in the groups I–VI during post-monsoon season. In pre-monsoon sample groups, its variations were 24.00–400.00, 84.00–484.00, 196.00–412.00, 40.00–400.00, 292.00–53.6.00 and 208.00–504.00 mg/l, respectively. The maximum allowable limit of bicarbonate in consumption of water is 600 mg/l as per the World Health Organization (WHO 2011) guidelines. During post-monsoon season, variations in the chloride ion concentration were 23.44–172.98, 20.55–694.78, 70.90–847.26, 53.17–519.56, 88.63–673.27 and 106.35–106.35 mg/l, respectively, in sample groups of I–VI. Its variation in the pre-monsoon sample groups of I–VI was 20.00–112.00, 32.00–536.00, 20.00–664.00, 24.00–312.00, 84.00–192.00 and 44.00–324.00 mg/l, respectively. In this region, nitrate concentration exceeded the permissible limit of 45 mg/l making the groundwater not suitable for drinking purposes in many locations. The possible sources of nitrates are agricultural activities, animal wastes from poultry farms and septic tank leakages. The occurrence of excessive nitrate in the drinking groundwater may increase the prevalence of stomach cancer and capability risks to babies and conceived women (Narsimha and Sudarshan 2016; Rao 2006). During post-monsoon season, variations in the nitrate concentration were 0.10–124.00, 0.10–148.00, 1.85–160. 30–157.00, 0.20–136.00 and 35.00–35.00 mg/l, respectively, in I–VI groups of samples. In the pre-monsoon sample groups, the observed variations of nitrate were 6.00–22.00, 6.00–37.00, 15.00–42.00, 6.00–35.00, 24.00–37.00 and 20.00–28.00 mg/l. Sulphate ion variations in the six groups of groundwater samples of post-monsoon season were 0.56–13.01, 0.71–47.05, 2.32–41.84, 2.49–51.84, 1.84–53.70 and 34.53–34.53 mg/l, respectively. Concentrations of sulphate ions in the pre-monsoon sample groups were 16.00–205.00, 30.00–410.00, 160.00–452.00, 78.00–275.00, 154.00–215.00 and 132.00-280.00 mg/l. Variations in the phosphate ion concentration during post-monsoon were 0.01–3.12, 0.01–1.58, 0.08–0.71, 0.03–1.45, 0.01–0.63 and 0.10–0.10 mg/l, respectively, in I–VI sample groups. In the pre-monsoon season, the observed variations of phosphate in the sample groups I–III were, respectively, 0.00–0.20, 0.00–0.25, 0.00–0.15 mg/l. In the remaining sample groups, the concentration of phosphate was zero. The important sources of sulphate and phosphate ions are the fertilizers used in the agricultural fields.
Sources of fluoride
Literature studies indicate that the average value of fluoride in the earth crust is 625 mg/kg in various types of rocks (Mukherjee and Singh 2018; Tavener and Clark 2006; Edmunds and Smedley 2005). It is reported by various researchers that the important source of inorganic fluoride in groundwater is the fluoride-bearing minerals in rocks (Mukherjee and Singh 2018; Dey et al. 2012; Kalisinska and Palczewska-Komsa 2011; Camargo 2003). Fluoride ions reach the groundwater through weathering process. The granitic and gneissic rocks comprising of fluoride minerals such as apatite, hornblende, muscovite, biotite and amphiboles are the main resources for forming fluoride in the groundwater (Vithanage and Bhattacharya 2015). In the study area, hornblende–biotite gneiss is the important rock type, which possesses lot of fluoride-forming minerals as reported by (Vithanage and Bhattacharya 2015). In addition to the above geogenic source, applications of synthetic fertilizers in the agricultural fields may also contribute significant amount of fluoride ions to groundwater (Vithanage and Bhattacharya 2015). The schematic diagram indicating the sources of fluoride in the study area is shown in Fig. 3.
The schematic diagram showing the sources of fluoride contamination in groundwater (modified after Karunanidhi et al. 2019)
Rock–water Interaction
In hard rock terrain, groundwater chemistry is chiefly controlled by the groundwater interaction include aquifer minerals when its flows (Subramani et al. 2010). Groundwater has unique composition due to various subsurface hydrogeochemical processes such as water–rock interaction during rainfall infiltration and prolonged storage in the aquifer matrix, dissolution of mineral species and groundwater flow, etc. (Hem 1985). Therefore, hydrogeochemical processes that are responsible for changing the groundwater chemistry vary with respect to time and space. In the study area, groundwater level fluctuation was also high due to the usage of groundwater resources for drinking and irrigation purposes (Figs. 4 and 5). Gibbs plot was extensively used to find out the groundwater composition and to establish the lithological character of aquifers. It depicted three different fields like rock–water interaction, evaporation dominance and precipitation dominance (Gibbs, 1970). Major role of Gibbs plot is to understand the geochemical interactions and the mechanism of control in the chemistry of the groundwater (Adimalla et al. 2018; Adimalla and Venkatayogi 2018; Dişli 2017; Kouakou et al. 2017; Narsimha and Sudarshan 2017; Li et al. 2016). The Gibbs plot indicated that majority of the groundwater samples of the study area (about 90%) fall in the rock–water interaction field and few samples (about 10%) fall in the evaporation field during post- and pre-monsoon seasons (Fig. 6). It conveys that leaching of ions from the rocks control the major ion groundwater chemistry of this region (Subramani et al. 2010). Adimalla and Venkatayogi (2017) have also stated that rock weathering is the important source for high fluoride in groundwater of Medak region, Telangana State, India. Thomas et al. (2016) have conducted study in Ghana and reported that water–rock interaction including chemical weathering mainly influence the chemistry of groundwater. Excess of fluoride in groundwater comprises low ranges of calcium due to fluoride release from the mass through rock–water interaction as shown by the equations below
Spatial distribution of fluoride contamination
The presence of high amount of fluoride ion in groundwater makes its unsuitability for consumption purposes (Duraisamy et al. 2018). Since it is not possible to find out other alternative sources of fluoride-free water, dental fluorosis risks are higher in the study region. Fluoride concentration in the post-monsoon groundwater samples varied from 0.01 to 2.50 mg/l, whereas in the pre-monsoon samples it varied from 0.01 to 3.30 mg/l (Tables 2 and 3). As per the international standards, fluoride levels above 1.5 mg/l in drinking water are considered as hazardous (WHO 2011). The high fluoride levels are mostly related to interaction of water with rocks (Vithanage and Bhattacharya 2015; Robinson and Kapo 2003; Moore 2004; Dissanayake 1991; Stormer and Carmichael 1970; Apambire et al. 1997). Spatial distribution of fluoride concentrations in groundwater during post- and pre-monsoon seasons was plotted using GIS, which are presented in Figs. 7 and 8, respectively. During post-monsoon season, fluoride concentration was less than 0.49 mg/l at 17 locations (Well Nos. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 21, 29, 37 and 48). It ranged from 0.50 to 0.99 mg/l at 18 locations (Well Nos. 14, 15, 17, 18, 19, 22, 27, 30, 31, 46, 49, 50, 51, 52, 55, 56, 57 and 60). At 12 locations (Well Nos. 20, 25, 26, 28, 32, 35, 36, 40, 41, 44, 58 and 61) fluoride concentration varied between 1.00 and 1.49 mg/l. The concentration exceeded 1.5 mg/l and was below 2 mg/l at 6 locations (Well Nos. 24, 34, 42, 45, 47 and 53). Its concentration was between 2.00 and 2.49 mg/l at 7 locations (Well Nos. 16, 23, 33, 38, 39, 43 and 54), whereas it exceeded 2.49 mg/l at one location (Well No. 59) during the post-monsoon season. Similarly during pre-monsoon season, fluoride in groundwater of the study region was less than 0.49 mg/l at 15 locations (Well Nos. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 22, 23 and 41). Twenty sampling locations (Well Nos. 14, 16, 17, 19, 21, 27, 28, 29, 34, 35, 37, 38, 39, 43, 48, 49, 54, 56, 59 and 61) had the fluoride concentration ranges between 0.5 and 0.99 mg/l. Fluoride concentration was between 1.00 and 1.49 mg/l at 10 locations (Well Nos. 15, 20, 30, 32, 33, 47, 50, 51, 58 and 60) and between 1.50 and 1.99 mg/l at 8 locations (Well Nos. 24, 26, 36, 42, 45, 46, 52 and 53). At 2 locations (Well Nos. 13 and 18), its concentration ranged from 2.00 to 2.49 mg/l, whereas at 6 locations (Well Nos. 25, 31, 40, 44, 55 and 57) it exceeded 2.49 mg/l.
The results highlight that the fluoride concentration in groundwater of Shanmuganadhi River basin was high during pre-monsoon season when compare with post-monsoon season. It clearly indicates the dilution mechanism, which considerably reduces the concentration of fluoride due to monsoon rainfall infiltration (Subramani et al. 2010). Eventually, water level rise decreases the concentration of fluoride in many locations. Therefore, recharge of groundwater through artificial rainwater harvesting techniques will certainly improve the groundwater quality of this region. The spatial distribution maps indicate that 14 samples of post-monsoon season and 16 samples of pre-monsoon exceeded the maximum allowable limit of 1.5 mg/l for drinking purposes (WHO 2011). The maps further indicate that wells located in the charnockite formation were having very low fluoride content in groundwater when compare with the wells located in the hornblende–biotite gneiss formation. Out of 16 wells located in the charnockite formation, 14 wells were having very low (Class I) fluoride concentration (< 0.49 mg/l) during pre-monsoon season. One well was having low (Class II) concentration of fluoride (0.50–0.99 mg/l) and only one well exceeded the permissible limit of 1.5 mg/l. However, the concentration of fluoride in this well was less than 1.99 mg/l, which represents Class IV (High) category. Interestingly, this well was also located very close to the contact between charnockite and hornblende–biotite gneiss. During the post-monsoon season, out of 16 wells situated in the charnockite terrain, 12 wells were having very low (Class I) fluoride content (< 0.49), 2 wells were having low (Class II) fluoride content (0.49–0.99 mg/l), one well was having high (Class IV) fluoride content (1.50–1.99 mg/l) and one well was having extremely high (Class VI) fluoride content (2.00–2.49 mg/l). Similar to the pre-monsoon season, high and extremely high fluoride content wells were located very close to the contact between charnockite and gneissic formation.
Correlation between fluoride and other hydrogeochemical parameters
The correlation coefficient is used for the analysis of relationship among the physico-chemical variables in groundwater (Pearson 1896; Wu et al. 2014). According to Batabyal (2014), correlation coefficient is normally used to identify the relationship among the two variables. Nowadays, correlation matrices prepared using software packages are widely used in hydrogeochemical studies. Subramani et al. (2005) have prepared correlation matrices to understand the relationship among various hydrogeochemical parameters of post- and pre-monsoon seasons in river basin of Chithar, Tamil Nadu, India. In the current investigation, fluoride ion concentration in groundwater of Shanmuganadhi River basin was correlated with the major geochemical parameters such as pH, calcium, sodium and bicarbonate by plotting scatter plots. The statistical results (Tables 4 and 5) indicated the significant relationship among the geochemical parameters in the six different groups of fluoride. The minimum concentrations of the geochemical parameters were comparatively more in the higher classes of fluoride during post- and pre-monsoon seasons. Similarly, mean and maximum concentrations of most of the geochemical parameters of post-monsoon season were more in the higher fluoride classes. However, the mean and maximum concentrations of geochemical parameters of pre-monsoon season were more in the moderate fluoride class (Class III). The scatter diagrams prepared for post- and pre-monsoon seasons are illustrated in Figs. 9 and 10. In addition to the scatter diagrams, correlation matrices were prepared separately for post- and pre-monsoon seasons (Tables 6 and 7) to understand the relationship among the geochemical parameters. According to Adams et al. (2001) strong, moderate and weak positive correlations were represented by the correlation coefficient ranges from 0.70 to 1.00, from 0.30 to 0.70 and from 0.00 to 0.30, respectively. Similarly strong, moderate and weak negative correlations were represented by the correlation coefficient ranges from − 0.70 to − 1.00, from − 0.30 to − 0.70 and from 0.00 to − 0.30, respectively.
Strong positive correlation was exhibited among EC versus TDS and Mg2+ versus Na+ and Cl− during post-monsoon season. F− exhibited moderate positive correlation with EC, TDS, Mg2+ and SO42− during post-monsoon. During pre-monsoon season, fluoride exhibited moderate positive correlation with EC, TDS, K+, Na+, HCO3−, SO42− and NO3+. TDS exhibited strong to good positive correlation with all the ions in pre-monsoon season. It clearly shows that decrease in the water level raises the concentration of all the ions in groundwater. The high fluoride concentrations in groundwater of this region were found with high Na+, Mg2+, HCO3−, SO42− and NO3+ contents (Guo et al. 2007; Jacks et al. 1993; Handa 1975). Fluoridated endemic areas generally acquire a lot of bicarbonate in drinking water samples; therefore, Shanmuganadhi basin area is under fluoride endemic nature (Raju 2012; Viswanathan et al., 2009).
Probable fluorosis risk zones
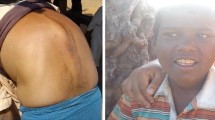
The excess fluoride in drinking groundwater is the reason for dental fluorosis and crippling fluorosis, whereas the disorder can case mottling of the teeth and ligament calcification (Kowalski 1999; Fawell et al. 2006; Mukherjee and Singh 2018; Karthikeyan et al. 1996; Dissanayake 1991). The results show that excess fluoride (> 1.5 mg/l) was observed in 23% of groundwater samples during post-monsoon season and in 26% of groundwater samples during pre-monsoon season (Table 1), which causes dental fluorosis in this region (Fig. 11). The health survey carried out in the study area shows that dental fluorosis was observed in many villages especially in children. The computed total hazard index (THI) and hazard quotient (HQ) to evaluate the health risk due to excess fluoride in groundwater of this basin highlight that the total non-carcinogenic risk for children and adults (men) ranged from 0.02 to 7.66 and from 0.10 to 5.67, respectively (Karunanidhi et al. 2019). Besides, the total health index exceeded the suggested limit of HQ > 1 for children and men in 80% and 69% of the samples, respectively (Karunanidhi et al. 2019). Therefore, children are mainly affected due to tooth decay when compared with adults. The study finally identified 26 villages in Shanmuganadhi River basin as fluorosis risk areas, which are Kamarajnagar, Kiranur, Shanmugavaram, Tirukkavndan Valasu, Manur, Sandachetti Valasu, Puliyamarathu Settu, Puliyampatti, Saravanampatti, Undhrapatti, Kongur, Nachiyappagoundan Valasu, Melkaraipatti, Vadipatti, Chinnagandhipuram, Velampatti, Kothamangalam, Kolumaikondan, Perumalpudur Pirivu, Tumbalapatti, Andinaicken Valasu, Narikkalpatti, Periyamotanootu, Kondapanaicken Patti, Balasamuthiram and A.Kalayampatti (Fig. 11).
Conclusions
Geochemical evaluation of fluoride contamination in groundwater from Shanmuganadhi River basin, South India, was investigated in the present study. Groundwater samples and their quality parameters of post- and pre-monsoon seasons were grouped into six categories based on the concentrations of fluoride. First three categories such as ‘Very Low’, ‘Low’ and ‘Moderate’ (Classes I, II and III) are within the permissible limit for consumption. Out of 61 groundwater samples, 6 and 8 samples of post- and pre-monsoon represented ‘High’ fluoride (Class IV) category. Seven and two samples represented ‘Very High’ fluoride (Class V) category, respectively, during post- and pre-monsoon seasons. One groundwater sample of post-monsoon and six samples of pre-monsoon represented ‘Extremely High’ fluoride category (Class VI). Therefore, fluorosis risk to human due to continuous intake of 23% of samples in post-monsoon and 26% of samples in pre-monsoon is unavoidable. The spatial variation analysis indicated that fluoride concentration gradually increased from the southern part of the basin to the northern part during both monsoon seasons. Therefore, fluorosis risk is high in the northern part of the basin.
The Gibbs diagram shows that nearly 90% of the groundwater samples in Shanmuganadhi River basin signify the rock–water interaction field. Therefore, leaching of fluoride from the bedrocks contaminates the groundwater. The study indicates that fluorosis risk areas in Shanmuganadhi basin are confined to the hornblende–biotite gneiss formations. Groundwater in charnockite formation does not cause fluorosis because of lower concentration of fluoride, and its existance in hilly region. Fluoride contamination was comparatively higher during pre-monsoon season in both the rock types. The recharge of groundwater due to monsoon rainfall dilutes the fluoride concentration at many places of the study area. Shanmuganadhi River basin falls under fluoride endemic category since the groundwater samples of this region attain more bicarbonate. The study highlights that 26 villages in this basin are prone for fluorosis.
Recommendations
The study finally recommends the following three methods to avoid the risks of fluorosis in Shanmuganadhi River basin
-
Implementation of large-scale rain water harvesting in the basin to recharge the groundwater will reduce the fluoride concentration.
-
Treatment of fluoride-rich groundwater by the village administration before providing drinking water supply will reduce the fluorosis risk.
-
Adopting reverse osmosis (RO) system in the houses will provide safe water for drinking and cooking.
References
Adams, S., Titus, R., Pietersen, K., Tredoux, G., & Harris, C. (2001). Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. Journal of Hydrology,241, 91–103. https://doi.org/10.1016/S0022-1694(00)00370-X.
Adimalla, N., Vasa, S. K., & Li, P. (2018). Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: An insight of controlling factors of fluoride enrichment model. Modeling Earth System and Environment.. https://doi.org/10.1007/s40808-018-0443-z.
Adimalla, N., & Venkatayogi, S. (2017). Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environmental Earth Sciences,76, 45. https://doi.org/10.1007/s12665-016-6362-2.
Adimalla, N., & Venkatayogi, S. (2018). Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Applied Water Science. https://doi.org/10.1007/s13201-018-0682-1.
Aghapour, S., Bina, B., Tarrahi, M. J., Amiri, F., & Ebrahimi, A. (2018). Distribution and health risk assessment of natural fluoride of drinking groundwater resources of Isfahan, Iran, using GIS. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-018-6467-z.
Ahada, C. P. S., & Suthar, S. (2017). Assessment of human health risk associated with high groundwater fluoride intake in Southern Districts of Punjab, India. Exposure and Health. https://doi.org/10.1007/s12403-017-0268-4.
Ali, S. A., & Ali, U. (2018). Hydrochemical characteristics and spatial analysis of groundwater quality in parts of Bundelkhand Massif, India. Applied Water Science. https://doi.org/10.1007/s13201-018-0678-x.
Ali, S., Thakur, S. K., Sarkar, A., & Shekhar, S. (2016). Worldwide contamination of water by fluoride. Environmental Chemistry Letters,14, 291–315. https://doi.org/10.1007/s10311-016-0563-5.
Anandakumar, S., Subramani, T., & Elango, L. (2009). Major ion groundwater chemistry of Lower Bhavani River basin, Tamil Nadu, India. Journal of Applied Geochemistry,11(1), 92–101.
Apambire, W. B., Boyle, D. R., & Michel, F. A. (1997). Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environmental Geology,33, 13–24. https://doi.org/10.1007/s002540050221.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington: American Public Health Association/American Water Works Association/Water Environment Federation.
Aqeel, A., Al-Amry, A., & Alharbi, O. (2017). Assessment and geospatial distribution mapping of fluoride concentrations in the groundwater of Al-Howban basin, Taiz-Yemen. Arabian Journal of Geosciences. https://doi.org/10.1007/s12517-017-3069-y.
Aref, F., & Roosta, R. (2016). Assessment of groundwater quality and hydrochemical characteristics in Farashband plain, Iran. Arabian Journal of Geosciences. https://doi.org/10.1007/s12517-016-2781-3.
Batabyal, A. K. (2014). Correlation and multiple linear regression analysis of groundwater quality data of Bardhaman District, West Bengal, India. International Journal of Research in Chemistry Environment,4(4), 42–51.
Batabyal, A. K. (2018). Hydrogeochemistry and quality of groundwater in a part of Damodar Valley, Eastern India: An integrated geochemical and statistical approach. Stochastic Environmental Research and Risk Assessment,32(8), 2351–2368. https://doi.org/10.1007/s00477-018-1552-y.
Bhargava, D. S., & Killender, D. J. (1988). The technology of water resources in industries: A rational approach. Journal of Indian Water Works Association,20, 107–112.
Bhattacharya, P., Lesafi, F., Filemon, R., Ligate, F., Ijumulana, J., & Mtalo, F. (2016). Geogenic fluoride and arsenic contamination in the groundwater environments in Tanzania. EGU General Assembly Conference Abstracts,18, 16677.
Camargo, J. A. (2003). Fluoride toxicity to aquatic organisms: A review. Chemosphere,50, 251–264. https://doi.org/10.1016/S0045-6535(02)00498-8.
Chidambaram, S., Bala Krishna Prasad, M., Manivannan, R., Karmegam, U., Singaraja, C., Anandhan, P., et al. (2013). Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamilnadu (India). Environmental Earth Sciences,68, 333–342. https://doi.org/10.1007/s12665-012-1741-9.
Choi, A. L., Sun, G., Zhang, Y., & Grandjean, P. (2012). Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environmental Health Perspect,120(10), 1362–1368.
Chuah, C. J., Lye, H. R., Ziegler, A. D., Wood, S. H., Kongpun, C., & Rajchagool, S. (2016). Fluoride: A naturally-occurring health hazard in drinking-water resources of Northern Thailand. The Science of the Total Environment,545, 266–279.
Dar, M. A., Sankar, K., & Dar, I. A. (2011). Fluorine contamination in groundwater: A major challenge. Environmental Monitoring and Assessment,173, 955–968. https://doi.org/10.1007/s10661-010-1437-0.
Datta, P. S., Deb, D. L., & Tyagi, S. K. (1996). Stable isotope (18O) investigations on the processes controlling fluoride contamination of groundwater. Journal of Contaminant Hydrology,24(1), 85–96. https://doi.org/10.1016/0169-7722(96)00004-6.
Dean, H. T., & Elvove, E. (1937). Further studies on the minimal threshold of chronic endemic dental fluorosis. Public Health Reports (1896-1970), 52(37), 1249.
Dey, R. K., Swain, S. K., Mishra, S., Sharma, P., Patnaik, T., Singh, V. K., et al. (2012). Hydrogeochemical processes controlling the high fluoride concentration in groundwater: A case study at the Boden block area, Orissa, India. Environmental Monitoring and Assessment,184, 3279–3291. https://doi.org/10.1007/s10661-011-2188-2.
Dissanayake, C. B. (1991). The fluoride problem in the ground water of Sri Lanka - environmental management and health. International Journal of Environmental Studies, 38(2–3), 137–155.
Dişli, E. (2017). Hydrochemical characteristics of surface and groundwater and suitability for drinking and agricultural use in the upper Tigris River basin, Diyarbakır–batman, Turkey. Environmental Earth Sciences,76, 500. https://doi.org/10.1007/s12665-017-6820-5.
Duraisamy, S., Govindhaswamy, V., Duraisamy, K., Krishinaraj, S., Balasubramanian, A., & Thirumalaisamy, S. (2018). Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0183-z.
Edmunds, W. M., & Smedley, P. L. (2001). Fluoride in natural waters. In O. Selinus (Ed.), Essentials of medical geology (pp. 311–336). Dordrecht: Springer.
Edmunds, W. M., & Smedley, P. L. (2005). Fluoride in natural waters. In O. Selinus (Ed.), Essentials of medical geology (pp. 311–336). Dordrecht: Springer.
Fawell, J., Bailey, K., Chilton, J., Dahi, E., Fewtrell, L., & Magara, Y. (2006). Fluoride in drinking-water. London: IWA Publishing.
Francisca, M. M., Patrick, C. K., & Peter, G. N. (2017). Assessment of the impact of groundwater fluoride on human health: A case study of Makindu District in Kenya. Journal of Earth Science and Climate Change,2017(8), 4. https://doi.org/10.4172/2157-7617.1000396.
Gao, H., Jin, Y., & Wei, J. (2012). Health risk assessment of fluoride in drinking water from Anhui Province in China. Environmental Monitoring and Assessment, 185(5), 3687–3695. https://doi.org/10.1007/s10661-012-2820-9.
Gao, H. J., Jin, Y. Q., & Wei, J. L. (2013). Health risk assessment of fluoride in drinking water from Anhui Province in China. Environmental Monitoring and Assessment,185(5), 3687–3695. https://doi.org/10.1007/s10661-012-2820-9.
Garg, V. K., Suthar, S., Singh, S., Sheoran, A., Garima, M., & Jai, S. (2009). Drinking water quality in villages of southwestern Haryana, India: Assessing human health risks associated with hydrochemistry. Environmental Geology,58, 1329–1340.
Ghosh, A., Mukherjee, K., Ghosh, S. K., & Saha, B. (2013). Sources and toxicity of fluoride in the environment. Research on Chemical Intermediates,39, 2881–2915. https://doi.org/10.1007/s11164-012-0841-1.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science,17, 1088–1090.
Giridharan, L., Venugopal, T., & Jayaprakash, M. (2008). Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of river Cooum, Chennai, India. Environmental Monitoring and Assessment,143, 161–178. https://doi.org/10.1007/s10661-007-9965-y.
Gopalakrishnan, S. B., Viswanathan, G., & Ilango, S. S. (2012). Prevalence of fluorosis and identification of fluoride endemic areas in Manur block of Tirunelveli district, Tamil Nadu, South India. Applied Water Science,2, 235–243. https://doi.org/10.1007/s13201-012-0043-4.
Gowrisankar, G., Jagadeshan, G., & Elango, L. (2017). Managed aquifer recharge by a check dam to improve the quality of fluoride-rich groundwater: A case study from southern India. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-017-5910-x.
Guo, Q., Wang, Y., Ma, T., & Ma, R. (2007). Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. Journal of Geochemical Exploration, 93(1), 1–12. https://doi.org/10.1016/j.gexplo.2006.07.001.
Handa, B. K. (1975). Geochemistry and Genesis of Fluoride-Containing Ground Waters in India. Ground Water, 13(3), 275–281.
Hanipha, M. M., & Hussain, Z. A. (2013). Study of groundwater quality at Dindigul town, Tamil Nadu, India. International Research Journal of Environment Sciences,2, 68–73.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water (Vol. 2254). Reston: Department of the Interior, US Geological Survey.
Hema, S., Subramani, T., & Elango, L. (2010). GIS Study on vulnerability assessment of water quality in a part of Cauvery River. International Journal of Environmental Sciences,1(1), 1–17.
Irigoyen-Camacho, M., Pérez, A. G., González, A. M., & Alvarez, R. H. (2016). Nutritional status and dental fluorosis among schoolchildren in communities with different drinking water fluoride concentrations in a central region in Mexico. The Science of the Total Environment,541, 512–519. https://doi.org/10.1016/j.scitotenv.2015.09.085.
Jacks, G., Rajagopalan, K., Alveteg, T., & Jönsson, M. (1993). Genesis of high-F groundwaters, southern India. Applied Geochemistry, 8, 241–244.
Jadhav, S. V., Bringas, E., Yadav, G. D., Rathod, V. K., Ortiz, I., & Marathe, K. V. (2015). Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. Journal of Environmental Management,162, 306–325. https://doi.org/10.1016/j.jenvman.2015.07.020.
Jain, P. K. (1998). Hydrology and quality of groundwater Hirapur district, Sagar (M.P), India. Pollution Research,17(1), 91–94.
Jayaprakash, M., Giridharan, L., Venugopal, T., Krishna Kumar, S. P., & Periakali, P. (2008). Characterization and evaluation of the factors affecting the geochemistry of groundwater in Neyveli, Tamil Nadu, India. Environmental Geology,54, 855–867. https://doi.org/10.1007/s00254-007-0868-6.
Kalisinska, E., & Palczewska-Komsa, M. (2011). Teeth of the red fox Vulpes vulpes (L., 1758) as a bioindicator in studies on fluoride pollution. Acta Theriologica (Warsz),56, 343–351. https://doi.org/10.1007/s13364-011-0039-8.
Karthikeyan, G., Pius, A., & Apparao, B. V. (1996). Contribution of fluoride in water and food to the prevalence of fluorosis in areas of Tamil Nadu in south India. Fluoride,29, 151–155.
Karthikeyan, G., & Shanmugasundarraj, A. (2000). Isopleth mapping and in insitu Fluoride dependence on water quality in the Krishnagiri block of Tamil Nadu in South India. Fluoride,33, 121–127.
Karthikeyan, K., Nanthakumar, K., Velmurugan, P., Tamilarasi, S., & Lakshmanaperumalsamy, P. (2010). Prevalence of certain inorganic constituents in groundwater samples of Erode district, Tamilnadu, India, with special emphasis on fluoride, fluorosis and its remedial measures. Environmental Monitoring and Assessment,160, 141–155. https://doi.org/10.1007/s10661-008-0664-0.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Wu, J., & Srinivasamoorthy, K. (2019). Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Human and Ecological Risk Assessment: An International Journal,4, 5. https://doi.org/10.1080/10807039.2019.1568859.
Karunanidhi, D., Vennila, G., Suresh, M., & Subramanian, S. K. (2013). Evaluation of the groundwater quality feasibility zones for irrigational purposes through GIS in Omalur Taluk, Salem District, South India. Environmental Science and Pollution Research,20(10), 7320–7333. https://doi.org/10.1007/s11356-013-1746-2.
Keshavarzi, B., Moore, F., Esmaeili, A., & Rastmanesh, F. (2010). The source of fluoride toxicity in Muteh area, Isfahan, Iran. Environmental Earth Sciences,61(4), 777–786. https://doi.org/10.1007/s12665-009-0390-0.
KheradPisheh, Z., Ehrampoush, M., Montazeri, A., Mirzaei, M., Mokhtari, M., & Mahvi, A. (2016). Fluoride in drinking water in 31 provinces of Iran. Exposure and Health,8(4), 465–474. https://doi.org/10.1007/s12403-016-0204-z.
Kim, K., & Jeong, G. Y. (2005). Factors influencing natural occurrence of fluoride-rich groundwaters: A case study in the southeastern part of the Korean Peninsula. Chemosphere,58(10), 1399–1408. https://doi.org/10.1016/j.chemosphere.2004.10.002.
Kouakou, V. K., Obuobie, E., Banning, A., & Wohnlich, S. (2017). Hydrochemical characteristics of groundwater and surface water for domestic and irrigation purposes in Vea catchment, northern Ghana. Environmental Earth Sciences,76, 185. https://doi.org/10.1007/s12665-017-6490-3.
Kowalski, F. (1999). Fluoridation. Journal American Water Works Association,91, 4.
Kumar, M. G., Agarwal, A. K., & Rameshwar, B. (2008). Delineation of potential sites for water harvesting structures using remote sensing and GIS. Journal of the Indian Society of Remote Sensing,36(4), 323–334. https://doi.org/10.1007/s12524-008-0033-z.
Kut, K. M. K., Sarswat, A., Srivastava, A., Pittman, C. U., & Mohan, D. (2016). A review of fluoride in African groundwater and local remediation methods. Groundwater for Sustainable Development,2, 190–212.
Li, P., He, X., Li, Y., & Xiang, G. (2018). Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: A case study of Tongchuan, Northwest China. Exposure and Health. https://doi.org/10.1007/s12403-018-0278-x.
Li, P., Wu, J., & Qian, H. (2016). Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arabian Journal of Geosciences,9, 15. https://doi.org/10.1007/s12517-015-2059-1.
Li, X., Hou, X., Zhou, Z., & Liu, L. (2009). Distribution and 428 geochemical evolution of fluoride in groundwater of Taiyuan basin, China. In ICEET ‘09 proceedings of the 2009 international conference on energy and environment technology (Vol. 2, pp. 507–510).
Manikandan, S., Chidambaram, S., Ramanathan, A. L., Prasanna, M. V., Karmegam, U., Singaraja, C., et al. (2012). A study on the high fluoride concentration in the magnesiumrich waters of hard rock aquifer in Krishnagiri district, Tamilnadu, India. Arabian Journal of Geosciences,7, 273–285. https://doi.org/10.1007/s12517-012-0752-x.
Marghade, D., Malpe, D. B., & Zade, A. B. (2011). Geochemical characterization of groundwater from the northeastern part of Nagpur urban, Central India. Environmental Earth Sciences,62, 1419–1430.
Mondal, D., Dutta, G., & Gupta, S. (2016). Inferring the fluoride hydrogeochemistry and effect of consuming fluoridecontaminated drinking water on human health in some endemic areas of Birbhum district, West Bengal. Environmental Geochemistry and Health,38, 557–576. https://doi.org/10.1007/s10653-015-9743-7.
Moore, R. B. (2004). Quality of water in the fractured-bedrock aquifer of New Hampshire, U.S. Geological Survey Scientific Investigations Report. 2004–5093, 30.
Mukherjee, I., & Singh, U. K. (2018). Groundwater fluoride contamination, probable release, and containment mechanisms: A review on Indian context. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0096-x.
Nag, S. K., & Suchetana, B. (2016). Groundwater quality and its suitability for irrigation and domestic purposes: A study in Rajnagar Block, Birbhum District, West Bengal, India. Journal of Earth Science and Climate Change.,7, 337. https://doi.org/10.4172/2157-7617.1000337.
Narsimha, A., & Rajitha, S. (2018). Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Human and Ecological Risk Assessment: An International Journal. https://doi.org/10.1080/10807039.2018.1438176.
Narsimha, A., & Sudarshan, V. (2016). Contamination of fluoride in groundwater and its effect on human health: A case study in hard rock aquifers of Siddipet, Telangana State, India. Applied Water Science,2017(7), 2501–2512. https://doi.org/10.1007/s13201-016-0441-0.
Narsimha, A., & Sudarshan, V. (2017). Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana state, India. Applied Water Science,7, 2717–2725. https://doi.org/10.1007/s13201-016-0489-x.
Oruc, N. (2008). Occurrence and problems of high fluoride waters in Turkey: An overview. Environmental Geochemistry and Health,30(4), 315–323. https://doi.org/10.1007/s10653-008-9160-2.
Panneer, M., Sivakumar, R., & Senthilkumar, M. (2017). Fluoride hydrogeochemistry and its occurrence in drinking water in Morappur region of Dharmapuri District, South India. International Journal of Environmental Science and Technology,14(9), 1931–1944. https://doi.org/10.1007/s13762-017-1277-3.
Patel, P., Raju, N. J., Reddy, B. C. S. R., Suresh, U., Gossel, W., & Wycisk, P. (2016). Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin, Andhra Pradesh, India. Environmental Earth Sciences,75, 611. https://doi.org/10.1007/s12665-015-5108-x.
Patolia, P., & Sinha, A. (2017). Fluoride contamination in Gharbar Village of Dhanbad District, Jharkhand, India: Source identification and management. Arabian Journal of Geosciences. https://doi.org/10.1007/s12517-017-3164-0.
Pearson, K. (1896). Mathematical contributions to the theory of evolution, III. Regression, heredity and panmixia. Philosophical Transactions of the Royal Society of London,187, 253–318.
Periakali, P., Subramanian, S., Eswaramoorthi, S., Arul, B., Rao, N. R., & Sridhar, S. G. D. (2001). Distribution of fluoride in the ground water of Salem and Namakkal districts, Tamil Nadu. Journal of Applied Geochemistry,3, 120–132.
Prajapati, M., Jariwala, N., & Agnihotri, P. (2017). Spatial distribution of groundwater quality with special emphasis on fluoride of Mandvi Taluka, Surat, Gujarat, India. Applied Water Science,7(8), 4735–4742. https://doi.org/10.1007/s13201-017-0636-z.
Prasad, N. B. N. (1984). Hydrogeological studies in the Bhadra River basin. Ph.D. thesis, University of Mysore, Karnataka, India, p. 323.
Raj, D., & Shaji, E. (2017). Fluoride contamination in groundwater resources of Alleppey, southern India. Geoscience Frontiers,8(1), 117–124. https://doi.org/10.1016/j.gsf.2016.01.002.
Raju, N. J. (2012). Evaluation of hydrogeochemical processes in the Pleistocene aquifers of Middle Ganga Plain, Uttar Pradesh, India. Environmental Earth Sciences,65(4), 1291–1308.
Raju, N. J. (2016). Prevalence of fluorosis in the fluoride enriched groundwater in semi-arid parts of eastern India: Geochemistry and health implications. Quaternary International. https://doi.org/10.1016/j.quaint.2016.05.028.
Rao, N. C. R. (2003). Fluoride and environment—A review. In M. J. V. Bunch, M. Suresh, & T. V. Kumaran (Eds.), Proceedings of third international conference on environment and health. Chennai: York University.
Rao, N. S. (1997). The occurrence and behaviour of fluoride in the groundwater of the Lower Vamsadhara River basin, India. Hydrological Sciences Journal,42(6), 877–892. https://doi.org/10.1080/02626669709492085.
Rao, N. S. (2006). Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environmental Geology,51(4), 631–645. https://doi.org/10.1007/s00254-006-0358-2.
Rasool, A., Xiao, T., Baig, Z. T., Masood, S., Mostafa, K. M., & Iqbal, M. (2015). Co-occurrence of arsenic and fluoride in the groundwater of Punjab, Pakistan: Source discrimination and health risk assessment. Environmental Science and Pollution Research,22(24), 19729–19746. https://doi.org/10.1007/s11356-015-5159-2.
Robinson, G. R., & Kapo, K. E. (2003). Generalized lithology and lithogeochemical character of nearsurface bedrock in the New England Region. Reston: U.S. Geological Survey.
Sahu, P., Kisku, G. C., Singh, P. K., Kumar, V., Kumar, P., & Shukla, N. (2018). Multivariate statistical interpretation on seasonal variations of fluoride-contaminated groundwater quality of Lalganj Tehsil, Raebareli District (UP), India. Environmental Earth Sciences. https://doi.org/10.1007/s12665-018-7658-1.
Sajil Kumar, P. J. (2017). Grounding a natural background level for fluoride in a potentially contaminated crystalline aquifer in south India. Environmental Science and Pollution Research,24(34), 26623–26633. https://doi.org/10.1007/s11356-017-0239-0.
Sakram, G., Kuntamalla, S., Machender, G., Dhakate, R., & Narsimha, A. (2018). Multivariate statistical approach for the assessment of fluoride and nitrate concentration in groundwater from Zaheerabad area, Telangana State, India. Sustainable Water Resources Management. https://doi.org/10.1007/s40899-018-0258-0.
Salve, P., Maurya, A., Kumbhare, P., Ramteke, D., & Wate, S. (2008). Assessment of groundwater quality with respect to fluoride. Bulletin of Environmental Contamination and Toxicology,81(3), 289–293. https://doi.org/10.1007/s00128-008-9466-x.
Saraf, A. K., & Choudhury, P. R. (1998). Integrated remote sensing and GIS for groundwater exploration and identification of artificial recharge sites. International Journal of Remote Sensing,19(10), 1825–1841. https://doi.org/10.1080/014311698215018.
Satheeshkumar, S., Venkateswaran, S., & Kannan, R. (2017). Temporary fluoride concentration changes in groundwater in the context of impact assessment in the Vaniyar sub-basin, South India. Acta Geochimica,36(1), 112–123. https://doi.org/10.1007/s11631-016-0137-z.
Shaji, E., Viju, J., & Thambi, D. (2007). High fluoride in groundwater of Palghat District, Kerala. Current Science,92, 240–245.
Singh, S., Raju, N. J., & Ramakrishna, Ch. (2015). Evaluation of groundwater quality and its suitability for domestic and irrigation use in parts of the Chandauli-Varanasi region, Uttar Pradesh, India. Journal of Water Resource and Protection,7, 482–497.
Srinivasamoorthy, K., Vijayaraghavan, K., Vasanthavigar, M., Sarma, S., Chidambaram, S., & Anandhan, P. (2012). Assessment of groundwater quality with special emphasis on fluoride contamination in crystalline bed rock aquifers of Mettur region, Tamilnadu, India. Arabian Journal of Geosciences,5, 83–94. https://doi.org/10.1007/s12517-010-0162-x.
Stormer, J. C., & Carmichael, I. S. E. (1970). Villiaumite and the occurrence of fluoride minerals in igneous rocks. American Mineralogist,55, 126–134.
Subba Rao, N. (2011). High-fluoride groundwater. Environmental Monitoring and Assessment,176, 637–645.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River basin, Tamil Nadu, India. Environmental Geology,47, 1099–1110.
Subramani, T., Elango, L., & Rajmohan, N. (2010). Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environmental Monitoring and Assessment,162, 123–137.
Sudhakar, A., & Narsimha, A. (2013). Suitability and assessment of groundwater for irrigation purpose: A case study of Kushaiguda area, Ranga Reddy district, Andhra Pradesh, India. Advances in Applied Science Research,4(6), 75–81.
Susheela, A. K. (2003). Treatise on fluorosis (2nd ed.). Delhi: Fluorosis Res. Rural Dev. Found.
Tavener, S. J., & Clark, J. H. (2006). Fluorine: Friend or foe? A green chemist’s perspective. In A. Tressaud (Ed.), Fluorine and the environment: Agrochemicals, archaeology, green chemistry and water (Chapter 5) (pp. 177–202). Amsterdam: Elsevier.
Tejaswi, S. K., Shetty, S., Annapoorna, B., Pujari, S. C., Reddy, S., & Nandlal, B. (2013). A pioneering study of dental fluorosis in the libyan population. Journal of International Oral Health: JIOH,5, 67.
Thivya, C., Chidambaram, S., Rao, M. S., Thilagavathi, R., Prasanna, M. V., & Manikandan, S. (2015). Assessment of fluoride contaminations in groundwater of hard rock aquifers in Madurai district, Tamil Nadu (India). Applied Water Science,7, 1011–1023. https://doi.org/10.1007/s13201-015-0312-0.
Thomas, K. B., Opoku, F., Acquaah, S. O., & Akoto, O. (2016). Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environmental Earth Sciences,75, 489. https://doi.org/10.1007/s12665-015-5105-0.
Vennila, G., Subramani, T., & Elango, L. (2008). GIS based groundwater quality assessment of Vattamalaikarai basin, Tamil Nadu, India. Journal of Nature Environment and Pollution Technology,7(4), 585–592.
Vikas, C., Kushwaha, R., Ahmad, W., Prasannakumar, V., & Reghunath, R. (2013). Genesis and geochemistry of high fluoride bearing groundwater from a semi-arid terrain of NW India. Environmental Earth Sciences,68, 289–305.
Viswanathan, G., Jaswanth, A., Gopalakrishnan, S., Ilango, S. S., & Aditya, G. (2009). Determining the optimal fluoride concentration in drinking water for fluoride endemic regions in South India. Science of the Total Environment,407, 5298–5307. https://doi.org/10.1016/j.scitotenv.2009.06.028.
Vithanage, M., & Bhattacharya, P. (2015). Fluoride in the environment: Sources, distribution and defluoridation. Environmental Chemistry Letters,13, 131–147. https://doi.org/10.1007/s10311-015-0496-4.
Wasana, H. M., Perera, G. D., Gunawardena, P. D. S., Fernando, P. S., & Bandara, J. (2017). WHO water quality standards vs synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Scientific Reports,7, 42516. https://doi.org/10.1038/srep42516.
WHO. (2011). Guidelines for drinking water quality. Geneva: World Health Organization.
Wu, J., Li, P., Qian, H., Duan, Z., & Zhang, X. (2014). Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: Case study in Laoheba phosphorite mine in Sichuan, China. Arabian Journal of Geosciences,7(10), 3973–3982. https://doi.org/10.1007/s12517-013-1057-4.
Young, S. M., Pitawala, A., & Ishiga, H. (2011). Factors controlling fluoride contents of groundwater in north-central and north-western Sri Lanka. Environment and Earth Science,63, 1333–1342.
Zabin, S. A., Foaad, M., & Al-Ghamdi, A. Y. (2008). Noncarcinogenic risk assessment of heavy metals and fluoride in some water wells in the Al-Baha Region, Saudi Arabia. Human and Ecological Risk Assessment,14(6), 1306–1317. https://doi.org/10.1080/10807030802494667.
Acknowledgements
The authors are greatly indebted to the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India (File No. ECR/2017/000132 dated 18.07.2017) for providing the grants and support to carry out this work effectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aravinthasamy, P., Karunanidhi, D., Subramani, T. et al. Geochemical evaluation of fluoride contamination in groundwater from Shanmuganadhi River basin, South India: implication on human health. Environ Geochem Health 42, 1937–1963 (2020). https://doi.org/10.1007/s10653-019-00452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00452-x