Abstract
Varying ionic liquid, 1-ethyl 3-methyl imidazolium acetate, pretreatment incubation temperature on lignocellulosic biomass substrates, corn stover, switchgrass and poplar, can have dramatic effects on the enzymatic digestibility of the resultant regenerated biomass. In order to delineate the chemical and physical changes resulting from the pretreatment process and correlate changes with enzymatic digestibility, X-ray powder and fiber diffraction, 13C cross polarization/magic angle spinning nuclear magnetic resonance spectroscopy, and compositional analysis was completed on poplar, corn stover and switchgrass samples. Optimal pretreatment incubation temperatures were most closely associated with the retention of amorphous substrates upon drying of regenerated biomass. Maximal glucan to glucose conversion for 24 h enzyme hydrolysis was observed for corn stover, switchgrass and poplar at ionic liquid incubation temperatures of 100, 110 and 120 °C, respectively. We hypothesize that effective pretreatment temperatures must attain lignin redistribution and retention of xylan for optimal enzyme digestibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is an abundant carbon source which can provide a renewable platform for carbon based fuels and chemicals and unlike corn, wheat or soy is not a food source. Lignocellulose is comprised of three major components: cellulose, a polymer of glucose monomeric units (30–55 %); hemicellulose, a highly substituted polysaccharide mixture of pentose and hexose sugars (8–25 %); and lignin (10–35 %), a macromolecule consisting of phenyl propanoid subunits (Zhao et al. 2012). The cellulose component is highly crystalline whereas the hemicellulosic and lignin components are less structured. The hydrolysis of the polysaccharide fractions, cellulose and hemicellulose, to monomeric sugars forms a sugar platform for production of fuels and value-added chemicals through fermentation or chemical conversion. Monomeric sugars can be produced through a two-step biochemical process consisting of biomass pretreatment followed by enzymatic hydrolysis. Pretreatment of the biomass produces a more readily hydrolyzed substrate while enzymatic deconstruction of polysaccharides to monomeric sugars avoids the formation of sugar decomposition products that can be inhibitory to downstream fermentation steps. Structural and chemical changes in biomass during pretreatment have a profound effect on enzyme digestibility and downstream processing.
Lignocellulosic structure with highly crystalline cellulose imbedded in a matrix of less structured hemicellulose covalently cross linked with hydrophobic lignin creates a major barrier to conversion of polysaccharides to monomeric sugars. This is due largely to the inaccessibility of cellulose to water and enzymes. Properties of biomass that affect recalcitrance to enzyme hydrolysis include cellulose crystal morphology and crystallinity (Samayam et al. 2011; Cheng et al. 2011; Zhu et al. 2008; Puri 1984), lignin and hemicellulose content (Zhu et al. 2008; Selig et al. 2009; Bura et al. 2009), and particle size (Yeh et al. 2010). A number of chemical pretreatment technologies have been developed to counteract the natural recalcitrance of biomass to deconstruction. Some pretreatment technologies (i.e. acid pretreatment) can lead to the degradation of hemicellulose (Torget et al. 1988) resulting in the substantial loss of carbon and the formation of fermentation inhibitors (Chheda et al. 2007) as well as retention or enhancement of cellulose crystallinity (Pingali et al. 2010a, b).
Ionic liquid (IL) pretreatment is an alternative process that can largely retain hemicellulose and convert the native cellulose I allomorph to cellulose II or an amorphous substrate without the production of inhibitors, enabling greater access of polysaccharides for enzymatic deconstruction (Dadi et al. 2006; Li et al. 2010; Wang et al. 2011; Samayam and Schall 2010; Samayam et al. 2011). The ease of cellulose deconstruction is dependent on its structure. Amorphous cellulose is the most easily hydrolyzed followed by cellulose II (mercerized cellulose) then cellulose I (Wada et al. 2010; Samayam and Schall 2010). The transition from cellulose I to cellulose II has been found to be dependent upon IL treatment temperature and appears to vary with specific biomass feedstocks (Samayam et al. 2011; Cheng et al. 2011). It has been suggested that mercerization is furthered when lignin is redistributed as its glass transition temperature is approached (Wormald et al. 1996; Samayam et al. 2011). Lignin glass transition temperature is feedstock dependent and has been reported to range between 100 and 160 °C (Arora et al. 2010; Hatakeyama et al. 1982; Irvine 1985; Selig et al. 2007).
In order to systematically explore the dependecy of biomass structural changes with IL treatment temperature, a hardwood (poplar) and herbaceous (corn stover and switchgrass) substrates were pretreated at incubation temperatures ranging from 75 to 140 °C with the IL 1-ethyl-3-methyl imdizaolium acetate (EMIM-OAc) followed by IL displacement with water. Characteristics of the IL treated biomass were analyzed including cellulose crystallinity and morphology, composition, and enzymatic digestibility. Structural changes in cellulose were assessed using X-ray powder diffraction (XRD), X-ray fiber diffraction, and solid state cross polarization—magic angle spin 13C (CP/MAS) nuclear magnetic resonance (NMR) spectroscopy (Isogai et al. 1989; Park et al. 2009; Samayam et al. 2011). The enzyme digestibility of the pre-treated substrates was also assessed in order to identify optimal pretreatment temperatures for each substrate.
Materials and methods
Materials
1-ethyl 3-methyl imidazolium acetate (EMIM-OAc) was purchased from Sigma Aldrich (St. Louis, MO). Three different biomass sources were investigated: poplar, corn stover and switchgrass. Poplar samples were provided by the National Renewable Energy Laboratory (Golden, CO). Corn stover and switchgrass samples were provided by the United States Department of Agriculture (USDA, NCAUR Laboratory, Peoria, IL). Commercial enzyme mixture, Cellic CTec2, was obtained from Novozymes North America, Inc. (Franklinton, NC).
Methods
X-ray powder diffraction
Biomass samples were milled, sieved (−80/+20), and dried at ~45 °C in a vacuum oven. Biomass was added to EMIM-OAc at a biomass to ionic liquid ratio of 5 % (wt. biomass/wt. IL). The biomass samples were incubated at constant temperature ranging from 75 to 140 °C for 4 h with mixing. After the 4 h incubation, the biomass samples were washed with deionized water and centrifuged in an Eppendorf centrifuge (model 5810R) with a swinging bucket rotor at 4,000 rpm (3,220×g) for 10 min. The supernatant was removed and the washing process was repeated until the supernatant appeared visually colorless. Pretreated biomass was vacuum-filtered and dried at ~45 °C in a vacuum oven. Before XRD or CP/MAS NMR, biomass samples were ground to powder in a mortar and pestle. The resulting powder was analyzed on an X’pert powder diffractometer PAN 188 analytical with Cu Kα radiation and X’celerator detector with a range of 5°–38° and step size of 0.0334°.
X-ray fiber diffraction
Biomass samples cut into very fine splinters were fully immersed in EMIM-OAc in a glass vial and capped with a septum lid. The biomass samples were incubated followed by IL displacement with water as described above. The samples were then pressed between two microscope slides and placed into a desiccator to dry. A sample of dried biomass was mounted and X-ray diffraction data were collected at room temperature on a Rigaku FR-E diffractometer with copper Kα radiation and an R-Axis IV++ detector. Data were also collected at the Advanced Photon Source at Argonne National Laboratory, BioCARS BM-14-C, with an ADSC Q316 detector with a wavelength of 0.979 Å. Diffraction data from native and mercerized ramie fibers, cellulose I and cellulose II, respectively, were used to guide interpretation of diffraction data. Preparation of cellulose II is described in a previous publication (Samayam et al. 2011).
Solid state 13C CP/MAS NMR
Solid state 13C CP/MAS NMR spectra were collected on a Varian Unity Inova 600 MHz NMR with a magnetic field of 600 MHz and a 13C frequency of 150.84 Hz with a CP/MAS unit at room temperature. Data were collected at a spinning rate of 10 kHz and a contact time of 10 ms. The spectrum was collected for 215 transients.
Commercial enzyme characterization
The protein concentration of Cellic CTec2 was determined using the colorimetric Bradford protein assay with bovine serum albumin standards (Bradford 1976). The rate of hydrolysis of a filter paper substrate was measured via a 2,5-dinitrosalicylic acid assay and expressed as filter paper units, FPU (Miller 1959). The enzyme activity of Cellic CTec2 was also determined using other specific cellulosic and xylan substrates with measurement of release of soluble reducing sugars via a Nelson-Somogyi assay (Nelson 1944). Endoglucanase activity was measured using a carboxymethyl cellulose (CMC) substrate. Cellobiohydrolase activity was measured using crystalline cellulose (Avicel) and endoxylanase activity was measured using xylan (birchwood xylan). One unit of activity, IU, is the release one μmol of soluble sugar reducing ends per minute. β-glucosidase and β-xylosidase activity were measured by the release of 4-nitrophenol from p-nitrophenyl-glucopyranoside and p-nitrophenyl-xylopyranoside, respectively (Gilead and Shoham 1995). For the PNP assay, one unit of activity, IU, is the release one μmol of p-nitrophenol per minute.
Enzymatic hydrolysis
Cellic CTec2 (Novozymes) was added to the substrate at loadings of 5 FPU/g glucan (4 mg/g glucan) for poplar and 7 FPU/g glucan (5 mg/g glucan) for corn stover and switchgrass based on untreated substrate composition. These loadings correspond to an endoglucanase activity loading of 150–200 IU/g glucan. FPU and specific activities of Cellic CTec2 was used to hydrolyze well defined substrates as described previously and are summarized in Supplemental Table 1 (Samayam and Schall 2010; Barr et al. 2012).
Biomass was hydrolyzed in 50 mM citric acid buffer, pH 4.8, at 50 °C with a substrate loading of 1 % (w/v) with orbital shaking for 24 h. A supernatant sample was incubated at 100 °C for 5 min to stop the enzymatic hydrolysis. The released sugars were analyzed by high performance liquid chromatography (HPLC) with refractive index detection. Sugars were separated using a Bio-Rad (Richmond, CA) Aminex HPX-87P carbohydrate analysis column with an isocratic mobile phase of HPLC-grade water at a flow rate of 0.6 mL/min and a column temperature of 80 °C. Mixed sugar standards of known concentrations were used to generate standard curves to calculate the concentration of released sugars. Glucan and xylan converted to glucose and xylose, respectively, were reported as a percentage of theoretical yields of monomeric sugars based on glucan and xylan composition and recovery of pretreated substrates.
Compositional analysis
Carbohydrate and lignin content in the untreated and pretreated biomass were determined in triplicate using concentrated acid hydrolysis followed by dilute acid hydrolysis according to the standard laboratory analytical procedures developed by the National Renewable Energy Laboratory (NREL) standard LAP 002 protocol (Sluiter et al. 2008). Monomeric sugars were quantified by HPLC as described above.
Results and discussions
Crystallinity and crystal polymorph
Cellulose crystallinity can be estimated via a crystallinity index, CrI, from X-ray powder diffraction (XRD) data (Segal et al. 1959) (Table 1). All IL treated biomass substrates exhibit reduced crystallinity compared to the native substrates (~70 % reduction for poplar and 58 and 44 % reductions for corn stover and switchgrass, respectively). As the incubation temperature increases, the crystallinity decreases to minimum then increases at higher temperatures. The CrI temperature minimum varies from substrate to substrate and occurs at ~100 °C for poplar, 75 to 100 °C for corn stover and 110 °C for switchgrass.
Cellulose I (native substrates) XRD data exhibit an intense peak at 2θ ~ 22.6° corresponding to Miller index of 200 and a broad peak at ~15.5° comprised of a doublet peaks corresponding to Miller indices of 1–10 and 110, at 14.8° and 16.3° respectively. This doublet is more clearly seen near the beam center in fiber diffraction of untreated substrates and ramie controls (Supplemental Figure 1). In XRD data, the appearance of cellulose II is characterized by a broad XRD peak at ~20° formed by a doublet of peaks at 19.8° and 22.6° for Miller indices 110 and 020, respectively, and a singlet at a lower 2θ of ~12.1° for 1–10 (Samayam et al. 2011; Isogai et al. 1989). These diffraction features are again more clearly seen in fiber data with a singlet near the beam center and a doublet measured further from the beam center for mercerized ramie controls. A shift of XRD peaks from 2θ ~ 22° to ~20°, and 2θ from 15 to 12° in native and IL treated substrates is more apparent with increasing IL incubation temperature (Fig. 1).
X-ray powder diffraction patterns of native and IL-treated a poplar, b corn stover, and c switchgrass incubated in IL at the noted temperatures. Samples were dried before measurement of diffraction. XRD patterns indicate a conversion from cellulose I (native substrate) on the bottom progressing to cellulose II at the top (increasing IL treatment temperatures)
X-ray fiber data can often reveal finer structural details from diffraction. (Supplemental Figures 2–4). Poplar and corn stover fiber samples retain diffraction features consistent with cellulose I at IL incubation temperatures up to 75 °C particularly in the low angle reflections (near the beam center). However at an IL pretreatment temperature of 85 °C, corn stover loses much of the native diffraction features although a broad band of diffraction intensity corresponds more closely with cellulose I than cellulose II. In all samples, as temperatures increase the biomass appears largely amorphous with weak diffraction features. Overall, the fiber diffraction features appear weakest at an incubation temperature of 100 °C for poplar, at 85 °C for corn stover and 110 °C for switchgrass. However, once pretreatment temperatures are 120 °C and higher, diffraction features reappear, indicating increasing order. This is seen in the increase in intensity in the reflections closer to the beam center. We interpret these features as corresponding most closely with cellulose II for all substrates.
While weaker diffraction features are more apparent in fiber data compared to XRD, it is still difficult to identify transitions from cellulose I to cellulose II in the limited crystalline and largely amorphous IL treated substrates. Solid-state 13C CP/MAS NMR was used to further probe for the presence of cellulose I and cellulose II polymorphs. There are differences in the chemical shifts of the C4 and C6 carbons in the transition from cellulose I and cellulose II from 89.1–89.8 ppm to 88.7–88.8 ppm and from 65.5–66.2 ppm to 63.5–64.1 ppm, respectively (Isogai et al. 1989). Additionally, a small peak near 98 ppm appears in cellulose II, attributed to “the α-type anomeric C1 carbon of the reducing end glucose residue in the cellulose oligomers” (Isogai et al. 1989). These chemical shifts can be observed in cellulose I and cellulose II standards (Supplemental Figure 5).
Native biomass NMR spectra are shown with positions of the aforementioned key chemical shifts for cellulose I and II (Fig. 2). For all IL incubation temperatures the resulting biomass spectra appears to display a reduction in cellulose I features and a gradual increase in cellulose II features with increasing temperature. Chemical shifts associated with cellulose II appear to become dominant between 100 and 120 °C for poplar, between 75 and 100 °C for corn stover, and between 110 and 120 °C for switchgrass.
Cellulose crystal structural characterization using solid state 13C CP/MAS NMR of a native and recrystallized poplar, b native and recrystallized corn stover, and c native and recrystallized switchgrass. Spectra are offset to facilitate visualization. NMR spectra indicate a conversion from cellulose I (native substrate) on the bottom to cellulose II at the top. The C4 (89.1–89.8 ppm to 88.7–88.8 ppm) and C6 (65.5–66.2 ppm to 63.5–64.1 ppm) peaks indicative of the transition from cellulose I and cellulose II as well as a characteristic cellulose II peak at ~97 ppm are marked. The NMR spectrum for native biomass contains a single peak at ~88.9 ppm corresponding to the C4 peak for cellulose I
Composition
The overall biomass losses were determined gravimetrically by measuring the weight of the dry biomass before and after pretreatment (Table 1). Biomass loss to the IL or wash solutions increases with increasing incubation temperature for all substrates, as has been observed in other studies (Arora et al. 2010). Overall mass losses of ~20 % were higher for the herbaceous substrates than ~10 % for the hardwood poplar at pretreatment temperatures of 120 °C or less.
Lignin, xylan and glucan composition in biomass was measured allowing estimation of these component losses. Over 90 % of the glucan was recovered at all incubation temperatures explored for poplar and at temperatures <120 °C for corn stover and switchgrass. Xylan losses were higher than those of glucan for all substrates resulting in enrichment of the IL treated feedstock with glucan as incubation temperature increases. Cellulose II recrystallization appears more evident at higher incubation temperatures and is closely associated with higher xylan losses during IL pretreatment. It has been previously reported that increasing fractions of xylan and mannan components in biomass impede mercerization of cellulose I (Serkov et al. 1986; Serkov et al. 1983), consistent with our observed decreases in xylan losses and mercerization at lower ionic liquid incubation temperatures.
Losses of xylan and lignin were greater for the herbaceous substrates than the hardwood poplar for most incubation temperatures. Poplar and corn stover xylan losses were <10 % at incubation temperatures of 100 °C or under whereas switchgrass exhibited higher losses of ~30 % at an incubation temperature of 110 °C. For incubation temperatures of 120 °C or less below, poplar exhibited <10 % lignin losses, whereas corn stover and switchgrass lignin losses were approximately 20 and 30 %, respectively. At an IL treatment temperature of 120 °C, poplar xylan losses increased to ~20 %.
Enzymatic digestibility
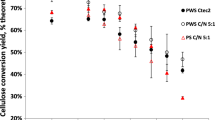
Ultimately, our aim is identify maxima in enzyme digestibility of biomass polysaccharides with respect to IL incubation temperature and explore linkages between biomass structural and compositional changes and digestibility. Enzyme digestibility was evaluated by measurement of conversion of glucan and xylan to monomeric sugars after 24 h of hydrolysis (Fig. 3). Enzyme loadings were selected to reach incomplete 24 h monomeric sugar conversions to discern potential maxima. The percent conversion to monomeric sugars was based on composition and mass of IL treated substrates to account for polysaccharide losses during pretreatment (Table 1). Consistent with prior reports (Samayam and Schall 2010; Samayam et al. 2011; Barr et al. 2012), ionic liquid pretreatment greatly improves polysaccharide hydrolysis when compared to hydrolysis of native biomass (Fig. 3). This improvement is observed even at the lowest incubation temperatures.
24 h hydrolysis of native and recrystallized a poplar, b corn stover, and c switchgrass. Solid/blue bars denote percent glucan to glucose conversion while hashed/red bars denote percent xylan to xylose conversion. Percent conversion is based on the recrystallized biomass composition. Samples without error bars are duplicates with deviations <10 % while those with error bars are triplicates. (Color figure online)
Maxima in the glucan and xylan conversion to monomeric sugars with respect to pretreatment temperatures are observed for all substrates (Fig. 3). For poplar, a maximum conversion of glucan (60 %) and xylan (70 %) to monomers occurs at 120 °C. The optimal pretreatment temperatures appear to be lower for the herbaceous crops than poplar. At the incubation temperatures explored, corn stover has a maximum in glucan (60 %) and xylan conversion (55 %) at 100 °C. Switchgrass has a maximum glucan conversion (45 %) at 110 °C. Glucan conversion assessed at incubation temperatures of 110 °C for poplar and corn stover and 100 °C for switchgrass yielded 24 h conversions of 55, 46 and 45 %, respectively, lower than the maxima (data not shown in Fig. 3).
The maximum for xylan conversion to xylose for switchgrass was at an IL incubation temperature of 140 °C. This is likely a result of the larger xylanase loading per gram of xylan. Losses of xylan during pretreatment at this temperature were ~80 % leading to a concomitant increase in loading of xylanase per gram of xylan. Similar xylan losses with EMIM-OAc treatment of switchgrass have been reported previously (Arora et al. 2010). Since unmodified CTec2 enzyme mixture was used, xylanase loadings were not adjusted independently of the cellulase loadings.
The temperatures of the glucan 24 h hydrolysis maxima appear to be consistent with low CrI substrates. Substrates are largely amorphous with evidence of some cellulose recrystallization and little to no residual cellulose I features. At incubation temperatures higher than the enzyme hydrolysis maxima, the polymorph exhibits exclusively cellulose II features as observed in both the NMR and diffraction data. This result was true for all three substrates examined. This is consistent with an expectation of relative ease in digestion of amorphous cellulose followed by cellulose II then cellulose I. Therefore, one of the strongest factors associated with IL pretreatment enhancement of biomass hydrolysis appears associated with increasing cellulose chain mobility, affecting a transformation of cellulose I to cellulose II at incubation temperatures above the hydrolysis maxima.
Deacetylation of hemicellulose has been reported in EMIM-OAc and other cellulose dissolving ILs at temperatures ranging from 60 to 120 °C (Çetinkol et al. 2010) (Labbé et al. 2012). Acetylation of lignin has also been reported in EMIM-OAc at 120 °C (Çetinkol et al. 2010). The deacetylation of xylan in conjunction with the acetylation of lignin may indicate reactions that sever hemicellulose-lignin covalent linkages. Interruptions of theses linkages combined with temperatures nearing the glass transition temperature of lignin may lead to redistribution of lignin and increased mobility of cellulose. Lignin coalescence outside the secondary cell wall in EMIM-OAc treated biomass has been reported previously in imaging studies (Singh et al. 2009).
Interruptions in the hemicellulose-lignin linkages may facilitate increased xylan solubilization during IL pretreatment. Increasing xylan losses are observed at increasing incubation temperatures (Table 1). At higher incubation temperature the xylan removal may lead to enhancement of cellulose recrystallization upon sample drying. Previous studies have shown that xylan can impede the mercerization of cellulose (Serkov et al. 1983, 1986). In order to retain a primarily amorphous cellulosic substrate even upon drying, retention of xylan during ionic liquid pretreatment may be desirable.
Conclusions
Based on examination of multiple biomass substrates, the optimal IL incubation temperature for maximizing 24 h enzyme hydrolysis ranges from 100 to 120 °C dependent upon substrate. Optimal glucan to glucose conversion occurred at 100 °C for corn stover, 110 °C for switchgrass, and 120 °C for poplar. While these temperatures vary among biomass sources, the properties of the dried regenerated biomass at each the optimal temperatures are similar. At the optimal incubation temperatures, X-ray powder and fiber diffraction techniques show minima in CrI or primarily amorphous material. Examination of NMR spectra indicated that the residual crystalline portion of the biomass at the optimal temperatures may be predominantly cellulose II with some cellulose I. Above these optima, xylan losses greatly increase, leading increasingly to crystalline cellulose II features upon drying. Up to the optimal temperatures, lignin losses are low. The necessity of elevated incubation temperatures may be associated with the need to reach temperatures where lignin coalescence and redistribution is possible. The maxima in IL incubation temperatures appear to be a balance between minimizing xylan losses and increasing cellulose fibril mobility and associated structural transitions.
References
Arora R, Manisseri C, Li C, Ong MD, Scheller HV, Vogel K, Simmons BA, Singh S (2010) Monitoring and analyzing process streams towards understanding ionic liquid pretreatment of switchgrass (Panicum virgatum L.). Bioenergy Res 3:134–145
Barr CJ, Mertens JA, Schall CA (2012) Critical cellulase and hemicellulase activities for hydrolysis of ionic liquid pretreated biomass. Bioresour Technol 104:480–485. doi:10.1016/j.biortech.2011.10.101
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3
Bura R, Chandra R, Saddler J (2009) Influence of xylan on the enzymatic hydrolysis of steam-pretreated corn stover and hybrid poplar. Biotechnol Progr 25:315–322
Çetinkol ÖP, Dibble DC, Cheng G, Kent MS, Knierim B, Auer M, Wemmer DE, Pelton JG, Melnichenko YB, Ralph J (2010) Understanding the impact of ionic liquid pretreatment on eucalyptus. Biofuels 1(1):33–46
Cheng G, Varanasi P, Li C-L, Liu H-B, Melnichenko YB, Simmons BA, Kent MS, Singh S (2011) Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis. Biomacromolecules 12(4):933–941
Chheda JN, Román-Leshkov Y, Dumesic JA (2007) Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem 9:342–350
Dadi AP, Varanasi S, Schall CA (2006) Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng 95(5):904–910
Gilead S, Shoham Y (1995) Purification and characterization of a-L-arabinofuranosidase from Bacillus stearothermophilus T-6. Appl Environ Microbiol 61(1):170–174
Hatakeyama T, Nakamura K, Hatakeyama H (1982) Studies on heat capacity of cellulose and lignin by differential scanning calorimetry. Polymer 23(12):1801–1804
Irvine G (1985) The significance of the glass transition of lignin in thermomechanical pulping. Wood Sci Technol 19(2):139–149
Isogai A, Usuda M, Kato T, Uryu T, Atalla RH (1989) Solid-state CP/MAS carbon-13 NMR study of cellulose polymorphs. Macromolecules 22(7):3168–3172. doi:10.1021/ma00197a045
Labbé N, Kline LM, Moens L, Kim K, Kim PC, Hayes DG (2012) Activation of lignocellulosic biomass by ionic liquid for biorefinery fractionation. Bioresour Technol 104:701–707
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101(13):4900–4906
Miller GL (1959) Use of dinitrosalylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Park S, Johnson D, Ishizawa C, Parilla P, Davis M (2009) Measuring the crystallinity index of cellulose by solid state 13C nuclear magnetic resonance. Cellulose 16(4):641–647. doi:10.1007/s10570-009-9321-1
Pingali SV, Urban VS, Heller WT, McGaughey J, O’Neill HM, Foston M, Myles DA, Ragauskas AJ, Evans BR (2010a) SANS study of cellulose extracted from switchgrass. Acta Crystallogr D Biol Crystallogr 66(11):1189–1193
Pingali SV, Urban VS, Heller WT, McGaughey J, O’Neill H, Foston M, Myles DA, Ragauskas A, Evans BR (2010b) Breakdown of cell wall nanostructure in dilute acid pretreated biomass. Biomacromolecules 11(9):2329–2335
Puri VP (1984) Effect of crystallinity and degree of polymerization of cellulose on enzymatic saccharification. Biotechnol Bioeng 26(10):1219–1222. doi:10.1002/bit.260261010
Samayam IP, Schall CA (2010) Saccharification of ionic liquid pretreated biomass with commercial enzyme mixtures. Bioresour Technol 101:3561–3566
Samayam IP, Hanson BL, Langan P, Schall CA (2011) Ionic-liquid-induced changes in cellulose structure associated with enhanced biomass hydrolysis. Biomacromolecules 12(8):3091–3098
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794
Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2007) Deposition of lignin droplets produced during dilute acid pretreatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog 23(6):1333–1339
Selig MJ, Vinzant TB, Himmel ME, Decker SR (2009) The effect of lignin removal by alkaline peroxide pretreatment on the susceptibility of corn stover to purified cellulolytic and xylanolytic enzymes. Appl Biochem Biotechnol 155:397–406
Serkov A, Klinova S, Vol’f L, Voitenko I (1983) Removal of hemicellulose during the mercerization process. Fibre Chem 15(2):127–130
Serkov A, Kuzicheva N, Fedotova V, Kruglova N (1986) Effect of hemicellulose on the productivity of mercerizing units and viscose filterability. Fibre Chem 17(5):364–366
Singh S, Simmons BA, Vogel KP (2009) Visualization of biomass solubilization and cellulose regeneration during ionic liquid pretreatment of switchgrass. Biotechnol Bioeng 104(1):68–75
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. LAP-002 NREL Analytical Procedure. National Renewable Energy Laboratory (NREL): Golden
Torget R, Himmel M, Wright J, Grohmann K (1988) Initial design of a dilute sulfuric acid pretreatment process for aspen wood chips. Appl Biochem Biotechnol 17(1):89–104. doi:10.1007/bf02779148
Wada M, Ike M, Tokuyasu K (2010) Enzymatic hydrolysis of cellulose I is greatly accelerated via its conversion to the cellulose II hydrate form. Polym Degrad Stab 95(4):543–548
Wang Y, Radosevich M, Hayes D, Labbe N (2011) Compatible ionic liquid-cellulases system for hydrolysis of lignocellulosic biomass. Biotechnol Bioeng 108(5):1042–1048
Wormald P, Wickholm K, Larsson PT, Iversen T (1996) Conversions between ordered and disordered cellulose. Effects of mechanical treatment followed by cyclic wetting and drying. Cellulose 3(1):141–152
Yeh A-I, Huang Y-C, Chen SH (2010) Effect of particle size on the rate of enzymatic hydrolysis of cellulose. Carbohydr Polym 79(1):192–199. doi:10.1016/j.carbpol.2009.07.049
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels, Bioprod Biorefin 6(4):465–482. doi:10.1002/bbb.1331
Zhu L, O’Dwyer JP, Chang VS, Granda CB, Holtzapple MT (2008) Structural features affecting biomass enzymatic digestibility. Bioresour Technol 99:3817–3828
Acknowledgments
Research funding was provided by the National Science Foundation grant #0933250, and National Science Foundation GK-12 Program, grant #DGE-0742395. Additional funding for undergraduate research opportunities (KC and GP) as well as access to Argonne National Laboratories Advanced Photon Source was from NSF REU grant 1004921. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract No. DE-AC02-06CH11357. Assistance in XRD and NMR was provided by Dr. Pannee Burckel, Instrumentation Center, and Dr. Yong Wah Kim, NMR Laboratory, University of Toledo.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barr, C.J., Hanson, B.L., Click, K. et al. Influence of ionic-liquid incubation temperature on changes in cellulose structure, biomass composition, and enzymatic digestibility. Cellulose 21, 973–982 (2014). https://doi.org/10.1007/s10570-013-0052-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0052-y