Abstract
In the current study effect of nicotine on expression of neurotrophins, brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) has been studied in hippocampus and frontal cortex during development of brain in rats. Neurotrophins are factors that help in development of brain among which BDNF and NGF are very important, expressed at different stages during the developmental process. Different sedatives are reported to alter the expression of these factors. In this study, three groups of neonate rats (1–5, 5–10 and 10–15 days age) were used each having 20 rats. Ten were subjected to a dose of 66 μg of nicotine while other ten received the same amount of saline at the same time interval. Then expression of the BDNF and NGF was observed in hippocampus and frontal cortex tissue using immunoassay. Western blotting was used to observe the presence of BDNF in hippocampus as well as frontal cortex. In all groups there was a significant decrease in concentration of neurotrophic factors where nicotine was applied as compared to control. The highest expression of BDNF and NGF in hippocampus and frontal cortex was observed in 10–15 days group (G3) and in 5–10 group (G2) as compared to the control, P < 0.01. It was concluded that exposure of neonate rats to nicotine causes a decrease in the expression of NGF and BDNF and it effects the development of brain in neonates that can further impair brain functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotine, an alkaloid that can affect many physiological functions of human body, is extracted commercially from the leaves of Nicotiana rustica and Nicotiana tabacum [1]. Humans use nicotine in the form of tobacco products where its physiological effects occur on interaction with nicotine receptors in body. Various pharmacological doses of nicotine and smoking can cause heart rate to accelerate, constriction of blood vessels and increase in blood pressure [2].
Nicotinic receptors are stimulated directly releasing dopamine, acetylcholine and neurotransmitters like norepinephrine and serotonin [3]. The effect of nicotine has been widely studied in animals and in adult rodents; it has been shown that memory performance can be enhanced on exposure to acute levels of nicotine [4]. Dose-dependent activity with respect to nicotine administration has also been reported where high doses result in hypoactivity while low doses result in hyperactivity [5]. Nordberg et al. [6] demonstrated that rat treated with nicotine at 10–16 days showed hypoactivity while rat treated with saline showed hyperactivity at adult age in response to an acute nicotinic injection. The intrinsic toxicity of nicotine has been defined and related to the hazardous outcomes of smoking [7]. However, after the administration of nicotine the response of body is quite complex and unpredictable [8].
During development of brain, neurotrophic factors are very important for proliferation, survival and development of neurons. These factors are also expressed during adulthood and help in regulation of survival and maintenance of neurons. Among different neurotrophic factors two important factors are brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF). Studies have shown that BDNF and NGF levels are at the highest during perinatal period where BDNF protein level is expressed at the highest concentration by 14 days after birth in hippocampus and NGF level reaches to its peak by 7 days after birth [9]. Both these factors also help in memory and learning, where administration of NGF for a period of 4 weeks is attributed to restore age-induced cholinergic atrophy and improvement of Morris water maze (MWM) performance [10]. The deficit in MWM performance has also been observed in rats that carry heterozygous NGF disruption which is mitigated on NGF infusion [11]. An increased BDNF level in hippocampus in adult animals is reported to increase long-term potentiation and also facilitates the phosphorylation of NMDA-NR1 [12] and NMDA-NR2 receptor subunits [13]. As data reported are from experiments on effect of BDNF on adult brain therefore it suggests the involvement of BDNF in learning and memory. The role of neurotrophins is also reported in the proper formation of synaptic circuitry of brain [14].
The objective of this study was to determine the effect of nicotine on expression of BDNF and NGF in hippocampus and frontal cortex in brain of neonate rats so that its effect on the development of brain can be established. This effect was studied in three different groups in three different time periods in both parts of the brain so that the time where development of brain is most effected (if any) could be explored.
Materials and methods
For study male and female Sprague-Dawly rats were obtained from Medical School of Lishui University. This study was approved by Animal Research Ethics Committee of Lishui Medical University and experiments were carried out in accordance with the Guidelines and Suggestions for the Care and Use of Laboratory Animals, Ministry of Science and Technology of the People’s Republic of China. After placing a male and female rat in hanging cages, female rats were moved to polycarbonate cages until parturition, where date of birth was considered as postnatal day (P)0. Then on P1 pups were culled to 20 male each for 3 experiments to be performed where 10 rats were experimental and 10 rats were control in each experiment.
-
nicotine-bi-tartrate was obtained from Sigma–Aldrich, St. Louis, MO and the pH of
-
nicotine base was adjusted to 7.0 in all the experiments. The administration of nicotine doses was confined in accordance with the doses human absorb through smoking of up to 10 cigarettes daily, that is, 66 µg nicotine base/kg body weight of rat [15]. Three groups of rats (20 pups each, 10 experimental and 10 control) received a dose of 66 µg/kg bodyweight of
-
nicotine base through single shot and control received saline 10 mg/kg bodyweight once daily where nicotine and saline groups, that is experimental and control groups received doses at the same time in the same quantity, group I from day 1 to 5 with day 0 as postnatal day, group II received the dose from day 5 to day 10, similarly group III received the dose from day 10 to day 15. Animals were removed on respective days from their cages and dissected in the dissection room where decapitation was performed and blood sampled. After rapid removal of brains from skull, hippocampus and frontal cortex were dissected on ice and then frozen on dry ice [16].
The defined protocol of the manufacturer (Promega, Madison, WI, USA) was used for BDNF and NGF immunoassay. To perform BDNF assay 9.99 ml of carbonate coating buffer at pH 9.7 was used and 10 µl of antiBDNF monoclonal antibody (mAb) was added in it. Then 100 µl of this mixture was added to polystyrene ELISA plate (Nunc, MaxiSorb) for incubation at 4 °C overnight. TBST buffer was used for washing all wells and then incubation at room temperature was carried out for 1 h, while to block non-specific binding, a block and sample 1× buffer along with deionized water mixture were added to each well and incubated for 1 h at room temperature. BDNF standard supplied from the manufacturer (1 µg/ml) was used to prepare the BDNF standard curve. 1:2,000 dilution of the standard was made in Block X sample 1× buffer to achieve a final concentration of 500 pg/ml. Prior to assay both hippocampus and frontal cortex were further diluted to 1:2. Both standard and samples were incubated for 2 h at shaking room temperature. Then antihuman BDNF pAB was added to each well plate and then incubated for 2 h at room temperature, and then incubation with antiIgY horseradish peroxidase (HRP) conjugate was performed for 1 h. Then 10 µl TMB one solution was added to each well and incubated for 10 min at room temperature. 100 µl of IN HCl was then added to each well to stop the reaction and results were read after 30 min.
For NGF analysis Promega kit (Madison, WI, USA) was utilized and a similar protocol as described above was followed. pg of neurotrophic factor per mg protein was the method used to express the contents of both BDNF and NGF. BCA protein assay kit (Pierce, Rockford, IL, USA) was used for the quantification of protein concentrations. Statistical analysis was carried out using SPSS software (SPSS, Chicago, IL, USA), one way ANOVA and P value of <0.01 was considered significant.
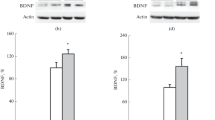
Western blots were performed on whole cell extracts according to the previously described studies [17]. Sample buffer was used to boil a 20 mg of each sample for 5 min before separation on 10–20 % SDS–polyacrylamide gel and then proteins were transferred to nitrocellulose membranes. 5 % nonfat dry milk dissolved in TBST was used to block non-specific immunoreactivity for 60 min. Anti BDNF antibody (Aviva Systems Biology, San Diego, CA; 1:700 dilution) was used to incubate the membranes overnight at 4 °C. Then after primary antibody incubation six washes (10 min at room temperature) in TBST were used before incubation with secondary antibody (HRP conjugated goat anti-rabbit IgG, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) followed by six washes and visualization through ECL detection system (Perkin-Elmer New England Nuclear, Waltham, MA). An integrated density value (IDV) was calculated after normalizing the intensity of each BDNF against the corresponding GAPDH control band using Gel Pro Analyzer 4.0 software and results are shown in Figs. 1, 2 for hippocampus and frontal cortex in all three groups, respectively.
Results
In both hippocampus and frontal cortex, there was an effect on the concentration of BDNF in samples where nicotine was applied as compared to saline controls, P < 0.01. The lowest concentration of BDNF was observed in G1 nicotine (day 1–day 5) 300 pg/mg, P < 0.01 while highest concentration, 500 pg/mg was found in both G2 nicotine (day 5–day 10), P < 0.01 and G3 nicotine (day 10–day 15), P < 0.01. Control samples treated with saline in all groups showed high concentration of BDNF as compared to experiment. In case of controls, the highest concentration was found in G3 (day 10–day 15) which was 700 pg/mg and a subsequent decline in other two groups was observed, that is, 600 pg/mg and 500 pg/mg in G2 and G1, P < 0.01 as compared to samples, respectively. All these results were observed analyzing hippocampus samples as shown in Fig. 3. In frontal cortex the concentration of BDNF was found lowest in G1 nicotine, 200 pg/mg, P < 0.01 as compared to control and P > 0.01 within the group; highest in G3 nicotine 540 pg/mg, P < 0.01 as compared to control and P > 0.01 within the group; and in G2 nicotine it was 510 pg/mg, P < 0.01 compared to control and P > 0.01 within the group. All controls had a greater concentration of BDNF in all the groups, G1 had 300 pg/mg, G2 640 pg/mg and G3 670 pg/mg, with P < 0.01 as compared to samples and P > 0.01 within the group as shown in Fig. 4.
Quantitative comparison of BDNF in all the groups in animals treated with nicotine and corresponding controls in hippocampus. a Graph showing a decrease in the expression of BDNF in hippocampus in group 1 (day 1–day 5) where P C < 0.01 as compared to control. b A decrease in the expression of BDNF was observed in group 2 (day 5–day 10) where P C < 0.01 as compared to control. c Effect of nicotine on expression of BDNF in group 3 (day 10–day 15) where quantity of BDNF is decreased significantly in experimental samples (P C < 0.01) as compared to control
Quantitative illustration of expression of BDNF in all three groups in frontal cortex. a A decrease in the expression of BDNF in frontal cortex in G1 (day 1–day 5) is observed P C < 0.01 as compared to corresponding control 200 pg/mg compared to 300 pg/mg in control group. b Expression of BDNF decreases in group 2 (day 5–day 10), P C < 0.01 in frontal cortex of experimental rats as compared to the control. c Highest amount of BDNF 540 pg/mg in experimental samples and 670 pg/mg in control samples is observed in G3 (day 10–day 15) where the amount of expression is decreased in experiments as compared to control P C < 0.01
In hippocampus it was analyzed that in G2 nicotine the highest concentration of NGF, 190 pg/mg (P < 0.01) was found; while in G1 nicotine and G3 nicotine it was 100 pg/mg (P < 0.01) and 170 pg/mg (P < 0.01), respectively, as compared to corresponding controls. In all the groups in control samples of hippocampus quantity of NGF was found greater as compared to nicotine-administered groups. In G2 control highest level, 310 pg/mg was found; while in G3 control 280 pg/mg; and in G1 control 200 pg/mg (P < 0.01 compared to samples) was analyzed in hippocampus shown in Fig. 5. In samples of frontal cortex, the highest concentration of NGF in nicotine-administered samples was found in G2, 130 pg/mg, (P < 0.01). In G3 and G1 it was analyzed to be 120 pg/mg and 70 pg/mg (P < 0.01). The control samples of frontal cortex also showed a high concentration of NGF, G2 showing 160 pg/mg, G3 150 pg/mg and G1 90 pg/mg (P < 0.01 compared to samples) shown in Fig. 6.
Graphic illustration of expression of NGF in hippocampus samples compared with control in all groups. a Significant decrease in the concentration of NGF in rats effected by nicotine was observed as compared to controls, P C < 0.01. b Highest concentration of NGF, 190 pg/mg is observed in nicotine group 2 (day 5–day 10) and its corresponding control (310 pg/mg) where P C < 0.01 for samples in comparison with corresponding controls. c In group 3 (day 10–day 15), 170 pg/mg of NGF is obtained compared to control, 280 pg/mg where P C < 0.01 for samples compared with corresponding controls
Graphic illustration of expression of NGF in frontal cortex of experimental and control animals. a In frontal cortex of experimental group G1 (day 0–day 5) a decrease in the expression of NGF is observed as compared to the corresponding controls, P C < 0.01. b Highest expression of NGF is observed in group 2 (day 5–day 10) in both experiment and control samples, however a significant decrease is observed in rats effected by nicotine as compared to controls, P C < 0.01. c In group 3 (day 10–day 15) again NGF expression is significantly in frontal cortex (P C < 0.01) compared to corresponding controls
In all the groups in both hippocampus and frontal cortex, western blot analyses were used to assess the quantitative comparison of expression of BDNF compared with GAPDH expression used as control. Expression of BDNF was found lower in the nicotine-administered rats as compared to the saline-exposed rats in the hippocampus and frontal cortex in G3 (mean ± SEM density values; hippocampus, Saline = 13.0 ± 2.12, Nicotine = 4.0 ± 1.29; t = 3.52, df = 6; P = 0.01, Fig. 1); frontal cortex, Saline = 21.5 ± 1.42, Nicotine = 8.95 ± 4.23, t = 3.26, df = 6, P = 0.02, Fig. 2). The bands showing western blot analyses are shown in Fig. 7 for hippocampus and Fig. 8 for frontal cortex.
Discussion
We concluded from this study that the exposure of neonatal rat to nicotine can affect the development of brain because expression of neurotrophins, BDNF and NGF is effected in male neonate rats. Previously different studies had been carried out in this aspect, Brown et al. [18] reported a significant decrease of NGF and BDNF in both hippocampus and frontal cortex because of nicotine, where neonate rats had been treated with saline and then effect of nicotine in adulthood was observed on them.
Both these factors had been studied widely in human and other experimental animals and it has been reported that NGF increases in the situations characterized by anxiety like parachute jump of male soldiers [19] or following the cessation of smoking [20]. Similarly different studies conducted on BDNF have established a link between increase in level of BDNF and efficacy of antidepressants [21]. The gene for neurotrophin BDNF is reported a risk locus of depression [22]. It is this reason that in patients suffering from depression and anxiety decreased blood level of BDNF has been observed and use of antidepressants reverted this level [23]. Different studies on rodents showed that expression of NGF and BDNF is confined to frontal cortex during the process of development and these two regions hold key for synaptic plasticity in developmental as well as adult stage [24]. Due to the importance of these two factors in the development of brain, we decided to carry out this study on neonates so that possible effects on the development can be studied and we observed that expression of both the factors is altered on exposure of neonates to nicotine as compared to control groups. Recently different studies have emphasized the disturbance caused in neurotrophic factors homeostasis in brain development. Different compounds also used in anesthesia like nitric oxide, isoflurane, midazolam and propofol resulted in a decrease in NGF involving an AKT pathway [25, 26]. It has been shown that exposure of rats to compounds used in anesthesia can cause neurodegeneration in developing brain and effects are prolonged that continue through the adulthood [27].
In the current study it has been observed that use of nicotine during development of brain in neonates can result in disturbance of brain development effecting BDNF and NGF expression in hippocampus and frontal cortex. To the best of our knowledge this is the first study in this aspect where effect of nicotine on the expression of two important neurotropic factors has been analyzed in neonatal brain, and therefore further investigations should be carried out and we may be able to better understand the pathways that are effected depending on the different concentrations of nicotine. The extension of this study to neonates that can be affected by passive smoking can be a promising aspect for a better understanding of the effect of this compound on brain development in neonates.
References
Henningfield JE, Woodson PP (1989) Dose related actions of nicotine on behavior and physiology: review and implications for replacement therapy for nicotine dependence. J Subst Abus 1:301–317
James JR, Nordberg A (1995) Genetic and environmental aspects of the role of nicotinic receptors in neurodegenerative disorders: emphasis on Alzheimer’s disease and Parkinson’s disease. Behav Genet 25:149–159
Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG (1989) Presynaptic modulation of transmitter release by nicotinic receptors. Prog Brain Res 79:157–163
Levin ED (2002) Nicotinic receptor subtypes and cognitive function. J Neurobiol 53:633–640
Nordberg A, Bergh C (1985) Effect of nicotine on passive avoidance behavior and motoric activity in mice. Acta Pharmacol Toxicol Copenh 56:337–341
Nordberg A, Zhang XA, Fredriksson A, Eriksson P (1991) Neonatal nicotine exposure induces permanent changes in brain nicotinic receptors and behavior in adult mice. Brain Res Dev Brain Res 63:201–207
General Surgeon (1988) The health consequences of smoking: nicotine addiction. United States Department of Health, Education, and Welfare. USA, Washington
Sherwood N, Kerr JS, Hindmarch I (1991) Effects of nicotine on short-term memory. In: Adlkofer F, Thurau K (eds) Effects of nicotine on biological systems. Birkhauser Verlag, Basel, pp 531–535
Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S (2001) Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience 103:739–761
Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH (1987) Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 329:65–68
Chen KS, Nishimura MC, Armanini MP, Crowley C, Spencer SD, Phillips HS (1997) Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J Neurosci 17:7288–7296
Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB (1997) Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-d-aspartate receptor subunit 1. Proc Natl Acad Sci USA 94(15):8191–8195
Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB (1998) BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal post-synaptic densities. Brain Res Mol Brain Res 55:20–27
Horch HW (2004) Local effects of BDNF on dendritic growth. Rev Neurosci 15:117–129
Russell MAH (1990) Nicotine intake and its control over smoking. In: Wonacott S, Russell MAH, Stolerman IP (eds) Nicotine Psychopharmacology: Molecular, Cellular and Behavioural Aspects. Oxford University Press, Oxford, pp 374–418
Williams MT, Herring NR, Schaefer TL, Skelton MR, Campbell NG, Lipton JW, McCrea AE, Vorhees CV (2007) Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘Foxy’) to adult rats: a new drug of abuse. Neuropsychopharmacology 32:1404–1420
Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bas CE, Terwilliger EF, Pierce RC, Cha JH (2010) Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci 30:11735–11744
Brown RW, Perna MK, Schaefer TL, Williams MT (2006) The effects of adulthood nicotine treatment on D2-mediated behavior and neurotrophins of rats neonatally treated with quinpirole. Synapse 59:253–259
Aloe L, Bracci-Laudiero L, Alleva E, Lambiase A, Micera A, Tirassa P (1994) Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc Natl Acad Sci USA 91(22):10440–10444
Lang UE, Gallinat J, Kuhn S, Jockers-Scherubl C, Hellweg R (2002) Nerve growth factor and smoking cessation. Am J Psychiatry 159:674–675
Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G (2005) Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 57:1068–1072
Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES (2002) Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry 7:579–593
Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Lyo M (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54:70–75
Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA (1997) Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78:431–448
Pesic V, Milanovic D, Tanic N, Popic J, Kanazir S, Jevtovic-Todorovic V, Ruzdijic H (2009) Potential mechanism of cell death in the developing rat brain induced by propofol anesthesia. Int J Dev Neurosci 27:279–287
Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V (2006) General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis 11:1603–1615
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiaoyu, W. The exposure to nicotine affects expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in neonate rats. Neurol Sci 36, 289–295 (2015). https://doi.org/10.1007/s10072-014-1934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1934-y