Abstract
Germinal matrix hemorrhage (GMH) refers to bleeding that derives from the subependymal (or periventricular) germinal region of the premature brain. GMH can induce severe and irreversible damage attributing to the vulnerable structure of germinal matrix and deleterious circumstances. Molecular mechanisms remain obscure so far. In this review, we summarized the newest preclinical discoveries recent years about GMH to distill a deeper understanding of the neuropathology, and then discuss the potential diagnostic or therapeutic targets among these pathways. GMH studies mostly in recent 5 years were sorted out and the authors generalized the newest discoveries and ideas into four parts of this essay. Intrinsic fragile structure of preterm germinal matrix is the fundamental cause leading to GMH. Many molecules have been found effective in the pathophysiological courses. Some of these molecules like minocycline are suggested active to reduce the damage in animal GMH model. However, researchers are still trying to find efficient diagnostic methods and remedies that are available in preterm infants to rehabilitate or cure the sequent injury. Merits have been obtained in the last several years on molecular pathways of GMH, but more work is required to further unravel the whole pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germinal matrix hemorrhage (GMH) occurs in the subependymal (or periventricular) germinal region of the premature brain (Fig. 1), and sometimes develops into intraventricular hemorrhage (IVH). It is very common in preterm infants, the incidence of which is usually disproportionate to the gestational age of premature (Hefti et al. 2016; Supramaniam et al. 2013). About 20–30% infants born with very low weight (birth weight < 1500 g) or gestational age < 28 weeks suffer GM-IVH (Coen 2013; Haines et al. 2013; de Bijl-Marcus et al. 2017). Occurrence of GM-IVH is highly related to the gestational age and birth weight, and it is in the first 4 days that GM-IVH typically occurs in immature infants (Itsiakos et al. 2016; Okazaki et al. 2013). Intrauterine demise is frequent in fetus with GMH (Sanapo et al. 2017). The mortality of infants with GM-IVH has dropped thanks to the development of diagnostic techniques and intensive care in recent decade, but it still induces severe and permanent damage on premature brain, leading to hydrocephalus, cerebral palsy, seizures, hemiplegia, learning disabilities, and so on (Haines et al. 2013; Vesoulis and Mathur 2017; Sheehan et al. 2017; Movsas et al. 2013; Hefti et al. 2016; Payne et al. 2013). Recent discoveries even indicate the decreased development of cerebellum, and the developmental retardation of preterm neonates is proportional to the grade of GM-IVH (Lee et al. 2016).
Researchers notice the tendency of getting injured during 22–36 weeks of gestation when the developing course start maximizing, accounting for a lot of neonatal neural disorders that develop in this specific gestation stage (Huang and Vasung 2014; Zhan et al. 2013). Attributing to the vulnerable structure, germinal matrix is highly exposed to insults such as hypoxia, hypocarbia, systemic or partial circulatory dysfunction, and electrolyte disturbances (Baburamani et al. 2012; Waitz et al. 2016). Moreover, maternal disorders, fetal disorders, and poisons during perinatology, such as maternal infection, drugs abuse, smoking, inherent diseases, lead to a higher risk that premature fetal suffer GM-IVH (Xiaoyu 2015).
Even if rescued from fatality, premature infants who suffered GM-IVH could inevitably have neural disabilities because these early injuries damage the functional area as well as interrupt normal maturation of nervous system (Panigrahy et al. 2012; Hinojosa-Rodriguez et al. 2017). Given the special condition of neonates, it is much of difficulty to precisely identify GM-IVH from limited clinical features, and thus paramount attention is laid on diagnostic technics, especially radiology and ultrasonography. Based on various clinical backgrounds and conditions of individual infants born immature, pediatrists grade these infants into 4 levels according to the extent of periventricular hemorrhage (Fig. 2). CT provides us a rapid acknowledgement of severe lesions. MRI provides much more details to quantify and grade the hemorrhage as well as confirm the lesion of ventricles and brain parenchyma, such as hydrocephalus and leukomalacia. Even transcranial ultrasonography (TUS) shows unsatisfactory sensitivity of diagnosing low-grade GMH in a few cases, it is increasingly efficient and convenient to detect intracranial lesions (Parodi et al. 2015) (Fig. 3). Color Doppler ultrasonography enjoys the advantages of sensitivity when it comes to congenital vascular disease (Vesoulis and Mathur 2017). Automated assessment of electroencephalography (EEG) developed by Iyer and the teammates is probable to detect GMH bedsides earlier and more sensible to some extent (Iyer et al. 2015).
Series of GM-IVH of different grades. a Grade I: the hematoma is limited inside the germinal matrix, or sometimes occurs within caudate nuclei. b Grade II: the bleeding bursts into ipsilateral ventricle with smooth CSF flowing course. c Grade III: hemorrhage of Grade II plus hydrocephalus. d Grade IV: hematoma breaks into parenchyma and causes intraparenchymal hemorrhage with or without intraventricular hemorrhage
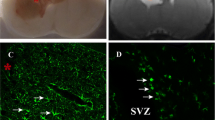
a A delayed ultrasonic image of a GMH case, grade I. A low-density cystic mass can be seen beneath the ependyma of this premature, and the ventricle remains intact. b A transcranial ultrasonography image taken 17 days after birth of a twin infant on gestation age of 29 + 3 weeks, with a concomitant preterm pneumorrhagia. The high-density lesion pointed by arrow shows the hematoma disrupting into lateral ventricle with significant ventriculomegaly
Nowadays survival of premature infant has increased a lot attributing to developing neonatal intensive therapeutic methods, but preterm-associated sequelae persist and become chronic problems to these little patients. Because of the difficulty in identically simulating the course of GM-IVH by proper animal model, what we understand so far is still unsatisfactory despite the long-term hard work of predecessors. More efficient diagnosis and therapies are needed to cure these patients.
Based on cellular experiments, there are several theories and pathways where some confirmed molecules work and will possibly become the therapeutic targets. In this paper, we attempt to review pathological and molecular developments of GM-IVH so far and introduce some newest discoveries about the mechanisms and have a prospect of potential treatments.
Pathological Anatomy: Fragilities of Germinal Matrix
Due to the fragility of brain tissue and immature respiratory function, preterm infants are highly exposed to brain injury caused by premature delivery even if they do not suffer GM-IVH (Gao et al. 2015). What is more, germinal matrix is a highly cellular and highly vascularized structure beneath ependymal (or periventricular) germinal region in the brain where cells migrate out during brain development. The proliferation and differentiation of neurons with specific functional potentials in the fetal rely on the specific parts of germinal vasculature (Ma et al. 2017). The histological structure is much more of vulnerability and complexity, to which researchers have been seeking the keys for decades.
Structural fragility of the germinal matrix is fundamentally what leads it to GM-IVH. Primarily, the parenchyma of basal lamina is relatively soft and fragile because of deficient fibronectin and collagen (Ballabh 2014). Secondly, intracranial vasculature of preterm neonates has the same innate immaturity as the vessels in other organs, which means that the vessels walls with endothelia much weaker than adult are more prone to rupture. Decreased expression of glial fibrillary acidic protein (GFAP) in the germinal matrix is very likely to decrease the strength of the cytoskeletal structure, and expose the delicate vasculature of germinal matrix into higher rupture risk (Lekic et al. 2015b). The structural variants of subependymal veins are also confirmed to bring about the brittleness of germinal matrix, as well as the inclination of thrombosis (Tortora et al. 2017; Raybaud et al. 2013). Besides, the highly vascularization adds to the fragility of germinal matrix as well, especially when the fetal encounters hypoxia (Lekic et al. 2015b). Furthermore, the premature vasculature lacks the auto-adaptability to modulate the lumen under fluctuant hemodynamics (Ma et al. 2017; Andreone et al. 2015; Lekic et al. 2015b). As a result, once encountered either external or internal environmental changes that lead to rapid fluctuation of blood pressure, these immature infants are in great danger of GM vascular rupture (Baburamani et al. 2012; Ballabh 2014).
Recently the disorder of hemodynamics also has been indicated to impede the normal coagulation course when vasculature gets injured, encouraging the occurrence of GM-IVH (Kuperman et al. 2013). Platelets dysfunction probably participates deeply into the pathogenesis (Coen 2013; Mitsiakos et al. 2016). Besides, based on specially immature vasculature of germinal matrix, preterm neonates bear much higher risk of thrombosis, if faced with platelet dysfunction simultaneously (Itsiakos et al. 2016; Brew et al. 2014). In addition, maternal condition affects immature fetal as well. The blood becomes hypercoagulable when the mother gets pregnant. Microthrombus originated from mother or placenta can possibly pass through placental barrier of highly porosity. What is more, a parent giving birth to premature baby is usually suffering some other antenatal disorders that have adverse impacts on the fetal. As a result, neonates may probably have been injured when it was still in womb.
Right after the germinal matrix bleeds, this periventricular lamina basalis gets injured because of structural fracture, mechanical compression and intracranial hypertension. Brain swelling immediately aggravates out of interstitial and cytotoxic edema (Michinaga and Koyama 2015). Secondary compressive ischemia, partially resulted from vasospasm and edema, occurs in peripheral nervous tissue as cerebral blood flow plunges. Some severe germinal hematoma with immense volume may lead to herniation, or break through ependymal layer, namely IVH. During these serial processes, destruction of neurons and axons will inevitably take place due to mechanical mass effect, hypoxia, ischemia and cytotoxicity. Even if the preterm neonates get rid of fatality, they are still subjected to many sequelae caused by GM-IVH.
Even though GM-IVH bears much resemblance to the adult intracranial hemorrhage, it holds some typical characters relevant to neurological growth. There appears to be a considerable proportion of leukomalacia following the survival in GM-IVH, and cortical maturation will inevitably get retarded (Okazaki et al. 2013; O’Dell et al. 2015). Even the infant cerebellar development gets impaired because of GM-IVH (Lee et al. 2016). Nonetheless, since there is still no animal model that is perfectly congruent with the real pathogenesis, our knowledge remains far from the whole mechanism of GM-IVH, even though we have known pretty much of the histopathological defection of neonatal brain.
Neuropathological Mechanism
Researchers have been looking for the mechanisms and molecular pathways that lead to injury of GM-IVH. However, what we achieved so far is not satisfied, which appears to be complicated and interweaving (Fig. 4). Overall, there are some major individual or serial molecules that occupy the core status. The authors try to introduce the prevalent theories of preterm GM-IVH with some new discoveries as follows.
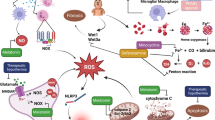
The complicated mechanisms of how GM-IVH injures the premature infant brain. The solid arrows and texts without a box illustrate the mainly extracellular mechanisms and molecules involved in the damaging course of GMH; the hollow arrows and texts inside boxes shows those mainly intracellular mechanisms and molecules involved. HOs: heme-oxygenases; S1PR: sphingosine-1-phosphate receptor; TLR: toll-like receptor; AMPK/Nrf2: adenosine monophosphate-activated protein kinase/nuclear factor erythroid 2-related factor 2.
Blood Components and Metabolites
Researchers have long confirmed the important role of blood components in intracranial hemorrhagic diseases, especially the hemoglobin metabolite, iron compounds, and thrombin (Strahle et al. 2014; Lekic et al. 2015a; Gao et al. 2014; Garton et al. 2016). Heme-oxygenase (HO) expressions are significantly increased in brain parenchyma by exogenous hemoglobin or protoporphyrin injection (Strahle et al. 2014). Iron imposes adverse impact on apoplexy infants mainly through encouraging fibrosis and sequent adhesion of arachnoid potentially aggravates brain edema (Guo et al. 2015; Klebe et al. 2014). Wnt signaling pathway is a well-known active target for cancer therapy (Tai et al. 2015), and researchers have testified that it is activated in fibrosis of many organs varying from skeleton to kidney (Cisternas et al. 2014). Meng’s and Kaur’s findings particularly highlight the relationship between iron and Wnt1/Wnt3a gene expression pathway. Iron suppresses renewal of neurons and enhances fibrotic process and gliocyte’s proliferation by stimulus to Wnt pathway, and further leads to post-hemorrhagic sequelae such as hydrocephalus (Meng et al. 2015; Kaur et al. 2013). Another research also implicates the active role of Wnt pathway in regulating the myelination of neonatal white matter, and the construction of synapses is disrupted through irregulating Wnt signaling (Back 2017). On another aspect, hepcidin, which inhibits the intracellular iron efflux of endotheliocytes, was described in adult animals as the effective molecule on cognitive impairment through Toll-like receptor 4 (TLR4)/MyD88 signaling pathway, which is the potential target of preterm-related injury (Xiong et al. 2016). Discoveries relevant to this process also indicate the effective role of iron on inflammatory factor (like interleukin-6), and oxidative stress after intracranial hemorrhage (Hu et al. 2016; Xiong et al. 2016; Vela 2018). It is intriguing of iron to be multifunctional on various interactive signaling pathways, which needs further study.
Thrombin is a well-proven agent to construct hydrocephalus animal model. The disruptive effect of thrombin to the blood brain barrier (BBB) has been described in GMH by Tao and the teammates (Tao et al. 2015). Apart from that, thrombin induces the phosphorylation of mitogen-activated protein kinase (MAPK) leading to disruption of tight-junction protein which is another key to break down BBB (Li et al. 2015). Lekic also led a research that unveiled the activation of proteinase-activated-receptors (PAR) signaling pathway resulted by thrombin, and cyclooxygenase (COX)-2 is promising to be the effective treatment for GM-IVH neonates (Lekic et al. 2015a; Cheng et al. 2014).
Given the researches based on animals and cells so far, we still need more evidence to explain the definite relationship among blood cell metabolites, molecular mechanisms, and secondary neurologic deficits of preterm neonates with GM-IVH.
Microglia and Inflammation
Inflammation has long been believed as one of the paramount mechanisms of hemorrhagic stroke, and microglia deeply participate in the inflammatory response in the signal transmitting course of GMH (Blaho et al. 2015; Shigemoto-Mogami et al. 2014; Supramaniam et al. 2013). Microglia have two differentiated states. One is a pro-inflammatory classically activated state (M1), and the other is an immune-dampening and tissue-regenerative alternatively activated state (M2)(Klebe et al. 2015). Microglia get activated and infiltrate into the subventricular zone including germinal matrix (Shigemoto-Mogami et al. 2014). This phenomenon becomes evident when hemorrhage happens in germinal matrix (Tang et al. 2015). A variety of inflammatory cytokines were decreased when the activated microglia are suppressed, verifying the inflammatory activity of microglia (Shigemoto-Mogami et al. 2014; Zhang et al. 2018; Wan et al. 2016). Peroxisome proliferator-activated receptor gamma (PPARγ) induces the microglia of GMH brain to transform into M2 state, contributing to attenuation of hemorrhagic brain inflammatory response (Flores et al. 2016). In addition, there exists CD36 expression in microgliocyte activated with PPARγ, which helps improve the long-term neurofunctional development after GMH (Flores et al. 2016).
Over a decade before, researchers have already confirmed the efficiency of celecoxib (an anti-inflammation) on premature animal model, reducing the risk of neonatal brain hemorrhage by proliferation of germinal matrix endotheliocytes. However, after the hemorrhage occurs, endothelial proliferation seems to be beneficial to the neurologic deficits by vascular endothelial growth factor (VEGF) treatment (Dzietko et al. 2013). VEGF and the downstream mediators are believed to be of great participation in the inflammatory courses, and anti-inflammatory drugs such as celecoxib turn out effective in attenuate the severity of GM-IVH (Yang et al. 2013; Phillips et al. 2013). Recently, Zhang et al. (2018) substantiated that GMH-induced inflammatory response by promoting ChemR23/CAMKK2/AMPK/Nrf2 pathway. In addition, aminomethyl phosphonic acid (AMPA) receptor pathway are believed active in preventing injured neurons from restoration, maturation, and regeneration through various inflammatory cytokines, and leading to apoptosis of neurons as well (Dohare et al. 2016). Several animal experiments, respectively, indicated MAPK family pathway is also involved, and cannabinoid receptor 2 (CB2R) is a functional target that ameliorates injury induced by GM-IVH (Tang et al. 2015, 2017; Tao et al. 2015; Li et al. 2015). Besides, they also found traces on oxidation stress during pathogenic progress of GM-IVH just as some other scientists did (Esiaba et al. 2016). According to this hypothesis, some researchers try to treat intracranial hemorrhage in adult by antioxidants like Resveratrol and hydrogen (Duan et al. 2016; Bonsack et al. 2017; Eckermann et al. 2012), which may be helpful in preterm GM-IVH. In conclusion, inflammation response is of great complexity interweaving with other molecular mechanisms, but it is still a promising breakthrough seeking effective therapies for GM-IVH.
Lymphocytes and Immunity
Regardless of the age, lymphocytes infiltrate into the lesion when the brain suffers an attack. There are many researchers devoting to finding out the deeper relationship among immunity, lymphocytes, and apoplectic neuropathology, including hemorrhagic and ischemic stroke in neonates (Doyle et al. 2015; Nazmi et al. 2018). Albertsson and the colleagues noticed a special type of immune response held by CD4+ T-helper (Th) cell in a mice model of hypoxia-ischemic stroke, some of which result in GM-IVH (Albertsson et al. 2014). As the first subset of T lymphocytes to emerge during ontogeny, γδT cells are confirmed to specifically take part in the injury of developing brain other than mature one. In both sepsis and ischemic brain injury, it is indicated that γδT cells induce the long-term neurologic deficit resulted by demyelination of and gliosis (Albertsson et al. 2018; Zhang et al. 2017). Besides, this kind of autoimmune response also found interweaving with broad-spectrum inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17). Not only does the immune system affect the expression of inflammatory cytokines, but the elevated cytokines such as Sphingosine-1-phosphate (S1P) also affect the proliferation and differentiation of lymphocytes (Albertsson et al. 2014). Inflammatory cytokines can also become the potential targets to develop immune modulators (Tsai and Han 2016). Several adult-related studies indicated that S1P and S1P receptor (S1PR) pathway take an active part in this course. Molecules as its antagonists can reduce the neuroinflammation by arresting lymphocyte egress from secondary lymphoid tissues in the central nervous system, and at the same time regulate macrophages dendritic cell functions (Tsai and Han 2016; Blaho et al. 2015). Immune system of fetus and preterm neonate is immature, so the immune response is possibly characteristic in comparison with adults. Altogether, more evidences are needed to define the effects of different types of lymphocytes and cytokines involved in the GM-IVH course.
Treatments and Preventions
The outcome of premature GM-IVH turns out quite pessimistic. In the acute stage of GM-IVH, intracranial hypertension is often what leads the afflicted infants to fatality. To decrease the intracranial pressure with dehydrator and corticosteroid is the essential part of treatment. Nevertheless, because of the fragile vasculature of germinal matrix, dehydration with mannitol is a double-edged sword that decompresses the cranial cavity as well as imperils the infant to higher risk of re-bleeding and renal failure. Surgical operation, mainly inclusive of trephination drainage and external ventricular drainage, is the last choice to save the life yet with awful prognosis. In the chronic stage out of danger, treatments aim at reducing the secondary injury caused by hemorrhage, attenuating the sequelae and facilitating the recovery of neural functions. Infant disabilities of sucking, swallowing, and coughing arise after GM-IVH, so their survival and quality of life can be deeply derogated (Laptook 2013). Consequently, this kind of complications should be carefully controlled by elaborative nursing in case of sudden accidents like choking.
In terms of medical treatments, many drugs seem to be influential to either vivo or vitro animal models. Oestradiol show the effects on ameliorating the neurologic outcomes by increasing the expression of neurotrophic factor (Firozan et al. 2014). Based on the theories introduced in Iron chelators such as minocycline, deferoxamine has a favorable reaction in vitro and animal experiments as well (Guo et al. 2015; Meng et al. 2015). Just in 2018, melatonin was reported protective in secondary brain injury induced by hemorrhage. It is variously effective in impacting apoptosis, oxidative stress, inflammation, DNA damage, brain edema, and BBB damage, and reducing mitochondrial membrane permeability transition pore opening (Wang et al. 2018). Simvastatin was found with potential impacts on upregulating CD36 expression, which probably promotes the absorption of intraventricular hemorrhage (Chen et al. 2017). Researchers also found the positive role of glibenclamide in reducing the expression of MMPs and thereby protect brain from further injury (Jiang et al. 2017). Even though researches showed positive effects in preclinical modes, how they work and whether they are curative in apoplectic patients are still unknown. Some other drugs targeted at pathways including PPAR, TGF-β, and CB2R also impress scientists very well (Tang et al. 2015; Flores et al. 2016; Tsai and Han 2016). But some of the mechanisms of how these medications wore are still controversial, so what authors mentioned need to confirm their curative effects by more evidences.
In the recent decade, stem cell dominates the treatment research of restoration after brain injury. Treatments are mainly characterized by types of stem cells and transplantation methods (Phillips et al. 2013). Mesenchymal stem cells deriving from placenta and umbilical cord planted intraventricularly take effect in reducing the hydrocephalus, and the reporters confirmed their assumption on anti-inflammatory effects according to the regulation of inflammation-involved cytokines (Ahn et al. 2013; Ding et al. 2017). Neural progenitor cells (NPCs) can be more beneficial to neuron regeneration for the functions of releasing neurotrophic factors, and differentiated types of cells are capable of performing corresponding functions (Bae et al. 2016). Besides, there are some traces that induced pluripotent stem (iPS) cells, which also have the likened value of treating the brain deficit, but it is an incomplete technics that need improving, especially the inclination of tumorigenesis (Li et al. 2014). Despite all the problems that stem cell scientists encounter today, stem cells therapy is one of the most promising approaches to GM-IVH.
Compared with post-hemorrhagic treatment, prediction and prevention seem to be more effective, but our knowledge on it goes hardly further than pathophysiology for decades. Since there are plenty of reports implying the risk factor of GM-IVH, the risk can be reduced by measures like controlling the maternal diseases and intensive care for newborn premature (Waitz et al. 2016). In the past some, pediatrists tried to reduce risk of GM-IVH by altering the head position in the hope of improving the hemodynamics and oxygen supply, but insufficient evidence has proven the feasibility so far (de Bijl-Marcus et al. 2017).
Ment and coworkers reported that methylenetetrahydrofolate reductase (MTHFR) variants may make neonates more vulnerable when encountering hypoxia (Ment et al. 2014). Szpecht and associates reported several intriguing discoveries about the impact of genotypes on GM-IVH. Infants with genotype GT eNOS 894G > T or MTHFR 1298A > C polymorphism suffer a higher risk of IVH born before 28+6 weeks of gestation (Szpecht et al. 2017b, a). These discoveries strongly demonstrate the definite genetic effect on the occurrence of GM-IVH, which holds a promising future of genetic diagnosis and prevention to GM-IVH.
Conclusion
GM-IVH in preterm infants is a disastrous disease with considerable fatality and morbidity, which is highly relevant to gestational age, maternal conditions, and delivering situations. Special vulnerability of germinal matrix pathologically leads to higher risk of GM-IVH. Pediatrists and scientists have been deeply looking for the keys to lowering the risk, reducing the mortality and attenuating the sequelae. Given the limited acknowledge of GM-IVH, there are only several methods we can choose to deal with it, while the advanced treatments such as neurotrophic drugs, iron chelators, NSAIDs, and stem cells therapy are still in research. But researchers are still in hope of bigger breakthroughs in this issue to promote the survival of GM-IVH.
References
Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, Oh WI, Park WS (2013) Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 44(2):497–504. https://doi.org/10.1161/STROKEAHA.112.679092
Albertsson AM, Bi D, Duan L, Zhang X, Leavenworth JW, Qiao L, Zhu C, Cardell S, Cantor H, Hagberg H, Mallard C, Wang X (2014) The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. J Neuroinflammation 11:153. https://doi.org/10.1186/s12974-014-0153-z
Albertsson AM, Zhang X, Vontell R, Bi D, Bronson RT, Supramaniam V, Baburamani AA, Hua S, Nazmi A, Cardell S, Zhu C, Cantor H, Mallard C, Hagberg H, Leavenworth JW, Wang X (2018) Gammadelta T cells contribute to injury in the developing brain. Am J Pathol 188(3):757–767. https://doi.org/10.1016/j.ajpath.2017.11.012
Andreone BJ, Lacoste B, Gu C (2015) Neuronal and vascular interactions. Annu Rev Neurosci 38:25–46. https://doi.org/10.1146/annurev-neuro-071714-033835
Baburamani AA, Ek CJ, Walker DW, Castillo-Melendez M (2012) Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair? Front Physiol 3:424. https://doi.org/10.3389/fphys.2012.00424
Back SA (2017) White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 134(3):331–349. https://doi.org/10.1007/s00401-017-1718-6
Bae DK, Park D, Lee SH, Yang G, Kyung J, Kim D, Shin K, Choi EK, Kim G, Hong JT, Kim SU, Kim YB (2016) Comparative effects of human neural stem cells and oligodendrocyte progenitor cells on the neurobehavioral disorders of experimental autoimmune encephalomyelitis mice. Stem Cells Int 2016:4079863. https://doi.org/10.1155/2016/4079863
Ballabh P (2014) Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol 41(1):47–67. https://doi.org/10.1016/j.clp.2013.09.007
Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, Proia RL, Steinman L, Han MH, Hla T (2015) HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523(7560):342–346. https://doi.org/10.1038/nature14462
Bonsack F, Alleyne CH Jr, Sukumari-Ramesh S (2017) Resveratrol attenuates neurodegeneration and improves neurological outcomes after intracerebral hemorrhage in mice. Front Cell Neurosci 11:228. https://doi.org/10.3389/fncel.2017.00228
Brew N, Walker D, Wong FY (2014) Cerebral vascular regulation and brain injury in preterm infants. Am J Physiol Regul Integr Comp Physiol 306(11):R773–R786. https://doi.org/10.1152/ajpregu.00487.2013
Chen Q, Shi X, Tan Q, Feng Z, Wang Y, Yuan Q, Tao Y, Zhang J, Tan L, Zhu G, Feng H, Chen Z (2017) Simvastatin promotes hematoma absorption and reduces hydrocephalus following intraventricular hemorrhage in part by upregulating CD36. Transl Stroke Res 8(4):362–373. https://doi.org/10.1007/s12975-017-0521-y
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y (2014) Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res 5(4):472–475. https://doi.org/10.1007/s12975-013-0288-8
Cisternas P, Vio CP, Inestrosa NC (2014) Role of Wnt signaling in tissue fibrosis, lessons from skeletal muscle and kidney. Curr Mol Med 14(4):510–522
Coen RW (2013) Preventing germinal matrix layer rupture and intraventricular hemorrhage. Front Pediatr 1:22. https://doi.org/10.3389/fped.2013.00022
de Bijl-Marcus KA, Brouwer AJ, de Vries LS, van Wezel-Meijler G (2017) The effect of head positioning and head tilting on the incidence of intraventricular hemorrhage in very preterm infants: a systematic review. Neonatology 111(3):267–279. https://doi.org/10.1159/000449240
Ding H, Zhang H, Ding H, Li D, Yi X, Ma X, Li R, Huang M, Ju X (2017) Transplantation of placenta-derived mesenchymal stem cells reduces hypoxic-ischemic brain damage in rats by ameliorating the inflammatory response. Cell Mol Immunol 14(8):693–701. https://doi.org/10.1038/cmi.2015.99
Dohare P, Zia MT, Ahmed E, Ahmed A, Yadala V, Schober AL, Ortega JA, Kayton R, Ungvari Z, Mongin AA, Ballabh P (2016) AMPA-kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J Neurosci 36(11):3363–3377. https://doi.org/10.1523/JNEUROSCI.4329-15.2016
Doyle KP, Quach LN, Sole M, Axtell RC, Nguyen TV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, Buckwalter MS (2015) B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci 35(5):2133–2145. https://doi.org/10.1523/JNEUROSCI.4098-14.2015
Duan X, Wen Z, Shen H, Shen M, Chen G (2016) Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev 2016:1203285. https://doi.org/10.1155/2016/1203285
Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM (2013) Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res 4(2):189–200. https://doi.org/10.1007/s12975-012-0221-6
Eckermann JM, Krafft PR, Shoemaker L, Lieberson RE, Chang SD, Colohan A (2012) Potential application of hydrogen in traumatic and surgical brain injury, stroke and neonatal hypoxia-ischemia. Med Gas Res 2(1):11. https://doi.org/10.1186/2045-9912-2-11
Esiaba I, Angeles DM, Holden MS, Tan JB, Asmerom Y, Gollin G, Boskovic DS (2016) Urinary allantoin is elevated in severe intraventricular hemorrhage in the preterm newborn. Transl Stroke Res 7(2):97–102. https://doi.org/10.1007/s12975-015-0405-y
Firozan B, Goudarzi I, Elahdadi Salmani M, Lashkarbolouki T, Rezaei A, Abrari K (2014) Estradiol increases expression of the brain-derived neurotrophic factor after acute administration of ethanol in the neonatal rat cerebellum. Eur J Pharmacol 732:1–11. https://doi.org/10.1016/j.ejphar.2014.02.041
Flores JJ, Klebe D, Rolland WB, Lekic T, Krafft PR, Zhang JH (2016) PPARgamma-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol Dis 87:124–133. https://doi.org/10.1016/j.nbd.2015.12.015
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G (2014) Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 34(6):1070–1075. https://doi.org/10.1038/jcbfm.2014.56
Gao J, Sun QL, Zhang YM, Li YY, Li H, Hou X, Yu BL, Zhou XH, Yang J (2015) Semi-quantitative assessment of brain maturation by conventional magnetic resonance imaging in neonates with clinically mild hypoxic-ischemic encephalopathy. Chin Med J 128(5):574–580. https://doi.org/10.4103/0366-6999.151646
Garton T, Keep RF, Wilkinson DA, Strahle JM, Hua Y, Garton HJ, Xi G (2016) Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl Stroke Res 7(6):447–451. https://doi.org/10.1007/s12975-016-0480-8
Guo J, Chen Q, Tang J, Zhang J, Tao Y, Li L, Zhu G, Feng H, Chen Z (2015) Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res 1594:115–124. https://doi.org/10.1016/j.brainres.2014.10.046
Haines KM, Wang W, Pierson CR (2013) Cerebellar hemorrhagic injury in premature infants occurs during a vulnerable developmental period and is associated with wider neuropathology. Acta Neuropathol Commun 1:69. https://doi.org/10.1186/2051-5960-1-69
Hefti MM, Trachtenberg FL, Haynes RL, Hassett C, Volpe JJ, Kinney HC (2016) A century of germinal matrix intraventricular hemorrhage in autopsied premature infants: a historical account. Pediatr Dev Pathol 19(2):108–114. https://doi.org/10.2350/15-06-1663-OA.1
Hinojosa-Rodriguez M, Harmony T, Carrillo-Prado C, Van Horn JD, Irimia A, Torgerson C, Jacokes Z (2017) Clinical neuroimaging in the preterm infant: diagnosis and prognosis. Neuroimage Clin 16:355–368. https://doi.org/10.1016/j.nicl.2017.08.015
Hu X, Tao C, Gan Q, Zheng J, Li H, You C (2016) Oxidative stress in intracerebral hemorrhage: sources, mechanisms, and therapeutic targets. Oxid Med Cell Longev 2016:3215391. https://doi.org/10.1155/2016/3215391
Huang H, Vasung L (2014) Gaining insight of fetal brain development with diffusion MRI and histology. Int J Dev Neurosci 32:11–22. https://doi.org/10.1016/j.ijdevneu.2013.06.005
Itsiakos G, Papathanasiou AE, Kyriakidis I, Karagianni P, Tsepis K, Tzimou I, Lazaridou E, Chatziioannidis I (2016) Intraventricular hemorrhage and platelet indices in extremely premature neonates. J Pediatr Hematol Oncol 38(7):533–538. https://doi.org/10.1097/MPH.0000000000000631
Iyer KK, Roberts JA, Hellstrom-Westas L, Wikstrom S, Hansen Pupp I, Ley D, Breakspear M, Vanhatalo S (2015) Early detection of preterm intraventricular hemorrhage from clinical electroencephalography. Crit Care Med 43(10):2219–2227. https://doi.org/10.1097/CCM.0000000000001190
Jiang B, Li L, Chen Q, Tao Y, Yang L, Zhang B, Zhang JH, Feng H, Chen Z, Tang J, Zhu G (2017) Role of glibenclamide in brain injury after intracerebral hemorrhage. Transl Stroke Res 8(2):183–193. https://doi.org/10.1007/s12975-016-0506-2
Kaur N, Chettiar S, Rathod S, Rath P, Muzumdar D, Shaikh ML, Shiras A (2013) Wnt3a mediated activation of Wnt/beta-catenin signaling promotes tumor progression in glioblastoma. Mol Cell Neurosci 54:44–57. https://doi.org/10.1016/j.mcn.2013.01.001
Klebe D, Krafft PR, Hoffmann C, Lekic T, Flores JJ, Rolland W, Zhang JH (2014) Acute and delayed deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke 45(8):2475–2479. https://doi.org/10.1161/STROKEAHA.114.005079
Klebe D, McBride D, Flores JJ, Zhang JH, Tang J (2015) Modulating the immune response towards a neuroregenerative peri-injury milieu after cerebral hemorrhage. J Neuroimmune Pharmacol 10(4):576–586. https://doi.org/10.1007/s11481-015-9613-1
Kuperman AA, Brenner B, Kenet G (2013) Intraventricular hemorrhage in preterm infants and coagulation–ambivalent perspectives? Thromb Res 131 Suppl 1:S35–S38. https://doi.org/10.1016/S0049-3848(13)70018-5
Laptook AR (2013) Neurologic and metabolic issues in moderately preterm, late preterm, and early term infants. Clin Perinatol 40(4):723–738. https://doi.org/10.1016/j.clp.2013.07.005
Lee W, Al-Dossary H, Raybaud C, Young JM, Morgan BR, Whyte HE, Sled JG, Taylor MJ, Shroff MM (2016) Longitudinal cerebellar growth following very preterm birth. J Magn Reson Imaging 43(6):1462–1473. https://doi.org/10.1002/jmri.25098
Lekic T, Klebe D, McBride DW, Manaenko A, Rolland WB, Flores JJ, Altay O, Tang J, Zhang JH (2015a) Protease-activated receptor 1 and 4 signal inhibition reduces preterm neonatal hemorrhagic brain injury. Stroke 46(6):1710–1713. https://doi.org/10.1161/STROKEAHA.114.007889
Lekic T, Klebe D, Poblete R, Krafft PR, Rolland WB, Tang J, Zhang JH (2015b) Neonatal brain hemorrhage (NBH) of prematurity: translational mechanisms of the vascular-neural network. Curr Med Chem 22(10):1214–1238
Li J, McDonald CA, Fahey MC, Jenkin G, Miller SL (2014) Could cord blood cell therapy reduce preterm brain injury? Front Neurol 5:200. https://doi.org/10.3389/fneur.2014.00200
Li L, Tao Y, Tang J, Chen Q, Yang Y, Feng Z, Chen Y, Yang L, Yang Y, Zhu G, Feng H, Chen Z (2015) A cannabinoid receptor 2 agonist prevents thrombin-induced blood–brain barrier damage via the inhibition of microglial activation and matrix metalloproteinase expression in rats. Transl Stroke Res 6(6):467–477. https://doi.org/10.1007/s12975-015-0425-7
Ma S, Santhosh D, Kumar TP, Huang Z (2017) A brain-region-specific neural pathway regulating germinal matrix angiogenesis. Dev Cell 41(4):366–381 e364. https://doi.org/10.1016/j.devcel.2017.04.014
Meng H, Li F, Hu R, Yuan Y, Gong G, Hu S, Feng H (2015) Deferoxamine alleviates chronic hydrocephalus after intraventricular hemorrhage through iron chelation and Wnt1/Wnt3a inhibition. Brain Res 1602:44–52. https://doi.org/10.1016/j.brainres.2014.08.039
Ment LR, Aden U, Lin A, Kwon SH, Choi M, Hallman M, Lifton RP, Zhang H, Bauer CR, Gene Targets for IVHSG (2014) Gene–environment interactions in severe intraventricular hemorrhage of preterm neonates. Pediatr Res 75(1–2):241–250. https://doi.org/10.1038/pr.2013.195
Michinaga S, Koyama Y (2015) Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci 16(5):9949–9975. https://doi.org/10.3390/ijms16059949
Mitsiakos G, Papathanasiou AE, Kyriakidis I, Karagianni P, Tsepis K, Tzimou I, Lazaridou E, Chatziioannidis I (2016) Intraventricular hemorrhage and platelet indices in extremely premature neonates. J Pediatr Hematol Oncol 38(7):533–538. https://doi.org/10.1097/MPH.0000000000000631
Movsas TZ, Pinto-Martin JA, Whitaker AH, Feldman JF, Lorenz JM, Korzeniewski SJ, Levy SE, Paneth N (2013) Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. J Pediatr 163(1):73–78. https://doi.org/10.1016/j.jpeds.2012.12.084
Nazmi A, Albertsson AM, Rocha-Ferreira E, Zhang X, Vontell R, Zelco A, Rutherford M, Zhu C, Nilsson G, Mallard C, Hagberg H, Lai JCY, Leavenworth JW, Wang X (2018) Lymphocytes contribute to the pathophysiology of neonatal brain injury. Front Neurol 9:159. https://doi.org/10.3389/fneur.2018.00159
O’Dell MC, Cassady C, Logsdon G, Varich L (2015) Cinegraphic versus combined static and cinegraphic imaging for initial cranial ultrasound screening in premature infants. Pediatr Radiol 45(11):1706–1711. https://doi.org/10.1007/s00247-015-3382-0
Okazaki M, Fukuhara T, Namba Y (2013) Delayed germinal matrix hemorrhage induced by ventriculoperitoneal shunt insertion for congenital hydrocephalus. J Neurosurg Pediatr 12(1):67–70. https://doi.org/10.3171/2013.4.PEDS12599
Panigrahy A, Wisnowski JL, Furtado A, Lepore N, Paquette L, Bluml S (2012) Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr Radiol 42(Suppl 1):S33–S61. https://doi.org/10.1007/s00247-011-2239-4
Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, Rossi A, Ramenghi LA (2015) Low-grade intraventricular hemorrhage: is ultrasound good enough? J Matern Fetal Neonatal Med 28(Suppl 1): 2261–2264. https://doi.org/10.3109/14767058.2013.796162
Payne AH, Hintz SR, Hibbs AM, Walsh MC, Vohr BR, Bann CM, Wilson-Costello DE, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (2013) Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr 167(5):451–459. https://doi.org/10.1001/jamapediatrics.2013.866
Phillips AW, Johnston MV, Fatemi A (2013) The potential for cell-based therapy in perinatal brain injuries. Transl Stroke Res 4(2):137–148. https://doi.org/10.1007/s12975-013-0254-5
Raybaud C, Ahmad T, Rastegar N, Shroff M, Al Nassar M (2013) The premature brain: developmental and lesional anatomy. Neuroradiology 55(Suppl 2):23–40. https://doi.org/10.1007/s00234-013-1231-0
Sanapo L, Whitehead MT, Bulas DI, Ahmadzia HK, Pesacreta L, Chang T, du Plessis A (2017) Fetal intracranial hemorrhage: role of fetal MRI. Prenatal Diagn 37(8):827–836. https://doi.org/10.1002/pd.5096
Sheehan JW, Pritchard M, Heyne RJ, Brown LS, Jaleel MA, Engle WD, Burchfield PJ, Brion LP (2017) Severe intraventricular hemorrhage and withdrawal of support in preterm infants. J Perinatol 37(4):441–447. https://doi.org/10.1038/jp.2016.233
Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K (2014) Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34(6):2231–2243. https://doi.org/10.1523/JNEUROSCI.1619-13.2014
Strahle JM, Garton T, Bazzi AA, Kilaru H, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G (2014) Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery 75(6):696–705. https://doi.org/10.1227/NEU.0000000000000524 discussion 706.
Supramaniam V, Vontell R, Srinivasan L, Wyatt-Ashmead J, Hagberg H, Rutherford M (2013) Microglia activation in the extremely preterm human brain. Pediatr Res 73(3):301–309. https://doi.org/10.1038/pr.2012.186
Szpecht D, Gadzinowski J, Seremak-Mrozikiewicz A, Kurzawinska G, Drews K, Szymankiewicz M (2017a) The role of FV 1691G> A, FII 20210G> A mutations and MTHFR 677C> T; 1298A> C and 103G> T FXIII gene polymorphisms in pathogenesis of intraventricular hemorrhage in infants born before 32 weeks of gestation. Child’s Nerv Syst. https://doi.org/10.1007/s00381-017-3460-8
Szpecht D, Gadzinowski J, Seremak-Mrozikiewicz A, Kurzawinska G, Szymankiewicz M (2017b) Role of endothelial nitric oxide synthase and endothelin-1 polymorphism genes with the pathogenesis of intraventricular hemorrhage in preterm infants. Sci Rep 7:42541. https://doi.org/10.1038/srep42541
Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, Lieu CH (2015) Targeting the WNT signaling pathway in cancer therapeutics. Oncologist 20(10):1189–1198. https://doi.org/10.1634/theoncologist.2015-0057
Tang J, Tao Y, Tan L, Yang L, Niu Y, Chen Q, Yang Y, Feng H, Chen Z, Zhu G (2015) Cannabinoid receptor 2 attenuates microglial accumulation and brain injury following germinal matrix hemorrhage via ERK dephosphorylation in vivo and in vitro. Neuropharmacology 95:424–433. https://doi.org/10.1016/j.neuropharm.2015.04.028
Tang J, Miao H, Jiang B, Chen Q, Tan L, Tao Y, Zhang J, Gao F, Feng H, Zhu G, Chen Z (2017) A selective CB2R agonist (JWH133) restores neuronal circuit after germinal matrix hemorrhage in the preterm via CX3CR1+ microglia. Neuropharmacology 119:157–169. https://doi.org/10.1016/j.neuropharm.2017.01.027
Tao Y, Tang J, Chen Q, Guo J, Li L, Yang L, Feng H, Zhu G, Chen Z (2015) Cannabinoid CB2 receptor stimulation attenuates brain edema and neurological deficits in a germinal matrix hemorrhage rat model. Brain Res 1602:127–135. https://doi.org/10.1016/j.brainres.2015.01.025
Tortora D, Severino M, Malova M, Parodi A, Morana G, Sedlacik J, Govaert P, Volpe JJ, Rossi A, Ramenghi LA (2017) Differences in subependymal vein anatomy may predispose preterm infants to GMH-IVH. Arch Dis Childhood Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2017-312710
Tsai HC, Han MH (2016) Sphingosine-1-phosphate (S1P) and S1P signaling pathway: therapeutic targets in autoimmunity and inflammation. Drugs 76(11):1067–1079. https://doi.org/10.1007/s40265-016-0603-2
Varghese B, Xavier R, Manoj VC, Aneesh MK, Priya PS, Kumar A, Sreenivasan VK (2016) Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury. Indian J Radiol Imaging 26(3):316–327. https://doi.org/10.4103/0971-3026.190421
Vela D (2018) Hepcidin, an emerging and important player in brain iron homeostasis. J Transl Med 16(1):25. https://doi.org/10.1186/s12967-018-1399-5
Vesoulis ZA, Mathur AM (2017) Cerebral autoregulation, brain injury, and the transitioning premature infant. Front Pediatr 5:64. https://doi.org/10.3389/fped.2017.00064
Waitz M, Nusser S, Schmid MB, Dreyhaupt J, Reister F, Hummler H (2016) Risk factors associated with intraventricular hemorrhage in preterm infants with </=28 weeks gestational age. Klin Padiatr 228(5):245–250. https://doi.org/10.1055/s-0042-111689
Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, Xi G (2016) Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease-activated receptor-1. Transl Stroke Res 7(6):478–487. https://doi.org/10.1007/s12975-016-0472-8
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G (2018) Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl Stroke Res 9(1):74–91. https://doi.org/10.1007/s12975-017-0559-x
Xiaoyu W (2015) The exposure to nicotine affects expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in neonate rats. Neurol Sci 36(2):289–295. https://doi.org/10.1007/s10072-014-1934-y
Xiong XY, Liu L, Wang FX, Yang YR, Hao JW, Wang PF, Zhong Q, Zhou K, Xiong A, Zhu WY, Zhao T, Meng ZY, Wang YC, Gong QW, Liao MF, Wang J, Yang QW (2016) Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation 134(14):1025–1038. https://doi.org/10.1161/CIRCULATIONAHA.116.021881
Yang D, Baumann JM, Sun YY, Tang M, Dunn RS, Akeson AL, Kernie SG, Kallapur S, Lindquist DM, Huang EJ, Potter SS, Liang HC, Kuan CY (2013) Overexpression of vascular endothelial growth factor in the germinal matrix induces neurovascular proteases and intraventricular hemorrhage. Sci Transl Med 5(193):193ra190. https://doi.org/10.1126/scitranslmed.3005794
Zhan J, Dinov ID, Li J, Zhang Z, Hobel S, Shi Y, Lin X, Zamanyan A, Feng L, Teng G, Fang F, Tang Y, Zang F, Toga AW, Liu S (2013) Spatial-temporal atlas of human fetal brain development during the early second trimester. Neuroimage 82:115–126. https://doi.org/10.1016/j.neuroimage.2013.05.063
Zhang X, Rocha-Ferreira E, Li T, Vontell R, Jabin D, Hua S, Zhou K, Nazmi A, Albertsson AM, Sobotka K, Ek J, Thornton C, Hagberg H, Mallard C, Leavenworth JW, Zhu C, Wang X (2017) gammadeltaT cells but not alphabetaT cells contribute to sepsis-induced white matter injury and motor abnormalities in mice. J Neuroinflamm 14(1):255. https://doi.org/10.1186/s12974-017-1029-9
Zhang Y, Xu N, Ding Y, Zhang Y, Li Q, Flores J, Haghighiabyaneh M, Doycheva D, Tang J, Zhang JH (2018) Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav Immunity 70:179–193. https://doi.org/10.1016/j.bbi.2018.02.015
Funding
This study was supported by the National Natural Science Foundation of China (81500992), Natural Science Foundation of Zhejiang (LQ16H090002), and Medical and Health Key Project of Zhejiang Province (2016RCA015).
Author information
Authors and Affiliations
Contributions
SC was the principal investigator. JL and YL wrote the paper and made the original figures. HZ revised the figures. CR handled the language and made some comments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Luo, J., Luo, Y., Zeng, H. et al. Research Advances of Germinal Matrix Hemorrhage: An Update Review. Cell Mol Neurobiol 39, 1–10 (2019). https://doi.org/10.1007/s10571-018-0630-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0630-5