Abstract
The relationship between adiposity and breast cancer risk and prognosis is complex, with associations that differ depending on when body size is assessed (e.g., pre- vs. postmenopausal obesity) and when breast cancer is diagnosed (i.e., pre- vs. postmenopausal disease). Further, the impact of obesity on risk differs by tumor hormone receptor status (e.g., estrogen (ER) and progesterone (PR) receptor) and, among postmenopausal women, use of exogenous hormones (i.e., hormone replacement therapy (HRT)). In the context of these complexities, this review focuses on associations between childhood and adolescent adiposity, general adiposity, weight changes (i.e., loss and gain), abdominal adiposity, and breast cancer risk and survival. Finally, we discuss potential mechanisms linking adiposity to breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adiposity
- Body fatness
- BMI
- Waist circumference
- Waist hip ratio
- Weight change
- Somatotype
- Breast cancer incidence
- Breast cancer survival
- Hormone receptor
- Estrogen receptor
- Progesterone receptor
1 Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide (1.67 million new cases in 2012) and a leading cause of cancer mortality [1]. Age, age at menarche, first full-term pregnancy and menopause, parity, use of hormone replacement therapy (HRT), alcohol consumption, and postmenopausal obesity are among the established risk factors for disease. Obesity is a modifiable risk factor, but the relationship between adiposity and breast cancer risk is complex, and differs by tumor characteristics, menopausal status, and exogenous hormone use. We describe the association between body size, from childhood through post-menopause, and breast cancer risk and survival, and discuss the mechanisms linking adiposity to breast cancer risk.
2 Body Size Across the Life Course and Breast Cancer Risk
Higher adiposity has opposing effects on breast cancer risk, depending on a woman’s menopausal status. Among premenopausal women, higher adiposity, in childhood or during adult life, is associated with decreased risk of breast cancer, while among postmenopausal women obesity increases risk [2, 3]. Interestingly, greater adiposity during childhood and adolescence is inversely associated with both hormone receptor-positive disease (i.e., tumors expressing estrogen (ER) and progesterone (PR) receptors) and receptor-negative disease. In contrast, obesity in adulthood has been found to be associated predominantly with ER+/PR+ breast cancer, and, in the case of postmenopausal obesity, with increased risk of ER+/PR+ disease only among women not using HRT. Of note, while the relationship between body size in pre- and postmenopausal women and subsequent breast cancer risk has been extensively investigated, it still remains somewhat uncertain until what point in the premenopausal period obesity is protective, and, similarly, from what point in the postmenopausal period obesity begins to show a deleterious effect.
2.1 Childhood Body Size
In several prospective cohort studies, larger recalled body size in childhood and adolescence has been found to be inversely associated with subsequent breast cancer risk, independently of current body mass index (BMI, kg/m2) [3]. In the largest and most comprehensive study to date, Baer et al. investigated recalled childhood body size (ages 5 and 10 years; Fig. 1) and breast cancer risk among 188,860 women in the Nurses’ Health Studies [4]. Women reporting larger body size in childhood had lower risk of breast cancer regardless of menopausal status at diagnosis and tumor hormone receptor status (e.g., RRs per 1-unit higher body size at age 5, premenopausal: 0.94 [0.90–0.97], postmenopausal: 0.93 [0.91–0.95]; RRs per 1-unit higher body size in adolescence: ER+/PR+: 0.92 [0.89–0.95], ER−/PR−: 0.85 [0.80–0.90]); results were essentially unchanged after controlling for BMI at age 18 or BMI at baseline recruitment (e.g., RRs per 1-unit higher body size at age 5, not adjusted for BMI at older ages RR: 0.93 (0.92–0.95), adjusted for current BMI, RR: 0.93 [0.91–0.95]), adjusted for BMI at age 18, RR: 0.95 [0.94–0.97]. Similar inverse associations have been observed in other prospective studies [3].
Somatotype pictogram to assess body shape at different ages. Reproduced from Stunkard et al. [94]
2.2 General Adiposity in Adulthood and Breast Cancer
2.2.1 BMI and Premenopausal Breast Cancer Risk
Consistent with larger childhood body size, obesity in adulthood is associated with lower breast cancer risk in premenopausal women [3], though this inverse association is observed only for hormone receptor-positive disease. For example, in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, a 5 kg/m2 higher BMI was associated with a 20 % decreased risk of ER+/PR+ breast cancer (RR: 0.80 [0.69–0.93]) among premenopausal women (age ≤ 49 years), but showed no association with ER−/PR− disease (RR: 0.90 [0.71–1.13], p heterogeneity = 0.44) [5]. Similar observations were made in the Nurses’ Health Study II (RRs, per 1-unit higher BMI, ER+: 0.91 [0.84–0.99]; ER−: 1.03 [0.91–1.15]) [6].
2.2.2 Premenopausal BMI and Postmenopausal Breast Cancer Risk
The protective effect of larger body size in early adulthood persists into the postmenopausal period [4, 7–10]. In the most recent prospective investigation, Fagherazzi et al. [7] observed a 14 % decrease in risk of postmenopausal breast cancer for women reporting larger body sizes at age 20–25 years (RR, ≥4 vs. 1 on pictogram: 0.86 [0.74–1.00]). These results are in line with findings from another large cohort using the somatotype pictogram [4] and prospective investigations evaluating breast cancer risk and BMI at age 18 [8–10]. The protective effect of premenopausal BMI on postmenopausal breast cancer risk is likely strongest for ER+/PR+ tumors; however, risk differences by hormone receptor status are not well characterized.
Of note, Fagherazzi et al. observed no association between later premenopausal body size (ages 35–40 years) and postmenopausal breast cancer risk (RR, ≥4 vs. 1 on pictogram: 0.98 [0.82–1.18]). Further data in prospective settings are required to clarify until what point larger premenopausal body size is inversely associated with postmenopausal breast cancer risk.
2.2.3 Postmenopausal BMI and Breast Cancer Risk
In contrast to the inverse association between larger early adult body size and breast cancer risk, higher postmenopausal BMI increases risk, though this association appears to be limited to ER+/PR+ disease among women not using HRT [5, 8, 10–12]. This differential effect by HRT use has been consistently observed across studies, with few exceptions [13]. In the EPIC cohort, risk was 28 % higher for every 5 kg/m2 higher BMI in women who had never used HRT, with no association observed among current HRT users (RRs ER+/PR+, HRT never users: 1.28 [1.18–1.38]; past users: 1.47 [1.26–1.72]; current users: 1.01 [0.91–1.12]). Further, in analyses cross-classified by both BMI and HRT use, higher BMI was associated with increased risk in a stepwise fashion among both never and past-HRT users, while risk of ER+/PR+ disease was more than twofold higher among current HRT users, regardless of BMI (all relative to HRT never users with BMI ≤ 22.5 kg/m2; Fig. 2).
BMI and risk of ER+ PR+ and ER− PR− tumors across HRT use categories, among postmenopausal women. HRT never users in BMI tertile1 serve as the reference category. BMI tertile cutpoints: T1: ≤22.5 kg/m2; T2: 22.6–25.8 kg/m2; T3: ≥25.9 kg/m2 [5]
Fat-derived estrogens are widely considered the principal mechanism through which postmenopausal obesity impacts breast cancer risk. Adipose tissue expresses high levels of aromatase and represents a major source of endogenous estrogens in the postmenopausal period. In turn, postmenopausal endogenous estrogens, as well as exogenous hormones (i.e., HRT), increase breast cancer risk [14–16]. It is plausible that fat-derived endogenous estrogens are only etiologically relevant for breast cancer in the context of the lower estrogen environment experienced by postmenopausal women not using HRT, and that obesity, and its actions via endogenous estrogens, is not etiologically relevant in the context of exogenous hormone use.
On balance, data to date do not support a relationship between BMI and ER−/PR− disease [5, 11, 13, 17]. In the EPIC cohort, BMI > 25.9 versus ≤22.5 kg/m2 was associated with an increased risk of ER−/PR− disease, but only among never HRT users (RR: 1.59 [1.08–2.34]) [5]. However, the impact of BMI on postmenopausal ER−/PR− disease is not fully understood, with few other studies examining risk of ER−/PR− disease by HRT use [12]. Possibly, some breast tumors presenting as receptor-negative tumors at diagnosis were still receptor-positive and hormone–response reflects earlier stages of development. There are limited prior data on obesity by other tumor classifications based on molecular phenotype [18], though existing data suggest obesity impacts androgen receptor (AR)-positive and androgen receptor-negative tumors similarly [19].
To date, it remains unclear precisely when in the postmenopausal period higher BMI begins to increase risk. Data from the EPIC cohort suggest BMI may become a relevant risk factor especially for breast cancer diagnosed at a more advanced, postmenopausal age. [5] evaluated risk in 5-year age bands (e.g., women 55–59 or 60–64 for both BMI assessment and diagnosis) to address the issue of timing (Fig. 3). In this investigation, higher BMI was significantly related to higher risk of ER+/PR+ disease among women aged 65 or older, but not significantly related to risk among women earlier in menopause (i.e., ages 55–64) [5].
Hazard ratios of ER+ PR+ and ER− PR− tumors for increases in BMI across age bands. All models are for a 5 kg/m2 increase in BMI and were stratified by age at recruitment and study center [5]
These results suggest that it may take several years after the onset of menopause before the inverse association of excess adiposity with breast cancer risk among premenopausal women is offset, and gradually turned into a positive association. The findings by [5]. are in agreement with a pooled analysis of prospective cohort data by van den Brandt et al. which suggested postmenopausal BMI increases breast cancer risk after age 65 [20], but contrast with findings in the Women’s Health Initiative which suggested stronger positive associations among younger postmenopausal women [10].
3 BMI and Breast Cancer-Specific Survival
Higher BMI is associated with poorer breast cancer-specific survival. A recent meta-analysis showed that overweight (25–29.99 kg/m2) and obese (≥30 kg/m2) women were at higher risk of breast cancer-specific mortality than normal weight women (18.5–24.99 kg/m2) (RRs, overweight: 1.11 [1.06–1.17]; obese: 1.35 [1.24–1.47]), whether BMI was assessed before diagnosis, within one year after diagnosis, and at least 12 months after diagnosis [21]. In contrast to investigations on BMI and breast cancer risk, studies on BMI and breast cancer survival generally do not suggest strong heterogeneity by menopausal status or tumor hormone receptor status [21].
4 Weight Changes
Independently of BMI, adult weight gain has been found to increase breast cancer risk in many epidemiologic studies. In addition to the possible independent effect of weight gain on risk, this observation underscores the importance of maintaining a healthy weight, or minimizing weight gain [22].
4.1 Weight Gain and Risk of Premenopausal Breast Cancer
Data with respect to weight gain and premenopausal breast cancer risk are sparse and results are inconsistent. In a recent meta-analysis of three prospective studies, weight gain was not associated with premenopausal breast cancer risk [22]. In contrast, weight gain was associated with increased risk of premenopausal breast cancer in two subsequent evaluations in the EPIC cohort and the Nurses’ Health Study (e.g., EPIC: RR, ≥0.83 kg/year weight gain vs. stable weight: 1.37 [1.02–1.85]) [23], and these associations persisted after adjustments for current [23] or average [24] BMI. In the Nurses’ Health Study, weight gain was only associated with PR− tumors (i.e., ER+/PR−, ER−/PR−) and not with ER+/PR+ disease [24]; in the EPIC study, analyses by hormone receptor status among the premenopausal women were not reported.
Both studies investigated whether the impact of weight gain differed by baseline BMI at the time of recruitment into the cohort, with contradictory results. In EPIC, results were similar regardless of baseline BMI (RR ≥ 0.83 kg weight gain vs. stable weight, BMI < 25 kg/m2: 1.23 [0.87–1.72]; BMI ≥ 25 kg/m2: 1.42 [0.76–2.65]; p for heterogeneity >0.05) [23]. In the Nurses’ Health Study, by contrast, weight gain was related to higher risk only among women leaner at baseline (RR per 11 kg weight gain, BMI < 25 kg/m2: 1.65 [1.35–2.02]; BMI ≥ 25 kg/m2: 1.02 [0.83–1.25]; p for heterogeneity <0.001) [24].
4.2 Weight Gain and Postmenopausal Breast Cancer Risk
As with overall postmenopausal obesity, adult weight gain is associated with postmenopausal breast cancer risk among women not using HRT (RRs per 5 kg increase, HRT nonusers: 1.11 [1.08–1.13]; HRT users: 1.01 [0.99–1.02]) [22] and according to recent meta-analyses appears to be more strongly associated with ER+/PR+ disease (RR: 2.33 [2.05–2.60]) than with ER−/PR− disease (RR: 1.34 [1.06–1.63]) [25]. However, it should be noted that two subsequent prospective analyses observed similar results regardless of HRT use and for both ER+/PR+ and ER−/PR− tumors [23, 24]. In most prospective studies, weight gain was associated with postmenopausal breast cancer risk irrespective of body size at baseline [24, 26–29], though three large studies, including recent analyses in the EPIC cohort and Women’s Health Initiative, reported stronger associations among women leaner at baseline [13, 23, 30].
4.3 Weight Loss and Breast Cancer Risk
Data to date do not support a relationship between weight loss and breast cancer risk, with most investigations observing no association [13, 23, 24, 26, 28, 31], or risk reductions only in subgroups of postmenopausal women (i.e., >10 kg weight loss after menopause and only in never HRT users (RR: 0.43 [0.21–0.86] [30]); >1 kg weight loss between ages 45–55 years (RR: 0.5 [0.3–0.9] [29]).
4.4 Weight Change and Breast Cancer-Specific Survival
Weight gain after breast cancer diagnosis is common [32, 33], but whether weight gain impacts breast cancer-specific survival is not fully understood. Weight gain of >10 % body weight was suggestively associated with increased risk of breast cancer-specific mortality in a recent meta-analysis (RR: 1.17[1.00–1.38]) [32]; it remains to be determined whether the effect of weight gain differs depending on baseline BMI. Clinical trials are underway to assess the effect of post-diagnosis weight loss on disease-free survival [34].
5 Visceral Adiposity: Waist Circumference/Waist-Hip Ratio
While imaging studies suggest that BMI is a valid parameter of general adiposity, it does not reflect the visceral fat compartment well [35]. Waist circumference (WC) and waist–hip ratio (WHR) provide measures of body fat distribution and are proxy measures of abdominal subcutaneous fat and of visceral adiposity. Independent of BMI, higher WC and WHR increase risk of several chronic diseases including other cancers, cardiovascular disease, and all-cause mortality [36–38] and accumulating evidence suggests WC and WHR may be risk factors for breast cancer [2, 39]. Whether the relationship between WC and WHR and breast cancer risk differs by tumor hormone receptor subtype has not been thoroughly explored in prior studies, though limited data to date suggest heterogeneity by tumor subtypes.
5.1 WC/WHR and Risk of Premenopausal Breast Cancer
On balance, data support a positive association between WHR and premenopausal breast cancer risk. Each 0.1 unit higher WHR was related to 8 % higher risk of premenopausal breast cancer in a recent meta-analysis of 12 studies [40]. Few studies have investigated WC and WHR and premenopausal breast cancer by hormone receptor subtype, and results are conflicting. After adjusting for BMI, WC and hip circumference (HC) were not associated with premenopausal ER+/PR+ or ER−/PR− breast cancer in the EPIC cohort [5], whereas higher WC, HC, and WHR was related to higher risk of ER−breast cancer risk in the Nurses’ Health Study II (e.g., WC ≥ 87 cm vs. <69 cm, RR: 2.75 [1.15–6.54], multivariable models including adjustment for BMI) [41]. Consistent with the results from EPIC, in the Nurses’ Health Study no association was observed for premenopausal ER+/PR+ disease.
5.2 WC/WHR and Risk of Postmenopausal Breast Cancer
Prospective investigations suggest higher WC and WHR increase postmenopausal breast cancer risk. A meta-analysis restricted to cohort studies observed a 5 % increase in risk with each 8 cm higher WC (RR: 1.05 [1.00–1.10]) and a 19 % increase in risk of with each 0.1 unit higher WHR (RR: 1.19 [1.10–1.28]) [2]. However, data to date are not consistent [5, 9, 10, 26, 42–46].
Investigations on the relationship between WHR and WC with postmenopausal breast cancer classified by hormone receptor subtype are limited [5, 44, 45, 47]. In the largest study to date, from the EPIC cohort, larger WC was not associated with ER+/PR+ or ER−/PR− breast cancer risk in BMI-adjusted models (ER+/PR+, n = 3586; ER−/PR−, n = 1021); WHR was not included in this investigation [5]. In contrast, higher WC was related to higher risk of ER+ breast cancer, but not triple negative disease (i.e., ER−/PR−/HER2−), in the Women’s Health Initiative (RRs WC > 95 vs. <76 cm, ER+: 1.34 [1.09–1.64] n = 2610 cases; triple negative: 0.66 [0.37–1.20], n = 307 cases; after adjustment for BMI); WHR was not associated with either subtype [47]. Other prospective studies [44, 45] observed no association between WHR and WC and postmenopausal breast cancer by ER status. While there is some evidence that the association between WC and breast cancer risk may differ by HRT use [5, 47], this is not well characterized.
5.3 WC and WHR and Breast Cancer-Specific Survival
The impact of abdominal adiposity on survival after a breast cancer diagnosis is not established; however, data to date do not support an association between WC and breast cancer-specific mortality [48, 49], and the evidence for WHR is weak (e.g., RR, ≥0.867 vs. <0.763: 1.27 (0.98–1.65), ptrend = 0.04; n = 11,351 breast cancer cases) [48].
Body fat distribution versus general adiposity: Limitations of anthropometric exposure assessments
While general adiposity is well reflected by BMI, imaging data show that this is not the case for visceral adiposity (Fig. 4) [35, 50]. The use of alternative anthropometric parameters more directly reflecting visceral adiposity (e.g., WC and WHR) may not fully resolve this issue; however, anthropometric measures of adiposity in most large prospective investigations are limited to BMI, WC, and WHR.
Individuals with similar age, gender, BMI and same % body fat. Reproduced from Thomas et al. [95]
An additional pitfall with the use of anthropometric parameters, especially in studies on breast cancer survival, is that muscle mass is not sufficiently captured [51]. It is plausible that sarcopenic adiposity, characterized by reduced muscle mass and abdominal fat accumulation, rather than overall adiposity, is related to worse prognosis in breast cancer patients [51, 52]. Although there is a lack of prospective patient trials, studies on other cancer types clearly indicate that both visceral fat accumulation and sarcopenic adiposity may be much more relevant for cancer progression than general adiposity [51]. Imaging studies to assess the effects of visceral and sarcopenic adiposity in breast cancer patients, as well as in the general population, are needed to achieve a better understanding of the role of different types of adiposity in cancer development and to facilitate a more precise quantification of risk estimates.
6 Potential Biologic Mechanisms
6.1 Endogenous Sex Hormones
Numerous observations document the relationships between breast cancer development and endogenous sex hormone metabolism. Cancer registry data worldwide show that before the average age at menopause of about 50 years breast cancer incidence rates increase more strongly with age than after the age of 50 [53]. Also, an older age at menopause, indicative of a longer cumulative period of premenopausal steroid hormone levels, is associated with increased risk of breast cancer; this is more strongly the case for ER+ than for ER− tumors [54]. These observations point to tumor-enhancing effects of ovarian sex hormones, notably estradiol, and possibly progesterone. Anti-estrogenic pharmacologic treatments with selective estrogen receptor modulators (SERMs) [55] or aromatase inhibitors (AIs) reduce the risk of ER+ breast tumor recurrences among cancer patients, and have also been shown to reduce first occurrence of breast tumors among high-risk women [56]. Conversely, the postmenopausal use of hormone replacements—especially combined estrogen-plus-progestin regimens, but to a lesser extent also regimens based on estrogen only—increases the risk of breast cancer, and this increase in risk consistently has been found to be stronger among women who are comparatively leaner and have a lower endogenous synthesis of estrogens [5, 47, 57].
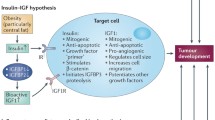
Especially in postmenopausal women, the impact of adiposity on concentrations of circulating sex steroid hormones likely represents the main mechanism linking adiposity and breast cancer risk. Aromatase is a key enzyme in the synthesis and metabolism of sex steroid hormones (Fig. 5).
Aromatase activity is well known to be upregulated in the context of obesity, and adipose tissue is a major source of estrogens in postmenopausal women [58, 59]. Adiposity is consistently associated with higher concentrations of estradiol and estrone (e.g., estradiol, BMI ≥ 30 kg/m2: 54.9 pmol/L; BMI < 22.5 kg/m2: 30.0 pmol/L; [60]) in postmenopausal women [60–64]. Beyond total estrogen concentrations, obesity is associated with lower sex hormone-binding globulin (SHBG) (e.g., BMI ≥ 30 kg/m2: 29.6 nmol/L; BMI < 22.5 kg/m2: 52.8 nmol/L) [60], resulting in higher concentrations of bioavailable estradiol, as well as bioavailable testosterone, unbound to SHBG.
Prospective epidemiologic investigations have consistently documented direct associations between circulating endogenous estrogens (i.e., estradiol, estrone) and postmenopausal breast cancer risk [14–16]. In a pooled analysis of eight cohort studies (total of 624 incident cases of breast cancer), the Endogenous Hormones and Breast Cancer Collaborative Group evaluated the mediation effect of postmenopausal sex steroid hormones on the association between BMI and postmenopausal breast cancer. In this analysis, which included only women not using HRT at the time of blood donation, a 5 kg/m2 unit higher BMI was associated with a 18 % increase in breast cancer risk (RR: 1.18 [1.06–1.16]) before adjusting for hormone concentrations. This increase in risk was attenuated and no longer statistically significant after adjusting for estrogens, with the strongest attenuations observed for estradiol and free estradiol (e.g., RRs, after estradiol adjustment: RR: 1.07 [0.95–1.20]; after free estradiol adjustment: RR: 1.02 [0.89–1.17]) [60]. Very similar findings were noted in a study within the EPIC cohort (613 incident cases of breast cancer), again in support of a mediation effect of estradiol and free estradiol on the association between other anthropometric measures and breast cancer risk [61].
With regard to premenopausal breast cancer, the mechanisms underlying the inverse associations between childhood and adult life overall adiposity and risk are less well understood. One postulated mechanism [65] refers to reductions in ovarian progesterone synthesis, especially among more obese women, as consequence of obesity-induced ovarian hyperandrogenism [66]. This hypothesis gains some support from epidemiologic observations that, among postmenopausal women, use of combined estrogen-plus-progestin HRT induces a stronger increase in breast cancer risk than the use of estrogen-only formulations [57]. However, prospective studies have not observed significant associations between higher serum progesterone levels and increased breast cancer risk among premenopausal women [67, 68], although in the same studies a significant reduction in progesterone levels was observed for women with BMI > 30 kg/m2 [67]. For estrogens, prospective studies among premenopausal women have shown a positive association between higher circulating estrogen levels and breast cancer risk [67, 68], as well as a weak inverse association between BMI and circulating total, but not bioavailable (calculated free, estradiol concentrations) [67].
Besides estrogens, prospective studies have shown increases in breast cancer risk among both pre- and postmenopausal women who have higher blood concentrations of androgenic steroid hormones, including androstenedione and testosterone [67–69]. Like estrogens, blood levels of androgens also show positive associations with BMI, among both pre- and postmenopausal women.
6.2 Insulin
Obesity and visceral adiposity are associated with insulin resistance, resulting in hyperinsulinemia and the metabolic syndrome [70], and insulin has been implicated in breast cancer due to its mitogenic and anti-apoptotic effects as a growth factor [70–73]. High insulin levels, furthermore, lead to increased levels of bioactive (free) IGF-I due to downregulation of IGF-binding proteins −1 and −2, and also lead to increased blood levels of bioavailable testosterone and estradiol by downregulating the hepatic synthesis and blood concentrations of SHBG. Finally, in at least a subgroup of susceptible women, hyperinsulinemia can cause overstimulation of ovarian androgen synthesis, which in a small percentage (3–5 %) of premenopausal women can result in anovulatory menstrual cycles coupled with impaired progesterone synthesis (polycystic ovary syndrome; PCOS) [66, 74].
The above various observations have led to speculations that, in addition to alterations in estrogen metabolism, hyperinsulinemia could provide a major physiologic link between excess adiposity and breast cancer development [73, 75]. Prospective studies, however, have yielded somewhat conflicting results regarding the relationship between insulin levels and risk of breast cancer [76–80]. A study within the EPIC cohort (1141 cases, 2204 matched controls), which included only women who did not use any exogenous hormones (i.e., oral contraceptives; HRT) at the time of blood donation, found a variable, age-dependent association of breast cancer risk with C-peptide, a marker for pancreatic insulin secretion, and breast cancer risk [78]. Elevated serum C-peptide levels were associated with a reduced risk of breast cancer diagnosed up to the age of 50 years, but with an increase of breast cancer risk among women above 60 years of age, mimicking the age-dependent association of breast cancer risk with BMI [78]. A study within the Women’s Health Initiative cohort (835 cases, 816 controls) showed a positive association of serum insulin concentrations with breast cancer risk among postmenopausal women not using HRT (top vs. bottom quartile, RR: 2.48 [1.38–4.47]), but no association among HRT users (e.g., estrogen and progesterone HRT, RR: 1.15 [0.34–3.84]), again mimicking associations observed between breast cancer risk and BMI [76]. Further, within this subgroup, insulin was most strongly associated with ER+ disease (top vs. bottom quartile, RR for ER+: 3.23 [1.62–6.49], ptrend = 0.001; ER−: 1.37 [0.57–3.25], ptrend = 0.99). Finally, a study within the Nurses’ Health Studies (1084 cases, 1785 controls) showed an approximately 50 % increase in risk of invasive breast cancer for the top versus bottom quartile of serum C-peptide; associations were similar after adjustment for free estradiol and sex hormone-binding globulin [79]. In this latter study, however, the association was stronger for ER− disease (RR: 2.0 [1.2–3.6]) than ER+ disease (RR: 1.4 [1.0–2.0]), contrary to the associations observed in the WHI cohort. Furthermore, in contrast to data from the EPIC cohort, associations were similar for breast cancers diagnosed before and after menopause (top vs. bottom quartile, RR, postmenopausal women: 1.4 [0.79–2.5]: premenopausal women: 1.5 [1.1–2.0]). Taken together, while experimental evidence supports growth promoting effects of insulin on breast cancer cells [72], epidemiologic associations between serum insulin or C-peptide and breast cancer risk are inconsistent with regard to menopausal status, users and nonusers of HRT, and tumor ER status. Thus, at present the evidence for insulin as an independent physiologic link between adiposity and breast cancer development, further to estrogens, remains limited.
6.3 Insulin-like Growth Factor 1 (IGF-1)
IGF-I is a peptide with high structural homology to insulin, and plays a central role in regulating anabolic (growth) processes as a function of available energy and elementary substrates, particularly amino acids. IGF-I has well-documented effects on cell proliferation, differentiation, and apoptosis. Further, higher IGF-I may both increase sex steroid hormone synthesis and decrease SHBG concentrations, resulting in higher concentrations of circulating free estradiol and free testosterone [81].
Epidemiologic investigations support a role for IGF-I in breast cancer risk. A pooled analysis of nested case–control data from 17 prospective cohorts, including a total of 4790 incident cases of breast cancer and 9428 matched controls, showed a modest positive association of breast cancer risk with blood levels of IGF-I (top vs. bottom quintiles, RR: 1.28 [1.14–1.44], p < 0.0001). This association did not vary significantly by menopausal status at blood collection, but was restricted to ER+ (RR: 1.38 [1.14–1.68]). No association was seen for ER-negative tumors (RR: 0.80 [0.57–1.13]) (p for heterogeneity = 0.007) [82, 83]. A complementary study within the EPIC cohort (938 breast cancer cases and 1394 matched controls) observed similar findings (top vs. bottom quartiles, IGF-I and ER+ disease, RR: 1.41 [1.01–1.98]), and no association for ER− tumors [83]. These observations suggest synergism between IGF-I and estrogens in promoting breast tumor development—an interpretation that is in line with experimental findings [84]. Epidemiologic data, however, did not indicate any clear evidence for interaction between IGF-I and BMI, or between IGF-I and serum estrogen levels, among either post- or premenopausal women [82, 83].
Although the association of higher IGF-I with higher risk of ER-positive breast tumors is well documented, this association can provide only a marginal explanation for the observed epidemiologic relationships of breast cancer risk with adiposity. First of all, the association of IGF-I with breast cancer risk is relatively weak. More importantly, however, the relationship between BMI and serum total IGF-I concentration is nonlinear and shows modest increases in IGF-I with BMI increasing from less than 18 to about 26 kg/m2, followed by a progressive drop in IGF-I concentrations as BMI levels increase further [82, 83, 85]. A more direct, linear relationship may exist between BMI and serum levels of free IGF-I, a small fraction (1–2 %) of circulating IGF-I that is unbound to IGF-binding proteins, rather than with total IGF-I [81, 86], and one might anticipate a stronger association of free IGF-I with breast cancer risk. However, due to complexities associated with assays for free IGF-I only few prospective studies on the relationship of free IGF-I with breast cancer risk have been conducted so far, and did not document any clear association [76].
6.4 Chronic Inflammation
Obesity has been characterized as a condition of low-grade chronic inflammation [87]. To a large extent, the inflammation is the result of increased macrophage infiltration of adipose tissue. Adipocytes and macrophages synergize to increase the production of inflammatory mediators, including the pro-inflammatory cytokines including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [88]. The pro-inflammatory cytokines contribute to the development of obesity-related insulin resistance and hyperinsulinemia [89], and also play a key role in upregulating aromatase expression and activity [90].
Recent studies have shown that overweight and obesity-associated aromatase expression and activity (i.e., hormone alterations) correlate with inflammation in the breast tissue of women, and, additionally, that increased levels of cyclo-oxygenase-2 (COX2) and its product prostaglandin E2 (PGE2) contribute to elevated aromatase expression in inflamed breast tissue of obese women [91]. The inflammation was strongly related to the presence of crown-like structures, formed by macrophages around large, lipid-filled adipocytes which are present in the breasts of obese women, and the severity of breast inflammation, defined as the CLS-B (crown-like structure) index, was found to correlate with both body mass index and adipocyte size [92]. COX2 is also frequently expressed in breast tumors and correlates with tumor size and a worse disease-free interval. Observations suggest that PGE2 produced by the tumor directly increases aromatase activity in immediately surrounding adipose tissue [90]. The local production of estrogens, in turn, can further stimulate proliferation of the tumorous breast epithelium. Besides increasing aromatase activity and estrogen synthesis in breast tissue, the inflammatory response in adipose tissue also causes the systemic increase in circulating estrogen levels observed in obese women.
6.5 Summary: Biologic Mechanisms
Taken together, epidemiologic, clinical and experimental data indicate a dominant role of estrogens in the development of ER+ breast cancer, the breast tumor subtype most clearly associated with adiposity. Overweight and obesity may drive breast cancer development by increasing estrogen synthesis in adipose tissue. This increase is largely the result of an inflammatory response of adipose tissue, which is characterized by macrophage infiltration and the production of pro-inflammatory cytokines and PGE2. Recent findings suggest that the obesity–inflammation–aromatase axis is present in the breast tissue of most overweight and obese women, and is likely to contribute to the increased risk of hormone receptor-positive breast cancer and the worse prognosis of obese patients with breast cancer. In addition to increasing aromatase activity and estrogen synthesis in breast tissue, the inflammatory response in adipose tissue also causes the systemic increase in circulating estrogen levels observed in obese women. Increased circulating levels of insulin and bioactive IGF-I may contribute to breast tumor development by increasing bioavailable estrogen levels, or directly as growth factors (Fig. 6).
7 Summary and Conclusions
Higher adiposity has opposing effects on breast cancer risk, depending on the window of exposure (Table 1). Among premenopausal women, higher adiposity, in childhood or during adult life, is associated with decreased risk of both hormone receptor-positive and hormone receptor-negative disease. Conversely, postmenopausal obesity increases risk of hormone receptor-positive disease, though only among women not using HRT. It still remains uncertain until what point in the premenopausal period obesity is protective, and, similarly, from what point in the postmenopausal period onwards obesity begins showing a deleterious effect. Higher BMI is associated with poorer breast cancer-specific survival, irrespective of menopausal status or hormone receptor status of the tumor. As with postmenopausal BMI, weight gain increases risk of postmenopausal disease, though these effects may be limited to women not using HRT and appear to be predominantly impact risk of ER +/PR + disease. Data to date support a weak but significant positive association between WHR and pre- and postmenopausal breast cancer risk, with suggestive heterogeneity by tumor subtypes.
While the strengths of risk associations with respect to postmenopausal breast cancer risk may seem modest, it has been argued that approximately 10 % of all postmenopausal breast cancer cases worldwide, and up to 14 % of cases in North America and Europe, can be attributed to high BMI, given the high global prevalence of adiposity [93]. These observations underscore the relevance of maintaining a healthy weight, or minimizing weight gain, as a strategy for modulating breast cancer risk.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Bray F (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC cancer base No. 11. International Agency for Research on Cancer, Lyon. http://globocan.iarc.fr. Accessed 15 Dec 2015
World Cancer Research Fund/American Institute for Cancer Research (2007) Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR
Amadou A, Hainaut P, Romieu I (2013) Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol 2013:906495. doi:10.1155/2013/906495
Baer HJ, Tworoger SS, Hankinson SE, Willett WC (2010) Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol 171(11):1183–1194. doi:10.1093/aje/kwq045
Ritte R, Lukanova A, Berrino F, Dossus L, Tjonneland A, Olsen A, Overvad TF, Overvad K, Clavel-Chapelon F, Fournier A, Fagherazzi G, Rohrmann S, Teucher B, Boeing H, Aleksandrova K, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Quiros JR, Buckland G, Sanchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Sund M, Lenner P, Bueno-de-Mesquita B, van Gils CH, Peeters PH, Krum-Hansen S, Gram IT, Lund E, Khaw KT, Wareham N, Allen NE, Key TJ, Romieu I, Rinaldi S, Siddiq A, Cox D, Riboli E, Kaaks R (2012) Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res (BCR) 14(3):R76. doi:10.1186/bcr3186
Michels KB, Terry KL, Willett WC (2016) Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med 166:2395–2402
Fagherazzi G, Guillas G, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S (2013) Body shape throughout life and the risk for breast cancer at adulthood in the French E3 N cohort. Eur J Cancer Prev (The Official Journal European Cancer Prevention Organisation) 22(1):29–37. doi:10.1097/CEJ.0b013e328355ec04
Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC (1997) Dual effects of weight and weight gain on breast cancer risk. JAMA (The Journal of the American Medical Association) 278(17):1407–1411
Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L (2007) A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomark Prev 16(9):1795–1802. doi:10.1158/1055-9965.EPI-07-0336
Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson JA, Margolis KL, Muti PC (2002) Obesity, body size, and risk of postmenopausal breast cancer: the women’s health Initiative (United States). Cancer Causes Control 13(8):741–751
Renehan AG, Zwahlen M, Egger M (2015) Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 15(8):484–498. doi:10.1038/nrc3967
Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A (2014) Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 36:114–136. doi:10.1093/epirev/mxt010
Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF, Urrutia RP, Knudtson J, Anderson GL (2015) Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol 1(5):611–621. doi:10.1001/jamaoncol.2015.1546
Key T, Appleby P, Barnes I, Reeves G, Group EHaBCC (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94(8):606–616
James RE, Lukanova A, Dossus L, Becker S, Rinaldi S, Tjønneland A, Olsen A, Overvad K, Mesrine S, Engel P, Clavel-Chapelon F, Chang-Claude J, Vrieling A, Boeing H, Schütze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Krogh V, Panico S, Tumino R, Sacerdote C, Rodríguez L, Buckland G, Sánchez M-J, Amiano P, Ardanaz E, Bueno-de-Mesquita B, Ros MM, van Gils CH, Peeters PH, Khaw K-T, Wareham N, Key TJ, Allen NE, Romieu I, Siddiq A, Cox D, Riboli E, Kaaks R (2011) Postmenopausal serum sex steroids and risk of hormone receptor-positive and -negative breast cancer: a nested case-control study. Cancer Prev Res 4(10):1626–1635. doi:10.1158/1940-6207.CAPR-11-0090
Zhang X, Tworoger SS, Eliassen AH, Hankinson SE (2013) Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat 137(3):883–892. doi:10.1007/s10549-012-2391-z
Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, Fasching PA, Hein R, Spurdle AB, Blows F, Driver K, Flesch-Janys D, Heinz J, Sinn P, Vrieling A, Heikkinen T, Aittomaki K, Heikkila P, Blomqvist C, Lissowska J, Peplonska B, Chanock S, Figueroa J, Brinton L, Hall P, Czene K, Humphreys K, Darabi H, Liu J, Van ‘t Veer LJ, van Leeuwen FE, Andrulis IL, Glendon G, Knight JA, Mulligan AM, O’Malley FP, Weerasooriya N, John EM, Beckmann MW, Hartmann A, Weihbrecht SB, Wachter DL, Jud SM, Loehberg CR, Baglietto L, English DR, Giles GG, McLean CA, Severi G, Lambrechts D, Vandorpe T, Weltens C, Paridaens R, Smeets A, Neven P, Wildiers H, Wang X, Olson JE, Cafourek V, Fredericksen Z, Kosel M, Vachon C, Cramp HE, Connley D, Cross SS, Balasubramanian SP, Reed MW, Dork T, Bremer M, Meyer A, Karstens JH, Ay A, Park-Simon TW, Hillemanns P, Arias Perez JI, Menendez Rodriguez P, Zamora P, Benitez J, Ko YD, Fischer HP, Hamann U, Pesch B, Bruning T, Justenhoven C, Brauch H, Eccles DM, Tapper WJ, Gerty SM, Sawyer EJ, Tomlinson IP, Jones A, Kerin M, Miller N, McInerney N, Anton-Culver H, Ziogas A, Shen CY, Hsiung CN, Wu PE, Yang SL, Yu JC, Chen ST, Hsu GC, Haiman CA, Henderson BE, Le Marchand L, Kolonel LN, Lindblom A, Margolin S, Jakubowska A, Lubinski J, Huzarski T, Byrski T, Gorski B, Gronwald J, Hooning MJ, Hollestelle A, van den Ouweland AM, Jager A, Kriege M, Tilanus-Linthorst MM, Collee M, Wang-Gohrke S, Pylkas K, Jukkola-Vuorinen A, Mononen K, Grip M, Hirvikoski P, Winqvist R, Mannermaa A, Kosma VM, Kauppinen J, Kataja V, Auvinen P, Soini Y, Sironen R, Bojesen SE, Orsted DD, Kaur-Knudsen D, Flyger H, Nordestgaard BG, Holland H, Chenevix-Trench G, Manoukian S, Barile M, Radice P, Hankinson SE, Hunter DJ, Tamimi R, Sangrajrang S, Brennan P, McKay J, Odefrey F, Gaborieau V, Devilee P, Huijts PE, Tollenaar RA, Seynaeve C, Dite GS, Apicella C, Hopper JL, Hammet F, Tsimiklis H, Smith LD, Southey MC, Humphreys MK, Easton D, Pharoah P, Sherman ME, Garcia-Closas M (2011) Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst 103(3):250–263. doi:10.1093/jnci/djq526
Barnard ME, Boeke CE, Tamimi RM (2015) Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta 1856(1):73–85. doi:10.1016/j.bbcan.2015.06.002
Zhang X, Eliassen AH, Tamimi RM, Hazra A, Beck AH, Brown M, Collins LC, Rosner B, Hankinson SE (2015) Adult body size and physical activity in relation to risk of breast cancer according to tumor androgen receptor status. Cancer Epidemiol Biomark Prev 24(6):962–968. doi:10.1158/1055-9965.EPI-14-1429
van den Brandt PA (2000) Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 152(6):514–527. doi:10.1093/aje/152.6.514
Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals Oncol (Official Journal of the European Society for Medical Oncology/ESMO) 25(10):1901–1914. doi:10.1093/annonc/mdu042
Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL (2015) Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 107(2). doi:10.1093/jnci/djv088
Emaus MJ, van Gils CH, Bakker MF, Bisschop CN, Monninkhof EM, Bueno-de-Mesquita HB, Travier N, Berentzen TL, Overvad K, Tjonneland A, Romieu I, Rinaldi S, Chajes V, Gunter MJ, Clavel-Chapelon F, Fagherazzi G, Mesrine S, Chang-Claude J, Kaaks R, Boeing H, Aleksandrova K, Trichopoulou A, Naska A, Orfanos P, Palli D, Agnoli C, Tumino R, Vineis P, Mattiello A, Braaten T, Borch KB, Lund E, Menendez V, Sanchez MJ, Navarro C, Barricarte A, Amiano P, Sund M, Andersson A, Borgquist S, Olsson A, Khaw KT, Wareham N, Travis RC, Riboli E, Peeters PH, May AM (2014) Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study. Int J Cancer 135(12):2887–2899. doi:10.1002/ijc.28926
Rosner B, Eliassen AH, Toriola AT, Hankinson SE, Willett WC, Natarajan L, Colditz GA (2015) Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat 150(3):643–653. doi:10.1007/s10549-015-3344-0
Vrieling A, Buck K, Kaaks R, Chang-Claude J (2010) Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat 123(3):641–649. doi:10.1007/s10549-010-1116-4
Ahn J, Schatzkin A, Lacey JV, Albanes D, Ballard-Barbash R, Adams KF, Kipnis V, Mouw T, Hollenbeck AR, Leitzmann MF (2007) Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med 167(19):2091–2102. doi:10.1001/archinte.167.19.2091
Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE (2004) Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomark Prev 13(2):220–224
Lahmann PH, Schulz M, Hoffmann K, Boeing H, Tjonneland A, Olsen A, Overvad K, Key TJ, Allen NE, Khaw KT, Bingham S, Berglund G, Wirfalt E, Berrino F, Krogh V, Trichopoulou A, Lagiou P, Trichopoulos D, Kaaks R, Riboli E (2005) Long-term weight change and breast cancer risk: the European prospective investigation into cancer and nutrition (EPIC). Br J Cancer 93(5):582–589. doi:10.1038/sj.bjc.6602763
Radimer KL, Ballard-Barbash R, Miller JS, Fay MP, Schatzkin A, Troiano R, Kreger BE, Splansky GL (2004) Weight change and the risk of late-onset breast cancer in the original Framingham cohort. Nutr Cancer 49(1):7–13. doi:10.1207/s15327914nc4901_2
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE (2006) Adult weight change and risk of postmenopausal breast cancer. JAMA 296(2):193–201. doi:10.1001/jama.296.2.193
Alsaker MD, Janszky I, Opdahl S, Vatten LJ, Romundstad PR (2013) Weight change in adulthood and risk of postmenopausal breast cancer: the HUNT study of Norway. Br J Cancer 109(5):1310–1317. doi:10.1038/bjc.2013.403
Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML (2015) Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst 107(12). doi:10.1093/jnci/djv275
Makari-Judson G, Braun B, Jerry DJ, Mertens WC (2014) Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol 5(3):272–282. doi:10.5306/wjco.v5.i3.272
Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W (2014) Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev 15(9):749–768. doi:10.1111/obr.12190
Neamat-Allah J, Wald D, Husing A, Teucher B, Wendt A, Delorme S, Dinkel J, Vigl M, Bergmann MM, Feller S, Hierholzer J, Boeing H, Kaaks R (2014) Validation of anthropometric indices of adiposity against whole-body magnetic resonance imaging–a study within the German European prospective investigation into cancer and nutrition (EPIC) cohorts. PLoS ONE 9(3):e91586. doi:10.1371/journal.pone.0091586
Prineas RJ, Folsom AR, Kaye SA (1993) Central adiposity and increased risk of coronary artery disease mortality in older women. Ann Epidemiol 3(1):35–41. doi:10.1016/1047-2797(93)90007-Q
Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE (1998) Abdominal adiposity and coronary heart disease in women. JAMA (The Journal of the American Medical Association) 280(21):1843–1848
van Noord PAH, Seidell JC, Tonkelaar IDEN, Halewijn EABVAN, Ouwehand IJ (1990) The relationship between fat distribution and some chronic diseases in 11 825 women participating in the DOM-project. Int J Epidemiol 19(3):564
World Cancer Research Fund/American Institute for Cancer Research (2010) Continuous update project report. Food, nutrition, physical activity, and the prevention of breast cancer
Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P (2013) Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 14(8):665–678. doi:10.1111/obr.12028
Harris HR, Willett WC, Terry KL, Michels KB (2011) Body fat distribution and risk of premenopausal breast cancer in the Nurses’ Health Study II. J Natl Cancer Inst 103(3):273–278. doi:10.1093/jnci/djq500
Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JAE, Rosner B, Speizer FE, Hankinson SE (1999) Waist circumference, waist: hip ratio, and risk of breast cancer in the Nurses’ health study. Am J Epidemiol 150(12):1316
Kaaks R, Van Noord PA, Den Tonkelaar I, Peeters PH, Riboli E, Grobbee DE (1998) Breast-cancer incidence in relation to height, weight and body-fat distribution in the Dutch DOM cohort. Int J Cancer 76(5):647–651
Fagherazzi G, Chabbert-Buffet N, Fabre A, Guillas G, Boutron-Ruault MC, Mesrine S, Clavel-Chapelon F (2012) Hip circumference is associated with the risk of premenopausal ER-/PR- breast cancer. Int J Obes 36(3):431–439. doi:10.1038/ijo.2011.66
Sellers TA, Davis J, Cerhan JR, Vierkant RA, Olson JE, Pankratz VS, Potter JD, Folsom AR (2002) Interaction of waist/hip ratio and family history on the risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Am J Epidemiol 155(3):225–233
Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw K-T, Tehard B, Berrino F, Tj nneland A, Bigaard J, Olsen A, Overvad K, Clavel-Chapelon Fo, Nagel G, Boeing H, Trichopoulos D, Economou G, Bellos G, Palli D, Tumino R, Panico S, Sacerdote C, Krogh V, Peeters PHM, Bueno-de-Mesquita HB, Lund E, Ardanaz E, Amiano P, Pera G, Quir s JR, Mart nez C, Tormo MaJ, Wirf lt E, Berglund Gr, Hallmans Gr, Key TJ, Reeves G, Bingham S, Norat T, Biessy C, Kaaks R, Riboli E (2004) Body size and breast cancer risk: findings from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 111(5):762–771. doi:10.1002/ijc.20315
Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Vitolins M, Kabat GC, Rohan TE, Li CI (2011) Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomark Prev 20(3):454–463. doi:10.1158/1055-9965.EPI-10-0974
Kwan ML, John EM, Caan BJ, Lee VS, Bernstein L, Cheng I, Gomez SL, Henderson BE, Keegan TH, Kurian AW, Lu Y, Monroe KR, Roh JM, Shariff-Marco S, Sposto R, Vigen C, Wu AH (2014) Obesity and mortality after breast cancer by race/ethnicity: the California breast cancer survivorship consortium. Am J Epidemiol 179(1):95–111. doi:10.1093/aje/kwt233
George SM, Bernstein L, Smith AW, Neuhouser ML, Baumgartner KB, Baumgartner RN, Ballard-Barbash R (2014) Central adiposity after breast cancer diagnosis is related to mortality in the health, eating, activity, and lifestyle study. Breast Cancer Res Treat 146(3):647–655. doi:10.1007/s10549-014-3048-x
Shuster A, Patlas M, Pinthus JH, Mourtzakis M (2012) The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 85(1009):1–10. doi:10.1259/bjr/38447238
Sheean P, Hoskins K, Stolley M (2012) Body composition changes in females treated for breast cancer: a review of the evidence. Breast Cancer Res Treat 135(3):663–680. doi:10.1007/s10549-012-2200-8
James FR, Wootton S, Jackson A, Wiseman M, Copson ER, Cutress RI (2015) Obesity in breast cancer—what is the risk factor? Eur J Cancer 51(6):705–720. doi:http://dx.doi.org/10.1016/j.ejca.2015.01.057
Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME (2014) How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst 106(8). doi:10.1093/jnci/dju165
Collaborative Group on Hormonal Factors in Breast C (2012) Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13(11):1141–1151. doi:10.1016/S1470-2045(12)70425-4
Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, Dowsett M, Forbes JF, Ford L, LaCroix AZ, Mershon J, Mitlak BH, Powles T, Veronesi U, Vogel V, Wickerham DL, Group SCoBCO (2013) Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381(9880):1827–1834. doi:10.1016/S0140-6736(13)60140-3
Olin JL, St Pierre M (2014) Aromatase inhibitors in breast cancer prevention. Ann Pharmacother 48(12):1605–1610. doi:10.1177/1060028014548416
Narod SA (2011) Hormone replacement therapy and the risk of breast cancer. Nat Rev Clin Oncol 8(11):669–676. doi:10.1038/nrclinonc.2011.110
Lorincz AM, Sukumar S (2006) Molecular links between obesity and breast cancer. Endocr Relat Cancer 13(2):279–292. doi:10.1677/erc.1.00729
Bulun SE, Chen D, Moy I, Brooks DC, Zhao H (2012) Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab 23(2):83–89. doi:10.1016/j.tem.2011.10.003
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Group EHBCC (2003) Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95(16):1218–1226
Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, Biessy C, Vineis P, Sacerdote C, Berrino F, Panico S, Tumino R, Palli D, Nagel G, Linseisen J, Boeing H, Roddam A, Bingham S, Khaw KT, Chloptios J, Trichopoulou A, Trichopoulos D, Tehard B, Clavel-Chapelon F, Gonzalez CA, Larranaga N, Barricarte A, Quiros JR, Chirlaque MD, Martinez C, Monninkhof E, Grobbee DE, Bueno-de-Mesquita HB, Ferrari P, Slimani N, Riboli E, Kaaks R (2006) Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int J Cancer 118(11):2832–2839. doi:10.1002/ijc.21730
Vaidya D, Dobs A, Gapstur SM, Golden SH, Cushman M, Liu K, Ouyang P (2012) Association of baseline sex hormone levels with baseline and longitudinal changes in waist-to-hip ratio: multi-ethnic study of atherosclerosis. Int J Obes. doi:10.1038/ijo.2012.3
Baglietto L, English DR, Hopper JL, MacInnis RJ, Morris HA, Tilley WD, Krishnan K, Giles GG (2008) Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat 115(1):171–179. doi:10.1007/s10549-008-0069-3
Danforth KN, Eliassen AH, Tworoger SS, Missmer SA, Barbieri RL, Rosner BA, Colditz GA, Hankinson SE (2010) The association of plasma androgen levels with breast, ovarian and endometrial cancer risk factors among postmenopausal women. Int J Cancer 126(1):199–207. doi:10.1002/ijc.24709
Dowsett M, Folkerd E (2015) Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat 149(1):1–4. doi:10.1007/s10549-014-3211-4
Ehrmann DA, Barnes RB, Rosenfield RL (1995) Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16(3):322–353. doi:10.1210/edrv-16-3-322
Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P (2013) Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14(10):1009–1019. doi:10.1016/S1470-2045(13)70301-2
Kaaks R, Tikk K, Sookthai D, Schock H, Johnson T, Tjonneland A, Olsen A, Overvad K, Clavel-Chapelon F, Dossus L, Baglietto L, Rinaldi S, Chajes V, Romieu I, Boeing H, Schutze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Tumino R, Ricceri F, Mattiello A, Buckland G, Ramon Quiros J, Sanchez MJ, Amiano P, Chirlaque MD, Barricarte A, Bas Bueno-de-Mesquita H, van Gils CH, Peeters PH, Andersson A, Sund M, Weiderpass E, Khaw KT, Wareham N, Key TJ, Travis RC, Merritt MA, Gunter MJ, Riboli E, Lukanova A (2014) Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status—results from the EPIC cohort. Int J Cancer 134(8):1947–1957. doi:10.1002/ijc.28528
Key TJ, Appleby PN, Reeves GK, Travis RC, Brinton LA, Helzlsouer KJ, Dorgan JF, Gapstur SM, Gaudet MM, Kaaks R, Riboli E, Rinaldi S, Manjer J, Hallmans G, Giles GG, Le Marchand L, Kolonel LN, Henderson BE, Tworoger SS, Hankinson SE, Zeleniuch-Jacquotte A, Koenig K, Krogh V, Sieri S, Muti P, Ziegler RG, Schairer C, Fuhrman BJ, Barrett-Connor E, Laughlin GA, Grant EJ, Cologne J, Ohishi W, Hida A, Cauley JA, Fourkala EO, Menon U, Rohan TE, Strickler HD, Gunter MJ, Endogenous H, Breast Cancer Collaborative G (2015) Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanalysis of eighteen prospective studies. Steroids 99(Pt A):49–55. doi:10.1016/j.steroids.2014.09.001
Cowey S, Hardy RW (2006) The metabolic syndrome: a high-risk state for cancer? Am J Pathol 169(5):1505–1522. doi:10.2353/ajpath.2006.051090
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol (Official Journal of the American Society of Clinical Oncology) 20(1):42–51
Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev 8(12):915–928
Kaaks R (1996) Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control 7(6):605–625
Norman RJ, Dewailly D, Legro RS, Hickey TE (2007) Polycystic ovary syndrome. Lancet 370(9588):685–697. doi:10.1016/S0140-6736(07)61345-2
Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D (2013) Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia 18(3–4):277–289. doi:10.1007/s10911-013-9303-7
Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD (2009) Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 101(1):48–60. doi:10.1093/jnci/djn415
Kabat GC, Kim M, Caan BJ, Chlebowski RT, Gunter MJ, Ho GY, Rodriguez BL, Shikany JM, Strickler HD, Vitolins MZ, Rohan TE (2009) Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer 125(11):2704–2710. doi:10.1002/ijc.24609
Verheus M, Peeters PH, Rinaldi S, Dossus L, Biessy C, Olsen A, Tjonneland A, Overvad K, Jeppesen M, Clavel-Chapelon F, Tehard B, Nagel G, Linseisen J, Boeing H, Lahmann PH, Arvaniti A, Psaltopoulou T, Trichopoulou A, Palli D, Tumino R, Panico S, Sacerdote C, Sieri S, van Gils CH, Bueno-de-Mesquita BH, Gonzalez CA, Ardanaz E, Larranaga N, Garcia CM, Navarro C, Quiros JR, Key T, Allen N, Bingham S, Khaw KT, Slimani N, Riboli E, Kaaks R (2006) Serum C-peptide levels and breast cancer risk: results from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 119(3):659–667. doi:10.1002/ijc.21861
Ahern TP, Hankinson SE, Willett WC, Pollak MN, Eliassen AH, Tamimi RM (2013) Plasma C-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer Epidemiol Biomark Prev 22(10):1786–1796. doi:10.1158/1055-9965.EPI-13-0375
Keinan-Boker L, Bueno-de-Mesquita HB, Kaaks R, van Gils CH, Van Noord PAH, Rinaldi S, Riboli E, Seidell JC, Grobbee DE, Peeters PHM (2003) Circulating levels of insulin-like growth factor I, its binding proteins -1,-2, -3, C-peptide and risk of postmenopausal breast cancer. Int J Cancer 106(1):90–95. doi:10.1002/ijc.11193
Kaaks R, Lukanova A (2001) Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 60(1):91–106
Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Roddam AW (2010) Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol 11(6):530–542. doi:10.1016/S1470-2045(10)70095-4
Kaaks R, Johnson T, Tikk K, Sookthai D, Tjonneland A, Roswall N, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Dossus L, Rinaldi S, Romieu I, Boeing H, Schutze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Grioni S, Tumino R, Sacerdote C, Panico S, Buckland G, Arguelles M, Sanchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Bueno-de-Mesquita HB, van Gils CH, Peeters PH, Andersson A, Sund M, Weiderpass E, Gram IT, Lund E, Khaw KT, Wareham N, Key TJ, Travis RC, Merritt MA, Gunter MJ, Riboli E, Lukanova A (2014) Insulin-like growth factor I and risk of breast cancer by age and hormone receptor status—a prospective study within the EPIC cohort. Int J Cancer 134(11):2683–2690. doi:10.1002/ijc.28589
Hawsawi Y, El-Gendy R, Twelves C, Speirs V, Beattie J (2013) Insulin-like growth factor—oestradiol crosstalk and mammary gland tumourigenesis. Biochim Biophys Acta 1836(2):345–353. doi:10.1016/j.bbcan.2013.10.005
Crowe FL, Key TJ, Allen NE, Appleby PN, Overvad K, Gronbaek H, Tjonneland A, Halkjaer J, Dossus L, Boeing H, Kroger J, Trichopoulou A, Zylis D, Trichopoulos D, Boutron-Ruault MC, de Lauzon-Guillain B, Clavel-Chapelon F, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, van Gils CH, Peeters PH, Gram IT, Rodriguez L, Jakszyn P, Molina-Montes E, Navarro C, Barricarte A, Larranaga N, Khaw KT, Rodwell S, Rinaldi S, Slimani N, Norat T, Gallo V, Riboli E, Kaaks R (2011) A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European prospective investigation into cancer and nutrition (EPIC). Ann Hum Biol 38(2):194–202. doi:10.3109/03014460.2010.507221
Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H (1995) Free insulin-like growth factors in human obesity. Metabolism 44(10 Suppl 4):37–44
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev 4(8):579–591. doi:10.1038/nrc1408
Despres JP, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444(7121):881–887. doi:10.1038/nature05488
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846. doi:10.1038/nature05482
Simpson ER, Brown KA (2013) Obesity and breast cancer: role of inflammation and aromatase. J Mol Endocrinol 51(3):T51–T59. doi:10.1530/JME-13-0217
Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ (2012) Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov 2(4):356–365. doi:10.1158/2159-8290.CD-11-0241
Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ (2011) Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 4(7):1021–1029. doi:10.1158/1940-6207.CAPR-11-0110
Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I (2015) Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol 16(1):36–46. doi:10.1016/s1470-2045(14)71123-4
Stunkard AJ, Sorensen T, Schulsinger F (1983) Use of the Danish adoption register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 60:115–120
Thomas EL, Frost G, Taylor-Robinson SD, Bell JD (2012) Excess body fat in obese and normal-weight subjects. Nutr Res Rev 25(1):150–161. doi:10.1017/S0954422412000054
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Fortner, R.T., Katzke, V., Kühn, T., Kaaks, R. (2016). Obesity and Breast Cancer. In: Pischon, T., Nimptsch, K. (eds) Obesity and Cancer. Recent Results in Cancer Research, vol 208. Springer, Cham. https://doi.org/10.1007/978-3-319-42542-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-42542-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42540-5
Online ISBN: 978-3-319-42542-9
eBook Packages: MedicineMedicine (R0)