Abstract
Quality of groundwater is concerned with various processes such as precipitation, weathering and dissolution of mineral, cation exchange and ground water exploitation. Present study aims to interpret the seasonal variation of groundwater quality contaminated with fluoride. Sixty water samples were collected during pre- and post-monsoon seasons from Lalganj Tehsil of Raebareli District, UP (India). Multivariate statistical analyses such as factor analysis (FA), principal component analysis (PCA), cluster analysis and correlation matrix were applied on 14 hydro-chemical constituents, i.e., pH, EC, TDS, TBDT, TH, bicarbonate (BiC), NO3−, SO42−, F−, Cl−, Ca2+, Mg2+, Na+ and K+. Factor analysis explained that TH, Mg2+, SO42−, Cl−, EC, TDS and F− were significantly loaded parameters during both seasons that influence the groundwater quality due to weathering and dissolution of mineral during aquifer recharging. The source of F− appears to be geogenic due to the alluvial sediments deposited in the geological past as no hard rock terrain was present in the nearby areas that were dominantly made up of mud with pocket of sand. The cumulative variance obtained from PCA suggested that the groundwater quality in pre-monsoon was better than that of post-monsoon. Correlation explained that EC of groundwater completely depends on the dissolved solids. Dendrogram of cluster analysis indicated that parameters were grouped into four clusters on the basis of similarity, during both seasons. Values of Mg+, F−, TH, and TDS exceeded more than the drinking water standard during both seasons. It will bring alertness to the people regarding whether the groundwater is suitable or unsuitable for drinking purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is an elixir of life because it is an essential and major constituent of all living things. Groundwater is a key source of drinking water among freshwater resources and their need for different purposes is growing day by day due to rapid growth of population, rapid urbanization with change in lifestyle, growing industrialization and agricultural activities, which have deteriorated the surface water and groundwater quality in the last few decades (Gajbhiye et al. 2014; Sharma et al. 2014). Groundwater forms the major resource of fresh water and provides for about 88% of the drinking water need in rural areas of India. The population has widely dispersed in the rural area where accessibility of infrastructure for the treatment and transportation of surface water are not possible (Jain et al. 2010). In India, most of the villagers suffer health disorders due to occurrences of F− and As in drinking water and F− contamination is far more widespread than that of As contamination in the country (Subba Rao 2011). Low level of fluoride is required by human system as it is helpful in preventing dental carries but ingestion of excessive amounts of fluoride may cause dental and skeletal fluorosis, joint pain, restriction of mobility, and probably increase the risk of some bone fractures (Pathak et al. 2012) and non-skeletal fluorosis; excessive consumption of fluoride may lead to muscle fibre degeneration, deformities in RBCs, excessive thirst, headache, skin rashes, nervousness, neurological manifestations, depression, gastrointestinal problems, urinary tract malfunctioning, nausea, abdominal pain, tingling sensation in fingers and toes, reduced immunity, repeated abortions or still births, male sterility, etc. (Meenakshi and Maheshwari 2006). The quality of water is a combination of its physical, chemical, biological and radiological characteristics. Water is an important component and a key factor for the maintenance of a well-balanced environment (Ntengwe 2006). In India, excessive exploitation of groundwater has put 66 million people at risk of a high level of fluoride in 22 states and around 10 million people in 6 states at risk of arsenic (Ghosh 2007). Many states of India faced problems due to high level of salinity and hardness in groundwater, e.g., coastal areas of Andhra Pradesh, Tamil Nadu, Gujarat, NCR (National Capital Region of Delhi) and western Uttar Pradesh. Andhra Pradesh, Jharkhand, Rajasthan, Goa, and Orissa had higher concentration of iron and nitrate in groundwater (Srikanth 2009).
Fluorine and fluoride are interchangeable in generic term, and fluorine is the lighter member of the halogen group and most electronegative of all elements. Fluoride enrichment in groundwater takes place mainly through leaching and weathering of the fluoride-bearing minerals (fluorite, apatite, biotite, hornblende, etc.) present in hard rock terrains (basalts, granites, gneisses, schists, charnockites, khondalites, etc.) and anthropogenic sources, i.e., chemical fertilizers, sewage, sludge (Subba Rao et al. 2017). Fluorosis is widespread in many parts of the world predominantly in mid-latitude regions. Of the 85 million tons of fluoride deposits in the earth crust worldwide, 12 million tons of fluoride are found in India. Known fluoride belts on land include one that stretches from Syria through Jordan, Egypt, Libya, Algeria, Sudan and Kenya, and another that stretches from Turkey through Iraq, Iran, Afghanistan, India, Northern Thailand, China and similar belts in the America and Japan also (WHO 2005). The endemic fluorosis is predominant in many states including Andhra Pradesh, Rajasthan, Gujarat, Bihar, Delhi, Haryana, Karnataka, Madhya Pradesh, Chhattisgarh, Maharashtra, Odisha, Punjab, Tamil Nadu, Telangana and Uttar Pradesh in the country. Andhra Pradesh is one of the first states in India, where fluoride was first detected in drinking water at Nellore district in 1937 (Ayoob and Gupta 2006). For groundwater, inland salinity and excessive fluoride had become a problem in many districts of Uttar Pradesh including Unnao, Agra, Mathura, Ghaziabad and Aligarh (CGWB 2010). Long-term taking of contaminated groundwater can pose serious ill effects on human health. Groundwater having low pH can cause gastrointestinal disorders. pH < 6.5 causes corrosion of metal pipes, resulting in the release of toxic metals such as Zn, Pb, Cd, and Cu (Trivedi and Goel 1986). EC of groundwater is an important parameter for irrigation and industrial purposes and total dissolved solids help out to the portability of groundwater.
In recent years, many studies have been done using principal components analysis in the interpretation and grouping of water quality parameters. Gajbhiye (2015) utilized principal components technique conducted on two-point source of municipal waste (Urdana Nala and Moti Nala) in Jabalpur, India. Bhat et al. (2014) have used this technique for pollution source identification in the Sukhnag stream, Kashmir, India. Environmental researchers referred that PCA is more highly consistent than factor analysis and it is a mathematical technique without any assumption. PCA is designed to decrease the number of variables to a small number of indices while endeavouring to keep the relationships between the original data sets. In this study, PCA was used to understand and group the water quality parameters. The Euclidean distance usually gives the similarity between two samples, and a distance can be represented by the difference between transformed values of the samples (Otto 1998).
The present hydro-chemical investigation has an objective to evaluate the quality of groundwater for its protection, management, and quality conservation because it is very important to assess the water quality not only for its present use but also for future consumption or sustainable development. However, information about the status of groundwater quality and their impact on urban and industrial development is available for some districts such as Unnao, Ghaziabad, Sonbhadra, Varanasi, and Deoria (Misra and Mishra 2007; Umar et al. 2006; Singh et al. 2012; Raju et al. 2009, 2011; Bhardwaz and; Singh 2011), but such evidence is lacking for many districts of Uttar Pradesh including Raebareli. Multivariate statistical analyses such as PCA have been used to provide a quantifiable relatedness of water quality data sets, and studies have confirmed the usefulness of multivariate analysis techniques for the evaluation and interpretation of groundwater quality parameters (Singh et al. 2004, 2009) and identifying critical water quality issues and possible sources of pollution (Singh et al. 2005; Kumar and Riyazuddin 2008; Sargaonkar et al. 2008; Rao et al. 2010).
Materials and methods
Study area
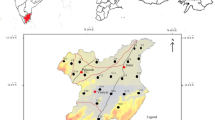
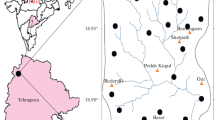
Lalganj is a Tehsil of Raebareli District (UP) lying between 26°09′53.18″N latitude and 80°58′14.89″E longitude located on Lucknow to Raebareli road at a distance of 84 km from Lucknow given in Fig. 2. Raebareli District is 285–295 feet above the sea level and satuated at Indo-Gangetic river basin. The district is drained by the Ganga, Sai and their tributaries. The slope, in general, is from northwest to southeast. Being the part of the Gangetic plain, the district shows the geology of recent origin and exposes ordinary Gangetic Alluvium. The alluvial sediments of Ganga Basin have been classified as Older Alluvium and Newer Alluvium; the former consists of sediments which were formed in distant past and are partly undergoing denudation, while the latter is under its process of formation (Khanna 1992). The Older Alluvium is made up of massive beds of clay of a pale reddish brown colour, very often yellowish with kankar (calcrete) present in between the clay layers. The Newer Alluvium is light coloured and poor in calcareous matter. It is noted for kankar, reh and brick earth deposits. On the basis of geology, soil topography climate and natural vegetation the district is divided into four sub-micro regions. The climate of Lalganj is semi-arid with 923 mm average rainfall and maximum and minimum temperatures are 44.2 and 8.3 °C, respectively. Lalganj Tehsil extends up to 709.362 km area. According to census 2011, total population of study area is 4,98,136 which consists of 2,57,163 males and 2,40,973 females. The population of 0- to 6-year age group is 60,320 with 31,713 males and 28,607 females (Fig. 1).
Water sampling and analysis
Groundwater samples were collected for hydro-chemical investigation during pre- and post-monsoon of 2016 (60 × 2 samples) from Lalganj Tehsil, Raebareli District, Uttar Pradesh, India. The groundwater samples were collected in pre-washed polyethylene plastic containers (1 L capacity for physicochemical and 500 ml for metals analysis). All samples were collected after 5-min pumping of the hand pump and bore well, rinsing the bottles thrice. Samples were preserved and stored for analysis as outlined in standard methods for the examination of water and wastewater (WEF 2012). Fourteen water quality parameters were analysed, most of which are included in the drinking water standards of WHO (2008) and IS (2012). Samples were transported and analysed in the laboratory of CSIR-Indian Institute of Toxicology Research, Lucknow, India. Total hardness (TH) as CaCO3, bicarbonate (BiC) and chloride (Cl) was analysed by titrimetric method. Electrical conductivity (EC) is measured by ion meter, total dissolve solids (TDS) are quantified by gravimetric method and turbidity (TBDT) was estimated using turbidity meter. Sodium, potassium, calcium, and magnesium were measured, using a flame emission mode by AAS (AAS, Model GBC Avanta-Sigma, Australia). Sulphate (SO42) and nitrate (NO3−) were analysed using spectrophotometer (Dynemica UV–VIS, SB-10). Fluoride was measured by Fluoride Ion Selective Electrode (Orion 4 Star) made by Thermo Fisher Scientific. 10 ml sample was taken in a plastic beaker, 1 ml TISAB-III (total ionic strength adjustment buffer) solution was added after calibration of fluoride meter. For calibration of fluoride meter, 10 ml of different concentrations of standard solution (0.01, 0.1, 1.0, 10, 100 ppm F−) was taken in differ plastic beakers and then 1 ml TISAB-III was added and the calibration button of fluoride meter was pressed. After the analysis of five samples, the meter was checked by dipping in standard solution.
Quality control and quality assurance
Chemicals were used throughout the analytical study grade (AR) chemicals (Merck and Sigma-Aldrich, Germany) without any further purification or disturbance. The glassware was washed with dilute nitric acid (1.15 N) followed by several portions of distilled water. The quality assurance measures included meticulous infectivity control (stringent washing/cleaning procedures), monitoring of blank levels of solvents, equipment, and other materials. All reagents and calibration standards for analysis were prepared using Milli-Q water. All analyses were carried out in triplicate and all stock and standard solutions were stored at 4 °C before its use for analysis.
Results and discussion
Quality criteria for drinking purpose
Groundwater of Lalganj Tehsil was alkaline in nature. The average pH in pre-monsoon and post-monsoon in 2016, respectively, 7.51 and 8.02 (Table 1), is within the safe limit (6.5–8.5), prescribed for drinking water by IS (2012) and WHO (2008). Regular consumption of drinking water containing nitrate > 45 mg/L can cause a number of health disorders, such as blue baby syndrome or methaemoglobinaemia in infants, gastric cancer, goitre, birth malformations and hypertension (Majumdar and Gupta 2000; Murali et al. 2011). The concentration of NO3 in the groundwater samples was 99 and 96% in pre-monsoon and post-monsoon seasons, respectively, which was within the limit for drinking water standard (Table 1). Average concentration of sulphate ion varied from 81 to 123 mg/L which was within the prescribed limits (Table 1). In combination with Na+ and Mg2+, SO42− also exerts a cathartic effect on digestive tracts (Subba Rao 2006). The concentration of TDS in study area ranged from 216 to 3254 mg/L. In the groundwater, 51% samples were more than the recommended limit (500 mg/L) in pre-monsoon while 98% samples in post-monsoon. High concentration of TDS may cause gastrointestinal irritation in the consumers. Total hardness depends on the concentration of Ca2+ and Mg2+ and usually expressed as CaCO3. Calcium (Ca2+) and magnesium (Mg2+), the important parameters for total hardness, ranged from 5 to 133 and 5 to 221 mg/L, respectively. 59.01% of the samples in pre-monsoon season exceeded the WHO’s acceptable limits for magnesium in drinking water, while this percentage was 77.77 for the post-monsoon season. The principal natural sources of hardness in groundwater are sedimentary rocks. In general, hard waters originate in areas with thick topsoil and limestone formations. The total hardness ranged from 26 to 736 mg/L, and mean value exceeded drinking water standard during both seasons as shown in Table 1. In the study area, the quantity of sodium in 95% of the samples groundwater samples varies from 11 to 296 mg/L and the average concentration in both seasons was well within the prescribed limit of WHO. Total alkalinity is the sum of bicarbonate and carbonate, and carbonate was absent in groundwater of the study area. Indian standard suggested that an acceptable limit of total alkalinity in drinking water is 200 mg/L. However, in large quantities, it imparts a bitter taste to the water. The alkalinity in the groundwater samples of Lalganj Tehsil ranged from 156 to 1146 mg/L. In pre-monsoon, 5% of the samples exceeded the acceptable limit of 200 mg/L, while this percentage was 7 in post-monsoon.
Chloride in the groundwater samples of the study area varied from 7 to 658 mg/L. 13% of the samples in pre-monsoon and 15% in post-monsoon exceeded the acceptable level stipulated by WHO. The surplus concentration of Cl− in drinking water gives a salty taste and had a laxative effect on people not accustomed to it (Subba Rao 2006). Deficiency of fluoride in drinking water below 0.5 mg/L contributes to tooth caries while an excess of over 1.5 mg/L causes fluorosis (IS 2012), but within the limit, it becomes an important element for healthy teeth and bones. F− concentration was varied from 0.44 to 17.2 mg/L in the groundwater samples of the study area (Lalganj Tehsil). The concentration of fluoride in study area was observed to be more than the permissible limit of IS and WHO standard (Table 1). 49 and 60.66% of water sample of pre-monsoon and post-monsoon were affected by high fluoride concentration. Lalganj Tehsil is part of Indo-Gangetic region. Pandey (2001) and CGWB (1999) reported the highest contents of fluoride in groundwater of Indo-Gangetic basin. The geology of the Indo-Gangatic basin is devoid of any hard rocks, alluvial deposits of mud, sand, and clays with fluoride-bearing minerals i.e., muscovite and biotite (Kumar and Saxena 2011). So, the source of fluoride in groundwater of the study area was geogenic. Kanaujia et al. 2013 also found high level of fluoride in Dalmau block of Rabareli District connected with Lalganj. Shaji et al. (2007) suggest that high fluoride in groundwater is mainly derived from the hornblende–biotite gneiss. The fluoride released into the groundwater could be the weathering product of mica minerals, probably of muscovite or biotite or both. It is also known that biotite is an alumino-silicate mineral containing magnesium, iron and potassium; thus the enrichment of these elements also suggests the active weathering of biotite.
Factor analysis/principal component analysis
The principal component analysis is performed to extract the most important factors affecting the water quality described by 14 physicochemical parameters from 60 locations of the study area. Due to the complex associations between physicochemical parameters, it was tough to draw clear conclusions but principal component analysis extracts the information and explains the variables. Physicochemical data were generally normalized to drop misclassification due to the diverse order of magnitude and range of variation of the analytical parameter. The rotation of the factors was executed by the Varimax with Kaiser normalization. 14 physicochemical parameters during both seasons were selected for FA. The physicochemical parameters were reduced to three and four PCs having values greater than 1 and together they account for 62.26 and 79.087% total cumulative variance of the data set for pre- and post-monsoon seasons given in Table 2. Any principal components or factors with an eigenvalue greater than 1 are considered significant (Liang and Yu 2000). In this study, remaining factors have eigenvalue less than unity (Fig. 2).
Rotation sums of squared loadings measuring the degree of closeness between the variables and the values of PCs are given in Fig. 3 and Table 3. The factor loading includes both positive and negative loadings. Loadings between 0 and ± 0.49 indicate weak correlation, between ± 0.5 and ± 0.74 indicate moderate correlation, if more than ± 0.75 are considered, it indicate strong correlation and loadings close to ± 1 indicate the highest correlation (Lui et al. 2003). During pre-monsoon, PC 1 holds 34.498% variance of the total cumulative variance; it has strong loading of TDS, TH, SO42−, and Mg2+, while having moderate loading of NO3− and Cl−. PC 2 having 15.879% variance of the total cumulative variance has strong loaded with F− while moderate loading on bicarbonate and Na+. PC3 explains 11.884% variance of the total cumulative variance and it has strong loading with turbidity but moderate loading with K+. During post-monsoon, PC1 is strongly positively loaded with TDS, EC, TH, SO42−, Mg2+, and Cl− while moderately loaded with NO3− and Ca2+. These parameters strongly influence the quality of the groundwater with 39.883% variance. PC2 contributes to 20.40% variance of the total cumulative variance in the data set and F−, bicarbonate, and Na+ were highest loaded variables as compare to pre-monsoon. PC3 was strongly loaded with K+ and moderately loaded with turbidity; this parameter illuminates 10.073% variance of the total cumulative variance. PC 4 was strongly loaded with pH and moderately loaded with EC; both parameters account for 8.783% variance of the total cumulative variance.
Spearman’s correlation matrix
Spearman’s correlation matrix was applied for finding the relation within the physicochemical parameter. The one-tailed bivariate correlation was carried out to investigate the relationship between fluoride and physicochemical parameters shown in Table 4. In statistical analysis of groundwater samples, bicarbonate, SO42−, TDS, EC and Na were found to have significant correlation with F− during post-monsoon while bicarbonate, EC and Na had significant correlation with F− during pre-monsoon. Correlation matrix confirmed that after monsoon, minerals dissolved with water during percolation and increase the concentration of physicochemical parameters.
Hierarchical cluster analysis
HCA is a powerful data mining technique, which classifies variables into clusters on the basis of similarities within a group and dissimilarities between different groups. Physicochemical variable classification was performed by the use of cluster analysis. Hierarchical cluster analysis was performed after careful consideration of available combinations of similarity/dissimilarity measurements. Physicochemical data were run by Ward’s method for similarity measurement which provided visually meaningful dendrogram and distinguished the groups. The dendrogram has classified the fourteen variables into two broad groups which were further divided into minor clusters as shown in Fig. 4. The first broad group was further divided into two sub-groups (TH) and (BiC) and second sub-group divided into two minor groups (turbidity, F−, pH, K+, NO3−, Ca2+) and (Mg2+, SO42− Na+, Cl−). The second group of variables was divided into two groups, EC and TDS, during pre-monsoon. In post-monsoon, the first broad group was further divided into two sub-groups, BiC formed first sub-group and the second sub-group was further divided into two minor groups: turbidity, F−, pH, K+, NO3−, Ca2+ and Mg2+, Na+, Cl− SO42−, TH. The second broad group of variables was divided into two groups, EC and TDS during pre-monsoon. The dendrogram visually shows during both seasons; the main variables are Na+, Cl−, TH, SO42−, and bicarbonate.
Seasonal variation due to leaching activity
Seasonal variation in the concentrations of hydro-chemical constituents (Table 1) is mainly due to leaching. For example, variation in the average concentrations of Na+ (78–89 mg/L) and Cl− (86–123 mg/L) from pre- to post-monsoons is more prominent, due to dissolution of minerals bearing these ions during recharge of an aquifer by rainfall same as for turbidity. There is a significant variation of NO3− and SO42−, reflecting the involvement of anthropogenic sources such as excessive use of fertilizers in agricultural activities from pre-monsoon to post-monsoon. The concentration of HCO3− (380–437 mg/L) was more than the concentration of TH (262–281 mg/L) in the groundwater; this relation suggests that the non-carbonate hardness (TH > BiC) is a more significant character in post-monsoon than in pre-monsoon due to increase of Cl−, SO42− and NO3− ions during rainy season. Table 1 shows that the concentration of Ca2+ is similar in pre- and post-monsoons. This is governed by ion exchange between Na+ and Ca2+or precipitation of CaCO3− (Subba Rao 2002), suggesting an enrichment of Na+ relative to Ca 2+ and attaining a higher concentration of Mg than Ca 2+ in the groundwater. The results proposed two factors responsible for K+ and Mg2+ were more dominant in post-monsoon, first factor was excess application of NPK fertilizer and second factor was dissolution of mineral during percolation of rain water, investigated by Khan et al. (2012) and Mohapatra et al. (2011).
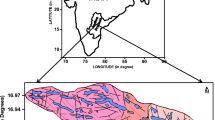
Variation in the concentration of F− during pre- to post-monsoon (2.21–2.29 mg/L) indicates the role of leaching activity (Subba Rao 2003). Special distribution of fluoride in groundwater of Lalganj Tehsil for pre- and post-monsoons is given in Fig. 5 a, b. Free circulation of water caused by rainfall and/or irrigation in the weathered products dissolves and leaches the minerals, contributing fluoride to the groundwater (Subba Rao 2008; Shaji et al. 2007). pH and bicarbonate both showed a significant positive relation with F− while TH showed a negative relation with F−, which specify a greater affinity of F− with bicarbonate and pH rather than with TH (Table 4). The positive relation between F− with pH suggested its comeback from a dissolution of silicate mineral and potentially also degassing of CO2 (Subba Rao 2011). An equivalent relation between F− and bicarbonate leads to a more conducive environment for the mobilization of F−. Higher concentration of F− with lower concentration of TH was explained as the Ca2+ and Mg2+ ions in the TH precipitate as carbonates and/or involve an ion exchange of Ca2+ with Na+ (Gaciri and Davis 1993; Hem 1991). Table 1 shows a higher concentration of TDS in post-monsoon than pre-monsoon, because of leaching of various salts into the post-monsoon groundwater by infiltrating recharge waters. Hence, the results evidently show that there is evidence of monsoon effect on the mean values of groundwater quality after recharging of the aquifer (Table 5).
Conclusion
The present hydro-geochemical investigations reflect that the weathering of fluoride-bearing minerals is responsible for the high level of fluoride in groundwater at Lalganj Tehsil near Ganga basin. In the majority of sampling locations, about 49% (pre-monsoon) and 60.66% (post-monsoon) samples exceeded permissible limit of 1.5 mg/L, representing Na-HCO3− type water, while fluoride < 1.5 mg/L shows Ca-HCO3− signature. Correlation matrix also indicates that Na, EC and bicarbonate were significant with fluoride during pre-monsoon while SO42−, bicarbonate, TDS, EC and Na were significant due to weathering of minerals in post-monsoon. Results of FA illustrated that the groundwater quality in pre-monsoon is better than post-monsoon because of high variance found in physico-chemical data of post-monsoon as compared to pre-monsoon. Results of groundwater are compared with WHO and Indian Standard where Mg, F−, TH, TDS exceeded drinking water standard value during both seasons. In this study, a very high concentration of fluoride in groundwater of up to 17.20 mg/L was measured. Continuous use of groundwater for drinking purpose without its amelioration by the population of the study area may expose them to very high fluoride content through drinking water and they may suffer from dental fluorosis, skeletal fluorosis and non-skeletal fluorosis. This study could help to generate existing baseline data about groundwater quality of the Ganga Basin Lalganj Tehsil, which will bring alertness to people about its purity and quality importance and also help the government of UP in installing the de-fluoridation setup. Proper measures such as amelioration of fluoride before use and rainwater harvesting are suggested for this area.
References
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev in Environ Sci Technol 36:433–487
Bhardwaj V, Singh DS (2011) Surface and groundwater quality characterization of Deoria District, Ganga Plain, India. Environ Earth Sci 63:383–395
Bhat SA, Gowhar M, Sayar Y, Pandit AK (2014) Statistical assessment of water quality parameters for pollution source identification in Sukhnag stream: an inflow stream of lake Wular (Ramsar Site), Kashmir Himalaya. J Ecosyst. https://doi.org/10.1155/2014/898054
CGWB (1999) High fluoride groundwater in India occurrences, genesis and remedies. Ministry of Water Resources, Government of India, 29
CGWB (2010) Groundwater quality in shallow aquifers of India. Central ground water board, Faridabad India 117.
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412. https://doi.org/10.1016/0022-1694(93)90201-J
Gajbhiye S, Mishra SK, Pandey A (2014) Simplified sediment yield index model incorporating parameter. Arab J Geosci 8:1993–2004. https://doi.org/10.1007/s12517-014-1319-9
Gajbhiye S, Sharma SK, Awasthi MK (2015) Application of principal components analysis for interpretation and grouping of water quality parameters. Inter J Hybrid Inf Technol 8(4):89–96
Ghosh A (2007) Current knowledge on the distribution of arsenic in groundwater in five states of India. J Environ Sci Health Part A 42:1–12
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water. In: United States Geological Survey professional paper. Scientific Publisher, Jodhpur. https://pubs.usgs.gov/wsp/wsp2254/pdf/wsp2254a.pdf
IS (2012) Guidelines for drinking water. Bureau of Indian standard (BIS: 10500) Second Revision. http://cgwb.gov.in/Documents/WQ-standards.pdf
Jain C, Bandyopadhyay A, Bhadra A (2010) Assessment of groundwater quality for drinking purpose, District Nainital, Uttarakhand,India. Environ Monit Assess 166(1–4):663–676
Kanaujia S, Bharat Singh AB, Singh SK (2013) Mapping of fluoride endemic areas in Raebareli District, Uttar Pradesh, India. Chem Sci Trans 2(4):1411–1417. https://doi.org/10.7598/cst2013.539
Khan MMA, Umar R, Baten MA, Lateh H, Kamil AA (2012) Seasonal variations in groundwater quality: a statistical approach. Int J Phys Sci 7(25):4026–4035
Khanna SP (1992) Hydrogeology of central ganga plain, U. P. Gangetic Plain. Tera Incognita, pp 23–27
Kumar AR, Riyazuddin P (2008) Application of chemometric techniques in the assessment of groundwater pollution in a suburban area of Chennai city, India. Curr Sci 94(8):1012–1022
Kumar S, Saxena A (2011) Chemical weathering of the indo-gangetic alluvium with special reference to release of fluoride in the groundwater, Unnao District, Uttar Pradesh. J Geol Soc India 77:459–477
Liang YZ, Yu RQ (2000) Chemometrics central. South University Press, Changsha
Lui CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Tiwan. Sci Total Environ 313(1–3):77–89
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42(1):28–39
Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater 137:456–463
Misra AK, Mishra A (2007) Study of quaternary aquifers in Ganga plain, India: focus on groundwater salinity, fluoride and fluorosis. J Hazard Mater 144:438–448
Mohapatra PK, Vijay R, Pujari PR, Sundaray SK, Mohanty BP (2011) Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri city, India: a multivariate statistical approach. Water Sci Technol 64(4):809–817
Murli K, Swasthik RD, Elangovan R (2011) Assessment of ground water quality in Coimbatore south Taluk, Coimbatore district, India. WQI Approach 10(4):521–524
Ntengwe FW (2006) Pollutant loads and water quality in streams of heavily populated and industrialized towns. Phys Chem Earth 31:832–839
Otto M (1998) Multivariate methods. In: Kellner R, Mermet JM, Widmer HM (eds) Analytical chemistry. Wiley-VCH, Weinheim
Pandey AK (2001) A report on study of fluoride contamination in ground water in parts of Unnao district, U. P. (Annual Action Plan: 1996–97). Central Ground Water Board, Ministry of Water Resources, Govt. of India, 45
Pathak RP, Sharma P, Vyas S, Mahure NV, Kumar R, Murari R (2012) Detection of fluoride contamination in the surface and sub-surface water near thermal power station. Int J Eng Sci 1(1):44–47
Raju JN, Dey S, Das K (2009) Fluoride contamination in groundwaters of Sonbhadra district, Uttar Pradesh, India. Curr Sci 96:979–985
Raju JN, Shukla UK, Ram P (2011) Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast urbanizing center in Uttar Pradesh, India. Environ Monit Assess 173:279–300
Rao YRS, Keshari AK, Gosain AK (2010) Evaluation of regional groundwater quality using PCA and geostatistics in the urban coastal aquifer, East Coast of India. Int J Environ Waste Manag 5(1–2):163–180
Sargaonkar AP, Gupta A, Devotta S (2008) Multivariate analysis of groundwater resources in Ganga-Yamuna basin (India). J Environ Sci Eng 50(3):215–222
Shaji E, Bindu VJ, Thambi DS (2007) High fluoride in groundwater of Palghat District. Kerala Curr Sci 92(2):240–245
Sharma SK, Gajbhiye S, Nema RK, Tignath S (2014) Assessing vulnerability to soil erosion of a watershed of tons River basin in Madhya Pradesh using remote sensing and GIS. Int J Environ Res Dev 4(2):153–164
Singh KP, Malik A, Singh VK, Mohan D, Sinha S (2004) Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Analytica Chimia Acta 550(1–2):82–91
Singh SK, Singh CK, Kumar KS, Gupta R, Mukherjee S (2005) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly District of Uttar Pradesh, India. J Hydrol Hydromech 57(1):45–54
Singh KP, Malik A, Mohan D, Sinha S (2009) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38:3980–3992
Singh VK, Bikundia DS, Sarswat A, Mohan D (2012) Groundwater quality assessment in the village of Lutfullapur Nawada, Loni, District Ghaziabad, Uttar Pradesh, India. Environ Monit Assess 184:4473–4488
Srikanth R (2009) Challenges of sustainable water quality management in rural India. Curr Sci 3:317–325
Subba Rao N (2002) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41:552–562
Subba Rao N (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur District, Andhra Pradesh, India. Hydrol Sci J 48:835–847
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ Geol 49:413–442
Subba Rao N (2008) Factors controlling the salinity in groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Monit Assess 138(1–3):327–341
Subba Rao N (2011) High-fluoride groundwater. Environ Monit Assess 176:637–645. https://doi.org/10.1007/s10661-010-1609-y
Subba Rao N, Surya Rao P, Dinakar A, Nageswara Rao PV, Marghade D (2017) Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Appl Water Sci 7:1467–1478. https://doi.org/10.1007/s13201-015-0338-3
Trivedi RK, Goel PK (1986) Chemical and biological methods for water pollution studies. Environmental Publication, Karad
Umar R, Khan MMA, Absar A (2006) Groundwater hydrochemistry of a sugarcane cultivation belt in parts of Muzaffarnagar district, Uttar Pradesh India. Environ Geol 49:999–1008
WEF (2012) Standard methods for examination of water and wastewater, 22nd edn. American Public Health Association, Washington, DC
WHO (2005) Water-related diseases, fluorosis; the disease and how it affects people World Health Organization, Geneva, Switzerland. http://www.who.int/water sanitation health/diseases/fluorosis/en
WHO (2008) Guidelines for drinking water quality incorporating first addendum to third edition. Recommedation Geneva 1:595
Acknowledgements
The authors are thankful to Professor Alok Dhawan, Director, CSIR-IITR, Lucknow, India for providing all necessary facilities for this work. This research did not receive any specific grant from funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahu, P., Kisku, G.C., Singh, P.K. et al. Multivariate statistical interpretation on seasonal variations of fluoride-contaminated groundwater quality of Lalganj Tehsil, Raebareli District (UP), India. Environ Earth Sci 77, 484 (2018). https://doi.org/10.1007/s12665-018-7658-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7658-1