Abstract
Obesity is a risk factor for the development of new cases of breast cancer and also affects survival in women who have already been diagnosed with breast cancer. Early studies of obesity and breast cancer survival have been summarised in two meta-analyses, but the latest of these only included studies that recruited women diagnosed as recently as 1991. The primary aim of this study was to conduct a meta-analysis that included the more recent studies. A systematic search of MEDLINE, EMBASE and CINAHL was conducted to identify original data evaluating the effects of obesity on survival in newly diagnosed breast cancer patients. Adjusted hazard ratios (HR) from individual studies were pooled using a random effects model. A series of pre-specified sensitivity analyses were conducted on factors such as overall versus breast cancer survival and treatment versus observational cohort. The meta-analysis included 43 studies that enrolled women diagnosed with breast cancer between 1963 and 2005. Sample size ranged from 100 to 424168 (median 1192). The meta-analysis showed poorer survival among obese compared with non-obese women with breast cancer, which was similar for overall (HR = 1.33; 95% confidence interval (CI): 1.21, 1.47) and breast cancer specific survival (HR = 1.33; 95% CI: 1.19, 1.50). The survival differential varied only slightly, depending on whether body mass index (1.33; 1.21, 1.47) or waist–hip ratio (1.31; 1.08, 1.58) was used as the measure of obesity. There were larger differences by whether the woman was pre-menopausal (1.47) or post-menopausal (1.22); whether the cohort included women diagnosed before (1.31) or after 1995 (1.49); or whether the women were in a treatment (1.22) or observational cohort (1.36), but none of the differences were statistically significant. Women with breast cancer, who are obese, have poorer survival than women with breast cancer, who are not obese. However, no study has elucidated the causal mechanism and there is currently no evidence that weight loss after diagnosis improves survival. Consequently, there is currently no reason to place the additional burden of weight loss on women already burdened with a diagnosis of cancer. Further research should concentrate on assessing whether factors such as diabetes or type of chemotherapy modify the obesity effect and on understanding the causal mechanism, in particular the role of relative under-dosing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In post-menopausal women, obesity, as measured by body mass index (BMI) or waist–hip ratio (WHR), is a risk factor for the development of new cases of breast cancer [1]. In pre-menopausal women, the evidence is less clear-cut: some reviews have reported a weak negative association with general obesity, as measured by BMI [2, 3]; whereas others have reported a positive association with central obesity, as measured by WHR [4, 5]. Obesity also seems to affect survival in women who have already been diagnosed with breast cancer; early studies have been summarised in two meta-analyses.
The first of these included 14 studies [6] which enrolled women diagnosed with breast cancer between 1968 and 1989. It reported a pooled estimate for the hazard ratio (HR) for obese versus non-obese women (based on BMI) of 1.6 (95% confidence interval (CI) 1.4, 1.8). The second meta-analysis [7] included 12 studies which enrolled patients from 1968 to 1991. The pooled estimate of the HR (based on BMI) was also 1.6 (95% CI 1.2, 2.0). There are now more than 40 published studies on the association between obesity and breast cancer survival (including a few studies that used WHR). Consequently, the primary aim of this study was to conduct a meta-analysis that included the more recent studies. Our hypothesis was that women who were obese at the time of diagnosis of invasive breast cancer, had worse overall or breast cancer specific survival than non-obese women.
Methods
Search strategy
A systematic search of MEDLINE, EMBASE and CINAHL, from inception to December 2009, was used to identify studies evaluating the effects of obesity on survival in cancer patients. The search strategy included key terms for cancer (neoplasms OR cancer OR tumour), AND obesity (body size OR overweight OR bodyweight OR obesity), AND survival or prognosis (survival analysis OR survival rate OR proportional hazards model OR survival OR prognosis). All search terms were ‘exploded’ in conjunction with using a keyword search. The search was limited to English language papers that included adults (age ≥ 18 years). The reference lists of all eligible articles and reviews were also scanned to identify additional relevant studies. All cancer studies were included so as not to miss any results for breast cancer that might have been reported as part of a larger study that included other cancer types. Based on our knowledge of the literature, we anticipated that most of the identified studies would be cohort studies; however, all study designs were in scope.
Study selection
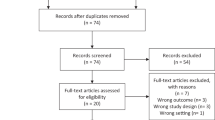
Studies were eligible for inclusion if they contained original data analysis, evaluated overall survival or breast cancer specific survival in a cohort of newly diagnosed breast cancer patients; and additionally assessed obesity status at or around the time of first diagnosis. We did not include studies that assessed the relationship between survival and weight gain or weight loss after diagnosis. We concentrated on survival as the outcome, rather than recurrence; our reasoning was that date of death is more likely to be measured accurately and that results for survival are easier to interpret than those for recurrence [8–11]. Figure 1 describes the search and study selection process.
Study selection. Note: exclusion criteria A—studies that did not evaluate a prognostic outcome (all-cause mortality or breast cancer specific survival/mortality) in breast cancer patients, exclusion criteria B—studies that did not measure obesity status at or around the time of breast cancer diagnosis, exclusion criteria C—studies which did not report original data, exclusion criteria D—studies which used recurrent breast cancer cases (rather than newly diagnosed cases); exclusion criteria E—studies which contained a repeated analysis in a single population
Data extraction and quality assessment
Articles were reviewed independently by two of us and data items extracted as per Supplementary Table 1. Disagreements were settled by consensus or a third reviewer for adjudication. To assess quality we abstracted information on the study population, measurement of obesity, ascertainment of survival and adjustment for confounding (Supplementary Table 2). If more than one adjusted HR was reported, the most adjusted HR was extracted.
Data synthesis and statistical analysis
To avoid overlapping patient populations, comparisons between studies were made on recruitment years, data source and geographic location. If a patient population was found to overlap, the data with the most comprehensive population was included. This resulted in the exclusion of one article from the meta-analysis [12].
Adjusted HR estimates were pooled using random effects meta-analysis [13]. I 2 was used to assess heterogeneity across studies [14]. Pre-specified analyses were conducted to examine differences in survival for obese versus non-obese women with breast cancer in different strata defined according to how survival was measured (overall or breast cancer specific), how obesity was measured (BMI or WHR), study design (treatment cohort or observational cohort), period of diagnosis (pre-1995 or post-1995) and menopausal status. These stratified analyses were conducted by comparing pooled HRs in each stratum, and P-values for each comparison were obtained using random effects meta-regression [15]. Publication bias was assessed using a funnel plot and trim-and-fill method [16]. All analyses were conducted using Stata 10 [17].
Funding source
There was no external funding for this study. Resources were provided by the authors’ institutions.
Results
The literature search identified 3566 citations of which 45 met the criteria for inclusion in the systematic review. The main reason for exclusion was that the study evaluated breast cancer incidence or mortality among cohorts of women defined according to obesity status, without breast cancer at inception; rather than assessing survival in cohorts of patients newly diagnosed with breast cancer (Fig. 1).
Description of studies
The 45 studies included women diagnosed with breast cancer across the years 1963–2005. Thirty-nine were observational cohorts, while six were treatment cohorts. The studies had been conducted in the United States (23), Europe (11), Canada (4), Asia (2), Africa (1), Australia (1), New Zealand (1) and multiple countries (2). Sample size ranged from 100 to 424168, with a median of 1192.
Three of the studies used both BMI and WHR, 40 used BMI alone, one used WHR alone and one used tables of ideal weight for height. Seventeen of the studies used height and weight based on self-report; in the others BMI was based on height and weight, which were measured by investigators or obtained from medical records. In two studies the WHR measurements were obtained by investigators, and in the other two studies WHR was estimated using self-reported data. A variety of cut points were used to categorise BMI. Most common was ≥30 kg/m2 versus <25 kg/m2, which was used in 12 of the 43 studies that reported BMI. Other various cut points were either based on quartiles or tertiles of the observed distribution of BMI for that particular study. Slightly different cut points were used for each of the four studies that reported WHR (Supplementary Table 1). Studies used death registries (n = 28), medical records (n = 3) and active follow-up (n = 8) to ascertain vital status and six did not report the method of outcome ascertainment.
All studies had a median follow-up time of at least 4 years, with two studies having a median follow-up time of more than 14 years. Adjustment for potential confounding was not consistent across studies: four studies only provided unadjusted results and another 10 did not account for at least age (or menopausal status) and stage (using, for instance, restriction, stratification or statistical modelling). Some studies adjusted for prognostic factors such as oestrogen receptor (ER) status, progesterone receptor (PR) status, tumour grade and treatment (e.g. tamoxifen use); others did not (Supplementary Table 1). Only one study adjusted for diabetes [18] and one other study excluded patients with diabetes [19].
Meta-analysis
One of the identified studies [20] could not be included in the meta-analysis because it reported odds ratios. It was a study of 301 post-menopausal women diagnosed with breast cancer from 1977 to 1985. The adjusted odds ratio was 1.37 (95% CI: 0.73, 2.56) for BMI ≥ 27 kg/m2 versus BMI < 27 kg/m2, indicating worse survival for obese patients. Statistical adjustment was for age, stage, ER status and level of treatment.
Another study [21] could not be included in the meta-analysis because it reported 5- and 10-year survival, rather than HRs. This was a follow-up of a phase II trial of adjuvant chemotherapy in women with node-positive breast cancer. Obesity was defined as BMI ≥ 30 kg/m2 and obese women had lower 10 year survival (42% survival) than women of normal (BMI < 30) weight (53%).
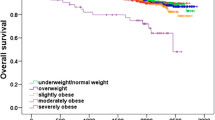
The meta-analysis of the remaining 43 studies showed poorer survival among obese compared with non-obese women with breast cancer, which was similar for overall survival (HR = 1.33; 95% CI: 1.21, 1.47) (Fig. 2) and breast cancer specific survival (HR = 1.33; 95% CI: 1.19, 1.50) (Fig. 3). The survival differential varied only slightly, depending on whether BMI (1.33; 1.23, 1.44) or WHR (1.31; 1.14, 1.50) was used as the measure of obesity. There were larger differences by whether the woman was pre-menopausal (1.47) or post-menopausal (1.22); whether the cohort included women diagnosed before (1.31) or after 1995 (1.49); or whether the women were in a treatment (1.22) or observational cohort (1.36), but none of the differences were statistically significant (Table 1).
Meta-analysis and pooled hazard ratio of the effect of obesity on breast cancer specific survival in breast cancer patients. Note: 15 studies provided 16 estimates. a Pre-menopausal women only, b study reported HR per one unit increase in BMI as a continuous variable and was converted to HR per 5 unit increase in BMI for the purpose of this meta-analysis, c post-menopausal women only
There was arguably a lack of medium-sized, negative studies on the funnel plot (Supplementary Figure 1), however, accounting for this using the trim-and-fill method gave a HR = 1.23 (95% CI: 1.11, 1.37), indicating that the detrimental effect of obesity on breast cancer survival is robust to potential publication bias.
Because studies used different methods to account for confounding, we conducted a series of post hoc sensitivity analyses, as opposed to our pre-specified sensitivity analyses. After excluding univariate estimates (n = 4) the HR for overall survival was 1.29 (1.18, 1.42). We then excluded all studies that did not adjust (or restrict) at least by age or menopausal status, in addition to stage at diagnosis (n = 10) and obtained HR 1.30 (1.21, 1.40). Next, we excluded studies that did not additionally adjust for ER, PR or HER-2 status (n = 12) and obtained HR 1.28 (1.16, 1.40).
We also compared HRs for studies that measured height and weight to obtain BMI (n = 25) and those that used self-reported height and weight (n = 17). There was little difference in the HR for measured 1.38 (1.23, 1.55); or self-report 1.40 (1.12, 1.76).
A potential source of heterogeneity is that different studies used different cut points for BMI and WHR (Supplementary Table 1). We conducted on post hoc sensitivity analysis on just the 18 studies that used the WHO definition of obesity (30+ kg/m2) versus the reference category of <25 kg/m2 (12 studies) or 18.5–24.9 (4 studies) or 20–24.9 (2 studies) and obtained HR = 1.35. We then analysed just the 12 studies that used <25 versus 30+ and obtained HR = 1.37. We could not do a similar analysis for WHR because all four of these studies used different cut points (Supplementary Table 1).
Discussion
In this meta-analysis of 43 studies, survival among obese women with breast cancer was worse than survival among non-obese women (HR = 1.33). The pooled estimate of a 33% increase in the rate of death among obese women is smaller than the 60% increase (HR = 1.6) reported by the two earlier meta-analyses [6, 7]. This regression-to-the-truth effect is common as evidence accumulates [22].
The obesity effect was larger in pre-menopausal (1.47) than post-menopausal (1.22), women but the difference was not statistically significant. Post hoc power calculations indicate that more than 50 studies with a sample size of 1192 (the median sample size in this meta-analysis) would be needed in each stratum to show that a HR of 1.47 was statistically significantly different from 1.22. Therefore, obesity might be a stronger determinant of survival in pre-menopausal as opposed to post-menopausal women, but this meta-analysis did not have the statistical power to show it definitively.
Most studies used BMI as a definition of obesity. However, BMI is a relationship of weight to height, not necessarily fatness, and definitions of obesity or ‘leanness’ based on BMI may not be accurate predictors of response to metabolic stress [23]. There was only a minor difference according to whether BMI (1.33) or WHR (1.31) was used as the measure of obesity, however, only four analyses used WHR. Also, we could not pool results for WHR by menopausal status because only one study stratified by menopausal status (pre-menopausal HR = 1.2; post-menopausal HR = 3.3) [24], and one study included only pre-menopausal women (HR = 1.52) [25]. The other two studies did not stratify by menopausal status (HR = 1.38; HR = 1.1) [26, 27].
In randomised control trials (RCT), doses of chemotherapy are given according to a pre-specified protocol, whereas in everyday practice, clinicians may reduce the dose in obese women because of concern for toxicity if appropriate dose for body surface area (BSA) is given; applying empiric dose reductions to ‘cap’ the BSA at 2 m2, or using ideal rather than actual body weight to calculate BSA [28, 29]. The results of this meta-analysis suggest, but do not definitively show, that some of the obesity effect might be due to such under-dosing. The pooled HR from treatment cohorts (1.22) was less than from observational cohorts (1.36), but the difference was not statistically significant. There were only seven analyses of treatment cohorts, therefore, as for menopausal status, the lack of a statistically significant difference might reflect low statistical power rather than the absence of a real effect.
The identified studies enrolled women diagnosed with breast cancer over a long period: 1963–2005, but the obesity effect did not vary much over that time. Specifically, the obesity effect was slightly higher post-1995 (HR = 1.49) compared to pre-1995 (1.31), but the difference was not statistically significant.
Limitations
It is possible that not all relevant studies were included, however, the search strategy was broad and references from each of the included studies were checked.
Only non-randomised cohort studies or secondary, post hoc analyses of treatment cohorts were identified for inclusion. Meta-analyses of such studies are prone to biases and confounding factors that are inherent in the original studies [30]. When extracting data we took the most adjusted estimate. Sensitivity analyses excluding studies that did not adjust for at least stage, age (or menopausal status), ER status or the use of hormone replacement therapy (HRT) suggested that the obesity effect was robust to potential confounding.
Mechanism of action of obesity on breast cancer survival
When we excluded studies that did not adjust for stage (and age or menopausal status) we obtained HR = 1.31. Therefore, advanced stage at diagnosis cannot be the explanation for the poor survival of obese patients despite the knowledge that obese women undergo less breast cancer screening [31].
The obesity effect was of similar size for overall survival and breast cancer specific survival (HR for both 1.33). This result is not consistent with the hypothesis that obese women with breast cancer have poorer overall survival because of a higher risk of non-cancer causes of death [32].
Obese women are at higher risk for a second primary breast cancer [33] and this could lead to poorer breast cancer specific survival, unrelated to an increased risk of death from the first breast cancer. However, this is unlikely to account for all the increased risk of breast cancer specific death, identified in this meta-analysis, because the risk of a second primary is small, even for obese women. For example, in the recent study by de Azambuja et al. [34], only 3.1% of obese women developed a second primary breast cancer within 5 years of their first primary; the corresponding percentage for non-obese women was 2.2%.
There are two other hypotheses (i.e. besides more advanced stage and higher risk of second primary) which may explain why obese breast cancer patients have worse survival. Firstly, obese patients may have more biologically aggressive tumours. Obesity is associated with the upregulation of a number of cellular proliferation pathways [35], and consequently obese patients may have increased tumour cell proliferation and metastasis due to an undefined adipokine effect on tumour cells [36]. For example, leptin, an adipocytokine, is produced mainly by white adipose tissue and is known to act as a growth factor in cancer, including cancer of the breast [37]. It promotes angiogenesis, potentially directly stimulating growth of breast cancer cells, possibly translating to reduced survival [38]. Insulin-like Growth Factor-1 (IGF1) also inhibits apoptosis, and higher fasting insulin concentrations are correlated with increased recurrences and decreased survival in breast cancer [19].
It has also been postulated that adipose tissue production of oestrogens may contribute to more biologically aggressive ER-positive tumours in post-menopausal women because BMI is directly related to circulating oestrogen levels [39]. However, in studies of women receiving anti-hormonal treatment, BMI did not appear to be an independent prognostic factor, suggesting that excessive endogenous oestrogens are not responsible for the adverse effect of obesity on survival [11, 40].
Secondly, several studies have shown that obese patients are more likely to receive reduced doses of chemotherapy compared to normal weight women. A large retrospective cohort study of breast cancer patients conducted by Griggs et al. [41] found that overweight and obese women were more likely to receive intentionally reduced doses of chemotherapy compared with women of normal weight, despite several studies finding that obese women receiving full doses of chemotherapy experienced no more toxicity than normal weight patients [42, 43]. Obese patients may also have a relative reduction in cancer treatment due to comorbidities such as cardiovascular or renal disease or local toxicity from radiation therapy. A related hypothesis is that obesity has an effect on metabolism of cytotoxic drugs; for example, through an effect on the p450 system [44].
Implications for further research
There was limited information available about the possible joint effect of diabetes and obesity on survival. Only one study [18] adjusted for diabetes (in addition to stage, age, ER status, smoking status and hypertension) and reported adjusted HR = 1.14 for overall survival and 1.16 for breast cancer specific survival. Another study excluded diabetes [19] and reported adjusted HR = 1.78 (overall survival). A recent meta-analysis of four studies examining effect of diabetes on overall survival among women with breast cancer reported a pooled HR of 1.61 [45]. However, the studies did not adjust or stratify by obesity, which shares many risk factors with diabetes so the effect of one on the other is relevant and worthy of further investigation.
There was also limited information about whether the obesity effect varies by different chemotherapy regimens. Eight studies adjusted for whether the women received any chemotherapy at all, without specifying the type of chemotherapy (i.e. any chemotherapy: yes/no) and without providing analyses stratified into “chemotherapy” and “no chemotherapy” (Supplementary Table 1). Given the broad, non-specific nature of this statistical adjustment and the lack of stratified analyses, it was not possible to explore the issue of obesity–chemotherapy interaction using the currently available evidence.
Several studies adjusted for prognostic factors such as HER-2 status, ER status, PR status, p53 and HRT-use but as for type of chemotherapy, no studies presented stratified analyses, so it was not possible to assess whether these prognostic factors modified the effect of obesity on survival and further work along these lines would also be useful.
It is currently unclear whether post-diagnosis weight reduction modifies the association between obesity at diagnosis and poor survival. A recent RCT showed that reducing dietary fat intake improves relapse-free survival of breast cancer, despite only a modest effect on weight [46]. A RCT assessing the effect of a weight loss and physical activity intervention on survival from breast cancer is also planned [47].
Conclusion
Based on the results of this meta-analysis, women should be advised that keeping their weight within normal limits will have benefits should they develop breast cancer. However, no study has elucidated the causal mechanism and there is currently no evidence that weight loss after diagnosis improves survival. Consequently, there is currently no reason to place the additional burden of weight loss on women already burdened with a diagnosis of cancer. Further research should concentrate on assessing both whether factors such as diabetes, ER status or type of chemotherapy regimen modify the obesity effect and on understanding the causal mechanism, in particular the role of relative under-dosing.
References
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578
Vainio H, Kaaks R, Bianchini F (2002) Weight control and physical activity in cancer prevention, international evaluation of evidence: International Agency for Research on Cancer. Eur J Cancer Prev 11(Suppl 2):S94–S100
van den Brandt P, Spiegleman D, Yaun S et al (2000) Pooled analysis of prospective cohort studies on height weight and breast cancer risk. Am J Epidemiol 152:514–527
Connolly BS, Barnett C, Vogt KN, Li T, Stone J, Boyd NF (2002) A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutr Cancer 44(2):127–138
Harvie M, Hooper L, Howell AH (2003) Central obesity and breast cancer risk: a systematic review. Obes Rev 4(3):157–173
Goodwin PJ, Esplen MJ, Winocur J, Butler K, Pritchard KI (1995) Development of a weight management program in women with newly diagnosed locoregional breast cancer. 11th international congress of psychosomatic obstetrics and gynaecology, Basel, Switzerland
Ryu SY, Kim CB, Nam CM, Park JK, Kim KS, Park J et al (2001) Is body mass index the prognostic factor in breast cancer?: a meta-analysis. J Korean Med Sci 16(5):610–614
Fleming TR, DeMets DL (1996) Surrogate end points in clinical trials: are we being misled? Ann Intern Med 125(7):605–613
Pollock BH (2009) Cheaper, faster, better: chasing the elusive clinical trial end point. J Clin Oncol 27(17):2747–2748
Sridhara R, Johnson JR, Justice R, Keegan P, Chakravarty A, Pazdur R (2010) Review of oncology, hematology drug product approvals at the US Food, Drug Administration between July 2005, December 2007. J Natl Cancer Inst 102(4):230–243
Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP (2003) Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst 95(19):1467–1476
Majed B, Moreau T, Asselain B (2009) Overweight, obesity and breast cancer prognosis: optimal body size indicator cut-points. Breast Cancer Res Treat 115(1):193–203
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 27(7414):557–560
van Houwelingen HC, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21(4):589–624
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2):455–463
StataCorp (2009) Stata/SE 10.1 for Windows. Stata Corporation, College Station, Texas, USA
Reeves KW, Faulkner K, Modugno F, Hillier TA, Bauer DC, Ensrud KE et al (2007) Body mass index and mortality among older breast cancer survivors in the study of osteoporotic fractures. Cancer Epidemiol Biomarkers Prev 16(7):1468–1473
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y et al (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20(1):42–51
Katoh A, Watzlaf VJ, D’Amico F (1994) An examination of obesity and breast cancer survival in post-menopausal women. Br J Cancer 70(5):928–933
Vitolins MZ, Kimmick GG, Douglas Case L (2008) BMI influences prognosis following surgery and adjuvant chemotherapy for lymph node positive breast cancer. Breast J 14(4):357–365
Ioannidis J, Lau J (2001) Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses. Proc Natl Acad Sci USA 98(3):831–836
Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG et al (2006) Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 368(9536):666–678
Borugian MJ, Sheps SB, Kim-Sing C, Olivotto IA, Van Patten C, Dunn BP et al (2003) Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol 158(10):963–968
Abrahamson PE, Gammon MD, Lund MJ, Flagg EW, Porter PL, Stevens J et al (2006) General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev 15(10):1871–1877
Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M et al (2008) Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer 123(9):2188–2194
Tao MH, Shu XO, Zhi XR, Gao YT, Zheng W (2006) Association of overweight with breast cancer survival. Am J Epidemiol 163(2):101–107
Madarnas Y, Sawka CA, Franssen E, Bjarnason GA (2001) Are medical oncologists biased in their treatment of the large woman with breast cancer? Breast Cancer Res Treat 66(2):123–133
Srokowski TP, Fang S, Hortobagyi GN, Giordano SH (2009) Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol 27(13):2170–2176
Egger M, Schneider M, Davey Smith G (1998) Spurious precision? Meta-analysis of observational studies. BMJ 316(7125):140–144
Wee CC, McCarthy EP, Davis RB, Phillips RS (2004) Obesity and breast cancer screening. J Gen Intern Med 19(4):324–331
Dignam JJ, Mamounas EP (2004) Obesity and breast cancer prognosis: an expanding body of evidence. Ann Oncol 15(6):850–851
Li CI, Daling JR, Porter PL, Tang MT, Malone KE (2009) Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor-positive invasive breast cancer. J Clin Oncol 27(32):5312–5318
de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M et al (2010) The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02–98 trial. Breast Cancer Res Treat 119(1):145–153
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4(8):579–591
Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92(4):720–729
Somasundar P, McFadden DW, Hileman SM, Vona-Davis L (2004) Leptin is a growth factor in cancer. J Surg Res 116(2):337–349
Hu X, Juneja SC, Maihle NJ, Cleary MP (2002) Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94(22):1704–1711
Tchernof A, Despres JP (2000) Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res 2(11–12):526–536
Lee CW, Norris B, Moravan V, Olivotto I (2003) Does body mass index influence the effectiveness of adjuvant tamoxifen in postmenopausal women with early stage breast cancer? Breast Cancer Res Treat 82(Suppl 1):(Abstr 309)
Griggs JJ, Sorbero ME, Lyman GH (2005) Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 165(11):1267–1273
Hunter RJ, Navo MA, Thaker PH, Bodurka DC, Wolf JK, Smith JA (2009) Dosing chemotherapy in obese patients: actual versus assigned body surface area (BSA). Cancer Treat Rev 35(1):69–78
Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC et al (1996) Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol 14(11):3000–3008
Kotlyar M, Carson SW (1999) Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther 37(1):8–19
Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL et al (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 300(23):2754–2764
Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK et al (2006) Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 98(24):1767–1776
Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A et al (2009) Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst 101(9):630–643
Barnett GC, Shah M, Redman K, Easton DF, Ponder BAJ, Pharoah PDP (2008) Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol 26(20):3310–3316
Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU (1994) Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med 120(1):18–25
Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam CM et al (2004) Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol 15(6):875–884
Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B et al (2008) Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 19(10):1319–1328
Carmichael AR, Bendall S, Lockerbie L, Prescott RJ, Bates T (2004) Does obesity compromise survival in women with breast cancer? Breast 13(2):93–96
Chang S, Alderfer JR, Asmar L, Buzdar AU (2000) Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat 64(2):157–163
Cleveland RJ, Eng SM, Abrahamson PE, Britton JA, Teitelbaum SL, Neugut AI et al (2007) Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev 16(9):1803–1811
Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN et al (2008) Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res 14(6):1718–1725
den Tonkelaar I, de Waard F, Seidell JC, Fracheboud J (1995) Obesity and subcutaneous fat patterning in relation to survival of postmenopausal breast cancer patients participating in the DOM-project. Breast Cancer Res Treat 34(2):129–137
Dignam JJ, Wieand K, Johnson KA, Raich P, Anderson SJ, Somkin C et al (2006) Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat 97(3):245–254
Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB et al (1994) Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA 272(12):947–954
Enger SM, Bernstein L (2004) Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer 90(11):2138–2141
Ewertz M, Gillanders S, Meyer L, Zedeler K (1991) Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer 49(4):526–530
Greenberg ER, Vessey MP, McPherson K, Doll R, Yeates D (1985) Body size and survival in premenopausal breast cancer. Br J Cancer 51(5):691–697
Hebert JR, Hurley TG, Ma Y (1998) The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat 51(1):17–28
Holmberg L, Lund E, Bergstrom R, Adami HO, Meirik O (1994) Oral contraceptives and prognosis in breast cancer: effects of duration, latency, recency, age at first use and relation to parity and body mass index in young women with breast cancer. Eur J Cancer 30A(3):351–354
Jain M, Miller AB (1994) Pre-morbid body size and the prognosis of women with breast cancer. Int J Cancer 59(3):363–368
Kroenke CH, Chen WY, Rosner B, Holmes MD (2005) Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23(7):1370–1378
Labidi SI, Mrad K, Mezlini A, Ouarda MA, Combes JD, Ben Abdallah M et al (2008) Inflammatory breast cancer in Tunisia in the era of multimodality therapy. Ann Oncol 19(3):473–480
Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M et al (2008) Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol 26(25):4072–4077
Loi S, Milne RL, Friedlander ML, McCredie MRE, Giles GG, Hopper JL et al (2005) Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 14(7):1686–1691
Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B (2008) Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat 111(2):329–342
Mason BH, Holdaway IM, Stewart AW, Neave LM, Kay RG (1990) Season of tumour detection influences factors predicting survival of patients with breast cancer. Breast Cancer Res Treat 15(1):27–37
Mohle-Boetani JC, Grosser S, Whittemore AS, Malec M, Kampert JB, Paffenbarger RS Jr (1988) Body size, reproductive factors, and breast cancer survival. Prev Med 17(5):634–642
Moon HG, Han W, Noh DY (2009) Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 27(35):5899–5905
Newman SC, Lees AW, Jenkins HJ (1997) The effect of body mass index and oestrogen receptor level on survival of breast cancer patients. Int J Epidemiol 26(3):484–490
Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ et al (2009) Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev 18(5):1403–1409
Petrelli JM, Calle EE, Rodriguez C, Thun MJ (2002) Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control 13(4):325–332
Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L et al (2007) Greater survival after breast cancer in physically active women with high vegetable–fruit intake regardless of obesity. J Clin Oncol 25(17):2345–2351
Reeves GK, Patterson J, Vessey MP, Yeates D, Jones L (2000) Hormonal and other factors in relation to survival among breast cancer patients. Int J Cancer 89(3):293–299
Rosenberg L, Czene K, Hall P (2009) Obesity and poor breast cancer prognosis: an illusion because of hormone replacement therapy? Br J Cancer 100(9):1486–1491
Saxe GA, Rock CL, Wicha MS, Schottenfeld D (1999) Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat 53(3):241–253
Vatten LJ, Foss OP, Kvinnsland S (1991) Overall survival of breast cancer patients in relation to preclinically determined total serum cholesterol, body mass index, height and cigarette smoking: a population-based study. Eur J Cancer 27(5):641–646
Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA (2005) Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 14(8):2009–2014
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors contributed to the design of the study and data extraction. MP performed the meta-analyses. All authors contributed to writing the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Protani, M., Coory, M. & Martin, J.H. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 123, 627–635 (2010). https://doi.org/10.1007/s10549-010-0990-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0990-0