Abstract
The occurrence of dental/skeletal fluorosis among the people in the study area provided the motivation to assess the distribution, severity and impact of fluoride contamination in groundwater of Bankura district at Simlapal block, West Bengal, India. To meet the desired objective, groundwater samples were collected from different locations of Laxmisagar, Machatora and Kusumkanali regions of Simlapal block at different depths of tube wells in both pre- and post-monsoon seasons. Geochemical results reveal that the groundwaters are mostly moderate- to hard-water type. Of total groundwater samples, 37% are situated mainly in relatively higher elevated region containing fluoride above 1.5 mg/L, indicating that host aquifers are severely affected by fluoride contamination. Machatora region is highly affected by fluoride contamination with maximum elevated concentration of 12.2 mg/L. Several symptoms of fluorosis among the different age-groups of people in Laxmisagar and Machatora areas are indicating consumption of fluoridated water for prolonged period. The groundwater samples were mainly Na–Ca–HCO3 type and rock dominance indicating the dissolution of minerals taking place. Ion exchange between OH− ion and F− ion present in fluoride-bearing mineral is the most dominant mechanism of fluoride leaching. High concentration of Na+ and HCO3 − increases the alkalinity of the water, providing a favorable condition for fluoride to leach into groundwater from its host rocks and minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride contamination in groundwater is a worldwide problem. Around 200 million people, from 25 nations, are at risk due to fluoride enrichment in groundwater (Kut et al. 2016; Hallett et al. 2015; Ayoob and Gupta 2006). Groundwater is the major source of drinking water in the rural areas of developing countries like India (Selvakumar et al. 2017; Bhattacharya et al. 1997). Since last few decades, fluoride contamination in groundwater has become a serious threat (Kim et al. 2012; Yadav and Khan 2010; Yadav and Kumar 2010; Sabal and Khan 2008; Wang et al. 1999). Chronic injection of high fluoride contaminated water may cause dental fluorosis and in extreme cases, skeletal fluorosis (Raju 2016). Totally, twenty states of India including 43 blocks of seven districts of West Bengal are suffering due to elevated fluoride concentration in groundwater (Kumar et al. 2016; Datta et al. 2014; Majumdar 2011; Dutta et al. 2006). According to joint action plan of Public Health Engineering Department (PHED) with The United Nations Children’s Fund (UNICEF) 2006, it was identified that seven districts of West Bengal, i.e., Bankura, Purulia, Birbhum, Maldah, Uttar Dinajpur, Dakshin Dinajpur and South 24 Parganas, were having elevated fluoride concentration above the permissible limit 1.5 mg/L as recommended by World Health Organization (WHO) and Bureau of Indian Standard (BIS) (Gupta and Misra 2016; Mondal et al. 2014; Majumdar 2011; Adler and World Health Organization 1970; Amini et al. 2008). Simlapal block is one of the fluoride-affected areas of Bankura district (Samal et al. 2015). The aquifers are generally contaminated with fluoride from the fluoride-bearing rocks and minerals present in the subsurface layers (Alarcón-Herrera et al. 2013). Due to unavailability of appropriate data of the subsurface layers and aquifers, the contaminant transport mechanism remains obscure. Several researches have been carried out since last few decades to understand the principal factors responsible for occurrence and mobility of fluoride in aquifer (Ayenew 2008). The geographical areas associated with high fluoride contamination are mainly characterized by arid and semiarid climatic conditions (Edmunds and Smedley 2013). The factors controlling the dissolution and leaching of fluoride-bearing minerals are pH, temperature, the degree of water–rock interaction, long groundwater residence time, presence of complex ions and solubility of fluoride-bearing minerals (Apambire et al. 1997; Raj and Shaji 2017). In India, fluorite, fluorapatite, mica (biotite, muscovite), amphiboles, hornblende, topaz, rock phosphate, cryolite and hydroxylapatite are mainly responsible for fluoride enrichment in groundwater (Patel et al. 2014; Chakrabarti and Ray 2013; Agrawal et al. 1997). The presence of excessive concentrations of fluoride in groundwater may persist for years, decades or even centuries and can reach the food system (Dey and Giri 2016; Peckham and Awofeso 2014; Manarelli et al. 2014). The villagers residing in the affected areas are consuming fluoridated water due to the unavailability of other drinking water sources. The main aim of the present research is to delineate the safe and unsafe aquifers and to prepare spatiotemporal variation map of fluoride contamination of the ‘study area,’ i.e., Simlapal block, to facilitate the groundwater management. Another objective of this work is to understand the leaching and mobilization process of fluoride in groundwater system using statistical analysis of the physicochemical parameters.
Study area
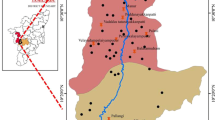
Bankura district is geomorphologically divided into three zones. The western part is dominated by hilly hard rock. The eastern part contains sedimentary alluvial plains. The central part consists of both rocky and alluvial formation (Chakrabarti and Bhattacharya 2013). Simlapal block is located in eastern part of the district where the study was conducted. In the present study, groundwater sampling was carried out from Laxmisagar, Kusumkanali and Machatora regions to check the severity of fluoride contamination in groundwater of Simlapal block. It is situated in Khatra subdivision of Bankura district and geographically extended from 22°59′38.84″N to 22°50′34.42″N latitude and from 86°55′20.15″E to 87°13′06.10″E longitude. It is surrounded by Taldangra block in the north, Khatra block in the west, Raipur block in the southwest and West Midnapore district in the east. The annual average rainfall in the study area is about 1400 mm/year, and average temperature during summer and winter is 40 and 10 °C, respectively. The study area is a drought-prone zone with undulated land profile. The sampling locations of the study area are shown in Fig. 1.
The spatial distribution of fluoride illustrates that western part, which is situated at higher elevated area (Fig. 1), is mainly affected by fluoride contamination as shown in Fig. 2. This area is more likely to contain fluoride-bearing minerals from where the fluoride is getting leached into the groundwater and spread over the lower elevated zone (eastern part of study area). In the southwestern part of the study area, fluoride concentration is within the permissible limit.
From the preliminary investigation, several symptoms of fluorosis were observed among the different age-group of people in Laxmisagar and Machatora areas (shown in Fig. 3), which is the indication of consumption of fluoride-contaminated aquifer water for prolonged period. The results suggest that fluoride contamination level is predominant in higher elevated zone, i.e., Laxmisagar and Machatora regions (Fig. 1), which leads to mobilization of fluoride from higher to lower elevation zone.
Materials and methods
To understand the distribution of fluoride contamination level in the study area, the water sampling was carried out from 43 numbers of different locations during both pre-monsoon and post-monsoon seasons. The groundwater samples were collected randomly from the different locations of the study area from the tube wells generally installed by PHEDWB (Public Health Engineering Department, West Bengal). The groundwater samples were collected and analyzed in the field after few minutes of purging to get fresh aquifer water. In the field, pH, TDS, EC and temperature were measured using Multi-Parameter PCSTestr 35 (Eutech), and ORP was measured using ORPTestr 10 (Eutech). For analysis of other physicochemical parameters, the groundwater samples were collected and stored in two separate 500-ml airtight polyethylene bottles to facilitate the cations and anions analysis. Samples were acidified using 50% HNO3 for lowering the pH below 2 to preserve the cations. No acidification was required for anions and fluoride estimations. The coordinates of sampling locations were identified using GPS Trimble Juno SA. The cations (Ca2+, Mg2+, Na+, K+) were analyzed using AAS (Atomic Absorption Spectrophotometer), PerkinElmer 900T, and anions (SO 2−,4 NO3 −, F−, CO3 2−,HCO3 − and Cl−) were estimated as per standard method (APHA 2005). Alkalinity values of the unacidified samples were determined by the titrimetric method as per specified in standard method (APHA 2005). In titration method, the maximum deviation can be observed of 1 mg CaCO3/L in the groundwater samples (APHA 2005). The chloride concentrations of the samples were estimated using argentometric method with a relative standard deviation of 4.2% and a relative error of 1.7% (APHA 2005). Sulfate and nitrate were estimated by spectrophotometric method using UV–visible spectrophotometer (Model No: UV2300) with the photometric accuracy of ± 0.002 Å. Fluoride was estimated using ion-selective electrode (Orion 4 Star, Thermo Scientific) by following standard method. To restrict the interference of other ions, buffer solution (TISAB III, Orion) was added at 1:10 ratio to the samples and standards as specified by standard method. After addition of TISAB III, the fluoride concentrations of the samples were immediately measured. The detection limit of the ion-selective electrode is 0.02 ppm for fluoride measurement. Sodium fluoride (AR Grade, Merck) was used to prepare standard and stock solutions. Analytical-grade chemicals were used for the standard preparation and also to conduct all desired experiments. The plasticwares were used to store the stock solutions and also to conduct the laboratory experiments. Deionized water was used for the experiments.
For mineralogical analysis, the soil sample was borrowed from an under-constructed open pit at approx. 1 m depth from the Sarabaska region. The sample was collected in two different sealed plastic bags to prevent the oxidation and reduction and stored immediately at 4 °C temperature. Initially, the sample was dried in hot air oven at 110 °C temperature for 24 h; thereafter, soil sample was ground using mortar and pestle and finally sieved through 75-µm sieve. This powdered sample (less than 75 µm) was used for the analysis in XRD (X-ray diffractometry).
To understand the source and distribution of fluoride contamination in the study area, the measured data were interpreted and plotted using kriging method in Surfer 9 and TNT MIPS 2013 as shown in Fig. 2.
Results and discussion
Part 1: Physicochemical analysis of water sample
Based on the analysis, 37% of total groundwater samples collected during pre-monsoon season contain fluoride above 1.5 ppm (permissible limit set by WHO and BIS), whereas during post-monsoon season the contamination level is reduced to 30% of the total groundwater sample. It may be possible that infiltration of rainwater reduced the magnitude of the fluoride contamination level in relatively lower elevation zone (Raju 2016). The maximum concentration of fluoride (12.2 mg/L) was observed during post-monsoon at Sarabaska village in Machatora region, which is situated at apparently higher elevated area. During post-monsoon season, the fluoride concentration increased due to infiltration of rainwater through fluoride enriched rocks and minerals strata which are present apparently just below the ground surface, enhanced the dissolution mechanism in reducing condition (He et al. 2013). Along with fluoride, other geochemical parameters were estimated and correlated to understand the leaching mechanism and mobilization technique in the aquifer. The variation in wider range of descriptive statistics of analytical data as listed in Table 1 suggests that the aquifers are heterogeneous in nature.
Figure 4 illustrates the Piper plots of geochemical parameters of groundwater samples collected during pre-monsoon and post-monsoon seasons. Piper plot shows most of the aquifer water is of Ca–HCO3 type. In the post-monsoon samples, significant heterogeneity can be seen, which may be due to infiltration of rainwater and dissolution of minerals from host rocks.
Interactions between groundwater and aquifer matrix have a significant role on water chemistry, which is also useful to understand the origin of groundwater (Olaka et al. 2016) as well as the source of F− in groundwater. As the study area experiences semiarid climatic condition, evaporation can play a significant role in hydrogeochemical process. Hence, Gibbs ratio is used to understand and differentiate the influences of rock–water interaction, evaporation and precipitation, on water chemistry (Gibbs 1970). Average ratio of 0.464 and 0.38 for Na+/(Na+ + K+), 0.163 and 0.467 for Cl−/(Cl− + HCO3 −), 362 and 372 mg/L for TDS during Pre-monsoon and post-monsoon respectively, represent the sediment–water interaction, primarily controlling the major ion chemistry of groundwater irrespective of season (Chae et al. 2007). Gibbs plot (Fig. 5a, b) illustrates that the origin of the major ions present in the groundwater samples is mainly rock dominant, indicating that the subsurface layers can be considered as the controlling factor of the leaching mechanism (Zabala et al. 2016).
The groundwater samples collected from the study area were analyzed for geochemical parameters, and the results reveal that the comparatively greater depth (> 150 ft) of tube wells is mainly affected by fluoride contamination. The groundwater has longer retention period in case of deeper aquifers resulting in more dissolution of minerals (Beg et al. 2011). The pH value of the samples varies between ranges (5.4–10.5). The maximum pH of 10.5 was found in Sarabaska where the highest fluoride contamination was observed. This sample contains higher amount of Cl− (392 mg/L), EC (1086 µs), TDS (760 mg/L) and Na+ (153 mg/L), most negative ORP (− 120) and very less amount of Ca2+ (2.6 mg/L) and Mg2+ (BDL), indicating that the alkaline nature of groundwater accelerates the leaching of fluoride from its host rocks in higher reducing environment (Li et al. 2015; Beg et al. 2011; Saxena and Ahmed 2001; Handa 1975). The samples with fluoride concentration above permissible limit contain higher TDS (760 mg/L) and EC (1070 µs) values indicating the enrichment of groundwater with higher amount of dissolution of minerals. It is the evidence of dissolution of fluoride along with minerals from host rocks into groundwater (Ambade and Rao 2012; Singh 2002).
The sodium concentration was observed to be lying between 7.07 and 103.65 mg/L during pre-monsoon season and 5.35–153.15 mg/L during post-monsoon showing a wide range of variation. The samples with higher fluoride concentration were also having higher sodium concentration. The positive correlation of the scatter plot (Fig. 6) between fluoride and sodium indicates affinity of fluoride toward Na+ ion which helps in dissolution of fluoride from CaF2 and sustain in the aqueous solution (Chandio et al. 2015; Guo et al. 2007; Saxena and Ahmed 2001). Saxena and Ahmed (2001) suggested the following reactions as shown in Eqs. 1 and 2 might be taking place during the weathering of rock-bearing minerals (Saxena and Ahmed 2001).
In the above reaction, Na–HCO3-rich water enhances the dissolution of CaF2 to release fluoride into groundwater with time.
The negative correlation (R 2 = − 0.85) between fluoride and calcium (Ca2+) in Laxmisagar region as shown in electronic supplementary material (Fig. A1 and Fig. A2) might be due to the formation of precipitate called CaF2 causing the fluoride to precipitate out from the groundwater (Li et al. 2015; Farooqi et al. 2007; Gupta et al. 2006). The concentration of magnesium (Mg2+) was found to be varying from below detectable limit (BDL) to 72.0 mg/L. The magnesium also negatively correlates (as shown in electronic supplementary material Fig. A3) with fluoride. A significant and positive correlation of fluoride was observed with bicarbonate concentration in Kusumkanali (R 2 = 0.69) and Machatora (R 2 = 0.81) regions. Chandio et al. (2015) and Handa (1975) suggested that alkalinity favors the dissolution of fluoride into groundwater system from fluoride-bearing minerals (Chandio et al. 2015; Handa 1975).
Part 2: Mineralogical analysis of soil sample
The data generated from XRD analysis were plotted using OriginPro8, and the peaks were identified (shown in Fig. 7). According to JCPDS (Joint Committee on Powder Diffraction Standards) database, the peaks (26.7125, 45.375 and 24.8525) match with the peaks of muscovite 2M1 K0.86Al1.94 (Al0.965Si2.895O10) ((OH)1.744F0.256). It is the evidence of the presence of muscovite in the soil sample.
From the XRD analysis of soil sample, muscovite was identified in two soil samples. The presence of muscovite may be due to recrystallization or hydrolysis of Al-bearing minerals, but apart from such secondary processes muscovite can also form directly (Deer et al. 1992). The groundwater sample of Sarabaska was found to contain higher amount of Na+ (153 mg/L) and pH (10.5), which leads to excess OH− concentration in groundwater. In the presence of Na+, higher amount of OH− ions having same radius and charge often replaces the exchangeable fluoride from its corresponding minerals (Rafique et al. 2015; Gupta et al. 2012) as shown in Eq. 3.
Results concluded that OH− ion attributes to the leaching mechanism of fluoride into groundwater system from muscovite.
Conclusion
The groundwater of the study area is observed to be Ca–HCO3 type from the Piper diagram. The groundwater of northwestern part of the study area is highly contaminated with fluoride concentration. Fluoride shares positive relationship with bicarbonate and sodium concentrations, whereas calcium concentration shares negative relationship. Mineralogical analysis of soil sample of the Sarabaska region confirms the presence of fluoride-bearing mineral, i.e., muscovite 2M1, from which the fluoride is getting dissolved in groundwater. Ion exchange between OH− ion and F− ion present in fluoride-bearing mineral (e.g., muscovite) is the most dominant mechanism of fluoride leaching. Concentration of bicarbonate and sodium in groundwater increases the alkalinity of the water providing a favorable condition for fluoride to leach into groundwater from its mineralogical composition, whereas calcium concentration tries to precipitate out the fluorides present in the groundwater by forming CaF2. But high Na+ and HCO3 − concentration prohibits the precipitation of CaF2 in aqueous medium, and fluoride is also leached out from CaF2. Fluoride above the permissible limit is mostly observed from the tube wells with deeper depth where the residence time of the groundwater is longer. Increased residence time of groundwater also increases the weathering or dissolution of host rocks. From the Gibbs diagram also it became clear that the ions present in the groundwater are due to the rocks present beneath the earth surface.
References
Adler P, World Health Organization (1970) Fluorides and human health
Agrawal V, Vaish A, Vaish P (1997) Groundwater quality: focus on fluoride and fluorosis in Rajasthan. Curr Sci 73:743–746
Alarcón-Herrera MT et al (2013) Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: genesis, mobility and remediation. J Hazard Mater 262:960–969
Ambade B, Rao CM (2012) Assessment of groundwater quality with a special emphasis on fluoride contamination in Rajnandgaon district of Chhattisgarh state in central India. Int J Environ Sci 3:851
Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Møller KN, Sarr M, Johnson CA (2008) Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42:3662–3668
Apambire W, Boyle D, Michel F (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol 33:13–24
APHA, AWWA, WPCF (2005) Standard methods for examination of water and wastewater, 21st edn. APHA, AWWA, WPCR, New York, p 1
Ayenew T (2008) The distribution and hydrogeological controls of fluoride in the groundwater of central Ethiopian rift and adjacent highlands. Environ Geol 54:1313–1324
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Beg M, Srivastav S, Carranza E, de Smeth J (2011) High fluoride incidence in groundwater and its potential health effects in parts of Raigarh District, Chhattisgarh, India. Curr Sci 100:750–754
Bhattacharya P, Chatterjee D, Jacks G (1997) Occurrence of arsenic-contaminated groundwater in alluvial aquifers from delta plains, Eastern India: options for safe drinking water supply international. Water Res Dev 13:79–92
Chae GT, Yun ST, Mayer B, Kim KH, Kim SY, Kwon JS, Kim K, Koh YK (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385:272–283
Chakrabarti S, Bhattacharya H (2013) Inferring the hydro-geochemistry of fluoride contamination in Bankura district, West Bengal: a case study. J Geol Soc India 82:379–391
Chakrabarti S, Ray S (2013) Fluoride contamination in a hard rock terrain: a case study of Purulia district, West Bengal, India. J Chem Biol Phys Sci 3:2931–2941
Chandio TA, Khan MN, Sarwar A (2015) Fluoride estimation and its correlation with other physicochemical parameters in drinking water of some areas of Balochistan, Pakistan. Environ Monit Assess 187:1–9
Datta A, Chakrabortty A, De Dalal S, Lahiri S (2014) Fluoride contamination of underground water in West Bengal, India. Fluoride 47:241–248
Dey S, Giri B (2016) Fluoride fact on human health and health problems: a review. Med Clin Rev 2:1–6
Deer WA, Howie RA, Zussman J (1992) An introduction to the rock-forming minerals, vol 2. Longman Scientific & Technical, Hong Kong
Dutta R, Saikia G, Das B, Bezbaruah C, Das H, Dube S (2006) Fluoride contamination in groundwater of Central Assam, India. Asian J Water Environ Pollut 3:93–100
Edmunds WM, Smedley PL (2013) Fluoride in natural waters. In: Selinus O (ed) Essentials of medical geology. Springer, Dordrecht, pp 311–336
Farooqi A, Masuda H, Kusakabe M, Naseem M, Firdous N (2007) Distribution of highly arsenic and fluoride contaminated groundwater from east Punjab, Pakistan, and the controlling role of anthropogenic pollutants in the natural hydrological cycle. Geochem J 41:213–234
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Guo Q, Wang Y, Ma T, Ma R (2007) Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J Geochem Explor 93:1–12
Gupta R, Misra AK (2016) Groundwater quality analysis of quaternary aquifers in Jhajjar District, Haryana, India: focus on groundwater fluoride and health implications. Alex Eng J. https://doi.org/10.1016/j.aej.2016.08.031
Gupta S, Banerjee S, Saha R, Datta J, Mondal N (2006) Fluoride geochemistry of groundwater in Nalhati-1 block of the Birbhum district, West Bengal, India. Fluoride 39:318
Gupta S, Mondal D, Bardhan A (2012) Geochemical provenance and spatial distribution of fluoride in Groundwater in parts of Raniganj coal field, West Bengal, India. Arch Appl Sci Res 4(1):292–306
Hallett B, Dharmagunawardhane H, Atal S, Valsami-Jones E, Ahmed S, Burgess W (2015) Mineralogical sources of groundwater fluoride in Archaen bedrock/regolith aquifers: mass balances from southern India and north-central Sri Lanka. J Hydrol Reg Stud 4:111–130
Handa B (1975) Geochemistry and genesis of fluoride-containing ground waters in India. Ground Water 13:275–281
He J, An Y, Zhang F (2013) Geochemical characteristics and fluoride distribution in the groundwater of the Zhangye Basin in Northwestern China. J Geochem Explor 135:22–30
Kim S-H, Kim K, Ko K-S, Kim Y, Lee K-S (2012) Co-contamination of arsenic and fluoride in the groundwater of unconsolidated aquifers under reducing environments. Chemosphere 87:851–856
Kumar M, Das A, Das N, Goswami R, Singh UK (2016) Co-occurrence perspective of arsenic and fluoride in the groundwater of Diphu, Assam, Northeastern India. Chemosphere 150:227–238
Kut KMK, Sarswat A, Srivastava A, Pittman CU, Mohan D (2016) A review of fluoride in african groundwater and local remediation methods. Groundw Sustain Dev 2:190–212
Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165
Manarelli M, Delbem ACB, Lima T, Castilho F, Pessan JP (2014) In vitro remineralizing effect of fluoride varnishes containing sodium trimetaphosphate. Caries Res 48:299–305
Majumdar KK (2011) Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health 55:303–308
Mondal D, Gupta S, Reddy D, Nagabhushanam P (2014) Geochemical controls on fluoride concentrations in groundwater from alluvial aquifers of the Birbhum district, West Bengal, India. J Geochem Explor 145:190–206
Olaka LA, Wilke FD, Olago DO, Odada EO, Mulch A, Musolff A (2016) Groundwater fluoride enrichment in an active rift setting: Central Kenya Rift case study. Sci Total Environ 545:641–653
Patel SC, Khalkho R, Patel SK, Sheikh JM, Behera D, Chaudhari S, Prabhakar N (2014) Fluoride contamination of groundwater in parts of eastern India and a preliminary experimental study of fluoride adsorption by natural haematite iron ore and synthetic magnetite. Environ Earth Sci 72:2033–2049
Peckham S, Awofeso N (2014) Water fluoridation: a critical review of the physiological effects of ingested fluoride as a public health intervention. Sci World J. https://doi.org/10.1155/2014/293019
Rafique T, Naseem S, Ozsvath D, Hussain R, Bhanger MI, Usmani TH (2015) Geochemical controls of high fluoride groundwater in Umarkot sub-district, Thar Desert, Pakistan. Sci Total Environ 530:271–278
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, southern India. Geosci Front 8:117–124
Raju NJ (2016) Prevalence of fluorosis in the fluoride enriched groundwater in semi-arid parts of eastern India: geochemistry and health implications. Quat Int 443:265–278
Sabal D, Khan T (2008) Fluoride contamination status of groundwater in Phulera tehsil of Jaipur district, Rajasthan. J Environ Biol 29:871–876
Samal AC, Bhattacharya P, Mallick A, Ali MM, Pyne J, Santra SC (2015) A study to investigate fluoride contamination and fluoride exposure dose assessment in lateritic zones of West Bengal, India. Environ Sci Pollut Res 22:6220–6229
Saxena V, Ahmed S (2001) Dissolution of fluoride in groundwater: a water–rock interaction study. Environ Geol 40:1084–1087
Selvakumar S, Ramkumar K, Chandrasekar N, Magesh N, Kaliraj S (2017) Groundwater quality and its suitability for drinking and irrigational use in the Southern Tiruchirappalli district, Tamil Nadu, India. Appl Water Sci 7:411–420
Singh AK (2002) Quality assessment of surface and subsurface water of Damodar river basin. Indian J Environ Health 44:41–49
Wang X, Kawahara K, Guo X-J (1999) Fluoride contamination of groundwater and its impacts on human health in Inner Mongolia area. J Water Supply Res Technol AQUA 48:146–153
Yadav AK, Khan P (2010) Fluoride and flurosis status in groundwater of Todaraisingh area of district Tonk (Rajasthan, India): a case study. Int J Chem Environ Pharmaceut Res 1:6–11
Yadav S, Kumar R (2010) Assessment of ground water pollution due to fluoride content and water quality in and around Tanda Taluka of Rampur district, Uttar Pradesh, India. J Chem Pharm Res 2:564–568
Zabala M, Manzano M, Vives L (2016) Assessment of processes controlling the regional distribution of fluoride and arsenic in groundwater of the Pampeano Aquifer in the Del Azul Creek basin (Argentina). J Hydrol 541:1067–1087
Acknowledgements
We would like to acknowledge the National Institute of Technology, Durgapur, for providing infrastructure. We are thankful to all faculty members, staff members and student of the Department of Earth and Environmental Studies, NIT, Durgapur, for the endless support. We are also thankful to the villagers, lived in the study area, for their valuable help during sampling.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mondal, S., Kumar, S. Investigation of fluoride contamination and its mobility in groundwater of Simlapal block of Bankura district, West Bengal, India. Environ Earth Sci 76, 778 (2017). https://doi.org/10.1007/s12665-017-7122-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-7122-7