Abstract
While many kinetic models have been developed for the enzymatic hydrolysis of cellulose, few have been extensively applied for process design, optimization, or control. High-solids operation of the enzymatic hydrolysis of lignocellulose is motivated by both its operation decreasing capital costs and increasing product concentration and hence separation costs. This work utilizes both insights obtained from experimental work and kinetic modeling to develop an optimization strategy for cellulose saccharification at insoluble solids levels greater than 15% (w/w), where mixing in stirred tank reactors (STRs) becomes problematic. A previously developed model for batch enzymatic hydrolysis of cellulose was modified to consider the effects of feeding in the context of fed-batch operation. By solving the set of model differential equations, a feeding profile was developed to maintain the insoluble solids concentration at a constant or manageable level throughout the course of the reaction. Using this approach, a stream of relatively concentrated solids (and cellulase enzymes) can be used to increase the final sugar concentration within the reactor without requiring the high initial levels of insoluble solids that would be required if the operation were performed in batch mode. Experimental application in bench-scale STRs using a feed stream of dilute acid-pretreated corn stover solids and cellulase enzymes resulted in similar cellulose conversion profiles to those achieved in batch shake-flask reactors where temperature control issues are mitigated. Final cellulose conversions reached approximately 80% of theoretical for fed-batch STRs fed to reach a cumulative solids level of 25% (w/w) initial insoluble solids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
- ξ :
-
extent of cellulose conversion (grams insoluble solids removed per gram initial cellulose)
- ρ :
-
liquid phase density (grams liquid phase per liter liquid phase)
- Φ:
-
NPL objective function
- ϕ :
-
discretized NPL objective function
- C :
-
cellulose mass concentration (grams per kilogram of slurry)
- cb:
-
cellobiose liquid phase concentration (grams per liter of liquid phase)
- CB:
-
cellobiose mass concentration (grams per kilogram of slurry)
- D :
-
dilution rate (per hour)
- E 1 :
-
all endoglucanases (EGs) and cellobiohydrolases (CBHs) mass concentration (grams per kilogram of slurry)
- E 2 :
-
β-glucosidase mass concentration (grams per kilogram of slurry)
- E 1 feed :
-
all EGs and CBHs mass concentration in the feed (grams per kilogram of slurry)
- E 2 feed :
-
β-glucosidase mass concentration in the feed (grams per kilogram of slurry)
- f :
-
continuous function
- F :
-
total mass fed to reactor (kilogram of slurry)
- F1:
-
feeding policy 1
- F2:
-
feeding policy 2
- g :
-
glucose liquid phase concentration (grams per liter of liquid phase)
- G :
-
glucose mass concentration (grams per kilogram of slurry)
- G feed :
-
glucose mass concentration in the feed (grams per kilogram of slurry)
- k :
-
discrete time index
- r 1 :
-
rate of cellulose conversion to glucose (grams per kilogram slurry per hour)
- r 2 :
-
rate of cellulose conversion to cellobiose (grams per kilogram slurry per hour)
- r 3 :
-
rate of cellobiose conversion to glucose (grams per kilogram slurry per hour)
- r :
-
desired control trajectory
- S cumul. :
-
cumulative or “equivalent” insoluble solids fed to reactor (grams insoluble solids per gram slurry)
- S I,0 :
-
initial insoluble solids (grams insoluble solids per gram slurry)
- S I :
-
insoluble solids (grams insoluble solids per gram slurry)
- S IF :
-
insoluble solids in the feed stream (grams insoluble solids per gram slurry)
- S I,SP :
-
insoluble solids set point or maximum insoluble solids (grams insoluble solids per gram slurry)
- t :
-
time (hour)
- t 0 :
-
initial time (hour)
- t f :
-
final time (hour)
- u :
-
control variable
- V :
-
working “volume” of the reactor (kilogram slurry)
- V 0 :
-
initial working “volume” of the reactor (kilogram slurry)
- X :
-
vector of state variables
- x 0 :
-
initial value of state variables
- x cell :
-
initial cellulose fraction in the solid phase (grams initial cellulose per gram insoluble solids)
Introduction
Petroleum-derived fuels are currently the primary energy source used for transportation. As worldwide and domestic energy demands increase, petroleum reserves remain finite. To achieve long-term energy sustainability, renewable and environmentally responsible alternatives to fossil fuels should be developed. Energy derived from lignocellulosic biomass (plant material such as trees, grasses, and agricultural crops) represents one such energy source that is both renewable and abundant. It can be used for steam and electricity generation, converted into liquid or gaseous fuels, or used for the production of chemicals. Ethanol derived from lignocellulose represents one of only a few renewable transportation fuels having the potential to displace gasoline on a large scale [1].
One of the more promising approaches to the conversion of lignocellulosic biomass to ethanol involves dilute acid pretreatment, followed by an enzymatic hydrolysis of cellulose to glucose, and mixed sugar fermentation to ethanol [2]. Many kinetic models have been developed for the enzymatic hydrolysis of cellulose or cellulosic biomass to glucose and cellobiose [3–8]. Such models are typically motivated by one of two objectives. The first objective is to demonstrate an understanding of a physical process by proposing a model structure that suitably fits the experimental data. Another goal is to develop an application-based model (either mechanistic or empirical) for process design, simulation, or control.
“High-solids” enzymatic saccharification can be roughly defined as beginning at the insoluble solids level (S I) where significant levels of free liquid are no longer present in the slurry such that the separation of a liquid and solid phase from the suspension is not spontaneous. The implementation of a high-solids saccharification process is motivated by the potential to realize significant economic advantages over a conventional low solids process as a consequence of: lower capital costs due to reduced volume; lower operating costs due to less energy required for heating and cooling; lower downstream processing costs due to higher product concentrations; reduced disposal and treatment costs due to lower water usage [9]. Techno-economic models have been used to approximate the potential cost savings. The work of Wingren et al. [10] estimated that increasing the insoluble solids from 5% to 8% (all solids are given on a w/w basis) in a simultaneous saccharification and fermentation (SSF) process for bioconversion of softwood to ethanol reduces the operating costs by 19%. Design and modeling work at the National Renewable Energy Laboratory (NREL) considers a 2,000 ton/day corn stover to ethanol plant using dilute-acid pretreatment at 30% insoluble solids followed by enzymatic hydrolysis at 20% total solids (10–12% insoluble solids, dependent on amount of biomass solubilization in addition to 8–10% soluble solids generated during pretreatment), with subsequent fermentation of pentoses and hexoses to ethanol [11]. Preliminary calculations using this model suggest that due to energy and capital cost reductions, the minimum ethanol selling price decreases by $0.10/gal when the total solids concentration to the saccharification unit operation increases from 20% to 30%.
While in situ native cellulase systems in wood-degrading microorganisms have been reported to hydrolyze cellulose at insoluble solids values as high as 68–76% [12], industrial enzymatic saccharification reactions are ultimately limited by processing constraints. With increasing insoluble solids, the viscosity of the slurry increases sharply, and viscosity for pretreated corn stover (PCS) solids has been shown to be highly non-Newtonian [13]. Preliminary work in our laboratory has shown that approximately 12–15% insoluble solids represents the upper limit at which slurries of unhydrolyzed PCS solids can be mixed effectively in stirred tank reactors (STRs). Besides only insoluble solids level, the composition of insoluble and soluble components can exert a strong influence on the mixing rheology depending on the physical properties of the compound (e.g., degree of polymerization). Performing the saccharification reaction at high insoluble solids introduces a new set of process-related problems associated with slurry mixing and cellulase enzyme effectiveness. Enzyme-dependent factors include sugar inhibition [14] and reaction temperature [15]. At increasing levels of solids, sugar inhibition of enzymes may become more important due to the increasing difficulty in diffusion of sugars away from the catalytic site. At the same time, this hinders the ability of the enzyme to reach the reaction site. Another issue associated with increasing insoluble solids is the increasing difference between the liquid phase concentration (gram per liter of liquid) of a component and the overall concentration of the same component in the slurry (grams per kilogram of slurry); the latter is more useful for tracking two-phase reactions such as cellulose hydrolysis, but calculation requires the measurement or estimation of the insoluble solid concentration of the slurry.
High-solids reactor configurations have been developed in the past for lignocellulosic conversion technologies in the areas of high-solids pretreatment [2, 16, 17] and high-solids SSF [9, 18–20]. For the enzymatic saccharification reaction during SSF, a variety of reactor types have been used to try to circumvent the problem of challenging slurry rheology. Examples include fermentation shake flasks [19, 21], pilot-scale helical ribbon impeller reactors [18], a horizontally mounted paddle-impeller reactor [20], a vertically mounted paddle-impeller reactor [22], and a horizontally revolving reactor developed from a laboratory ball mill [9]. Temperature control is an important issue because the enzymatic reaction is highly sensitive to temperature, and local hot spots within the reactor, such as heat transfer surfaces, could significantly alter the reactivity of the enzymes [15]. Most laboratory-scale reactor systems require either a large reservoir of temperature-controlled air (incubator) or water (water bath) to circumvent the difficulty of maintaining accurate temperature measurement and feedback control.
While a number of batch saccharification studies have been conducted at insoluble solids levels of 15% or higher [9, 18, 20], there are also several documented high-solids fed-batch saccharification studies [19, 21, 23]. All of these batch and fed-batch studies used SSF to offset the recognized effects of glucose and cellobiose inhibition.
A number of strategies have been reported for fed-batch enzymatic saccharification of cellulose, although all of these are based on ad hoc approaches to feeding rather than application of a rigorously designed fed-batch feeding policy. These motivations underlying previous work fall into three general categories, the first of which is enzyme recycle. Pristavka et al. [24] used a fill and draw approach (a type of fed-batch operation) for high-solids saccharification of steam-exploded willow. For this, a high-solids slurry was enzymatically saccharified, a portion of the liquid phase was then removed, and more solids were added such that a portion of enzyme adsorbed to the solids could be reused. Total sugar concentrations including glucose and hemicellulosic sugars from pretreatment averaged 120 g/l. Another enzyme recycle approach is to add only pretreated solids but no enzymes. As the cellulose is hydrolyzed, enzymes should be released back into the liquid such that more cellulosic biomass can be added to the reactor.
The second category of past work used fed-batch SSF [10, 25] to mitigate the inhibitory effects of compounds in hydrolyzate liquors on the fermentative microorganism. Since hydroxymethylfurfural and furfural can be biologically detoxified by Saccharomyces cerevisiae over time, fed-batch fermentation can result in slurries with higher final equivalent levels of these inhibitors than could be tolerated by the microorganism if the entire slurry were added initially. Besides offsetting the effects of fermentation inhibition, the effective insoluble solids were increased from 3% to 5% or to 8.4% for the two studies, respectively. Borden et al. [26] also used a fed-batch approach to mitigate the effects of inhibitors in the hydrolyzate during SSF to acetic acid by Clostridium thermoaceticum.
The third motivation for fed-batch saccharification is to utilize feeding to increase the cumulative insoluble solids level during saccharification to overcome reactor mixing. In the work reported by Ballesteros et al. [21] on SSF using Kluyveromyces marxianus, the effective cumulative insoluble solids level was increased from 10% to 20%, which the reactor would have been incapable of handling initially, by using a fed-batch approach that also had the benefit of reducing the effects of inhibitors in the hydrolyzate. Due to problems encountered in mixing wet oxidization-treated corn stover at insoluble solids greater than 12%, Varga et al. [19] used a fed-batch hybrid hydrolysis and fermentation approach to achieve high ethanol yields at a 17% cumulative insoluble solids loading. Fan et al. [23] used a fed-batch approach to minimize the effects of mixing bleached Kraft paper sludge in an SSF reactor by feeding a stream of 30% insoluble solids to achieve a reactor solids content of 12%. Mohagheghi et al. [9] compared high-solids batch to fed-batch SSF and determined that a ball-mill reactor system achieved similar ethanol yields and productivities relative to batch operation.
Kleman et al. [27] identify a number of open- and closed-loop control options for developing a robust feeding policy. These are based on controlling substrate levels for fed-batch fermentations using either indirect or direct closed-loop control. The indirect closed-loop control approach to fed-batch has been applied to the fermentation of dilute acid-pretreated hydrolyzate liquors of hardwoods and softwoods based on measurement of off-gas composition and calculation of the CO2 evolution rate (CER). Control strategies have been developed to change feed rate based on changes in CER [28–29] as well as to estimate and control sugar concentration [30]. The direct closed-loop approach to fed-batch control used in our previous work [31] was based on high-performance liquid chromatography (HPLC) measurements of reactor conditions coupled to a predictive controller based on a kinetic model of the process.
An open-loop approach can be used to develop a feeding policy without requiring online measurements of insoluble solids but rather only model predictions. Since open-loop control schemes rely solely on process models or speculation on the physical parameters governing the process, these control methods are only as accurate as the model. Using a system of model differential equations, an exact analytical solution for a feeding profile can be obtained by developing and solving an “optimal control” problem. Optimal control fed-batch operation is often applied to cell cultures in bioreactors for a variety of objectives such as the maximization of the production of cell mass, amino acids, enzymes, and bioengineered products such as penicillin, ethanol, and recombinant proteins [32–34].
Using a mechanistic understanding of the kinetics of enzymatic saccharification as a basis, the goals of the present work are to apply an open-loop fed-batch approach to an STR to increase the cumulative level of insoluble solids to the reactor. This approach mimics the performance of a reactor at a higher insoluble solids loading while maintaining the operating characteristics of a reactor at a lower solids loading. These approaches are implemented using a thoroughly developed system of equations converting measured liquid-phase concentrations in the biphasic compositions to mass-basis concentrations and using this to estimate and track insoluble solids. The capabilities of fed-batch systems are explored using both mass balance methods and dynamic simulations. Two strategies from the dynamic simulations are applied experimentally offline to demonstrate the effectiveness of the approach.
Materials and Methods
Cellulosic Substrate
Corn stover was pretreated by dilute sulfuric acid in NREL’s continuous pilot-scale vertical reactor as reported elsewhere [2]. The solids (by dry mass 53.2% cellulose, 31.7% lignin, 7.0% other hemicellulose sugars, 7.0% ash, 3.7% protein, 1.1% acetate) are washed with greater than ten times their total mass in deionized (DI) water during multiple cycles of centrifuging at 15,800×g to remove interstitial hydrolyzate liquors. Washed solids are used to minimize any inhibition effects from sugars and other soluble components released during pretreatment and entrained in the solids.
Enzymatic Saccharification
For the enzymatic saccharification experimental work, two scales of reactors were used: shake-flask reactors and a bench-scale STR, run in batch and fed-batch modes, respectively. The cellulase/β-glucosidase enzyme mixture used for this study was Spezyme CP (Genencor International, Palo Alto, CA, USA). Total protein was assayed at 106.6 mg/ml (Bio-Rad assay), and the activity was determined to be 0.27 FPU/mg of protein using NREL LAP-006: Measurement of Cellulase Activities (http://www.eere.energy.gov/biomass/analytical_procedures.html). The enzyme loading is 40 mg protein/g cellulose corresponding to 10.7 FPU/g cellulose. A Na-citrate buffer (pH 4.8) at 0.05 mol/kg (based on the final working mass for fed-batch reactors) is used to maintain the pH. Enzyme, DI water, and citrate buffer are filter sterilized before use. Washed PCS is air dried to approximately 45% insoluble solids, and the insoluble solids content is determined in triplicate using a 105°C drying oven.
For shake-flask batch reactors, PCS is added and weighed before autoclaving. After autoclaving, the shake flasks are weighed again so that any water lost could be made up. Temperature is maintained at 45°C in a rotary shaking incubator. For fed-batch STRs, the 7-l vessels (Bioflo 3000, New Brunswick Scientific, Edison, NJ, USA) are insulated and equipped with two large marine impellers (impeller/vessel diameter ratio of 0.68), which are maintained at 400 rpm. The temperature is maintained at 45°C by water circulating to a heating jacket from a temperature-controlled water bath (maximum T = 52°C). These reactors are autoclaved empty and allowed to cool in a sterile biological hood. The appropriate amounts of solids are autoclaved with the amount of water lost during sterilization determined and added back. Solids and other sterile components are next added to the reactors and allowed to mix and reach steady-state temperature before enzyme is added. Feeding policies are based on the solution to the optimal control problem as outlined in the “Modeling and Theoretical Aspects” section. Feeding is performed by adding both autoclaved solids and filter-sterilized enzyme at discrete times, with samples for HPLC measurements taken both prior to and after feeding. Enzyme loading is 40 mg protein per gram cellulose (10.7 FPU/g cellulose), and enzyme is fed proportionally to the amount of PCS solids added. This can be considered as equivalent to the same amount of enzyme required for a given amount of PCS as would be used in an equivalent batch reaction. Cellulose conversion for fed-batch reactors is determined based on the percentage of the final cumulative cellulose converted to glucose and cellobiose using the mathematical approach developed in a later section.
Sampling and Data Analysis
Samples are removed from reactors with disposable 5 ml pipettes from which the tips were broken off in order to accommodate the high level of insoluble solids. Slurry samples are transferred to 2.5 ml microcentrifuge tubes, and the pH is measured. The samples are then centrifuged at 10,000 rpm for 5 min. The supernatant is decanted, diluted 1:5 in water, and syringe filtered into HPLC vials for subsequent analysis of sugar composition. HPLC analysis is performed on all saccharification samples to determine the glucose, xylose, and cellobiose concentrations. An Agilent 1100 series HPLC (Agilent Technologies, Palo Alto, CA, USA) equipped with a differential refractive index detector is used. This is equipped with an Aminex HPX-87H organic acid column (Bio-Rad Laboratories, Hercules, CA, USA) operating at 55°C with a 0.01-N H2SO4 mobile phase at a flow rate of 0.6 ml/min. For both batch and fed-batch experiments, cellulose conversions is calculated based on measured glucose and cellobiose. Component concentrations obtained from HPLC measurement are converted to grams per kilogram concentrations based on the estimated insoluble solids level in the reactor determined from initial insoluble solids levels and sugar concentrations.
Modeling and Theoretical Aspects
Calculation of Conversion, Mass Concentrations, and Insoluble Solids
Cellulose conversion is commonly used as a measure of the effectiveness of enzymatic hydrolysis of cellulose. One common method for determining cellulose conversion has been defined as the “summative approach” [35]. This approach relies on measurements of glucose, cellobiose, alternatively soluble cellodextrin oligomers, and ethanol in the case of SSF to estimate the cellulose that has been enzymatically hydrolyzed. The equation for this approach to determine the extent of cellulose conversion (ξ) while considering only glucose and cellobiose gives:
where all variables are defined in the Nomenclature.
While the insoluble solids level can be measured, the summative method permits the solids level to be determined from the change in sugar concentrations relative to the initial level (net sugar produced). This change in sugar levels can be related stoichiometrically to the amount of cellulose removed from the solid phase to estimate an insoluble solids level. A conversion factor is necessary due to the addition of one molecule of water across each β-1,4 bond that is hydrolyzed in the case of cellulosic glucan. Furthermore, since the sugars (glucose and cellobiose) are in solution, the determination of both component mass concentrations and total insoluble solids by the summative approach at any given sample time cannot be estimated independently of each other and requires the simultaneous solution to the following system of linear equations assuming a known liquid phase density:
Solving in terms of the insoluble solids with only measured variables appearing in the right-hand sides of Eqs. 2–4 gives:
where the glucose and cellobiose liquid phase concentrations are the only measured variables.
Liquid fraction density is another important consideration that becomes more significant in high-solids saccharification. The liquid fraction contains high concentrations of sugar, and the density cannot be assumed to be the same as water. A mixture of sugars is present in the liquid phase that varies in composition during enzymatic hydrolysis. Due to this, it would be impractical to determine correlations between liquid phase density and sugar concentrations for a wide range of sugar compositions. Since this work primarily uses washed solids and glucose is the most abundant sugar, glucose is considered as the primary density-affecting sugar. A correlation based on glucose data developed from Weast [36] relates density (grams liquid per liter liquid) to sugar concentration (grams sugar per liter liquid):
Mass Balance Equations for Prediction of Fed-Batch Capabilities
Mass balances were performed on the reaction system to evaluate the potential of using a fed-batch approach. A key assumption was that the insoluble solids (S I) are fed at the same rate as they are hydrolyzed in order to maintain a constant level of insoluble solids throughout the process or S I,0 = S I(t) = S I(t final). The mass balance equation on insoluble solids at any time point gives:
Which assuming S I,0 = S I(t final) gives:
with the solution for the feeding rate normalized to the initial reactor volume as:
The cumulative or equivalent insoluble solids (S cumul.) is the total amount of insoluble solids initially present plus those fed to the reactor. This can be considered as the level of initial insoluble solids that would be present if all of the solids were added initially and the reactor was operated in batch mode to enable comparison of fed-batch performance with batch reactor performance on an equivalent basis. A separate balance on the insoluble solids results in the solution to S cumul. as:
Using these mass balance equations, the plots in Figs. 1 and 2 were developed. Figure 1 demonstrates how feeding can increase the level of cumulative insoluble solids for constant conversion contours; up to 27% at 85% cellulose conversion by maintaining a reactor at 15% insoluble solids. This is to say that if the solids were fed at the same rate as they were hydrolyzed and the reactor were maintained at 15% insoluble solids, the cumulative insoluble solids or equivalent initial insoluble solids that would be present in a batch fermentation would be 27%. Another important consideration for the fed-batch reactor is the change in reactor volume associated with the feeding of solids. Figure 2 depicts how the most important parameter for the change in reactor volume, the insoluble solids in the feed (S IF), affects the reactor volume for feeding as given in Fig. 1 at the 85% cellulose conversion contour. The major finding is that both the reaction volume and the level to which reactors can be fed are ultimately limited by S IF. The solutions to these equations are also constrained by the physical limitations of the reaction system. As these results are determined independent of reaction rate, some of the solutions obtained may be physically unrealizable. Examples of this are cellulose conversions that are predicted at greater than 85% and unrealistically long retention times where enzymes may, in practice, cease to be active.
Mass balance predictions of the reactor volume changes (at 85% cellulose conversion) required for fed-batch operation as in Fig. 1
Development of the Optimal Control Problem
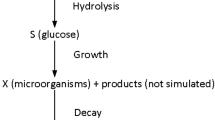
For kinetic studies, the mechanistic model of Kadam et al. [6], developed for dilute sulfuric acid-pretreated corn stover, was used in this study. The model provides rate expressions for concentrations of glucose, cellobiose, and cellulose and considers Langmuir adsorption of cellulase enzyme and competitive inhibition of enzymes by glucose and xylose. The kinetic model was implemented in MATLAB (The MathWorks, Natick, MA, USA) and was modified to consider changes in volume for fed-batch operation as well as introducing several new state variables: insoluble solids concentration, enzyme concentration, and total reaction mass. Because cellulose is the primary insoluble component undergoing solubilization, the rate of change of insoluble solids is assumed to be identical to the rate of cellulose hydrolysis. In addition to these new variables, all of the other kinetic equations needed modification to account for the feeding and the change in reaction mass. A new variable that needed to be introduced to account for the changing mass and concentration due to a feed stream was the dilution rate (D), defined as the feed flow rate per reactor mass. Modified model equations are listed in Table 1, where all of the expressions for rate terms used in this model and the parameters within the rate terms are found in Kadam et al. [6]. It should be considered that the base kinetic model was developed using CPN cellulase (Iogen, Ottawa, Ontario, Canada), while this experimental work used a Genencor cellulase and PCS produced under similar but not identical conditions. An additional consideration for a fed-batch model is the residence time distribution of the substrate over time and changing reactivity of the substrate. Because not all cellulose is equally accessible to enzymatic attack, the rate can be dependent on the amount of substrate already utilized. While the substrate residence time is properly considered in the fed-batch model, the distribution of the substrate reactivity may introduce some error as well as the fact that the PCS was produced in a different pretreatment batch.
A general form for the continuous-time nonlinear system that is approximated by the model system in the optimal control problem can be described by the following system of equations as:
where x(t) and u(t) are the vectors of state variables and control variables, respectively. A continuous form for the objective function for this algorithm seeks to minimize the difference between the desired process trajectory and the predicted process trajectory, giving the minimization algorithm as:
subject to:
where r(t) is the desired output trajectory of the controlled variable and x i (t) is the controlled variable. The optimal path for u(t) can be determined by reformulating the problem in terms of a Hamiltonian equation and developing a set of adjoint equations in order to solve the optimization problem under the conditions of the Pontryagin maximum principle [37]. Another approach is to discretize this continuous form of the objective function by subdividing the time region into K equal intervals as:
and the discretized form of the minimization algorithm generated becomes a series of optimization tasks as:
subject to:
Using this approach, only K values for the control variable u(k) are required. These control actions are fixed over each time interval, resulting in a straightforward optimization problem. For this method, as the number of time steps is more finely discretized, the calculated profile approaches the continuous optimal profile [32].
Fed-Batch Saccharification Model Simulation
In order to develop an optimal fed-batch saccharification strategy, variables and parameters that are affected by the feeding must be identified. Many options can be considered for the development of such a fed-batch strategy, and some of those applied in the past are discussed in the “Introduction” section. One barrier to developing a high-solids saccharification process in STRs is rheological challenges manifested as limitations in the ability to maintain effective mass and heat transfer. Previous work in our laboratory (unpublished) has demonstrated using PCS solids that the saccharification reaction is scalable from shake flasks to bench-scale STRs with respect to reaction rate and extent of reaction at insoluble solids levels of 15% or less provided proper attention is given to agitator and heat jacket design. Above this level, STRs exhibit significant problems with mixing and temperature control. Based on this finding, the fed-batch approach control objective is to optimize reactor conditions (insoluble solids levels) at conditions that enable adequate mixing and temperature control. Although a number of variables exist that can be altered to achieve this objective during the process of feeding, the feed rate is the only variable that is free to be changed.
Using the kinetic model, a feeding policy was developed based upon controlling the insoluble solids below a defined critical value during the saccharification reaction. This is possible by feeding a stream of PCS solids and cellulase at a rate that approximately matches the rate at which cellulose in the reactor is depolymerized and solubilized. Using the kinetic model equations, the rate of change for insoluble solids can be determined with the set of initial operating conditions and a feed rate.
To determine a solution for the feeding policy, the set of equations must be integrated over time with the value for feed rate manipulated in order to satisfy the control objective for insoluble solids level. For this, the rate of change of insoluble solids level (Eq. 11) can be either set to 0 as:
or it can be maintained to achieve a specified trajectory [S I,SP(t)] as a function of the insoluble solids and sugar levels:
since sugar level can be correlated to the substrate conversion and consequently to slurry rheology. To achieve the control objectives of either Eqs. 22 or 23, the objective function of the optimization problem (Eq. 21) becomes:
while the constraints become the system of model equations in Eqs. 11 through 17.
Using this optimal control algorithm, fed-batch feeding policies were developed by generating a set of feeding curves over various reactor solids concentrations and initial conditions generated to determine within the theoretical physical limitations, constraints of the systems, and the potential for using a fed-batch approach. Ultimately, physical limitations constrain the cumulative insoluble solids level to which a reactor can be fed, as discussed previously.
Results
Kinetic Model Simulations
Using the kinetic model and fed-batch optimization approach outlined above, simulations were performed to gauge which variables significantly affect process kinetics to aid in developing a practical experimental protocol. Kinetic simulations were performed assuming an enzyme loading of 40 mg protein per gram of cellulose (10.7 FPU per gram of cellulose for Spezyme CP) and a cellulose/β-glucosidase ratio of 0.975:0.025. Figure 3 shows four feeding policies developed from a MATLAB simulation to maintain insoluble solids at a constant level for a feed stream of 45% insoluble solids, with the target cumulative insoluble solids in the reactor specified as 25%. The results indicate that long residence times (more than 300 h) are required when solids are controlled at 15% or lower because both more solids and entrained liquid must be added to the reactor to reach these higher solids levels during the course of the reaction when controlling at lower insoluble solids levels. The water content of the feed stream solids (insoluble solids) is one variable that strongly affects fed-batch reaction performance. Fed-batch simulations (results not shown) for the same feeding requirements (i.e., identical requirements for initial conditions and final insoluble solids concentrations) indicate that increasing the insoluble solids level in the feed increases both the rate of cellulose conversion (due to faster feeding) and especially the time required to attain an equivalent level of insoluble solids. The increased rate at high cellulose concentrations is due to the approximately first-order kinetics of the enzymatic reaction, where higher reactant concentrations result in faster reaction kinetics. These simulation results also indicate that higher insoluble solids levels in the feed result in both smaller reactor volumes (Fig. 2) and shorter residence times to achieve a given feeding objective (Fig. 3). The reason for this is that feeding a lower solids stream adds more water and, in so doing, effectively dilutes the product (glucose) in the reactor.
Another feeding policy option is to use a non-constant insoluble solids level for the control trajectory. Our previous unpublished findings determined that the extent of enzymatic digestion of PCS solids (in addition to insoluble solids concentration) affects the mixing rheology in STRs. After enzymatic hydrolysis, higher levels of glucan-depleted solids could be mixed effectively relative to the same level of glucan-rich solids, presumably due to the removal of long-chained polymeric cellulose and hydrophilic hemicellulose. This should decrease slurry viscosity that confounds the mixing by removing network-forming cellulose that results in a high-yield stress and decreases the water-absorbing capacity of these solids. We implemented this scheme with insoluble solids initially maintained at 12% and then increased the insoluble solids loading to about 15% over the course of the saccharification reaction to achieve an equivalent cumulative solids loading of 25%. The rate of increase in the insoluble solids set point (S I,SP) is proportional to the amount of glucose produced. This will result in both a faster feeding time than if the solids were maintained only at 12% and allow better mixing to be achieved than if the reactor were maintained at 15%. The final glucose and cellobiose concentrations at the end of saccharification was used to estimate the cumulative insoluble solids. Based on a linear increase in insoluble solids from 12% to 15% with respect to soluble sugars, the following empirically derived relation was developed:
This was derived estimating the best-case final total soluble sugar concentration when feeding was complete (approximately 140 g/kg of sugar produced from 25% insoluble solids). This approach (F1) was one of the two feeding policies selected for experimental validation of the modeling results. The other (F2) was based on controlling the insoluble solids at 15%, which was shown in simulation to have relatively rapid performance using a feed stream of 45% insoluble solids. As shown in Fig. 3, this range of feeding choices covers both the benefits of higher solids operation, while trying to minimize the drawbacks associated with the reactor solids limitations, and long reaction times. Figure 4 shows the two feeding policies (F1 and F2) chosen for experimental testing in the bench-scale STRs, while important characteristics and properties of the feeding policies are outlined in Table 2. As seen in Fig. 4, the model predicts that by maintaining the insoluble solids level at 15% as in F2, solids can be fed up to 25% cumulative insoluble solids within 120 h while F1 requires 216 h.
Experimental Implementation of Fed-Batch
The two feeding policies simulated in Fig. 4 were performed experimentally in bench-scale STRs. Batch saccharification in shake flasks was also performed in triplicate at 25% initial insoluble solids under similar conditions as a control and to determine maximum saccharification potential. The shake flask data, without the limitations for temperature control imposed by the reaction vessel, are meant to be considered as the “best case” for these reaction conditions since these can be assumed to have uniform temperature throughout the saccharification due to the reaction being performed in an air-temperature-controlled shaking incubator. However, reaction rate limitations due to increased resistance to mass transfer at high insoluble solids cannot be ruled out in shake flasks, since other work in our laboratory implicates mass transfer as a rate-limiting factor in these reaction systems at very high solids concentrations (greater than 19% insoluble solids, data not shown). The calculated insoluble solids concentration profiles for both feeding policies are depicted in Fig. 5. The discrete feeding times were every 24 h with two on the first day due to the rapid initial hydrolysis rate until the model-determined feeding period was complete. It was assumed that this discrete feeding would improve the mixing relative to a continuous feeding since most of the residence time is spent below the insoluble solids set point (maximum insoluble solids level) determined by the feeding policy. The experimental concentration profiles closely approximate the profiles simulated/projected using the model (Fig. 4), although for F1 the actual insoluble solids never reached more than 14% insoluble solids in the reactor due to the “best-case” assumption of the feeding policy. Both fed-batch reactors were able to maintain low enough levels of insoluble solids that temperature control did not present an apparent problem.
The profiles of the liquid phase glucose and cellobiose concentrations and cellulose conversion levels are plotted in Fig. 6a, b. The “saw-tooth” pattern for the fed-batch reactors is due to the change in volume and subsequent dilution of reactor components after addition of the feed. The final sugar concentration and conversion values for both F1 and F2 feeding policies match the batch shake flask results demonstrating that both reactor systems are capable of reaching approximately 80% cellulose conversion under the test conditions. This conversion represents approximately the maximum digestibility of this substrate at any insoluble solids level due to effectiveness of the pretreatment rather than due to sugar inhibition. The cellobiose concentrations were particularly high for all cases due to the high concentrations of glucose, which would be more inhibitory to β-glucosidases, and impede this portion of the reaction. Supplementation of the original enzyme preparation with β-glucosidase would likely improve the cellobiose hydrolysis. These results experimentally validate that achieving equivalent results using fed-batch operation need not require significantly longer residence times than a batch reaction. The reactor using feeding policy F1 shows a slower saccharification rate than F2 due to the longer time required for feeding, while F2 shows more rapid rates than were predicted by simulation due to unavoidable errors in the model as well as differences between the experimental system in this work and the model system as discussed previously.
Discussion
While high solid cellulose saccharification holds promise from a process economics perspective, such an approach presents a number of challenges for both the reaction and the reactor system, particularly with handling high-solids biomass slurries. Reaction-derived challenges include sugar inhibition, mass transfer, and longer residence times. Advantages associated with a high-solids saccharification approach include higher product titer, higher reactor volumetric productivity, and concomitant lower capital and costs for saccharification and downstream operations that such an approach would permit.
Process modeling was used both to provide insights into the process performance and was used to optimize the process for experimental implementation. The modeling portion of this work used mass balance and kinetic models to examine the potential advantages of a fed-batch system relative to a batch process. It was determined that the principal advantage of a fed-batch saccharification process is that cumulative solids level can be increased in a reactor during feeding, thereby extending the operational solids capacity of a given reactor. Using the kinetic model, reaction limitations could be identified more clearly and used to choose an experimental operating strategy such as the ones selected. As outlined in Fig. 1, the mass balance model demonstrated that, independent of the kinetics and limitations imposed by the feed stream insoluble solids level, an enzymatic saccharification reactor could be maintained at, for example, 15% insoluble solids while being fed a high-solids PCS stream to achieve a cumulative insoluble solids concentration of 27% assuming 85% cellulose conversion. Such an approach exploits the solubilization of cellulose during enzymatic hydrolysis (depolymerization of cellulose to glucose, cellobiose, and soluble dextran oligomers) to increase the solids loading to the reactor, which would not have been capable of handling it if the solids were all added initially. A previously developed kinetic model was modified to consider fed-batch operation and used to characterize the kinetic requirements and time-dependent limitations of a fed-batch process and to use the insights gained from this model to identify potential feeding policies capable of achieving what was predicted by the mass balance model. Two of these feeding policies were applied experimentally to test the accuracy of the simulation results. Comparison of the accuracy of the model prediction to the experimental data indicated that, although the model was slightly lacking in robustness, the generalization properties were acceptable enough to demonstrate an effective high-solids saccharification process based on the kinetic and physical limitations determined from the model.
The experimental results validated that a well-designed fed-batch approach could be used to allow an STR reactor capable of handling PCS slurries at approximately 15% insoluble solids, the approximate upper limit for the reactor system used, to operate at cumulative initial insoluble solids as high as the set goal of 25%, while achieving high cellulose conversions without significantly increasing the reaction time. It was also demonstrated that a kinetic model could be applied in an open-loop optimal control scheme to determine a fed-batch feeding policy capable of facilitating mixing and temperature control within the reactor, while achieving these high cumulative insoluble solids levels. While shake-flask reactors may be suitable for laboratory-scale processing of high-solids slurries, at larger scales, reactors are ultimately limited by physical factors associated with these slurries. Because of this, shake-flask reactors may be considered as the “best-case” reaction system, since these do not have the limitations of temperature control and mixing encountered in STRs. When compared to batch saccharification results obtained in shake-flask reactors, the overall rates in fed-batch reactors were slower due to the shorter cumulative residence times, although the final conversion results were similar. This is significant since slurry handling by process equipment (pumping, mixing, temperature, and pH control) is greatly simplified by operating at a lower solids level while gaining the economic advantages of a high-solids process. Most importantly, the STR was able to achieve very high cumulative solids loadings (equivalent to 25% initial insoluble solids) that a reactor of this type would be incapable of handling otherwise, thereby improving its operating capability and range.
The implications of this work are that fed-batch saccharification enables high solids levels to be hydrolyzed at high conversions without the drawbacks associated with a high-solids slurry that would limit level of solids used in stirred tank reactors. Final liquid phase glucose concentrations greater than 130 g/l and estimated cellulose conversions of 80% were achieved in both reactors, which is industrially realistic. These high sugar levels are significant in that previous high-solids enzymatic saccharification work [9, 18–20] assumes sugar inhibition to be a severely limiting factor, necessitating an SSF approach. Not only does this work show that current enzyme preparations are capable of cellulolytic activity at these high glucose levels but that fed-batch can be used to perform these reactions at reasonable insoluble solids levels in STRs. Thus, SSF is not necessary as a means to offset sugar inhibition of enzymes and reactors can be operated at the optimum temperature for enzymatic hydrolysis. However, capital costs for reactors can be decreased by the combination of a fermentation and saccharification step if a better compromise on reactor temperature can be reached.
The long retention times required by this work also needs to be addressed. While 300 h may not be considered economically promising, this is the worst case at a relatively low enzyme loading (40 mg protein per gram cellulose). Faster rates were achieved for feeding policy F2, where cellulose conversions of nearly 80% were achieved in 168 h. These rates have the potential to be improved either using higher enzyme loadings or with improved enzyme preparations with increased tolerance to sugar inhibition.
A train of continuous stirred tank reactors is often the most practical reactor configuration for an enzymatic hydrolysis and fermentation process [11]. For a continuous process to operate using a high-solids feed stream such as was used in this work (25% insoluble solids), the first reactors in the series would require operation at insoluble solids levels much greater than 15%. However, just as a series of continuous reactors approximates a batch reactor, the reactor train could be operated by feeding concentrated fresh solids and enzymes to reactors further down the chain in an approximation of fed-batch operation. For this, the reactors would each operate at a lower insoluble solids level, while obtaining the benefits of higher effective solids loadings and higher product concentrations. Another process configuration that is suggested by this work is a separate utilization of the soluble hemicellulosic sugars and the glucan-rich insoluble solids from pretreatment, whereby enzymatic hydrolysis of the washed, inhibitor-free insoluble solids could be used to yield a high-purity glucose stream to be used as a feedstock for further processing.
While these results are encouraging, the experiments were performed using a substrate that was washed to remove pretreatment hydrolyzate liquor, providing an “ideal” substrate. One particularly problematic obstacle to enzymatic saccharification of full-slurry unwashed lignocellulose is the pronounced decrease in enzymatic effectiveness in the presence of pretreatment hydrolyzate liquors. Rather than the presence of toxic inhibitors such as phenolics and furans in the hydrolyzate slurry as is the case for fermentation inhibition, the inhibition of enzymatic hydrolysis is most likely due to inhibition by hemicellulosic sugars released during pretreatment and the nonproductive binding of enzymes to hydrophobic lignin-derived compounds [38]. Future studies should build upon this work under process-relevant conditions by characterizing the effects of pretreatment hydrolyzate liquors on enzymatic hydrolysis, particularly relevant for high-solids saccharification where inhibitors are more concentrated.
References
Sheehan, J., & Himmel, M. (1999). Biotechnology Progress, 15, 817–827.
Schell, D. J., Farmer, J., Newman, M., & McMillan, J. D. (2003). Applied Biochemistry and Biotechnology, 105–108, 69–85.
Calsavara, L. P., De Moraes, F. F., & Zanin, G. M. (1999). Applied Biochemistry and Biotechnology, 77–79, 789–806.
Chang, V. S., & Holtzapple, M. T. (2000). Applied Biochemistry and Biotechnology, 84–86, 5–37.
Converse, A. O., Ooshima, H., & Burns, D. S. (1990). Applied Biochemistry and Biotechnology, 24–25, 67–73.
Kadam, K. L., Rydholm, E. C., & McMillan, J. D. (2004). Biotechnology Progress, 20, 698–705.
Philippidis, G. P., & Hatzis, C. (1997). Biotechnology Progress, 13, 222–231.
Schell, D. J., Ruth, M., & Tucker, M. (1999). Applied Biochemistry and Biotechnology, 77–79, 67–81.
Mohagheghi, A., Tucker, M., Grohmann, K., & Wyman, C. (1992). Applied Biochemistry and Biotechnology, 33, 67–81.
Wingren, A., Galbe, M., & Zacchi, G. (2003). Biotechnology Progress, 19, 1109–1117.
Aden, A., Ruth, M., Ibsen, K., Jechura, J., Neefes, K., Sheehan, J., et al. (2002). NREL Technical Paper 510–32438, Golden, Colorado, USA.
Mandels, M., & Reese, E. T. (1965). Annual Review of Phytopathology, 3, 85–102.
Pimenova, N. V., & Hanley, T. R. (2003). Applied Biochemistry and Biotechnology, 105–108, 383–392.
Xiao, Z., Zhang, X., Gregg, D. J., & Saddler, J. N. (2004). Applied Biochemistry and Biotechnology, 115, 1115–1126.
Boer, H., & Koivula, A. (2003). European Journal of Biochemistry, 270, 841–848.
Alizadeh, H., Teymouri, F., Gilbert, T. I., & Dale, B. E. (2005). Applied Biochemistry and Biotechnology, 121–124, 1133–1141.
Zhu, Y., Lee, Y. Y., & Elander, R. T. (2004). Applied Biochemistry and Biotechnology, 117, 103–114.
De Bari, I., Viola, E., Barisano, D., Cardinale, M., Nanna, F., Zimbardi, F., et al. (2002). Industrial & Engineering Chemistry Research, 41, 1745–1753.
Varga, E., Klinke, H. B., Réczey, K., & Thomsen, A. B. (2004). Biotechnology and Bioengineering, 88, 567–574.
Jørgensen, H., Vibe-Pedersen, J., Larsen, J., & Felby, C. (2007). Biotechnology and Bioengineering, 96, 862–870.
Ballesteros, I., Oliva, J. M., Negro, M. J., Manzanares, P., & Ballesteros, M. (2002). Applied Biochemistry and Biotechnology, 98–100, 717–732.
Tengborg, C., Galbe, M., & Zacchi, G. (2001). Enzyme and Microbial Technology, 28, 835–844.
Fan, Z., South, C., Lyford, K., Munsie, J., van Walsum, P., & Lynd, L. R. (2003). Bioprocess and Biosystems Engineering, 26, 93–101.
Pristavka, A. A., Salovarova, V. P., Zacchi, Z., Berezin, I. V., & Rabinovich, M. L. (2000). Prikladnaa biohimia i mikrobiologia, 36, 278–86.
Söderström, J., Galbe, M., & Zacchi, G. (2004). Biotechnolgy Progress, 20, 744–749.
Borden, J. R., Lee, Y. Y., & Yoon, H. H. (2000). Applied Biochemistry and Biotechnology, 84–86, 963–970.
Kleman, G. L., Chalmers, J. J., Luli, G. W., & Strohl, W. R. (1991). Applied and Environmental Microbiology, 57, 910–917.
Rudolf, A., Galbe, M., & Liden, G. (2004). Applied Biochemistry and Biotechnology, 113–116, 601–617.
Taherzadeh, M. J., Niklasson, C., & Liden, G. (2000). Biotechnology and Bioengineering, 69, 330–338.
Nilsson, A., Taherzadeh, M. J., & Liden, G. (2002). Bioprocess and Biosystems Engineering, 25, 183–191.
Hodge, D. B., & Karim, M. N. (2002). Biotechnology Progress, 18, 572–579.
Lee, J. H., Lim, H. C., Yoo, Y. J., & Park, Y. H. (1999). Bioprocess Engineering, 20, 137–146.
Hong, J. (1986). Biotechnology and Bioengineering, 28, 1421–1431.
Modak, J. M., Lim, H. C., & Tayeb, Y. J. (1986). Biotechnology and Bioengineering, 28, 1396–1407.
Chung, Y. C., Bakalinsky, A., & Penner, M. H. (1997). Applied Biochemistry and Biotechnology, 66, 249–262.
Weast, R. C. (1985). CRC handbook of chemistry and physics, (66th ed.). Boca Raton: CRC.
Gamkrelidze, R. V. (1978). Principles of optimal control theory. New York: Plenum.
Palonen, H., Tjerneld, F., Zacchi, G., & Tenkanen, M. (2004). Journal of Biotechnology, 107, 65–72.
Acknowledgement
The US Department of Energy (DOE) supported this work through the Office of the Biomass Programs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hodge, D.B., Karim, M.N., Schell, D.J. et al. Model-Based Fed-Batch for High-Solids Enzymatic Cellulose Hydrolysis. Appl Biochem Biotechnol 152, 88–107 (2009). https://doi.org/10.1007/s12010-008-8217-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8217-0