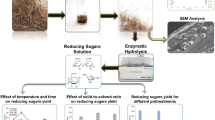
Abstract
This chapter discusses the treatment of palm oil empty fruit bunch using ionic liquids (ILs) pretreatment. By mixing IL and cellulase enzyme (IL-E) in a single pot, the empty fruit bunch (EFB) was pretreated with simultaneous fermentation. Choline acetate [Ch][Ac], which has excellent biological compatibility and renewability, has been used for pretreatment. Chemical analysis, electron scanning microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR) were used to characterize the EFB and its hydrolysate. After 24 and 48 h of enzymatic hydrolysis, sugar yield improved from 0.058 g/g EFB in the crude (untreated) sample to 0.283 and 0.62 06 g/g in the IL-E phase. EFB hydrolysate demonstrates suitability for the production of ethanol (EtOH) with a yield of 0.275 g EtOH/g EFB in the presence of [Ch][Ac] without additional nutrients, compared to the low yield without IL pretreatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
The palm oil industry produces noteworthy amounts of lignocellulosic palm oil biomass (OPB). Approximately 21.625 tonnes of biomass per year can be generated per hectare of oil palm plantation. Chew and Bhatia (2008) stated that 50.31% (fronds) and 20.44% (EFB) is reported to have the highest levels of cellulose. The key components of oil palm EFB (OPEFB) are lignin (17.6%) and hollow cellulose (82.45%). OPEFB is high in carbohydrate content which can be derived for producing mannose, ethanol, animal feed, and xylose as it is known to be a cheap source of carbohydrates (Ishola et al., 2014). Thanks to their composition, these agro-industrial substances are alternative tools for the development of biofuels and can be used for the production of sugar and the bioconversion of different forms of products (Zainan et al., 2013). While cellulose is the most important component of lignocellulosic substances, it is covered by hemicellulose and lignin (Laureano-Perez et al., 2005), which complicates the process of hydrolysis using cellulases. As a result, the pretreatment of lignocellulose for hydrolysis is the most critical stage in the effective use of biomass (Hahn-Hägerdal et al., 2006).
ILs have been implemented as useful solvents for the pretreatment of lignocellulose, enabling hydrogen attachments to be disrupted and allowing molecules’ exposure to cellulase hydrolysis. Despite this, ILs can induce a loss of cellulase function, which is controlled by the analysis to ensure that cellulase can tolerate the effects of ILs. As reported by Wang and colleagues (Wang et al., 2011), who inspected ILs with yellow polar biomass saccharification at 15% [EMIM]Ac, some commercial cellulases were active in a few ILs. Switchgrass treatment was also conveyed in 10–20% of [EMIM]Ac before enzymatic hydrolysis (Shi et al., 2013). Moreover, in order to detect IL-enzyme consistency in cholinium-based ILs, several researchers have researched cellulase stability (Ninomiya et al., 2015a). On the other hand, single-pot hydrolysis is an interesting bioethanol conversion process that combines lignocellulosic substance and IL treatment. Simple in situ lignocellulose conversion could result from recovery, which could help develop an integrated ethanol production system.
Researchers have used a number of methods to extract EFB sugar, including enzymatic treatment, sodium hydroxide and steam (Choi et al., 2013), high temperature diluted sulphuric acid (Chong et al., 2013) and ILs treatment (Ninomiya et al., 2015a). Treatment with acid and alkali, on the other hand, induces the release of HMF and furfural, which are both toxic and hinder microbial growth. Many ILs are versatile substances that create less by-products while preserving enzyme stability and allowing microorganisms to grow naturally (Reddy, 2015).
Enzymatic hydrolysis precedes the extraction of sugar from lignocellulose by a multiple-step process that includes cellulose regeneration by washing, according to the majority of previous research. For instance, Nothofagus pumilio (Lienqueo et al., 2016), rice straw (Poornejad et al., 2014), bagasse (Ninomiya et al., 2015b), and Eucalyptus globulus Labill are fermented to generate ethanol from the hydrolysate. This research, however, focused on the synthesis of IL and cellulase in a single vessel by using EFB. In addition, a detailed analysis was conducted before and after hydrolysis to assess the hydrolysate’s suitability for yeast fermentation. Predictably, this strategy will provide a clear understanding of the immediate step effect of IL-cellulase (IL-E) on the conversion of lignocellulose into sugar and thus into ethanol.
3.2 A Brief Perspective of Ionic Liquid
Scientists working in many different fields of study have come to recognize the peculiar characteristics of ILs over the past decade and to acknowledge the promise of these new materials. They are non-toxic and reusable replacement to volatile organic compounds (VOCs), which attract researchers and professional interests as potential pretreatment solvents for lignocellulosic biomass prior to further processing (Moniruzzaman & Goto, 2018). The ability of ammonium salts to break down cellulose was first acknowledged in 1934. The use of ILs for biomass production (which typically involves cellulose) is now a well-established and well-researched field (Graenacher, 1934; Macfarlane et al., 2017).
Using ILs as solvents or co-solvents can solve substrates’ low solubility and materials during wood delignification in aqueous solutions. This is primarily due to enzyme entry issues within concrete substrates and low solubility of substrates and materials, such as lignin, which dignifies slowly in aqueous systems (Sousa et al., 2009). Unfortunately, some ILs, such as hydrophilic ILs can have adverse effects on enzyme structure which can cause the deactivation of the enzyme when used for enzyme delignification. By increasing the solubility of substrates and materials, the overall process efficacy can be increased. Therefore, such an effect can be balanced. Prior to enzymatic hydrolysis and delignification, it is widely acknowledged that ILs can be used to successfully pretreat lignocellulosic materials (Elgharbawy et al., 2016a).
3.2.1 Pretreatment Process for Lignocellulosic Biomass
Pretreatment, enzyme hydrolysis, fermentation and ethanol separation are the four steps involved in converting lignocellulose into bioethanol. To effectively extract hemicellulose and lignin, inexpensive approach that improves cellulose hydrolysis is used as the first step in the pretreatment process. Pretreatment is the process of modifying biomass in order to speed up and increase the yields of cellulose and hemicellulose enzymatic hydrolysis. This involves removing lignin, which increases surface area while decreasing cellulose crystallinity. In contrast to pure lignin, extracting natural lignin in wood is more difficult owing to its complex structure and strong intramolecular reactions with lignocellulose. Due to the presence of strong covalent bonds, it is more vulnerable to breakdown than other lignocellulosic elements. ILs are effective in treating hemicellulose and lignin, as the crystallinity of cellulose is decreased (Elgharbawy et al., 2016b). Sun et al. (2009) observed that a wide variety of substrates, like softwood and hardwood, can be dissolved using ILs.
Enzyme adsorption into cellulose particles and enzyme-substratum complexes (ES) aggregation that are further correlated with several enzymes and substrate factors are basically two stages of cellulose enzyme-catalyzed hydrolysis (Chandra et al., 2012). Compound inhibition (cellobiose and D-glucose), adsorption, synergy and thermal stability are factors to consider when it comes to enzymes. Meanwhile, external and internal cellulose surface proximity, hemicellulose and lignin, degree of polymerisation (DP), and cellulose crystallinity are substrate related variables (Zhao et al., 2009). Zhao et al. (2009) hypothesized that cellulose is more accessible to regenerated cellulose since IL-regenerated cellulose is less crystalline compare to untreated cellulose and prevents quicker hydrolysis of the enzyme. Elgharbawy et al. (2016c) stated that during the fermentation process, lignocellulose biomass, cellulose, and hemicellulose are predominantly hydrolyzed to sugar monomers and eventually converted to alcohol, hydrogen, or methane.
3.2.2 Ionic Liquid–Cellulase Compatible Systems
Multi-stage pretreatment of lignocellulosic materials with different ILs, accompanied by IL removal and recovery of cellulose, followed by hydrolysis process using enzymes was highlighted in several reports (Bian et al., 2014; Elgharbawy et al., 2016b). Researchers have also studied hydrolysis in single-stage processing of bioethanol production that combines lignocellulosic substances pretreatment and enzymatic hydrolysis.
This is because it was possible to customize ILs to adapt to a specific reaction. In addition, the thermal tolerance of the enzymes in ILs enables a reaction to being carried out at higher temperatures. Researchers tend to regenerate IL-biomass cellulose before enzymatic hydrolysis to mitigate the adverse effect of ILs on enzymatic hydrolysis (Elgharbawy et al., 2016c; Moniruzzaman et al., 2010).
Elgharbawy and colleagues (Elgharbawy et al., 2016a) suggest that combining ILs with cellulase in a system necessarily requires proper component selection as well as reaction control conditions. The following are typical characteristics of the enzyme with ILs (IL-E):
-
i.
Using a broad molecular structure to reduce the basic nature and nucleophilicity of H-bonding.
-
ii.
It has a wide range of ether and/or hydroxyl groups to improve water affinity, H-bond basicity for mild enzyme behaviors and enzyme viscosity (Moniruzzaman et al., 2010).
3.3 Objective of Experiment
The primary aim is to design a cellulase-compatible IL system for the development of bioethanol from EFB in a single reactor. Therefore, the primary aim of the suggested study is as follows:
-
1.
To select a cellulase compatible with ionic liquid (IL-E), which can be used for single-step EFB hydrolysis.
-
2.
To determine the effect of IL-E on the characteristics of the substrate on untreated and treated EFB.
-
3.
To maximize factors that affect the E-IL mechanism and biomass dissolution in a single stage.
-
4.
To enhance bioethanol fermentation by mixing fermentable sugars derived from IL-E with yeast (Saccharomyces cerevisiae).
-
5.
To investigate the kinetics of EFB saccharification and bioethanol production in the IL-E system.
3.5 Methodology
3.5.1 Production of PKC-Cel
As mentioned earlier in our study, locally produced cellulase was obtained as a PKC fermentation product (Elgharbawy et al., 2016a). For the primary medium used for processing, the cellulase enzyme was labeled ‘PKC-Cel’. The base medium was a solid-state fermentation of palm kernel cake. Fermentation occurred after inoculating samples of Trichoderma reesei (RUTC30) suspension for seven days at room temperature (30.0 ± 2 °C). The crude enzyme was extracted with a sodium citrate buffer (pH 4.8 ± 0.2) before centrifuge, micro-filtration, and ultra-filtration. The activity of the retentate-containing enzyme was determined. Whatman No. 1 was used as a substrate material to monitor the hydrolysis of filter paper units (FPU). One unit of FPU was estimated to release 1 mol per mL enzyme per minute of glucose. A glucose standard curve was used to calculate the amount of glucose produced. Sodium carboxymethyl cellulose (CMC) has been studied as a substrate for endo-b-1,4-D-glucanase (Salvador et al., 2010). As Ghose (1987) states, one unit of CMCase is defined as the quantity of enzyme produced by 1 mol of glucose per minute. On both filter paper and CMCase, the assay was performed.
3.5.2 Enzymatic Hydrolysis of EFB
The ground EFB was seived into three different particle dimensions, as suggested by the arrangement (200, 450, and 600 µm) (Retsch AS 300). For EFB pretreatment, separate biomass loading percentages of volatile solids (percent VS) were used by ignition the sample at 550 °C for 30 min. To facilitate the pretreatment, 1.875 g of EFB were weighted in a glass vial, and 5 g of IL was added, followed by a 60 min incubation at 75 °C. In order to achieve a 10% IL buffer solution (v/v), the sample was mixed with pH 4.8 of sodium citrate buffer after incubation before cooling to room temperature. After that, 45 FPU/g EFB of ultra-filtered (UF) cellulase was prepared. The DNS assay was used to measure the released sugar concentration after 48 h of enzyme reaction. Elgharbawy et al. (2016a) and (2016b) have described methods and techniques in prior studies.
3.5.3 Characterization of the Native and Treated EFB for IL-E Evaluation
The EFB’s chemical constituents were identified with some modifications in accordance with the criteria mentioned in the NREL test techniques, as published by Ninomiya et al. (2015a).
3.5.4 Structural Carbohydrates and Sugar Determination
By mixing 2 mL of 72% (v/v) H2SO4 aqueous solution with 0.1 g of the sample for 2 h at room temperature, then mixed with 75 mL of water and autoclaving around 121 °C for 15 min, hemicellulose and lignin contents were determined. The solid residue was used to calculate the weight at 100 °C after 12 h of drying to determine acid-insoluble lignin (AIL) after filtering the acid-diluted hydrolysate. The amount of acid-soluble lignin (ASL) was calculated at 205 nm with a UV absorbance of 110 L/g/cm.
The total of AIL and ASL was calculated as the sum of all lignin (Ninomiya et al., 2015c). As seen in Eq (3.1), the quantity of hemicellulose was calculated in terms of the content of xylose:
By using monomeric sugar concentration such as anhydrous xylose and arabinose correction factors (0.88 or 132/150), the ratios obtained from the respective polymeric sugars can be determined (Nieves et al., 2016). The quality of untreated and refined EFB cellulose was measured using standard microcrystalline cellulose using the anthrone technique (Updegraff, 1969). Using phenol-sulfuric acid analysis, the quantity of biomass carbohydrates was calculated (Dubois et al., 1956).
The total soluble sugar in the solution was evaluated by dissolving 100 mg of EFB in 100 mL of distilled water, and the total free sugar was collected and filtered using filter paper (Whatman No. 1).
The acquired filtrate was analyzed using the phenol-sulfuric technique. Following the report of Salvador et al. (2010), total glucose, galactose, mannose, and arabinose reduction in sugar (TRS) were determined. A COSMOSIL SUGAR-D column (4.6 mm l. D. × 250 mm) for high-performance liquid chromatography (HPLC) comprised of high purity porous spherical silica gel was used to analyze monosaccharides and oligosaccharides. Primary and tertiary amines are the main components of the stationary phase. In hydrolyzed samples, arabinose, glucose, mannose, galactose, and xylose occur on the sugar solution. Syringe filter nylon (0.2 µm) was used to purify each sample (1.0 mL) into a transparent HPLC glass vial. Deionized water was used as a solvent to make four different standards of each sugar. A syringe filter was used to transfer standard dilutions to HPLC vials. In the Waters 600 (Hampton, USA) system, HPLC was used with a refractive index detector (RID) fitted with an acetonitrile mobile phase with deionized water ratio of 70:30, 75:25, and 80:20 in a set of 1.0 mL/min flow rate at 30 °C. The mobile phase was passed through a membrane filter before running the HPLC to prevent column blockage.
The concentration of acetic acid (CH3COOH) was calculated using an Agilent 1200 HPLC process with RID and column of REZEX ROA (Phenomenex, USA) (California, USA). With isocratic elution, sulfuric acid (5 mmol/L) was used as a mobile step at a 0.6 mL/min flow rate at ambient temperature. Using variety of acetic acid levels with 20 μL of sample injection, standard curves were produced. To maximize the main column’s longevity, an identical packed guard column was used. Institute of Systems Biology (INBIOSIS) of Universiti Kebangsaan Malaysia (UKM), situated in Selangor (Bangi), Malaysia, kindly gathered the results of the research.
3.5.4.1 Morphological Structural Changes
The samples of treated and native (untreated) were characterized using SEM. Both samples were stored at −80 °C after being dried using a freeze dryer (LABCONCO). Dry samples were used for observation using the Hitachi SU1510 SEM Back-Scatter Detector (BSE) Mode at 500X, 1000X and 5000X magnifications for both treated and untreated samples. The analysis was carried out at UKM-MTDC Smart Technology Center (Quasi-S Sdn. Bhd), Bangi, Malaysia.
3.5.4.2 Fourier Transform Infrared Spectroscopy (FTIR)
The hydrolysate, IL-treated, and raw EFB were measured using FTIR after 72 h frozen dry in the range of 4000–5000 cm−1 at 4 cm−1 resolution with 16 scans. In the absorbance mode, the spectral results were reported as a function of the wavenumber.
3.5.4.3 Swelling Capacity
For one hour, a dry sample (0.1 g) was put in a non-woven fiber bag and immersed in distilled water to calculate pretreated and native EFB swelling capacity (Noori & Karimi, 2016). The swelling capacity was estimated by calculating the different dry weight (W1) and swollen weight (W2) materials using Eq. (3.2):
3.5.4.4 Enzyme Adsorption and Desorption
In 15 mL citrate buffered centrifuge tubes, 400 FPU/g cellulase was diluted up to 5.0 mL with 0.05 g of substrate samples (native or pretreated) to 50 mM in pH 4.8. For two hours, all tubes were mixed at 100 rpm and incubated around 4 °C. After centrifugtion, the Bradford assay was used to measure the supernatant protein content of unabsorbed cellulase (15 min, 4000 rpm, 4 °C). Prepared samples were diluted at 4 °C with a 5 mL citrate buffer for enzyme desorption estimation and incubated for 2 h at 4 °C for determination of protein content after centrifugation. Equation 3.3 show the calculation of total desorption of cellulase percentage:
where after 2 h, C is the total amount of adsorbed cellulase to solid pretreated (mg/g), and D is the sum of unadsorbed proteins (mg/g) (Noori & Karimi, 2016).
3.5.4.5 Crystallinity Index
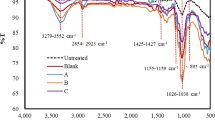
The effect of EFB structure pretreatment was determined using FTIR adsorption analysis. The 1430 cm−1 absorption band represents hydrolysis-resistant cellulose I, while the 896 cm−1 band is easily hydrolyzed by cellulose II (Kljun et al., 2011).
3.5.4.6 Determination of Total Nitrogen (TN) and Total Phosphorus (TP)
After hydrolysis, the overall nitrogen content of the samples was determined using HACH technique 10071 (Test ‘N Tube Vials). The molybdovanadate reagent was employed to calculate phosphorus in the samples.
3.5.4.7 Determination of Minerals
Referred to the UNIPEQ-MTDC Technology Centre, UKM (Ref: ULUKM/1787/16), in-house Method (No. STP/Chem/A13 Microwave Digestion-AAS) was used to evaluate the content of calcium (Ca), sodium (Na), copper (Cu) and potassium (K). By using atomic absorption spectrophotometry (AAS), zinc (Zn), iron (Fe) and manganese (Mn) were investigated. Including the standards, 0.2% nitric acid was used to prepare all samples.
3.5.4.8 Determination of Total Phenolic Compounds (TPC)
The MultiskanTM GO Microplate Spectrophotometer is used to determine total phenolic compounds (TPC) in specimens at 760 nm by using the Folin-Ciocalteu method (Wolfe et al., 2003). In mg per 100 g of sample, the standard curve of gallic acid solutions prepared was used to measure the absorption of gallic acid. For each sample, triplicate assay samples were analyzed.
3.5.4.9 Determination of Furfural
To assess the furfural content, the phenylhydrazine solution technique was used and the absorbance was recorded at 446 nm (Zarei, 2009). The exact methods were followed for the standard preparation, except the sample solution was substituted with various concentrations of furfural solution (0.5, 1.5, and 2.0 g/L).
3.5.4.10 Fermentation to Ethanol
On the autoclaved hydrolysate (100 mL), fermentation was performed in a 250 mL Erlenmeyer flask. After chilling, 2.5 mL of prepared inoculum (Saccharomyces cerevisiae) was inoculated, tightly covered with a cotton lid, and incubated at 30 °C at 150 rpm. After 72 h, the broth was centrifuged at 8000 rpm for 15 min, and the EtOH concentration was evaluated using chromic acid procedure (Caputi et al., 1968) and then subjected to GC/MS. In the GC/MS, a DB-WAX column (122–7032) was used, as well as an Agilent CTC-PAL autosampler (7890A GC and 5975C MS) fitted with a headspace sampling module capillary column (Agilent Technologies, Wokingham, UK) using 25 cm/s helium gas carrier at 150 °C. Using the DNS assay, the average sugar consumption was monitored for 84 h. To extract ILs from EtOH, distillation was used, where EtOH is obtained at the receiver vessel.
3.6 Results and Discussion
3.6.1 Characterization of the Native (Untreated) and Treated Substrate to Evaluate the Effect of IL-E
3.6.1.1 Pretreatment and Saccharification of EFB
Table 3.4 displays the effects of a 24-hour IL hydrolysis to determine which solvent can achieve the maximum level of sugar. To be subjected to EFB hydrolysis, [Ch][Ac] and [Ch][Bu] are used in sample pretreatment. Conversion variations between pretreated and native EFB demonstrated that the structure has been weakened by IL pretreatment and has increased the enzyme’s sensitivity towards the substrate.
During pretreatment, [Ch][Ac] and [Ch][Bu] were found to be completely aligned with PKC-Cel in this study, as confirmed by a decrease in lignin level. Likewise, with the presence of ILs in the environment, there was a rise in saccharification. An amount of 0.14 ± 0.05 g of glucose was obtained with [Ch][Ac] from 1 g of EFB. When [Ch][Bu] was used, the production was slightly lower.
3.6.1.2 Changes in Cellulose, Hemicellulose, and Lignin
The hydrolysis-accessible native EFB cellulose content was estimated around 2.1 ± 1.0% (w/w) and when ILs were applied, the cellulose content increased. Table 3.4 reveals that [Ch][Ac] has the highest cellulose, with 32.6 ± 1.5% (w/w) (accessible for hydrolysis after 24 h). Without pretreatment with ILs, improvements in hemicellulose tended to be null.
The overall native EFB lignin content was estimated at 23.6 ± 0.6% (w/w) with a further substantial decrease of 16.81 ± 0.35% (w/w) in [Ch][Ac]. [Ch][Ac] was 31.7 ± 0.1 g/L, while the total native EFB hydrolyzed sugar was 10.02 ± 0.1 g/L. For [Ch][Ac] after hydrolysis, the maximum detectable glucose concentration was 16.57 0.5 g/L in specific case.
3.6.1.3 Scanning Electron Microscopy (SEM) for Morphological Structural Changes
SEM was used to investigate the structure and morphology. Figures 3.1 and 3.2 represent EFB native samples and those [Ch][Ac] and [Ch][Bu] treated micrograph, respectively. The study of the pretreated samples showed slight variations, while sample structure in both IL yielded identical findings. At first glance, a distorted surface was noticeable for both ILs, which seemed to be robust and unbending in contrast to the native EFB. Cellulose strands were detected after an hour by examining the IL-pretreated samples. Figure 3.1 displays comparable EFB images of [Ch][Bu] and PKC-Cel that is equivalent to [Ch][Ac] images. Both ILs had distinct native EFB morphologies after IL-cellulase integration and pretreatment. Differences have been predicted to be observed due to the deterioration of lignin and decreased crystallinity of cellulose that interrupts the tissue system. Nonetheless, this has been discovered in both FTIR study and biomass chemical analysis. Figures 3.1b and 3.2b demonstrate how the surface is extended and disrupted in contrast to the native EFB. The relationship with the integrated framework became apparent. The native EFB surface was rough and solid, Figs. 3.1a and 3.1a but it gradually began to be damaged in the presence of the IL.
Morphology and characterization of surface modification confirmed that there was a compact immaterial pore structure in EFB-pretreated samples. As seen in Figs. 3.1c and 3.2c, the pretreated EFB had more open structures.
3.6.1.4 Fourier Transform Infrared Spectroscopy (FTIR)
Figure 3.3 displays both [Ch][Ac] and [Ch][Bu] spectrum of FTIR on native, hydrolyzed and IL-pretreated specimens. In [Ch][Ac] pretreated samples, the absorbance bands have a lower intensity range of approximately 1505–1557 cm−1. 1503–1556 cm−1 was the result observed in [Ch][Bu]. Furthermore, pretreatment with ILs triggers the disruption of EFB lignin. The presence of cellulose was found when the absorbance increased between 1430 and 896 cm−1 in contrast to native EFB (Nomanbhay et al., 2013).

(Reprinted by permission from Springer Nature: Springer, 3 Biotech, Elgharbawy et al., 2018)
FTIR spectra of a native (untreated) EFB at the absorbance bands of 500–4000 cm−1, b[Cho]Bu-pretreated EFB in the enzymatic system (IL-E), c[Cho]OAc-pretreated EFB in the enzymatic system (IL-E)
Changes in [Ch][Ac] EFB treated sample were also observed at 1720–1740 cm−1 and a comparable range was observed in [Ch][Bu] EFB untreated sample. The decreasing spectra of sugar peaks ranging from 1707 to 1584 cm−1 and 2947 to 3633 cm−1 were integrated with PKC-Cel after [Ch][Ac] and [Ch][Bu] pretreatment. Cellulose II recorded the band at 895 cm−1, which is highly acquiescent to hydrolysis.
3.6.1.5 Chemical Analysis of the Pretreated‑Hydrolyzed EFB
Chemical analysis was also used after hydrolysis to gather data on the output of the sample, in addition to FTIR and SEM analysis. As shown in Table 3.5, carbon, nitrogen sources and minerals were included in the hydrolysate produced.
The average phosphorus in EFB was 11.8 mg/g contributing total weight of EFB around 0.98%. In this analysis, the metal ions Mg2+ (0.103 mg/L), Mn2+ (0.171 mg/L) and Zn2+ (0.183 mg/L) was obtained with 0.3 g/L of nitrogen content. Baharuddin et al. (2011) calculated the dry weight of EFB cellulose, lignin and hemicellulose at 50.3, 18 and 26.1%, respectively. For total nitrogen, phosphorus and potassium, the dry weight of the EFB were 1, 0.6 and 2.3%, respectively.
Producing 120 g/L of EtOH, Mg2+ (0.05 g/L), Mn2+ (0.04 g/L) and Zn2+ (0.01 g/L) were found as best EtOH supplements for sweet sorghum juice (Deesuth et al., 2012). The findings prove that they are consistent with the reported evidence.
According to a relevant study, furfural acid (2.1 ± 0.5 g/L) and CH3COOH (0.62 ± 0.05 g/L) did not affect EtOH production (Ylitervo et al., 2013). The peak CH3COOH concentration was 3.0 g/L, which comes within an adequate range for metabolism inhibition (Palmqvist & Hahn-Hägerdal, 2000). The total phenolic compounds (0.36 0.05 mg/g) did not fall in the fermentation suppression range. Free amino nitrogen may be replaced by an internal source of nitrogen through hydrolysis. The study showed that total nitrogen (TN) was found to be 0.237 g/L (1.27% w/w), while total protein (TP) is 0.3 g/L (2.12% w/w). Despite the low concentrations, the information reported on EFB is still comparable, showing that dry protein weight was approximately 2.3 ± 0.1%.
In Fig. 3.4, glucose (45.47%) was the most prevalent component in the mixture followed by xylose (19.65%) after investigated by HPLC in the hydrolysate monosaccharides of sugar portion. Low sugars presented in the hydrolysate included mannose (0.45%), galactose (0.16%) and arabinose (0.087%). Galactose and arabinose contributed to the lowest levels attained in this term.

(Reprinted by permission from Springer Nature: Springer, 3 Biotech, Elgharbawy et al., 2018)
Detailed analysis of sugar components for raw EFB and its hydrolysate obtained after treatment in the IL-E system. Pretreatment time and temperature: 60 min and 75 °C, hydrolysis time: 48 h, IL concentration 10%, PKC-Cel loading 45 FPU/g EFB, and temperature 50 °C
EFB generated 22.0 ± 0.4% (w/w) lignin insoluble, 24.2 ± 0.5% (w/w) MGX (mannan, galactan and xylan), 36.6 ± 0.6% (w/w) glucan and 1.2 ± 0.1% (w/w) arabin when tested for carbohydrate content. The remaining was produced in the form of crude protein and extractive (Kim et al., 2015). The findings of further research are compatible with the most recent published studies.
3.6.1.6 Swelling Capacity and Crystallinity Index (CrI)
As seen in Table 3.6, Crystallinity (CrI), [Ch][Bu] was more effective than [Ch][Ac] at reducing cellulose crystallinity, decreasing the CrI value by integrating both IL and PKC-Cel into a single framework. [Ch][Bu], in addition to [Ch][Ac], however has a complex thermal expansion and standard enthalpy. This indicates [Ch][Ac] has higher molecular energy (Muhammad et al., 2012).
Figure 3.5 demonstrate maximum cellulase adsorption was detected in EFB, followed by (79.3 ± 2.8) g/L [Ch][Ac] and (81.4 ± 3.3) g/L [Ch][Bu]. The IL pretreatment can soften the compounds and extract them from EFB. In contrast to the crude samples, the desorption rate of cellulase increased in the IL-E treated samples.
3.6.1.7 Fermentation of Hydrolysate to Ethanol
After 48 h of 0.62 ± 0.06 g/g EFB hydrolysis, the average content of sugar is estimated around 70–75 g/L. For fermentation media, EtOH production was used for hydrolysate. Figure 3.6 demonstrates the profile concentration of ethanol and sugar over 84 h duration of the fermentation period. When a higher number of cells produces, so does the amount of ethanol. Production of EtOH hit its peak at 72 h and then decreased after 72 h. After 48 h, a sudden decline in sugar concentration was detected, while EtOH had begun to rise drastically. The EtOH yield was obtained around 0.4178 g/L/h. As observed in the recent study, the production of EtOH was 0.275 g/g of EFB. Without additional supplement after 72 h, 34.58 g/L of EtOH output was obtained.
The EtOH yield observed was 0.127 g EtOH/g EFB for the raw EFB, while 0.275 g EtOH/g EFB was produced by the IL-E system; 87.94% of the theoretical output. In different pretreatment conditions, the findings achieved in the present research were contrasted with the reported data.
For pretreatment with Eucalyptus, [EMIM][Ac] was used, which was then given 0.172 g EtOH/g biomass after Novo-Celluclast hydrolyzed (Lienqueo et al., 2016). Without supplementation, results showed the ability to transform EFB hydrolysate to EtOH.
3.6.2 Discussion
3.6.2.1 Pretreatment and Saccharification of EFB
The ability to recycle and immobilize IL during the reaction and the use of reaction in a single vessel can help commercialize this approach in the future, despite the high IL’s high ratio to biomass in this lab-scale study. The findings are similar to research done without any ILs on biomass hydrolysis. Pretreated weed biomass enzymatic hydrolysis was achieved under continuous solid loading with mechanical agitation and sonication. Sonication has been shown to accelerate weed biomass hydrolysis. However, lowering sugar levels to 35–40 g/L required a total of 10 h of treatment (Borah et al., 2016). Pretreated P. hysterophorus biomass delignified by sulphuric acid was enzymatically hydrolyzed, then achieved approximately 400 mg of sugar/g of substrate. Considering the shorter time and enzyme intake, the findings obtained in the current study (EFB 600 mg/g) were higher in both cases (Singh et al., 2015).
Saccharification was supported in part by the distribution of lignin and hemicellulose. ILs anions act as hydrogen bond acceptors by interfering and degrading the hydroxyl group’s structure in cellulose. Meanwhile, the cations interact with lignin and also with the interactions of π-π with hydrogen bonding (Asakawa et al., 2015). The inhibitory effect of PKC-Cel between lignocellulose and component of IL should be minimized as a result of these interactions. Zhao (2016) investigated whether hydrate cosmotropic occurs when enzymes are inactivated due to a lack of water or anions borderline (such as chloride and acetate) in high H-bond basic ILs. As a result, in low-water ILs (1%), cosmotropic anion activating enzymes like Cl− and Ac− in diluted aqueous solutions to become enzyme-inactivating agents. Perhaps this can explain PKC-resistance cellulase during the hydrolysis process to the IL-enzyme solution.
Previous studies by Elgharbawy et al. (2016a) found the highest production of sugar with [Ch][Ac]. The study yielded samples treated with [Ch][Ac] containing 22.5 g/L xylose, 0.09 g/L arabinose and 24 g/L glucose. Untreated EFB hydrolysis released 0.50 g/L (xylose), 0.13 g/L (arabinose) and 0.12 g/L (glucose) under the same conditions of 45 FPU/g EFB, at 50 °C for 24 h of enzyme hydrolysis without IL pretreatment. The sugar test shows that hydrolysate contains a number of sugars, including glucose, fructose, galactose, ribose and glyceraldehyde.
IL anions and hydroxyl hydrogen particles interact in the cellulose chain by forming hydrogen bonds. The hydrolytic rate rises and more sugar is produced as these bonds interact with the cellulose H-bonding mechanism. Furthermore, owing to IL application, the degree of crystallinity and polymerization decreases, hence weakening the composition of biomass (Yang & Fang, 2015).
Because of EFB pore enlargement and lignin disruption, IL’s effect was three times greater than native samples due to the increase of cellulose hydrolysis (Sun et al., 2013). The structure of cellulose was subjected to polymorphic modifications during and after treatment. The structural improvements made it possible for EFB to be hydrolyzed by enzymes. Furthermore, cellulose I is a hydrolysis-resistant crystalline form. Enzymatic hydrolysis would be more susceptible to cellulose I conversion to other types of cellulose (amorphous structures or cellulose II) (Samayam et al., 2011).
3.6.2.2 Changes in Cellulose, Hemicellulose, and Lignin
In this analysis, the ability of ILs to interrupt the development of lignocellulosic biomass was demonstrated. Pretreatment of ILs affects hydrolysis, which affects the production of sugar and glucose. The content of the cellulose evaluated differed. The elimination of lignin triggers the hydrolysis of biomass, and the pretreatment with ILs carries out a significant and important task. The elimination of lignin makes the hydrolysis stage easier.
3.6.2.3 Scanning Electron Microscopy (SEM) for Morphological Structural Changes
The IL and PKC-Cel pretreatments resulted in a smooth and swollen structure and surface changes that exposed the cellulose fiber in one process. In general, as seen in the following sections, lignocelluloses with the fewest structure crystalline are ideally suited for hydrolysis by the enzyme.
3.6.2.4 Fourier Transform Infrared Spectroscopy (FTIR)
EFB treatment with hemicellulose, the structure of AFEX and lignin was reported to be between 1800 and 900 cm−1 in a report published by Abdul et al. (2016). The peak modifications were predicted by the aromatic ring of lignin (C = C vibration). Particularly, EFB lignin degradation is facilitated by [Ch][Bu] and [Ch][Ac]. Cellulose (C-H bonding) and hemicellulose (C-O-C band vibration) were identified as causes of reduced peak intensity. Furthermore, EFB absorbance (1738 cm−1) in hemicelluloses is related to carbonyl and carboxylic bonds (Abdul et al., 2016).
3.6.2.5 Chemical Analysis of the Treated‑Hydrolyzed EFB
It is worth noting that the yeast’s ability to produce EtOH is dependent on a range of factors, including nutrients and microbial strains. Carbon and nitrogen are important supplements to the media. Nitrogen is essential for the growth of yeast and affects ethanol generation as well as tolerance of EtOH. Micronutrients, in addition to nitrogen and carbon, are needed for cell growth and fermentation.
Zn2+, Mg2+, and Mn2+ are essential trace elements for the development of yeast and the processing of ethanol. Zinc influences cell growth as well as yeast cell metabolism. The Zn2+-supplemented culture had improved the production and resistance of ethanol. Magnesium is necessary for yeast cell formation and cell production and enzymatic activity as a cofactor enzyme. It has a beneficial effect on EtOH production as it lowers proton by stabilizing the bilayer and interacting with the phospholipid layer for anion to enter the plasma layer. This promotes an enhancement in the resistance of ethanol yeast. The Mn2+ metal ion is a crucial element in the metabolism of S. cerevisiae, since it is a part of some proteins that make fermentation-related enzymes like carboxylase pyruvate (Deesuth et al., 2012).
During pretreatment and hydrolysis, microorganism-inhibiting compounds such as CH3COOH, carbohydrate-derived compounds, and lignin by-products such as furfural and phenolic acids may form.
CH3COOH may be released into the medium after solubilization and hemicellulose hydrolysis. Furfural is formed as xylose is degraded in acidic environments, but comparatively high amounts can be obtained by thorough pretreatment with lignocellulose (Palmqvist et al., 1999).
Furfural reduction may result in its respective alcohol being catalyzed by alcohol dehydrogenase, which has a less inhibitory impact (Dabirmanesh et al., 2012). By reducing the effect of inhibition, high cell concentrations will lead to faster yeast cell bioconversion in the presence of furfurals. Without significant changes in ethanol processing, saccharomyces cerevisiae can tolerate elevated furfural concentrations at elevated cell concentrations. The yeast successfully released ethanol in a culture medium containing 17.0 g/L furfural (Ylitervo et al., 2013).
In the current research, much of the cellulose has been broken down into glucose (99%). The enzymatic method showed the efficacy of hydrolysis based on the analysis. The transition rate from hemicellulose to xylose was 78.8%, with the rest being arabinose, galactose, and mannose. The total sugar produced may result from unreacted sugars or monosaccharides which have not been identified by the HPLC.
The presence of mannose, xylose, arabinose, and galactose in the mixture fit the composition of the EFB as reported. The components obtained were determined to EFB initial used, as shown in Fig. 3.7. In hydrolysate, several EFBs were broken down into xylose and glucose, yielding 1.23 g of sugar. With just 0.5% error, the experimental yield for the conversion of cellulose to glucose was 87.3% of the theoretical yield.

(Source Elgharbawy et al., 2018)
Detailed biomass balance of the EFB pretreatment and hydrolysis in IL-E system. The process starts with 1.875 g of the EFB
3.6.2.6 Swelling Capacity and Crystallinity Index (CrI)
Pretreatment at low temperatures allowed the structure to swell and cellulose crystallinity to decrease, while high-temperature pretreatment produced large pores and disrupted the biomass (Noori & Karimi, 2016). IL can degrade the hydrogen bonds in the cellulose system, allowing enzymes to hydrolyze cellulose I into cellulose II. IL accessing the amorphous area of the cellulose substance induces swelling, which weakens hydrogen bonds, increases the portability of the cellulose chain, and decreases crystalline components. The greater the EFB swelling, the greater availability that enables hydrolysis.
Cations enter and pass through tiny pores in cellulose during IL pretreatment, improving the pretreated lignocellulose polyionic character. The cation can acts as a counter-charge to COO in structure, causing the material to swell (Reddy, 2015). Reducing the crystallinity of cellulose dissolution is a perfect way to enhance IL enzymatic hydrolysis at low temperatures (Xu et al., 2017). The primary effect of IL on lignocellulose at high temperatures is destruction in the structure which contributes to the decomposition of hemicellulose and lignin (Soudham et al., 2015).
IL pretreatment increased cellulase desorption by improving the surface area and reducing the content of lignin, resulting in successful hydrolysis. The tendency of lignin to irreversibly absorb cellulase is one of the potential causes of cellulase’s deactivation. In the presence of IL, hydrolysis will also decrease the content of lignin because it can bind to IL, exposing cellulose that can facilitate cellulose desorption while also increasing hydrolysis (Zhao, 2016).
3.6.2.7 Fermentation of Hydrolysate to Ethanol
Fermentation has not hindered the development of yeast cells in the presence of IL. The graph also shows that as the incubation duration grew, the optical density corresponding to the output increased as well. Choline has been reported as a vitamin B compound and is commonly used as a dietary supplement; ILs that rely on it are known to be environmentally safe and thus considered non-toxic (Ossowicz et al., 2016).
3.7 Conclusion
In the IL-E process, an integrated IL ([Ch][Ac]) of EFB pretreatment and PKC-Cel (cellulase) yielding theoretical glucose output of around 87.3%. After 48 h in the [Ch][Ac]-enzyme method, about 99% of glucose was converted from cellulose. The hydrolysate of EFB was evaluated as a potential EtOH refining process. Furthermore, IL’s presence in the fermentation medium had no detrimental effect on yeast cell growth due to vitamin B compound containing in IL.
References
Abdul, P. M., Jamaliah, M. J., Suhaida, H., Masturah, M., Nabilah, L., Hassan, O., Venkatesh, B., Dale, B. E., & Mohd, T. M. N. (2016). Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Journal of Bioresource Technology, 211, 200–208.
Asakawa, A., Kohara, M., Sasaki, C., Asada, C., & Nakamura, Y. (2015). Comparison of choline acetate ionic liquid pretreatment with various pretreatments for enhancing the enzymatic saccharification of sugarcane bagasse. Industrial Crops and Products, 71, 147–152. https://doi.org/10.1016/j.indcrop.2015.03.073.
Baharuddin, A. S., Rahman, N. A., Shan, U. K., Hassan, M. A., Wakisaka, M., & Shirai, Y. (2011). Evaluation of pressed shredded empty fruit bunch (EFB)-palm oil mill effluent (POME) anaerobic sludge based compost using Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) analysis. African Journal Biotechnology, 10, 8082–8289.
Bian, J., Peng, F., Peng, X., Xiao, X., Peng, P., & Xu, F. (2014). Effect of [Emim] Ac pretreatment on the structure and enzymatic hydrolysis of sugarcane bagasse cellulose. Carbohydrate Polymers, 100, 211–217. https://doi.org/10.1016/j.carbpol.2013.02.059.
Borah, A. J., Agarwal, M., Poudyal, M., Goyal, A., & Moholkar, V. S. (2016). Mechanistic investigation in ultrasound induced enhancement of enzymatic hydrolysis of invasive biomass species. Bioresource Technology, 213, 342–349. https://doi.org/10.1016/j.biortech.2016.02.024.
Caputi, A., Ueda, M., & Brown, T. (1968). Spectrophotometric determination of ethanol in wine. American Journal of Enology and Viticulture, 19, 160–165.
Chandra, R., Takeuchi, H., & Hasegawa, T. (2012). Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renewable and Sustainable Energy Reviews, 16(3), 1462–1476. https://doi.org/10.1016/j.rser.2011.11.035.
Chew, T. L., & Bhatia, S. (2008). Catalytic processes towards the production of biofuels in a palm oil and oil palm biomass-based biorefinery. Bioresource Technology, 99, 7911–7922. https://doi.org/10.1016/j.biortech.2008.03.009.
Choi, W. I., Park, J. Y., Lee, J. P., Oh, Y. K., Park, Y. C., Kim, J. S., Park, J. M., Kim, C. H., & Lee, J. S. (2013). Optimization of NaOH catalyzed steam pretreatment of empty fruit bunch. Biotechnology for Biofuels, 6, 170.
Chong, P. S., Jahim, J. M., Harun, S., Lim, S. S., Sahilah, A. M., Osman, H., & Mohd, T. M. N. (2013). Enhancement of batch biohydrogen production from prehydrolysate of acid treated oil palm empty fruit bunch. International Journal of Hydrogen Energy, 38, 9592–9599.
Dabirmanesh, B., Khajeh, K., Ranjbar, B., Farideh, G., & Heydari, A. (2012). Inhibition mediated stabilization effect of imidazolium based ionic liquids on alcohol dehydrogenase. Journal of Molecular Liquids, 170, 66–71. https://doi.org/10.1016/j.molliq.2012.03.004.
Deesuth, O., Laopaiboon, P., Jaisil, P., & Laopaiboon, L. (2012). Optimization of nitrogen and metal ions supplementation for very high gravity bioethanol fermentation from sweet sorghum juice using an orthogonal array design. Energies, 5, 3178–3197.
Dubois, M., Gilles, K. A., Hamilton, J. K., Hamilton, P. A., Reber, P. A., & Fred, S. (1956). Colorimetric method for determination of sugar and related substances. Analytical Chemistry, 28, 350–356. https://doi.org/10.1021/ac60111a017.
Elgharbawy, A. A., Alam, M. Z., Kabbashi, N. A., Moniruzzaman, M., & Jamal, P. (2016a). Implementation of definite screening design in optimization of in situ hydrolysis of EFB in cholinium acetate and locally produced cellulose combined system. Waste Biomass Valorization. https://doi.org/10.1007/s12649-016-9638-6 (LB—Elgharbawy 2016).
Elgharbawy, A. A., Alam, M. Z., Kabbashi, N. A., Moniruzzaman, M., & Jamal, P. (2016b). Evaluation of several ionic liquids for in situ hydrolysis of empty fruit bunches by locally-produced cellulase. 3 Biotech, 6, 128. https://doi.org/10.1007/s13205-016-0440-8.
Elgharbawy, A. A., Alam, Z., Moniruzzaman, M., & Goto, M. (2016c). Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochemical Engineering Journal, 109, 252–267. https://doi.org/10.1016/j.bej.2016.01.021.
Elgharbawy, A. A., Alam, M. Z., Moniruzzaman, M., Kabbashi, N. A., & Jamal, P. (2018). Chemical and structural changes of pretreated empty fruit bunch (EFB) in ionic liquid-cellulase compatible system for fermentability to bioethanol. 3 Biotech, 8(5), 236. https://doi.org/10.1007/s13205-018-1253-8.
Ghose, T. K. (1987). Measurement of cellulase activities. International Union of Pure and Applied Chemistry, 59, 257–268. https://doi.org/10.1351/pac198759020257.
Graenacher, C. (1934). Patent No. 1,943,176.
Hahn-Hägerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Liden, G., & Zacchi, G. (2006). Bioethanol: The fuel of tomorrow from the residues of today. Trends in Biotechnology, 24, 549–556.
Ishola, M. M., Isroi, & Taherzadeh, M. J. (2014). Effect of fungal and phosphoric acid pretreatment on ethanol production from oil palm empty fruit bunches (OPEFB). Bioresource Technology, 165, 9–12. https://doi.org/10.1016/j.biortech.2014.02.053.
Kim, D. Y., Um, B. H., & Oh, K. K. (2015). Acetic acid-assisted hydrothermal fractionation of empty fruit bunches for high hemicellulosic sugar recovery with low byproducts. Applied Biochemistry and Biotechnology, 176, 1445–1458.
Kljun, A., Benians, T. A. S., Goubet, F., Meulewaeter, F., Knox, J. P., & Blackburn, R. S. (2011). Comparative analysis of crystallinity changes in cellulose I polymers using ATR-FTIR, X-ray diffraction, and carbohydrate-binding module probes. Biomacromolecules, 12, 4121–4126. https://doi.org/10.1021/bm201176m.
Laureano-Perez, L., Teymouri, F., Alizadeh, H., & Dale, B. E. (2005). Understanding factors that limit enzymatic hydrolysis of biomass. Applied Biochemistry and Biotechnology, 124, 1081–1099. https://doi.org/10.1385/abab:124:1-3:1081.
Lienqueo, M. E., Ravanal, M. C., Pezoa-Conte, R., Cortinez, V., Martinez, L., Niklitschek, T., Salazar, O., Carmona, R., Garcia, A., Hyvarinen, S., Maki-Arvela, P., & Mikkola, J.-P. (2016). Second generation bioethanol from eucalyptus globulus Labill and Nothofagus pumilio: Ionic liquid pretreatment boosts the yields. Industrial Crops and Products, 80, 148–155.
MacFarlane, D. R., Kar, M., & Pringle, J. M. (2017). An introduction to ionic liquids. In Fundamentals of ionic liquids: From chemistry to application (pp. 1–25).
Moniruzzaman, M., & Goto, M. (2018). Ionic liquid pretreatment of lignocellulosic biomass for enhanced enzymatic delignification. In: Itoh T, & Koo Y-M (Eds.), Application of ionic liquids in Biotechnology, (Vol. 168, pp. 61–77). Springer International Publishing, Cham. https://doi.org/10.1007/10_2018_64. PMID: 29744542.
Moniruzzaman, M., Nakashima, K., Kamiya, N., & Goto, M. (2010). Recent advances of enzymatic reactions in ionic liquids. Biochemical Engineering Journal, 48, 295–314. https://doi.org/10.1016/j.bej.2009.10.002.
Muhammad, N., Hossain, M, I., Man, Z., El-Harbawi, M., Bustam, M. A., Noaman, Y. A., Alitheen, N. B. M., Ng, M. K., Hefter, G., & Yin, C.-Y. (2012). Synthesis and physical properties of choline carboxylate ionic liquids. Journal of Chemical & Engineering Data, 57, 2191–2196.
Nieves, D. C., Ruiz, H., De Cárdenas, L. Z., Alvarez, G. M., Aguilar, C. N., Ilyina, A., & Hernandez, J. L. M. (2016). Enzymatic hydrolysis of chemically pretreated mango stem bark residues at high solid loading. Industrial Crops and Product, 83, 500–508. https://doi.org/10.1016/j.indcrop.2015.12.079.
Ninomiya, K., Kohori, A., Tatsumi, M., Osawa, K., Endo, T., Kakuchi, R., Ogino, C., Shimizu, N., & Takahashi, K. (2015a). Ionic liquid/ultrasound pretreatment and in situ enzymatic saccharification of bagasse using biocompatible cholinium ionic liquid. Bioresource Technology, 176, 169–174. https://doi.org/10.1016/j.biortech.2014.11.038.
Ninomiya, K., Ogino, C., Ishizaki, M., Yasuda, M., Shimizu, N., & Takahashi, K. (2015b). Effect of post-pretreatment washing on saccharification and co-fermentation from bagasse pretreated with biocompatible cholinium ionic liquid. Biochemical Engineering Journal, 103, 198–204. https://doi.org/10.1016/j.bej.2015.08.002.
Ninomiya, K., Omote, S., Ogino, C., Kuroda, K., Noguchi, M., Endo, T., Kakuchi, R., Shimizu, N., & Takahashi, K. (2015c). Saccharification and ethanol fermentation from cholinium ionic liquid-pretreated bagasse with a different number of post-pretreatment washings. Bioresource Technology, 189, 203–209. https://doi.org/10.1016/j.biotech.2015.04.022.
Nomanbhay, S. M., Hussain, R., & Palanisamy, K. (2013). Microwave-assisted alkaline pretreatment and microwave assisted enzymatic saccharification of oil palm empty fruit bunch fiber for enhanced fermentable sugar yield. Journal of Sustainable Bioenergy Systems, 3, 7–17.
Noori, M. S., & Karimi, K. (2016). Detailed study of efficient ethanol production from elmwood by alkali pretreatment. Biochemical Engineering Journal, 105, 197–204. https://doi.org/10.1016/j.bej.2015.09.019.
Ossowicz, P., Janus, E., Szady-Chełmieniecka, A., & Rozwadowski, Z. (2016). Influence of modification of the amino acids ionic liquids on their physico-chemical properties: Ionic liquids versus ionic liquids supported Schiff bases. Journal of Molecular Liqiud, 224, 211–218. https://doi.org/10.1016/j.molliq.2016.09.111.
Palmqvist, E., Grage, H., Meinander, N. Q., & Hahn-Hägerdal, B. (1999). Main and interaction effects of acetic acid, furfural and p hydroxybenzoic acid on growth and ethanol productivity of yeast. Biotechnology and Bioengineering, 63, 46–55. https://doi.org/10.1002/(SICI)1097-0290(19990405)63.
Palmqvist, E., & Hahn-Hägerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresource Technology, 74, 25–33. https://doi.org/10.1016/s09608524(99)001613.
Poornejad, N., Karimi, K., & Behzad, T. (2014). Ionic liquid pretreatment of rice straw to enhance saccharification and bioethanol production. Journal of Biomass to Biofuel, 1, 8–15. https://doi.org/10.11159/jbb.2014.002.
Reddy, P. (2015). A critical review of ionic liquids for the pretreatment of lignocellulosic biomass. South African Journal of Science, 111, 1–9.
Salvador, Â. C., Santos, M. D. C., & Saraiva, J. A. (2010). Effect of the ionic liquid [bmim] Cl and high pressure on the activity of cellulase. Green Chemistry, 12, 632–635.
Samayam, I, P., Hanson, B. L., Langan, P., & Schall, C. A. (2011). Ionic-liquid induced changes in cellulose structure associated with enhanced biomass hydrolysis. Biomacromolecules. https://doi.org/10.1021/bm200736a (LB—Samayam 2011).
Shi, W., Jia, J., Gao, Y., & Zhao, Y. (2013). Influence of ultrasonic pretreatment on the yield of bio-oil prepared by thermo-chemical conversion of rice husk in hot-compressed water. Bioresource Technology, 146, 355–362.
Singh, S., Agarwal, M., Bhatt, A., Goyal, A., & Moholkar, V, S. (2015). Ultrasound enhanced enzymatic hydrolysis of Parthenium hysterophorus: A mechanistic investigation. Bioresource Technology, 192, 636–645. https://doi.org/10.1016/j.biortech.2015.06.031.
Soudham, V, P., Raut, D, G., Anugwom, I., Brandberg, T., Larsson, C., & Mikkola, P. (2015). Coupled enzymatic hydrolysis and ethanol fermentation: Ionic liquid pretreatment for enhanced yields. Biotechnology for Biofuels, 8, 135. https://doi.org/10.1186/s13068-015-0310-3 (LB—Soudham 2015).
Sousa, L. D., Chundawat, S. P. S., Balan, V., & Dale, B. (2009). Cradle-to-grave assessment of existing lignocelluloses pretreatment technologies. Current Opinion Biotechnology, 20, 339–347.
Sun, N., Rahman, M., Qin, Y., & Maxim, M. L. (2009). Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate †‡. Green Chemistry, 11, 646–655. https://doi.org/10.1039/b822702k.
Sun, Y.-C., Xu, J.-K., Xu, F., & Sun, R.-C. (2013). Structural comparison and enhanced enzymatic hydrolysis of eucalyptus cellulose via pretreatment with different ionic liquids and catalysts. Process Biochemistry, 48, 844–852. https://doi.org/10.1016/j.procbio.2013.03.023.
Updegraff, D. M. (1969). Semimicro determination of cellulose inbiological materials. Analytical Biochemistry, 32, 420–424. https://doi.org/10.1016/S0003-2697(69)80009-6.
Wang, X., Li, H., Cao, Y., & Tang, Q. (2011). Cellulose extraction from wood chip in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Bioresource Technology, 102, 7959–7965.
Wolfe, K., Wu, X., & Liu, R. H. (2003). Antioxidant activity of apple peels. Journal of Agriculture and Food Chemistry, 51, 609–614.
Xu, A.-R., Wen, S., & Chen, L. (2017). Dissolution performance of cellulose in MIM plus tetrabutylammonium propionate solvent. Journal of Molecular Liquids, 246, 153–156. https://doi.org/10.1016/j.molliq.2017.09.065.
Yang, C.-Y., & Fang, T. J. (2015). Kinetics of enzymatic hydrolysis of rice straw by the pretreatment with a bio-based basic ionic liquid under ultrasound. Process Biochemistry, 50, 623–629. https://doi.org/10.1016/j.procbio.2015.01.013.
Ylitervo, P., Franzén, C, J., & Taherzadeh, M, J. (2013). Impact of furfural on rapid ethanol production using a membrane bioreactor. Energies, 6, 1604–1617. https://doi.org/10.3390/en6031604.
Zainan, N. H., Alam, Z., & Al-Khatib, M. F. (2013). Production of sugar by hydrolysis of empty fruit bunches using palm oil mill effluent (POME) based cellulases: Optimization study. African Journal of Biotechnology, 10, 18722–18727.
Zarei, A. R. (2009). Spectrophotometric determination of trace amounts of furfural in water samples after mixed micelle-mediated extraction. Acta Chimica Slovenica, 56, 322–328.
Zhao, H. (2016). Protein stabilization and enzyme activation in ionic liquids: Specific ion effects. Journal of Chemical Technology & Biotechnology, 91, 25–50. https://doi.org/10.1002/jctb.4837.
Zhao, H., Jones, C. L., Baker, G. A., Xia, S., Olubajo, O., Person, V. N., & Ridge, O. (2009). Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. Journal of Biotechnology, 139, 47–54. https://doi.org/10.1016/j.jbiotec.2008.08.009.
Acknowledgements
The project was funded by the Ministry of Higher Education (MOHE) in Malaysia under the FRGS-13-088-0329 research grant. We thank the Research Management Center, IIUM for the grant [RMCG20-021-0021RMCG20-021-0021]. We also thank the International Institute for Halal Research and Training (INHART), IIUM Department of Biotechnology Engineering and Ionic Liquid Research Center (CORIL), UTP for assisting us with the essential laboratory equipment. We are also thankful for the raw materials provided by Sime Darby Plantation (West Oil Mill).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Elgharbawy, A.A.M., Putra, S.S.S., Alam, M.Z., Moniruzzaman, M., Kabbashi, N.A., Jamal, P. (2021). Role of Ionic Liquids in the Processing of Lignocellulosic Biomass. In: Amid, A. (eds) Multifaceted Protocols in Biotechnology, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-030-75579-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-75579-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75578-2
Online ISBN: 978-3-030-75579-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)