Abstract
Investigation of presence of Uranium (U) in groundwater/drinking water is an active are of research due to its chemical and radiological toxicity as well as long-term health effects. The current study had the objective of estimating U as a naturally occurring radioactive element in groundwater samples and assessment of ingestion dose, when groundwater is the source of drinking water. The random sampling method was chosen for the collection of samples based on population density. The estimation of U was done using LED fluorimeter. Statistical tools were applied to analyze the data and its spatial distribution. The U concentrations in three blocks of urban Patna were well below the permissible limits suggested by different health agencies of the world. A correlation test was performed to analyze the association of U with other physiochemical parameters of water samples. It was found that the sulfate, chloride, calcium, hardness, alkalinity, TDS, salinity, and ORP were positively correlated, whereas fluoride, phosphate, magnesium, dissolved oxygen, and pH were negatively correlated with U concentrations. The ingestion dose due to U, occurring in groundwater, was found to vary from 0.2–27.0 μSv y−1 with a mean of 4.2 μSv y−1, which was well below the recommended limit of 0.1 mSv (WHO WHO Chron 38:104–108, 2012).Therefore, the water in this region is fit for drinking purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been increasing concern over uranium (U) in drinking water due to its chemical and radiological toxicity. In recent time, many researchers associate its renal effects especially on tubular cells of kidney (Arzuaga et al. 2010), bone effects (Kurttio et al. 2005) with various form of cancer (Radespiel-Tröger and Meyer 2013; Wagner et al. 2011) due to intake of U-contaminated drinking water.
Several studies in northern and western parts of India, estimated U and ingestion dose, clearly shows the dissolution of natural U in groundwater system, as a result of mineral dissolution or anthropogenic activity or their combined effect. Duggal and Sharma (2017) and Duggal et al. (2017b) reported elevated U concentration of geogenic nature and established correlation of U concentration with conductivity and total dissolved solids. They found the evidence of non-carcinogenic effects (chemical toxicity) of U in western Haryana and negligible carcinogenic risk probability due to U. Again in 2017, Duggal et al. (2017a) estimated U ingestion dose and its toxicity considering different age groups in northern Rajasthan and concluded that the probability of carcinogenic risk was negligible in this region due to intake of groundwater. Saini et al. (2016) have done a comparative statistical analysis to investigate ingestion dose of U in different regions of Punjab and reported 3–4 times higher U concentration in the south-western part as compared to that in the western part of Punjab and same values were found to be 17 times higher than the average value of north-east region of Punjab, which was as a result of urbanization and anthropogenic activity. Yadav et al. (2014) reported the correlation of U concentration increasing with the depth of water samples. The ingestion dose for infants was slightly higher than the other age groups as results of higher dose coefficient and their higher radio-sensitivity and the ingestion dose near the lower bound of the dose range. The higher concentration of U (only 4% samples) in Himachal Pradesh and Punjab was reported by (Rani et al. 2013).
U is a naturally occurring radioactive element with three isotopes U-238, U-235, and U-234 out of which 238U is the most abundant (99.28%) with low specific activity and a longer half-life (4.5 billion years). The concentration of individual isotopes cannot be assumed steady in terms of specific activity in natural condition (Smedley et al. 2006). U, in naturally occurring minerals, has two oxidation states with wide variation in their solubility. It occurs both as tetravalent and hexavalent forms and only the latter form is soluble (Cothern and Lappenbusch 1983). In groundwater, U primarily forms complexes with phosphate and carbonates, but at lower pH, it has an affinity towards fluorides and chloride and their complexes (Smedley et al. 2006). In the past, researchers (Kabir et al. 2014; Shin et al. 2016) have also reported U affinity towards sulfate and magnesium, indicating the effects of the dissolution of dolomitic limestone and gypsum. Brunt et al. (2004) reported strong affinity of U towards the fluorides in plutonic and metamorphic bedrock regions. In the environmental behavior of U, hydrolysis plays an important role and it competes with organic and inorganic complexation (Cothern and Lappenbusch 1983). The hydrothermal alteration, weathering, and erosion are the factors on which redistribution occurs in mineralized zones and dispersed deposits (Porcelli and Swarzenski 2003). The phenomenon of “roll-front U deposits” is produced as a result oxygenated groundwater flow in an aquifer, where initial conditions are reducing or slightly changing towards semi (oxic) condition, (e.g., dissolution/disseminating of iron sulphide minerals and organic matter into U(IV) state) (Smedley et al. 2006).
U in natural groundwater system depends upon several factors such as lithology, geomorphology, and other geological attributes of the region. Furthermore, the spatial variation of U mainly depends on geochemical factors (rock-water interaction) and its residence time in groundwater (Babu et al. 2008). Nevertheless, anthropogenic sources also contribute to its enrichment, which includes agricultural activities in the form of excessive fertilizer application, leaching from natural deposits, mining activities, the nuclear industry, and fertilizer manufacture (Smedley et al. 2006). The municipal discharges may release U from various sources, a factor which remains mostly unaddressed.
The World Health Organization had earlier recommended a reference level 15 μg l−1 for U, but at present, its permissible limit is 30 μg l−1 in drinking water (WHO 2012), and as per guidelines of India’s Atomic Energy Regulatory Board, it is 60 μg l−1 (AERB 2004).
Patna is the capital and the most populated city of Bihar with an urban agglomeration of 1803 persons per square kilometer. The prime source of water consumption is through groundwater only and it is supplied from the nearby Sone megafan aquifer. Sone flows along south to north along the western boundary of the area, where it joins Ganga at the north extreme (AQUIM 2017). The city of Patna is historic; it was the capital of ancient India and has a long history of groundwater usage. Very limited scientific information is available even about the basic water quality parameters of this region especially the distribution of U is unavailable. As per the authors’ knowledge, this study is the first of its kind for the central northeastern part of India.
This study was undertaken to estimate spatial distribution of U concentration in groundwater with its associated water quality parameters and to assess the ingestion dose (radiological and chemical) through the intake of groundwater at the most populated places.
Study area and regional hydrogeology
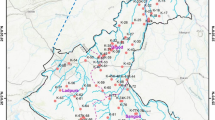
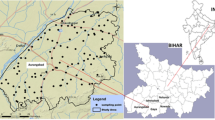
The study area as shown in (Fig. 1) lies between latitudes 25° 32′ 8.12″ to 25° 39′ 18.14″ N and longitudes 85° 0′ 16.83″ to 85° 16′ 2.37″ E and occupies 87 km2 of geographical area. The study area consists three blocks of Patna districts (“Patna Sadar,” “Phulwarisarif,” and “Danapur”). The topology of this region has the western part of the district sloping towards the north and north-east, with an altitude of land surface varying from 68 m in the south to 48 m in the north, and from 67 m in the west to 45 m in the east. The average annual precipitation in the district is 1076 mm with an average annual temperature is 26 °C (CGWB 2013).
This region is part of the middle Gangetic plain contains immensely thick alluvial deposit comprising various grades of clay, silt, sand, and gravel. It is characterized by the occurrence of various grades of sand admixed with kankars (nodules of CaCO3) in alluvial sediments, resulting in the formation of the prolific aquifer with spatially variable hydraulic properties (AQUIM 2017). The occurrence of kankar and fine sand at different places render the top clay zone as semi-pervious in nature. Therefore, groundwater occurs mainly under phreatic condition (CGWB 2013). The deeper aquifers of this region are made up of medium- to coarse-grained sand with occasional gravels. The local tube wells are bored to a depth till this layer is reached to draw drinking water. These deeper aquifers can yield up to 300 m3 hr−1 with the drawdown of 6 m and transmissivity ranges from 3786 m2 day−1 to 19,540 m2 day−1 (CGWB 2013). During the pre-monsoon season, the spatial distribution of water levels during this season reveals that the south-west and central parts of the district have groundwater levels ranging from 2 to 5 m bgl (below ground level), while in the eastern part, the depth of the water level is more than 10 m below bgl. In the post-monsoon season, the spatial distribution of water level shows groundwater levels in the range of 2 to 5 m bgl. In the southern, central, and eastern parts of the study area, the water level is more than 10 m bgl (CGWB 2013).
Methodology
Thirty-two water samples were collected across the three blocks of “Patna Sadar,” “Phulwarisarif,” and “Danapur” in the Patna district. The random sampling method was chosen for the collection of samples based on population density shown in Fig. 1. One-liter low-density polypropylene (LDP) bottles were used in sample collection. The bottles were decontaminated by repeated soaking in 5 M (molarity) of nitric acid followed by 2 M nitric acid and then rinsing with distilled water. The bottles were conditioned using Millipore water prior to collection of the samples. Samples were collected during the month of June 2016 (pre-monsoon) from several locations (Fig. 1). To minimize sample contamination during collection, filtration, storage, and handling, standard protocol has been followed (APHA 2005). Only bore well/hand pump was chosen for sampling and they were flushed for least 2 min before taking the sample. At the time of sample collection, two bottles of the sample were taken, one acidified with 1 N (normality) nitric acid to minimize the effect of wall deposition for U analysis, another one for water quality analysis. Samples were stored in the refrigerator (under 4 °C) until analysis was completed. Samples were analyzed after filtration with 0.45-μm cellulose filters for U and spectrophotometric analysis.
Experimental technique
U estimation
The naturally occurring U present in the groundwater sample was estimated using Quantalase Uranium analyzer (Quantalase LF-2a). The working principle of LED fluorimeter is based on fluorescence of uranyl ion; more detail could be found (Sahu et al. 2014). The fluorescence yield varies for different complexes of U ions. Therefore, an inorganic reagent, Fluren (fluorescence enhancing reagent) is added to the sample to convert all the complexes into a single form having the same fluorescence yield. The Fluren solution consists of 5% sodium pyrophosphate (Na4P2O7.10H2O), which is prepared in distilled water by adjusting the pH value to 7.0 by slowly adding diluted (10% vol/vol) phosphoric acid (Rathore et al. 2001).
The LED fluorimeter was calibrated with standard samples containing 1, 3, 5, 7, and 10 μg.l−1 of U against observed values of fluorescence. The upper detection limit of U concentration in drinking water samples using fluorimeter was 30 μg l−1. Higher concentration samples needed appropriate dilution before analysis. The instrument has a minimum detection limit (MDL) of 0.1 ± 10% μgl−1 (Sahu et al. 2014).
Determination of physiochemical parameters
The in- situ parameters (electrical conductivity (EC), dissolved oxygen (DO), pH, oxidation-reduction potential (ORP), total dissolved solids (TDS), salinity, and temperature) were measured using Thermo Scientific Orion VERSA STAR pH/ ISE/ Conductivity/ RDO/ Dissolved Oxygen Benchtop Multiparameter Meter Kit at field during sampling. The selected anions in water, phosphate, nitrate, and sulfate were determined following the Stannous Chloride Method, Screening Method, and Turbidimetry Method respectively using a UV spectrophotometer (Thermo Scientific Evolution 200). The chloride ions were determined using Mohr’s method. For total hardness, calcium and magnesium were estimated using a standard EDTA titration method. All procedures followed for analysis were as per standard methods for the examination of water and wastewater (APHA 2005).
Results and discussion
Analysis of results
The groundwater in this region was chemically of Ca-HCO3 type with the approximately low amount of TDS (3rth quantile <422.50 mg l−1), which is below the desirable limit (500 mg l−1) at approximately neutral pH (7.17) (Table 1) (BIS 2012). The elevated value of calcium (3rth quantile > 200 mg l−1), magnesium (3rth quantile > 100 mg l−1) also support the fact that, the groundwater quality is of Ca-HCO3 type (Table 1). Groundwater of such quality typically occurs in medium to deep wells (groundwater depth > 10 m bgl during pre-monsoon). An elevated value of EC (> 1000 mg l−1) was also found in some of the samples in this region but most of them were found to be under the permissible limits (BIS 2012). The DO (1.85–3.92 mg l−1) and ORP (−22.8–24.5 mV) values ranged between oxic to the suboxic condition. The observed concentrations of nitrate (NO3-N < 45 mg l−1) indicate pollution not by anthropogenic activity like the use of fertilizers but resulting due to leaching of mineral deposits (Shin et al. 2016).
The level of U concentration in groundwater samples varied from 0.1 to 14.5 μg l−1 with an average of 2.3 μg l−1 and most of the samples have U level less than permissible values suggested by the internationally accredited agencies such as AERB, USEPA, and WHO (AERB 2004; USEPA 2011; WHO 2012).
Statistical relationship
A Pearson’s correlation matrix was built, in order to assess the dependence of the variables (i.e., concentration of various water quality parameters) using open source R statistical software (Peterson et al. 2014). The correlation coefficient matrix of U with other physicochemical species is presented in Table 2. Significant correlations were found between U and other parameters. A strong positive correlation coefficient (0.78) with a p value < 0.05 was found between U and sulfate (Fig. 2). U correlations with sulfate and calcium signify the dissolution effects of gypsum (CaSO4.2H2O) and dolomitic limestone (Ca.(Mg).(CO3)2) (Shin et al. 2016). This finding implies that the occurrence of U was partially associated with the background water composition, sedimentary rocks beneath the subsurface, and Ca-HCO3-enriched groundwater. Therefore, this statistical finding with U may imply the weathering and dissolution process of minerals in the vadose zone or might be excess exploitation of groundwater rather than anthropogenic sources, which accelerate the leaching process in this region as a result of the dissolution process. The elevated U components (or precipitate) relate to these primary and/or secondary minerals, which may be released to the groundwater by roll-front U deposit actions (Smith et al. 1996). The oxidizing condition (positive ORP value and DO) can also favor the release and transport of U in aquifers (Baik et al. 2007; Smith et al. 1996). In addition, relatively high correlation coefficients between U and calcium imply the potential formation of calcium uranyl carbonate complexes that strengthen the U mobility by decreasing its sorption efficiency (Fox et al. 2006; Shin et al. 2016). The negative correlation of U with nitrate and phosphate illustrate the absence of chemical fertilizer and its complexes (Saini et al. 2016) and support the fact that, sampling was done on non-agricultural land.
Distribution fits for U data
In probabilistic statistical modeling, fitting distributions to the dataset is time-consuming but a very common trade-off process. This requires judgment and expertise for distribution choice, parameter estimation, and quality of fit to any data. In this section, fittdistrplus package of R (Delignette-Muller and Dutang 2015) was used to analyze and assess the variability of U dataset, for selecting the best-fit distribution.
The observations exhibit heteroscedasticity of the data, supported by the fact of higher kurtosis (Table 1), having a heavy tail (Fig. 3). Among the several distribution fits, lognormal, Weibull and Gamma distribution were the best fit (see Fig. 3). Based on likelihood fit (Fig. 3), gamma distribution seemed to be the best fit distribution for U data in this particular dataset based on goodness-of-fit statistics and goodness-of-fit criteria (Table 3). Finding the distribution of any data is reasonably necessary, so that it can be transformed to normal distribution, because most of the geostatistical methods work based on this principle and it would help in the selection of appropriate data transformation methods and enhance the model prediction (inverse distance weighted (IDW) methods and spatial statistical (kriging) model). That would be the scope of the work in this regard; in this case, IDW methods is used due to the limitation of data to validate the model.
Spatial statistical analysis
The spatial deterministic techniques was used for describing and modeling spatial data, to assess spatial patterns, distribution, trends, processes, and relationships based on area, length, proximity, orientation, or spatial relationships (Fischer and Getis 2009). The following spatial statistical analyses were performed (using ArcGIS 10.3) on U and its associated data.
Mean center
To assess the central tendency, the mean center tool was used, which indicates the geometric center, in the form of average X and average Y coordinate for a geographic region. The mean center point of the U dataset has found to be X (85.156) and Y (25.590) for this study region (Fig. 4).
Standard distance
The standard distance tools measure the spatial distribution of geographic features around their geometric center as calculated in the above section, which provides information about feature dispersion and orientation. The standard distance for population in the study area was computed, which is shown in Fig. 5. It showed that 60% data fell under the weighted mean center of the population and 40% on another side. The estimated standard distance was found 7.5 km, which was needed while selecting fixed-length methods for inverse distance weighted (IDW) spatial modeling.
Direction distribution
Direction distribution is represented by a standard ellipsis to summarize the spatial characteristics of the geographical features such as trends in particular direction and to measures whether the distribution of features exhibits any directional trends for spatial autocorrelation. It also assists in selecting the number of neighbors based on surrounding points for spatial modeling (IDW). All these estimated water quality parameters, in which they are correlated, were found to be directed in the same direction, i.e., west to east (Fig. 6), while for non-correlated parameters, a slight difference in the direction towards the north-west in direction distribution (Fig. 7).
Spatial distribution of U and its associated parameters
The spatial analyst modeling tool (ArcGIS 10.3) was used, to predict unknown U concentration from known sample points for the geographical area based on spatial autocorrelation/interpolation (IWD) fixed-length method. From analogy, it is easy to see that the values of points close to sampled points were more likely to be similar than those farther apart on the weighted distance. Such methods are helpful for risk assessment based on spatial variability and finding the hot spots. In this study, we applied deterministic inverse distance weighting interpolation for predicting U as well as its correlated parameters for finding the pattern of their distribution after generating raster surface. The resulting map showed an elevated level of U was found towards the eastern portion of Patna Sadar and comparatively low towards the mid-southern part of Phulwari Sharif (Fig. 8). More or less a similar pattern was also observed in the case of sulfate, calcium, and TDS towards the eastern portion of Patna Sadar (Figs. 9, 10, 11, 12, and 13). For nitrate, an elevated concentration was found at Patna Sadar with some elevated patches towards the north-west section of the study area.
Ingestion dose evaluation
U is a series of the heavy radioactive element. It delivers a dual kind of radiation dose, firstly due to radioactive property resulting in carcinogenic risk and the other due to being a heavy metal resulting in non-carcinogenic risks (chemical toxicity).
Radiological risk evaluation
The Radiological Risk is expressed in the terms of excess cancer risk (ECR), which evaluated based on following Eqs. (1, 2, 3) (EPA 2000).
In this study, 25.29 Bq/mg is taken as mass to activity conversion factor, which is the combined effect of aqueous-phase U isotopes present in water sample (Table 4) (Sahoo et al. 2010). Since 234U occurs in the 238U radioactive series in secular equilibrium with the same specific activity, the same has been considered despite the lower mass abundance in natural matrix (Sahoo et al. 2010). The different ratio of 234U/238U was observed by other researchers, which ranges from 1.07–2.60 (Pietrzak-Flis et al. 2004) and 0.98–1.43 (Kronfeld et al. 2004). Mass to activity conversion factor for U is calculated in Table 4.
In this case, the risk coefficient of U is taken as 1.19 × 10−9 Bq−1, life expectancy is 63.7 years, 23,250 days as total exposure duration, and water intake rate is 4.5 l.day−1, which is the upper bound level for adult Indian population (Dang et al. 1994).
The estimated value of ECR in this region varied from 3.2 × 10−7 to 4.6 × 10−5 with the mean 7.3 × 10−6, which is significantly lower as compared to the value (1.67 × 10−4) of maximum permissible limit for excess cancer risk (AERB 2004) (see Table 5).
Chemical toxicity evaluation
The non-carcinogenic risk is estimated in terms of lifetime average daily dose (LADD) for U through intake of water,which is calculated by Eq. (4) (EPA 2000).
To estimate LADD, the following parameters DIR (daily intake rate) of water (4.5 l day−1), EF (exposure frequency) as 350 days per year, AD is average exposure duration (63.7 years), LE (life expectancy) is 63.7 years, and ABW (average body weight) is 51.5 ± 8 kg for adult Indian population (Dang et al. 1994) is considered.
A descriptive statistic of observed lifetime daily dose (LADD) due to U ingestion is presented in Table 5. The wide variation of LADD was observed which ranges from 0.01 to 1.04 μg kg−1 day−1 as result of non-uniform distribution of U concentration in drinking water.
Hazard quotient (HQ) is also used to calculate the extent of the harm, which is calculated by using Eq. (5) (EPA 2000).
The reference dose (RD) is consider 4.53 μg kg−1 day−1, proposed by AERB. Form chemical toxicity point of view, the HQ greater than 1 is not acceptable for drinking purpose. The mean HQ value 0.04 was observed for this region, which is significantly lower than 1. Therefore, there is no harm due to intake of groundwater for this region.
The annual effective dose (AED) is estimated as whole body dose, which is calculated using eq. (6 &7) (EPA 2000). The effective dose per unit intake ( ED1) is taken as 4.5 × 10−8Sv Bq−1 (EPA 2000).
The wide variation AED were observed (see Table 5), which ranges from 0.2–27.0 μSv year−1 with the mean value of 4.2 μSv year−1, which is well below the recommended permissible limit of 0.1 mSv year−1 (WHO 2012) (see Table 5).
Conclusion
This present investigation determined the spatial distribution of u in groundwater and evaluated the potential health risks due to ingestion of water in three blocks of urban Patna. Large spatial variations (0.10–14.45 μg l−1) of U concentrations were observed in drinking water samples. From a water quality point of view, most of the water quality parameters were well within the permissible limit, recommended by the Bureau of Indian Standards (BIS 2012) except calcium, magnesium, and total hardness. From the spatial analysis, it was observed that the consistent elevated value of water quality existed towards the eastern part of the study region (Patna Sadar) with few elevated spots in Phulwarisharif and Danapur blocks. In the case of U, a non-uniform variation of concentration was also observed throughout the regions with elevated values towards the eastern part of Patna Sadar block. The elevated concentration in this part may be due to Ca-HCO3-enriched groundwater in (sub) oxic condition, which aids in increasing U concentration and its mobility. Since the study region was fully urbanized, dissolution of minerals might be the prime cause for higher concentrations of U, which resulted in the low concentration as well as negative correlation with nitrate and phosphate at these elevated points. Therefore, the U concentration in groundwater should be consistently monitored at these locations including the adjacent areas to investigate the root cause; that would be the scope of this research. The ingestion dose assessment, carcinogenic risk (ECR), and non-carcinogenic (chemical toxicity risk) revealed that the mean radiological and chemical toxicity risk is negligible and well below the prescribed permissible limits (AERB 2004; WHO 2012) (see Table 5). The committed effective dose as whole body, due to U intake through drinking water, was found to vary from 0.2–27.0 μSv y−1, with an average value of 4.3 μSv y−1, which is well below the recommended limit of 0.1 mSv (WHO 2012). The mean HQ value of 0.04 was observed, which is significantly lower than 1. Therefore, groundwater of this region is fit for drinking purposes.
References
AERB (2004) Drinking water specifications in India. Department of Atomic Energy, Govt. of India
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
AQUIM (2017) Aquifer mapping—watershed , Patna District, Bihar http://www.aquiferindia.org/About_AQUIM_Watershed_Patna.aspx. 2017, 25 Apr
Arzuaga X, Rieth SH, Bathija A, Cooper GS (2010) Renal effects of exposure to natural and depleted uranium: a review of the epidemiologic and experimental data. J Toxicol Environ Health B 13:527–545
Babu M, Somashekar R, Kumar S, Shivanna K, Krishnamurthy V, Eappen K (2008) Concentration of uranium levels in groundwater. Int J Environ Sci Technol 5:263–266
Baik M-H, Park C-K, Cho W-J (2007) Migration and retardation properties of uranium through a rock fracture in a reducing environment. J Nucl Fuel Cycle Waste Technol (JNFCWT) 5:113–122
BIS (2012) Indian standards specifications for drinking water. Bureau of Indian Standards, New Delhi
Brunt R, Vasak L, Griffioen J (2004): Fluoride in groundwater: probability of occurrence of excessive concentration on global scale. Igrac: international groundwater resources assessment Centre. UNESCO. Report No. SP
CGWB (2013): Ground Water Information Booklet,Patna District, Bihar State,Central Ground water Board,Ministry of Water Resources,(Govt. of India)
Cothern RC, Lappenbusch WL (1983) Occurrence of uranium in drinking water in the US. Health Phys 45:89–99
Dang H, Jaiswal D, Parameswaran M, Krishnamony S (1994) Physical, anatomical, physiological and metabolic data for reference Indian man—a proposal. Bhabha Atomic Research Centre, Mumbai
Delignette-Muller ML, Dutang C (2015) Fitdistrplus: an R package for fitting distributions. J Stat Softw 64:1–34
Duggal V, Sharma S (2017) Chemotoxicity and radiotoxicity risk assessment from exposure to uranium in groundwater from western Haryana, India. Int J Pure Appl Phys 13:107–112
Duggal V, Rani A, Mehra R, Saini K, Bajwa B (2017a) Assessment of age-dependent radiation dose and toxicity risk due to intake of uranium through the ingestion of groundwater from Northern Rajasthan, India. Toxicol Environ Chem 99:516–524
Duggal V, Sharma S, Saini K, Bajwa BS (2017b) Assessment of carcinogenic and non-carcinogenic risk from exposure to Uranium in groundwater from Western Haryana, India. J Geol Soc India 89:663–668
EPA (2000): Edition of the drinking water standards and health advisories. US Environmental Protection Agency Washington, DC
Fischer MM, Getis A (2009): Handbook of applied spatial analysis: software tools, methods and applications. Springer Science & Business Media
Fox PM, Davis JA, Zachara JM (2006) The effect of calcium on aqueous uranium (VI) speciation and adsorption to ferrihydrite and quartz. Geochim Cosmochim Acta 70:1379–1387
Kabir ML, Park Y, Lee J-Y (2014) Chemical characteristics of groundwater in carbonate rock areas of Korea. J Soil Groundw Environ 19:7–15
Kronfeld J, Godfrey-Smith D, Johannessen D, Zentilli M (2004) Uranium series isotopes in the Avon Valley, Nova Scotia. J Environ Radioact 73:335–352
Kurttio P, Komulainen H, Leino A, Salonen L, Auvinen A, Saha H (2005) Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environ Health Perspect 113:68
Peterson BG, Carl P, Boudt K, Bennett R, Ulrich J, Zivot E, Lesstel M, Balkissoon K, Wuertz D (2014): PerformanceAnalytics: Econometric tools for performance and risk analysis. R package version 1.4. 3541
Pietrzak-Flis Z, Kaminska I, Chrzanowski E (2004) Uranium isotopes in public drinking water and dose assessment for man in Poland. Radiat Prot Dosim 113:34–39
Porcelli D, Swarzenski PW (2003) The behavior of U-and Th-series nuclides in groundwater. Rev Mineral Geochem 52:317–361
Radespiel-Tröger M, Meyer M (2013) Association between drinking water uranium content and cancer risk in Bavaria, Germany. Int Arch Occup Environ Health 86:767–776
Rani A, Singh S, Duggal V, Balaram V (2013) Uranium estimation in drinking water samples from some areas of Punjab and Himachal Pradesh, India using ICP-MS. Radiat Prot Dosim 157:146–151
Rathore D, Tarafder P, Kayal M, Kumar M (2001) Application of a differential technique in laser-induced fluorimetry: simple and a precise method for the direct determination of uranium in mineralised rocks at the percentage level. Anal Chim Acta 434:201–208
Sahoo S, Mohapatra S, Chakrabarty A, Sumesh C, Jha V, Tripathi R, Puranik V (2010) Determination of uranium at ultra trace level in packaged drinking water by laser fluorimeter and consequent ingestion dose. Radioprotection 45:55–66
Sahu S, Maity S, Bhangare R, Pandit G, Sharma D (2014): Determination of uranium in ground water using different analytical techniques, Bhabha Atomic Research Centre
Saini K, Singh P, Bajwa BS (2016) Comparative statistical analysis of carcinogenic and non-carcinogenic effects of uranium in groundwater samples from different regions of Punjab, India. Appl Radiat Isot 118:196–202
Shin W, Oh J, Choung S, Cho B-W, Lee K-S, Yun U, Woo N-C, Kim HK (2016) Distribution and potential health risk of groundwater uranium in Korea. Chemosphere 163:108–115
Smedley P, Smith B, Abesser C, Lapworth D (2006): Uranium occurrence and behaviour in British groundwater
Smith B, Powell J, Gedeon R, Amro H (1996): Groundwater pollution by natural radionuclides: an evaluation of natural and mining contamination associated with phosphorite (Jordan)
USEPA (2011): United States Environmental Protection Agency,Edition of the Drinking Water Standards and Health Advisories. Washington, DC. Office of Water.
Wagner SE, Burch JB, Bottai M, Puett R, Porter D, Bolick-Aldrich S, Temples T, Wilkerson RC, Vena JE, Hébert JR (2011) Groundwater uranium and cancer incidence in South Carolina. Cancer Causes Control 22:41–50
WHO (2012) Guidelines for drinking-water quality. WHO Chron 38:104–108
Yadav AK, Sahoo SK, Mahapatra S, Kumar AV, Pandey G, Lenka P, Tripathi R (2014) Concentrations of uranium in drinking water and cumulative, age-dependent radiation doses in four districts of Uttar Pradesh, India. Toxicol Environ Chem 96:192–200
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOC 128 kb)
Rights and permissions
About this article
Cite this article
Kumar, D., Singh, A. & Jha, R.K. Spatial distribution of uranium and basic water quality parameter in the capital of Bihar and consequent ingestion dose. Environ Sci Pollut Res 25, 17901–17914 (2018). https://doi.org/10.1007/s11356-018-1922-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1922-5