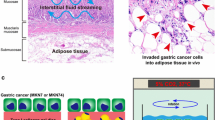
Abstract
Adipose tissue exists in the gastric submucosa and subserosa. Thus, adipose tissue stromal cells (ATSCs), which include mesenchymal stem cells (MSCs), seem critical for the progression of gastric cancer but their interaction with the cancer cells is unknown. We demonstrated an interaction between these cells, using immunohistochemistry, Western blot and the collagen gel invasion assay system, in which the adenocarcinoma cells (well and poorly differentiated types, MKN28 and MKN45, respectively) were cultured on a ATSC-embedded or ATSC-non-embedded gel. ATSCs promoted the expression of the growth marker, proliferation cell nuclear antigen but inhibited that of the apoptosis marker, single-stranded DNA, in the cancer cell types. ATSCs accelerated the invasion of only MKN28 into the gel and promoted the expression of mitogen-activated protein kinase (MAPK, pERK-1/2) but decreased that of the molecularly targeted protein, HER2, in the cancer cells. ATSCs did not affect the expression of the prostaglandin biosynthetic enzyme cyclooxgenase-2 (COX-2) in the cancer cells. The COX-2 inhibitor celecoxib did not affect the morphology or invasion of the cancer cells. The cancer cell types in turn promoted the display of the myofibroblast marker, α-smooth muscle actin, whereas they decreased that of some MSC markers, e.g., CD44 and CD105, in ATSCs. The data suggest that (1) ATSCs influence the progression of gastric cancer by increasing their growth/invasion and decreasing their apoptosis through MAPK activation in a COX-2-independent way; (2) ATSCs adversely affect HER2-targeted therapy; (3) the cancer cells induce the cancer-associated myofibroblast phenotype in ATSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer tissue consists in cancer cells and stromal cells such as fibroblasts (Radisky et al. 2007). The cancer-stromal interaction is essential for cancer biology (De Wever et al. 2008). Indeed, the cancer cell-fibroblast interaction plays important roles in the survival, growth, differentiation, invasion and metastasis of various cancer cell types, including gastric cancer cells (Semba et al. 2009). In the stomach, adipose tissue exists in both the submucosa and subserosa. Gastric cancer cells generated within the mucosa progressively infiltrate into the submucosal layers and possibly interact with adipose tissue stromal cells (ATSCs) at the invasion sites. ATSCs thus seem critical for the behavior and progression of gastric cancer cells but their interaction is unknown.
ATSCs are heterogeneous cell types, including preadipocytes and mesenchymal stem cells (MSCs; Wozniak et al. 2009). During the cancer-stromal interaction, the cancer-associated stromal cells develop within the tumor tissue and actively influence the behavior of the cancer cells (Guo et al. 2008). In addition, the cancer-associated fibroblasts are known to gain a myofibroblast phenotype (Gonda et al. 2010; Pietras and Ostman 2010) but the cancer-associated phenotype that gastric cancer cells induce in the ATSCs is unclear.
To address these issues, we first studied the effects of ATSCs on the apoptosis, growth, differentiation and invasion of gastric adenocarcinoma cells by using a collagen gel invasion assay system. The following growth-, differentiation-, invasion- and motility-related molecules have been analyzed by immunohistochemistry and Western blot: human epidermal growth factor receptor-related 2 (HER2; Zhang et al. 2009), mitogen-activated protein kinase (MAPK, pERK-1/2; Ohsawa et al. 2010), mucin 1 (MUC1), MUC4 (Ho et al. 1995), matrix metalloproteinase-1 (MMP-1), MMP-9 (Westermarck and Kahari 1999), trophinin (Chang et al. 2009) and filamin A (Cunningham 1992). We have also investigated the effects of the cancer cells on the MSC and on myofibroblast differentiation of ATSCs by using immunohistochemistry with some MSC markers, viz., CD44 and CD105 (Gomillion and Burg 2006) and the myofibroblast marker, α-smooth muscle actin (α-SMA; Pietras and Ostman 2010). Finally, we have examined the expression of the prostaglandin biosynthetic enzyme, cyclooxgenase-2 (COX-2), which might be involved in the mechanisms underlining the cell-fibroblast interaction in breast cancer (Hu et al. 2009).
Materials and methods
Cell lines and ATSC preparation
All procedures involving animal and human materials were performed in accordance with the regulations laid down by the ethical guidelines of Saga University. The human gastric adenocarcinoma cell lines, MKN28 (the well differentiated type) and MKN45 (the poorly differentiated type), were obtained from the Japanese Cancer Research Bank (JCRB, Tsukuba, Japan). ATSCs were isolated from subcutaneous adipose tissue of 1-week-old Wister rats and human gastric subserosal adipose tissue of three autopsy cases without any cancers, as described previously (Manabe et al. 2003; Satoh et al. 2005). The isolated ATSCs were primary-cultured in a 90-mm-dish and grew to subconfluency at 3-5 days. The cells isolated with trypsin were used. All of the subcultured ATSCs expressed the common mesenchymal marker, vimentin. The percentages of S-100-protein-positive preadipocytes, CD44/CD105-positive MSC-like cells and CD31/CD54-positive endothelial cells within the ATSCs were about 5%, 70% and 0.3%, respectively. These cell types were cultured and maintained in the following complete medium: Ham F-12 medium supplement with 10% fetal bovine serum and 50 μg/ml gentamicin.
Culture system
The collagen gel invasion assay system (Fig. 1) was set up as described previously (Satoh et al. 2005; Toda et al. 2005; Yamada et al. 1999). Briefly, 10 × 105 ATSCs were embedded in 1.0 ml acid-soluble type I collagen gel (Nitta Gelatin, Osaka, Japan). The ATSC-embedded gel was poured into a 30-mm-diameter culture dish (inner dish), the bottom of which was composed of a nitrocellulose membrane (Millicell-CM, Millipore, Bedford, Mass., USA). Then, 1 × 105 gastric adenocarcinoma cells were seeded onto the gel of the inner dish. This inner dish was placed into a 90-mm-diameter culture dish (outer dish) and the complete medium (see above) was added to both dishes.
Collagen gel invasion assay system. a Gastric adenocarcinoma cells are seeded onto an ATSC-embedded collagen gel layer in an inner dish with a nitrocellulose membrane as its base. The inner dish is placed in a larger outer dish and then the complete medium is added to both the dishes. Cells are fed by a sufficient medium from both the inner and outer dishes, because of the permeability of the nitrocellulose membrane. b, c As controls, the cancer cells or ATSCs, respectively, are cultured alone
Morphology and cancer cell invasion
At 7 and 14 days in culture, the culture assemblies were fixed with 4% paraformaldehyde, routinely processed and vertically embedded in paraffin. Deparaffinized sections were stained with hematoxylin and eosin (H&E). Cellular morphology was analyzed on the H&E-stained sections by light microscopy (Toda et al. 2005; Yamada et al. 1999). To detect preadipocytes, oil red O staining was carried out, as described previously (Sonoda et al. 2008). To observe basement membrane-related reticular fibers formed by the cancer cells, deparaffinized sections were subjected to a reticulin silver impregnation stain. The invasion of cancer cells into the collagen gel was examined, as described previously (Toda et al. 2005). The depth to which the cancer cells had invaded into the gel was measured from the basal line to the deepest cell invasive front in 10 randomly chosen non-contiguous and non-overlapping fields (at low power view, ×10 objective) by light microscopy.
Growth and apoptosis
Cell proliferation and apoptosis were examined by immunohistochemistry with mouse monoclonal antibodies against proliferating cell nuclear antigen (PCNA) and against single-stranded DNA (ssDNA; DakoCytomation, Kyoto, Japan), repectively, as previously described (Matsuyama et al. 2002). A total of 1000 cells were counted and the percentages of PCNA- and ssDNA-positive nuclei were calculated.
Immunohistochemistry
Cancer cells and ATSCs were identified by monoclonal antibodies against pancytokeratin AE1/AE3 and vimentin (DakoCytomation), respectively. ATSCs are heterogeneous cell types and include preadipocytes, MSCs and endothelial cells (Trayhurn 2007). In order to distinguish between these cell types, we used a rabbit polyclonal S-100 protein antibody (DakoCytomation) for preadipocytes, mouse monoclonal CD44 (Pierce Biotechnology, Rockford, Ill., USA) and CD105 antibodies (Novocastra Laboratories, North Tyneside, United Kingdom) for MSCs (Gomillion and Burg 2006) and rabbit polyclonal CD31 (Lab Vision, Fremont, Calif., USA) and mouse monoclonal CD54 (ICAM-1, Pierce Biotechnology) for endothelial cells (Mulligan et al. 1993). Mouse monoclonal α-SMA antibody (DakoCytomation) was used for the detection of the myofibroblast differentiation of ATSCs, mouse monoclonal MUC1 (NovoCastra Laboratories) and MUC4 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies for the mucin expression of cancer cells and monoclonal E-cadherin antibody (DakoCytomation) for the E-cadherin expression of cancer cells. To characterize the growth of the cancer cells, we examined the expression of HER2 and MAPK (pERK-1/2) by using mouse monoclonal antibodies against HER2 (DakoCytomation) or pERK-1/2 (Santa Cruz Biotechnology). To estimate the invasion and motility of the cancer cells, we investigated the expression of MMP-1, MMP-9 (Westermarck and Kahari 1999), trophinin (Chang et al. 2009) and filamin A (Cunningham 1992) by using rabbit polyclonal antibodies against MMP-1, MMP-9 and trophinin and mouse monoclonal antibodies against filamin A (Lab Vision). Given that COX-2 is suggested to be involved in the mechanisms underlining cell-fibroblast interactions in breast cancer (Hu et al. 2009), we studied the COX-2 expression of cancer cells and ATSCs by using the rabbit polyclonal COX-2 antibody (Abcam, Tokyo, Japan). The intensity of the immunohistochemical results was assessed by three pathologists and classified as negative, weak, moderate and strong staining. Immunohistochemistry was carried out on deparaffinized sections by an avidin–biotin complex immunoperoxidase method, as described previously (Toda et al. 1997). As positive controls, we performed immunohistochemistry according to the protocols supplied by the manufacturers by using various normal and cancer tissues of human or rat origin. The controls always gave positive results in a cell-type-specific manner. As negative controls, phosphate-buffered saline, normal rabbit IgG and mouse IgG were appropriately used instead of a primary antibody. The controls always gave negative results.
Western blot
The expression of pERK-1/2 and COX-2 by cancer cells (MKN28 and MKN45) was examined by Western blot under two conditions: with and without cell-cell contact between the cancer cells and ATSCs. To prepare the cells under conditions in which cell-cell contact was allowed, ATSCs and cancer cells were grown separately until confluent in a plastic dish and on a coverslip (Nalge Nunc International, Naperville, Ill., USA), respectively. The coverslip was then overlaid onto the surface of the ATSC-containing dish and cultured further for 6 h. To prepare the cells under conditions of no cell-cell contact, cancer cells were cultured in the double-dish culture system. In this system, ATSCs were cultured as a feeder layer on the bottom of the outer dish and the cancer cells were grown separately on inserts with a 0.4-μm pore size (ThinCert cell culture inserts, Greiner bio-one). When the cells were confluent, these inserts were placed on the underlying ATSCs and cultured for 24 h. The cancer cells under the two conditions described above were subsequently scraped from the coverslips and inner dishes. They were homogenized in 1 mM sodium orthovanadate supplemented with protease inhibitors (Protease Inhibitor Cocktail Set, BoehringerMannheim, Tokyo, Japan). Protein (20 μg) was loaded onto 4%-12.5% Bis-TRIS Gel (Bio-rad) for SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane, which was incubated overnight at 4°C with the antibody against pERK-1/2 (200× dilution) or against COX-2 (1000× dilution; same antibodies as used in immunohistochemistry). The antigen on the membrane was visualized according to the protocols supplied by the manufacturer (WesternBreeze, chromogenic Western blot immunodetection kit, Invitrogen,, Calif., USA). The signal intensity was analyzed by densitometry. The results are presented as a ratio of the control values.
Effects of MEK and COX-2 inhibitors on the morphology and invasion of cancer cells
We examined the effects of the MEK and COX-2 inhibitors, PD98059 (50 μM; Sigma-Aldrich, St. Louis, Mo., USA) and celecoxib (100 μM; EMD Bioscience, Calif., USA), respectively, on the morphology and invasion of gastric cancer cells either alone or with ATSCs. The inhibitors were added to the culture medium every 2 days for 2 weeks. The morphology and invasion of cancer cells were analyzed by the same methods as described above.
Cancer-associated phenotype of ATSCs
To elucidate the cancer-associated phenotype of ATSCs, we examined the expression of some MSC markers, viz., CD44 and CD105 and the myofibroblast marker, α-SMA, in ATSCs grown with or without the gastric cancer cells by using immunohistochemistry as described above. Culture assemblies fixed with paraformaldehyde were vertically cut into four slices and then the four slices were embedded in paraffin in one cassette. Sections (6 μm thick) were prepared from this paraffin block, deparaffinized and prepared for immunohistochemistry. We counted a total of 1000 cells in non-overlapping fields of the immunohistochemically stained sections by using a 10× objective. The percentages of CD44-, CD105- and α-SMA-positive cells were calculated. Finally, we carried out Western blot for α-SMA, according to the same method as given above, in cells grown under conditions of no cell-cell contact between cancer cells and ATSCs.
Statistical analysis
The date obtained from five independent experiments were analyzed by the Student t-test. Values represent means±SD. P < 0.05 was considered significant.
Results
Effects of ATSCs on the morphology of cancer cells and their invasion into collagen gel
The well and poorly differentiated adenocarcinoma cell lines, MKN28 and MKN45 cells, respectively, each formed a stratified layer on ATSC-non-embedded collagen gel (Fig. 2). MKN28 cells organized a tight epithelial layer (Fig. 2), whereas MKN45 formed a loose epithelial layer (Fig. 2). On the ATSC-embedded gel, both MKN28 and MKN45 cells organized similar epithelial structures to those above but the stratification magnitude of the cancer cell types with ATSCs was greater than that of the cell types without ATSCs (Fig. 2). MKN28 cells with ATSCs began to penetrate the gel at 9 days in culture and thereafter apparently invaded the gel by 14 days in culture (Figs. 2, 3). In contrast, neither MKN28 cells alone nor MKN45 with or without ATSCs infiltrated into the gel (Figs. 2, 3). These phenomena showed no differences between rat subcutaneous and human subserosal ATSCs. Thus, rat ATSCs were mainly used in the following studies.
Morphology of cancer cells cultured with or without rat ATSCs at 14 days. In monocultures without ATSCs, MKN28 cells (a) formed a tight epithelial stratified layer, whereas MKN45cells (c) organized a loose layer. In cocultures with ATSCs, MKN28 (b) and MKN45 cells (d) formed similar epithelial structures but the stratification magnitude of both cancer cell types with ATSCs (b, d) was greater than that of the cell types without ATSCs (a, c). Interestingly, only MKN28 cells cultured with ATSCs infiltrate into the gel (arrowhead in b)
Effects of ATSCs on the invasion of cancer cells into collagen gel at 14 days. Invasion depth of cancer cells was determined as described in Materials and methods. a Coculture of MKN28 cells and ATSCs (arrows basement membrane, vertical bar invasion depth). MKN28 cells with ATSCs (a) infiltrated into the gel, whereas MKN45 cells even with ATSCs (b) never did. c ATSCs significantly induce the invasion of MKN28 cells into the gel, whereas invasion occurs in neither MKN28 cells alone nor MKN45 cells with and without ATSCs. a, b Reticulum silver impregnation stain
Effects of ATSCs on cancer cell growth and apoptosis
ATSCs significantly increased the PCNA expression of MKN28 and MKN45 cells (Fig. 4a-d). In contrast, ATSCs significantly decreased the ssDNA expression of MKN28 and MKN45 cells (Fig. 4e-h).
Proliferation (a-d) and apoptosis (e-h) of cancer cells cultured with or without ATSCs at 14 days, as analyzed by immunohistochemistry with PCNA and ssDNA, respectively, in nuclei (arrowheads representative results) of cancer cells (brown). ATSCs more significantly promote the PCNA expression in MKN28 and MKN45 cells than in these cell types cultured alone (a-d; top graph, *P < 0.05, MKN28; **P < 0.001, MKN45), whereas ATSCs more significantly decrease the ssDNA expression of MKN28 and MKN45 cells than in these cell types cultured alone (e–h; lower graph, *P < 0.05, MKN28 and MKN45)
Effects of ATSCs on the mucin expression of cancer cells
ATSCs clearly decreased the expression of MUC1 and MUC4 in MKN28 cells, whereas they did not affect that of MUC1 and MUC4 in MKN45 cells (Fig. 5).
Effects of ATSCs on the MUC-1 (a, b, e, f) and MUC-4 (c, d, g, h) expression in MKN28 (a-d) and MKN45 (e-h) cells as shown by immunohistochemistry. ATSCs decrease the MUC-1 and MUC-4 expression of MKN28 cells; note that both MUC-1 and MUC-4 are weakly expressed in MKN28 cells with ATSCs. In contrast, ATSCs do not affect the MUC-1 and MUC-4 expression of MKN45 cells
Effects of ATSCs on the invasion-related molecule and HER2 expression of cancer cells
ATSCs increased the expression of trophinin and filamin A in both MKN28 and MKN45 cells but decreased their expression of E-cadherin (Fig. 6). However, ATSCs did not affect the expression of MMP-1 and MMP-9 in either MKN28 or MKN45 cells, although the cancer cell types with or without ATSCs notably expressed the markers (Fig. 6). Interestingly, ATSCs clearly decreased the cell-membrane-localized expression of HER2 in both MKN28 and MKN45 cells (Fig. 7).
Effects of ATSCs on MMP-1 (a-d), MMP-9 (e-h), trophinin (i-l), filamin A (m-p) and E-cadherin (q-t) expression of MKN28 and MKN45 cells as shown by immunohistochemistry. The intensity of the staining was classified as negative (i), weak (j), moderate (m) and strong (n). MMP-1 (a-d) and MMP-9 (e-h) expression of MKN28 and MKN45 cells showed no difference between the cell types with ATSCs and without ATSCs. Both MKN28 and MKN45 cells cultured with ATSCs expressed trophinin (i-l) and filamin A (m-p) more prominently than those without ATSCs. Both MKN28 and MKN45 cells cultured with ATSCs expressed E-cadhirin (q-t) more weakly than those without ATSCs
Effects of ATSCs on the MAPK and COX-2 expression of cancer cells
Immunohistochemistry revealed that ATSCs increased the expression of pERK-1/2 in both MKN28 and MKN45 cells (Fig. 8a-d). In contrast, the cancer cell types with or without ATSCs readily expressed COX-2 (Fig. 8e-h). The immunohistochemical result for pERK-1/2 was supported by Western blot data obtained from cancer cells grown under conditions of direct cell-cell contact with ATSCs (Fig. 8i, k), whereas this was not the case in the absence of cell-cell contact (data not shown). On the other hand, the immunohistochemical result of COX-2 was supported by Western blot under conditions with or without the cell-cell contact (Fig. 8j, k). This suggested that cell-cell contact was critical for the ATSC-promoted pERK-1/2 expression of the cancer cells.
Effects of ATSCs on the pERK-1/2 and COX-2 expression of MKN28 and MKN45 cells as demonstrated by immunohistochemistry and Western blot. Both MKN28 (b) and MKN45 cells (d) cultured with ATSCs (b, d) expressed pERK-1/2 more strongly than those cultured without ATSCs (a, c). The presence or absence of ATSCs did not affect the COX-2 expression of either MKN28 (e, f) or MKN45 (g, h) cells. Under all conditions, the cancer cell types clearly expressed COX-2. Representative Western blots (i, j) of pERK-1/2 (44/42 kDa) and COX-2 (70 kDa) expression in both MKN28 and MKN45 cells cultured with or without ATSCs supported the immunohistochemical results above. These Western blot analyses that were carried out with samples derived from cancer cells allowed direct cell-cell contact with ATSCs. k Densitometry analyses
Effects of MEK and COX-2 inhibitors on cancer cell morphology and invasion
The MEK inhibitor PD98059 clearly inhibited the cellular stratification of MKN28 cells with or without ATSCs, whereas it only slightly inhibited that of MKN45 cells (Fig. 9a-h). Moreover, the inhibitor inhibited the invasion of MKN28 cells grown with ATSCs into the gel (Fig. 9d). The COX-2 inhibitor celecoxib did not affect the morphology or invasion of the two gastric cancer cell types with or without ATSCs (Fig. 9i-p).
Effects of MEK and COX-2 inhibitors on the morphology and invasion of cancer cells at 14 days in culture. MKN28 cells treated with the MEK inhibitor PD98059 (50 μM) with (b) or without (d) ATSCs were less stratified than the nontreated cells with (c) or without (a) ATSCs. The inhibitory effect of the agent on the stratification of MKN45 with (g, h) or without (e, f) ATSCs was mild. PD98056-treated MKN28 cells with ATSCs (d) did not infiltrate into the gel, whereas the nontreated cells with ATSCs (c) did infiltrate. The MEK inhibitor thus seemed to prohibit the growth and/or invasion of the cancer cell types. The COX-2 inhibitor celecoxib (100 μM) had no such effects on MKN28 cells (i-l) or MKN45 cells (m-p) with or without ATSCs
Effects of cancer cells on the cancer-associated phenotype of ATSCs
To elucidate the cancer-associated phenotype of ATSCs, we examined the expression of some MSC markers, viz., CD44 and CD105 and the myofibroblast marker, α-SMA, in ATSCs grown with or without the gastric cancer cells, by using immunohistochemistry. The intensity of the CD44 and CD105 expression of ATSCs cultured alone was strong, whereas that of ATSCs cultured with the cancer cells was weak. The intensity of the α-SMA expression of ATSCs alone was strong, whereas that of ATSCs grown with the cancer cells ranged widely from weak to strong. The number of all those ATSCs that expressed CD44, CD105, or α-SMA weakly to strongly was counted. The presence of MKN28 and MKN45 cells significantly decreased the number of CD44- or CD105-positive cells in ATSCs, whereas the presence of the cancer cell types significantly increased that of α-SMA-positive cells in ATSCs (Fig. 10). Western blot data supported the immunohistochemical results for α-SMA expression (for a representative case of Western blot, see Fig. 10h).
Effects of MKN28 and MKN45 cells on the CD44, CD105 and α-SMA expression of ATSCs as revealed by immunohistochemistry and Western blot. The presence of CD44 (a, d), CD105 (b, e) and α-SMA (c, f) appears as brown staining (arrowheads) in ATSCs cultured with (d–f) or without (a–c) MKN28 cells. MKN28 and MKN45 cell types significantly decrease the number of CD44- and CD105-positive ATSCs (g, *P < 0.001), whereas the two cell types significantly increase the number of α-SMA-positive ATSCs (g, *P < 0.001). A representative case of Western blot with regard to α-SMA expression of ATSCs grown with MKN45 cells is shown in h
Discussion
Here, we have shown, for the first time, the active interactions between gastric adenocarcinoma cells and ATSCs. Namely, ATSCs promote and suppress the growth/invasion and the apoptosis of the cancer cells, respectively, enabling them to activate MAPK (pERK-1/2). Notably, ATSCs cause the cancer cells to inhibit the expression of HER2, which is a key molecule for the molecular-targeted therapy of advanced gastric cancer (Lee et al. 2010). In turn, gastric cancer cell types induce the cancer-associated myofibroblast phenotype in ATSCs. These results suggest that ATSCs might play critical roles in the progression and HER2-targeted therapy of gastric cancer through the cancer-promoted myofibroblast phenotype.
In this study, we have not clarified in detail the basic mechanisms underling the gastric cancer cell-ATSC interaction. Recently, the increased expression of COX-2 has been demonstrated in various cancer tissues, including gastric, esophageal, colorectal, pancreatic, hepatic and mammary cancers (Antonacopoulou et al. 2008; Bai et al. 2010; Hu et al. 2009; Jimenez et al. 2010; Tucker et al. 1999). Furthermore, COX-2 plays critical roles in tumor development and progression. Indeed, one notable study has demonstrated that the fibroblast-induced upregulation of COX-2 in breast cancer cells leads to the development of aggressive cancer cells with their increased motility and invasion under the influence of cancer-stromal interaction (Hu et al. 2009). However, our current investigation has shown that ATSCs do not enable gastric adenocarcinoma cells to increase COX-2 expression. In our study, the COX-2 inhibitor, celecoxib, also does not affect the growth, apoptosis and invasion of gastric cancer cells cultured with or without ATSCs. These results suggest that ATSCs but not fibroblasts, affect the behavior of gastric cancer cells in a COX-2-independent way.
Recently, MAPK has been suggested to be involved in the growth-signaling pathway of various cell types (Dent et al. 2003). In our study, ATSCs enable gastric cancer cells to increase their MAPK (pERK-1/2) expression. One interesting investigation has shown that hypoxia-induced activation of MAPK allows gastric cancer cells to enhance growth and suppress apoptosis (Toda et al. 2005). In addition, several studies have demonstrated that irradiated fibroblasts cause the bystander cells of normal and neoplastic origin to activate MAPK and COX-2 (Kamochi et al. 2008; Liu et al. 2010). However, our current data indicate that the MAPK activation of gastric cancer cells is not linked to their COX-2 expression. Together, these results suggest that ATSCs promote and inhibit the growth and apoptosis of gastric cancer cells, respectively, through MAPK activation of the cancer cells in a COX-2-independent way. Finally, we have demonstrated that ATSCs promote MAPK expression in the cancer cells only under conditions of cell-cell contact. Moreover, our preliminary study has shown that the ATSC-conditioned medium does not apparently replicate the ATSC-induced morphology of gastric cancer cell types. Thus, direct cell-cell contact mechanisms are considered to be critical for gastric cancer-ATSC interaction. This suggests that the cell-cell contact is critical for the ATSC-promoted pERK-1/2 expression of gastric cancer cells. Further studies are now needed to clarify in more detail the cell-cell contact-mediated mechanisms underling cancer-stromal interaction.
The molecular-targeted agent herceptin, a humanized monoclonal antibody targeting the HER2/neu antigen, has been used as a first-line cancer therapy in HER2-overexpressing breast cancer patients (Wu et al. 2009). Herceptin is also suggested to be effective in the treatment of gastric cancer with HER2 overexpression (Corsini et al. 2003). Interestingly, we have shown, for the first time, that ATSCs decrease the HER2 expression of gastric cancer cells. Concomitantly with the infiltration of gastric cancer cells into the submucosa, in which adipose tissue exists, the cancer cells might interact with ATSCs at the invasion sites and, as a result, reduce their HER2 expression. Thus, ATSCs might adversely affect HER2-targeted therapy. Although the mechanism regarding the ATSC-induced downregulation of HER2 is still unclear from this study, the clarification of the mechanism might lead to the development of a new molecular-targeted therapy for gastric cancer.
The invasion and metastatic spread of cancer cells are regulated by various factors such as proteolytic, locomotive and angiogenic agents (De Wever and Mareel 2003; Kopfstein and Christofori 2006; Mareel and Leroy 2003). In this study, ATSCs appear to promote the expression of trophinin (Chang et al. 2009) and filamin A (Cunningham 1992) in both well and poorly differentiated adenocarcinoma cell types of the stomach, whereas they decrease the expression of E-cadherin (Wijnhoven et al. 2000) in both cell types. In contrast, ATSCs do not affect the expression of MMP-1 and MMP-9 (Westermarck and Kahari 1999) in the cancer cells. Interestingly, ATSCs allow the well differentiated cancer cells to infiltrate into collagen gel, whereas they never stimulate the poorly differentiated ones to do so. These results suggest that the ATSC-affected expression of trophinin, filamin A and E-cadherin of the gastric cancer cells is involved in their invasion mechanisms but the stromal invasion mechanism of the well differentiated cancer cells is different from that of the poorly differentiated cancer cells. In addition, despite the finding of MMP-1 and MMP-9 in the cancer cells grown with or without ATSCs in this study, the structure of the collagen gel layer is retained. Thus, the production of these matrix-degrading molecules by the cancer cells grown with or without ATSCs might be low.
Here, we have evaluated cancer cell growth by using immunohistochemistry for PCNA. However, PCNA might be expressed in some non-dividing cells because of its multifunctionality and long half-life (Naryzhny 2008). To assess this possibility, we have carried out immunohistochemistry for another growth marker, Ki-67. Our preliminary study has shown that the numbers of Ki-67-positive cancer cell types grown with or without ATSCs are well correlated with those for PCNA-positive cells, although the PCNA-positive numbers are higher than those for Ki-67-positive cells. Thus, PCNA is considered to be a useful growth marker, at least for the gastric cancer cell types tested here.
In this study, gastric cancer cells seem to allow ATSCs to enhance their expression of the myofibroblast marker, α-SMA (Pietras and Ostman 2010) and to reduce that of some MSC markers, viz., CD44 and CD105 (Gomillion and Burg 2006). In general, cancer-associated fibroblasts are suggested to express the myofibroblast phenotype (Pietras and Ostman 2010; Semba et al. 2009). Our results thus suggest that gastric cancer cells enable ATSCs to gain a myofibroblast phenotype. Given that our system stably recreates the cancer-stromal interaction, our method might be a useful tool for studying the pathophysiology of cancer-associated stromal cells.
References
Antonacopoulou AG, Tsamandas AC, Petsas T, Liava A, Scopa CD, Papavassiliou AG, Kalofonos HP (2008) EGFR, HER-2 and COX-2 levels in colorectal cancer. Histopathology 53:698–706
Bai XM, Jiang H, Ding JX, Peng T, Ma J, Wang YH, Zhang L, Zhang H, Leng J (2010) Prostaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cells. Life Sci 86:214–223
Chang XZ, Yu J, Zhang XH, Yin J, Wang T, Cao XC (2009) Enhanced expression of trophinin promotes invasive and metastatic potential of human gallbladder cancer cells. J Cancer Res Clin Oncol 135:581–590
Corsini C, Mancuso P, Paul S, Burlini A, Martinelli G, Pruneri G, Bertolini F (2003) Stroma cells: a novel target of herceptin activity. Clin Cancer Res 9:1820–1825
Cunningham CC (1992) Actin structural proteins in cell motility. Cancer Metastasis Rev 11:69–77
De Wever O, Mareel M (2003) Role of tissue stroma in cancer cell invasion. J Pathol 200:429–447
De Wever O, Demetter P, Mareel M, Bracke M (2008) Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 123:2229–2238
Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S (2003) MAPK pathways in radiation responses. Oncogene 22:5885–5896
Gomillion CT, Burg KJ (2006) Stem cells and adipose tissue engineering. Biomaterials 27:6052–6063
Gonda TA, Varro A, Wang TC, Tycko B (2010) Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol 21:2–10
Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M (2008) Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem 283:19864–19871
Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA (1995) Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res 55:2681–2690
Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K (2009) Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci USA 106:3372–3377
Jimenez P, Piazuelo E, Cebrian C, Ortego J, Strunk M, Garcia-Gonzalez MA, Santander S, Alcedo J, Lanas A (2010) Prostaglandin EP2 receptor expression is increased in Barrett’s oesophagus and oesophageal adenocarcinoma. Aliment Pharmacol Ther 31:440–451
Kamochi N, Nakashima M, Aoki S, Uchihashi K, Sugihara H, Toda S, Kudo S (2008) Irradiated fibroblast-induced bystander effects on invasive growth of squamous cell carcinoma under cancer-stromal cell interaction. Cancer Sci 99:2417–2427
Kopfstein L, Christofori G (2006) Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci 63:449–468
Lee MS, Cha EY, Thuong PT, Kim JY, Ahn MS, Sul JY (2010) Down-regulation of human epidermal growth factor receptor 2/neu oncogene by corosolic acid induces cell cycle arrest and apoptosis in NCI-N87 human gastric cancer cells. Biol Pharm Bull 33:931–937
Liu L, Zhang H, Sun L, Gao Y, Jin H, Liang S, Wang Y, Dong M, Shi Y, Li Z, Fan D (2010) ERK/MAPK activation involves hypoxia-induced MGr1-Ag/37LRP expression and contributes to apoptosis resistance in gastric cancer. Int J Cancer 127:820–829
Manabe Y, Toda S, Miyazaki K, Sugihara H (2003) Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol 201:221–228
Mareel M, Leroy A (2003) Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev 83:337–376
Matsuyama A, Toda S, Yamada S, Inokuchi A, Sugihara H (2002) Effects of irradiation on biological behavior of carcinoma cells under carcinoma-stromal cell interaction and air-liquid interface: a possible model for testing radiosensitivity of carcinoma of the upper aerodigestive tract using a collagen gel culture system. Pathol Res Pract 198:469–478
Mulligan MS, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA (1993) Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol 142:1739–1749
Naryzhny SN (2008) Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci 65:3789–3808
Ohsawa R, Miyazaki H, Niisato N, Shiozaki A, Iwasaki Y, Otsuji E, Marunaka Y (2010) Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J Cell Physiol 223:764–770
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316:1324–1331
Radisky DC, Kenny PA, Bissell MJ (2007) Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 101:830–839
Satoh S, Toda S, Inokuchi A, Sugihara H (2005) A new in vitro model for analyzing the biological behavior of well-differentiated squamous cell carcinoma. Pathol Res Pract 201:27–35
Semba S, Kodama Y, Ohnuma K, Mizuuchi E, Masuda R, Yashiro M, Hirakawa K, Yokozaki H (2009) Direct cancer-stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous-type gastric carcinoma cells. Br J Cancer 101:1365–1373
Sonoda E, Aoki S, Uchihashi K, Soejima H, Kanaji S, Izuhara K, Satoh S, Fujitani N, Sugihara H, Toda S (2008) A new organotypic culture of adipose tissue fragments maintains viable mature adipocytes for a long term, together with development of immature adipocytes and mesenchymal stem cell-like cells. Endocrinology 149:4794–4798
Toda S, Matsumura S, Fujitani N, Nishimura T, Yonemitsu N, Sugihara H (1997) Transforming growth factor-beta1 induces a mesenchyme-like cell shape without epithelial polarization in thyrocytes and inhibits thyroid folliculogenesis in collagen gel culture. Endocrinology 138:5561–5575
Toda S, Yamada S, Aoki S, Inokuchi A, Sugihara H (2005) Air-liquid interface promotes invasive growth of laryngeal squamous cell carcinoma with or without hypoxia. Biochem Biophys Res Commun 326:866–872
Trayhurn P (2007) Adipocyte biology. Obes Rev 8 (Suppl 1):41–44
Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ 3rd (1999) Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 59:987–990
Westermarck J, Kahari VM (1999) Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 13:781–792
Wijnhoven BP, Dinjens WN, Pignatelli M (2000) E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 87:992–1005
Wozniak SE, Gee LL, Wachtel MS, Frezza EE (2009) Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 54:1847–1856
Wu M, Jung L, Cooper AB, Fleet C, Chen L, Breault L, Clark K, Cai Z, Vincent S, Bottega S, Shen Q, Richardson A, Bosenburg M, Naber SP, DePinho RA, Kuperwasser C, Robinson MO (2009) Dissecting genetic requirements of human breast tumorigenesis in a tissue transgenic model of human breast cancer in mice. Proc Natl Acad Sci USA 106:7022–7027
Yamada S, Toda S, Shin T, Sugihara H (1999) Effects of stromal fibroblasts and fat cells and an environmental factor air exposure on invasion of laryngeal carcinoma (HEp-2) cells in a collagen gel invasion assay system. Arch Otolaryngol Head Neck Surg 125:424–431
Zhang XL, Yang YS, Xu DP, Qu JH, Guo MZ, Gong Y, Huang J (2009) Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg 33:2112–2118
Acknowledgements
We thank Messrs. H. Ideguchi, S. Nakahara and F. Mutoh and Mrs. M. Nishida for their helpful technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology for Scientific Research (nos. 18591871, 20592023 to S.T.; 20590728 to K.F.) and personal grants from Koike Hospital, Sasebo Chuo Hospital, Nagasaki Rosai Hospital and Yamada Clinic (to S.T.).
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nomoto-Kojima, N., Aoki, S., Uchihashi, K. et al. Interaction between adipose tissue stromal cells and gastric cancer cells in vitro. Cell Tissue Res 344, 287–298 (2011). https://doi.org/10.1007/s00441-011-1144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1144-3