Abstract
The significant increase in the incidence of obesity represents the next global health crisis. As a result, scientific research has focused on gaining deeper insights into obesity and adipose tissue biology. As a result of the excessive accumulation of adipose tissue, obesity results from hyperplasia and hypertrophy within the adipose tissue. The functional alterations in the adipose tissue are a confounding contributing factor to many diseases, including cancer. The increased incidence and aggressiveness of several cancers, including colorectal, postmenopausal breast, endometrial, prostate, esophageal, hematological, malignant melanoma, and renal carcinomas, result from obesity as a contributing factor. The increased morbidity and mortality of obesity-associated cancers are attributable to increased hormones, adipokines, and cytokines produced by the adipose tissue. The increased adipose tissue levels observed in obese patients result in more adipose stromal/stem cells (ASCs) distributed throughout the body. ASCs have been shown to impact cancer progression in vitro and in preclinical animal models. ASCs influence tumor biology via multiple mechanisms, including the increased recruitment of ASCs to the tumor site and increased production of cytokines and growth factors by ASCs and other cells within the tumor stroma. Emerging evidence indicates that obesity induces alterations in the biological properties of ASCs, subsequently leading to enhanced tumorigenesis and metastasis of cancer cells. As the focus of this review is the interaction and impact of ASCs on cancer, the presentation is limited to preclinical data generated on cancers in which there is a demonstrated role for ASCs, such as postmenopausal breast, colorectal, prostate, ovarian, multiple myeloma, osteosarcoma, cervical, bladder, and gastrointestinal cancers. Our group has investigated the interactions between obesity and breast cancer and the mechanisms that regulate ASCs and adipocytes in these different contexts through interactions between cancer cells, immune cells, and other cell types present in the tumor microenvironment (TME) are discussed. The reciprocal and circular feedback loop between obesity and ASCs and the mechanisms by which ASCs from obese patients alter the biology of cancer cells and enhance tumorigenesis will be discussed. At present, the evidence for ASCs directly influencing human tumor growth is somewhat limited, though recent clinical studies suggest there may be some link.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Adipose tissue
Adipose tissue is distributed throughout the human body. In adults, it is localized in subcutaneous and visceral depots, bone marrow, intraarticular regions, and ectopic sites such as intra-hepatic and intra-muscular. Adipose tissue was viewed as a passive organ that served primarily as an energy reservoir for decades [1, 2]. However, discovering that the first adipose tissue produced cytokine with systemic actions, leptin, led to the re-classification of adipose tissue as an endocrine organ [3, 4]. Additional adipokines have been subsequently identified, including adiponectin, omentin, and resistin, all produced by adipocytes. Adipokines play central roles in the regulation of metabolism. Adipose tissue also secretes numerous cytokines, including IL-6, TNF-\(\mathrm{\alpha }\), IL-1\(\upbeta\), IL-8, and MCP-1, which are pro-inflammatory [5, 6]. These cytokines are believed to be produced by the non-adipocyte cells in the adipose depot. Historically, adipose tissue has been categorized as either white adipose tissue (WAT) or brown adipose tissue (BAT). However, three distinct types of adipose tissue have been described in humans: white adipose tissue (WAT), brown adipose tissue (BAT), and beige adipose tissue.
Depending on their location and activity, WAT depots are categorized as either visceral (around the organs) or subcutaneous (between the muscle and the dermal fascia). The visceral WAT stores excess energy and provides physical protection to the organs. WAT is composed of monolocular adipocytes and primarily functions as an energy storage depot that also produces adipokines. The principal function of subcutaneous WAT is to store excess triglycerides and release free fatty acids during extended periods of fasting, starvation, or exercise. It has also been suggested that subcutaneous WAT functions as a buffer during the intake of dietary lipids to protect the organs against the lipotoxicity of free fatty acid oxidation [7].
In contrast, BAT, which is rich in mitochondria, oxidizes chemical energy to produce heat, through the actions of mitochondrial uncoupling protein‐1 (UCP1), as a defense against hypothermia [8, 9]. The thermogenic regulation of BAT can be induced by shivering and non-shivering mechanisms. BAT is abundant in infants, but as humans age BAT levels decrease. In adults, BAT is primarily located in the supraclavicular, paravertebral, mediastinal, para-aortic, and suprarenal regions of the body. Adipocytes in brown adipose tissue are more closely related to skeletal muscle than in white adipose tissue [10,11,12].
Beige (“brite” or “brown/white”) adipose tissue appears to be a hybrid between white adipose tissue and brown adipose tissue, the biologic capabilities of both. It functions for energy storage, but it can express UCP1, which indicates that it can have thermogenic activity. Unlike classical BAT, which is derived from a myogenic factor 5 (MYF5) muscle‐like cellular lineage, the beige adipocytes lack Myf5 expression. From a developmental perspective, it appears to be most similar to white adipose tissue [13, 14]. Beige adipose depots are often located in the same regions where white adipose depots are found [15, 16].
2 Mesenchymal stromal cells
Mesenchymal stromal cells, also known as multipotent stromal cells or medicinal signaling cells (MSCs), are localized throughout the body. They have been described as cellular components of most major organ systems. MSCs are currently under development as therapeutic candidates for many human diseases. Their therapeutic potential is derived from their natural ability to maintain tissue homeostasis as part of their native biology. MSCs can sense and respond to signals within the body and migrate to areas of tissue injury to facilitate repair [17,18,19]. Upon arrival to the area of damage, MSC may differentiate into components of the injured tissue. However, data supporting differentiation as a primary repair mechanism mediated by MSCs is minimal. However, MSCs’ primary therapeutic effects lie in their potent immunomodulatory effects and ability to secrete factors that promote tissue repair [20, 21].
The two main questions concerning adult stem cell sources are the specifics of the physiology of the cell donor and the tissue location from which the cells are isolated. With regard to location, bone marrow is the most common source of MSCs currently under investigation in preclinical and clinical trials. However, MSCs have been isolated and characterized from various tissue sources, including bone marrow, adipose tissue, umbilical cord, dental pulp, placenta, amniotic fluid, and Wharton’s jelly [22,23,24,25].
3 Adipose tissue–derived mesenchymal stromal cells (ASCs)
In contrast to bone marrow, MSCs can be isolated from subcutaneous adipose tissue with few potential complications and a significantly increased yield in cell numbers compared to bone marrow isolation. The most common source of adipose tissue processed for ASC isolation is subcutaneous white adipose tissue isolated from the abdomen, thigh, or hips/buttocks, generally during plastic surgery. While adipose tissue is composed primarily of mature adipocytes, considerable cellular heterogeneity can be found in all depots. The diverse populations of cells found in adipose tissue depots include preadipocytes, pericytes (3–5%), endothelial cells (10–20%), granulocytes (10–15%), monocytes (5–15%), and lymphocytes (10–15%) and adipose tissue–derived mesenchymal stem cells (ASCs, 15–30%) [26]. Together, these cells make up a communication network that regulates the activity and function of adipose tissue depots. In addition, ASCs are routinely isolated from adipose tissue’s stromal vascular fraction (SVF). The SVF is generated by processing adipose tissue, generally collected by liposuction, collagenase digestion, and centrifugation, resulting in the removal of the mature adipocytes, debris, and free lipid. Thus, the SVF contains circulating blood cells, fibroblasts, pericytes, endothelial cells, and ASCs [26]. The ASCs are generated by plating the SVF cells, allowing the adherent ASCs to expand. ASCs are a relatively homogenous population of spindle-shaped, fibroblast-like cells that are expanded out after 7–14 days of culture after SVF is plated onto standard cell culture surfaces. ASCs are capable of long-term expansion in vitro to large numbers and cryopreserved for future use. Over 400,000 liposuction procedures are performed annually, with up to 3 L of lipoaspirate discarded after each procedure, ensuring an ample supply of starting material from which to isolate the ASCs.
ASCs are ideal candidates for use in tissue engineering and regenerative medicine applications, as they have been reported to differentiate into multiple cell types, including mesodermal origin cells and tissues such as osteocytes, chondrocytes, and adipocytes. However, one of the most potent properties of ASCs is their inherent ability to modulate immune system activity in inflammatory environments [27,28,29,30,31]. ASCs have been shown to influence the functional properties of primary immune cells, either through direct or indirect interactions. One of the initial discoveries that ASCs influence the biology of immune cells was their ability to alter T cell activity in vitro and in vivo [32,33,34,35,36]. ASCs also inhibit B-cell proliferation and the production of immunoglobulins and suppress B-cell functions [37, 38]. In addition, ASCs also efficiently inhibit natural killer cell activation, resulting in impaired cytotoxicity processes [39,40,41]. The anti-inflammatory properties of ASCs also impact macrophages. The introduction of ASCs into an inflammatory environment shifts the balance of pro-inflammatory (M1) macrophages and anti-inflammatory (M2) macrophages both in vitro and in vivo [42, 43]. Exposure of macrophages to ASCs or conditioned media from ASCs increases the number of macrophages expressing proteins of M2 lineage cells, such as CD206 and Arg1. It decreases the levels of pro-inflammatory mediators such as IL-6, IL1b, and MCP1 in the macrophages.
The cell surface cluster of differentiation (CD) antigens found on ASCs has been described. Many of the CD antigens analyzed are canonical markers for mesenchymal stem cells. To date, no ASC-specific CD marker or antigen has been described that identifies a cell as an ASC. In general, analysis of both positive and negative CD antigens ensures that the isolated cells are mesenchymal stem cells and that other cell lineages are not contaminating the preparation. Human ASC expresses the traditional mesenchymal stem cell surface markers, including the cell adhesion molecules CD29, CD44, CD146, and CD166; the receptor molecules CD90 and CD105; and the GPI anchored enzyme CD73. In addition, ASC should not express hematopoietic lineage cell surface antigens, such as CD11b, CD13, CD14, CD19, and CD45. They are also negative for the endothelial markers CD31 and HLA-DR [26]. ASC are often reported to express CD34 in low-passage cultures. However, the expression decreases with the serial passage of the cells [44, 45]. The expression of CD34 is dependent on the passage number and the culture conditions used. In addition, ASCs do not express the adhesion marker CD106 or the sialoglycoprotein podocalyxin (PODXL), which makes them unique from bone marrow-derived MSCs (BMSCs) [26].
ASCs are considered relatively immune-privileged because they do not express HLA-DR (major histocompatibility complex class II, MHC II) and low levels of MHC class I, which provides them with reduced immunogenic levels in vivo [46, 47]. In addition, ASCs do not express the primary costimulatory molecules for T and B cells, including CD80, CD86, CD40, and CD40L. A significant amount of data demonstrates this when ASCs are tested in mixed lymphocyte reactions (MLR) in vitro. However, despite the general thought that ASCs may be immune-privileged, studies have failed to demonstrate long-term persistence following infusion in vivo in any model. Additionally, ASCs have recently been shown to induce complement pathways in vivo, suggesting that they are not entirely immune evasive.
The ability to effectively isolate, efficiently expand, and cryopreserve ASCs has permitted researchers to generate banks of cell lines from numerous adipose tissue depots independent of age, gender, disease state, or obesity status. Much of our understanding of ASC biology and function has resulted from direct comparisons of the properties of ASCs from various donor types that have been made both in vitro and in vivo.
4 Obesity and health consequences
Currently, over 42% of adults in the USA are obese, and the incidence of severe obesity (typically represented by a body mass index (BMI) of 40 or more) continues to increase (https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf). According to the World Health Organization statistics, obesity rates across the globe have nearly tripled since 1975. It is estimated that approximately 39% of adults are overweight and 13% are obese, and the incidence in children is climbing rapidly (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). The distinction between being overweight and obese is determined by the BMI, calculated based on the height and weight of an individual. For example, an individual with a BMI of 24.9 to 29.9 is considered overweight. In contrast, a person with a BMI greater than 30.0 is defined as obese. While all adipose depot sites can increase in volume, only WAT accumulation and expansion correlate with the increased risk of developing numerous diseases, including heart disease, metabolic syndrome, stroke, and cancer [48,49,50]. The World Cancer Research Fund has pioneered meta-analyses addressing the impacts of obesity on cancer incidence and mortality. They have released three reports describing the association between food, nutrition, and cancer (www.wcrf.org). The initial report released in 1997 spurred intense research interest in the connections between obesity, nutrition, and cancer. The research resulted in a follow-up report that was a collaborative effort between the World Cancer Research Fund (WCRF) and its affiliates, including the American Institute for Cancer Research (AICR). The second report summarized the new evidence linking obesity and physical activity; food, nutrition, physical activity, and cancer. The third report, published in 2020, presented a follow-up assessment and interpretation of the rapidly expanding scientific literature on diet, nutrition, physical activity, and cancer. The core message from these reports indicates that high levels of adiposity and obesity drive increased rates of multiple cancers, including colorectal, postmenopausal breast, and renal carcinomas. Furthermore, additional linkages between obesity and cancer have been identified in both sexes, including endometrial, prostate, esophageal cancers, malignant melanoma, and hematological malignancies [51,52,53,54,55,56,57,58].
While several factors increase the incidence of breast cancer, obesity is one of the most critical risk factors for breast cancer in postmenopausal women. Multiple studies have demonstrated that postmenopausal breast cancer positively correlates with a high-BMI. Approximately 18–20% of postmenopausal breast cancer cases are attributable to obesity [57, 58]. The data suggests an increased incidence of breast cancer in obese patients, especially for estrogen receptor-positive (ER+) tumors [59]. Obesity in breast cancer patients is also associated with poor prognosis, increased risk of recurrence, resistance to chemotherapy, and worsened outcomes for pre- and postmenopausal women [60,61,62]. Surprisingly, these statistics suggest that obesity is the primary risk factor for postmenopausal breast cancer deaths in the USA [63]. Furthermore, it is correlated with the incidence of breast cancer and mortality due to poor prognosis and outcomes [64,65,66,67]. Obesity affects the prognosis by multiple mechanisms, including enhanced tumorigenesis, metastasis, and drug resistance [68, 69]. Furthermore, recent studies have demonstrated a stronger correlation between abdominal obesity and breast cancer [70,71,72]. To understand the tumor-promoting factors in obese tissue that alter cancer progression, individual groups have focused on ASCs.
5 Impacts of obesity on ASCs
Data suggest that the adipose tissue environment in obese adults alters the biology of ASCs. Obesity alters the physiology of adipose tissue, resulting in a local environment of chronic inflammation, high levels of immune cells, hypoxia, and altered production of adipokines. The obesity-associated physical expansion of adipose tissue increases the distance between the adipocytes and the vasculature, resulting in localized hypoxia because the adipocyte expansion exceeds the diffusion distance of oxygen with the tissue [73, 74]. The oxygen content in obese adipose tissue is nearly zero at distances of 100 μm or more from the vasculature, indicating that the local environment of obese adipose tissue is potently hypoxic [75]. Despite the substantial increase in adipose tissue volume associated with obesity, neither cardiac output nor total blood flow to the adipose tissue increases [76, 77]. In obese mice, reduced blood perfusion and hypoxia appear to be limited to WAT [78]. The hypoxic environment in obese adipose tissue results in increased hypoxia‐induced factor 1‐alpha (HIF‐1α) and increased angiogenesis; however, the response cannot compensate for the growing adipocytes, resulting in chronic inflammation [79, 80]. Chronic low‐grade inflammation may result in high levels of pro‐inflammatory cytokines, chemokines, protease, and protease inhibitors.
Zhang and colleagues revealed that a higher number of ASCs could be isolated from the WAT of obese mice than in lean mice, possibly due to the increased volume of WAT in obese mice [81]. Consistent with Zhang et al., Bellows and colleagues found an increased frequency of ASCs in the circulation of obese patients compared to lean patients [82, 83]. In addition, ASCs isolated from obese women have an increased potential to traffic the tumor compared to the ASCs isolated from lean women [84].
ASCs isolated from donors with different BMIs display marked differences in their secretome, angiogenic potential, and migration/invasion capacities [84, 85]. ASCs isolated from abdominal subcutaneous adipose tissue of obese individuals demonstrated increased migration/invasion resulting from inherent increases in the expression of calpain-4, calpastatin, and MMP-15 [84]. Our group data also indicates that the levels of pro-inflammatory cytokines produced by ASCs from obese individuals are markedly upregulated, such that ASCs from obese donors may contribute to the inflammatory environment in obesity [86].
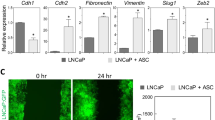
Our studies also demonstrate that obesity alters the gene expression profile of ASCs (Fig. 1). Gene expression of ASCs from obese donors revealed marked upregulation in the mRNA for leptin (LEP), leptin receptor (LEPR), sortilin 1 (SORT1), thyrotropin-releasing hormone (TRH), melanin-concentrating hormone 1 (MCHR1), peroxisome proliferator-activated receptor-gamma (PPAR-\(\gamma\)), peroxisome proliferator-activated receptor-gamma coactivator 1-α (PPARGC1A), and thyroid hormone receptor-β (THRB) [86]. ASCs from obese donors produce high levels of pro-tumorigenic factors, including leptin, IL-1, IL-6, IL-12, PDGF-A, TNF-\(\alpha\) leukemia inhibitory factor (LIF), intercellular adhesion molecule 1 (ICAM-1), and granulocyte-colony stimulating factor (GCSF) compared to ASCs from lean individuals [87].
Adipose stromal cells (ASCs) possess distinct biologic properties as a consequence of being localized the obese adipose tissue and contribute to worse outcomes for breast cancer. In the context of healthy adipose tissue, adipose stem cells primarily possess an anti-inflammatory gene expression profile when activated, have normal levels of metabolic function and have decreased ability to differentiate into cancer-associated fibroblasts (CAFs). However, ASCs from lean donors stimulate the growth of BC cell lines in vitro and tumorigenicity of xenografted tumors in vivo. ASCs isolated from subcutaneous adipose tissue of obese individuals have disparate biologic properties including the increased production of pro-inflammatory cytokines, enhanced pro-tumorigenic signaling, increased ability to become CAFs and enhanced ability to mediate remodeling of the tumor extracellular matrix (ECM). The ASCs isolated from obese adipose tissue reservoirs express pro-tumorigenic elements such leptin, resistin, IL-6, CXCL5, TNF-a, and MCP-1, which mediate the migration, invasion, and metastasis of tumor cells. ASCs from obese donors markedly increase the growth of BC cell lines in vitro and enhance both the tumorigenicity and metastasis of cancer cells in xenografted tumors in vivo
In preclinical models, obesity also increases the capacity of ASCs to differentiate into cancer/carcinoma-associated fibroblasts, or CAFs. CAFs are found in more invasive tumors and enhance cancer cell proliferation, invasion, and metastasis [88, 89]. Obesity exacerbates this trend: following exposure to breast cancer cells, obese ASCs expressed higher CAF markers, including alpha-smooth muscle actin (\(\alpha\)-SMA), NG2, FAP, and FSP, compared to ASCs from lean individuals [90]. This is another example of how crosstalk between breast cancer cells and stromal cells creates a pro-tumor environment: cancer cells transform stromal cells into CAFs, and CAFs secrete unique cytokines and growth factors, such as CXCL12, VEGF, PDGF, and hepatocyte growth factor [91]. Co-culture with obese ASCs resulted in breast cancer cells having increased mesenchymal phenotype and proliferative capacity compared to those cultured with lean ASCs, indicating an increased tumor growth and metastasis capacity. Furthermore, substantial evidence demonstrates that CAFs are essential regulators of the cancer immune response and promote immune evasion of cancer cells [92]. Together, these studies highlight the ability of obese ASCs to remodel the tissue environment, specifically eliciting enhanced remodeling in the obese tumor environment.
6 Obesity impacts on extracellular matrix (ECM) in adipose tissue
Tissues are composed of an array of structural proteins that provide support and scaffolding to resident cells. Additionally, matrix proteins mediate essential processes, including cell adhesion, cell recruitment, biomechanical signals, and serve as a reservoir for growth factors. Previous studies have reviewed and identified the matrix composition of adipose tissue. The adipose tissue matrix comprises fiber-forming collagens (I, II, III, V), basement membrane (collagen IV), fibronectin, laminins, and filament-forming collagen, collagen VI [93, 94]. From these reviews, it has been highlighted that collagen VI, in particular, is collagen specific for adipocytes [93, 95]. Diseases such as cancer and obesity can result in extensive matrix remodeling regarding molecular composition, mechano-transduction, and fiber orientation [96,97,98,99,100]. To date, meaningful parallels exist between the obese tissue and the cancer matrix [97]. Specifically, both tissue environments possess alterations that favor the release of pro-inflammatory signals (TGF-β, MCP1), M2 macrophage recruitment, stiffening of the matrix, and enhanced expression of matrix proteins that promote stemness [96, 97, 100, 101]. Recent reviews on obesity and tumor matrix have highlighted the parallels of matrix stiffening between obese tissue and tumors [97].
Recently, the matrix proteins enhanced in obese tissues have been reviewed [93, 94]. It is well documented that collagen VI is an essential mediator of adipocyte growth and is markedly improved in obese tissue [93,94,95]. One study has observed COL6A3 as being repressed in tissue samples and that leptin negatively regulated COL6A3 expression [102]. Others have suggested that this may partly be due to visceral/omental and subcutaneous fat differences. Apart from this study, the collagen VI genes elevated in obesity demonstrate COL6A3 to have exceptionally high levels in human adipose tissues [95]. Studies in obese mouse models and in vitro studies in 3T3-LT cell lines indicate that collagen VI regulates adipocyte stress forces, adipocyte size, and adipogenic differentiation [103, 104]. This is observed through knockout experiments that show that loss of collagen VI in obese mouse models resulted in an increased adipocyte size [103]. The decreased stress on adipocytes mediated by the loss of matrix tension forces around the adipocyte results in increased size, reduced inflammatory profile, and loss of immune recruitment, specifically decreased infiltration of macrophages. In accordance with this, others have demonstrated that collagen VI is associated with inflammation and macrophage regulation, precisely that collagen VI expression enhances the generation of the M2 phenotype [95, 105]. Specifically, high levels of COL6A3 are associated with increased macrophage presence in adipose tissue, which was evident through staining CD68-positive macrophages in human adipose tissue [95]. Further studies comparing obese and lean human tissues demonstrated that obese tissue samples had elevated collagen VI and macrophage levels, evident through enhanced expression of CD68 mRNA levels [106] and CD68+ cells [95]. Knocking out the expression of COL6A3 in 3T3-L1 adipocytes and obese mice resulted in inflammation loss [104]. In vitro co-culture studies show that collagen VI production is enhanced through the co-culture of M2 macrophages with adipocytes [105]. Given the positive correlation between the expression of collagen VI and M2 macrophage recruitment, it is not surprising that collagen VI is identified as being associated with cancer progression [107,108,109].
Collagen VI and its role in cancer have recently been reviewed [107]. Collagen VI level is elevated in colon, breast, pancreatic, and ovarian tumors. In breast cancer, collagen VI expression was observed near adipocytes. The invasive tumor front and RNA sequencing demonstrated that COL6A1 and COL6A3 RNA expression is associated with poor patient survival in ER-negative patient samples [107, 110]. Collagen VI is currently well described as being highly expressed in obese fibrotic tissues compared to lean and aiding as a mediator for M2 immune recruitment and pro-inflammatory adipokine and TGF-β release [106]. Tumors are often described as wounds that never heal, with increased regions of fibrosis and recruitment of M2 macrophages [111, 112]. Furthermore, tumors demonstrate a similar role for collagen VI in macrophage recruitment and inflammation through the regulation of the TGF-β axis [107]. Therefore, it may be interesting to determine if there is a role for collagen VI expression, macrophage recruitment and release of TGF-β, and adipocyte release of pro-inflammatory adipokines such as MCP in solid tumors [107]. Currently, collagen VI is a suggested therapeutic target for fibrosis in other diseases [113]. In addition to modulating immune recruitment, recent studies have demonstrated that collagen VI has an observed role in cancer stemness [109] in ovarian cancer [109]. It is associated with increased invasion, where COL6A3 KO resulted in the loss of cell invasion of SKOV3 cells [109]. Evaluation of breast cancer tumors demonstrated that more aggressive tumors had elevated stiffness at the stromal interface and more aggressive phenotypes were associated with increased macrophage infiltration and TGF-β signaling [114].
Additional collagen proteins are observed to be elevated in obese tissues. In normal tissues, collagen V and IV act as regulators of adipogenesis [94]. The basement membrane collagen IV is elevated in obese tissue samples derived from subcutaneous adipose tissue compared to lean tissue. Studies in breast cancer in vitro have demonstrated that collagen IV regulates proliferation in ER+ breast cancer cells [110]. Similar to collagen VI, collagen IV expression correlated with TGF-β1 expression [115]. Collagen V is elevated in obese tissue samples and is associated with M2 macrophages, which induce collagen V expression in adipocytes [106]. High-COL5A1 and 5A2 mRNA expression in breast cancer is associated with poor patient survival in ER-negative tumors [110].
Periostin (POSTN) is elevated in the visceral fat of mice fed a high-fat diet (HFD) and is a regulator of obesity inflammation [116]. Mice fed a HFD that did not express POSTN had larger adipocyte size and decreased pro-inflammatory gene expression levels of MCP1 and TNFα compared to wild-type mice on a HFD. Similar to other obesity-associated matrix proteins, POSTN is associated with macrophage recruitment. However, unlike collagens V and VI, POSTN production is believed to be mediated by macrophages and not adipocytes and macrophages use POSTN production for cellular motility.
Other matrix proteins observed to be elevated in obese tissues are those commonly associated with fibrosis (collagen I, fibronectin, and hyaluronic acid) [97]. Cancer ECM has been well described and characterized as a mediator of breast progression primarily through matrix density and stiffness [117, 118]. A recent review highlights that for all ECM proteins associated with increasing stemness, of interest to obesity, ECM proteins elevated in obese tissue are observed to regulate stemness [108]. Collagen type IV, COL VI, POSTN, TNC promoted in obese tissue visceral adipose tissue-linked with inflammation [119]. To date, parallels exist between matrix remodeling in obesity and cancer. Furthermore, many matrix proteins enhanced in obese tissues promote immune macrophage recruitment and cancer stem-like phenotypes. A deeper understanding of the obese tissue matrix will help determine whether obesity increases the severity and tumorigenesis of certain cancers.
7 ASCs are components of the tumor stroma
The microenvironment of a tumor is a highly complex network comprised of extracellular matrix (ECM), fibroblasts, and a variety of nonmalignant cell types, including immune system cells, cancer-associated fibroblasts (CAFs), myofibroblasts, vascular cells, and mesenchymal stromal cells. ASCs are an integral component of the tumor microenvironment (TME) [120,121,122,123,124]. It is clear that cell–cell and cell–ECM interactions are bidirectional and result in alterations to the gene expression profiles and biological activities of cancer cells that drive tumorigenesis, metastasis and, in some cases, chemoresistance. For example, ASCs and their derived products mediate critical endocrine signaling functions that contribute to normal breast physiology and cancer-associated pathological processes [125, 126]. ASCs play a central role in tumorigenesis, tumor growth, and metastasis by producing trophic factors secreted into the local microenvironment and interacting with tumor cells and immune system cells in various cancer types.
The inherent immunomodulation properties of ASCs are integral to cancer progression. Tumors represent non-healing wounds that usurp the innate wound healing response to promote growth [127, 128]. Interactions with the immune system have direct effects on tumorigenicity and growth. For example, regulatory T cells (CD4+CD25+FoxP3+ cells) that protect the host from autoimmune disease generation are often hijacked by cancers to suppress tumor-associated immune activity [129]. As discussed above, ASCs drive the generation of T regulatory cells in vivo.
ASCs can be mobilized and recruited to the tumor from local and distant WAT depots. Upon reaching the tumor, some of the ASCs are converted to CAFs and others remain ASCs, and both are integrated into the tumor stroma [130, 131]. In an elegant study, Zhang and colleagues demonstrated that ASCs are effectively recruited to xenografted tumors via systemic circulation, where they persist and promote tumor growth. The tumor produces CXCL1 and CXCL 8, which drives the ASC migration via CXCR1 and CXCR2 to tumors [132]. CAFs play a key role in the biology and severity of the tumor. More aggressive cancers have a higher number of CAFs [133,134,135,136]. CAFs originate from diverse cell populations, including normal-resident fibroblasts and ASCs; these cells are transformed into CAFs when exposed to tumor-derived factors [92, 137]. ASCs exposed to cancer cells or tumor cell–conditioned media express tenascin‐C and α‐SMA, characteristic of CAFs [131]. Evidence from tumor xenograft model studies reveals that bone marrow-derived mesenchymal stem cells are recruited, or home to tumors upon injection in vivo and persist within the tumors [138,139,140,141]. In vivo studies have confirmed that simultaneous co‐injection of primary breast cancer and ASCs into nude mice results in the integration of ASCs into the tumor stroma, thereby increasing tumor volume and vascularity [142,143,144].
Not all recruited ASCs are converted to CAFs. Tumor-associated ASCs can influence the growth rate, aggressiveness, tumor cell migration, invasion, and metastasis via their secretome. ASCs secrete high levels of tumorigenic factors, cytokines, and growth factors within the TME, including leptin, IL-6, C-X-C motif chemokine ligand 4 (CXCL4), CXCL5, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) [87]. ASCs, respond to the breast cancer-derived secretome by increasing the expression of multiple tumor‐promoting factors, including stromal cell–derived factor 1 (SDF‐1, CXCL12), vascular endothelial growth factor (VEGF), chemokine ligand 5 (CCL5), platelet‐derived growth factor D (PDGF‐D), and transforming growth factor-beta (TGF‐β) [130, 131, 145,146,147,148]. ASCs participate in the recruitment of macrophages to the tumor by releasing chemokine ligand 2 (CCL2). Once in the tumor, the macrophages produce CXCL12 to promote growth by enhancing tumor-associated angiogenesis [149]. ASCs also secrete various pro-angiogenic growth factors and chemokines, including VEGF, bFGF, c-kit, PDGF, and SDF-1, allowing increased blood supply to support tumorigenesis [150,151,152,153,154]. ASCs produce large amounts of extracellular matrix molecules. Evidence shows that ASC-produced fibronectin alters breast ECM stiffness in breast cancer tumors [155]. ASCs also alter the ECM with the tumors by promoting myofibroblast differentiation via mechano-transduction [96].
Additionally, it has been demonstrated that ASCs enhanced the migration of several different cancers, including breast, colon, prostate, gastric, and head and neck tumors [137, 142, 156,157,158,159]. In addition, data indicate that implanting spheroids, or organoids, formed with breast cancer cells and ASCs in vivo increased the number of lung metastases [160,161,162]. The evidence indicates that ASCs are recruited to the tumor microenvironment, enhancing the tumorigenicity (cancer cell proliferation), metastasis, and potentially angiogenesis within the tumor.
8 Impacts of ASCs on tumor ECM
ASCs have also been shown to express extracellular matrix remodeling proteins and upregulate these proteins’ expression by cancer cells. Remodeling proteins, such as MMP-2, MMP-9, and tissue inhibitor metalloprotease 1 (TIMP-1), are associated with tumor progression and metastasis [163, 164]. Specifically, MMP-2 and MMP-9 are proteolytic enzymes that digest-type IV collagen, a principal constituent of the basement membrane; invasion through the basement membrane is essential in metastasis, particularly for epithelial tumors (carcinomas) [165,166,167]. TIMPs are tissue inhibitors of MMPs. These proteins not only inhibit MMPs and can play an inhibitory role in cancer but also have been shown to promote some aspects of cancer [168, 169]. For example, TIMPs have increased cell proliferation and promoted angiogenesis [169, 170].
ASC-conditioned media induced fibronectin expression, α‐SMA, and vimentin in breast cancer cells, which are markers of EMT [146]. These results correlated with increased expansion of CD44 high/CD24 low cancer stem cells (CSCs) and anchorage-independent growth of cancer cells, leading to EMT of cancer cells [146]. Furthermore, an association between CCL5 secretion by ASCs and elevated levels of MMP‐9 activity within the tumor microenvironment has been described, leading to increased tumor invasion [171]. ASC‐derived IL‐6 and IL‐8 have also increased migration, invasion, and anchorage-independent growth of breast cancer cell lines, including MDA‐MB‐231, T47D, and MCF7 cells [158, 172]. These initial studies laid the groundwork for the TME and ASCs in regard to tumor growth and progression. Since this time, additional studies by us and others have further defined the role ASCs play in enhancing cancer progression. Including identifying ASCs as mediators of metastasis and drug resistance.
9 Breast cancer
Breast cancer (BC) accounts for approximately 30% of all new cancer diagnoses in women and is the most frequently diagnosed cancer in women worldwide [173, 174]. Within the USA, breast cancer incidence rates continue to increase by 0.5% per year, partially due to declines in fertility and increased body weight [174]. Furthermore, the declining mortality rates in previous years slowed in 2021 due to rapidly rising distant-stage breast cancer in younger age groups [173, 175]. BC is broadly categorized (1) based on receptors expressed by breast tumors or (2) molecular intrinsic subtypes, representing the heterogeneity within this cancer type. Clinical subtype classification is based on the expression or amplification of receptors within breast cancer cells: estrogen receptor-positive (ER+) and HER2/Neu receptor amplified subtypes can be treated with HER2/Neu-targeted or endocrine-targeted therapies, while triple-negative breast cancer (TNBC) is the umbrella term for the remaining breast cancer subtypes [176]. Molecular intrinsic subtypes of breast cancers classify tumors based on the expression of gene and protein patterns that dictate tumor behavior and better represent the intra-tumoral and environmental heterogeneity of breast cancers [177, 178]. TNBC and the basal-like molecular subtypes of breast cancer are biologically and clinically aggressive, with high metastasis rates, drug resistance, and tumor recurrence [179]. Metastatic breast cancer is often treated palliatively to prolong life [176]. Chemotherapy is the mainstay of treatment for early-stage and advanced TNBC disease, often accompanied by radiation and/or surgical resection [180, 181]. Regardless of treatment, residual disease after completion of chemotherapy dictates prognosis, survival, and recurrence [180]. The presence of residual disease and both acquired and inherited resistance to chemotherapy regimens and targeted therapies remains a difficult obstacle in the discovery of effective therapeutic regimens for all subtypes of breast cancer.
Environmental and epidemiologic factors contributed to the development and progression of breast cancer and acquired and inherited genetic mutations. Modifiable risk factors include diet, alcohol, physical activity, BMI, and smoking in all breast cancers. Non-modifiable risk factors in all cancers include older age, White ethnicity, and postmenopausal status [182, 183]. These risk factors vary depending on the specific subtypes of breast cancer (e.g., ER+, HER2+, TNBC). For example, in TNBC cases, there is a greater incidence in women under the age of 40 [184]. Additionally, women with TNBC were significantly more likely to be non-Hispanic Black or Hispanic [184]. Non-Hispanic Black women with late-stage TNBC have the worst overall survival (5-year survival of 14%) compared to women of other ethnicities [184]. Across all age groups in the USA, Black women are more likely to have TNBC compared to White populations [185]. Other epidemiologic risk factors for TNBC include reproductive factors such as younger ages at menarche and first full-term pregnancy, higher parity, and shorter duration of breastfeeding [186]. Higher body mass index and waist-to-hip ratio have also been associated with increased incidence of breast cancer throughout all subtypes, demonstrating that obesity and the presence of adipocytes and the inflammatory process may play significant roles in the progression of the disease [72, 187].
10 The impact of leptin expression on cancer
The gene expression profiles have been compared as part of the analysis of ASCs from lean and obese donors. Our analysis revealed that leptin was one of the most differentially expressed genes in ASCs from obese donors, upregulated more than 200-fold and leptin expression appeared to be regulated by exposure to estrogen [86]. Leptin is an adipokine produced primarily in WAT that regulates energy balance. Leptin production and secretion follow circadian rhythm patterns and fluctuate according to nutritional status. The expression levels of leptin vary in proportion to the amount of adipose tissue, with higher expression levels at increased BMIs. The physiologic activity of leptin is broad, with its primary activity being the regulation of energy homeostasis, but it also has a role in neuroendocrine function, metabolism, regulation of immune function, and bone metabolism [188, 189]. In addition, leptin is an anorexigenic and pro-inflammatory factor that links the neuroendocrine and immune systems.
Leptin impacts several cancer types, including bladder, lung, breast, prostate, testicular, ovarian, large B-cell lymphoma, mesothelioma, pancreatic, kidney, colorectal, liver, AML, and thyroid cancer [190]. Increased leptin and leptin receptor expression are associated with worse prognosis, increased morbidity, mortality, metastasis, and cancer recurrence [191,192,193,194]. Leptin enhances proliferation, migration, and invasion of cancer cells while decreasing their adhesion and apoptosis. Leptin also enhances angiogenesis and inflammation in tumors [195, 196].
Leptin levels have been linked to breast cancer aggressiveness and epidemiological studies have demonstrated worse clinical outcomes in breast cancer patients with elevated leptin levels [197,198,199,200]. Exogenously delivered leptin has been shown to increase the proliferation of breast cancer cells [201,202,203,204,205]. Leptin upregulates aromatase and ERα, likely targeting the estrogen axis [206, 207]. Leptin promotes the survival of cancer cells by activating multiple signaling pathways. In breast cancer cells, leptin functions through the JAK2‐STAT3, PI3K‐AKT, ERK1/2, and activator protein 1 (AP‐1) pathways, increasing the expression of proteolytic enzymes that are required in tumor growth, metastasis, and neoangiogenesis [208,209,210]. In estrogen receptor‐positive human breast cancer cell lines, leptin has been shown to exert its influence through the activation of the MAPK pathway [208]. Thus, high levels of leptin resulting from obesity may increase breast cancer incidence.
11 ASCs and breast cancer
Numerous studies have demonstrated a positive correlation between adult BMI and postmenopausal breast cancer and an increased incidence of breast cancer, especially for ER+ tumors [59]. ASCs enhance the growth and proliferation of breast cancer cells, regardless of whether they originate from lean or obese donors [158, 160, 211] (Fig. 1). In addition, ASCs increase a murine breast cancer cell line’s proliferation and enhance cancer cells’ invasion and metastasis [160]. Utilizing ASCs isolated from the breast and abdominal adipose tissue, Walter and colleagues demonstrated that the secretion of IL-6 from ASCs enhanced the migration and invasion of breast cancer cells in vitro [158].
Studies have described the interaction between ASCs and breast cancer cells, but only recently have the mechanism(s) of this interaction been thoroughly investigated. While all ASCs enhance the growth of breast cancer cell lines and tumors, our group and others have reported that ASCs from obese individuals have novel biology resulting from the obese environment in adipose tissue that drives cancer to even worse outcomes. Evidence from our team suggests that obesity induces significant changes in the biological properties of ASCs and that these alterations enhance breast cancer tumorigenesis.
ASCs stimulated by cancer cells secrete a wide range of cytokines, chemokines, and growth factors that, in turn, increase the proliferation of breast cancer cells in an ASC/cancer cell reciprocal feedback loop [212]. ASCs isolated from obese donors with increased leptin expression drive the proliferation and migration of breast cancer cells in vitro [86, 196]. The genetic knockdown of leptin expression in ASCs significantly reduced tumor volume. Obese ASCs treated with estrogen markedly increased breast cancer cell proliferation and tumor growth of breast cancer cell lines in vitro and in vivo in the context of xenograft models. The exposure of breast cancer cells to obese ASCs also altered their gene expression profiles. Altered expression of genes regulating cell cycle, apoptotic, angiogenesis, metastasis, and adhesion was observed. Breast cancer cell lines co-cultured with ASCs from obese donors demonstrated increased expression of genes involved in the epithelial-to-mesenchymal transition (EMT) and metastasis (SERPINE1, MMP-2, and IL-6). The gene expression profile of BC lines co-cultured with ASCs from obese donors in which leptin expression was blocked by an siRNA did not display similar induction for the expression of these genes [196]. SERPINE1 is a serine protease inhibitor that limits the activity of matrix metalloproteases in the extracellular matrix microenvironment [213]. SERPINE1 also binds to the extracellular matrix protein vitronectin. The binding of SERPINE1 to vitronectin results in the detachment of the tumor cells from the ECM, enhancing the mobility of the cells [214]. Likewise, MMP-2 expression correlates with increased metastasis and poorer clinical prognosis [163, 215, 216]. Furthermore, MMP-2 expression also indicates the aggressiveness of breast cancer tumors [217, 218]. Elevated expression of MMP-2 in cancer cells was related to smaller tumors, while expression of MMP-2 by the stromal cells was associated with enhanced aggressiveness [217]. In other studies by our team, the elevated expression of SERPINE1 and MMP-2 metastatic factors in ASCs correlated with the increased incidence of metastatic lesions in the lung and liver of mice in xenograft studies. Furthermore, SERPINE1 and MMP2 were reduced in tumors formed with cancer cells and leptin shRNA obASCs, relative to tumors formed with cancer cells and control shRNA. These results suggest that leptin affects the overexpression of these key metastatic factors within the tumor.
Other direct co-culture studies revealed the upregulation of CDKN2A, a cell cycle regulator, and GSTP1, a gene responsible for detoxifying drugs, upregulated in multi-drug-resistant breast cancer [219]. While breast cancer–stimulated ASCs secrete numerous cytokines to promote proliferation, a recent analysis revealed that an increase in chemokine C-X-C ligand 5 (CXCL5) was most noticeable. The neutralization of CXCL5 reversed the stimulatory effects that ASCs had on breast cancer cell (BCC) proliferation [220]. Furthermore, Sakurai et al. have demonstrated that crosstalk between these cells within the tumor microenvironment promotes proliferation and migration. This group found that BCCs stimulate ASCs to secrete cytokines by upregulating the expression of S100A7, a small calcium-binding protein [221].
The results from these studies demonstrate that the local obese microenvironment induces significant alterations to the biological properties of ASCs and that these alterations enhance ER+ breast cancer tumorigenesis via estrogen-dependent pathways [137]. Inhibiting leptin expression using leptin-neutralizing antibodies or shRNA reduced the impact of obese ASCs on breast cancer cell proliferation in vitro [196]. Furthermore, obese ASCs have been shown to alter the expression of several essential regulatory genes involved in the cell cycle, apoptosis, angiogenesis, EMT, and metastasis [222]. The dysregulation in the expression of molecular markers involved in the central processes in breast cancer cells is associated with poorer prognosis in patients [163, 215, 216]. It is clear that the secretome of ASCs strongly influences breast cancer behavior.
Triple-negative breast cancers (TNBCs) are a clinically aggressive subtype associated with higher mortality. Patients with TNBC are likely younger when they develop breast cancer and more likely to have a recurrence and metastasis within the first 3 years [223, 224]. TNBCs are defined by the lack of targetable receptors (estrogen, progesterone, and EGFR2 amplification). TNBCs cannot be treated with the small-molecule–targeted therapies developed for receptor-positive tumors. The result is increased rates of recurrence and metastasis, making TNBCs more challenging to treat. Patients with TNBC have worse 5-year survival rates than patients with other BC subtypes [225]. Concerning TNBC, our data suggest that non-estrogen-driven leptin promotes metastasis but not tumorigenesis of cell lines and patient-derived xenografts (PDX) in vivo [226]. Metastasis in TNBC cell lines is induced by the upregulation of EMT genes and promoting migration in vitro [226]. The pro-metastatic effects of ASCs from obese donors were dependent upon leptin expression. The reduction in leptin expression levels via delivery of shRNA abrogated the metastatic effects both in vitro and in vivo. Our group has also demonstrated that leptin enhances EMT in TNBC through upregulation of Serpine1, Twist1, Snai2, IL-6, PTGS2, CCL5, and CD90 [226]. These data indicate that in obese donors, ASCs are a primary source of leptin in cancer. In the context of TNBC in obese patients, leptin may be a promising target to inhibit BC metastasis.
12 ASCs in other cancer types
12.1 ASCs in colon cancer
Colorectal cancer (CRC) is the third most common cause of cancer-related death worldwide and is made worse by obesity [173]. However, colon cancer metastasis and the molecular mechanism(s) that drive it remain poorly defined. CRC is also impacted by the paracrine signaling effects of ASCs [227]. IL-6 produced by ASCs acts on CRC cells by activating the JAK2/STAT3 pathway to increase sphere generation and cell growth and upregulate self-renewal gene expression [227]. Furthermore, the cancer cells promoted the increased secretion of IL-6 from ASCs [227]. Other research groups have demonstrated that ASC-secreted trophic factors IL-6 and HGF enhance the proliferation and number [228, 229].
In turn, the CRC-CSC secrete neurotrophins, including nerve growth factor (NGF) and neurotrophin-3 (NT-3), that recruit ASCs to the tumor, where they go transdifferentiation along endothelial lineage, which allows the cancer cells to acquire a metastatic phenotype [230]. ASCs enhance the metastatic capacity of CRC cells by inducing epithelial-mesenchymal transition (EMT)-associated genes in a contact-dependent manner. Reciprocally, the CRC cells induced the expression of metastasis-related factors and cytokines, such as FGF10, VEGFC, and matrix metalloproteinases (MMPs) via Wnt signaling in the ASCs [231]. Additional studies have demonstrated that senescent ASCs have a more significant impact on CRC cell proliferation than nonsenescent ASCs [232]. Senescence can induce a senescent-associated secretory phenotype, serving as a tumor-supporting cell in the tumor microenvironment. One example is the secretion of Galectin 3 from senescent ASCs, which activates the mitogen-activated protein kinase (MAPK) pathway in the CRC cells [232]. Another group showed that neuregulin 1 via the human epidermal growth factor receptor 3 (HER3) receptor acts as a paracrine signal from ASCs that promotes CRC invasion, survival, and tumorigenesis [148]. While CRC severity is linked to obesity, it does not appear studies looking at the interplay between ASCs based on obesity status (lean vs. obese) and CRC cells have been published as of yet, so it is not clear if CRC cells respond to the impacts of obesity similar to other cancers, though hypothesizing that they would seem logical.
12.2 ASCs in prostate cancer
Prostate cancer (PC) is the most common cancer and the second leading cause of cancer death among men in the USA. PC is typically a slow-growing tumor and tumorigenesis is driven by the periprostatic adipose tissue [157, 233, 234]. While the mechanism(s) influencing tumorigenesis have not been fully defined, it is becoming evident that the recruitment of ASCs out of the periprostatic adipose tissue into the prostate occurs and is driven by chemokines secreted by PC cells [235]. Multiple studies have demonstrated that the interactions between ASCs and PC are altered by obesity [236]. PC patients have a much higher number of ASCs in periprostatic adipose tissue than nearby visceral tissue [236]. The results of an in vivo study performed in rodents in which PC3 cells were implanted in one flank and ASCs implanted in the opposite flank of athymic mice cells consistently had larger tumors than control animals in which no ASCs were implanted [159]. Additionally, ASCs were localized within the tumor, suggesting that the PC cells recruited the ASCs to the tumor. The tumor localized ASCs expressed CXCR4, suggesting that the ASCs migrated to the tumor using a CXCL12 (SDF-1)/CXCR4 and CXCR7 axis, similar to findings reported in breast cancer. Within the PC tumor microenvironment, ASCs increase tumor vascularity and promote tumor growth by upregulating fibroblast growth factor 2 expression [159]. The role of the pro-inflammatory CXCL12-CXCR4/CXCR7 signaling axis was further defined in an obesity-driven mouse model of myc-induced prostate cancer [237]. The analysis of SVF cells isolated from periprostatic white adipose tissue of obese HiMyc mice revealed a dramatic increase in CXCL12 mRNA expression levels. Further data indicated increased CXCL12 protein levels and the exposure of PC cell lines to CXCL12 resulted in enhanced migration and invasion. Also, higher CXCR4 and CXCR 7 were found in PC cell lines derived from tumors in the HiMyc animals. Direct evidence of the role of CXCL12 signaling in ASC recruitment in the context of PC was recently published [238]. CXCL12 expression was localized to stromal cells expressing platelet-derived growth factors receptors (PDGRF). Creating tissue-specific knockouts in which CXCL12 expression was inhibited in PDGFR+ cell lineages inhibited tumor growth and EMT.
Data on the impact(s) that obesity has on prostate tumors has been limited to date. A recent study demonstrated that PC cells’ enhanced recruitment of ASCs to prostate tumors in obese patients is attributed to the differential secretion of CXCL1 and CXCL8 [239]. In cell culture models, CXCL1 and CXCL8 attract ASCs by signaling to CXCR1 [239]. While CXCL8 expression is obesity-independent, CXCL1 expression is obesity-dependent, and CXCR1 is expressed at higher levels in obASCs than lnASCs [239]. Together, these results highlight how obesity alterations in the tumor microenvironment result in multidirectional paracrine signaling between ASCs and tumor cells.
It has also been suggested that prostate cancer patient-derived ASCs (pASCs) undergo a neoplastic transformation when treated with PC-conditioned media or PC-derived exosomes [240]. The pASCs demonstrated genetic instability, mesenchymal-to-epithelial transition (MET), and oncogenic transformation, forming prostate-like neoplastic lesions in vivo. Moreover, the cells recapitulated the tumors upon transplantation into secondary recipients [240]. In addition, the exosomes derived from PC cells were found to contain oncogenic factors, including H-ras and K-ras transcripts, oncomiRNAs miR-125b, miR-130b, and miR-155, as well as the Ras superfamily of GTPases Rab1a, Rab1b, and Rab11a, which may contribute to the neoplastic reprogramming of pASCs [240, 241]. These novel ASC reprogramming findings may be specific to PC, as they have not been described in other tumor settings investigating the interplay between cancer cells and ASCs, nor have they been investigated in the context of obesity.
12.3 ASCs in ovarian cancer
Ovarian cancer (OC) is the most lethal cancer of the female reproductive system, and it ranks fifth in cancer deaths among women. OC is particularly troubling because it tends to metastasize and seed the peritoneum, conferring a worse prognosis with a 5-year survival rate of roughly 45% [242]. ASCs play a role in both metastasis and chemoresistance of OC. Multiple studies have demonstrated that ASCs promote OC’s more aggressive metastatic phenotype. These data suggest that the proximity of tumors to peritoneal and abdominal fat may increase the impact of the ASCs as components of the TME.
The trophic factors produced by ASCs increase proliferation, migration, and spheroid formation through various mechanisms [243]. The co-culture of ASCs isolated from the omentum of OC patients cultured with OC cell lines in vitro demonstrated enhanced proliferation, migration, and chemoresistance of the OC cells [244]. In addition, ASCs increase ovarian cancer proliferation and migration by secreting IL-6, activating the JAK2/STAT3 pathway [245]. ASCs also increases matrix metalloproteinase (MMP) expression, particularly MMP2 and MMP9, which degrade the extracellular matrix and promote invasion in ovarian cancer cells [231].
In mouse xenograft studies in vivo revealed that OC xenografts implanted with ASCs demonstrated increased tumor metastasis and growth in the peritoneal cavity and elevated the expression of MMP2 and MMP9. ASCs taken from patients with omental metastasis demonstrate the reverse Warburg effect, providing lactate substrate to cancer cells for oxidative phosphorylation [244]. A study investigating proteomic alterations in OC cells exposed to conditioned media from ASCs found that thymosin beta 4 x-linked (TMSB4X) was significantly increased in the OC cells [246]. The inhibition of TMSB4X in OC cells reduced tumorigenicity both in vitro and in vivo and demonstrates its role as a key factor driving the tumor-promoting effects in OC.
Similar to what has been described in BC, it appears that ASCs in ovarian cancer can be readily converted to CAFs. The culture of ASCs in the presence of epithelial OC cells resulted in increased expression of myofibroblast markers, including \(\alpha\)-SMA and fibroblast activation protein, via TGF-\(\upbeta\) signaling [247]. The generation of CAFs from the ASCs resulted in increased OC proliferation and invasion and the inhibition of TGF-\(\upbeta\) inhibits the CAF formation by ASCs in OC models. The tumor proliferation and metastasis of OC cells were duplicated in vivo in rodents.
Other studies have reported the potential of ASCs to increase the number of CSCs in the context of OC. ASCs express high levels of bone morphogenic proteins (BMPs), and it appears the expression of BMPs in ASCs is influenced by Hedgehog (HH) produced by the OC cells in a positive feedback loop. The HH appears to induce the levels of BMP4 in ASCs, which reciprocally increases the levels of HH in the OC cell lines [248, 249]. This BMP4-HH feedback loop induces chemoresistance in OC; however, the interruption of this pathway reverses the chemoresistance. The chemoresistance observed in OC is also partially induced by the secretome of ASCs.
Furthermore, it has been shown that the obesity status and fat location of ASCs also impact the extent that ASCs increase ovarian cancer progression. Obese and visceral ASCs have been shown to increase the growth of ovarian tumors [243]. ASCs from obese donors also increase vascularity and the number of inflammatory cells in ovarian tumors [243]. Additional studies on obesity, ASCs, and OC would provide greater insight into the tumorigenic effects obesity has on OC.
12.4 ASCs in multiple myeloma
In the setting of multiple myeloma (MM), ASCs from obese donors promoted the growth and metastasis of MM cell lines. Notably, ASCs from obese donors had an altered cytokine/adipokine profile, which led to increased cell adhesion and MMP2 expression in MM [250], consistent with studies evaluating the effect of ASCs on other cancer types [86, 137]. Additionally, leptin stimulates the proliferation of MM cells and decreases the efficacy of chemotherapies in this malignancy, so leptin produced by ASCs in the tumor microenvironment may promote MM progression [251]; however, more work is needed to identify ASC pathways and drivers of MM progression.
12.5 ASCs in osteosarcoma
Studies evaluating osteosarcoma (OS), a primary malignancy of bone that mostly affects children and young adults, have seen similar effects [252]. ASCs promote OS cell growth and metastasis through the upregulation of MMP2 and MMP9 through STAT3 activation. STAT3 inhibition attenuated these effects, decreased MMP2/9 expression, and prolonged survival in mice [252]. Although the upstream activators of STAT3 were not identified, STAT3 is activated by many of the aforementioned paracrine factors from ASCs. Recently, it was demonstrated that ASC exosomes drive the invasion, migration, and proliferation of OS cells and increase the expression of collagen beta (1-O) galactosyltransferase 2 (COLGALT2). The inhibition of COLGALT2 by shRNA in OS cells reduced OS cell invasion, migration, and proliferation in vitro [253]. More is needed OS to identify secreted factors contributing to these outcomes. However, studies with ASCs stratified based on the obesity status of the patient do not appear to have been published as of yet.
12.6 ASCs in cervical cancer
Limited studies have evaluated the effect of ASCs on cervical cancer. However, one study determined that S100A7, which has a known role in breast cancer proliferation and metastasis, also enhances cervical cancer cells’ migration, invasion, metastasis, and the epithelial-to-mesenchymal transition [254].
A study investigated the impact of ASCs on the proliferation and migration of cervical cancer cell lines [255]. The data suggest that both co-culture with ASCs and using an ASC-conditioned medium promoted the proliferation and invasion of cervical cancer cells in vitro. In addition, the data from this study revealed that the ASC-conditioned medium also promoted the growth of HeLa cells in xenograft models in vivo.
12.7 ASCs in bladder cancer
The interaction between ASCs and bladder cancer cell lines has been investigated. In one of the first reports, ASCs increased the production of IL-8 and IL-6 when co-cultured with bladder cancer cells [256]. This is believed to have played a role in reduced extracellular matrix adhesion and increased cell viability, invasion, and migratory ability. In addition, the conditioned media from ASCs increased the proliferation and viability of bladder cancer cell lines (5637 and HT-1376) [257].
In another study, bladder cancer cells expressing the cancer stem cell marker CD133 were isolated and treated with an ASC-conditioned medium [31]. The data indicate that the conditioned media increased the proliferation and viability of CD133 + and CD133 − subpopulations and the unsorted cancer cell line. These data suggest that bladder cancer stem cells may respond to ASCs and their soluble factors.
However, another study suggests that ASCs and ASC-conditioned media may inhibit the growth of bladder cancer cells (lines EJ and T24) [231]. Treatment of these cancer cell lines with ASC-conditioned medium decreased the viability and migration of the cancer cells. The data also indicated that the bladder cancer cells underwent apoptosis in response to the conditioned medium.
12.8 ASC gastric cancer
Finally, the interaction between gastric cancer cells and ASCs or ASC-conditioned media has been investigated in multiple studies. For example, adenocarcinoma cell lines (MKN28 and MKN45) were cultured on gels embedded with ASCs in vitro [258]. The data indicate that exposure to ASCs increased the growth and invasion of the cancer cells and decreased the apoptotic frequency of the cells by activating MAPK.
ASCs have been reported to increase cell proliferation, migration/invasion, and progression of gastric cancer cell line MGC-803 in vitro, which appeared to be mediated through the SDF-1/CXCR4 axis [259].
In another study investigating the impact of ASCs on gastric cancer, a single gastric cancer cell line, HGC-27, was exposed to ASC-conditioned media and it appeared that the growth of the cancer cell line was inhibited both in vitro and in vivo in co-transplantation tumorigenicity studies [260].
12.9 ASCs in head and neck cancer
While it has been reported that ASCs enhanced the migration of head and neck tumors, the impact of ASCs on head and neck cancer cells in vitro is not fully known. Sharaf and colleagues published data indicating that human ASCs promote the proliferation and migration of head and neck cancer cells [257]. In this study, ASC-conditioned media was tested for paracrine effects on the proliferation of the HNSCC tumor cell line, FaDu, using in vitro assays. The data demonstrated that ASC-conditioned media enhanced proliferation and migration/invasion of the FaDu cells. However, it is just a single cancer cell line.
In another study by Rowan and colleagues, ASCs and ASC-conditioned media were shown not to impact cell growth of two head and neck cancer cell lines, Cal-27 and SCC-4 [261]. However, additional data from this study indicated that co-administration of ASCs with these cancer cells in vivo did increase the number of micro-metastases in the brains of mice, suggesting that the enhanced migration observed in vitro may drive the formation of tumors in vivo.
However, a study published by Danan and colleagues investigated the impact of ASCs on the proliferation, survival and migration of eight head and neck squamous cell carcinomas (HNSCC) cell lines in vitro and in the context of a xenograft model in mice [262]. Their data suggest that ASCs on enhanced proliferation, migration, and survival of only a single line, denoted as SCC9.
While there is limited research investigating the role of ASCs in these less frequent malignancies, the results across these various cancers are consistent. ASCs produce trophic factors that influence biological properties, enhancing the proliferation, migration, and invasion in vitro and in vivo in xenograft models, as reported for several cancers. For most of the published data, it is evident that the alterations in the tumor cells drive further changes in the ASCs via cancer cell-produced macromolecules to enhance tumorigenicity and metastasis even more. This further strengthens the need to study the role of ASCs in the context of obesity within the tumor microenvironment to define further the molecular pathways through which ASCs from obese donors enhance tumor growth and metastasis to identify potential therapeutic targets.
13 Safety of ASC application and fat grafting in the context of cancer: clinical outcomes
The preclinical data indicate that ASCs can enhance tumor cell growth, proliferation, and migration, resulting in a more invasive and aggressive phenotype across multiple cancer types in vitro and in vivo. A primary concern with fat grafting used in plastic surgery applications and ASC infusions under investigation as a therapy in numerous diseases in human patients is the activation or re-activation of tumors in vivo, which would be the result of the cancer cell-stimulating paracrine factors released from ASCs. While not explicitly focused on the impacts of obesity, multiple analyses of clinical outcomes in patients infused with ASCs to treat numerous diseases do not concur with the preclinical findings reported. ASCs have been infused in large numbers of patients as potential treatments for various disorders, both oncological and non-oncological [263]. Analyses have also been performed on patients that underwent fat grafting or were infused with ASCs in non-oncologic clinical trials for different diseases [260, 264]. The outcomes reported from these studies do not demonstrate any increase in the incidence of tumor formation resulting from ASC infusions. The issue with many of these reports is that the patient numbers are small, the follow-up is not always long-term, and different cell types and routes of administration are commonly used.
ASC infusions have been utilized in numerous oncologic settings, ranging from radiotherapy-induced xerostomia, osteosarcoma, tissue reconstruction after sarcoma resection, laser-assisted pulmonary metastasectomy, and breast reconstruction in breast cancer patients, with encouraging clinical and safety outcomes [263]. Much of the data about the oncologic safety of autologous fat grafts and ASC infusions comes primarily from groups of female breast cancer patients who underwent reconstruction. The first reported case of harvesting fat using a liposuction technique with placement into the breasts was reported in 1987 [265]. Since that time, fat grafting or lipofilling in breast reconstruction has become one of the most commonly used procedures for breast reconstruction. Autologous fat grafting with ASCs or micronized adipose tissue has been widely used for breast reconstruction, including post quadrantectomy defects to total breast reconstructions [266, 267]. In 2007, a task force from the American Society of Plastic Surgeons (ASPS) analyzed clinical outcomes of fat grafting in patients. It concluded that fat grafting was safe with no malignancy risk [268]. In 2015, the ASPS put out a grade B recommendation which stated that fat grafting does not increase the risk for local recurrence even though strict adherence to radiological follow-up protocols and an adequate disease-free interval is mandatory (https://www.plasticsurgery.org/Documents/Health-Policy/Principles/principle-2015-post-mastectomy-fat-grafting.pdf).
Numerous case study reports, meta-analyses, and systematic reviews on the outcomes of fat grafting safety in patients in regard to oncologic outcomes have been published subsequently. However, most of these reviews focus on patients undergoing lipofilling for various reasons [263, 269,270,271,272,273,274]. A meta-analysis by Toyerskani and colleagues in 2017 focused on the outcomes of trials using ASC or ASC-derived products [271]. The study was focused on a total of 70 trials (with over 1400 patients) and included a broad spectrum of disorders being treated. However, the majority were case studies, meaning there was no control group for comparison. The focus on the oncologic outcomes was evaluated for studies administering cells in the setting of previous malignancy, which narrowed the set to five studies (all case series) with a follow‐up in the range of 3–12 months. The results indicated only a single case of local breast cancer recurrence out of 121 total patients in this analysis. Since the studies were primarily cased studies, meaning small numbers of patients with no control treatment, it is impossible to determine if the case of one breast cancer recurrence could be attributed to the ASC treatment.
One issue with most of the retrospective studies published to date is relatively short-term analyses of patients, typically only weeks to months in duration. However, another large retrospective study of lipofilling in breasts with matched controls was published by Kronowitz and colleagues in 2016 [275]. This study used data in which the duration of follow-up was either 44 months or 60 months. The study analyzed outcomes from 719 cancer patients, 639 underwent mastectomies treated with fat grafting, and 670 non-grafted patients. The data indicate no substantive difference between the groups in loco-regional, systemic, or second breast cancer recurrence rates.
In a study specifically focused on the analysis of outcomes of adipose fat transfer after breast cancer surgery, Waked and colleagues focused on the outcomes of 18 clinical trials performed between 1997 and 2011 [266]. Each trial had a minimum monitoring period of 2 years, and many were longer than 5 years. The team assessed the incidence of loco-regional recurrence (LRR) incidence rates in each of the 18 studies. The LRR was determined to range from 0–3.9% per year. In the clinical trials designed to include a matched control group, the LRR incidence between the two groups was no different, suggesting that autologous fat grafting does not induce an increased incidence of cancer regeneration in breast cancer patients after treatment.
The general conclusion from the case reports and retrospective analyses is that ASC infusions and fat grafting do not appear to result in the formation of tumors nor drive the activation of tumor cells, even in oncological patients, but additional studies are required. However, it is important to note that many studies reported a lack of control populations, and the analyses are for short durations. In addition, the groups are composed of patients with mismatched grades of tumors or are undersized in terms of patient numbers.
In 2020, a team from Korea published a retrospective cohort study, analyzing 339 patients who had undergone immediate reconstructive surgery after nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) [276]. From a total of 67 patients who had undergone fat grafting, 10 patients were confirmed to have a cancer recurrence, which resulted in a hazard ratio (HR) of 2.52 with a 95% confidence interval for fat grafting. These data suggest that lipofilling results in a higher risk of cancer recurrence, countering most published data.
The findings from a retrospective, monocentric, case–control study investigating the survival and local recurrence rate in women who had fat grafting for breast reconstruction due to breast cancer were recently published [277]. The control patients underwent breast reconstruction without fat grafting. The data indicate an increased risk of tumor recurrence locally after lipofilling in patients with invasive breast cancer. The findings of this study suggest that physicians may need to be aware of special considerations in treating women who have had invasive breast cancer. While additional studies like this need to be performed to bolster these findings, the data suggest that the level of invasiveness of breast cancer could determine the outcomes in patients in which lipofilling is for breast reconstruction.
Further studies are needed to evaluate tumor formation and recurrence more carefully before declaring the use of ASCs and adipose tissue to be completely safe. Also, more prospective, multicentric, randomized clinical studies with longer follow-ups are required to detect the potential risks underlying ASC clinical use and the specific at-risk categories of patients. In addition, clinical studies focused on large numbers of obese patients would help clarify the impact of obesity on the safety of ASC infusions.
14 Conclusions
This review aimed to provide insight into our current understanding of the role that ASCs play in the context of cancer and the impact of obesity on ASCs within the tumor and tumor microenvironment. It is evident that ASCs are key contributors to the processes of tumorigenesis and metastasis. ASCs are recruited to integrate into the tumor stroma and influence tumor biology either by differentiation into CAFs or by the macromolecules they produce as undifferentiated residents of tumors. The ASCs secretome influences the other cells within the tumor stroma and tumor cells. The biological properties of ASCs are significantly impacted by donor characteristics such as age, BMI, and health status, which impact the differentiation, growth, biological properties, and secretome of ASCs. Our group and others have demonstrated that ASCs are impacted by obesity and alter important biologic properties and their impacts on other cells and tissues, including cancer.
It is evident that obesity impacts numerous cancer types, including colorectal, postmenopausal breast, endometrial, prostate, esophageal, hematological, malignant melanoma, and renal carcinomas. However, the cellular interactions and molecular pathways underlying obesity and cancer are not yet fully defined. Recent work has demonstrated that obesity impacts tumor cells directly and induces changes in other cell types within the tumor, including the ASCs. ASCs from obese patients demonstrate altered gene expression profiles and biological properties compared to lean patients. The data collected indicate that ASCs from obese donors have a markedly increased potential to assist tumorigenesis and metastasis. With respect to breast cancer, ASCs isolated from obese women secrete a novel set of chemokines, cytokines, and growth factors. The higher levels of chemokines and cytokines drive cancer cell proliferation and migration, tumor migration, invasion, and metastasis to distant organs. Our data on breast cancer suggest that in obese individuals, leptin is a primary molecule driving changes in the BCC that result in enhanced tumorigenicity and metastasis. The ASCs from obese donors appear to drive metastasis via leptin-mediated pathway(s), with SERPINE1 and MMP-2 being primarily involved, though other macromolecules may play a role but have not yet been described.
In summary, the data generated in preclinical models investigating the interplay between ASCs and cancer cells indicate that ASCs from obese donors contribute to tumorigenesis and can also enhance metastasis. The ASCs in the tumor stroma may represent a novel avenue by which obesity influences the biology of cancer cells. The cells may also represent an undescribed avenue for developing new therapies to counter the effects of obesity on tumors.
15 Future avenues
Future studies on the effects of obesity on ASCs and understanding how obesity primes the ASCs to enhance tumorigenesis and/or metastasis will provide valuable insight into reducing cancer morbidity and mortality. Furthermore, additional studies investigating the potential interaction of ASCs with other cell types in the tumor microenvironment are warranted to determine if alterations in other cell types influence the biology of the tumor. As tumor-associated ASCs impact the biology of the tumor, regardless of BMI, targeting the ASCs to develop strategies to interrupt their communication with tumor cells is a novel area that has yet to be explored. Also, developing therapeutic strategies explicitly targeting the obesity and cancer axis represents an unexplored area that could be impactful.
References
Zwick, R. K., Guerrero-Juarez, C. F., Horsley, V., & Plikus, M. V. (2018). Anatomical, physiological, and functional diversity of adipose tissue. Cell Metabolism, 27(1), 68–83. https://doi.org/10.1016/j.cmet.2017.12.002
Harvey, I., Boudreau, A., & Stephens, J. M. (2020). Adipose tissue in health and disease. Open Biology, 10(12), 200291. https://doi.org/10.1098/rsob.200291
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., & Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505), 425–432. https://doi.org/10.1038/372425a0
Friedman, J. (2014). 20 years of leptin: Leptin at 20: An overview. Journal of Endocrinology, 223(1), T1-8. https://doi.org/10.1530/joe-14-0405
Caër, C., Rouault, C., Le Roy, T., Poitou, C., Aron-Wisnewsky, J., Torcivia, A., et al. (2017). Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Science and Reports, 7(1), 3000. https://doi.org/10.1038/s41598-017-02660-w
Coppack, S. W. (2001). Pro-inflammatory cytokines and adipose tissue. Proceedings of the Nutrition Society 60(3):349-356 https://doi.org/10.1079/pns2001110
Frayn, K. N., Arner, P., & Yki-Järvinen, H. (2006). Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays in Biochemistry, 42, 89–103. https://doi.org/10.1042/bse0420089
Klingenberg, M. (1990). Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends in Biochemical Sciences, 15(3), 108–112. https://doi.org/10.1016/0968-0004(90)90194-g
Puigserver, P., Herron, D., Gianotti, M., Palou, A., Cannon, B., & Nedergaard, J. (1992). Induction and degradation of the uncoupling protein thermogenin in brown adipocytes in vitro and in vivo. Evidence for a rapidly degradable pool. Biochem Journal, 284(Pt 2), 393–398.
Seale, P., Bjork, B., Yang, W., Kajimura, S., Chin, S., Kuang, S., et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature, 454(7207), 961–967. https://doi.org/10.1038/nature07182
Seale, P., Conroe, H. M., Estall, J., Kajimura, S., Frontini, A., Ishibashi, J., et al. (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of Clinical Investigation, 121(1), 96–105. https://doi.org/10.1172/jci44271
Wang, W., & Seale, P. (2016). Control of brown and beige fat development. Nature Reviews Molecular Cell Biology, 17(11), 691–702. https://doi.org/10.1038/nrm.2016.96
Wu, J., Boström, P., Sparks, L. M., Ye, L., Choi, J. H., Giang, A. H., et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 150(2), 366–376. https://doi.org/10.1016/j.cell.2012.05.016
Harms, M., & Seale, P. (2013). Brown and beige fat: Development, function and therapeutic potential. Nature Medicine, 19(10), 1252–1263. https://doi.org/10.1038/nm.3361
Pilkington, A. C., Paz, H. A., & Wankhade, U. D. (2021). Beige adipose tissue identification and marker specificity-overview. Front Endocrinol (Lausanne), 12, 599134. https://doi.org/10.3389/fendo.2021.599134
Sidossis, L., & Kajimura, S. (2015). Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. The Journal of Clinical Investigation, 125(2), 478–486. https://doi.org/10.1172/jci78362
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology, 8(9), 726–736. https://doi.org/10.1038/nri2395
Galipeau, J., & Sensébé, L. (2018). Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell, 22(6), 824–833. https://doi.org/10.1016/j.stem.2018.05.004
Bunnell, B. A. (2021). Adipose tissue-derived mesenchymal stem cells. Cells, 10(12), 3433. https://doi.org/10.3390/cells10123433
Prockop, D. J., & Oh, J. Y. (2012). Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Molecular Therapy, 20(1), 14–20. https://doi.org/10.1038/mt.2011.211
Regulski, M. J. (2017). Mesenchymal stem cells: “Guardians of Inflammation.” Wounds, 29(1), 20–27.
Zuk, P. A., Zhu, M., Mizuno, H., Huang, J., Futrell, J. W., Katz, A. J., et al. (2001). Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering, 7(2), 211–228. https://doi.org/10.1089/107632701300062859
Fukuchi, Y., Nakajima, H., Sugiyama, D., Hirose, I., Kitamura, T., & Tsuji, K. (2004). Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells, 22(5), 649–658. https://doi.org/10.1634/stemcells.22-5-649
Troyer, D. L., & Weiss, M. L. (2008). Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells, 26(3), 591–599. https://doi.org/10.1634/stemcells.2007-0439
Huang, G. T., Gronthos, S., & Shi, S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of Dental Research, 88(9), 792–806. https://doi.org/10.1177/0022034509340867
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15(6), 641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Gonzalez-Rey, E., Gonzalez, M. A., Varela, N., O’Valle, F., Hernandez-Cortes, P., Rico, L., et al. (2010). Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Annals of the Rheumatic Diseases, 69(1), 241–248. https://doi.org/10.1136/ard.2008.101881
Semon, J. A., Maness, C., Zhang, X., Sharkey, S. A., Beuttler, M. M., Shah, F. S., et al. (2014). Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Research & Therapy, 5(1), 2. https://doi.org/10.1186/scrt391
Zhang, S., Danchuk, S. D., Bonvillain, R. W., Xu, B., Scruggs, B. A., Strong, A. L., et al. (2014). Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury. Stem Cells, 32(6), 1616–1628. https://doi.org/10.1002/stem.1632
Luque-Campos, N., Contreras-López, R. A., Jose Paredes-Martínez, M., Torres, M. J., Bahraoui, S., Wei, M., et al. (2019). Mesenchymal Stem cells improve rheumatoid arthritis progression by controlling memory T cell response. Frontiers in Immunology, 10, 798. https://doi.org/10.3389/fimmu.2019.00798
Jiang, W., & Xu, J. (2020). Immune modulation by mesenchymal stem cells. Cell Proliferation, 53(1), e12712. https://doi.org/10.1111/cpr.12712
Le Blanc, K., Tammik, L., Sundberg, B., Haynesworth, S. E., & Ringdén, O. (2003). Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scandinavian Journal of Immunology, 57(1), 11–20. https://doi.org/10.1046/j.1365-3083.2003.01176.x
Krampera, M., Cosmi, L., Angeli, R., Pasini, A., Liotta, F., Andreini, A., et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells, 24(2), 386–398. https://doi.org/10.1634/stemcells.2005-0008
Batten, P., Sarathchandra, P., Antoniw, J. W., Tay, S. S., Lowdell, M. W., Taylor, P. M., et al. (2006). Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: Relevance to tissue engineering human heart valves. Tissue Engineering, 12(8), 2263–2273. https://doi.org/10.1089/ten.2006.12.2263
Le Blanc, K., Samuelsson, H., Lönnies, L., Sundin, M., & Ringdén, O. (2007). Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation, 84(8), 1055–1059. https://doi.org/10.1097/01.tp.0000285088.44901.ea
Selmani, Z., Naji, A., Zidi, I., Favier, B., Gaiffe, E., Obert, L., et al. (2008). Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells, 26(1), 212–222. https://doi.org/10.1634/stemcells.2007-0554
Bochev, I., Elmadjian, G., Kyurkchiev, D., Tzvetanov, L., Altankova, I., Tivchev, P., et al. (2008). Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biology International, 32(4), 384–393. https://doi.org/10.1016/j.cellbi.2007.12.007
Asari, S., Itakura, S., Ferreri, K., Liu, C. P., Kuroda, Y., Kandeel, F., et al. (2009). Mesenchymal stem cells suppress B-cell terminal differentiation. Experimental Hematology, 37(5), 604–615. https://doi.org/10.1016/j.exphem.2009.01.005
DelaRosa, O., Sánchez-Correa, B., Morgado, S., Ramírez, C., del Río, B., Menta, R., et al. (2012). Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev, 21(8), 1333–1343. https://doi.org/10.1089/scd.2011.0139
Spaggiari, G. M., Capobianco, A., Becchetti, S., Mingari, M. C., & Moretta, L. (2006). Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood, 107(4), 1484–1490. https://doi.org/10.1182/blood-2005-07-2775
Spaggiari, G. M., Capobianco, A., Abdelrazik, H., Becchetti, F., Mingari, M. C., & Moretta, L. (2008). Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood, 111(3), 1327–1333. https://doi.org/10.1182/blood-2007-02-074997
Geng, Y., Zhang, L., Fu, B., Zhang, J., Hong, Q., Hu, J., et al. (2014). Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Research & Therapy, 5(3), 80. https://doi.org/10.1186/scrt469
Song, W. J., Li, Q., Ryu, M. O., Ahn, J. O., Ha Bhang, D., Chan Jung, Y., et al. (2017). TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in Mice. Science and Reports, 7(1), 5187. https://doi.org/10.1038/s41598-017-04766-7
Traktuev, D. O., Merfeld-Clauss, S., Li, J., Kolonin, M., Arap, W., Pasqualini, R., et al. (2008). A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation Research, 102(1), 77–85. https://doi.org/10.1161/circresaha.107.159475
Suga, H., Matsumoto, D., Eto, H., Inoue, K., Aoi, N., Kato, H., et al. (2009). Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev, 18(8), 1201–1210. https://doi.org/10.1089/scd.2009.0003
Weiss, M. L., Anderson, C., Medicetty, S., Seshareddy, K. B., Weiss, R. J., VanderWerff, I., et al. (2008). Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells, 26(11), 2865–2874. https://doi.org/10.1634/stemcells.2007-1028
Sousa, B. R., Parreira, R. C., Fonseca, E. A., Amaya, M. J., Tonelli, F. M., Lacerda, S. M., et al. (2014). Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytometry Part A, 85(1), 43–77. https://doi.org/10.1002/cyto.a.22402
Fogelholm, M. (2010). Physical activity, fitness and fatness: Relations to mortality, morbidity and disease risk factors A systematic review. Obes Rev, 11(3), 202–221. https://doi.org/10.1111/j.1467-789X.2009.00653.x
Doyle, S. L., Donohoe, C. L., Lysaght, J., & Reynolds, J. V. (2012). Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proceeding of Nutrition Society 71(1):181-189 https://doi.org/10.1017/s002966511100320x
Pérez-Hernández, A. I., Catalán, V., Gómez-Ambrosi, J., Rodríguez, A., & Frühbeck, G. (2014). Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol (Lausanne), 5, 65. https://doi.org/10.3389/fendo.2014.00065
Bianchini, F., Kaaks, R., & Vainio, H. (2002). Overweight, obesity, and cancer risk. The lancet Oncology, 3(9), 565–574. https://doi.org/10.1016/s1470-2045(02)00849-5
Caldwell, S. H., Crespo, D. M., Kang, H. S., & Al-Osaimi, A. M. (2004). Obesity and hepatocellular carcinoma. Gastroenterology, 127(5 Suppl 1), S97-103. https://doi.org/10.1053/j.gastro.2004.09.021
Buschemeyer, W. C., 3rd., & Freedland, S. J. (2007). Obesity and prostate cancer: Epidemiology and clinical implications. European Urology, 52(2), 331–343. https://doi.org/10.1016/j.eururo.2007.04.069
Giovannucci, E., & Michaud, D. (2007). The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology, 132(6), 2208–2225. https://doi.org/10.1053/j.gastro.2007.03.050
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F., & Zwahlen, M. (2008). Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet, 371(9612), 569–578. https://doi.org/10.1016/s0140-6736(08)60269-x
Fader, A. N., Arriba, L. N., Frasure, H. E., & von Gruenigen, V. E. (2009). Endometrial cancer and obesity: Epidemiology, biomarkers, prevention and survivorship. Gynecologic Oncology, 114(1), 121–127. https://doi.org/10.1016/j.ygyno.2009.03.039
Reeves, K. W., Carter, G. C., Rodabough, R. J., Lane, D., McNeeley, S. G., Stefanick, M. L., et al. (2011). Obesity in relation to endometrial cancer risk and disease characteristics in the Women’s Health Initiative. Gynecologic Oncology, 121(2), 376–382. https://doi.org/10.1016/j.ygyno.2011.01.027
Crosbie, E. J., Roberts, C., Qian, W., Swart, A. M., Kitchener, H. C., & Renehan, A. G. (2012). Body mass index does not influence post-treatment survival in early stage endometrial cancer: Results from the MRC ASTEC trial. European Journal of Cancer, 48(6), 853–864. https://doi.org/10.1016/j.ejca.2011.10.003
Lauby-Secretan, B., Scoccianti, C., Loomis, D., Grosse, Y., Bianchini, F., & Straif, K. (2016). Body fatness and cancer–Viewpoint of the IARC working group. New England Journal of Medicine, 375(8), 794–798. https://doi.org/10.1056/NEJMsr1606602
Litton, J. K., Gonzalez-Angulo, A. M., Warneke, C. L., Buzdar, A. U., Kau, S. W., Bondy, M., et al. (2008). Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. Journal of Clinical Oncology, 26(25), 4072–4077. https://doi.org/10.1200/jco.2007.14.4527
Sánchez, L., Lana, A., Hidalgo, A., Rodríguez, J. M., Del Valle Mdel, O., Cueto, A., et al. (2008). Risk factors for second primary tumours in breast cancer survivors. European Journal of Cancer Prevention, 17(5), 406–413. https://doi.org/10.1097/CEJ.0b013e3282f75ee5
Niraula, S., Ocana, A., Ennis, M., & Goodwin, P. J. (2012). Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Research and Treatment, 134(2), 769–781. https://doi.org/10.1007/s10549-012-2073-x
Petrelli, J. M., Calle, E. E., Rodriguez, C., & Thun, M. J. (2002). Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes and Control, 13(4), 325–332. https://doi.org/10.1023/a:1015288615472
Schapira, D. V., Kumar, N. B., Lyman, G. H., & Cox, C. E. (1990). Abdominal obesity and breast cancer risk. Annals of Internal Medicine, 112(3), 182–186. https://doi.org/10.7326/0003-4819-112-3-182
Abrahamson, P. E., Gammon, M. D., Lund, M. J., Flagg, E. W., Porter, P. L., Stevens, J., et al. (2006). General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 15(10), 1871–1877. https://doi.org/10.1158/1055-9965.Epi-06-0356
Protani, M., Coory, M., & Martin, J. H. (2010). Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Research and Treatment, 123(3), 627–635. https://doi.org/10.1007/s10549-010-0990-0
Fortner, R. T., Katzke, V., Kühn, T., & Kaaks, R. (2016). Obesity and breast cancer. Recent Results in Cancer Research, 208, 43–65. https://doi.org/10.1007/978-3-319-42542-9_3
Carmichael, A. R. (2006). Obesity and prognosis of breast cancer. Obesity Reviews, 7(4), 333–340. https://doi.org/10.1111/j.1467-789X.2006.00261.x
Sestak, I., Distler, W., Forbes, J. F., Dowsett, M., Howell, A., & Cuzick, J. (2010). Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. Journal of Clinical Oncology, 28(21), 3411–3415. https://doi.org/10.1200/jco.2009.27.2021
Kumar, N. B., Cantor, A., Allen, K., & Cox, C. E. (2000). Android obesity at diagnosis and breast carcinoma survival: Evaluation of the effects of anthropometric variables at diagnosis, including body composition and body fat distribution and weight gain during life span, and survival from breast carcinoma. Cancer, 88(12), 2751–2757. https://doi.org/10.1002/1097-0142(20000615)88:12%3c2751::aid-cncr13%3e3.0.co;2-1
Borugian, M. J., Sheps, S. B., Kim-Sing, C., Olivotto, I. A., Van Patten, C., Dunn, B. P., et al. (2003). Waist-to-hip ratio and breast cancer mortality. American Journal of Epidemiology, 158(10), 963–968. https://doi.org/10.1093/aje/kwg236
Iwase, T., Wang, X., Shrimanker, T. V., Kolonin, M. G., & Ueno, N. T. (2021). Body composition and breast cancer risk and treatment: Mechanisms and impact. Breast Cancer Research and Treatment, 186(2), 273–283. https://doi.org/10.1007/s10549-020-06092-5
Harris, A. L. (2002). Hypoxia–A key regulatory factor in tumour growth. Nature Reviews Cancer, 2(1), 38–47. https://doi.org/10.1038/nrc704
Brahimi-Horn, M. C., & Pouysségur, J. (2007). Hypoxia in cancer cell metabolism and pH regulation. Essays in Biochemistry, 43, 165–178. https://doi.org/10.1042/bse0430165
Folkman, J., Hahnfeldt, P., & Hlatky, L. (2000). Cancer: Looking outside the genome. Nature Reviews Molecular Cell Biology, 1(1), 76–79. https://doi.org/10.1038/35036100
Blaak, E. E., van Baak, M. A., Kemerink, G. J., Pakbiers, M. T., Heidendal, G. A., & Saris, W. H. (1995). Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism, 44(2), 183–187. https://doi.org/10.1016/0026-0495(95)90262-7
Jansson, P. A., Larsson, A., & Lönnroth, P. N. (1998). Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. European Journal of Clinical Investigation, 28(10), 813–818. https://doi.org/10.1046/j.1365-2362.1998.00360.x
West, D. B., Prinz, W. A., Francendese, A. A., & Greenwood, M. R. (1987). Adipocyte blood flow is decreased in obese Zucker rats. American Journal of Physiology, 253(2 Pt 2), R228-233. https://doi.org/10.1152/ajpregu.1987.253.2.R228
Carroll, V. A., & Ashcroft, M. (2005). Targeting the molecular basis for tumour hypoxia. Expert Reviews in Molecular Medicine, 7(6), 1–16. https://doi.org/10.1017/s1462399405009117
Trayhurn, P. (2013). Hypoxia and adipose tissue function and dysfunction in obesity. Physiological Reviews, 93(1), 1–21. https://doi.org/10.1152/physrev.00017.2012
Zhang, Y., Daquinag, A. C., Amaya-Manzanares, F., Sirin, O., Tseng, C., & Kolonin, M. G. (2012). Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Research, 72(20), 5198–5208. https://doi.org/10.1158/0008-5472.Can-12-0294
Bellows, C. F., Zhang, Y., Chen, J., Frazier, M. L., & Kolonin, M. G. (2011). Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiology, Biomarkers & Prevention, 20(11), 2461–2468. https://doi.org/10.1158/1055-9965.Epi-11-0556
Bellows, C. F., Zhang, Y., Simmons, P. J., Khalsa, A. S., & Kolonin, M. G. (2011). Influence of BMI on level of circulating progenitor cells. Obesity (Silver Spring), 19(8), 1722–1726. https://doi.org/10.1038/oby.2010.347
Strong, A. L., Semon, J. A., Strong, T. A., Santoke, T. T., Zhang, S., McFerrin, H. E., et al. (2012). Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion. Stem Cells, 30(12), 2774–2783. https://doi.org/10.1002/stem.1229
Gealekman, O., Guseva, N., Hartigan, C., Apotheker, S., Gorgoglione, M., Gurav, K., et al. (2011). Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation, 123(2), 186–194. https://doi.org/10.1161/circulationaha.110.970145
Strong, A. L., Strong, T. A., Rhodes, L. V., Semon, J. A., Zhang, X., Shi, Z., et al. (2013). Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Research, 15(5), R102. https://doi.org/10.1186/bcr3569
Sabol, R. A., Giacomelli, P., Beighley, A., & Bunnell, B. A. (2019). Adipose stem cells and cancer: Concise review. Stem Cells, 37(10), 1261–1266. https://doi.org/10.1002/stem.3050
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013
Karagiannis, G. S., Poutahidis, T., Erdman, S. E., Kirsch, R., Riddell, R. H., & Diamandis, E. P. (2012). Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Molecular Cancer Research, 10(11), 1403–1418. https://doi.org/10.1158/1541-7786.Mcr-12-0307
Strong, A. L., Pei, D. T., Hurst, C. G., Gimble, J. M., Burow, M. E., & Bunnell, B. A. (2017). Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int, 2017, 9216502. https://doi.org/10.1155/2017/9216502
Plaks, V., Boldajipour, B., Linnemann, J. R., Nguyen, N. H., Kersten, K., Wolf, Y., et al. (2015). Adaptive immune regulation of mammary postnatal organogenesis. Developmental Cell, 34(5), 493–504. https://doi.org/10.1016/j.devcel.2015.07.015
Liu, T., Han, C., Wang, S., Fang, P., Ma, Z., Xu, L., et al. (2019). Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. Journal of Hematology & Oncology, 12(1), 86. https://doi.org/10.1186/s13045-019-0770-1
Mariman, E. C., & Wang, P. (2010). Adipocyte extracellular matrix composition, dynamics and role in obesity. Cellular and Molecular Life Sciences, 67(8), 1277–1292. https://doi.org/10.1007/s00018-010-0263-4
Datta, R., Podolsky, M. J., & Atabai, K. (2018). Fat fibrosis: Friend or foe? Journal of Clinic Investigation Insight, 3(19), e122289. https://doi.org/10.1172/jci.insight.122289
Pasarica, M., Gowronska-Kozak, B., Burk, D., Remedios, I., Hymel, D., Gimble, J., et al. (2009). Adipose tissue collagen VI in obesity. Journal of Clinical Endocrinology and Metabolism, 94(12), 5155–5162. https://doi.org/10.1210/jc.2009-0947
Seo, B. R., Bhardwaj, P., Choi, S., Gonzalez, J., Andresen Eguiluz, R. C., Wang, K., et al. (2015). Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Science Translational Medicine, 7(301), 301. https://doi.org/10.1126/scitranslmed.3010467
Druso, J. E., & Fischbach, C. (2018). Biophysical properties of extracellular matrix: Linking obesity and cancer. Trends in Cancer, 4(4), 271–273. https://doi.org/10.1016/j.trecan.2018.02.001
Ruiz-Ojeda, F. J., Méndez-Gutiérrez, A., Aguilera, C. M., & Plaza-Díaz, J. (2019). Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Internationl Journal of Molecular Science, 20(19), 4888. https://doi.org/10.3390/ijms20194888
Zhang, Y., Baloglu, F. K., Ziemer, L. E. H., Liu, Z., Lyu, B., Arendt, L. M., et al. (2020). Factors associated with obesity alter matrix remodeling in breast cancer tissues. Journal of Biomedial Optics, 25(1), 1–14. https://doi.org/10.1117/1.Jbo.25.1.014513
Rømer, A. M. A., Thorseth, M.-L., & Madsen, D. H. (2021). Immune modulatory properties of collagen in cancer. Frontiers in Immunology, 12, 791453. https://doi.org/10.3389/fimmu.2021.791453
Nallanthighal, S., Heiserman, J. P., & Cheon, D. J. (2019). The role of the extracellular matrix in cancer stemness. Frontiers in Cell and Developmental Biology, 7, 86. https://doi.org/10.3389/fcell.2019.00086
McCulloch, L. J., Rawling, T. J., Sjöholm, K., Franck, N., Dankel, S. N., Price, E. J., et al. (2015). COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology, 156(1), 134–146. https://doi.org/10.1210/en.2014-1042
Khan, T., Muise, E. S., Iyengar, P., Wang, Z. V., Chandalia, M., Abate, N., et al. (2009). Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Molecular and Cellular Biology, 29(6), 1575–1591. https://doi.org/10.1128/mcb.01300-08
Oh, J., Kim, C. S., Kim, M., Jo, W., Sung, Y. H., & Park, J. (2021). Type VI collagen and its cleavage product, endotrophin, cooperatively regulate the adipogenic and lipolytic capacity of adipocytes. Metabolism, 114, 154430. https://doi.org/10.1016/j.metabol.2020.154430
Spencer, M., Yao-Borengasser, A., Unal, R., Rasouli, N., Gurley, C. M., Zhu, B., et al. (2010). Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. American journal of physiology. Endocrinology and metabolism, 299(6), E1016-1027. https://doi.org/10.1152/ajpendo.00329.2010
Spencer, M., Unal, R., Zhu, B., Rasouli, N., McGehee, R. E., Jr., Peterson, C. A., et al. (2011). Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. Journal of Clinical Endocrinology and Metabolism, 96(12), E1990-1998. https://doi.org/10.1210/jc.2011-1567
Chen, P., Cescon, M., & Bonaldo, P. (2013). Collagen VI in cancer and its biological mechanisms. Trends in Molecular Medicine, 19(7), 410–417. https://doi.org/10.1016/j.molmed.2013.04.001
Nallanthighal, S., Heiserman, J. P., & Cheon, D.-J. (2019). The role of the extracellular matrix in cancer stemness. Frontiers in Cell and Developmental Biology, 7, 86. https://doi.org/10.3389/fcell.2019.00086
Ho, C. M., Chang, T. H., Yen, T. L., Hong, K. J., & Huang, S. H. (2021). Collagen type VI regulates the CDK4/6-p-Rb signaling pathway and promotes ovarian cancer invasiveness, stemness, and metastasis. American Journal of Cancer Research, 11(3), 668–690.
Byrne, C. E., Decombe, J. B., Bingham, G. C., Remont, J., Miller, L. G., Khalif, L., et al. (2021). Evaluation of extracellular matrix composition to improve breast cancer modeling. Tissue Engineering Part A, 27(7–8), 500–511. https://doi.org/10.1089/ten.TEA.2020.0364
Insua-Rodríguez, J., & Oskarsson, T. (2016). The extracellular matrix in breast cancer. Advanced Drug Delivery Reviews, 97, 41–55. https://doi.org/10.1016/j.addr.2015.12.017
Pan, Y., Yu, Y., Wang, X., & Zhang, T. (2020). Tumor-associated macrophages in tumor immunity. Frontiers in Immunology, 11, 583084. https://doi.org/10.3389/fimmu.2020.583084
Williams, L. M., McCann, F. E., Cabrita, M. A., Layton, T., Cribbs, A., Knezevic, B., et al. (2020). Identifying collagen VI as a target of fibrotic diseases regulated by CREBBP/EP300. Proceedings of the National Academy of Sciences 117(34):20753-20763 https://doi.org/10.1073/pnas.2004281117
Acerbi, I., Cassereau, L., Dean, I., Shi, Q., Au, A., Park, C., et al. (2015). Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb), 7(10), 1120–1134. https://doi.org/10.1039/c5ib00040h
Reggio, S., Rouault, C., Poitou, C., Bichet, J. C., Prifti, E., Bouillot, J. L., et al. (2016). Increased basement membrane components in adipose tissue during obesity: Links with TGFβ and Metabolic Phenotypes. Journal of Clinical Endocrinology and Metabolism, 101(6), 2578–2587. https://doi.org/10.1210/jc.2015-4304
Nakazeki, F., Nishiga, M., Horie, T., Nishi, H., Nakashima, Y., Baba, O., et al. (2018). Loss of periostin ameliorates adipose tissue inflammation and fibrosis in vivo. Science and Reports, 8(1), 8553. https://doi.org/10.1038/s41598-018-27009-9
Walker, C., Mojares, E., & Del Río Hernández, A. (2018). Role of extracellular matrix in development and cancer progression. International Journal of Molecular Science, 19(10), 3028. https://doi.org/10.3390/ijms19103028
Belgodere, J. A., King, C. T., Bursavich, J. B., Burow, M. E., Martin, E. C., & Jung, J. P. (2018). Engineering breast cancer microenvironments and 3D bioprinting. Frontiers in Bioengineering and Biotechnology, 6, 66. https://doi.org/10.3389/fbioe.2018.00066
Catalán, V., Gómez-Ambrosi, J., Rodríguez, A., Ramírez, B., Rotellar, F., Valentí, V., et al. (2012). Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. Journal of Clinical Endocrinology and Metabolism, 97(10), E1880-1889. https://doi.org/10.1210/jc.2012-1670
Kidd, S., Spaeth, E., Watson, K., Burks, J., Lu, H., Klopp, A., et al. (2012). Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE, 7(2), e30563. https://doi.org/10.1371/journal.pone.0030563
Miyazaki, Y., Oda, T., Mori, N., & Kida, Y. S. (2020). Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer-associated fibroblasts in vitro. FEBS Open Bio, 10(11), 2268–2281. https://doi.org/10.1002/2211-5463.12976
Miyazaki, Y., Oda, T., Inagaki, Y., Kushige, H., Saito, Y., Mori, N., et al. (2021). Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Science and Reports, 11(1), 4690. https://doi.org/10.1038/s41598-021-84058-3
Tan, H. X., Xiao, Z. G., Huang, T., Fang, Z. X., Liu, Y., & Huang, Z. C. (2020). CXCR4/TGF-β1 mediated self-differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and promoted colorectal carcinoma development. Cancer Biology & Therapy, 21(3), 248–257. https://doi.org/10.1080/15384047.2019.1685156
Brock, C. K., Hebert, K. L., Artiles, M., Wright, M. K., Cheng, T., Windsor, G. O., et al. (2021). A role for adipocytes and adipose stem cells in the breast tumor microenvironment and regenerative medicine. Frontiers in Physiology, 12, 751239. https://doi.org/10.3389/fphys.2021.751239
Bukowska, J., Alarcon Uquillas, A., Wu, X., Frazier, T., Walendzik, K., Vanek, M., et al. (2020). Safety and efficacy of human adipose-derived stromal/stem cell therapy in an immunocompetent murine pressure ulcer model. Stem Cells Development, 29(7), 440–451. https://doi.org/10.1089/scd.2019.0244
Bernard, J. J., & Wellberg, E. A. (2021). The tumor promotional role of adipocytes in the breast cancer microenvironment and macroenvironment. American Journal of Pathology, 191(8), 1342–1352. https://doi.org/10.1016/j.ajpath.2021.02.006
Dvorak, H. F. (2015). Tumors: Wounds that do not heal-redux. Cancer Immunology Research, 3(1), 1–11. https://doi.org/10.1158/2326-6066.Cir-14-0209
Dvorak, H. F. (2019). Tumors: Wounds that do not heal-a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Seminars in Thrombosis and Hemostasis, 45(6), 576–592. https://doi.org/10.1055/s-0039-1687908
Li, C., Jiang, P., Wei, S., Xu, X., & Wang, J. (2020). Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer, 19(1), 116. https://doi.org/10.1186/s12943-020-01234-1
Cho, J. A., Park, H., Lim, E. H., & Lee, K. W. (2012). Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. International Journal of Oncology, 40(1), 130–138. https://doi.org/10.3892/ijo.2011.1193
Jotzu, C., Alt, E., Welte, G., Li, J., Hennessy, B. T., Devarajan, E., et al. (2010). Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Analytical Cellular Pathology (Amsterdam), 33(2), 61–79. https://doi.org/10.3233/acp-clo-2010-0535
Zhang, Y., Daquinag, A., Traktuev, D. O., Amaya-Manzanares, F., Simmons, P. J., March, K. L., et al. (2009). White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Research, 69(12), 5259–5266. https://doi.org/10.1158/0008-5472.Can-08-3444
Shimoda, M., Mellody, K. T., & Orimo, A. (2010). Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Seminars in Cell & Developmental Biology, 21(1), 19–25. https://doi.org/10.1016/j.semcdb.2009.10.002
Hasegawa, T., Yashiro, M., Nishii, T., Matsuoka, J., Fuyuhiro, Y., Morisaki, T., et al. (2014). Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-β signaling. International Journal of Cancer, 134(8), 1785–1795. https://doi.org/10.1002/ijc.28520
Orimo, A., Gupta, P. B., Sgroi, D. C., Arenzana-Seisdedos, F., Delaunay, T., Naeem, R., et al. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell, 121(3), 335–348. https://doi.org/10.1016/j.cell.2005.02.034
Mueller, L., Goumas, F. A., Affeldt, M., Sandtner, S., Gehling, U. M., Brilloff, S., et al. (2007). Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. American Journal of Pathology, 171(5), 1608–1618. https://doi.org/10.2353/ajpath.2007.060661
Strong, A. L., Burow, M. E., Gimble, J. M., & Bunnell, B. A. (2015). Concise review: The obesity cancer paradigm: Exploration of the interactions and crosstalk with adipose stem cells. Stem Cells, 33(2), 318–326. https://doi.org/10.1002/stem.1857
Studeny, M., Marini, F. C., Champlin, R. E., Zompetta, C., Fidler, I. J., & Andreeff, M. (2002). Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Research, 62(13), 3603–3608.
Studeny, M., Marini, F. C., Dembinski, J. L., Zompetta, C., Cabreira-Hansen, M., Bekele, B. N., et al. (2004). Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. Journal of the National Cancer Institute, 96(21), 1593–1603. https://doi.org/10.1093/jnci/djh299
Nakamizo, A., Marini, F., Amano, T., Khan, A., Studeny, M., Gumin, J., et al. (2005). Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Research, 65(8), 3307–3318. https://doi.org/10.1158/0008-5472.Can-04-1874
Menon, L. G., Kelly, K., Yang, H. W., Kim, S. K., Black, P. M., & Carroll, R. S. (2009). Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells, 27(9), 2320–2330. https://doi.org/10.1002/stem.136
Zimmerlin, L., Donnenberg, A. D., Rubin, J. P., Basse, P., Landreneau, R. J., & Donnenberg, V. S. (2011). Regenerative therapy and cancer: In vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Engineering Part A, 17(1–2), 93–106. https://doi.org/10.1089/ten.TEA.2010.0248
Belmar-Lopez, C., Mendoza, G., Oberg, D., Burnet, J., Simon, C., Cervello, I., et al. (2013). Tissue-derived mesenchymal stromal cells used as vehicles for anti-tumor therapy exert different in vivo effects on migration capacity and tumor growth. Bmc Medicine, 11, 139. https://doi.org/10.1186/1741-7015-11-139
Kalimuthu, S., Zhu, L., Oh, J. M., Gangadaran, P., Lee, H. W., Baek, S. H., et al. (2018). Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin. International Journal of Medical Sciences, 15(10), 1051–1061. https://doi.org/10.7150/ijms.25760
Cho, J. A., Park, H., Lim, E. H., Kim, K. H., Choi, J. S., Lee, J. H., et al. (2011). Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecologic Oncology, 123(2), 379–386. https://doi.org/10.1016/j.ygyno.2011.08.005
Devarajan, E., Song, Y. H., Krishnappa, S., & Alt, E. (2012). Epithelial-mesenchymal transition in breast cancer lines is mediated through PDGF-D released by tissue-resident stem cells. International Journal of Cancer, 131(5), 1023–1031. https://doi.org/10.1002/ijc.26493
Tuxhorn, J. A., Ayala, G. E., Smith, M. J., Smith, V. C., Dang, T. D., & Rowley, D. R. (2002). Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical Cancer Research, 8(9), 2912–2923.
De Boeck, A., Hendrix, A., Maynard, D., Van Bockstal, M., Daniëls, A., Pauwels, P., et al. (2013). Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics, 13(2), 379–388. https://doi.org/10.1002/pmic.201200179
Arendt, L. M., McCready, J., Keller, P. J., Baker, D. D., Naber, S. P., Seewaldt, V., et al. (2013). Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Research, 73(19), 6080–6093. https://doi.org/10.1158/0008-5472.Can-13-0926
Castro-Oropeza, R., Vazquez-Santillan, K., Díaz-Gastelum, C., Melendez-Zajgla, J., Zampedri, C., Ferat-Osorio, E., et al. (2020). Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer. Science and Reports, 10(1), 14205. https://doi.org/10.1038/s41598-020-69907-x
Hutchings, G., Janowicz, K., Moncrieff, L., Dompe, C., Strauss, E., Kocherova, I., et al. (2020). The proliferation and differentiation of adipose-derived stem cells in neovascularization and angiogenesis. International Journal of Molecular Science, 21(11), 3790. https://doi.org/10.3390/ijms21113790
Preisner, F., Leimer, U., Sandmann, S., Zoernig, I., Germann, G., & Koellensperger, E. (2018). Impact of human adipose tissue-derived stem cells on malignant melanoma cells in an in vitro co-culture model. Stem Cell Reviews and Reports, 14(1), 125–140. https://doi.org/10.1007/s12015-017-9772-y
Salha, S., Gehmert, S., Brébant, V., Anker, A., Loibl, M., Prantl, L., et al. (2018). PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3K signaling pathway. Clinical Hemorheology and Microcirculation, 70(4), 543–551. https://doi.org/10.3233/ch-189319
Zhang, W., Torres-Rojas, C., Yue, J., & Zhu, B. M. (2021). Adipose-derived stem cells in ovarian cancer progression, metastasis, and chemoresistance. Experimental Biology and Medicine (Maywood), 246(16), 1810–1815. https://doi.org/10.1177/15353702211023846
Chandler, E. M., Saunders, M. P., Yoon, C. J., Gourdon, D., & Fischbach, C. (2011). Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Physical Biology, 8(1), 015008. https://doi.org/10.1088/1478-3975/8/1/015008
Samarajeewa, N. U., Yang, F., Docanto, M. M., Sakurai, M., McNamara, K. M., Sasano, H., et al. (2013). HIF-1α stimulates aromatase expression driven by prostaglandin E2 in breast adipose stroma. Breast Cancer Research, 15(2), R30. https://doi.org/10.1186/bcr3410
Ribeiro, R., Monteiro, C., Silvestre, R., Castela, A., Coutinho, H., Fraga, A., et al. (2012). Human periprostatic white adipose tissue is rich in stromal progenitor cells and a potential source of prostate tumor stroma. Experimental Biology and Medicine (Maywood), 237(10), 1155–1162. https://doi.org/10.1258/ebm.2012.012131
Walter, M., Liang, S., Ghosh, S., Hornsby, P. J., & Li, R. (2009). Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene, 28(30), 2745–2755. https://doi.org/10.1038/onc.2009.130
Lin, G., Yang, R., Banie, L., Wang, G., Ning, H., Li, L. C., et al. (2010). Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate, 70(10), 1066–1073. https://doi.org/10.1002/pros.21140
Muehlberg, F. L., Song, Y. H., Krohn, A., Pinilla, S. P., Droll, L. H., Leng, X., et al. (2009). Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis, 30(4), 589–597. https://doi.org/10.1093/carcin/bgp036
Rowan, B. G., Gimble, J. M., Sheng, M., Anbalagan, M., Jones, R. K., Frazier, T. P., et al. (2014). Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS ONE, 9(2), e89595. https://doi.org/10.1371/journal.pone.0089595
Li, W., Xu, H., & Qian, C. (2017). c-Kit-positive adipose tissue-derived mesenchymal stem cells promote the growth and angiogenesis of breast cancer. BioMed Research International, 2017, 7407168. https://doi.org/10.1155/2017/7407168
Nakopoulou, L., Tsirmpa, I., Alexandrou, P., Louvrou, A., Ampela, C., Markaki, S., et al. (2003). MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Research and Treatment, 77(2), 145–155. https://doi.org/10.1023/a:1021371028777
Quintero-Fabián, S., Arreola, R., Becerril-Villanueva, E., Torres-Romero, J. C., Arana-Argáez, V., Lara-Riegos, J., et al. (2019). Role of matrix metalloproteinases in angiogenesis and cancer. Frontiers in Oncology, 9, 1370. https://doi.org/10.3389/fonc.2019.01370
Curran, S., & Murray, G. I. (2000). Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. European Journal of Cancer, 36(13), 1621–1630. https://doi.org/10.1016/s0959-8049(00)00156-8
Coussens, L. M., Fingleton, B., & Matrisian, L. M. (2002). Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science, 295(5564), 2387–2392. https://doi.org/10.1126/science.1067100
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174. https://doi.org/10.1038/nrc745
Baker, A. H., Zaltsman, A. B., George, S. J., & Newby, A. C. (1998). Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. Journal of Clinical Investigation, 101(6), 1478–1487. https://doi.org/10.1172/jci1584
Park, H. Y., Kwon, H. M., Lim, H. J., Hong, B. K., Lee, J. Y., Park, B. E., et al. (2001). Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Experimental & Molecular Medicine, 33(2), 95–102. https://doi.org/10.1038/emm.2001.17
Hayakawa, T., Yamashita, K., Tanzawa, K., Uchijima, E., & Iwata, K. (1992). Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. Febs Letters, 298(1), 29–32. https://doi.org/10.1016/0014-5793(92)80015-9
Pinilla, S., Alt, E., Abdul Khalek, F. J., Jotzu, C., Muehlberg, F., Beckmann, C., et al. (2009). Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Letters, 284(1), 80–85. https://doi.org/10.1016/j.canlet.2009.04.013
Welte, G., Alt, E., Devarajan, E., Krishnappa, S., Jotzu, C., & Song, Y. H. (2012). Interleukin-8 derived from local tissue-resident stromal cells promotes tumor cell invasion. Molecular Carcinogenesis, 51(11), 861–868. https://doi.org/10.1002/mc.20854
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. Ca: A Cancer Journal for Clinicians, 71(1), 7–33. https://doi.org/10.3322/caac.21654
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Hendrick, R. E., Helvie, M. A., & Monticciolo, D. L. (2021). Breast cancer mortality rates have stopped declining in U.S. women younger than 40 years. Radiology, 299(1), 143–149. https://doi.org/10.1148/radiol.2021203476
Waks, A. G., & Winer, E. P. (2019). Breast cancer treatment: A review. Jama, 321(3), 288–300. https://doi.org/10.1001/jama.2018.19323
Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B., Shyr, Y., et al. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of Clinical Investigation, 121(7), 2750–2767. https://doi.org/10.1172/jci45014
Lehmann, B. D., & Pietenpol, J. A. (2014). Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. The Journal of Pathology, 232(2), 142–150. https://doi.org/10.1002/path.4280
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E., & Gianni, L. (2016). Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nature Reviews. Clinical Oncology, 13(11), 674–690. https://doi.org/10.1038/nrclinonc.2016.66
Killelea, B. K., Yang, V. Q., Mougalian, S., Horowitz, N. R., Pusztai, L., Chagpar, A. B., et al. (2015). Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: Results from the National Cancer Database. Journal of the American College of Surgeons, 220(6), 1063–1069. https://doi.org/10.1016/j.jamcollsurg.2015.02.011
Paradiso, A., & Singer, C. F. (2017). Therapeutic strategies in triple-negative breast cancer. Breast Care (Basel), 12(1), 6–7. https://doi.org/10.1159/000460238
Barnard, M. E., Boeke, C. E., & Tamimi, R. M. (2015). Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochimica et Biophysica Acta, 1856(1), 73–85. https://doi.org/10.1016/j.bbcan.2015.06.002
Arthur, R., Wassertheil-Smoller, S., Manson, J. E., Luo, J., Snetselaar, L., Hastert, T., et al. (2018). The combined association of modifiable risk factors with breast cancer risk in the Women’s Health Initiative. Cancer Prevention Research (Philadelphia), 11(6), 317–326. https://doi.org/10.1158/1940-6207.Capr-17-0347
Boyle, P. (2012). Triple-negative breast cancer: Epidemiological considerations and recommendations. Annals of Oncology, 23(Suppl 6), vi7–vi12. https://doi.org/10.1093/annonc/mds187
Plasilova, M. L., Hayse, B., Killelea, B. K., Horowitz, N. R., Chagpar, A. B., & Lannin, D. R. (2016). Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine (Baltimore), 95(35), e4614. https://doi.org/10.1097/md.0000000000004614
Lambertini, M., Santoro, L., Del Mastro, L., Nguyen, B., Livraghi, L., Ugolini, D., et al. (2016). Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treatment Reviews, 49, 65–76. https://doi.org/10.1016/j.ctrv.2016.07.006
Lee, K., Kruper, L., Dieli-Conwright, C. M., & Mortimer, J. E. (2019). The impact of obesity on breast cancer diagnosis and treatment. Current Oncology Reports, 21(5), 41. https://doi.org/10.1007/s11912-019-0787-1
Ramos-Lobo, A. M., & Donato, J., Jr. (2017). The role of leptin in health and disease. Temperature (Austin), 4(3), 258–291. https://doi.org/10.1080/23328940.2017.1327003
Francisco, V., Pino, J., Campos-Cabaleiro, V., Ruiz-Fernández, C., Mera, A., Gonzalez-Gay, M. A., et al. (2018). Obesity, fat mass and immune system: Role for leptin. Frontiers in Physiology, 9, 640. https://doi.org/10.3389/fphys.2018.00640
Lin, T. C., Huang, K. W., Liu, C. W., Chang, Y. C., Lin, W. M., Yang, T. Y., et al. (2018). Leptin signaling axis specifically associates with clinical prognosis and is multifunctional in regulating cancer progression. Oncotarget, 9(24), 17210–17219. https://doi.org/10.18632/oncotarget.24966
Cleary, M. P., Grossmann, M. E., & Ray, A. (2010). Effect of obesity on breast cancer development. Veterinary Pathology, 47(2), 202–213. https://doi.org/10.1177/0300985809357753
Grossmann, M. E., Ray, A., Nkhata, K. J., Malakhov, D. A., Rogozina, O. P., Dogan, S., et al. (2010). Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer and Metastasis Reviews, 29(4), 641–653. https://doi.org/10.1007/s10555-010-9252-1
Argolo, D. F., Hudis, C. A., & Iyengar, N. M. (2018). The impact of obesity on breast cancer. Current Oncology Reports, 20(6), 47. https://doi.org/10.1007/s11912-018-0688-8
Brown, K. A. (2021). Metabolic pathways in obesity-related breast cancer. Nature Reviews Endocrinology, 17(6), 350–363. https://doi.org/10.1038/s41574-021-00487-0
Kato, S., Abarzua-Catalan, L., Trigo, C., Delpiano, A., Sanhueza, C., García, K., et al. (2015). Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: An explanation for poor outcomes in obese women. Oncotarget, 6(25), 21100–21119. https://doi.org/10.18632/oncotarget.4228
Strong, A. L., Ohlstein, J. F., Biagas, B. A., Rhodes, L. V., Pei, D. T., Tucker, H. A., et al. (2015). Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Research, 17(1), 112. https://doi.org/10.1186/s13058-015-0622-z
García-Robles, M. J., Segura-Ortega, J. E., & Fafutis-Morris, M. (2013). The biology of leptin and its implications in breast cancer: A general view. Journal of Interferon and Cytokine Research, 33(12), 717–727. https://doi.org/10.1089/jir.2012.0168
Pan, H., Deng, L. L., Cui, J. Q., Shi, L., Yang, Y. C., Luo, J. H., et al. (2018). Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine (Baltimore), 97(27), e11345. https://doi.org/10.1097/md.0000000000011345
Ray, A., & Cleary, M. P. (2010). Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opinion on Therapeutic Targets, 14(4), 443–451. https://doi.org/10.1517/14728221003716466
Sánchez-Jiménez, F., Pérez-Pérez, A., de la Cruz-Merino, L., & Sánchez-Margalet, V. (2019). Obesity and breast cancer: Role of leptin. Frontiers in Oncology, 9, 596. https://doi.org/10.3389/fonc.2019.00596
Dieudonne, M. N., Machinal-Quelin, F., Serazin-Leroy, V., Leneveu, M. C., Pecquery, R., & Giudicelli, Y. (2002). Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochemical and Biophysical Research Communications, 293(1), 622–628. https://doi.org/10.1016/s0006-291x(02)00205-x
Laud, K., Gourdou, I., Pessemesse, L., Peyrat, J. P., & Djiane, J. (2002). Identification of leptin receptors in human breast cancer: Functional activity in the T47-D breast cancer cell line. Molecular and Cellular Endocrinology, 188(1–2), 219–226. https://doi.org/10.1016/s0303-7207(01)00678-5
Somasundar, P., Yu, A. K., Vona-Davis, L., & McFadden, D. W. (2003). Differential effects of leptin on cancer in vitro. Journal of Surgical Research, 113(1), 50–55. https://doi.org/10.1016/s0022-4804(03)00166-5
Yin, N., Wang, D., Zhang, H., Yi, X., Sun, X., Shi, B., et al. (2004). Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Research, 64(16), 5870–5875. https://doi.org/10.1158/0008-5472.Can-04-0655
Jardé, T., Caldefie-Chézet, F., Goncalves-Mendes, N., Mishellany, F., Buechler, C., Penault-Llorca, F., et al. (2009). Involvement of adiponectin and leptin in breast cancer: Clinical and in vitro studies. Endocrine-Related Cancer, 16(4), 1197–1210. https://doi.org/10.1677/erc-09-0043
Garofalo, C., Koda, M., Cascio, S., Sulkowska, M., Kanczuga-Koda, L., Golaszewska, J., et al. (2006). Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: Possible role of obesity-related stimuli. Clinical Cancer Research, 12(5), 1447–1453. https://doi.org/10.1158/1078-0432.Ccr-05-1913
Gelsomino, L., Giordano, C., Camera, G., Sisci, D., Marsico, S., Campana, A., et al. (2020). Leptin signaling contributes to aromatase inhibitor resistant breast cancer cell growth and activation of macrophages. Biomolecules, 10(4), 543. https://doi.org/10.3390/biom10040543
Catalano, S., Marsico, S., Giordano, C., Mauro, L., Rizza, P., Panno, M. L., et al. (2003). Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. Journal of Biological Chemistry, 278(31), 28668–28676. https://doi.org/10.1074/jbc.M301695200
Frankenberry, K. A., Skinner, H., Somasundar, P., McFadden, D. W., & Vona-Davis, L. C. (2006). Leptin receptor expression and cell signaling in breast cancer. International Journal of Oncology, 28(4), 985–993.
Gao, J., Tian, J., Lv, Y., Shi, F., Kong, F., Shi, H., et al. (2009). Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Science, 100(3), 389–395. https://doi.org/10.1111/j.1349-7006.2008.01053.x
Jotzu, C., Alt, E., Welte, G., Li, J., Hennessy, B. T., Devarajan, E., et al. (2011). Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cellular Oncology (Dordrecht), 34(1), 55–67. https://doi.org/10.1007/s13402-011-0012-1
Kucerova, L., Altanerova, V., Matuskova, M., Tyciakova, S., & Altaner, C. (2007). Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Research, 67(13), 6304–6313. https://doi.org/10.1158/0008-5472.Can-06-4024
Bajou, K., Noël, A., Gerard, R. D., Masson, V., Brunner, N., Holst-Hansen, C., et al. (1998). Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nature Medicine, 4(8), 923–928. https://doi.org/10.1038/nm0898-923
Hildenbrand, R., & Schaaf, A. (2009). The urokinase-system in tumor tissue stroma of the breast and breast cancer cell invasion. International Journal of Oncology, 34(1), 15–23.
Mendes, O., Kim, H. T., & Stoica, G. (2005). Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clinical & Experimental Metastasis, 22(3), 237–246. https://doi.org/10.1007/s10585-005-8115-6
Mendes, O., Kim, H. T., Lungu, G., & Stoica, G. (2007). MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clinical & Experimental Metastasis, 24(5), 341–351. https://doi.org/10.1007/s10585-007-9071-0
Pellikainen, J. M., Ropponen, K. M., Kataja, V. V., Kellokoski, J. K., Eskelinen, M. J., & Kosma, V. M. (2004). Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clinical Cancer Research, 10(22), 7621–7628. https://doi.org/10.1158/1078-0432.Ccr-04-1061
Ren, F., Tang, R., Zhang, X., Madushi, W. M., Luo, D., Dang, Y., et al. (2015). Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: A systematic review and meta-analysis. PLoS ONE, 10(8), e0135544. https://doi.org/10.1371/journal.pone.0135544
Kars, M. D., Işeri, O. D., & Gündüz, U. (2011). A microarray based expression profiling of paclitaxel and vincristine resistant MCF-7 cells. European Journal of Pharmacology, 657(1–3), 4–9. https://doi.org/10.1016/j.ejphar.2011.02.001
Zhao, Y., Zhang, X., Zhao, H., Wang, J., & Zhang, Q. (2018). CXCL5 secreted from adipose tissue-derived stem cells promotes cancer cell proliferation. Oncology Letters, 15(2), 1403–1410. https://doi.org/10.3892/ol.2017.7522
Sakurai, M., Miki, Y., Takagi, K., Suzuki, T., Ishida, T., Ohuchi, N., et al. (2017). Interaction with adipocyte stromal cells induces breast cancer malignancy via S100A7 upregulation in breast cancer microenvironment. Breast Cancer Research, 19(1), 70. https://doi.org/10.1186/s13058-017-0863-0
Sabol, R. A., Beighley, A., Giacomelli, P., Wise, R. M., Harrison, M. A. A., O’Donnnell, B. A., et al. (2019). Obesity-altered adipose stem cells promote ER+ breast cancer metastasis through estrogen independent pathways. International Journal of Molecular Science, 20(6), 1419. https://doi.org/10.3390/ijms20061419
Alluri, P., & Newman, L. A. (2014). Basal-like and triple-negative breast cancers: Searching for positives among many negatives. Surgical Oncology Clinics of North America, 23(3), 567–577. https://doi.org/10.1016/j.soc.2014.03.003
Wu, Q., Siddharth, S., & Sharma, D. (2021). Triple negative breast cancer: A mountain yet to be scaled despite the triumphs. Cancers (Basel), 13(15), 3697. https://doi.org/10.3390/cancers13153697
Dent, R., Trudeau, M., Pritchard, K. I., Hanna, W. M., Kahn, H. K., Sawka, C. A., et al. (2007). Triple-negative breast cancer: Clinical features and patterns of recurrence. Clinical Cancer Research, 13(15 Pt 1), 4429–4434. https://doi.org/10.1158/1078-0432.Ccr-06-3045
Sabol, R. A., Bowles, A. C., Côté, A., Wise, R., O’Donnell, B., Matossian, M. D., et al. (2019). Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Research, 21(1), 67. https://doi.org/10.1186/s13058-019-1153-9
Wei, H. J., Zeng, R., Lu, J. H., Lai, W. F., Chen, W. H., Liu, H. Y., et al. (2015). Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget, 6(10), 7713–7726. https://doi.org/10.18632/oncotarget.3481
Eterno, V., Zambelli, A., Pavesi, L., Villani, L., Zanini, V., Petrolo, G., et al. (2014). Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget, 5(3), 613–633. https://doi.org/10.18632/oncotarget.1359
Divella, R., De Luca, R., Abbate, I., Naglieri, E., & Daniele, A. (2016). Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. Journal of Cancer, 7(15), 2346–2359. https://doi.org/10.7150/jca.16884
Di Franco, S., Bianca, P., Sardina, D. S., Turdo, A., Gaggianesi, M., Veschi, V., et al. (2021). Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nature Communications, 12(1), 5006. https://doi.org/10.1038/s41467-021-25333-9
Chen, D., Liu, S., Ma, H., Liang, X., Ma, H., Yan, X., et al. (2015). Paracrine factors from adipose-mesenchymal stem cells enhance metastatic capacity through Wnt signaling pathway in a colon cancer cell co-culture model. Cancer Cell International, 15, 42. https://doi.org/10.1186/s12935-015-0198-9
Li, Y., Xu, X., Wang, L., Liu, G., Li, Y., Wu, X., et al. (2015). Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell & Bioscience, 5, 21. https://doi.org/10.1186/s13578-015-0012-3
Toren, P., & Venkateswaran, V. (2014). Periprostatic adipose tissue and prostate cancer progression: New insights into the tumor microenvironment. Clinical Genitourinary Cancer, 12(1), 21–26. https://doi.org/10.1016/j.clgc.2013.07.013
Tang, K. D., Liu, J., Jovanovic, L., An, J., Hill, M. M., Vela, I., et al. (2016). Adipocytes promote prostate cancer stem cell self-renewal through amplification of the cholecystokinin autocrine loop. Oncotarget, 7(4), 4939–4948. https://doi.org/10.18632/oncotarget.6643
Cozzo, A. J., Fuller, A. M., & Makowski, L. (2017). Contribution of adipose tissue to development of cancer. Comprehensive Physiology, 8(1), 237–282. https://doi.org/10.1002/cphy.c170008
Allott, E. H., Masko, E. M., & Freedland, S. J. (2013). Obesity and prostate cancer: Weighing the evidence. European Urology, 63(5), 800–809. https://doi.org/10.1016/j.eururo.2012.11.013
Saha, A., Ahn, S., Blando, J., Su, F., Kolonin, M. G., & DiGiovanni, J. (2017). Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives Myc-induced prostate cancer in obese mice. Cancer Research, 77(18), 5158–5168. https://doi.org/10.1158/0008-5472.Can-17-0284
Su, F., Daquinag, A. C., Ahn, S., Saha, A., Dai, Y., Zhao, Z., et al. (2021). Progression of prostate carcinoma is promoted by adipose stromal cell-secreted CXCL12 signaling in prostate epithelium. Npj Precis Oncol, 5(1), 26. https://doi.org/10.1038/s41698-021-00160-9
Zhang, T., Tseng, C., Zhang, Y., Sirin, O., Corn, P. G., Li-Ning-Tapia, E. M., et al. (2016). CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nature Communications, 7, 11674. https://doi.org/10.1038/ncomms11674
Abd Elmageed, Z. Y., Yang, Y., Thomas, R., Ranjan, M., Mondal, D., Moroz, K., et al. (2014). Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells, 32(4), 983–997. https://doi.org/10.1002/stem.1619
Fort, R. S., Mathó, C., Oliveira-Rizzo, C., Garat, B., Sotelo-Silveira, J. R., & Duhagon, M. A. (2018). An integrated view of the role of miR-130b/301b miRNA cluster in prostate cancer. Experimental Hematology & Oncology, 7, 10. https://doi.org/10.1186/s40164-018-0102-0
Naora, H., & Montell, D. J. (2005). Ovarian cancer metastasis: Integrating insights from disparate model organisms. Nature Reviews Cancer, 5(5), 355–366. https://doi.org/10.1038/nrc1611
Zhang, Y., Nowicka, A., Solley, T. N., Wei, C., Parikh, A., Court, L., et al. (2015). Stromal cells derived from visceral and obese adipose tissue promote growth of ovarian cancers. PLoS ONE, 10(8), e0136361. https://doi.org/10.1371/journal.pone.0136361
Nowicka, A., Marini, F. C., Solley, T. N., Elizondo, P. B., Zhang, Y., Sharp, H. J., et al. (2013). Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS ONE, 8(12), e81859. https://doi.org/10.1371/journal.pone.0081859
Kim, B., Kim, H. S., Kim, S., Haegeman, G., Tsang, B. K., Dhanasekaran, D. N., et al. (2017). Adipose stromal cells from visceral and subcutaneous fat facilitate migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Research and Treatment, 49(2), 338–349. https://doi.org/10.4143/crt.2016.175
Chu, Y., You, M., Zhang, J., Gao, G., Han, R., Luo, W., et al. (2019). Adipose-derived mesenchymal stem cells enhance ovarian cancer growth and metastasis by increasing thymosin beta 4X-linked expression. Stem Cells Int, 2019, 9037197. https://doi.org/10.1155/2019/9037197
Tang, H., Chu, Y., Huang, Z., Cai, J., & Wang, Z. (2020). The metastatic phenotype shift toward myofibroblast of adipose-derived mesenchymal stem cells promotes ovarian cancer progression. Carcinogenesis, 41(2), 182–193. https://doi.org/10.1093/carcin/bgz083
Thériault, B. L., Shepherd, T. G., Mujoomdar, M. L., & Nachtigal, M. W. (2007). BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis, 28(6), 1153–1162. https://doi.org/10.1093/carcin/bgm015
Fukuda, T., Fukuda, R., Tanabe, R., Koinuma, D., Koyama, H., Hashizume, Y., et al. (2020). BMP signaling is a therapeutic target in ovarian cancer. Cell Death Discovery, 6(1), 139. https://doi.org/10.1038/s41420-020-00377-w
Bullwinkle, E. M., Parker, M. D., Bonan, N. F., Falkenberg, L. G., Davison, S. P., & DeCicco-Skinner, K. L. (2016). Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer Letters, 380(1), 114–121. https://doi.org/10.1016/j.canlet.2016.06.010
Yu, W., Cao, D. D., Li, Q. B., Mei, H. L., Hu, Y., & Guo, T. (2016). Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget, 7(52), 86075–86086. https://doi.org/10.18632/oncotarget.13342
Wang, Y., Chu, Y., Yue, B., Ma, X., Zhang, G., Xiang, H., et al. (2017). Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget, 8(14), 23803–23816. https://doi.org/10.18632/oncotarget.15866
Wang, Y., Chu, Y., Li, K., Zhang, G., Guo, Z., Wu, X., et al. (2020). Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Frontiers in Cell Developmental Biology, 8, 353. https://doi.org/10.3389/fcell.2020.00353
Tian, T., Li, X., Hua, Z., Ma, J., Wu, X., Liu, Z., et al. (2017). S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial-mesenchymal transition. Oncotarget, 8(15), 24964–24977. https://doi.org/10.18632/oncotarget.15329
Zhai, Y., Wu, W., Xi, X., & Yu, R. (2020). Adipose-derived stem cells promote proliferation and invasion in cervical cancer by targeting the HGF/c-MET pathway. Cancer Management and Research, 12, 11823–11832. https://doi.org/10.2147/CMAR.S277130
Maj, M., Kokocha, A., Bajek, A., & Drewa, T. (2018). The interplay between adipose-derived stem cells and bladder cancer cells. Science and Reports, 8(1), 15118. https://doi.org/10.1038/s41598-018-33397-9
Maj, M., Kokocha, A., Bajek, A., & Drewa, T. (2019). The effects of adipose-derived stem cells on CD133-expressing bladder cancer cells. Journal of Cellular Biochemistry. https://doi.org/10.1002/jcb.28436
Nomoto-Kojima, N., Aoki, S., Uchihashi, K., Matsunobu, A., Koike, E., Ootani, A., et al. (2011). Interaction between adipose tissue stromal cells and gastric cancer cells in vitro. Cell and Tissue Research, 344(2), 287–298. https://doi.org/10.1007/s00441-011-1144-3
Zhao, B. C., Zhao, B., Han, J. G., Ma, H. C., & Wang, Z. J. (2010). Adipose-derived stem cells promote gastric cancer cell growth, migration and invasion through SDF-1/CXCR4 axis. Hepato-Gastroenterology, 57(104), 1382–1389.
Huang, R., Li, W., Zhao, Y., Yang, F., & Xu, M. (2020). Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: A meta-analysis. Medicine (Baltimore), 99(11), e19434. https://doi.org/10.1097/MD.0000000000019434
Hui, W., Litherland, G. J., Elias, M. S., Kitson, G. I., Cawston, T. E., Rowan, A. D., et al. (2012). Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Annals of the Rheumatic Diseases, 71(3), 455–462. https://doi.org/10.1136/annrheumdis-2011-200372
Danan, D., Lehman, C. E., Mendez, R. E., Langford, B., Koors, P. D., Dougherty, M. I., et al. (2018). Effect of adipose-derived stem cells on head and neck squamous cell carcinoma. Otolaryngology - Head and Neck Surgery, 158(5), 882–888. https://doi.org/10.1177/0194599817750361
Scioli, M. G., Storti, G., D’Amico, F., Gentile, P., Kim, B. S., Cervelli, V., et al. (2019). Adipose-derived stem cells in cancer progression: New perspectives and opportunities. International Journal of Molecular Science, 20(13), 3296. https://doi.org/10.3390/ijms20133296
Perez-Cano, R., Vranckx, J. J., Lasso, J. M., Calabrese, C., Merck, B., Milstein, A. M., et al. (2012). Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. European Journal of Surgical Oncology, 38(5), 382–389. https://doi.org/10.1016/j.ejso.2012.02.178
Bircoll, M. (1987). Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plastic and Reconstructive Surgery, 79(2), 267–271. https://doi.org/10.1097/00006534-198702000-00022
Losken, A., Pinell, X. A., Sikoro, K., Yezhelyev, M. V., Anderson, E., & Carlson, G. W. (2011). Autologous fat grafting in secondary breast reconstruction. Annals of Plastic Surgery, 66(5), 518–522. https://doi.org/10.1097/SAP.0b013e3181fe9334
Agha, R. A., Goodacre, T., & Orgill, D. P. (2013). Use of autologous fat grafting for reconstruction postmastectomy and breast conserving surgery: A systematic review protocol. British Medical Journal Open, 3(10), e003709. https://doi.org/10.1136/bmjopen-2013-003709
Gutowski, K. A. (2009). Current applications and safety of autologous fat grafts: A report of the ASPS fat graft task force. Plastic and Reconstructive Surgery, 124(1), 272–280. https://doi.org/10.1097/PRS.0b013e3181a09506
Gir, P., Oni, G., Brown, S. A., Mojallal, A., & Rohrich, R. J. (2012). Human adipose stem cells: Current clinical applications. Plastic and Reconstructive Surgery, 129(6), 1277–1290. https://doi.org/10.1097/PRS.0b013e31824ecae6
Silva-Vergara, C., Fontdevila, J., Weshahy, O., Yuste, M., Descarrega, J., & Grande, L. (2017). Breast Cancer recurrence is not increased with lipofilling reconstruction: A case-controlled study. Annals of Plastic Surgery, 79(3), 243–248. https://doi.org/10.1097/sap.0000000000001106
Toyserkani, N. M., Jørgensen, M. G., Tabatabaeifar, S., Jensen, C. H., Sheikh, S. P., & Sørensen, J. A. (2017). Concise review: A safety assessment of adipose-derived cell therapy in clinical trials: A systematic review of reported adverse events. Stem Cells Translational Medicine, 6(9), 1786–1794. https://doi.org/10.1002/sctm.17-0031
Waked, K., Colle, J., Doornaert, M., Cocquyt, V., & Blondeel, P. (2017). Systematic review: The oncological safety of adipose fat transfer after breast cancer surgery. Breast, 31, 128–136. https://doi.org/10.1016/j.breast.2016.11.001
Sorrentino, L., Regolo, L., Scoccia, E., Petrolo, G., Bossi, D., Albasini, S., et al. (2019). Autologous fat transfer after breast cancer surgery: An exact-matching study on the long-term oncological safety. European Journal of Surgical Oncology, 45(10), 1827–1834. https://doi.org/10.1016/j.ejso.2019.05.013
Fang, J., Chen, F., Liu, D., Gu, F., & Wang, Y. (2021). Adipose tissue-derived stem cells in breast reconstruction: A brief review on biology and translation. Stem Cell Research & Therapy, 12(1), 8. https://doi.org/10.1186/s13287-020-01955-6
Kronowitz, S. J., Mandujano, C. C., Liu, J., Kuerer, H. M., Smith, B., Garvey, P., et al. (2016). Lipofilling of the breast does not increase the risk of recurrence of breast cancer: A matched controlled study. Plastic and Reconstructive Surgery, 137(2), 385–393. https://doi.org/10.1097/01.prs.0000475741.32563.50
Chung, J. H., Kim, K. J., Jung, S. P., Park, S. H., & Yoon, E. S. (2021). Analysis of oncological safety of autologous fat grafting after immediate breast reconstruction. Gland Surgery, 10(2), 584–594. https://doi.org/10.21037/gs-20-645
De Berti, M., Goupille, C., Doucet, M., Arbion, F., Vilde, A., Body, G., et al. (2022). Oncological safety of autologous fat grafting in breast reconstruction after mastectomy for cancer: A case-control study. Journal of Gynecology Obstetrics and Human Reproduction, 51(1), 102257.
Acknowledgements
The authors would like to thank our current and former students and staff who worked very hard to perform the ASC characterization and breast cancer tumorigenesis studies that resulted in the central theme of this review article. Additionally, we thank our faculty colleagues for their input and scientific discussions on these topics. BioRender was used to generate the figure presented in this article.
Funding
Endowment Funds were paid for the studies and research projects of the authors and were provided through Tulane University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Bunnell is a co-founder and a Scientific Advisory Board member for BioAesthetics. All of the other authors do not have any conflicts to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bunnell, B.A., Martin, E.C., Matossian, M.D. et al. The effect of obesity on adipose-derived stromal cells and adipose tissue and their impact on cancer. Cancer Metastasis Rev 41, 549–573 (2022). https://doi.org/10.1007/s10555-022-10063-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10063-1