Abstract
Human health is strongly influenced by water quality which is threatened by the poor quality of polluted groundwater. In this study, the groundwater quality and its suitability for drinking have been studied in Lenjanat plain aquifer, Iran. Fifty-nine groundwater samples from study area were evaluated based on WHO and Iranian standards for drinking water. Groundwater samples from selected monitoring sources were sampled seasonally during 2009–2010. Physical and chemical parameters of groundwater such as electrical conductivity, pH, total dissolved solids, Ca2+, Na+, K+, Mg2+, HCO3 −, SO4 2−, Cl−, F− and NO3 − were determined. During the water quality index calculating process, the weight of each parameter is usually given by experts according to their practical experience, which is subjective, so much useful and valuable information about the water quality gets lost. In order to avoid personal judgments about the weight of parameters, an information entropy method was used to assign weight to each parameter. Calculation of entropy weighted water quality index (EWQI) for groundwater samples showed that in the wet season, over 57 and 74 % of samples were in the range of “excellent” to “medium” quality based on WHO and Iranian standards, respectively. Due to groundwater quality reduction during dry season, 42 and 62 % of samples were in the range of “excellent” to “medium” quality based on WHO and Iranian standards, respectively. The results indicate that application of the EWQI is very useful to help the public and decision-makers will be able to identify and to evaluate groundwater quality in Lenjanat, Iran.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is essential for life and a nominal supply of clean-safe drinking water is required for the sustenance of life. Human health is closely related with the groundwater quality and is threatened by the poor quality of groundwater caused by excessive application of fertilizers and unsanitary conditions (Godebo et al. 2011; Rango et al. 2012). In the past few decades, there has been a tremendous increase in the demand for fresh water due to the rapid growth of population and the accelerated pace of industrialization (Jiang et al. 2009; Ramakrishnaiah et al. 2009). Rapid urbanization which caused groundwater pollution has affected the availability and quality of groundwater due to its over exploitation and improper waste disposal (Eisena and Anderson 1979; Mohabansi et al. 2011). Groundwater quality depends on the quality of recharged water, atmospheric precipitation, inland surface water and subsurface geochemical processes (Broers and van der Grift 2004). Temporal changes in the origin and constitution of the recharged water, hydrologic and human factors may cause periodic changes in groundwater quality (Reza and Singh 2010). Inadequate environmental protection measures in the industries as well as waste dumps, coal washeries, coking coal plants, thermal power plants, steel, fertilizer and cement plants have resulted in significant water pollution (Chatterjee et al. 2010). Water pollution not only affects water quality but also threats human health, economic development and social prosperity (Milovanovic 2007). River basins are highly vulnerable to pollution due to absorption and transportation of domestic, industrial and agricultural waste water; therefore, it is significant to control water pollution and monitor water quality (Simeonov et al. 2003). Once groundwater is polluted, it is hard to stop the pollution and restore the water quality. It, therefore, becomes imperative to regularly monitor the quality of groundwater and to device ways and means to protect it (Vasanthavigar et al. 2010). The purpose of having drinking water quality guidelines and regulations is to ensure that all human beings within a country have access to safe drinking water. At present, there have been lots of methods for water quality evaluation such as fuzzy mathematics method, membership degree method, factor analysis method, gray modeling method and analytic hierarchy process method (Pei-Yue et al. 2010a). However, these methods cannot clearly express the water pollutant categories and we cannot explain whether the parameters involved in the evaluation meet the requirements of functional areas (Varnosfaderany et al. 2009).
Groundwater chemistry has been utilized as a tool to outlook water quality for various purposes. WQI is an important technique for demarcating groundwater quality and its suitability for drinking purposes. This method which attempts to provide a mechanism for presenting a cumulatively derived numerical expression defining a certain level of water quality is widely used in the world due to the capability of fully expressiing the water quality information. Some studies have been done by several researchers across the world in various fields such as groundwater quality assessment (Tiwari and Mishra 1985; Singh 1992; Rao 1997, 2006; Pesce and Wunderlin 2000; Mishra and Patel 2001; Edmunds et al. 2002; Nasiri et al. 2007; Simões et al. 2008; Jawad Alobaidy et al. 2010; Ishaku 2011; Rubio-Arias et al. 2012). However, during the WQI calculating process, the weight of each parameter is usually given by experts according to their practical experience, which is much subjective and a lot of useful and valuable information about the water quality gets lost.
In this paper, improved WQI with entropy weight was applied to the groundwater quality assessment in Lenjanat plain, Isfahan province, central Iran, to provide a general view of groundwater quality status in this region for drinking purpose. In Lenjanat plain, water quality has been adversely affected by human activities such as urban development, industry, residential and agricultural activities. World Health Organization (WHO) (1984, 1998) and Iranian standard (Water and Wastewater Co. of Tehran 2006) have been used to evaluate water quality changes of Lenjanat plain during wet and dry seasons of one year.
Study area
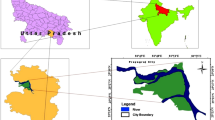
Lenjanat plain is located in the southwest of Isfahan province, Iran. Lenjanat plain, with approximately 1,170 km2 area and average altitude of 1,800 msl, is a sub-basin of Zayandehrud river. The climate of the study area varies from arid to semiarid. The geology and geographic location of the study area are shown in Fig. 1.
Lenjanat plain sediments are composed of Permian to Quaternary geological deposits (Geological Survey of Iran 1976a, b). The most important outcrop in the mountains is Jurassic shale and widely thick layers of Cretaceous limestone. The aquifer bedrock is Jurassic shale and, in some regions, Cretaceous limestone. Alluvial deposits are clay (at least 50 %), sand and gravel. Evaporite layers with gypsum and salt minerals have been deposited among the alluvium. The percentage of salt and clay deposits is higher in the bottom layers (Jafarian 1985; Ministry of Energy 1985; Parsabe Sepahan Andish 2008). Lenjanat alluvial aquifer is mainly recharged from limestone and crushed thick layers of Cretaceous rocks located from west to south mountain borders. General direction of groundwater flow is from west to east, following the overall basin slope. The maximum depth of the water table in the study area is 50 m (east of the area) and its minimum is about 1 m (adjacent to the river) (Nassery and Kayhomayoon 2013).
In the Lenjanat plain, industrial and agricultural water supply is provided by the Zayandehrud river and water for agriculture (except for agricultural land along the river) and livestock is obtained through groundwater resources. Agriculture is performed as double planting (spring and autumn) and flooding irrigation more used in the plain. In recent years, due to groundwater salinization, agricultural activities have been limited to planting of cereals (wheat, alfalfa and clover) in the Lenjanat plain.
Materials and methods
In this study, groundwater from 59 regional monitoring wells was sampled and analyzed seasonally during 2009–2010. Different sources such as agricultural and potable deep wells, rural dug wells, industrial and recreational facilities and drilled wells in the vicinity of pollution sources of urban and rural sewage discharge points were sampled. Studies were conducted for wet and dry seasons separately, and the results were combined finally. The standards for drinking purposes as recommended by WHO and Iran have been considered for the calculation of EWQI. In this study, 10 qualitative parameters including calcium (Ca2+), sodium (Na+), potassium (K+), magnesium (Mg2+), bicarbonate (HCO3 −), sulfate (SO4 2−), chloride (Cl−), fluoride (F−), nitrate (NO3 −) and electrical conductivity (EC) are used to evaluate the groundwater quality for drinking.
In the first step of investigation, the role and contribution of each parameter in water quality changes were described with the help of factor analysis, statistical characteristics of quality parameters and the regional geology. After preliminary studies, the improved method of water quality index (IWQI or EWQI) that uses entropy weighted value in calculations has been used to analyze groundwater quality. Information entropy is used to determine the weight of each parameter, which can reduce the error caused by ignoring the weight (Pei-Yue et al. 2010b). The concept of information entropy was first proposed in 1948 by Shannon (Shannon 1948). Shannon introduced the entropy concept into information theory by suggesting entropy as a measure of information or uncertainty. Shannon entropy expresses the degree of uncertainty implicated in predicting the output of a probabilistic event (Guey-Shin et al. 2011). Mathematically, there is a negative relationship between the amount of information entropy and the probability of occurrence. If the occurrence of an event can be precisely predicted, the probability value will be great, and inversely, the Shannon entropy will be small. Hence, information and uncertainty as dual terms that reveal the information gained are indirectly the amount of reduced uncertainty. Various fields of hydrology and water quality have recently applied entropy theory (Ozkul et al. 2000; Kawachi et al. 2001).
For computing EWQI, the following steps have been followed. In the first step, an entropy weight must be assigned to each parameter (Pei-Yue et al. 2010a; Jian-Hua et al. 2011). For calculating entropy weight when m water samples (i = 1, 2,…, m) are taken to evaluate the water quality and each sample is analyzed for “n” quality parameters (j = 1, 2,…, n), according to observed data, Eigen value matrix X can be constructed as follows:
Data pretreatment should be applied to eliminate the impact of different units of characteristic indices and different quantity grades of quality. According to attribution of every index, the feature indexes may be divided into four types: efficiency type, cost type, fixed type and interval type (Ding and Shi 2005). For the efficiency type, the construction function of normalization is
while for the cost type, the construction function of normalization is
After transformation, the standard-grade matrix Y can be obtained as shown below:
Then the ratio of index value of the j index and in i sample is
The information entropy is expressed by the formula as below
The smaller the value of e j is, the bigger the effect of j index. Then the entropy weight can be calculated with the following formula:
In the formula, ω j is defined as the entropy weight of j parameter. The second step for calculating EWQI is to assign a quality rating scale (q j ) for each parameter. The q j is calculated by the following formula:
where C j is the concentration of each chemical parameter in each water sample in mg/l, and S j is the limit for drinking groundwater of each parameter in mg/l according to quality standards for groundwater of Iran and WHO. The above equation ensures that if j parameter is totally absent in the water, the q j is 0, and when the amount of this parameter is just equal to its permissible value, the q j is 100. The EWQI can be calculated in the third step by the following formula:
According to EWQI, groundwater is classified into five ranks, ranging from “excellent water” to “extremely poor water” (Jian-Hua et al. 2011). The classification standards are listed in Table 1.
Results and discussion
Before calculation of EWQI for each sample, statistical properties of parameters are analyzed. The statistical characteristics of 59 samples of wet and dry seasons along with WHO and Iranian standards of drinking water are presented in Table 2. According to Table 2, the mean of Ca2+, Na+, Mg2+, SO4 2− and EC in both wet and dry seasons is more than desirable values for drinking water based on WHO and Iranian standards. Also, K+, HCO3 −, F−, Cl− and NO3 − ions have the smaller mean values than desirable values. Thus, as an initial conclusion, the contribution of Ca2+, Na+, Mg2+ and SO4 2− ions in increasing EC and in changes of groundwater quality is more than other ions and it is to some extent expected that the water quality is away from threshold ranks (i.e. “excellent” and “extremely poor”) and most of the samples are classified in three categories of 2, 3 and 4. The effect of geological structures (Fig. 1) in increasing concentrations of ions which mainly have natural origin, as a considerable matter in statistical results is visible.
The influence of each parameter or set of parameters in groundwater quality changes has been analyzed in detail through factor analysis, which is briefly described. Factor analysis is an extensively used multivariate statistical method to rearrange original variables into fewer underlying factors (also called common factors) to retain as much information contained in the original variables as possible (Ashley and Lloyd 1978; Suk and Lee 1999; Liu et al. 2008). Unlike original variables, factors are completely uncorrelated with each other. Hence, substituting these factors for the original variables can effectively reduce the overall complexity of large data (Guey-Shin et al. 2011). The Eigen value quantifies the contribution of a factor to total variance. This study performed factor analysis to determine the factors controlling regional groundwater composition. Factor extraction was carried out by principal components, where only Eigen values greater than one was retained. The factor loading matrix was rotated to obtain uncorrelated factors by varimax rotation (Akoyeton et al. 2010).
Factor analysis was performed on the normalized data sets (10 variables) separately for the wet and dry seasons in Lenjanat plain (Table 3). This study retained only factors with Eigen values that exceeded 1.0, and based on the absolute factor loadings, were >0.60 to determine predominant parameters of the common factors.
The results of the factor analysis show that 67.58 % of water quality changes in wet season are controlled by four factors. Factor 1 that explains about 25.58 % of total variance is in relation with Ca2+, Mg2+ and SO 24 . This factor can be considered as weathering of carbonate (calcite and dolomite) and evaporite (anhydrite and gypsum) minerals. Nassery and Kayhomayoon (2013) showed that the dissolution of carbonates (Mg2+/Ca2++Mg2+ <0.5) and gypsum (Ca2+/Ca2++SO 2− 4 = 0.5, HCO3 −/∑ anions <0.8) are the significant processes in the Lenjanat aquifer. Water recharge into Lenjanat aquifer is mainly from limestone formations and alluvial aquifer also contains an abundance of carbonate sediments containing calcium and magnesium resulting in the high concentrations of Ca2+ and Mg2+ in the groundwater. In Lenjanat plain, the Ca2+/(Ca2++SO4 2−) <0.5 is due to decrease of Ca2+ concentration in groundwater (calcite precipitation or ion exchange process), whereas Ca2+/(Ca2++SO4 2−) >0.5 is due to increase of Ca2+ concentration which results in dissolution of carbonate or silicate deposits (a source other than gypsum dissolution). Groundwater samples are undersaturated with respect to gypsum (negative saturation index) and gypsum dissolution, thus leading to an increased concentration of calcium and sulfate ions along groundwater flow direction (Nassery and Kayhomayoon 2013). In addition to gypsum dissolution, heavy use of sulfate fertilizers and agricultural return water can be considered as a secondary factor of increasing sulfate ion concentration in Lenjanat plain aquifer. The variables Na+ and EC have high positive loading of factor 2 that explains about 16.41 % of total variance. This factor indicates that the source of sodium may not be halite dissolution. Dissolution of a less soluble mineral such as albite (plagioclase) or cation exchange (natural softening) can be considered as a source of sodium ion. Due to negligible outcrops of igneous rocks and the abundance of silty-clay deposits in the plain, cation exchange is more likely (Nassery and Kayhomayoon 2013). In wet season, 14.89 % of groundwater chemistry variation is controlled by third factor. The factor loading of Cl− and F show high positive value on factor 3. Regarding the geological deposits in the study area, it can be assumed that the primary source of chloride in the groundwater is sodium chloride (directly from halite dissolution). Accordingly, in most samples, the dissolution of halite (Na+/Na++Cl− = 0.5) is predominant. High concentrations of chloride may also be due to excessive release of waste water in the vicinity the sampling sites. On the other hand, Nassery and Kayhomayoon (2013) by considering the concentration of Cl− ion with the presence of HCO3 − in the border of plain and negative slope close to the unit in Cl–Na+ versus Ca2++Mg2+–SO4 2−(HCO3 −+CO3 2−) concluded that cation exchange is a significant water composition-controlling process in this aquifer. Most of the fluoride found in groundwater is naturally occurring from the breakdown of rocks and soils or weathering and deposition of atmospheric volcanic particles. In Lenjanat plain, the origin of fluoride can also be considered from infiltration of agricultural return water contaminated with chemical fertilizers and waste water from industrial sources. Factor 4 that consists of two elements including K+ and NO3 − accounts for 10.68 % of total variance in wet season. Potassium can be released slowly upon dissolution of rocks or leaching of fertilizer in farm lands. The main source of nitrate in groundwater samples is leaching of nitrate with the percolating water due to the various agriculture and anthropogenic activities. Groundwater can also be contaminated by sewage and other wastes rich in nitrates.
In dry season, 62.16 % of water quality changes are controlled by three major factors. Factor 1 that explains 29.51 % of total variance is in relation with Ca2+, Mg2+ and NO3 −. As mentioned, the presence of Ca2+ and Mg2+ shows the geological impact and dominance of carbonate formations on groundwater quality. The existence of nitrate in this factor may be due to concentration of the NO3 − by decreasing in precipitation. Factor 2 accounts for 18.9 % of total variance including the parameters SO4 2−, F− and Cl−. Sulfate is a strong base that tends to form ion pairs or weak complexes with most mono- and bivalent cations (Langmuir 1997). Cations with higher capacities form covalent bonds with sulfate. Moreover, the solubility of Cl− is more than that of SO4 2−. Accordingly, increase in Cl− concentration due to dissolution of halite deposits leads to increase in salinity in groundwater flow direction, reverse cation exchange and Ca2+ concentration. As mentioned, predominantly gypsum dissolution increases SO4 2− in Lenjanat plain and as there is no precipitation occurrence in dry season, concentration of SO4 2− is less than wet season. Maybe the climatic change is the main cause of displacement of NO3 − and SO4 2− between principal components (factors) 1 and 2. The source of fluoride and chloride are similar to those discussed in the previous section (wet season). In dry season, factor 3 consists of two elements including Na+ and EC and accounts for 13.74 % of total variance. Similar to wet season, the main source of sodium in groundwater resources is caused by cation exchange.
By factors mentioned above, only 67.58 and 62.16 % of water quality changes are interpreted in, respectively, wet and dry seasons. This means there are another clandestine parameters and agents that can impact on water quality in Lenjanat plain.
Analysis of the effective factors in groundwater quality changes without considering EC shows that removing this parameter cannot lead to much change in total and cumulative variance of factors in wet and dry seasons (Table 4). Moreover, position of parameters showed no significant difference from the previous state.
After the above mentioned initial survey and analysis of the relationships between parameters and possible sources of their variability using factor analysis, the EWQI and quality rank of each water sample for drinking were calculated. In this case, after first step that was calculation of entropy value and entropy weight of each parameter, the WQI value was calculated according to rating scale of water quality. Finally, after multiplying WQI value and entropy weight of each parameter, the summation of these values is presented as EWQI for each sample (Eq. 9). The parameters with minimum entropy value and maximum entropy weight have the greatest impact on water quality (Guey-Shin et al. 2011; Jian-Hua et al. 2011). In Table 5, the calculation results of entropy and entropy weight for each parameter in wet and dry seasons are presented.
Results show that SO4 2− and then Cl−, F− and Mg2+ play the leading roles influencing the water quality change in wet season. K+ and Na+ have the same influence on groundwater quality and Ca2+ and NO3 − with equal entropy weight have the smaller impact than other parameters. In wet season, HCO3 − has the minimal influence on groundwater quality. In dry season, some parameters have more impact on water quality changes. In this case, Na+ and then Ca2+, F− and NO3 − have the greatest impact on water quality and finally SO4 2− and K+ are in next order with same influence. In dry season, HCO3 − has the minimal impact on groundwater quality, similar to wet season. Similar to results of factor analysis in both seasons, SO4 2−and NO3 − are among the most important parameters affecting groundwater quality. By considering that formations containing gypsum as the main source of SO4 2− and the rate of NO3 − produced by anthropogenic activities cannot change in a short period of time, the factor influencing these parameters are mostly controlled by the regional climate.
The magnitude of the sum of ranks was used to determine the stability of groundwater quality. The smaller value indicates a more unstable groundwater quality (Guey-Shin et al. 2011). Thus, in both wet and dry seasons, HCO3 − has the continuous small-scale changes. In addition to HCO3 −, NO3 − and Ca2+ have the continuous small-scale oscillations in wet season. In dry season, after HCO3 −, Mg2+ and Cl− show the continuous change in concentration.
EWQI determination using entropy weights and quality ranking of groundwater samples based on drinking water standards (WHO and Iran) was the main aim of this study. The calculated EWQI and quality ranking for each water sample in wet and dry seasons are presented in Tables 6 and 7, respectively. Ranking of samples in wet season showed that most of samples are placed in the “good” quality (rank 2). Twenty groundwater samples (33.90 %) and 25 samples (42.38 %) are classified in “good” quality categories based on WHO standard and Iranian standard, respectively. In addition, 2 and 5 sample are located in “excellent” quality rank (rank 1), 12 and 14 sample in “medium” quality rank (rank 3), 10 and 5 sample in “poor” quality rank (rank 4) and finally 15 and 10 sample in very “extremely poor” quality rank (rank 5), based on WHO and Iranian standards for drinking water, respectively. The brief numerical report of these analyses for wet season is presented in Table 8.
In dry season and according to WHO standard, 21 sample (35.60 %) had the “extremely poor” quality (rank 5), 13 sample (22.03 %) had the “poor” quality (rank 4), 15 sample (25.42 %) had the “medium” quality (rank 3), 9 sample (15.25 %) had the “good” quality (rank 2) and 1 sample (1.70 %) had the “excellent” quality (rank 1) for drinking. Analysis of samples using Iranian standard show a dramatic change in most cases so that over 30 % of samples are located in rank 2, and samples which had the “poor” and “extremely poor” quality in WHO standard are placed in better quality ranks (ranks 2, 3) according Iranian standard (Table 9).
Statistical analysis of samples’ quality ranking based on both standards shows the relative improvement in quality for wet rather dry season so that, based on WHO standard, over 57 % of samples had the “excellent”, “good” and “medium” quality, while this was reduced to 42 % in dry season. Based on Iranian standard, over 74 % of samples in wet season had the “excellent”, “good” and “medium” quality, and in dry season this was reduced to 62 %. The position of various ranks determined based on EWQI and according to WHO and Iranian standards for drinking water is showed in Figs. 2, 3.
Water quality in many parts of Lenjanat plain during wet and dry seasons shows a large quality change. Sometimes, 2 or 3 qualitative rank of groundwater quality changes is visible in both standards in this aquifer. Sampling situations with 2 or more order change in quality rank are displayed in Fig. 4. According to this map, the majority of sampling points with high-quality oscillation are located in urban, rural, residential, and industrial areas and in vicinity of Zayandehrud river.
Increasing the concentration of NO3 −, SO4 2−, Cl− and F− in groundwater is mainly due to infiltration of chemical fertilizers in agricultural areas, gypsum dissolution, cation exchange and leaking of the urban, rural, industrial wastewaters to underground, respectively. Under these conditions, concentration of solutes and groundwater pollutants can be changed by climatic factors such as precipitation as the most important agent (by change of dissolution rate and dilution of contaminants) in water quality oscillation.
Conclusion
The results revealed that the hydrochemical properties of the groundwater samples were controlled by rock/water interactions including ion exchange, dissolution of evaporation deposits (halite and gypsum) and precipitation/dissolution of carbonates. In addition to impact of geological formations, various agricultural and anthropogenic activities in industrial areas can change the hydrochemical properties of the water in Lenjanat aquifer.
The entropy weights of hydrochemical parameters show that the concentrations of SO4 2− and then Cl−, F− and Mg2+ play the leading roles influencing the groundwater quality in wet season. In dry season, Na+ and then Ca2+, F− and NO3 − are the most influencing parameters affecting the groundwater quality and the groundwater quality assessment results are greatly affected by the four parameters. In this plain, concentration of solutes and water pollutants in groundwater can be changed by climatic factors such as precipitation as the most important agent (by change of dissolution rate and dilution of contaminants) in water quality oscillation.
Entropy weight makes WQI perfect and makes the assessment results more reasonable. Based on calculation of entropy weighted water quality index in Lenjanat plain for wet season, relative improvement in water quality in both standards is obvious so that based on WHO standard over 57 % of samples had the “excellent”, “good” and “medium” quality, while in dry season this was reduced to 42 %. Based on calculation of EWQI using Iranian water quality standard, over 74 % of samples in wet season had the “excellent”, “good” and “medium” quality and in dry season, this was reduced to 62 %.
Using the entropy weighted in WQI calculation leads to prevention of personal judgments about weight of parameters. The calculation of EWQI shows that the EWQI method is an easy to use method for groundwater quality assessment and the assessment results are rational and are consistent with the results of field investigation. Results show that application of the EWQI is a very helpful tool that enables the public and decision makers to evaluate groundwater quality in Lenjanat plain, Iran.
References
Akoyeton IS, Ogundele FO, Soladoye O (2010) Characterization by factor analysis of chemical facies water in the coastal plain sand aquifers of Lagos, SW, Nigeria. Int J Acad Res 2(5):256–260
Ashley RP, Lloyd JW (1978) An example of the use of factor-analysis and cluster-analysis in groundwater chemistry interpretation. J Hydrol 39:355–364
Broers HP, van der Grift B (2004) Regional monitoring of temporal changes in groundwater quality. J Hydrol 296(1–4):192–220
Chatterjee R, Gourab T, Paul S (2010) Groundwater quality assessment of Dhanbad district, Jharkhand, India. B Eng Geol Environ 69:137–141
Ding SF, Shi ZZ (2005) Studies on incidence pattern recognition based on information entropy. J Inform Sci 31(6):497–502
Edmunds WM, Carrillo-Rivera JJ, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico City. J Hydrol 258:1–24
Eisena C, Anderson MP (1979) The effects of urbanization on ground-water quality: a case study. Ground Water 17:5
Geological Survey of Iran (1976a) Geological Map of Iran 1:100000 Series, Sheet 6354 Shahreza. Ministry of Industry and Mines
Geological Survey of Iran (1976b) Geological Map of Iran, 1:100000 Series, Sheet 6254 Riz-e-Lenjan. Ministry of Industry and Mines
Godebo TR, Merola BR, Kravchenko J, Vengosh A, Mccornick P (2011) Groundwater geochemistry, quality and its impact on human health: field based dental fluorosis assessment in the Main Ethiopian Rift. 4th International Conference on Medical Geology-Italy
Ishaku JM (2011) Assessment of groundwater quality index for Jimeta-Yola area, northeastern Nigeria. J Geol Min Res 3(9):219–231
Jafarian MA (1985) The project of soils studies in Mobarakeh Steel Co. Isfahan University, Isfahan
Jawad Alobaidy AHM, Abid HS, Maulood BK (2010) Application of water quality index for assessment of Dokan lake ecosystem, Kurdistan region, Iraq. J Water Resour Prot 2:792–798
Jiang Y, Wu Y, Groves C, Yuan D, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J Contam Hydrol 109(1–4):49–61
Jian-Hua W, Pei-Yue L, Hui Q (2011) Groundwater quality in Jingyuan plain, a semi-humid area in northwest China. E-J Chem 8(2):787–793
Kawachi T, Maruyama T, Singh VP (2001) Rainfall entropy for delineation of water resources zones in Japan. J Hydrol 246:36–44
Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall, New Jersey
Liu CW, Jang CS, Chen CP, Lin CN, Lou KL (2008) Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrol Process 22(3):376–383
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeastern Europe. Desalination 213:159–173
Ministry of Energy (1985) The report of geophysical studies in Mobarakeh (Isfahan). Department of Groundwater (in Persian)
Mishra PC, Patel RK (2001) Study of the pollution load in the drinking water of Rairangpur, a small tribal dominated town of North Orissa. Indian J Environ Ecoplanning 5(2):293–298
Mohabansi NP, Tekade PV, Bawankar SV (2011) Physico-chemical Parameters of Textile Mill Effluent Hinganghat, Dist. Wardha (M.S.). Curr World Environ 6(1):165–168
Nasiri F, Maqsiid I, Haunf G, Fuller N (2007) Water quality index: a fuzzy river pollution decision support expert system. J Water Resou Plan Manage 133:95–105
Nassery HR, Kayhomayoon Z (2013) Source of salinity in the groundwater of Lenjanat plain, Isfahan. Iran. Environ Earth Sci 68:413–427
Ozkul S, Harmancioglu NB, Singh VP (2000) Entropy-based assessment of water quality monitoring networks. J Hydrol Eng 5:90–100
Parsabe Sepahan Andish (2008) The project of contaminants recognition in Zarinshahr. thirteen volumes, Zarinshahr Municipality (in Persian)
Pei-Yue L, Hui Q, Jian-Hua W (2010a) Groundwater quality assessment based on improved water quality index in Pengyang plain, Ningxia, northwest China. E-J Chem 7(S1):S209–S216
Pei-Yue L, Jian-Hua W, Hui Q (2010b) Groundwater quality assessment based on entropy weighted osculating value method. Int J Environ Sci 1:4
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Cordoba City (Argentina) on Suquira river. Water Res 34:2915–2926
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in TumkurTaluk, Karnataka state, India. E-J Chem 6(2):523–530
Rango T, Kravchenko J, Atlaw B, McCornick PG, Jeuland M, Merola B, Vengosh A (2012) Groundwater quality and its health impact: an assessment of dental fluorosis in rural inhabitants of the Main Ethiopian Rift. Environ Int 43:37–47
Rao SN (1997) Studies on water quality index in hard rock terrain of Guntur district andhra Pradesh, India. National Seminar on Hydrology of Precambrian Terrains and Hard Rock Areas, pp 129–134
Rao SN (2006) Seasonal variation of groundwater quality in a part of Guntur District andhra Pradesh, India. Environmental Geol 49:413–429
Reza R, Singh G (2010) Assessment of ground water quality status by using water quality index method in Orissa, India. World Appl Sci J 9(12):1392–1397
Rubio-Arias H, Contreras-Caraveo M, Manuel Quintana R, Alfonso Saucedo-Teran R, Pinales-Munguia A (2012) An overall water quality index (WQI) for a man-made aquatic reservoir in Mexico. Int J Environ Res Public Health 9:1687–1698
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27(379–423):623–656
Shyu GS, Cheng BY, Chiang CT, Yao PH, Chang TK (2011) Applying factor analysis combined with kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. Int J Environ Res Public Health 8:1084–1109
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Simões FS, Moriera AB, Bisinoti MC, Gimenez SMN, Yabe MJS (2008) Water quality index as a simple indicator of aquaculture effects on aquatic bodies. Ecol Indic 8:476–484
Singh DF (1992) Studies on the water quality index of some major rivers of Pune, Maharashtra. Proc Acad Environ Biol 1(1):61–66
Suk H, Lee KK (1999) Characterization of a ground water hydrochemical system through multivariate analysis: clustering into ground water zones. Ground Water 37:358–366
Tiwari TN, Mishra MA (1985) A preliminary assignment of water quality index of major Indian rivers. Indian J Environ Prot 5:276–279
Varnosfaderany MN, Mirghaffary N, Ebrahimi E, Soffianian A (2009) Water quality assessment in an arid region using a water quality index. Water Sci Technol 60(9):2319–2327
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: thirumanimuttar sub-basin, Tamil Nadu, India. Environ Monit Assess 171(1–4):595–609
Water and Wastewater Co. of Tehran (2006) Drinking Water Standards (in Persian)
World Health Organization (WHO) (1984) Guideline of drinking quality. World Health Organization, Washington, DC, pp 333–335
World Health Organization (WHO) (1998) Guidelines for drinking water, vol 2, 2nd edn. Health criteria and other information genera Switzerland; pp 281–308
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amiri, V., Rezaei, M. & Sohrabi, N. Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat, Iran. Environ Earth Sci 72, 3479–3490 (2014). https://doi.org/10.1007/s12665-014-3255-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3255-0