Abstract
Deep eutectic solvents (DESs) are new and rapid emerging green solvents that have been gaining much attention lately. In this work, DES was prepared by mixing choline chloride with lactic acid with the molar ratio of 1:10 to pretreat empty fruit bunches. The effects of pretreatment temperature, pretreatment time and solid-to-solvent ratio were investigated. A maximum reducing sugars yield of 51.1% was obtained at the operating conditions of 100 °C for 1 h with solid-to-solvent ratio of 1:10 (w/v). The reducing sugars yield obtained for DES pretreatment was higher than dilute acid, alkaline and organosolv pretreatment suggesting DES pretreatment is a promising alternative to conventional pretreatment techniques. Furthermore, there was no reducing sugar loss during DES pretreatment. The outstanding DES pretreatment performance was further confirmed by both Fourier-transform infrared spectroscopy and scanning electron microscopy indicated that DES pretreatment was effective in altering biomass structure by disrupting the hydrogen bonding interactions within the chains of cellulose molecules and lignin extraction.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increase in consumption of energy and global climate change, alternatives to fuel sources have been developed to reduce the dependence on non-renewable sources of energy. Ethanol is an alternative fuel that can be produced from raw materials such as lignocellulosic biomass (Lynd 1996). Lignocellulosic biomass mainly consists of three main components: cellulose, hemicellulose and lignin. Due to the integral structural complexity of lignocellulosic fractions, pretreatment process is required to remove the hemicellulose and/or lignin in order to break down the recalcitrant structure for better hydrolysis performance in sugars production (Harmsen et al. 2010). The sugars will then undergo fermentation process to produce bioethanol. Hence, the transformation of lignocellulosic biomass into bioethanol consists of three main stages: (1) pretreatment of biomass, (2) hydrolysis process and (3) fermentation process (Mielenz 2001).
The most challenging process in bioethanol production is the pretreatment process due to complex matrix structure of lignocellulosic biomass (Kumar et al. 2016). An effective pretreatment is required to reduce the crystallinity of cellulose and to increase substrate porosity with lignin redistribution, so it could increase the exposure of cellulase enzyme to cellulose and hence increase the conversion rate in both hydrolysis and fermentation processes (Pan et al. 2005; Sun and Cheng 2002; Zheng et al. 2009). There are many pretreatment techniques including physical, chemical, physico-chemical and biological which serve the same purpose, i.e. to facilitate the conversion of biomass to bioethanol (Chiaramonti et al. 2012; Kumar et al. 2016, Mood et al. 2013; Mosier et al. 2005; Weil et al. 1994).
Among the pretreatment techniques, chemical pretreatments are widely explored by the researchers due to their effectiveness in biomass pretreatment (Bensah and Mensah 2013). Chemical pretreatment using ionic liquids (ILs) has been gaining much attention in the last decade due to effectiveness in biomass pretreatment and dissolution (Barr et al. 2014; Lee et al. 2013, 2014) along with their desired characteristics including low melting point, high thermal and chemical stability (Dománska and Bogel-Lukasik 2005), low vapor pressure (Kabo et al. 2004; Paulechka et al. 2003) and low flammability (Earle and Seddon 2009). Despite having much attractive properties, their non-environmentally benign, poor biodegradability, high cost, long synthesis and purification procedure making them impractical to be used for large industrial applications (Coleman and Gathergood 2010; Quijano et al. 2010).
Recently, deep eutectic solvents (DESs) have emerged as a new class of green solvent with many physicochemical properties similar to ILs (Dominguez de Maria and Maugeri 2011; Gorke et al. 2008; Zhang et al. 2012). DES is a homogeneous mix of two or three components at a specific molar ratio to form a eutectic mixture with melting point much lower than melting points of individual components (Loow et al. 2017; Zhang et al. 2012). Similar to ILs, DESs exhibit one of the most essential characteristics, which they are of low volatility making them very attractive to be used in commercialized processes as alternative sustainable solvents (Gorke et al. 2008). Their desired properties of biocompatible, highly biodegradable, easy to synthesis and low price (Dominguez de Maria and Maugeri 2011) also contribute to the suitability of DESs in pretreatment process of lignocellulosic biomass.
As DESs are mixtures of several anionic and/or cationic species, as well as non-ionic species, thus, many types of DESs could be synthesized and they can be classified into acid-based (e.g. choline chloride-lactic acid, ChCl/LA) and non-acidic based (e.g. choline chloride-glycerol, ChCl/G) (Smith et al. 2014). It has been reported that non-acidic based DESs appeared to be ineffective unless the pretreatment is carried out under severe conditions such as high temperatures and long duration hours (Procentese et al. 2018; Xu et al. 2016), while acid based DESs with monocarboxylic acid as hydrogen bond donor to be combined with a hydrogen bond acceptor reported to be effective in biomass pretreatment (Tang et al. 2017; Thi and Lee 2019).
Therefore, this work focuses on acid-based DES, in particular, choline chloride-lactic acid (ChCl/LA) with its reported effectiveness in lignin extraction and enzymatic hydrolysis (Li et al. 2017; Thi and Lee 2019; Zhang et al. 2016). ChCl/LA with molar ratio of 1:10 was employed in pretreating empty fruit bunches (EFB) as it was reported to outperform ChCl/LA with different molar ratios in terms of sugars and lignin extraction (Zhang et al. 2016; Li et al. 2017). EFB was chosen as raw material as it serves as a cheap indigenous source of energy in view of its high abundancy and non-food competitiveness. In addition, EFB was reported to have high cellulose content, which is beneficial in sugars production (Sudiyani et al. 2013). Potential parameters including pretreatment temperature, pretreatment time and solid-to-solvent ratio were investigated. The performance of DES pretreatment on EFB was evaluated by comparing the reducing sugars yield from enzymatic hydrolysis of pretreated EFBs, reducing sugars loss during pretreatment process, structural and morphology changes for different pretreatment methods including dilute acid, alkaline and organosolv pretreatments.
Materials and methods
Materials
EFB were donated by Kwantas Oil Sdn Bhd, Lahad Datu, Sabah, Malaysia. Raw EFB was ground and screened through mesh size of 0.5 mm. The ground EFB was dried in an oven (AX 120, Carbolite, United Kingdom) at 60 °C for 24 h to remove its moisture content. Chemicals including choline chloride (98.0% purity), lactic acid (88.0% purity), sulfuric acid (95.0–97.0% purity), ethanol (95.0% purity) and sodium hydroxide (analytical grade reagent) were purchased from Friendemann Schmidt, Malaysia. Onozuka R-10 cellulase enzyme (from Trichoderma viride) was purchased from Merck, Germany.
Synthesis of DES
Choline chloride (ChCl) and lactic acid (LA) with molar ratio of 1:10 were weighted and the DES solution was prepared by continuously mixing both chemicals at 350 rpm at 60 °C until a homogeneous colourless liquid was formed. In view of DES’s hygroscopic nature, the mixing was carried out in a fully sealed container. This is to prevent the contact of DES with moisture, as the presence of moisture in DES could alter its purity, chemical structures and physical properties, and hence, affecting its pretreatment performance (Chen et al. 2019; García et al. 2015).
DES pretreatment
ChCl/LA was added to a test tube consisted of EFB sample in solid-to-solvent ratio of 1:10 (w/v). The test tube with the content was placed in an oil bath (MC, Julabo, Germany) and pretreatment was carried out under pre-set operating conditions. The operating conditions were in temperatures range of 80–140 °C, for 0.5–5 h with solid-to-solvent ratio of 1:5, 1:10 and 1:15. After the pretreatment, pretreated biomass was filtered from its supernatant and washed with distilled water to ensure complete removal of residual DES. The pretreated solid residue was dried at 60 °C for 24 h prior to enzymatic hydrolysis, structural characterization using Fourier-transform infrared spectroscopy (FT-IR) and morphology study using scanning electron microscope (SEM). The supernatant from filtration process was proceeded with dinitrosalicylic (DNS) analysis (Miller 1959) for reducing sugar loss determination.
Performance of DES in pretreating EFB was evaluated by replacing DES with 0.5 M of sulfuric acid (dilute acid pretreatment), 0.5 M of sodium hydroxide (alkaline pretreatment) and 60% (v/v) of ethanol (organosolv pretreatment). Pretreatment was also conducted without any solvent but distilled water which served as control experiment. All the pretreatments were carried out at same operating conditions of 100 °C at 1 h. All experiments were conducted in duplicates.
Enzymatic hydrolysis
Enzymatic hydrolysis was conducted by mixing 0.02 g of pretreated EFB with Onozuka R-10 cellulase solutions maintained at pH 4.8 using 50 mM sodium acetate buffer. The test tube with its content was placed in a water bath (TW 12, Julabo, Germany) preset at 50 °C for 48 h. After 48 h, samples were boiled and centrifuged (2-6E, Sigma, Germany). The liquid product, known as hydrolysate was collected and subjected to DNS assay. Reducing sugars yield was calculated using Eq. (1):
Fourier-transform infrared (FT-IR) analysis
Dried pretreated EFB was characterized by conducting FT-IR analysis (Nicolet iS5, Thermo Fisher Scientific, United States). The analysis was performed for untreated EFB as well as all pretreated EFBs including DES, dilute acid, alkaline, organosolv pretreatment and control experiment. All spectra were recorded in the absorption band made over the range of 4000–800 cm−1 at a spectra resolution of 4 cm−1 and 64 scans per sample. FT-IR spectra was also utilized to determine total crystallinity index (TCI) of the pretreated EFBs. TCI of the pretreated EFBs was determined by measuring the ratio of peak heights at wavelength 1373–2900 cm−1 (Nelson and O’Connor 1964).
Scanning electron microscopy (SEM) analysis
Surface morphology study of untreated and pretreated EFBs was conducted using scanning electron microscope (SEM) (S-3400N, Hitachi, Japan). The images were obtained under 15 kV acceleration voltage and magnification of 1000×.
Results and discussion
Effect of pretreatment temperature and time
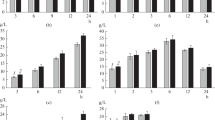
EFB applied in this work contains 76.7% carbohydrates in the form of cellulose and hemicellulose, 23.2% of lignin and minimal amount of ash, as determined in previous study (Thi and Lee 2019). The high content of carbohydrates which could be converted to reducing sugars via hydrolysis is a viable biomass feedstock in this study. To investigate the effect of pretreatment temperature and time on enzymatic hydrolysis, DES pretreatment with ChCl/LA ratio of 1:10 was carried out at temperatures of 80 °C, 100 °C, 120 °C and 140 °C for 5 h. Samples were collected during the course of pretreatment. The performance of pretreatment was based on reducing sugars yield obtained from enzymatic hydrolysis of pretreated EFB, as illustrated in Fig. 1. As shown in the figure, reducing sugars yield for pretreatment under temperature of 80 °C and 100 °C were higher than pretreatment conducted at higher temperatures of 120 °C and 140 °C. The low reducing sugars yield at high pretreatment temperatures was attributed to biomass degradation (Tang et al. 2017) and DES degradation (Chen et al. 2018; Skulcova et al. 2017). Despite DES has high thermal stability, when DES is to endure high temperature for a period of time, the thermal stability will be affected. According to Skulcova et al. (2017), 10% weight loss of solvent DES (ChCl/LA) was observed when the DES was heated at 120 °C for 2.5 h. This suggested less effective of DES in treating the EFB.
Besides, clumping of pretreated biomass was observed when pretreatment was conducted at high temperatures of 120 °C and 140 °C. According to Rodriguez et al. (2019), esterification of lactic acid and hydroxyl groups of choline chloride took place at elevated temperature. Hence, the occurrence of biomass clumping was suspected due to esterification reaction of the lactic acid with hydroxyl groups of the biomass when pretreatment was conducted at high temperatures. Clumping of biomass caused significant reduction in biomass surface area exposed for enzymatic hydrolysis, the reduced mass transfer during hydrolysis led to low conversion of biomass to reducing sugars (Du et al. 2017; Hodge et al. 2009).
As illustrated in Fig. 1, at temperature of 120 °C, reducing sugars yield increased when pretreatment time prolonged from 0.5 to 2 h. This ensured sufficient time is allocated for DES to interact with the cellulose of biomass and hence to break down the recalcitrance structure of the biomass (Li et al. 2017). However, further increment in the pretreatment time resulted in decrement of reducing sugars yield due to the increased of pretreatment severity resulting biomass degradation, DES degradation and clumping of biomass. At high temperature of 140 °C, it can be observed that the reducing sugars yield decreased with increasing pretreatment time. Despite high temperature and long pretreatment time promote better interaction between DES and biomass (Li et al. 2017; Zhang et al. 2016) the high degree of biomass and DES degradation along with reduction in surface area due to clumping, affected the conversion of biomass to reducing sugars.
A maximum reducing sugars yield of 51.1 ± 3.7% was achieved at 100 °C for 1 h. At these conditions, no clumping of pretreated biomass was observed. The most plausible reason for remarkable reducing sugars yield obtained could be due to well-balanced effects of cellulose crystallinity reduction with least biomass and DES degradation for enhanced enzymatic hydrolysis. However, when the pretreatment time was further prolonged, the reducing sugars yield decreased as the effect of biomass and DES degradation along with surface area reduction were greater than the positive interactive effect between DES and the biomass. The highly interactive effect of these parameters suggesting the importance of process parameters study and selection to ensure effective biomass pretreatment and enzymatic hydrolysis for sugars production.
Effect of solid-to-solvent ratio
The pretreatment temperature and time that produced the highest reducing sugars yield were further subjected to solid-to-solvent ratio study. Three different solid-to-solvent ratios of 1:5 (w/v), 1:10 (w/v) and 1:15 (w/v) were investigated and their results are plotted in Fig. 2. It is observed that reducing sugars yield increased when solid-to-solvent ratio increased from 1:5 (w/v) to 1:10 (w/v). The increased in DES amount led to better pretreatment with increasing digestibility of cellulose during enzymatic hydrolysis. This is because the amount of DES available for reaction with biomass increases with the increase in ratio. However, an optimum solid-to-solvent ratio is essential as a decrement in reducing sugars yield was observed when the solid-to-solvent ratio was further increased to 1:15 (w/v). Although DES assisted in biomass pretreatment, its presence in high concentration could lead to biomass degradation. In this study, solid-to-solvent ratio of 1:10 (w/v) was sufficient for high reducing sugars production while preventing biomass degradation.
Performance evaluation for DES pretreatment
Performance evaluation on DES pretreatment was conducted by replacing DES with other pretreatment medium. In this study, dilute acid (0.5 M dilute sulfuric acid), alkaline (0.5 M sodium hydroxide) and organosolv (60% v/v ethanol) pretreatments were selected due to their promising pretreatment performance (Huijgen et al. 2012; Thi and Lee 2019; Wang et al. 2012). Water was included as control for this study. All pretreatment processes were performed at 100 °C with 1:10 solid-to-solvent for 1 h. The reducing sugars yield obtained from enzymatic hydrolysis of pretreated EFB is shown in Fig. 3. Among all pretreatment solvents, DES exhibited the highest enzymatic hydrolysis efficiency with maximum reducing sugars yield of 51.1 ± 3.7%. This was due to effective delignification and cellulose disruption which will be discussed later in Sect. Structural characterization of pretreated EFBs. Alkaline pretreatment gave the second highest reducing sugars yield of 50.1 ± 2.5% where it is the most comparable pretreatment method in view of its effectiveness in lignin dissolution leading to high reducing sugars yield.
Both dilute acid and organosolv pretreatment had similar performance to control where they exhibited relatively low sugar yields as compared to DES pretreatment. Poor performance of dilute acid pretreatment was attributed to the loss of hemicellulose fractions from EFB residues and ineffective lignin removal. This observation will be discussed further in sugar loss analysis and structural characterization study. There are reports found that high temperature of 160 °C to 200 °C is needed for an effective organosolv pretreatment (Huijgen et al. 2012; Park et al. 2017). This explained the low reducing sugars yield attained for organosolv pretreatment in this study as the pretreatment was carried out at 100 °C. Therefore, DES pretreatment showed advantage of operating under milder condition with significantly enhanced enzymatic hydrolysis rate as compared to organosolv pretreatment. Despite the similar performance of DES and alkaline in pretreating EFB, DES pretreatment is preferred as no additional operational unit required for neutralization process and its environmentally benign properties (Brodeur et al. 2011; Loow et al. 2016). These collectively suggested the feasibility of DES in pretreating biomass.
Statistical analysis was included to investigate the significance level of pretreatment methods on reducing sugars yield. Null hypothesis H0 of no differences in different chemical pretreatment methods was performed to further verify on the significance of different chemical pretreatment methods on obtained reducing sugars yield. The experiments were run with five different chemical pretreatment methods with two replicates for each method. According to Table 1, F0 with value of 65.5 was to be compared to an appropriate upper-tail percentage point of F4,4 distribution. To use a fixed significance level approach, the value of α = 0.05 was selected. As F0.05,4,4 = 6.39, F0 > F0.05,4,4. Therefore, the null hypothesis H0 was rejected, and it can be concluded that pretreatment method significantly affects the reducing sugars yield.
Reducing sugars loss analysis
High sugars loss during pretreatment process is unfavourable as the reducing sugars yield obtained in enzymatic hydrolysis of pretreated biomass will be lesser. Therefore, supernatant obtained from DES pretreatment was subjected to reducing sugar loss analysis by measuring its sugars content in supernatant obtained from DES pretreatment. Result showed that there were no sugars detected in the supernatant. Similarly, there were no sugars loss for alkaline and organosolv pretreatments. However, low sugars loss was demonstrated in dilute acid pretreatment (0.1 ± 0.0 mg/ml). The presence of reducing sugars in the supernatant suggested the solubilisation of hemicellulose in dilute acid pretreatment. It had been reported in previous studies that the hemicellulose could be completely hydrolysed during dilute acid pretreatment, releasing monomeric sugars from the cell wall matrix into liquid supernatant depending on the pretreatment condition (Chen et al. 2010). Since the present work focuses on maximizing the conversion of EFB to reducing sugars through enzymatic hydrolysis of pretreated solid residue, retaining the carbohydrates including cellulose and hemicellulose during pretreatment is important.
Structural characterization of pretreated EFBs
FT-IR analysis was carried out to evaluate the changes in the functional groups of the biomass. The FT-IR spectra for DES, acid, alkaline, organosolv and water pretreatment is shown in Fig. 4. Difference on the transmittance was observed for untreated EFB with all pretreated EFBs. Generally, those bands that were mainly assigned to cellulose and lignin were the regions being affected the most. The band of 3333 cm−1 which assigned to O–H stretching intramolecular hydrogen bonds of cellulose (Isroi et al. 2012), and other bands that assigned to cellulose (2896 and 898 cm−1) showed more distinct peaks for pretreated EFBs compared to untreated EFB. This indicates that the cellulose content in the pretreated EFBs were richer as compared to untreated EFB. Among the pretreatment methods, DES gave the most distinct peaks, indicating the highest cellulose content in its pretreated biomass. The disappearance of 1512 cm−1 band, which assigned to stretching of aromatic ring in lignin (Isroi et al. 2012) in DES-pretreated EFB spectrum, suggested the ability of DES pretreatment in lignin removal.
For the case of alkaline pretreatment, noticeable changes can be observed for bands 1731 and 1239 cm−1. These bands attributed to the ester linkage of carboxylic group of the ferulic and ρ-coumaric acids of lignin (Sun et al. 2005). The absence of these two bands after alkaline pretreatment indicates the cleavage of lignin side chains, demonstrating the effectiveness of alkaline pretreatment in delignification to improve cellulose digestibility. In other pretreatment methods such as organosolv, dilute acid and water pretreatments, their pretreated biomass did not show as distinct changes from untreated EFB as compared to DES and alkaline pretreated biomass. This could further instill the outperformed of enzymatic hydrolysis for DES pretreated biomass as reported earlier in Sect. Performance evaluation for DES pretreatment. Besides demonstrating excellent performance in lignin extraction, DES pretreatment caused enrichment of cellulose content in pretreated biomass. These factors resulted in the high reducing sugars yield obtained for DES pretreatment. Thus, ChCl/LA based DES with the molar ratio of 1:10 proved to be a favorable alternative to other conventional pretreatment methods including dilute acid, alkaline and organosolv in pretreating biomass EFB.
Total crystallinity index (TCI) of untreated and pretreated EFBs
Crystallinity of the pretreated biomass could be used in evaluating the performance of the pretreatment. In this study, TCI values were used as an indicator of biomass crystallinity. Table 2 shows the TCI values of untreated EFB and EFB pretreated with various solvents. It can be observed that all pretreated biomass showed reduction in TCI values as compared to the untreated biomass. DES pretreated biomass exhibited the lowest TCI value of 0.926 further suggesting its effectiveness in disrupting the crystalline regions in the cellulose. Previous studies had reported that reduction in crystallinity resulted in a higher rate of bioconversion for the lignocelluloses (Laureano-Perez et al. 2005; Nelson and O’Connor 1964) as the cellulose are more accessible to enzymatic hydrolysis at lower degrees of crystallinity. Unlike DES pretreatment in which the solvent interacts with the hydrogen bonds of the cellulose and disrupts the cellulose structure, alkaline pretreatment process mainly disrupts the lignin structure of the lignocellulose (Chen et al. 2013; Yoshida et al. 2008) and this explained its higher TCI value. As both DES and alkaline pretreatments attained similar reducing sugars yield (as reported earlier in Sect. Performance evaluation for DES pretreatment), both the pretreated biomass showed different values of TCI suggesting that crystallinity alone may not necessarily give a good projection on the pretreatment efficacy. Other factors that affecting the pretreatment efficiency are degree of polymerization, specific surface area for enzyme accessibility, cellulose sheathing by hemicelluloses, lignin and acetyl content (Mood et al. 2013; Pan et al. 2006).
Morphology study of pretreated EFBs
SEM analysis was conducted to study on the effect of pretreatments on the morphology changes in EFB. Figure 5 shows SEM images of untreated and all pretreated EFBs. All pretreatment methods had made the EFBs more susceptible to enzymatic attacks by altering the structures and increasing the porosity of biomass. It can be observed from Fig. 5 that the untreated EFB shows relatively smooth surface without degradation whereas all the pretreated EFBs show porous surfaces where the fiber structures were altered and disrupted. DES, dilute acid and alkaline pretreatments gave more severe impact on the structures of biomass.
From Fig. 5b, it was evident that the DES pretreated EFB fiber structure appeared porous with disruptive surfaces as compared to the untreated EFB which appeared smooth and intact. The disruption of fibrils is essential in decreasing the recalcitrant nature of lignocellulosic biomass and also in increasing the cellulose accessibility by cellulase enzyme. In the case of alkaline pretreatment (Fig. 5d), disaggregation of the cell wall bundles can be observed. This structural change could be related to delignification that enhanced the separation of vascular bundles (Lima et al. 2013). Although dilute acid pretreatment caused high porosity and severe alteration in the biomass structure, the reducing sugars loss during the pretreatment resulted in the low reducing sugars yield obtained for enzymatic hydrolysis. As compared to other pretreatment methods, organosolv pretreated EFB consisted larger portion of smooth surface and lesser distortion can be observed, suggesting lesser cellulose can be attacked by the enzymes. This further clarified the results obtained at Sect. Performance evaluation for DES pretreatment where organosolv pretreatment at 100 °C for 1 h was not as effective as other pretreatment methods. Combined with the FT-IR analysis, the results showed that both DES and alkaline pretreatment are effective in altering the structure of biomass by breaking the hydrogen bonds between cellulose.
Conclusion
This work demonstrated that pretreatment of EFB using acid based ChCl/LA DES in molar ratio of 1:10 was affected by pretreatment temperature, time and solid-to-liquid ratio. A maximum reducing sugars yield of 51.1 ± 3.7% was obtained at 100 °C for 1 h pretreatment with the solid-to-solvent ratio of 1:10 (w/v). At these conditions, the effect of DES and biomass degradation as well as the reduction in surface area for enzymatic hydrolysis were well balanced for an effective pretreatment process. Among the pretreatment methods investigated, DES pretreatment gave the highest reducing sugars yield. The high reducing sugars yield is due to its effectiveness in hydrogen bonding disruption in cellulose and in delignification. In conclusion, DES could be a promising alternative pretreatment technique in view of its outstanding performance in enzymatic hydrolysis and other desirable characteristics such as non-volatility, non-flammability, biodegradability, biocompatibility and cost effective.
References
Barr CJ, Hanson BL, Click K, Perrotta G, Schall CA (2014) Influence of ionic-liquid incubation temperature on changes in cellulose structure, biomass composition, and enzymatic digestibility. Cellulose 21:973–982
Bensah EC, Mensah M (2013) Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovation. Int J Chem Eng 2013:1–21
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S (2011) Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res 2011:1–17
Chen W-H, Tu Y-J, Sheen HK (2010) Impact of dilute acid pretreatment on the structure of bagasse for bioethanol production. Int J Energy Res 34:265–274
Chen Y, Stevens MA, Zhu Y, Holmes J, Xu H (2013) Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol Biofuels 6:8
Chen W, Xue Z, Wang J, Jiang J, Zhao X, Mu T (2018) Investigation on the thermal stability of deep eutectic solvents. Acta Phys Chim Sin 34:904–911
Chen Y, Yu D, Chen W, Fu L, Mu T (2019) Water absorption by deep eutectic solvents. Phys Chem Chem Phys 21:2601–2610
Chiaramonti D, Prussi M, Ferrero S, Oriani L, Ottonello P, Torre P, Cherchi F (2012) Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 46:25–35
Coleman D, Gathergood N (2010) Biodegradation studies of ionic liquids. Chem Soc Rev 39:600–637
Dománska U, Bogel-Lukasik R (2005) Physicochemical properties and solubility of alkyl-(2-hydroxyethyl)-dimethylammonium bromide. J Phys Chem B 109:12124–12132
Dominguez de Maria P, Maugeri Z (2011) Ionic liquids in biotransformation: from proof-of-concept to emerging deep-eutectic-solvents. Curr Opin Chem Biol 15:220–225
Du J, Cao Y, Liu G, Zhao J, Li X, Qu Y (2017) Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour Technol 229:88–95
Earle MJ, Seddon KR (2009) Ionic liquids: green solvents for the future. Pure Appl Chem 72:1391–1398
García G, Aparicio S, Ullah R, Atilhan M (2015) Deep eutectic solvents: physicochemical properties and gas separation applications. Energy Fuels 29:2616–2644
Gorke JT, Srienc F, Kazlauskas RJ (2008) Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem Commun 10:1235–1237
Harmsen PFH, Huijgen WJJ, López LMB, Bakker RRC (2010) Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Wageningen UR—Food & Biobased Research, Wageningen
Hodge DB, Karim MN, Schell DJ, McMillan JD (2009) Model-based fed-batch for high solids enzymatic cellulose hydrolysis. Appl Biochem Biotechnol 152:88–107
Huijgen WJJ, Smit AT, de Wild PJ, den Uil H (2012) Fractionation of wheat straw by prehydrolysis, organosolv delignification and enzymatic hydrolysis for production of sugars and lignin. Bioresour Technol 114:389–398
Isroi Ishola MM, Millati R, Syamsiah S, Cahyanto MN, Niklasson C, Taherzadeh MJ (2012) Structural changes of oil palm empty fruit bunch (OPEFB) after fungal and phosphoric acid pretreatment. Molecules 17:14995–15002
Kabo GJ, Blokhin AV, Paulechka YU, Kabo AG, Shymanovich MP, Magee JW (2004) Thermodynamic properties of 1-butyl-3-methylimidazolium hexafluorophosphate in the condensed state. J Chem Eng Data 49:453–461
Kumar AK, Parikh BS, Pravakar M (2016) Natural deep eutectic solvent mediated pretreatment of rice straw: bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ Sci Pollut Res 23:9265–9275
Laureano-Perez L, Teymouri F, Alizadeh H, Dale BE (2005) Understanding factors that limit enzymatic hydrolysis of biomass. Appl Biochem Biotechnol 124:1081–1099
Lee KM, Ngoh GC, Chua ASM (2013) Process optimization and performance evaluation on sequential ionic liquid dissolution-solid acid saccharification of sago waste. Bioresour Technol 130:1–7
Lee KM, Ngoh GC, Chua ASM, Yoon LW, Ang TN, Lee M-G (2014) Comparison study of different ionic liquid pretreatments in maximizing total reducing sugars recovery. BioResources 9:1552–1564
Li T, Lyu G, Liu Y, Lou R, Lucia LA, Yang G, Chen J, Saeed HAM (2017) Deep eutectic solvents (DESs) for the isolation of willow lignin (Salix matsudana cv. Zhuliu). Int J Mol Sci 18:2266
Lima MA, Lavorente GB, da Silva HKP, Bragatto J, Rezende CA, Bernardinelli OD, Eduardo RD, Gomez LD, McQueen-Mason SJ, Labate CA, Polikarpov I (2013) Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: a potentially valuable source of fermentable sugars for biofuel production—part 1. Biotechnol Biofuels 6:75–92
Loow Y-L, Wu TY, Md. Jahim J, Mohammad AW, Teoh WH (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23:1491–1520
Loow Y-L, New EK, Yang GH, Ang LY, Foo LYW, Wu TY (2017) Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 24:3591–3618
Lynd LR (1996) Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment, and policy. Annu Rev Energy Environ 21:403–465
Mielenz JR (2001) Ethanol production from biomass: technology and commercialization status. Curr Opin Microbiol 4:324–329
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J Appl Polym Sci 8(1964):1325–1341
Pan X, Xie D, Gilkes N, Gregg DJ, Saddler JN (2005) Strategies to enhance the enzymatic hydrolysis of pretreated softwood with high residual lignin content. Appl Biochem Biotechnol 124:1069
Pan X, Gilkes N, Saddler JN (2006) Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 60:398–401
Park YC, Kim TH, Kim JS (2017) Effect of organosolv pretreatment on mechanically pretreated biomass by use of concentrated ethanol as the solvent. Biotechnol Bioprocess Eng 22:431–439
Paulechka YU, Kabo GJ, Blokhin AV, Vydrov OA, Magee JW, Frenkel M (2003) Thermodynamic properties of 1-butyl-3-methylimidazolium hexafluorophosphate in the ideal gas state. J Chem Eng Data 48:457–462
Procentese A, Raganati F, Olivieri G, Russo ME, Rehmann L, Marzocchella A (2018) Deep eutectic solvents pretreatment of agro-industrial food waste. Biotechnol Biofuels 11:37
Quijano G, Couvert A, Amrane A (2010) Ionic liquids: applications and future trends in bioreactor technology. Bioresour Technol 101:8923–8930
Rodriguez NR, van den Bruinhorst A, Kollau LJBM, Kroon MC, Binnemans K (2019) Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustain Chem Eng 7:11521–11528
Skulcova A, Majova V, Haz A, Kreps F, Russ A, Jablonsky M (2017) Long-term isothermal stability of deep eutectic solvents based on choline chloride with malonic or lactic or tartaric acid. Int J Sci Eng Res 8(7):2249–2252
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Sudiyani Y, Styarini D, Triwahyuni E, Sudiyarmanto Sembiring KC, Aristiawan Y, Abimanyu H, Han MH (2013) Utilization of biomass waste empty fruit bunch fiber of palm oil for bioethanol production using pilot-scale unit. Energy Procedia 32:31–38
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Sun XF, Xu F, Sun RC, Fowler P, Baird MS (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res 340:97–106
Tang X, Zuo M, Li Z, Liu H, Xiong C, Zheng X, Sun Y, Hu L, Liu S, Lei T, Lin L (2017) Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. Chemsuschem 10:2696–2706
Thi S, Lee KM (2019) Comparison of deep eutectic solvents (DES) on pretreatment of oil palm empty fruit bunch (OPEFB): cellulose digestibility, structural and morphology changes. Bioresour Technol 282:525–529
Wang Z, Li R, Xu J, Marita JM, Hatfield RD, Qu R, Cheng JJ (2012) Sodium hydroxide pretreatment of genetically modified switchgrass for improved enzymatic release of sugars. Bioresour Technol 110:364–370
Weil J, Westgate P, Kohlmann K, Ladisch MR (1994) Cellulose pretreatments of lignocellulosic substrates. Enzyme Microb Tehnol 16:1002–1004
Xu G-C, Ding J-C, Han R-Z, Dong J-J, Ni Y (2016) Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour Technol 203:364–369
Yoshida M, Liu Y, Uchida S, Kawarada K, Ukagami Y, Ichinose H, Kaneko S, Fukuda K (2008) Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinesis to monosaccharides. Biosci Biotechnol Biochem 72:805–810
Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F (2012) Deep eutectic solvents: synthesis, properties and applications. Chem Soc Rev 41:7108–7146
Zhang C-W, Xia S-Q, Ma P-S (2016) Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour Technol 219:1–5
Zheng Y, Pan Z, Zhang R (2009) Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol Eng 2:51–68
Acknowledgments
This work was supported by the UCSI University Pioneer Scientist Incentive Fund (Grant Number: Proj-In-FETBE-043).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sai, Y.W., Lee, K.M. Enhanced cellulase accessibility using acid-based deep eutectic solvent in pretreatment of empty fruit bunches. Cellulose 26, 9517–9528 (2019). https://doi.org/10.1007/s10570-019-02770-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02770-w