Abstract
Sustainable groundwater quality is a key global concern and has become a major issue of disquiets in most parts of the world including Bangladesh. Hence, the assessment of groundwater quality is an important study to ensure its sustainability for various uses. In this study, a combination of multivariate statistics, geographical information system (GIS) and geochemical approaches was employed to evaluate the groundwater quality and its sustainability in Joypurhat district of Bangladesh. The results showed that the groundwater samples are mainly Ca–Mg–HCO3 type. Principal component analysis (PCA) results revealed that geogenic sources (rock weathering and cation exchange) followed by anthropogenic activities (domestic sewage and agro-chemicals) were the major factors governing the groundwater quality of the study area. Furthermore, the results of PCA are validated using the cluster analysis and correlation matrix analysis. Based on the groundwater quality index (GWQI), it is found that all the groundwater samples belong to excellent to good water quality domains for human consumption, although iron, fluoride and iodide contaminated to the groundwater, which do not pose any significant health hazard according to World Health Organization’s and Bangladesh’s guideline values. The results of irrigation water quality index including sodium adsorption ratio (SAR), permeability index and sodium percentage (Na %) suggested that most of the groundwater samples are good quality water for agricultural uses. The spatial distribution of the measured values of GWQI, SAR, Fe (iron), EC (electrical conductivity) and TH (total hardness) were spatially mapped using the GIS tool in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sustainable groundwater quality is essentially vital for human consumption, and agricultural purposes in any region, while a recent study revealed “extensive contamination” possesses more threat to sustainable groundwater supply than depletion (Macdonald et al. 2016). The sustainability of groundwater quality in the context of Bangladesh is reiterated and highlighted with significant importance in recent literature (Hossain et al. 2010; Biswas et al. 2014; Bhuiyan et al. 2015). The evaluation of groundwater quality is not only necessary to know the suitability but also for planning the management of groundwater in a more sustainable way to meet the existing and future demands for drinking and irrigation uses. However, the surface water flow in Bangladesh is also at risk due to the disruption of trans-boundary river courses which has existed on Indian strip. Moreover, the erratic rainfalls also cause adverse effects on the groundwater quality and chemistry in the northwestern Bangladesh.
The excessive uses of groundwater resulting in depletion of groundwater table and degradation of the groundwater quality through anthropogenic processes have led to severe socioeconomic implications in northwestern region of Bangladesh (Shahid et al. 2015; Rahman et al. 2017). In addition, geological characteristics, rock–water interaction, dissolution/evaporation due to rainfall are the major geogenic processes controlling the groundwater quality in this region. Although several studies have focused on groundwater quality in Bangladesh (Saha et al. 2009; Afroza et al. 2009), published work concerning the influence of geogenic and anthropogenic activities on changes of the hydrogeochemical composition in natural water is limited. However, there is no unique solution for sustainable groundwater quality management, and several factors influence climatic, hydro-geologic and socioeconomic conditions that vary with region to region. The groundwater quality index is a plausible option to know the status of the groundwater quality either sustained or unsustainable way in any region.
Many researchers have endeavored to develop various groundwater quality indices (GWQIs) for evaluating water quality; the selection of GWQIs is based upon the groundwater input variables and the obtained outcomes (Singh et al. 2005; Vasanthavigar et al. 2010). The GWQI is an effective tool for measuring not only the suitability of drinking water, but also to formulate the information on groundwater quality in an area. However, most of the previous research works have been conducted in northwestern regions, especially in Natore, Bogra, and Dinajpur districts in Bangladesh (Mridha et al. 1996; Islam and Shamsad 2009; Hakim et al. 2009) where they have focused on the physico-chemical characteristics of groundwater quality and its suitability for human consumption and agriculture uses. Groundwater quality and its sustainability in Joypurhat district of Bangladesh by using integrated hydro-chemical techniques along with the GIS and multivariate statistical approaches are yet to be carried out.
It has been observed that statistical techniques are widely used for assessing the groundwater quality and quantity in the worldwide. Among various statistical techniques, multivariate statistics has been extensively applied for source identification of groundwater pollution in most regions of the world including Bangladesh ((Halim et al. 2010; Molla et al. 2015; Bhuiyan et al. 2016). In addition, spatial analysis shows the heterogeneity of groundwater quality in spatial extents. The combined approaches of multivariate statistics and spatial interpolation technique can provide the reliable results in groundwater contamination history. Since the spatial distribution of groundwater contaminants in any area is controlled by geochemical heterogeneity, the spatial interpolation technique is an option for measuring the value at unknown sampling sites to exhibit groundwater pollution (Webster and Oliver 2001; Hossain et al. 2007; Bhattacharya et al. 2011). In this study, the inverse distance weighting (IWD) interpolation technique has been employed to demonstrate the spatial variation of parameters of groundwater samples (Isaaks and Srivastava 1989; Islam et al. 2017).
The status of the drinking and irrigation water quality is important to know for the better insights into the sustainability of groundwater for domestic, agriculture and other purposes. However, a very limited research has been undertaken on groundwater quality assessment in the study region. Therefore, the integrated geochemical and multivariate statistical approaches are the widely used methods that help to understand the geochemical factor controlling the groundwater geochemistry and quality (Kumar et al. 2009; Liu et al. 2003; Bodrud-Doza et al. 2016; Bhuiyan et al. 2016; Alharbi et al. 2017). Considering above-mentioned aspects, Joypurhat district of the northwest Bangladesh has been chosen as the study site for a detailed investigation of groundwater quality and its sustainability using the integrated approach of the geochemical method and the GIS with multivariate statistics. The paper is organized into 4 sections. The material and method are given in the next Sect. 2. Section 3 contains the result and discussion parts which include the characteristics of groundwater quality, ionic relationship, factor controlling the groundwater quality, status of drinking and irrigation water quality and also spatial analysis. Conclusion is presented in Sect. 4.
2 Material and method
2.1 Study area
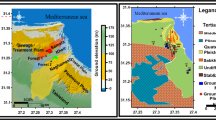
Panchbibi upazila (a small administrative unit), situated in northwestern region of Bangladesh, Joypurhat district has been chosen for this study, which is positioned between 25°02′N to 25°16′N latitude and 88°58′E to 89°12′E longitude (Fig. 1). The Joypurhat district is the part of Tista floodplain that experiences drought condition. Surface is generally flat low laying topography, with a 30 m elevation from mean sea level (Afroza et al. 2009). The study area lies in the sub-humid area of northwest Bangladesh and is vulnerable to different potential threats like growing population, rapid urbanization and small industries. It is occupied by a range of geological formation containing from the DupiTila Sandstone Formation to recent deposits. The DuptiTila Sandstone Formation is divided into semi-consolidated to unconsolidated formation. The sediment is deposited as an older alluvial fan of the Chiri, Haraboti and small Jamuna River. The groundwater quality is highly dominated by the old Brahmaputra and Teesta Rivers of Bangladesh. Aquifer system is mainly semi-confined in nature and originated in Plio-Pleistocene age. This area is characterized by hot summer monsoon climate. The climate is suitable for living and non-living ones and no distinguishable variation in temperature. The mean temperature is 26 °C, and the annual normal rainfall is about 1400 mm.
2.2 Sample design and analytical procedure
Water samples were obtained from local government and engineering department (LGED) at 20 sites from the study area during March 2014. Stratified sampling method was applied to collect water samples of the shallow tube wells whose depths vary from 20 to 85 m (Fig. 1). The collected groundwater samples were transferred into pre-cleaned 500 ml polythene bottles for analysis of physico-chemical parameters. Samples were sent to the laboratory in Dhaka for the details physico-chemical analysis such as pH, EC, and TDS, and major cations and anions (Ca2+, Mg2+, Na+, K+, Fe, Cl−, SO4 2−, HCO3 −, I− and F−). Chemical variables were investigated by the standard techniques followed by APHA (2005). The pH and EC were analyzed during on-site sample measurement by using Hanna HI 8633 portable meters calibrated with standard solutions. The Ca2+, Mg2+, Cl− and HCO3 − were computed by acidimetric titration (Chopra and Kanwar 1980), Na+ and K+ were determined by flamephotometry, I−, F−, SO4 2− were estimated by ion chromatography, and Fe was determined by atomic absorption spectrophotometer. Geochemical assessment of the groundwater samples was performed by using AquaChem (version 3.6) software which was used to classify the groundwater types and to show geochemical diagrams.
2.3 Statistical techniques
Descriptive statistical methods were applied to interpret the statistical variables (maximum, minimum, mean and standard deviation, variance) for groundwater quality data set. The correlation matrix (CM) analysis was carried out to define the degree of pair with two parameters in this study. The terms “significant/strong”, “moderate” and “insignificant” are applied to Pearson’s correlation matrix (CM) analysis based on the approach proposed by Liu et al. (2003), and it indicates to the values as >0.75, 0.75–0.50 and 0.50–0.30, respectively. Multivariate statistical approaches are very useful in attaining significant information from hydro-chemical dataset in the groundwater system. In this study, the multivariate statistical approaches including principal component analysis (PCA), factor analysis (FA) and cluster analysis (CA) were successfully applied to investigate the hydro-chemical data of groundwater. In order to understand the geochemical processes and the sources of major anions and cations in the groundwater, hydro-chemical data were subjected to PCA that allowed to group them based on their inherited properties. The PCA was performed with an orthogonal Kaiser’s Varimax rotation to make the factors more interpretable without changing the original mathematical dataset (Mertler and Vannatta 2005). The varimax rotation can successfully reduce the contribution of less significant parameters in the groundwater quality obtained from the PCA. The first PCA was accounting for the highest variance in the dataset followed by the next PCA and so on. Additionally, the cluster analysis (CA) was employed to identify similar groups or clusters based on similar characteristics within the class and dissimilar characteristics among various classes. The results of attaining multivariate statistical methods were assessed by the R-mode, Q-mode analyses, scree plot and dendrogram based on Ward’s method. Physico-chemical analysis results of groundwater in the study area were analyzed statistically. All the statistical analyses were performed by using the SPSS software version 22.0.
However, currently, various spatial interpolation techniques such as kriging method, inverse distance weighted method and so on were used for predicting and to measure the spatial variability of the groundwater dataset. Out of those interpolation techniques, the inverse distance weighted (IDW) method was deployed for the spatial analysis in this study because of its easiness and estimate accuracy compared to other interpolation methods like kriging (Gorai and Kumar 2013). This method is in-built within ArcGIS. The spatial distribution maps of groundwater dataset were done by using the ArcGIS software (version 10.2).The justification for using the IDW method is that it computes the spatially interpolated values very fast and accurately.
2.4 Water quality analysis
The groundwater quality was calculated by using the groundwater quality index (GWQI) with respect to national and international standards by Eq. (1), given by Vasanthavigar et al. (2010).
where \({q_i}\) is groundwater quality rating point, \({C_i}\) is concentrations of each parameter, and \({S_i}\) is WHO standard (Eq. 2). Then, \({\text{S}}{{\text{I}}_{\text{i}}}\) is the sub-index of ith chemical parameters.
The following equations have been adopted for parameters analysis of irrigation water quality indices.
The total hardness is calculated by Eq. 2
Also MH (magnesium hazard) is measured by Eq. 3
Kelley’s ratio (KR) is given by Eq. 4
The electrical conductance is a function of TDS which given by Eq. 5
The sodium adsorption ratio (SAR) is defined by Richard (1954) in the below Eq. 6
Residual sodium bicarbonate can be expressed in Eq. 7
The Na+ (sodium percentage) or SSP is calculated by the formula in Eq. 8
The permeability indices (P1) is calculated by the following Eq. 9
Ionic concentrations can be expressed by the meq/L. All physico-chemical parameter is matched with national and international standards to examine the sustainability of groundwater quality in the study area.
3 Result and discussions
3.1 Characterization of groundwater quality
Groundwater quality depends on several aspects including level of weathering of the different rock, quality of the aquifer and effect of peripheral pollution sources. These aspects and their relations consequence create complex groundwater chemistry (Aksever et al. 2016). To measure water quality, groundwater datasets were derived from various depths (20–85 m) during the dry season in March 2014, and it was evaluated. The descriptive statistics of physico-chemical parameters of groundwater samples from 20 sites are displayed in Table 1. From Table 1, it can be said that the 14 parameters and their standard deviation are varied noticeably. The results showed a wide range of variation in analyzed physico-chemical parameters in the groundwater. The pH values of the groundwater samples were ranged from 7.4 to 8.8 with a mean of 7.93, indicating the slightly alkaline in nature. According to WHO (2011) and Bangladesh standards (DoE 1997), the pH value of drinking water will be within 6.5 and 8.5. The finding is echoed by the similar work (Bhuiyan et al. 2015) where pH value is found in 7.53 on average in the northwestern part of Bangladesh. The EC values of water were varied from 163 to 865 μS/cm with a mean value of 422.62 μS/cm. The TDS values were ranged from 104.32 to 553.60 mg/L with a mean value of 270.48 mg/L. In accordance with the WHO (2011) and USEPA (2012), the analyzed samples of TDS values are suited for drinking water uses. The high variation on physical parameters may be due to inconsistency with underlying rocks each other. The Ca2+, HCO3 − and EC values of the studied samples were augmented because of deep movement, long residence time and continuous relation with rock types, recharging from the relatively high areas. Predominant ions in the water are Ca2+, Na+ and HCO3 −, respectively. HCO3 − anion was exceeded more than 50% of total anions of the investigated samples. The sequential order for abundance ions of groundwater was: major cations Ca2+ > Na+ > Mg2+ > K+ and major anions HCO3 − > Cl− > CO3 − > SO4 − > F− > I−. The cation exchange and dissolution of carbonate (dolomite) may be ascribed to the high Ca+ concentration in the aquifer. The K+ values are generally low as compared with Ca2+, Na+ and Mg2 in the aquifer system. The high Ca2+ and Mg2+ concentrations in groundwater could be causing the water to be considered as hard type of water. The higher Cl− concentration of groundwater may be caused a corrosion of septic tanks.
Hydro-chemical facies is a very important feature for identifying groundwater hydrochemistry. A trilinear Piper diagram is a popular technique for primary characterization of water quality (Piper 1944). This technique is useful in showing and categorizing chemical data in a single form for fast interpretation of groundwater types. The plots of Piper diagram indicates that Ca2+, Mg2+, HCO3 − play a vital role in defining groundwater type. The main groundwater type is Ca–Mg–HCO3, suggesting dominance of alkaline earths (Ca2+, Mg2+) and weak acid (HCO3 −) (Fig. 2). It is fresh water that occupies the sands of Joypurhat aquifer. This finding is consistent with the previous results of Hossain et al. (2010) for groundwater resources in northern part of Pabna district, Bangladesh. The plotting on Chadha diagram (Chadha 1999) confirms that Ca–Mg–HCO3 is major groundwater type in the study area samples (Fig. 3). The Ca–Mg–HCO3 water type is related to areas where the base rock–groundwater inter-relation is the major reasons for difference in the groundwater chemistry from the hydrologic basins (Yidana 2010). However, the Ca–Mg–HCO3 facies is derived from the dissolution of calcite which exists on the limestone of Eocene age in the aquifer. Furthermore, the molar ratio Ca/HCO3 1:05 is equivalent to 1, supporting the source of these ions in the aquifer system (Faye et al. 2005).
The Piper (1944) plots for groundwater samples of the study
In addition, Gibbs’s graph was used to attain a better understanding into geochemical processes on the groundwater chemistry in a region (Sivasubramanian et al. 2013). The values of physico-chemical parameters are plotted on the Gibb’s (1970) graph in the weight ratio of groundwater Na+/(Na+ + Ca2+), Cl−/(Cl− + HCO3 −) and TDS (e.g., weathering of rock, precipitation and evaporation). The Gibb’s graph indicates that rock-dominance zone is controlled by the processes of carbonate mineral dissolution within the aquifer (Fig. 4). Dissolution of rock is predominant process involving the explanation of groundwater hydrochemistry. The rock dominance on the groundwater chemistry is confirmed by the investigation of the geochemistry and also the computation of Hounslow ratios (Hounslow 1995): when the value of Cl/∑anion = 0.2, less than 0.8 indicates mainly weathering of rock, especially, carbonate dissolution (Rahman et al. 2017).
The Chadha (1999) plots showing the groundwater types
3.2 Ionic relationship in groundwater quality
Pearson’s Correlation matrix (CM) study was carried out using a bivariate statistics to determine the relationships between two sets of parameters through the linear correlation study. The results obtained from the correlation analyses are presented in Table 2. The pairs of Na+ and SO4 2− (r = 0.57), I− and HCO3 − (r = 0.59) showed a significant positive correlations, whereas the pair of Mg2+ and HCO3 − (r = −0.41) depicted negative correlation. The values of Ca2+ and K+ (r = 0.67) showed significant positive correlation, while the paired value of Mg2+ and Ca2+ (r = −0.03) exhibited a very insignificant negative correlation. The escape of Mg2+ and Ca2+ ions from parent materials of dolomite and calcite was the major sources of alkaline earth enrichment in the groundwater samples. The values of F− and HCO3 − (r = 0.50) indicated a strong moderate positive correlation. A moderate positive correlation was also observed between the paired of I− and pH (r = 0.47), I− and K+ (r = 0.48). A moderate negative correlation was also revealed in the values of Ca2+ and pH (r = −0.37), Mg2+ and HCO3 − (r = −0.41). The moderate negative correlation between Mg2+ and HCO3 − confirmed the alkaline earth and weak acidic anion exceeded than other materials in the groundwater.
The Na+/Cl− ratio was used to show the mechanism of water salinity, rock–groundwater interaction, and anthropogenic activities. Most of the datasets are plotted to the line of 1:1 (Fig. 5a) explained by Na+ and Cl− indicating a continuous line from rainfall and unpolluted groundwater (Panno et al. 1999). The Na+ and HCO3 − show an increasing concentration of groundwater indicating the weathering of carbonate rock occurs in the aquifer system (Fig. 5b). The Ca2+ and Na+ show that the ratio is equal to 0.5 or 1.1 line, suggesting that carbonate dissolution (Fig. 5c). The concentration of Ca2+ + Mg2+ and HCO3 − indicates the possibility of dolomite dissolution with calcite precipitation in groundwater (Fig. 5d). This finding is in good agreement with the earlier studies of Afroza et al. (2009) who reveals that dolomite dissolution occurs in the northwestern region of Bangladesh. The gentle slope line suggests a few changes occurred in the ionic chemistry and also indicating dissolution of carbonate minerals and some nearby (Zhou et al. 2012). The greater concentration of HCO3 − might be occurred in the groundwater because of the greater evaporation. The Ca2+ + Mg2+ and TDS gentle slopes follow the 1:1 line showing Na+ and K+ ions contribute to the increase in TDS (Sivasubramanian et al. 2013) (Fig. 5e). The plot of I− and F− concentrations that equal amount of sample above and below the 1:1 line reveals shorter residence time in aquifers system with lack of fluoride rich rocks such as biotite, apatite and amphibole which possess lower fluoride in the groundwater samples (Fig. 5f). This phenomenon also suggests the possibility of the presence of carbonate rock in the study areas which is confirmed by low positive correlation between them. Hence enrichment of Ca2+, Mg2+ and HCO3 − concentrations in carbonate rock tends to have the lowest concentration of I− and F− in the groundwater. The high Ca2 + and Mg2 + ratios and HCO3 − of the studied groundwater also confirm the calcite and dolomite dissolution exist in the aquifer, according to Eqs. 10 and 11, respectively.
Gibbs (1970) plot showing the mechanism governing groundwater chemistry
The samples of a ratio equal to 1 show that dolomite dissolution happened, while the remaining samples have a ratio >1 indicating calcite dissolution (Bhuiyan et al. 2015; Hassen et al. 2016). Hence, weathering of carbonate rock may be possibly influenced to the groundwater quality in the designated area.
3.3 Source of ions and factors controlling in groundwater quality
In this study, principal component analysis (PCA) was attained on groundwater samples of 20 sites and 14 physico-chemical parameters to explore the relations among the ions and trace metals. The PCA of analyzed samples was applied to differentiate the major contribution of sources either geogenic and anthropogenic which governed the water quality. This analysis also helps to find out information on datasets about sources of ion and factor controlling in groundwater quality. Factors with eigenvalues exceeding one were only considered for the study. Seven PCA were taken out from groundwater quality parameters, which represented 90.69% of total variance in the study area. A scree plot was used to demonstrate a major slope change after the fourth eigenvalues (Fig. 6a). The PCA loadings obtained after varimax rotation from groundwater quality variables are shown in Table 3. The first 3 PCs contribute 46.74% of the total variance in the present study (Fig. 6b).
In this study, PC1 represented 16.57% of total variance in groundwater geochemistry. The PC1 was observed by strong position loading on EC in studied S-2, S6–8, S-10, S-13 samples. The highest value of EC confirms geogenic process, which is an important factor controlling in overall geochemistry. This leads to an accumulation of salts in soils (Drever 1997). These salts reach the groundwater through the infiltrated recharge water. Thus, these are the genetic source of salts in the groundwater. So, EC indicates water salinity index; PC1 is revealed as the salinity controlled process. The PC2 exhibited 16.39% of total variance in groundwater quality. The high loading of Ca2+ and K+ in S1, S15, S17 sample sites, showing geogenic process in groundwater aquifer. This affects to the water quality and might be influenced by ionic or reverse ionic exchange in the study area (Loni et al. 2014).
The PC3 exhibited a high positively loaded of Mg2+, F− and HCO3 − and were widely distributed in S1–3, S-7, S-9, S15, S-19 sample locations, indicating the contribution of rock–water interaction in shallow unconfined aquifer. The high positive score of F− might be originated from dissolution of fluro-pyrites, fluorite, various silicate bearing minerals. However, the agricultural fertilizer is also the source of F− in the groundwater (Hem 1991). Furthermore, F− indicated a moderate positive correlation with HCO3 −. This correlation demonstrated that both F− and HCO3 − are believed to be geogenic source rather than anthropogenic activities. The PC4 denoted 13.57% of total variance with strong positively loaded on pH, I− and HCO3 −, showing the alkalinity controlled process in groundwater. The high value of pH may be contributed to be dissolution of carbonate rock in groundwater. The high HCO3 − value may be caused by long-term irrigation practices that circulate the water within the soil/weathered zone. The PC5 accounted for 12.16% of variance in groundwater quality. A high positive loading was depicted on Na+ and SO4 2− in S-14, S-16 and S19–20 sample locations. The high values of Na+, SO4 2− ions are mainly anthropogenic sources like as the wastes, and agro-chemicals (Todd 1980; Hem 1991) or originate from animal and domestic wastes in the study areas (Panno et al. 1999). However, the base rock and groundwater interaction within the aquifer (Hackley 2002) contribute Na+, SO4 2− ions to the groundwater (Subba et al. 2014). These are the additional sources to elevate the concentrations of Na+, SO4 2− ions in the groundwater. The PC6 demonstrated the high positive values of Mg2+ and CO3 − in the S-5, S-8–9, S12–13, S-18 sampling site, indicating ionic exchange geochemistry. The high Mg2+ value found in this PC6, which came from associated limestone rock, led to increase Mg2+ value in the area. Another possible source of Mg2+ could be dolomite dissolution (Lasaga 1984). The PC7 represented 8.44% of total variance in the studied groundwater parameters. The very high positive loading value of Cl− in S-2, S-5 sites, indicating the effect of anthropogenic factors such as agronomic practices and fertilizer uses and also, the dissolution of fluid inclusions and evaporates (Jiang et al. 2009).
R-mode CA was applied to predict physico-chemical parameters grouping in the groundwater samples, and the findings are exhibited in Fig. 7. Ions/parameters belong to the same cluster, indicating the same source. Seven clusters are found in the study area. Cluster 1 included EC, which might be explained by natural sources. Cluster 2 consists of F− showed the weathering of rock into the aquifer. Cluster 3 contains I−, HCO3 −, pH reflected by the dissolution of carbonate minerals. Cluster 4 donates by the K+, Ca2+, and Fe indicating rock–water interaction. Cluster 5 explains by the effect of domestic sewage and agrochemical pollution (Omo-Irabor et al. 2008). Cluster 6 included Mg2+ and CO3 − elucidated by also rock–water interaction in groundwater. Cluster 7 consists of Cl− indicating anthropogenic activities. Despite, some variation in cluster analysis, the CA results mostly good agreed with that of PCA findings. These results agree with the previous findings of Bhuiyan et al. (2016) who have shown that clustering analyses are influenced by both point and nonpoint sources in the Lakshmipur district, Bangladesh.
Q-mode cluster analysis (CA) was used to recognize the spatial resemblances and location grouping among the sample sites. Similar characteristics and interactions of groundwater parameters were grouped together a low linkage distances, whereas different parameters were connected at higher linkage distances. Cluster I has six samples (S-3, S-5, S-9, S-14, S-15, S-19) at low linkage distance which is linked to cluster II 14 samples (S1–2, S-4, S6–8, S10–13, S1–18, S-20) at the higher linkage distances (Fig. 8). It is not surprising that most of the samples follow the high linkage distance rather than low linkage distance.
3.4 Status of drinking water quality
The standard values set by WHO (1993), USEPA (1975) and Bangladesh’s standard (DoE 1997) with comparison of studying groundwater samples are displayed in Table 4. From Table 4, it inferred that all the values of studied groundwater samples indicate suitability for drinking purposes and groundwater does not pose any threat to human health according to WHO and Bangladesh standard except the Fe2+, I− and F− values. The lowest concentration of I− may lead to iodine deficiency disorder in human beings living in the study area (BGS 2000). This finding is quite different to those of the previous study (Hossain et al. 2010), in which they found good suitability for all samples for drinking water uses in the northwestern part of Bangladesh.
Groundwater quality index (GWQI) was applied to assess the status of water quality for drinking water purpose in the study area. The TDS, Na+, Fe Cl−, F− and SO4 2− ions/parameters are assigned to the maximum weight of 4, while HCO3 − ion is designated to the minimum weight of 1 for assessing groundwater quality (Srinivasamoorthy et al. 2008). Total 14 parameters/ions of determination were employed to evaluate drinking water quality (Table 5). The calculated GWQI values of the studied samples were ranged from 37.22 to 78.03. The value less than 50 means excellent water quality type and 50–100 is a good quality water type, 100–200 poorer quality, and more than 200 indicates very poor quality of water type for drinking purpose. All the groundwater sample belongs to excellent to good water quality type (Table 6).
3.5 Status of irrigation water quality
The EC and TDS values were ranged from 163 to 865 us/cm and 104.32 to 553.6 mg/l with the mean and standard deviation of 422.72 ± 163.72 and 270.48 ± 104.78, respectively (Table 1). The high TDS values may be due to the ionic exchange and the long residence time of water. Similar conditions were reported on the Bogra city in the vicinity of the study area (Zakir et al. 2011).The water containing residual sodium bicarbonate (RSBC) <5, 5–10 and <10 meq/L are regarded as safe, marginal and unsatisfactory categories(Gupta and Gupta 1987). The residual sodium bicarbonate (RSBC) values varied from −1.38 to 0.21 with an average of −0.49, indicating the water is fit for agricultural uses. The Na+ percentage values plotted on Wilcox’s diagram (Wilcox 1955), and all the sample values varied from 11 to 22 with an average of 14, suggesting good to excellent quality (Fig. 9). Total hardness (TH) values range from 131 to 178 ppm with a mean value of 153 ppm of CaCO3 (Table 7). Kelley’s ratio (Kelley 1963) (KR) showed an equal balance between Na+, Ca2+ and Mg2+ ions in the samples. When KR ratio >1, it indicates additional Na+ presence in the samples. KR values <1 in all the samples, suggesting excellent irrigation water quality. In the present study, MAR values were varied from 6.23 to 15.76 with a mean value of 10.92, depicting no harmful effect to soil for irrigation in the area (Table 7). The results showed that Mg/Ca ratios were varied from 0.07 to 0.19 with an average of 0.12. Similarly, Na/Ca ratio was varied from 0.15 to 0.31 with a mean value of 0.20. Both results suggest the groundwater free from threat of soil infiltration problem. The Na/Ca and Mg/Ca ratios <3 indicate no threat of infiltration problem for groundwater (Table 7). These irrigation quality indices results are consistent with the findings of Sarkar and Hassan 2006; Islam and Shamsad 2009; Hakim et al. 2009, where they found in northwestern, western part and northern part of the Bangladesh. But in these studies EC, TH, RSBC values are only observed to be fit for agricultural purposes, and this study meets suitability for all irrigation water indices in the Joypurhat district.
The sodium adsorption ratio (SAR) is usually considered as a robust index for irrigated agricultural practices (Ayers and Westcot 1985). The SAR values were ranged from 0.34 and 0.65 with an average of 0.43, showing excellent quality water for irrigation uses (Table 7). A similar condition was reported by Rahman et al. (2017) in the western part of Rajshahi district. In contrast, Mridha et al. (1996) and Khan et al. (1989) stated that slightly higher SAR values found in the Barind area, especially in Natore district and the northwestern region of Bangladesh compared to the present study. The obtained water data were plotted on the salinity diagram by Richards (1954) demonstrated that more than 90% of the studied samples fall in the categories of C2 S1, suggesting intermediate salinity and small sodium hazard for irrigation purposes (Fig. 10). WHO (1998) reported that the suitability of water for irrigation was based on the PI (permeability index). Three water classes are used on the Doneen chart (1964): class I is good quality of irrigation water and is regarded low PI; class II is intermediate quality which is suitable for irrigation, while class III is absolutely unfit for irrigation. The PI values were varied from 45 to 68% with a mean value of 56% (Table 7). According to PI values, the groundwater falls into class II (more than 90%) that indicates the best quality of the water for irrigation uses (Fig. 11).
Wilcox (1955) diagrams showing the rating of groundwater samples for irrigation purposes
US salinity laboratory’s (1954) plots for groundwater of the study area
3.6 Spatial analysis
The IDW interpolation technique was used to generate the spatial distribution maps of each parameter of groundwater dataset for this study. Many researchers, including Hossain et al. (2007); Adhikary et al. (2010); Bhattacharya et al. (2011); Gorai and Kumar (2013); Bodrud-Doza et al. (2016) and Islam et al. (2017), have discussed the spatial variability of groundwater quality in different regions of the world. The spatial distribution maps of GWQI showed the excellent water quality values in the central and southern part, and poor quality values exhibited in the southeastern and southern region of the study area (Fig. 12). Poor quality water may be due to leaching of ions, rapid infiltration of rainwater into the groundwater system, direct discharge of effluents in the aquifer. Such finding is echoed by the similar work of Islam et al. (2015), where the poor water quality observes in several locations of the northern Bogra district, Bangladesh, indicating detrital health effected by using drinking water. The spatial map of EC revealed that the northern part was observed much higher groundwater salinity than the southern one. Moreover, the high EC values were found in the northern region of the basin (Fig. 13a). This means that the shallow groundwater table condition with upstream drainage basin may be working as a contributing factor of the higher EC values. Further, the central part was influenced by the high salinity level due to leaching of Chiri river flow into the groundwater aquifer. The high Fe values were detected in the western region, while low Fe values were found in central and eastern part (Fig. 13b). The dissolution of lithogenic materials by penetrating water, carbonate rock and water interaction is the main reason for high Fe values (Singh et al. 2011). It was noticed that the highest SAR values were evident in the western and southern part, whereas the low SAR values were found in the western part (Fig. 13c). The spatial map of TH is alike to that of SAR distribution map. The higher TH values found in the southern part, and lower TH values in the western and eastern part (Fig. 13d). High TH values are possibly due to complex ionic exchange development within an aquifer system. The finding is in good agreement with the result of Bahar and Reza (2010), who conducted the groundwater quality study in the southeastern Bangladesh. However, the source of these elevated TH values deserves further investigated (Fig. 13).
Permeability Index (Doneen 1964) plots for groundwater of the study area
4 Conclusion
In the present study, descriptive statistics, multivariate statistical technique and geochemical technique were applied to assess the major factors controlling in groundwater quality and its sustainability at the Joypurhat district of Bangladesh. The statistical results demonstrated that the abundance of major cations was in the order of Ca2+> Na+> Mg2+>K+, while the dominant major anions trend in the study area was in the following order: HCO3 − > Cl− > CO3 − > SO4 − > F− > I−. The major water type of the groundwater is Ca–Mg–HCO3 hydro-chemical facies. The Gibbs diagrams showed that groundwater chemistry is mostly rock-dominance zone in the study area. Seven PCA were extracted from groundwater quality parameters that represented 90.69% of total variance. The geogenic processes (rock weathering and ionic exchange) followed by anthropogenic factors (domestic waste, agricultural fertilizers and agrochemical) were responsible for governing the groundwater chemistry. The outcomes of these processes are validated by using the cluster analysis (CA) and correlation matrix (CM) analysis. An assessment of groundwater quality for using GWQI revealed that all the sampling locations are excellent to good quality water for drinking uses in the study area. Although groundwater is mostly suited for human consumption, the excessive iron and insignificant amount of fluoride and iodide concentrations are the main contaminants which could create a health problem. Thus, the proper remedial measures should be required to these parameters to prevent the more contamination of groundwater. On the other hand, the results of irrigation water quality index such SAR, Na %, and PI indicated that the sampling groundwater fits for agriculture uses.
However, the jeopardizing impact on groundwater quality of limited data is one of the main hindrances for sustainable resource allocation. The spatial distribution maps of the studied samples can provide a reliable information for local decision makers in a more sustainable way. Sustainability of groundwater quality can be achieved by incorporating the management of groundwater and surface water, including effects on groundwater-driven aquifer system; integrating a long-term strategy; harmonizing relation between the environment, society and economy. It is anticipated that this study provides adequate primary information on physico-chemical parameters, water quality indices, probable source of ions, factors contributing the groundwater quality and spatial variability of Joypurhat district in Bangladesh. This study will be a useful guide for policy makers to take appropriate initiatives for sustainable groundwater quality management.
References
Adhikary, P. P., Chandrasekharan, H., Chakraborty, D., & Kamble, K. (2010). Assessment of groundwater pollution in West Delhi, India using geostatistical approach. Environmental Monitoring and Assessment, 167(1–4), 599–615.
Afroza, R., Mazumder, Q. H., Jahan, C. S., Kazi, M. A. I., Ahsan, M. A., & Al-Mansur, M. A. (2009). Hydrochemistry and origin of salinity in groundwater in parts of Lower Tista Floodplain, Northwest Bangladesh. Journal of the Geological Society of India, 74, 223–232.
Aksever, F., Davraz, A., & Bal, Y. (2016). Assessment of water quality for drinking and irrigation purposes: A case study of Başköy springs (Ağlasun/Burdur/Turkey). Arabian Journal of Geosciences, 9, 748. doi:10.1007/s12517-016-2778-y.
Alharbi, O. A., Loni, O. A., & Zaidi, F. K. (2017). Hydrochemical assessment of groundwater from shallow aquifers in parts of Wadi Al Hamad, Madinah, Saudi Arabia. Arabian Journal of Geosciences, 10, 35. doi:10.1007/s12517-016-2805-z.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington: American Public Health Association.
Ayers, R. S., & Westcot, D. W. (1985). Water quality for agriculture, irrigation and drainage (Paper No. 29). Rome: FAO.
Bahar, M. M., & Reza, M. S. (2010). Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environmental Earth Sciences, 61, 1065–1073.
BGS. (2000) British Geological survey. Water Quality Fact Sheet: Iodine (p. 4).
Bhattacharya, P., Mukherjee, A., & Mukherjee, A. B. (2011). Arsenic contaminated groundwater of India. In J. Nriagu (Ed.), Encyclopedia of environmental health (pp. 150–164). Amsterdam: Elsevier. doi:10.1016/B978-0-444-52272-6.00345-7.
Bhuiyan, M. A. H., Bodrud-Doza, M., Islam, A. R. M. T., Rakib, M. A., Rahman, M. S., & Ramanathan, A. L. (2016). Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environmental Earth Sciences, 75(12), 1020. doi:10.1007/s12665-016-5823-y.
Bhuiyan, M. A. H., Ganyaglo, S., & Suzuki, S. (2015). Reconnaissance on the suitability of the available water resources for irrigation in Thakurgaon District of northwestern Bangladesh. Applied Water Science, 5(3), 229–239. doi:10.1007/s13201-014-0184-8.
Biswas, R. K., Roy, D. K., Islam, A. R. M. T., Rahman, M. M., & Ali, M. M. (2014). Assessment of drinking water related to arsenic and salinity hazard in Patuakhali district, Bangladesh. International Journal of Advanced Geosciences, 2(2), 82–85. doi:10.14419/ijag.v2i2.3011.
Bodrud-Doza, M., Islam, A. R. M. T., Ahmed, F., Das, S., Saha, N., & Rahman, M. S. (2016). Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Science, 30(1), 19–40. doi:10.1016/j.wsj.
Chadha, D. K. (1999). A proposed new diagram for geochemical classification of natural water and interpretation of chemical data. Hydrogeology Journal, 7, 431–439.
Chopra, S. L., & Kanwar, J. S. (1980). Analytical agricultural chemistry. New Delhi: Kalyan Publishers.
DoE. (1997). The environment conservation rules 1997. Dhaka: Government of the People’s Republic of Bangladesh.
Doneen, L. D. (1964). Notes on water quality in agriculture, water science and engineering. Davis: University of California.
Drever, J. I. (1997). The geochemistry of natural waters (3rd ed.). Englewood: Prentice Hall.
Faye, S., Maloszewski, P., Stichler, W., Trimborn, P., Cissé Faye, S., & Cissé Faye, S. (2005). Groundwater salinization in the Saloum (Senegal) delta aquifer: minor elements and isotopic indicators. Science of the Total Environment, 343, 243–259.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 795–840.
Gorai, A. K., & Kumar, S. (2013). Spatial distribution analysis of groundwater quality index using GIS: A case study of Ranchi Municipal Corporation (RMC) area. Geoinformatics & Geostatistics: An Overview, 1, 2. doi:10.4172/2327-4581.1000105.
Gupta, S. K., & Gupta, I. C. (1987). Management of saline soils and waters. New Delhi: Oxford and IBH Publishing Company.
Hackley, K. C. (2002). A chemical and isotopic investigation of the groundwater in the mahomet bedrock valley aquifer: age, recharge and geochemical evolution of the groundwater. Ph.D. Thesis, University of Illinois, Urbana-Champaign (p. 152).
Hakim, M. A., Juraimi, A. S., Begum, M., Hasanuzzaman, M., Uddin, M. K., & Islam, M. M. (2009). Suitability evaluation of groundwater for irrigation, drinking and industrial purposes. American Journal of Environmental Science, 5, 413–419. doi:10.3844/ajessp.2009.413.419.
Halim, M. A., Majumder, R. K., Nessa, S. A., Oda, K., Hiroshiro, Y., & Jinno, K. (2010). Arsenic in shallow aquifer in the eastern region of Bangladesh: insights from principal component analysis of groundwater compositions. Environmental Monitoring and Assessment, 61, 453–472. doi:10.1007/s10661-009-0760-9.
Hassen, I., Hamzaoui-Azaza, F., & Bouhlila, R. (2016). Application of multivariate statistical analysis and hydrochemical and isotopic investigations for evaluation of groundwater quality and its suitability for drinking and agriculture purposes: case of Oum Ali-Thelepte aquifer, central Tunisia. Environmental Monitoring and Assessment, 188, 135. doi:10.1007/s10661-016-5124-7.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural waters, Book 2254 (3rd ed.). Jodhpur: Scientific Publishers.
Hossain, F., Hill, J., & Bagtzoglou, A. C. (2007). Geostatistically based management of arsenic contaminated ground water in shallow wells of Bangladesh. Water Resource Management, 21, 1245–1261. doi:10.1007/s11269-006-9079-2.
Hossain, G., Howladar, M. F., Nessa, L., Ahmed, S. S., & Quamruzzaman, C. (2010). Hydrochemistry and classification of groundwater resources of Ishwardi Municipal Area, Pabna District, Bangladesh. Geotechnical Geological Engineering, 28, 671–679. doi:10.1007/s10706-010-9326-4.
Hounslow, A. W. (1995). Water quality data. Analysis and interpretation. New York: Lewis.
Isaaks, E. H., & Srivastava, R. M. (1989). An introduction to applied geostatistics. New York: Oxford University Press.
Islam, A. R. M. T., Rakib, M. A., Islam, M. S., Jahan, K., & Patwary, M. A. (2015). Assessment of health hazard of metal concentration in groundwater of Bangladesh. American Chemical Science Journal, 5(1), 41–49. doi:10.9734/ACSj/2015/13175.
Islam, M. S., & Shamsad, S. Z. K. M. (2009). Assessment of irrigation water quality of Bogra district in Bangladesh. Bangladesh Journal of Agriculture Research, 34, 597–608.
Islam, A. R. M. T., Shen, S., Bodrud-Doza, M., Rahman, M. A., & Das, S. (2017). Assessment of trace elements of groundwater and their spatial distribution in Rangpur district, Bangladesh. Arabian Journal of Geosciences, 10, 95. doi:10.1007/s12517-017-2886-3.
Jiang, Y., Wu, Y., Groves, C., Yuan, D., & Kambeis, P. (2009). Natural and anthropogenic factors affecting the groundwater quality in the Nandongkarst underground river system in Yunan, China. Journal of Contamination Hydrology, 109, 49–61.
Kelley, W. P. (1963). Use of saline irrigation water. Soil Science, 95, 355–391.
Khan, L. R., Dutta, S. C., & Biswas, M. R. (1989). Quality of groundwater for irrigation in the northwestern region of Bangladesh. Bangladesh Journal of Agriculture Research, 14, 45–54.
Kumar, S. K., Rammohan, V., Sahayam, J. D., & Jeevanandam, M. (2009). Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 159(1–4), 341–351.
Lasaga, A. C. (1984). Chemical kinetics of water–rock interactions. Journal Geophysical Research, 89(B6), 4009–4025.
Liu, C. W., Lin, K. H., & Kuo, Y. M. (2003). Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Science of the Total Environment, 313(1), 77–89.
Loni, O. A., Zaidi, F. K., Alhumimidi, M. S., Alharbi, O. A., Hussein, M. T., Dafalla, M., et al. (2014). Evaluation of groundwater quality in an evaporation dominant arid environment; a case study from Al Asyah area in Saudi Arabia. Arabian Journal of Geosciences . doi:10.1007/s12517-014-1623-4.
Macdonald, A. M., Bonsor, H. C., Ahmed, K. M., et al. (2016). Groundwater quality and depletion in the Indo-Gangetic basin mapped from in situ observation. Nature Geoscience. doi:10.1038/NGEO2791.
Mertler, C. A., & Vannatta, R. A. (2005). Advanced and Multivariate statistical methods: Practical application and interpretation (3rd ed., p. 120). CA.: Glendale.
Molla, M. A., Saha, N., Salam, S. A., & Rakib-uz-Zaman, M. (2015). Surface and groundwater quality assessment based on multivariate statistical techniques in the vicinity of Mohanpur, Bangladesh. International Journal of Environmental Health Engineering, 4, 18. doi:10.4103/2277-9183.157717.
Mridha, M. A. K., Rashid, M. H., & Talukder, K. H. (1996). Quality of groundwater for irrigation in Natore district. Bangladesh Journal of Agriculture Research, 21, 15–30.
Omo-Irabor, O. O., Olobaniyi, S. B., Oduyemi, K., & Akunna, J. (2008). Surface and groundwater water quality assessment using multivariate analytical methods: a case study of the Western Niger Delta, Nigeria. Physics and Chemistry of the Earth, 33, 666–673.
Panno, S. V., Hackley, K. C., & Greenberg, S. E. (1999). A possible technique for determining the origin of sodium and chloride in natural waters: preliminary results from a site in northeastern Illinois. In (Abstract) in Environmental horizons 2000, conference proceedings (p. 57).
Piper, A. M. (1944). A graphic procedure in geochemical interpretation of water analysis. American Geophysical Union Transom, 25, 914–923.
Rahman, M. A. T. M. T., Saadat, A. H. M., Islam, M. S., Al-Mansur, M. A., & Ahmed, S. (2017). Groundwater characterization and selection of suitable water type for irrigation in the western region of Bangladesh. Applied Water Science, 7(1), 233–243. doi:10.1007/s13201-014-0239-x.
Richards, L. A. (US Salinity Laboratory). (1954). Diagnosis and improvement of saline and alkaline soils. US Department of Agriculture hand book (p. 60).
Saha, S. K., Kumar, U., & Rahman, M. (2009). An assessment of groundwater chemistry of Barind Tract in Bangladesh with special reference to carbonate weathering. Asian Journal of Water Environment Pollution, 6, 51–58.
Sarkar, A. A., & Hassan, A. A. (2006). Water quality assessment of a groundwater basin in Bangladesh for irrigation use. Pakistan Journal of Biological Science, 9(9), 1677–1684.
Shahid, S., Wang, X. J., Rahman, M. M., Hasan, R., Harun, S. B., & Shamsuddin, S. (2015). Spatial assessment of groundwater over-exploitation in Northwestern Districts of Bangladesh. Journal Geological Society of India, 85, 463–470.
Singh, K. P., Malik, A., & Sinha, S. (2005). Water quality assessment and apportionment of pollution sources of Gomti River (India) using multivariate statistical techniques: a case study. Anal Chemical Acta, 538, 355–374.
Singh, C. K., Shashtri, S., & Mukherjee, S. (2011). Integrating multivariate statistical analysis with GIS for geochemical assessment of groundwater quality in Shiwaliks of Punjab, India. Environmental Earth Science, 62, 1387–1405. doi:10.1007/s12665-010-0625-0.
Sivasubramanian, P., Balasubramanian, N., Soundranayagam, J. P., & Chandrasekar, N. (2013). Hydrochemical characteristics of coastal aquifers of Kadaladi, Ramanathapuram District, Tamilnadu, India. Applied Water Science, 3, 603–612.
Srinivasamoorthy, K., Chidambaram, S., Prasanna, M. V., Vasanthavihar, M., Peter, J., & Anandhan, P. (2008). Identification of major sources controlling groundwater chemistry from a hard rock terrain—A case study from Mettur taluk, Salem district, Tamil Nadu, India. Journal Earth System Science, 117(1), 49–58.
Subba, R. N., Vidyasagar, G., Surya, R. P., & Bhanumurthy, P. (2014). Assessment of Hydrogeochemical processes in a coastal region: Application of multivariate statistical model. Journal Geological Society of India, 84, 494–500.
Todd, D. K. (1980). Groundwater hydrology. New York: Wiley.
USEPA. (1975). Water programs, national interim primary drinking water regulations. Federal Register, 40(248), 59566–59587.
USEPA. (2012). Integrated risk information system. United States Environmental Protection Agency. http://cfpub.epa.gov/ncea/iris/index.cfm?fuseactioniris.showSubstanceList. Accessed December 2016.
Vasanthavigar, M., Srinivasamoorthy, K., Vijayaragavan, K., et al. (2010). Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environmental Monitoring Assessment. doi:10.1007/s10661-009-1302-1.
Webster, R., & Oliver, M. A. (2001). Geostatistics for environmental scientists. New York: Wiley.
WHO. (1993). Guidelines for drinking water quality (2nd ed., Vol. 1, p. 188). Geneva: WHO.
WHO. (1998). Guidelines for drinking-water quality, 2nd edn. Addendum to v.1–2.
WHO. (2011). Guidelines for drinking water quality (4th ed.). Geneva Switzerland: World Health Organization.
Wilcox, L. V. (1955). Classification and use of irrigation water. Washington, DC: US Department of Agriculture.
Yidana, S. M. (2010). Groundwater classification using multivariate statistical methods: Birimian Basin, Ghana. Journal Environmental Engineering, 136, 1379–1388.
Zakir, H. M., Begum, K., Mohiuddin, K. M., & Arafat, M. Y. (2011). Quality assessment of waters of Bogra city area, Bangladesh. Journal of Agroforestry Environment, 5(2), 21–26.
Zhou, T., Wu, J., & Peng, S. (2012). Assessing the effects of landscape pattern on river water quality at multiple scales: a case study of the Dongjiang River watershed, China. Ecological Indicator, 23, 166–175.
Acknowledgements
The authors would like to acknowledge the authority of the Department of Disaster Management, Begum Rokeya University, Rangpur, Bangladesh and the Nanjing University of Information Science and Technology, Nanjing, China, for all other forms of support for this study. The authors are also thankful to the anonymous reviewers for improving the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interests about the publication of this manuscript.
Rights and permissions
About this article
Cite this article
Islam, A.R.M.T., Shen, S., Haque, M.A. et al. Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environ Dev Sustain 20, 1935–1959 (2018). https://doi.org/10.1007/s10668-017-9971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-017-9971-3