Abstract
Obesity is associated with poor survival after breast cancer diagnosis in individual studies and meta-analyses. Evidence regarding associations of obesity with breast cancer-specific survival (BCSS) and overall survival (OS) in relation to hormone receptor status, or BCSS in relation to menopausal status has not been evaluated in a previous meta-analysis. In this study, we conducted a meta-analysis of the association of obesity with OS and BCSS in relation to hormone receptor status and menopausal status. MEDLINE, EMBASE, and COCHRANE databases from the first record to December 2011 and presentations made at major international meetings in the last 5 years were searched. We included observational or interventional studies reporting hazard ratios (HRs) of obesity with OS and/or BCSS in relation to hormone receptor and/or menopausal status. Twenty-one studies qualified, meeting the above criteria. The pooled HR for OS in heavier versus lighter women was 1.31 (95 % CI 1.17–1.46) for estrogen receptor/progesterone receptor (ER/PgR) positive cancers; 1.18 (95 % CI 1.06–1.31) for ER/PgR negative cancers; and the difference between the two groups was not significant (p = 0.31). The pooled HR for OS in heavier versus lighter women was 1.23 (95 % CI 1.07–1.42) for premenopausal women and 1.15 (95 % CI 1.06–1.26) for post-menopausal women, and the difference between the two groups was not significant (p = 0.57). Comparable pooled HRs for BCSS were 1.36 (95 % CI 1.20–1.54) for ER/PgR positive cancers and 1.46 (95 % CI 0.98–2.19) for ER/PgR negative cancers; and 1.18 (95 % CI 0.82–1.70) for pre-menopausal women and 1.38 (95 % CI 1.11–1.71) for post-menopausal women, also without significant group differences. Results were similar after adjustment for BMI measurement technique, years of follow-up, or study design. These findings led us to conclude that there is no evidence showing that the association of obesity with breast cancer outcome differs by hormone receptor or menopausal status. This has implications for studies of weight loss interventions in the adjuvant BC setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Obesity poses a major public health burden and, if the current trend continues, more than 50 % of the world’s population will be obese by the year 2030 [1]. Obesity is associated with increased risk of post-menopausal breast cancer, and some reports suggest central obesity may be associated with increased risk of premenopausal breast cancer [2–4]. Numerous studies, and three recent meta-analyses, have reported an association of obesity with poor breast cancer outcomes [5–7]. The most recent meta-analysis included patients diagnosed with breast cancer as recently as 2005 and showed a modest reduction in overall survival (OS) in obese patients, an association that was independent of menopausal status. Associations of OS with obesity in relation to hormone receptor status and that of breast cancer-specific survival (BCSS) in relation to hormone receptor and menopausal statuses were not examined; BCSS is an important outcome as it excludes obesity-associated deaths that occur as a result of non-breast cancer-related causes.
Several mechanisms for an effect of obesity on breast cancer outcomes have been proposed. Potential indirect mechanisms include presentation at a more advanced stage, chemotherapy underdosing, or an enhanced toxicity leading to reduced compliance. Direct mechanisms include hyperinsulinemia (occurring in the presence of insulin resistance), leading to activation of insulin receptor and the PI3K signaling pathway; increased inflammation; altered adipocytokine profile (increased leptin and decreased adiponectin) which can exert stimulatory effects on breast cancer cells, as well as increased levels of sex hormones such as estrogens leading to the increased signaling through estrogen receptors (ERs) [8, 9]. Obesity has been associated with increased expression of the aromatase enzyme (relevant in postmenopausal women) and with an inflammatory state in mouse models and humans [10, 11]. Furthermore, obesity has been reported to significantly influence the efficacy of treatment with aromatase inhibitors likely through influencing aromatase availability [12]. Cross-talk between growth factor (e.g., ER) and insulin signaling pathways [13] may also lead to treatment resistance and poor outcomes.
We have undertaken a series of meta-analyses to explore the associations of obesity with both OS and BCSS in relation to hormone receptor and menopausal statuses.
Methods
Search criteria
A comprehensive search of MEDLINE, EMBASE, and COCHRANE databases from the earliest record in the databases to December, 2011 was performed. Key words included POPULATION: exp Breast Neoplasms/or (exp Carcinoma/and exp breast/). EXPOSURE: body mass index (BMI)/or waist circumference/or waist-hip ratio (WHR)/or exp obesity/or body weight/or overweight. STUDY TYPES: cohort studies/or longitudinal studies/or follow-up studies/or prospective studies/or case–control studies/or retrospective studies/or cross-sectional studies. OUTCOMES: prognosis/or disease-free survival/or medical futility/or treatment outcome/or treatment failure/or disease progression/or morbidity/or incidence/or prevalence/or mortality/or cause of death/or fatal outcome/or survival rate/or survival analysis/or disease-free survival/or proportional hazards model/or exp risk. Hand searches of the reference lists of all pertinent reviews were undertaken. Presentations made at ASCO Annual Meetings, ASCO Breast Cancer Symposium, and San Antonio Breast Cancer Symposium in the last 5 years were also searched.
Identification of studies
Reports of observational or intervention studies involving newly diagnosed breast cancer populations that compared OS or BCSS in overweight and/or obese versus normal weight patients were included if they contained the following information: (i) OS and/or BCSS reported according to ER or progesterone receptor(PgR) status; and/or (ii) OS and/or BCSS reported according to menopausal status; (iii) Measurement of body size around the time of diagnosis, reported as BMI or WHR, to allow classification as overweight and/or obese versus normal weight; and (iv) explicit reporting of the hazard ratio (HR) associating body size with OS and/or BCSS. Provision of an estimate of relative risk (RR) at a single time point did not satisfy this criterion. Case-series, case reports, and other studies without a comparator, editorials, reviews, animal studies, and in vitro studies were excluded.
Data extraction
Studies were reviewed for relevance based on study design, types of participants, exposure and outcome measures. Reasons for exclusion of studies were recorded. Data were extracted using standardized data collection forms by two authors [SN and AO] independently, and any discrepancies that arose were resolved by consensus. The quality of the included studies was rated according to selection of study population, comparability of study groups, and outcome assessment based on Newcastle–Ottawa quality assessment scale [14] with modifications. When necessary, additional information was sought by correspondence with the authors of the studies.
Hormone receptor positive was defined as having positive expression of ER and/or PgR. Hormone receptor negative was defined as negative expression of both ER and PgR. Of note, most studies were conducted in the era when “low positive” (i.e., <10 % hormone receptor expression) was considered negative. OS was defined as time from breast cancer diagnosis to death from any cause, and BCSS was defined as time from diagnosis of breast cancer to death due to breast cancer or following a breast cancer-related event. HRs for the association of body size with OS and BCSS were extracted for inclusion in meta-analyses. Clinical heterogeneity of included studies was assessed before performing the meta-analysis.
Statistical analysis
Data were extracted and combined for meta-analysis using the RevMan 5.1 analysis software (The Cochrane Collaboration, Copenhagen, Denmark). Pooled estimates of HR outcomes were computed using the random-effects model [15] according to the generic inverse variance approach [16]. In this method, studies are weighted by the standard error for their individual HR rather than by sample size alone. Statistical heterogeneity was assessed by I 2 statistics [17]. Because the RevMan software cannot perform meta-regression, random effects meta-regression [18], conducted in R with the “metafor” package [19], was used to compare groups defined by hormone receptor and menopausal statuses. The sensitivity of these results to the following pre-defined factors: BMI ascertainment (self-reported vs investigator measured), follow-up duration (median years of follow-up ≤ vs >7), and study design (observation vs treatment cohort) was tested by adding each of these variables in turn to each meta-regression model as an adjusting variable. Owing to the small number of studies reporting BCSS, the sensitivity analyses were performed only for the OS outcome.
Results
Characteristics of the studies
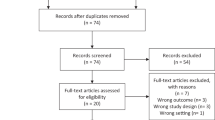
The search strategy identified 3403 citations; 95 % of these were excluded after reviewing the title. The remaining 160 citations were retrieved and reviewed as possibly relevant. After full text review, 21 contained the required information and were included in the meta-analysis [20–41] (Fig. 1). The main reason for exclusion of studies was unavailability of HR for the association of obesity with BCSS and/or OS in defined hormone receptor or menopausal status subgroups. We identified five studies that used various methods other than HR to report association of obesity with breast cancer outcome; these were excluded from the meta-analysis [42–46]. A summary of excluded studies [43, 44, 47–75], including the reasons for exclusion, is provided in Appendix Table 2.
Characteristics of the included studies are summarized in Table 1; the quality rating of these studies is provided in Appendix Table 3. Twelve of the 21 included studies were observational cohorts, and nine were interventional studies. Sample size ranged from 177 to 14709. Meeting presentations [40] provided necessary data for three interventional studies [37–39]. Three studies were included after the authors provided additional information not included in the original publication [28, 30, 35]. All studies used BMI to characterize body size; however, the cut-point used to analyze obesity varied; the most common was ≥30 vs ≤25 kg/m2. Body size was measured by investigators in 13 studies; in the remaining 8 studies body size was self-reported or the measurement method was not stated. Thirteen studies reported BMI associations with OS in hormone receptor positive cancers and 12 in hormone receptor negative cancers; 7 in pre-menopausal and 9 in post menopausal women. Seven studies reported the association of BMI with BCSS in ER/PgR-positive patients, 6 in ER/PgR-negative patients, 4 in pre-menopausal patients and 4 in post-menopausal patients. Median follow-up was less than 5 years in three studies (around 4 years), between 5 and 10 years in 10 studies and more than 10 years in 8 studies. Assessment of publication bias using techniques such as funnel plot was not done because of the small number of studies in each category.
Overall survival in relation to obesity
The pooled HR for the association of obesity (vs no obesity) with OS in hormone receptor positive breast cancer (Fig. 2) was 1.31 [95 % CI 1.17–1.46] in the 13 studies reporting this association. The pooled HR for the association of obesity (vs no obesity) with OS in hormone receptor negative breast cancer was 1.18 [95 % CI 1.06–1.31] in the 12 studies reporting this association. The combined HR was 1.25 (95 % CI 1.16–1.35). There was no evidence that the association of obesity with OS differed in hormone receptor positive and hormone receptor negative cancers (p = 0.31) and this did not change after adjusting in turn for BMI measurement technique, years of follow-up, and study design (receptor status p = 0.33, 0.25, 0.31, respectively, after adjustment). Similarly, HR for OS was 1.23 (95 % CI 1.07–1.42) for premenopausal women in seven studies reporting this association and 1.15 (95 % CI 1.06–1.26) for post-menopausal women in nine studies that reported this association (Fig. 3). The combined HR was 1.19 (95 % CI 1.10–1.28). There was no evidence of subgroup difference in association of OS with obesity between pre and post menopausal women (p = 0.57), and this did not change after adjusting in turn for BMI measurement technique, years of follow-up, and study design (receptor status p = 0.64, 0.33, and 0.64, respectively, after adjustment).
Breast cancer-specific survival in relation to obesity
The pooled HR for the association of obesity (vs no obesity) with BCSS in hormone receptor positive breast cancer was 1.36 [95 % CI 1.20–1.54] in the 7 studies examining this association. The pooled HR for the association of obesity (vs no obesity) with BCSS in hormone receptor negative breast cancer was 1.46 [95 % CI 0.98 to 2.19] in the 6 studies reporting this association. There was no evidence that these associations differed in hormone receptor positive and negative cancers (p = 0.95). HRs for the association of obesity (vs no obesity) with BCSS were 1.18 [95 % CI 0.82–1.70] in pre-menopausal women (4 studies) and 1.38 [95 % CI 1.11–1.71] in post-menopausal women (4 studies). There was no evidence that these associations differed in pre-and postmenopausal women (p = 0.35) (Figs. 4, 5).
Discussion
Although obesity has been associated with poor OS in previous studies, our meta-analysis is the first to our knowledge to demonstrate that the association of obesity with poor OS and that BCSS do not appear to differ in hormone receptor positive (vs hormone receptor negative) breast cancer (p for differences of 0.31 and 0.95, respectively).
Our finding that the decrease in OS and BCSS in those patients with obesity appears unrelated to the expression of hormone receptors is not consistent with the observation that a dietary intervention that was designed to reduce fat intake (and was also associated with modest weight loss) may have had a greater effect on relapse-free survival in women with ER/PR negative breast cancer than in those with ER/PR positive breast cancer (HR 0.44, 95 % CI 0.25–0.77 and HR 0.83, 95 % CI 0.58–1.17, respectively, interaction p = 0.15) [76]. This inconsistency may reflect the nature of the dietary fat intervention used in this study (as opposed to a weight loss intervention) or the play of chance (the interaction was not significant); it is also possible the pattern of prognostic associations of obesity at diagnosis may not predict which subgroups will benefit from weight loss interventions. From a biological point of view, our failure to identify differential effects of obesity in hormone receptor positive and negative breast cancers suggest that pathways not related to sex hormones, such as insulin or insulin-like growth factor (IGF) signaling pathways, may contribute to effects of obesity on breast cancer outcomes.
Our observation of a lack of evidence that the association of obesity with poor OS and BCSS differ by hormone receptor (p = 0.31 and 0.95, respectively) and menopausal status (p = 0.57 and 0.35, respectively) extends results of a previous meta-analysis reported by Protani et al. [6] that reported the association of menopausal status relation to OS but did not explore association of hormone receptor status on OS and BCSS or menopausal status on BCSS. In addition, we included more recent studies and exclusively limited our analysis to studies reporting the interaction of hormone receptor and/or menopausal status in association of obesity to outcome of breast cancer. Nevertheless, the overall effect size HRs for OS and BCSS in obese versus non-obese patients obtained in our study are comparable to that obtained by Protani et al.
Our observation that obesity is associated with poor BCSS (in addition to OS) suggests that prognostic associations of obesity are not due to death from causes other than breast cancer in overweight and/or obese patients. Our analyses of potential effects of the method of BMI ascertainment (investigator vs self-report), duration of follow-up, and study type (interventional vs observational) suggest that our findings were robust to the methodology used in the included studies.
The association of obesity with breast cancer outcomes is complex, and its underlying basis is likely multi-factorial. Although indirect causes, such as diagnosis at a more advanced stage or inadequate treatment of obese patients may contribute in some patients, poorer outcomes in obese women have been reported after these factors have been considered. Increased adiposity is associated with higher aromatase activity and higher estrogen levels in postmenopausal women and higher estrogen levels have been associated with worse breast cancer outcomes in this group [77]—it has also been suggested that a higher aromatage inhibitor dose be used in obese post-menopausal women with breast cancer [41]. However, obesity-associated estrogen levels are unlikely to be important mediators of obesity effects in premenopausal women (in whom most of the estrogen is derived from the ovaries), in women with hormone receptor negative breast cancer, or in those receiving tamoxifen. Emerging mechanistic research [78–80] in the clinical and preclinical settings has identified a group of obesity-associated physiologic factors associated with poor breast cancer outcomes and having plausible biologic mechanisms. These include higher circulating levels of insulin (and possibly IGFs), greater systemic and/or local inflammation and altered adipocytokines (higher leptin and lower adiponectin). High levels of insulin or c-peptide have been established to be associated with poor cancer outcomes [81–86]. Non-diabetic women with insulin levels in the highest (vs lowest) quartile have a doubled risk of recurrence and tripled risk of death, effects that persist after consideration of tumor and treatment-related factors [30]. Evidence exists, although less strong, for the other factors. Many of these physiologic disruptions occur together as part of the obesity-associated insulin resistance syndromes [87]. Our recent observation [88] that insulin may be most important early after diagnosis (first 5 years) and leptin may be more important long term underscores the complexity of obesity effects in breast and other cancers and highlights the need for additional research.
Strengths of our research include the broad literature search process, the evaluation of study quality, and the restriction of our analyses to pre-specified associations and subgroups. Obese and non-obese patients were fairly comparable in all studies. Major confounding factors such as age, stage (or nodal status), tumor size, and treatment received were adjusted for in most studies (Table 1). The ascertainment of outcome was through record linkage or direct inquiry in all the studies except in one where authors did not report on how the survival data were collected [34]. Follow-up duration was adequate in the majority of the studies. Losses to follow-up was reported and were acceptable (<20 %) when reported (5 studies). Finally, included studies were carried out in diverse locations around the world and included a variety of population of breast cancer contributing to generalizability of the results.
Limitations of our research include our analysis of published study-level data, rather than analysis of patient-level data. In addition, most included studies were not specifically designed to examine prognostic effects of obesity—as a result of which body size was often obtained through self-report, and it was not clear whether it was measured with the same rigor in all studies. Differences in categorizing obesity across studies likely contributed to heterogeneity in our meta-analysis. Two studies used populations that were highly selective sub-groups of breast cancer patients [26, 27], and this might have compromised the generalizability of our results. The failure of the majority of studies that have examined the association of obesity with breast cancer outcomes to report associations by menopausal or hormone receptor status reduced the number of studies that could be included in our meta-analyses, lowered the power, might have introduced bias, and thereby reduced generalizability of our findings. The lack of statistically significant differences, especially for BCSS, might reflect lower power rather than the absence of a real effect. Our meta-analysis is prone to biases that were present in the parent studies [89]. In addition, differing approaches in the management of breast cancer patients by hormone receptor and/or menopausal status may have confounded the associations we identified. Most included studies were from the era when expression of <10 % of ER or PR was considered negative; this differs from current practice. Finally, our study focused on baseline BMI which is likely to vary over time, and the effect of change in BMI to prognosis of breast cancer is not captured in our analysis. This is particularly important as there are concerns regarding adverse prognostic implications of change in BMI after diagnosis of breast cancer [48, 90]. In addition, evidence suggests that a very low BMI with breast cancer has a worse prognosis as compared with their normal weight counterparts [91, 92]; evaluation of such effect through this study is outside the scope of this analysis.
In summary, our results did not find evidence that the associations of obesity with either BCSS or OS differ by the menopausal status of the patient or the hormone receptor status of the cancer. Any differences that exist are likely to be quantitative rather than qualitative, based on the HRs we have observed. Our findings are consistent with a contribution of non-estrogenic mediators, such as insulin, inflammation, or altered adipokine profiles, to prognostic effects of obesity. Additional research is needed to investigate these mediators, in addition to estrogens. Our findings have relevance for future research in that they suggest intervention trials targeting weight loss or that physiologic mediators of obesity should include both hormone receptor positive and negative cancers and both pre-and postmenopausal women.
References
Kelly T et al (2008) Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 32(9):1431–1437
Renehan AG et al (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578
van den Brandt PA et al (2000) Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 152(6):514–527
Harvie M, Hooper L, Howell AH (2003) Central obesity and breast cancer risk: a systematic review. Obes Rev 4(3):157–173
Goodwin P et al (1995) Development of a weight management program in women with newly diagnosed locoregional breast cancer. In: 11th international congress of psychosomatic obstetrics and gynaecology, Basel, Switzerland
Protani M, Coory M, Martin JH (2010) Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 123(3):627–635
Ryu S et al (2001) Is body mass index the prognostic factor in breast cancer?: a meta-analysis. J Korean Med Sci 16(5):610–614
Hursting SD et al (2008) Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab 22(4):659–669
Renehan AG, Roberts DL, Dive C (2008) Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem 114(1):71–83
Subbaramaiah K et al (2011) Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 4(3):329–346
Morris PG et al (2011) Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 4(7):1021–1029
Pfeiler G et al (2011) Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol 29(19):2653–2659
Bowers L et al. (2010) Obesity promotes breast cancer progression and tamoxifen resistance via cross-talk between growth factor and estrogen signaling pathways. In: San Antonio breast cancer symposium 2010 [PD09-06]
Wells G et al (2011) The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Assessed on January 20, 2011
Deeks J, Higgins J, Altman D (2006) Analysing and presenting results: cochrane handbook for systematic reviews of interventions 4 2 5. In: The cochrane library. John Wiley and Sons, Chichester
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
van Houwelingen HC, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21(4):589–624
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48
de Azambuja E et al (2010) The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat 119(1):145–153
Berclaz G et al (2004) Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol 15(6):875–884
Conroy SM et al (2011) Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat 129(2):565–574
Chang S et al (2000) Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat 64(2):157–163
Chen X et al (2010) Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat 122(3):823–833
Dal Maso L et al (2008) Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer 123(9):2188–2194
Dignam JJ et al (2003) Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst 95(19):1467–1476
Dignam JJ et al (2006) Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat 97(3):245–254
Daling JR et al (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92(4):720–729
Enger SM, Bernstein L (2004) Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer 90(11):2138–2141
Goodwin PJ et al (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20(1):42–51
Keegan THM et al (2010) Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat 123(2):531–542
Kwan ML et al. (2011) Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Research & Treatment
Loi S et al (2005) Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 14(7):1686–1691
Majed B et al (2008) Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat 111(2):329–342
Vitolins MZ, Kimmick GG, Case LD (2008) BMI influences prognosis following surgery and adjuvant chemotherapy for lymph node positive breast cancer. Breast J 14(4):357–365
Whiteman MK et al (2005) Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 14(8):2009–2014
Sparano JA et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671
Davidson NE et al (2005) Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188). J Clin Oncol 23(25):5973–5982
Fetting JH et al (1998) Sixteen-week multidrug regimen versus cyclophosphamide, doxorubicin, and fluorouracil as adjuvant therapy for node-positive, receptor-negative breast cancer: an Intergroup study. J Clin Oncol 16(7):2382–2391
Sparano J et al. (2010) Obesity at diagnosis is associated with inferior outcomes in hormone receptor positive breast cancer. In: San Antonio breast cancer symphosium 2010 [S2-1]
Sestak I et al (2010) Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol 28(21):3411–3415
den Tonkelaar I, Seidell JC, Collette HJ (1995) Body fat distribution in relation to breast cancer in women participating in the DOM-project. Breast Cancer Res Treat 34(1):55–61
Demirkan B, Alacacioglu A, Yilmaz U (2007) Relation of body mass index (BMI) to disease free (DFS) and distant disease free survivals (DDFS) among Turkish women with operable breast carcinoma. Jpn J Clin Oncol 37(4):256–265
Maehle BO, Tretli S (1996) Pre-morbid body-mass-index in breast cancer: reversed effect on survival in hormone receptor negative patients. Breast Cancer Res Treat 41(2):123–130
Mohle-Boetani JC et al (1988) Body size, reproductive factors, and breast cancer survival. Prev Med 17(5):634–642
Lu Y et al (2011) Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol 29(25):3358–3365
Jain MG et al (2005) Body mass index and mortality in women: follow-up of the Canadian National Breast Screening Study cohort. Int J Obes 29(7):792–797
Kroenke CH et al (2005) Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23(7):1370–1378
den Tonkelaar I et al (1995) Obesity and subcutaneous fat patterning in relation to survival of postmenopausal breast cancer patients participating in the DOM-project. Breast Cancer Res Treat 34(2):129–137
Abrahamson PE et al (2006) General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev 15(10):1871–1877
Barnett GC et al (2008) Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol 26(20):3310–3316
Bastarrachea J et al (1994) Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med 120(1):18–25
Borugian MJ et al (2003) Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol 158(10):963–968
Caan BJ et al (2008) Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 19(10):1319–1328
Carmichael AR et al (2004) Does obesity compromise survival in women with breast cancer? Breast 13(2):93–96
Cleveland RJ et al (2007) Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev 16(9):1803–1811
Dawood S et al (2008) Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res 14(6):1718–1725
Eley JW et al (1994) Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA 272(12):947–954
Greenberg ER et al (1985) Body size and survival in premenopausal breast cancer. Br J Cancer 51(5):691–697
Hebert JR, Hurley TG, Ma Y (1998) The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat 51(1):17–28
Holmberg L et al (1994) Oral contraceptives and prognosis in breast cancer: effects of duration, latency, recency, age at first use and relation to parity and body mass index in young women with breast cancer. Eur J Cancer 30A(3):351–354
Katoh A, Watzlaf VJ, D’Amico F (1994) An examination of obesity and breast cancer survival in post-menopausal women. Br J Cancer 70(5):928–933
Labidi SI et al (2008) Inflammatory breast cancer in Tunisia in the era of multimodality therapy. Ann Oncol 19(3):473–480
Litton JK et al (2008) Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol 26(25):4072–4077
Mason BH et al (1990) Season of tumour detection influences factors predicting survival of patients with breast cancer. Breast Cancer Res Treat 15(1):27–37
Moon HG, Han W, Noh DY (2009) Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 27(35):5899–5905
Newman SC, Lees AW, Jenkins HJ (1997) The effect of body mass index and oestrogen receptor level on survival of breast cancer patients. Int J Epidemiol 26(3):484–490
Nichols HB et al (2009) Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev 18(5):1403–1409
Petrelli JM et al (2002) Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control 13(4):325–332
Pierce JP et al (2007) Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 25(17):2345–2351
Reeves GK et al (2000) Hormonal and other factors in relation to survival among breast cancer patients. Int J Cancer 89(3):293–299
Rosenberg L, Czene K, Hall P (2009) Obesity and poor breast cancer prognosis: an illusion because of hormone replacement therapy? Br J Cancer 100(9):1486–1491
Saxe GA et al (1999) Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat 53(3):241–253
Tao MH et al (2006) Association of overweight with breast cancer survival. Am J Epidemiol 163(2):101–107
Vatten LJ, Foss OP, Kvinnsland S (1991) Overall survival of breast cancer patients in relation to preclinically determined total serum cholesterol, body mass index, height and cigarette smoking: a population-based study. Eur J Cancer 27(5):641–646
Chlebowski RT et al (2006) Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 98(24):1767–1776
Rock CL et al (2008) Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomark Prev 17(3):614–620
Mulligan AM et al (2007) Insulin receptor is an independent predictor of a favorable outcome in early stage breast cancer. Breast Cancer Res Treat 106(1):39–47
Law JH et al (2008) Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 68(24):10238–10246
Goodwin PJ et al (2005) Is leptin a mediator of adverse prognostic effects of obesity in breast cancer? J Clin Oncol 23(25):6037–6042
Duggan C et al (2011) Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol 29(1):32–39
Emaus A et al (2010) Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat 121(3):651–660
Erickson K et al (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29(1):54–60
Irwin ML et al (2011) Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol 29(1):47–53
Ma J et al (2008) Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 9(11):1039–1047
Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8(12):915–928
Doyle SL et al (2011) Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc 1–9
Goodwin PJ et al (2011) Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol
Egger M, Schneider M, Davey Smith G (1998) Spurious precision? Meta-analysis of observational studies. BMJ 316(7125):140–144
Thivat E et al (2010) Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer 10:648
Chauvet B et al (1990) Prognostic significance of breast relapse after conservative treatment in node-negative early breast cancer. Int J Radiat Oncol Biol Phys 19(5):1125–1130
Marret H et al (2001) Low body mass index is an independent predictive factor of local recurrence after conservative treatment for breast cancer. Breast Cancer Res Treat 66(1):17–23
Acknowledgment
We would like to thank Drs. Prakesh Shah and Joseph Beyene at University of Toronto for their valuable suggestions during preparation of this manuscript.
Conflict of interest
This is to confirm that none of the authors of the above manuscript have conflict of interest of any kind which may arise from being named as an author on the manuscript.
It is to certify that (i) all financial support or benefits received by me, by any member of my immediate family, or any individual or entity with whom or with which I have a significant relationship from any commercial source related directly or indirectly to the scientific work reported in the article have been disclosed and have been included in the submitted manuscript, (II) neither I, nor any member of my immediate family, nor any individual or entity with whom or with which I have a significant relationship has a financial interest in the subject matter discussed in the manuscript, except as disclosed (I understand an example of such a financial interest would be a stock interest in any business entity which is included in the subject matter of the manuscript or which sells a product relating to the subject matter of the manuscript.), (III) all funding sources supporting the work and all institutional or corporate affiliations are acknowledged in a footnote, and (Iv) I have had full access to all the data in the study (if applicable) and thereby accept full responsibility for the integrity of the data and the accuracy of the data analysis.
Signed by: Saroj Niraula, Alberto Ocana, Marguerite Ennis and Pamela J. Goodwin
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niraula, S., Ocana, A., Ennis, M. et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 134, 769–781 (2012). https://doi.org/10.1007/s10549-012-2073-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2073-x