Abstract
Consumption of poor quality water causes serious human health hazards. Therefore, it is very crucial to investigate factors influencing the quality of groundwater and its suitability for drinking purpose. In the present study, groundwater quality of the Dhenkanal district of Odisha, India was characterized and the spatial distribution of different water quality parameters were analyzed using the multivariate statistics, entropy theory, and geostatistics techniques. In the present study 112 number of groundwater tube well samples were collected from the study area. The entropy theory revealed that SO42−, Mg+2 and Cl− were the most influencing parameters. A similar observation was also observed based on the correlation coefficient analysis. Groundwater quality index (GWQI) and entropy-weighted water quality index (EWQI) classifications indicated that 78.57 and 43.75% of the collected groundwater samples were categorized under excellent water quality, whereas, the rest of the samples were varying from good to medium drinking water quality. In addition, the result of EWQI classification offers more realistic assessment than that of GWQIs owing to its high precision, simplicity and without application of artificial weight. The correlation coefficient between Ca+2 and HCO3−, Mg+2 and PO4− were significantly high which might be due the presence of CaHCO3 and MgPO4 in the groundwater samples. The GWQI revealed a weak spatial dependence of groundwater quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is one of the major sources of drinking water in the planet (Li et al. 2016). However, wide spread pollution of groundwater seriously deteriorated its quality rather its quanitities. In general, quality of ground water primarily governed by dissolution of rocks, soil water interaction, degrees of precipitation, quality of discharge and recharge of water (Wagh et al. 2017). Due to the potential toxicity effects of uranium on human health particularly on kidney and in bone tissues thus,traces of naturally occurring uranium in groundwater is a deep concern (Kumar et al. 2018a). The permissible limit of uranium in drinking water is 30 μg l−1 (WHO 2011). Dhenkanal is a semi-arid region, the main stream of this population depend on open dugwells or tube well water for domestic application. The rapid and unplanned urbanization, leaching of industrial waste, agricultural runoff, environmental changes and climatic variations in the study area influence the quality of groundwater. Therefore, the characterization of groundwater quality and its regular monitoring is very crucial for sustainable groundwater resource management. In literature, various techniques have been applied for the assessment of groundwater quality and decision-making (Nihalani and Meeruty 2020; Gosetti et al. 2019). However, groundwater quality index (GWQI) is a very popular and widely used method (Yan et al. 2014). Although GWQI technique has been effectively employed by many researchers, however, the weights of the groundwater quality parameters are assigned based on the judgment of expert, as a result, a lot of valuable data get ignored (Amiri et al. 2014). Further, GWQI technique deals with a lot of prejudice and uncertainty associated with various environmental issues. For instance, without any valid scientific explanation in the index equations some of the parameters can influence the result of GWQIs drastically; consequently, it is very difficult to take an appropriate decision (Islam et al. 2017a). Similarly, a set of pair analysis model (Feng et al. 2014), kriged model (Fallah et al. 2019), fuzzy logic model (Kamrani et al. 2016), multivariate statistics (Pan et al. 2019), and analytic hierarchy procedure (Hosseinimarandi et al. 2014) have been employed to evaluate the feasibility of water quality. However, in these methods too many factors are required and the quality of groundwater cannot be determined appropriately.
Therefore, it is very crucial to develop an innovative groundwater quality indexing approach, which can offer correct and exact information on decision-making. The entropy-weighted water quality index (EWQI) is a potential tool to analyze the quality of groundwater properly and can reveals the relationship between two samples (Islam et al. 2017a). Therefore, comparative study involving GWQI and EWQI classifications is one of the most promising strategy (Gorgij et al. 2017; Su et al. 2018; Amiri et al. 2014). In contrast, geostatistical model like IDW (inverse distance weighting) is a quick, deterministic, and exact local interpolator (Johansson 2000) and has been widely applied to analyze the water quality parameters and its spatial variations. Analyzing the water quality parameters and their spatial distribution is very crucial for decision making in any particular constituency. However, it has not been investigated yet in the Dhenkanal district of Odisha, India, using the GWQI, EWQI and IDW methods along with the geostatistical approaches. Thus, in this study, the ground water quality ranks and its spatial variations have been evaluated.
Material and methods
Study area description
Dhenkanal district is located in the central part of Odisha. It lies between latitude: 20° 29′ to 21° 11′ North and longitude: 85° 58′ to 86° 2′ East. The total geographical area of Dhenkanal district is 4452 sq.km. As per 2011 census report, the population density is 268 (per sq. km). Most of the area is occupied with dense forest and hills. The climatic condition is hot and dry, semi-humid type. The average annual rainfall of the district is 1472 mm. The soil of the study area is predominantly sandy loam, red lateritic and alluvial in nature.The major source of water is primarily from deep and shallow aquifers extracted through deep and shallow wells (Groundwater information booklet of Dhenkanal distinct 2013).
Groundwater sampling and analysis
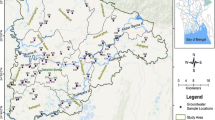
All the chemicals and reagents used in the present study were either of laboratory grade or of the analytical grade procured from Sigma Aldrich USA, Hi-media (India) and Merck (India). Systematic grid sampling technique was adopted in the present study to avoid any bias in the sampling. Smaller is the grid size, better is the resolution and interpretation. Considering the cost-effectiveness of the analysis an optimized grid size of 6 × 6 km2 was implemented in the present geochemical study which is better than the national and international recommendations. Samples were collected adjacent to the population habitats as the objective of the present study to assess the level of contaminations and their impact on the public. Therefore, the distance between the samples varies while the average distance is around 6 km between the two samples. The grid map of the study area is shown in Fig. 1. Based on the grid map 112 number of groundwater samples were collected in previously acid washed (15% v/v HNO3) high-density polyethylene (HDPE) bottles during the month of April to June. Prior to the collection of water samples, these tube wells were pumped for few minutes to remove any stagnant water.
In situ parameters such as; pH, temperature, dissolved oxygen, electrical conductivity, salinity, oxidation reduction potential (ORP) and total dissolved solids (TDS) were estimated in the spot of the sampling location employing portable multi-parameter water analysis kit (Model; Q40d Dual input Cat:8506000 + MTC10101, Make HACH CO, USA). A hand-held Global Positioning System was used to record the geographic position of the sampling point (Model Etrex 20 and Make; Gramin). Similarly, a radiation survey meter (Model PM-1405, Make-Polymaster, Belarus, Ltd) was employed to detect gamma radiations from each sampling point.
In order to estimate the concentration of uranium in the ground water samples, a 100 ml of water sample was acidified using nitric acid followed by filtration using 0.45 µm membrane filter. Then the concentration of uranium was measured by LED Fluorimeter by adding fluorescent enhancing agent (Model: LumexFluorate −02 −4M, Make:LumexFluorate, Russia). The concentrations of uranium in the collected samples were further rechecked in Health Physics Unit of Jaduguda and BARC, Mumbai, India. In order to estimate other water quality parameters, a 500 ml of sample without acidification was filtered by using 0.45 µm membrane filter. Concentration of nitrate was measured using ion selective electrode methods (Model; Orion Star A214 pH/ISE bench top meter and Orion nitrate electrode Model: Orion 9796 BNOP Make:Thermo Fischer scientific USA). Sulfate concentration was estimated using gravimetric techniques as described by CPCB India. Hardness, alkalinity and chloride concentrations were analyzed by titration method (APHA 2005). While phosphate concentration was measured using ascorbic acid method (APHA 2005). Fluoride concentration was estimated using ion selective electrode methods (Model: Orion Star A214 pH/ISE bench top meter USA, Fluoride ion selective Electrode Model: 9609BNWP, Make:Thermo Fischer scientific USA). A double beam UV-visible spectrophotometer was used for all the spectrophotometric measurements (Make: USA Model; Thermo Evolution 220). The experiments were performed in triplicate and the average results are reported with a standard deviation of ±2.4%.
Analysis of water quality parameters using water quality indices
Entropy water quality index (EWQI)
Entropy theory is based on the measurement of uncertainty. EWQI method reveals the arbitrariness of an event in terms of mathematical expression, and over comes any technical difficulty arises due to application of other techniques for evaluation of groundwater quality. In the present investigation, EWQI has been utilized to characterize the groundwater quality due to its reliability, high precision and simplicity (Amiri et al. 2014; Gorgij et al. 2017; Islam et al. 2017b). In general, three steps are used to compute the EWQI which signifies the weight of each parameter as shown in equation (1) (Li et al. 2010).
The eigenvalue matrix X is determined in the first step, X is related to hydro-chemical parameters of m number of water samples in equation. 1. The matrix X, can be expressed as standard-grade matrix Y, as shown below in equation 2
The parameter index value ratio of j and ith sample can be determined as Eq. 3 given below.
The information entropy, ej, can be expressed by Eq. 4.
The effectiveness of the parameter, j index increases with decrease in the amount of entropy. The entropy weight, wj, of each parameter can be determined from Eq. 5 as shown below.
In the second step the quantitative rating scale, qj for each parameter is expressed as shown in Eq. 6.
Where, Sj denotes the Bureau of Indian Standard (BIS) water quality permissible limit of the parameter j (mgl−1) and Cj represents the concentration of the parameter j (mgl−1). The calculation of EWQI can be made in the third step by employing Eq. 7.
Accordingly the groundwater quality can be categorized into five different ranks varying from extremely poor to excellent (Wu et al. 2011).
Groundwater quality index
The combined effect of diffrent groundwater physico-chemical parameters on the drinking water quality can be successfully analyzed by GWQI method (Bodrud-Doza et al. 2016). In this study, the standards for the drinking water quality were used as per the recommendation of BIS (2012). The quality of groundwater was analyzed according to Vasanthavigar et al. (2010) as shown in Eq. 8.
Where,Wi represents the relative weight, qi denotes water quality rating, Si is the Indian standard and Ci stands for concentrations of each parameter. SIi represents the sub-index of ith parameters. According to Kamrani et al. (2016), the groundwater quality can be characterized into five different classes/ranks on the basis of GWQI values as presented in Table 1.
Inverse distance weighting (IDW)
The geo-statistical model reveals the spatial distribution of the groundwater quality parameters. The universal IDW (inverse distance weighing) model is one of the most favorable geo-statistical methods used for mapping of groundwater quality. Numerous geospatial interpolation techniques are available for converting point data into spatially interpolated thematic maps. IDW, kriging and Semivariagram are the popular techniques for this purpose. Among these, IDW technique is extensively used because of its quick, deterministic, and exact local interpolator functions (Johansson 2000). IDW is a deterministic technique which determines the interpolated values by the weighted mean of the data on the basis of an inverse function of the distance between monitoring stations and weights depend on an arbitrary exponent between the interpolated value (Webster and Oliver 2011). In this method, the identical weight is allocated to every individual observation in a search radius and without discontinuity when the weighting exponent is larger than zero (Webster and Oliver 2011). The predicted values at an arbitrary non-measured location are computed as per the equation given below.
Where, n is the total number of measured points used in the interpolation. Zi is the distance between two known point, d represents the distance between the predicted points. The IDW technique is known as an exact interpolator, since the predicted location matches with observed location, the related weight is infinite; as a result at that location the forecast value is identical to the observed value. For this study, QGIS software was applied to produce IDW maps. In the present study, Pearson’s correlation analysis was used to find out the correlation between the different water quality parameters. In the correlation coefficient analysis, different terms were used such as; insignificant, moderate and significant which were associated with the absolute values.
Results and discussion
Statistical analysis of water quality parameters
Electrical conductivity (EC)
EC and TDS indicate the salt concentration in water and have vital role both in drinking and agriculture application (Bayatvarkeshi et al. 2020). In the study area, EC was varied from 37.5–1912 μS cm −1 with an average value of EC as 560 μS cm−1. The maximum values of EC is well corroborated with many reported literatures (Kumar et al. 2015). It is also reported that the electrical conductance in groundwater of Angul district, Odisha varies in the range of 94–2195 μS cm−1 (Keesari et al. 2019). In more than 72% of the collected groundwater samples the value of EC was less than 750 μS cm−1, and thus are coming under the acceptable category of “B” (BIS 2012). The maximum permissible limit of EC in drinking water is 1400 μm hos cm−1 (Saxena and Saxsena 2015) and 5 number of collected samples were exceeded this value. On the other hand, none of the groundwater samples were exceeded the permissible limit of EC as recommended by BIS i.e., 2250 μS cm−1 (group D) for the agricultural application (BIS 2012; Zeid et al. 2018). In general, increase in electrolytes concentration in water enhance its electrical conductivity. In the study area, the major source of salinity is primarily due to the dilution of rock salt which is also augmented by the long term irrigation. Gomez (2020) studied the quality of groundwater from the Santiago del estero province, in Argentina and reported a EC value of 228–6770 μS cm−1. The mines of Mahanadi Coal Field (MCL), aluminum smelter of NALCO, NTPC’s power plants etc. use water from the Brahmani river and its tributaries and finally discharge thousands gallons of wastewater to the river, containing suspended solids, TDS, ash, phosphorus, fluorides, ammonia, urea and different acids (Sahoo et al. 2016).
Further, the concentration of chloride varied from 12.5–391 mg l−1, which is well within the observations reported in literature, for example; 1.6–317.9 mg l−1 (Hossain and Patra 2020), 15.57–523.11 mg l−1 (Wang et al. 2019). More than 97% of the collected groundwater samples contain chloride concentration within the BIS permissible limit of 250 mg l−1. The chloride distribution is quite identical to that of electrical conductivity. The obtained trend may be due to the geogenic (chloride-rich minerals) rock-water interaction. The elevated concentration of chloride in some groundwater samples could be attributed to the excessive uses of inorganic fertilizer, effluents of septic tank, leachates of landfill and industrial effluent which percolate and pollute the groundwater (Ahada and Suthar 2017).
Nitrate, phosphate and sulfate profile
Nitrate is a predominant redox-sensitive water quality parameter. The minimum and maximum nitrate concentration in the study area was 2.7 and 102 mg l−1 respectively. Around 92% of the water samples the nitrate concentration was below the permissible limit as recommended by WHO (2011) and only 8% of water samples the concentration of nitrate exceeded the permissible limit of 45 mgl-1. Similar concentration of nitrate has been reported in numerous literature for example; 0.02–110.1 mg l−1 (Hossain and Patra 2020), 0.24–85 mg l−1 (Kumar et al. 2015), 0.1–147 mg l−1 (Keesari et al. 2019). When concentration of nitrate in drinking water exceeds more than 45 mg l−1, it causes blue baby syndrome or methamoglobinemia in infants (Singha et al. 2017). In addition, higher concentration of nitrate causes serious multiple sclerosis and thyroid problems (Ahada and Suthar 2018). In few samples, the elevated level of nitrate may be due to the widespread use of nitrogen (N)-fertilizer in agriculture field and leading to the percolation of unused fertilizer into the groundwater (Srivastava and Ramanathan 2018). Similarly, septic tanks, organic and inorganic waste disposal are the major anthropogenic sources of nitrate pollution in groundwater. Further, the occurrence of different types of rocks such as; sedimentary, metamorphic, granite, quartz and their interactions with water might have accelerated the weathering of these rocks and consequently enhanced the level of nitrate and ammonium ions in the groundwater (Amadi 2010). In general, concentration of nitrate is less than 45 mg l−1 in basaltic aquifers. On the other hand, its concentration varies from 22.2 to 178 mg l−1 in granitic aquifers (Khandare 2013). Therefore, contamination of nitrate is frequent in basic zone of extrusive granite formation.
The sulfate concentration in the study area varied from 5.3 to 233 mg l−1. In more than 96% of water samples, concentration of sulfate was below the permissible limit of 200 mg l−1 (WHO 2011). Similar concentration of sulfate has been reported in literatures for instance; 25.5–256 mg l−1 (Wang et al. 2019), 50–132 mg l−1 (Sahoo et al. 2016), 1.2–207 mg l−1 (Keesari et al. 2019). In the study area, the higher sulfate concentration can be attributed to the weathering of sulfate-bearing mineral (Islam et al. 2017a). In general, the occurrence of phosphate in natural water is very low. In the study area the concentration of phosphate was varied from BDL (below detactable limit) to 4.3 mg l−1. In some of the groundwater samples, the elevated concentration of phosphate might be attributed to the application of fertilizers in agricultural land, disintegration of organic waste matterials, agriculture runoff and washerman activities.
Hardness
The total hardness in the study area was in the range of 40–300 mg l−1. As per BIS the maximum acceptable limit of total hardness is 200 mg l−1, whereas WHO (2011) recommended its limit to 100 mg l−1. The concentration of calcium varied in the range of 20–200 mg l−1,while that of magnesium varied from minimum detection limit (MDL) to 220 mg l−1. Approximately 3.5% of samples possessed a high concentration of total hardness (≥200 mg l−1). This observation might be attributed to the dissolution of multivalent cations from sedimentary rocks present in some part of the study area (along the western border of Dhenkanal district nearer toTalcher region). In general, hardness of water increases due to the seepage and surface runoff activities. Further, the elevated hardness in a few groundwater samples might be due to the increase in evapotranspiration and decrease in the volume of water during the summer season (Mahananda et al. 2010). Keesari et al. (2016) reported identical results of hardness profile in the range of 43–672 mgl−1 in Angul and Talcher zone of Odisha.
Alkalinity
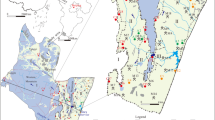
The estimated alkalinity in the study area ranged from 40 to 500 mg l−1. Applying IDW interpolation method, the spatial distribution of biocarbonate alkalinity profile in the study area has been constructed as shown in Fig. 2. Among the alkalinity species, bicarbonate alkalinity was predominantly observed in ground water samples (Fig. 2). In the stdy area high bicarbonate concentration in water facilitated the dissolution and weathering of minerals like plagioclase, metamorphic and sedimentary rocks. When the concentration of bicarbonate exceeded 400 mg l−1, the weathering and dissolution of metamorphic and sedimentary rocks also accelerated. Similar groundwater alkalinity profile has been reported in numerous literature from different parts of India, for instance; 212.67–408 mg l−1 (Kumar et al. 2015), 153 to 475 mg l−1 (Saxena and Saxena 2015), 205–542 mg l−1 (Sahoo et al. 2016). The median pH of the study area was estimated to be 6.8 which revealed that the collected water samples were not alkaline in nature and possessed lower concentration of CO32−. The pH profile of the study area is in conjunction with other reported literature for instance; 6.60–7.10 (Kumar et al. 2015), 7–7.3 (Sahoo et al. 2016) and 7.0 to 8.1 (Saxena and Saxena 2015).
Fluoride
The frequency distribution of fluoride is shown in Fig. 3. In general, when the concentration of fluoride is <0.5 mgl−1 in drinking water induces dental caries, whereas, at concentration more than 1.5 mg l−1 causes dental and skeletal fluorosis (Olaka et al. 2016; Singha et al. 2019). In the present study, 30% of the collected water samples possessed fluoride concentration less than 0.5 mg l−1, which is below the prescribed limit set by WHO (2011). On the other hand, in 5.3% of samples the concentration of fluoride were exceeding the WHO permissible limit. However, the estimated median fluoride concentration was 0.9 mg l−1, which indicates that the overall level of risk associated with flouride is minimum. Similar observation on the concentration of fluoride (0.01–2.8 mg l−1) has been reported in literature (Gomez et al. 2020). The higher fluoride concentration in the study area might be due to the application of synthetic phosphate fertilizer coupled with pesticides as well as dissolution of metamorphic rock under alkaline pH. Similar observations on the leaching of phosphatic fertilizers leading to increase in concentration of fluoride in groundwater have also been reported by Ramteke et al. (2018) and Dartan and Taspinar (2017). This finding is also in good agreement with many reported literature that fluoride contamination in Angul district is due to the weathering of rocks and minerals. The spatial distribution of fluoride in the study area using the IDW interpolation method is represented in Fig. 4.
Uranium profile
The concentration of uranium in the study area was varied from <0.2 to 55.7 ppb. In 91% of the water samples the concentration of uranium was less than MDL (0.2 ppb), which is well below the permissible limit prescribed by WHO (2011). Figure 5 demonstrated the the distribution of uranium in the study area. It was observed that except in one sample the concentration of uranium was well below to the permissible limit set by WHO (2011). Generally in groundwater, the concentration of uranium increases along the water flow direction, the Brahmani river flows towards southeast (SE) direction of Angul district. This might be one of the possible reason for occurrence of higher uranium concentration in western part of Dhenkanal district, particularly at Ghagaramunda (24.8 ppb, D10), Kendumunda (55.7 ppb, D24), Medhapada (29.9 ppb D31). Using IDW interpolation method the spatial distribution of uranium in Dhenkanal has been generated as shown in Fig. 6. A few samples namely, Kendumunda, Gagaramunda and Mendhapada were possessed a high concentration of uranium. These sampling points are located near the border area of Talcher region of Angul district. The presence of uranium in this region might be due to the talchir formation (presence of lower gondwana rocks with coal also known as Barakarformation). Because of the presence of sedimentary and metamorphic rock, coal formation in Talcher with slightly acidic pH which facilitates the dissolution of rock and minerals in the groud water. Further, the high concentration of uranium in Kendumunda (D24), and Mendhapada (D31) might be due to the presence of high concentration of calcium hardness (60–100 mgl−1) along with nitrate (25–35 mgl−1), which influenced the solubility properties of uranium and slowdown the transport of uranium in groundwater. Whereas the high concentration of uranium in Gagaramunda (D10) might be due the elevated concentration of bicarbonate alkalinity (340 mg l−1). This is attributed to the fact that at elevated concentration of biocarbonate alkalinity facilitates the dissolution of minerals and thus increases the concentration of uranium in the groundwater. Interstingly all observations were well within the drinking water limit as recommended by Atomic Energy Regulatory Board, the national nuclear regulatory agency (AERB DAE 2004).
Descriptive statistical analysis of data
The physico-chemical parameters such as; TDS, EC, pH, DO, ORP, Ca2+, Mg2+, HCO3−, Cl−, SO42− and NO3−, were statistically analyzed and are presented in Table 2. The preliminary evaluation as shown in Table 2 revealed that, EC, Cl−, SO42−, Ca +2, Mg+2 and total alkalinity have the maximum mean concentrations among the water quality parameters followed by NO3−. As per WHO guidelines, all the collected groundwater samples can be categorized under freshwater category on the basis of mean concentration of TDS (289.52 ± 216.68 mg l−1), SO42− (65.03 ± 57.46), PO43− (1.19 ± 1.02), U (1.17 ± 6.37) as shown in Table 2. The skewness for the majority of the water quality parameters was also under acceptable range (standard range ± 2). In contrast, the skewness of uranium was greater than that of the standard range, which signified that the data was heavily skewed and not normal (Islam et al. 2018). Moreover, uranium, Cl−, Ca+2 and NO3− ions demonstrated a high positively skewed data sets that governs the groundwater hydrology and geology of the study area. Similarly, incase of kurtosis, NO3−, Cl−, uranium, Ca+2 and ORP were categorized in the leptokurtic scale (values are greater than 3), whereas, the remaining ions were identified in the platykurticscale (values are less than 3) as shown in Table 2.
Inter-relation of parameters with entropy
The relationship between entropy weight and information entropy values with the water quality parameters has been evaluated. In general, the water quality parameters with a maximum entropy weight and minimum information entropy value exhort highest effects on overall quality of groundwater (Wu et al. 2011; Gorgij et al. 2017). The entropy value reduces any bias obtained by neglecting the artificial weights (Amiri et al. 2014). Table 3 represents the estimated groundwater quality weight and entropy weight for the investigated water quality parameters. From the Table 3, it can be observed that uranium has the maximum entropy weight of 0.4937 and minimum entropy information of 0.3354, which clearly revealed a significant impact of uranium on the quality of groundwater. The effects of other physico-chemical parameters on quality of groundwater are evaluated in the following order: U > SO42− > Mg+2 > total hardness > total alkalinity > TDS > Cl−> F−. It is clearly understood from the Table 3 that DO and pH possessed a negligible impact on overall quality of groundwater. However, this observation differs from reported literature that HCO3− is the most influential water quality parameter (Gorgij et al. 2017). Whereas, Amri et al. (2014) reported that SO42− is the predominant anion during the postmonsoon period. In contrast, the groundwater weight in most of the water quality parameters are estimated to be 4 with relative significance of water quality criteria (Islam et al. 2017b; Bodrud-Duza et al. 2016; Vasanthavigar et al. 2010). The elevated concentration of NO3− not only contributed by anthropogenic sources but also altered due to the climatic conditions. For example, change in precipitation pattern, temperature and concentration of atmospheric CO2 have influence on the agricultural NO3− source and agricultural yield (Stuart et al. 2011). However, the major water quality parameters such as; U > SO42− > Mg+2 > total hardness > total alkalinity > TDS > Cl−> F− have a greater effect on GWQI calculation with higher entropy weights, consequently yielded higher negative impact on quality of ground water. Thus, special attention is essential to these water quality parameters which plays a major role in contamination of the groundwater in the study area.
Characterization of groundwater quality ranks
The values of EWQI and the assigned ranks of collected groundwater samples were calculated by employing Eqs. 1–7. Similarly, the GWQI values of the groundwater samples were determined using employing Eq. 8. The calculated values of EWQI and GWQI are utilized to analysze and interpret the quality of groundwater samples. The quality of groundwater for drinking purpose has been assessed on the basis of Indian Standards as shown in Tables 4 and 5. The calculated mean and range of EWQIs of the water samples were 35.74 and 20.04 to 82.19, respectively. In general, the critical limit of EWQIs is 100 therefore,none of the samples were exceeding the critical limit. Moreover, 24 number of groundwater samples (21.42%) out of 112 were categorized as under rank 2 (good-quality drinking water), 88 number of samples (78.57%) were classified as excellent-quality water (rank 1) for drinking purpose (Table 4). None of the groundwater sample were falling under poor-quality water category (rank 4), which implies that most of these samples are suitable for drinking purpose. The estimated GWQI values are varied from 27.04–121.07 with an average of 56.76 as given in Table 5. Generally, the critical limit of GWQI value is 100 for drinking purpose (Vasanthavigar et al. 2010). The results revealed that 1.8% of the groundwater samples surpassed the critical limit (Table 5). It was also observed that maximum number of samples in the study area coming under good to excellent quality rank based on the EWQI values than that of GWQI classification. In terms of GWQI values, 98% groundwater samples were categorized under the good to excellent water quality ranks. Out of 112 groundwater samples, 2 samples(1.8%) are categorized under rank 3 (medium quality water), which indicates the requirement of a suitable pretreatment before application for drinking purpose (Table 4). It is also observed that 71 number of samples have an equal rank between the GWQI and EWQI classifications. There are few variations in the ranking of water quality using GWQI and EWQI classification. This deviation might be due to the assignment of artificial weight in the determination of water quality using GWQI technique. Therefore, the EWQI analysis of groundwater for drinking purposes offers more consistent results than that of the GWQIs technique. Further, Pearson’s correlation coefficient was applied to elucidate the relationship between the different waterquality indices and physico-chemical parameters. The details of correlation study of the GWQIs and EWQIs is shown in Table 6. For instance, the EWQI values of the collected groundwater have considerable positive correlation between total hardness (r = 0.854) and TDS (0.720). In addtion, EWQIs values have been positively correlated to total alkalinity (r = 0.414), EC (r = 0.631), Cl− (r = 0.437) and Uranium (r = 0.299), which clearly indicates the moderate impact of these factors on the overall groundwater quality. The concentration of fluoride, DO, nitrate and pH exhibited an insignificant correlation (Table 6). Similarly, the GWQI values have appreciable positive correlation with TDS (r = 0.659), total hardness (r = 0.827), total alkalinity (0.463), EC (r = 0.659) and Cl (r = 0.452). On the other hand, the values of GWQIs are insignificantly correlated with uranium (r = 0.033), fluoride (r = 0.270) and DO (r = 0.206).Thus, the adopted water quality index analysis indicates that both in EWQIs and GWQIs reveal a reasonable positive correlation with TDS, total hardness, total alkalinity, EC, and Cl− in the study area.
This fact suggest that anthropogenic sources particularly leaching of domestic and small industrial wastewater affect the overall quality of groundwater (Su et al. 2018). Similar, the exhistence of significant correlation between Mg+2 and Ca+2 hardness and water quality index has also been reported by Islam et al. (2017a) for Sylhet district of Bangladesh. Therefore, the results of these correlation coefficients offered a meaningful mathematical approach into the resemblance between the physico-chemical parameters that influence the quality of water in the study area. The result of EWQI and GWQI techniques shows relatively identical developments for the majority of the collected water samples. In the present investigation, the GWQI values exhibited elevated spatial variability, while EWQI values demonstrated lesser spatial variability. Overall, the quality of groundwater in the major parts of Dhenkanal district reveals a hefty spatial variation. The elevated concentrations of uranium and other ions such as; Cl−, SO42−, NO3− and Mg+2 are typically accountable in polluting the groundwater which might be due to the extensive application of fertilizers for cultivation purposes as well as infiltration of rural and urban wastewater.
Correlation study
In order to determine the influence of physico-chemical water quality parameters on the ground water quality, the Pearson correlation analysis was applied as shown in Table 7. From the Table 7 it can be observed that pH exhibited a positive correlation with electrical conductivity with a correlation coefficient of 0.360 while TDS is positively correlated with EC with a correlation coefficient of 0.641. However, correlation between total hardness and chloride was slightly positive with a correlation coefficient of 0.255. The occurrence of positive significant correlation can be attributed to man made or natural sources (Haloi and Sarma 2012). For instance; the correlation between EC and Cl− with a significant positive correlation coefficient of 0.247 might be originated due to the local geology. The estimated correlation coefficient between magnesium and phosphate was high (0.269), which reveals the occurrence of magnesium mainly in the form of MgPO4. On the other hand, correlation coefficient value between magnesium (Mg+2) and sulfate was insignificant (−0.062). Similarly, the correlation between nitrate and magnesium was quite insignificant, thus availability of Mg(NO3)2 seems to be very scarce in the collected groundwater samples. The correlation coefficient between calcium and bicarbonate alkalinity is significantly high (0.377), which strongly reveals the availability of calcium bicarbonate in the collected water sample. The concentration of fluoride is strongly correlated with alkalinity with a corelation coefficient value of 0.245 (Jabal et al. 2014). However, in some pairs of water quality parameters, the estimated correlation coefficient found to be insignificant, for example; uranium and sulfate (−0.035), fluoride and nitrate (−0.007), chloride-fluoride (−0.015) and fluoride and calcium (0.021). Similar observations have been reported by numerous researchers that concentration of fluoride is inversely proportional to calcium (Kumar et al. 2018b; Raj and Shaji 2017). Likewise, existence insignificant correlations were observed in other water quality parameters which clearly reveal that the sources of these physico-chemical groundwater parameters are not influneced by others (Kamrani et al. 2016).
Conclusion
The present study reveals the dominance of major cations and anions in the following order, SO42− > Mg+2 > total hardness > total alkalinity > TDS > Cl− and are typically accountable for the ground water pollution. This is primarily due the of extensive application of fertilizers for cultivation purposes, infiltration of rural and urban wastewater. All the groundwater samples possessed uranium within the drinking water limit prescribed by AERB, which are well supported by correlation analysis. Further, in most of the ground water samples, the result of EWQI and GWQI techniques are well corroborated to each other. Only 1.8% of the groundwater samples surpassed the critical limit (100) of GWQI (rank 3 medium), however, such water samples can be utilize for drinking purposes by adopting necessary pre-treatment strategy. In the IDW model, a slightly spatial dependency was monitored, which reveals the weak natural and anthropogenic influences governing the quality of groundwater. Spatial distribution of uranium and water quality parameters reveals that most of the high index values were typically present in the north-western region of Dhenkanal. Thus, special attention is very crucial to prevent further contamination of groundwater in the western parts of the study area. Most of the collected groundwater samples the water quality parameters meet the requirements as recommended by the WHO guidelines.The present investigation offers not only a broad information on the quality of the drinking water of Dhenkanal district for policy-making, but also a significant reference for regional water pollution control authority.
Data availability
All data generated or analyzed during this study are included in this manuscript
References
AERB DAE (2004) Atomic Energy Regulatory Board, Department of Atomic Energy). Drinking Water Specifications in India. Atomic Energy Regulatory Board, Mumbai, India
Ahada CP, Suthar S (2018) Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab India. Environ Sci Pollut Res 25:25336–25347. https://doi.org/10.1007/s11356-018-2581-2
Ahada CPS, Suthar S (2017) Hydrochemistry of groundwater in North Rajasthan, India: chemical and multivariate analysis. Environ Earth Sci 76:203. https://doi.org/10.1007/s12665-017-6496-x
Amadi AN (2010) Effects of urbanization on groundwater quality: a case study of Port-Harcourt, Southern Nigeria. Nat Appl Sci J 11(2):143–152
Amiri V, Rezaei M, Sohrabi N (2014) Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat, Iran. Environ Earth Sci 72:3479–3490. https://doi.org/10.1007/s12665-014-3255-0
APHA (2005) Standard methods for the examination of water and wastewater, 21th edn. APHA, AWWA and WEF, Washington DC
Bayatvarkeshi M, Imteaz MA, Kisi O, Zarei M, Yaseen ZM (2020) Application of M5 model tree optimized with Excel Solver Platform for water quality parameter estimation. Environ Sci Pollut Res https://doi.org/10.1007/s11356-020-11047-w
BIS (2012) Bureau of Indian Standards, drinking water specification (second revision) ICS 13.060.20, IS 10500:2012 New Delhi.
Bodrud-Doza M, Islam ARMT, Ahmed F, Samiran D, Narottam S, Rahman MS (2016) Characterization of groundwater qualityusing water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci 30:19–40. https://doi.org/10.1016/j.wsj.2016.05.001
Dartan G, Taspinar F (2017) Analysis of fluoride pollution from fertilizer industry and phosphogypsum piles in agricultural area. Jr Ind Poll Cont 33:662–669
Fallah B, Richter A, Ng KTW, Salama A (2019) Effects of groundwater metal contaminant spatial distribution on overlaying kriged maps. Environ Sci Pollut Res 26:22945–22957. https://doi.org/10.1007/s11356-019-05541-z
Feng LH, Sang GS, Hong WH (2014) Statistical prediction of changes in water resources trends based on set pair analysis. Water Resour Manag 28:1703–1711. https://doi.org/10.1007/s11269-014-0581-7
Gomez KR, Pasquali CEL, Gonzalez GP, Hernando PF, Martínez RMG (2020) Statistical evaluation of fluoride contamination in groundwater resources of Santiago del Estero Province, Argentina. Geosci Front 11:2197–2205. https://doi.org/10.1016/j.gsf.2020.02.018
Gorgij AD, Kisi O, Moghaddam AA, Taghipour A (2017) Groundwater quality ranking for drinking purposes, using the entropy method and the spatial autocorrelation index. Environ Earth Sci 76:269. https://doi.org/10.1007/s12665-017-6589-6
Gosetti F, Robotti E, Bolfi B, Mazzucco E, Quasso F, Manfredi M, Silvestri S, Facchi A, Marengo E (2019) Monitoring of water quality inflow and outflow of a farm in Italian Padana plain for rice cultivation: a case study of two years. Environ Sci Pollut Res 26:21274–21294. https://doi.org/10.1007/s11356-019-05155-5
Groundwater information booklet of Dhenkanal distinct, govt. of India ministry of water resources central ground water board south eastern region Bhubaneswar May (2013). http://cgwb.gov.in/District_Profile/Orissa/Dhenkanal.pdf
Haloi N, Sarma HP (2012) Heavy metal contaminations in the groundwater of Brahmaputra flood plain: An assessment of water quality in Barpeta District, Assam (India). Environ Monit Assess 184(10):6229–6237. https://doi.org/10.1007/s10661-011-2415-x
Hossain M, Patra PK (2020) Water pollution index—a new integrated approach to rank water quality. Ecol Indic 117:106668. https://doi.org/10.1016/j.ecolind.2020.106668
Hosseinimarandi H, Mahdavi M, Ahmadi H, Motamedvaziri B, Adelpur A (2014) Assessment of groundwater quality monitoring network using cluster analysis, Shib-Kuh Plain, Shur Watershed, Iran. J Water Resour Protect 6(6):618–624. https://doi.org/10.4236/jwarp.2014.66060
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017a) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24:26350–26374. https://doi.org/10.1007/s11356-017-0254-1
Islam ARMT, Shen S, Bodrud-Doza M, Rahman MA, Das S (2017b) Assessment of trace elements of groundwater and their spatial distribution in Rangpur district, Bangladesh. Arab J Geosci 10(4):95. https://doi.org/10.1007/s12517-017-2886-3
Islam ARMT, Shen S, Haque MA, Bodrud-Doza M, Maw KW, Habib MA (2018) Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approache. Environ Dev Sustain 20:1935–1959. https://doi.org/10.1007/s10668-017-9971-3
Jabal MSA, Abustan I, Rozaimy MR, Al-Najar H (2014) Fluoride enrichment in groundwater of semi arid urban area: Khan Younis City, southern Gaza Strip (Palestine). J Afr Earth Sci 100:259–266. https://doi.org/10.1016/j.jafrearsci.2014.07.002
Johansson B (2000) Areal precipitation and temperature in the Swedish Mountains—an evaluation from a hydrological perspective. Hydrology Res 31(3):207–228. https://doi.org/10.2166/nh.2000.0013
Kamrani S, Rezaei M, Amiri V, Saberinasr A (2016) Investigating the efficiency of information entropy and fuzzy theories to classification of groundwater samples for drinking purposes: Lenjanat Plain, Central Iran. Environ Earth Sci 75:1370. https://doi.org/10.1007/s12665-016-6185-1
Keesari T, Sinha UK, Kamaraj P, Sharma DA (2019) Groundwater quality in a semi-arid region of India: Suitability for drinking, agriculture and fluoride exposure risk. J Earth Syst Sci 128(24):1–14. https://doi.org/10.1007/s12040-018-1049-6
Keesari T, Sinha UK, Deodhar A, Krishna SH, Ansari A, Mohokar H, Dash A (2016) High fluoride in groundwater of an industrialized area of Eastern India (Odisha): inferences from geochemical and isotopic investigation. Environ Earth Sci 75(14):1–17. https://doi.org/10.1007/s12665-016-5874-0
Khandare HW (2013) Scenario of nitrate contamination in groundwater: Its causes and prevention. Int J Chem Tech Res 5(4):1921–1926
Kumar N, Kumar S, Poonam, Singh DP (2015) Ground water quality evaluation at suburban areas of Lucknow, U.P. Int J Environ Sci 6(3):376–387. https://doi.org/10.6088/ijes.6042
Kumar D, Singh A, Jha RK (2018a) Spatial distribution of uranium and basic water quality parameter in the capital of Bihar and consequent ingestion dose. Environ Sci Pollut Res 25:17901–17914. https://doi.org/10.1007/s11356-018-1922-5
Kumar S, Venkatesh AS, Singh R, Udayabhanu G, Saha D (2018b) Geochemical signatures and isotopic systematics constraining dynamics of fluoride contamination in groundwater across Jamui district, Indo-Gangetic alluvial plains, India. Chemosphere 205:493–505. https://doi.org/10.1016/j.chemosphere.2018.04.116
Li P, Li X, Meng X, Li M, Zhang Y (2016) Appraising groundwater quality and health risks from contamination in a semiarid region of north west China. Expo Health 8:361–379. https://doi.org/10.1007/s12403-016-0205-y
Li P, Wu J, Qian H (2010) Groundwater quality assessment based on entropy weighted osculating value method. Int J Environ Sci 27(3):31–34
Nihalani S, Meeruty A (2020) Water quality index evaluation for major rivers in Gujarat. Environ Sci Pollut Res https://doi.org/10.1007/s11356-020-10509-5
Olaka LA, Wilke FD, Olago DO, Odada EO, Mulch A, Musolff A (2016) Groundwater fluoride enrichment in an active rift setting: Central Kenya Rift case study. Sci Total Environ 545:641–653. https://doi.org/10.1016/j.scitotenv.2015.11.161
Pan C, Ng KTW, Richter A (2019) An integrated multivariate statistical approach for the evaluation of spatial variations in groundwater quality near an unlined landfill. Environ Sci Pollut Res 26:5724–5737
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, southern India. Geosci Front 8:117–124. https://doi.org/10.1016/j.gsf.2016.01.002
Ramteke LP, Sahayam AC, Ghosh A, Rambabu U, Reddy MR, Popat KM, Rebary B, Kubavat D, Marathe KV, Ghosh PK (2018) Study of fluoride content in some commercial phosphate fertilizers. J Fluor Chem 210:149–155. https://doi.org/10.1016/j.jfluchem.2018.03.018
Sahoo M, Mahananda MR, Seth P (2016) Physico-chemical analysis of surface and groundwater around Talcher Coal Field. J Geosci Environ Prot 4(2):26–37. https://doi.org/10.4236/gep.2016.42004
Saxena U, Saxena S (2015) Correlation study on physico-chemical parameters and quality assessment of groundwater of bassi tehsil of district Jaipur, Rajasthan, India. Int J Environ Sci Technol 1(1):78–91
Singha S, Pasupuleti S, Durbha KS, Singh SS, Singh R, Venkatesh AS (2019) An analytical hierarchy process-based geospatial modeling for delineation of potential anthropogenic contamination zones of groundwater from Arang block of Raipur district, Chhattisgarh, Central India. Environ Earth Sci 78(694):1–19. https://doi.org/10.1007/s12665-019-8724-z
Singha S, Pasupuleti S, Singh S, Villuri VGK (2017) An integrated approach for evaluation of groundwater quality in Korba district, Chhattisgarh using Geomatic techniques. J Environ Biol 38(5):865–872. https://doi.org/10.22438/jeb/38/5/MRN-600
Srivastava SK, Ramanathan AL (2018) Geochemical assessment of fluoride enrichment and nitrate contamination in groundwater in hard-rock aquifer by using graphical and statistical methods. J Earth Syst Sci 27:104. https://doi.org/10.1007/s12040-018-1006-4
Su H, Kang W, Xu Y, Wang J (2018) Assessing groundwater quality and health risks of nitrogen pollution in the Shenfu mining area of Shaanxi Province, Northwest China. Expo Health 10:77–97. https://doi.org/10.1007/s12403-017-0247-9
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamil Nadu, India. Environ Monit Assess 171(1–4):595–609. https://doi.org/10.1007/s10661-009-1302-1
Wagh VM, Panaskar DB, Muley AA, Mukate SV (2017) Groundwater suitability evaluation by CCME WQI model for Kadava river basin, Nashik, Maharashtra, India. Model Earth Syst Environ 3:557–565. https://doi.org/10.1007/s40808-017-0316-x
Wang Z, Chen H, Li F (2019) Identifying spatial heterogeneity of groundwater and its response to anthropogenic activities. Environ Sci Pollut Res 26:29435–29448. https://doi.org/10.1007/s11356-019-06121-x
Webster R, Oliver MA (2011) Geostatistics for environmental scientists. Wiley, New York, NY, pp 330
World Health Organisation (2011) Guidelines for drinking water quality. 4th World Health Organization, Geneva, 10.1080/10937404.2010.509015
Wu J, Li P, Qian H (2011) Groundwater quality in Jingyuan county, a semi-humid area in Northwest China. J Chem 8(2):787–793. https://doi.org/10.1155/2011/163695
Yan J, Liu J, Ma M (2014) In situ variations and relationships of water quality index with periphyton function and diversity metrics in Baiyangdianlake of China. Ecotoxicology 23(4):495–505. https://doi.org/10.1007/s10646-014-1199-5
Zeid SAM, Seleem EM, Salman SA, Abdel-Hafiz MA (2018) Water quality index of shallow groundwater and assessment for different usages in El-Obour city, Egypt. J Mater Environ Sci 9(7):1957–1968
Acknowledgement
This study was funded by the financial support received from the BRNS, DAE, Government of India, under National Uranium Project of BARC, Mumbai, for carrying out this research work. No. 36(4)114/19/2014-BRNS/1169-I.
Author information
Authors and Affiliations
Contributions
MMS: Conduct experiments and collected data, Analyzed sample and Data, Writing draft manuscript, & Editing. SR: Formal analysis, Visualization, Review & Editing. AD: Conceptualization, Supervision, Review & Editing. NKS: Conceptualization, Supervision, Writing- review and editing, Funding acquisition, project administration, Funding resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naik, M.R., Barik, M., Prasad, K.V. et al. Hydro-geochemical analysis based on entropy and geostatistics model for delineation of anthropogenic ground water pollution for health risks assessment of Dhenkanal district, India. Ecotoxicology 31, 549–564 (2022). https://doi.org/10.1007/s10646-021-02442-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02442-1