Abstract
Background
Tumor-infiltrating lymphocytes are an important component of the tumor microenvironment (TME) in breast cancer. They have been linked with tumor pathogenesis in advanced stages. However, little is known about their contribution in early phases. In this study, we analyzed the infiltration of leukocytes and cancer stem cells (CSC) in tumors from patients with early breast cancer.
Methods
Samples of blood and tumor tissue from 30 patients with breast cancer were collected, and the number of dendritic cells (DC), T cells, and CSC were analyzed by flow cytometry.
Results
Tumor-infiltrating CD4 and CD8 T cells expressed higher levels of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) compared with peripheral T cells. Regulatory T cells (Treg) were enriched in tumors and overexpressed glucocorticoid-induced TNFR-related protein and CTLA-4. Tumor Treg had a positive correlation with the amount of myeloid DC (mDC) and disease progression. The CD8/Treg ratio was associated with lymph node metastasis and tumor stages. The main subset of DC in early breast tumors was mDC, while plasmacytoid DC were almost absent. CSC were present in most tumors with higher frequencies in patients with lymph node metastasis. CSC were also associated with the amount of tumor-infiltrating Treg.
Conclusion
Early breast cancer has an inflammatory milieu characterized by mDC, Treg, and CSC infiltration. The frequencies of Treg, CSC and CD8/Treg ratio were associated with disease progression. The composition of leukocytes and the presence of CSC in early breast tumors should be considered for the development of new therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor development depends on a complex and orchestrated network of interacting cells that form the TME. In recent years, in several types of cancer, it has been observed that one of the determining components in the pathogenesis and response to treatments is the immune system. Activated cytotoxic CD8 T cells and NK cells have been associated with a good prognosis; in contrast, the presence of Treg have a detrimental effect on several cancers [1, 2]. Antigen-presenting cells (APC) also play an essential role in tumor pathogenesis. The major subpopulations of dendritic cells (DC) are myeloid DC (mDC) and plasmacytoid DC (pDC); both populations have been identified in the leukocyte fraction that infiltrates breast cancer tumors. Infiltration of DC in breast tumors is correlated with higher tumor grade and lymph node involvement, and pDC are associated with poor survival in advanced tumors [3]. Both populations of DC are associated with the induction and activation of Treg [4] and Th-2 responses [5, 6], which are linked with tumor development and bad prognosis in breast cancer [6, 7] and other tumors such as pancreatic and ovarian cancer [8, 9]. The contribution of these APC has been mainly described in aggressive tumors and in advanced stages of the disease, where they generate a tolerogenic microenvironment for tumor antigens through the induction and activation of suppressive cells [2]. Nevertheless, the infiltration of immune cells in the early stages of tumor development is less well studied in human breast cancer.
In addition to leukocytes, cancer cells are critical for the progression of the disease. These cells are a heterogeneous population showing distinct phenotypic and functional properties, limiting therapeutic efficacy and treatment outcomes. One of the central populations of cancer cells associated with tumor progression, metastasis, and recurrence is the CSC, also known as cancer initiating cells. CSC are highly tumorigenic due to their tumor-initiating, self-renewal, and differentiation abilities. Accumulating evidence indicates that this cell population is resistant to standard therapies, including chemotherapy and radiotherapy [10, 11]. CSC in solid tumors were initially described in breast cancer and were defined as CD45−CD44+CD24− cells [12]; a more recent study has defined these cells as CD45−CD90highCD24− cells [13]. These cells showed a higher capacity to form tumors in immunodeficient mice, and the tumors were highly invasive and metastatic [13]. Although more studies are required, these data suggest that CSC in breast cancer play an important role in tumor metastasis and recurrence. Breast cancer is a highly treatable disease if caught in the primary stages; however, the risk of recurrence is still high and could be associated with the presence of CSC. Therefore, it will be essential to determine the presence of these cells in the early stages and their possible association with prognostic factors. Additionally, the interaction of CSC and the immune infiltrate seems to be critical in tumor progression according to some studies that show that immune cells can favor the niche for CSC in the TME [13], and the fact that CSC have been associated with immunoregulatory mechanisms [11]. For those reasons, in this study, we aimed to analyze the frequency and correlation of CSC and the immune infiltrate in breast tumors at early stages of the disease.
Materials and methods
Patients
Samples of peripheral blood and tumor tissue from 30 patients with breast cancer who had surgical treatment and did not receive chemotherapy or radiation treatment before surgery were collected between October 2015 and February 2017 at the Gineco-Obstetrics Hospital of the Mexican Medical Social Security Institute (IMSS). The clinicopathologic characteristics of the patients are summarized in Table 1. Written informed consent was obtained from each patient before tissue donation. The protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committees of the IMSS and FES Iztacala.
Cell suspensions
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Lymphoprep, AXIS SHIELD). Cell count and viability were assessed by Trypan blue exclusion on a hemocytometer. Single-cell suspensions from tumor tissue were obtained by tissue digestion. Fresh tissue was cut into small pieces and digested with 0.5 mg/ml of collagenase (Cat. C5138, Sigma-Aldrich) and 0.2 mg/ml of DNase I (Cat. 10104159001, Roche) for 30 min at 37 °C. After digestion, tissues were mechanically disrupted, and cell suspensions were filtered through cell strainers. Finally, dead cells, granulocytes, and erythrocytes were removed by density gradient centrifugation.
Flow cytometry
Single-cell suspensions obtained from the clinical samples were analyzed by flow cytometry. Due to the limited number of cells obtained from the tumor samples, not all cell populations could be analyzed in each sample. Cells were incubated with monoclonal antibodies labeled with different fluorochromes for 10 min at room temperature protected from light. Then, cells were washed to remove excess antibody and analyzed on a BD LSRFortessa flow cytometer, and the data were analyzed with Flowjo software. For intracellular staining (FoxP3 and CTLA-4), cells were treated with the FoxP3 staining buffer set from Thermo Fisher Scientific. Anti-human antibodies used for flow cytometry: FITC-labeled anti-Lineage (CD3, CD14, CD16, CD19, CD20, CD56) cocktail-1 (Cat. 340546, BD Biosciences) and anti-CD24 (eBIOSN3, Cat. 11-0247, Thermo Fisher Scientific), Alexa Fluor 488-labeled anti-GITR (110416, Cat. FAB689G, R and D Systems), PE-labeled anti-ICOS-L (MIH12, Cat. 12-5889, Thermo Fisher Scientific), CTLA-4 (eBio20A, Cat. 12-1528, Thermo Fisher Scientific) and anti-CD127 (eBioRDR5, Cat. 12-1278, Thermo Fisher Scientific), PerCP-labeled anti-HLA-DR (L243 Cat. 347402, BD Biosciences), anti-CD44 (IM7, Cat. 65-0441, Tonbo biosciences), and anti-CD8 (SK-1, Cat. 340693, BD Biosciences), APC-labeled anti-FoxP3 (236A/E7 Cat. 17-4777), anti-CTLA-4 (Mab11, Cat. 17-7349) from Thermo Fisher Scientific, anti-CD11c (S-HCL-3, Cat. 340714, BD Biosciences), and anti-CD90 (BioLegend. 328114, 5610), APC-Cy7-labeled anti-CD4 (RPA-T4, Cat. 25-0049, Tonbo biosciences), PeCy7-labeled anti-CD3 (UCTH1, Cat. 25-0038) and anti-CD123 (6H6, Cat. 25-1239) from Thermo Fisher Scientific, V450-labeled anti-CD45 (HI30, Cat. 75-0459, Tonbo biosciences) and e-Fluor 450-labeled anti-FoxP3 (236A/E7, Cat. 48-4777, Thermo Fisher Scientific). The identification of Treg was performed by the expression of CD3, CD4, FoxP3, and the absence of CD127. The pDC subset was identified as Lineage—(CD3−/CD19−/CD20−/CD56−/CD14−/CD16−)/HLD-DR+/CD123+ cells, whereas mDC were Lin−/HLD-DR+/CD11c+. CSC were identified as CD45−CD24−CD90highCD44+ cells.
Statistical analysis
The differences between paired groups of data were analyzed according to their distribution by either t test or Wilcoxon matched pairs test. Differences between non-paired groups of patients were analyzed by either t test or Mann–Whitney test. Correlation analysis was performed by either Pearson or Spearman test. All the statistical analysis was carried out using GraphPad Prism Software and XLSTAT. P values less than 0.05 were considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Clinicopathologic features of patients
Invasive ductal carcinoma was the main form of breast cancer observed (19/30, 63%), followed by invasive lobular carcinoma (5/30, 17%). Importantly, early clinical stages were predominant, IA (7/30, 23%), IIA (11/30, 37%), IIB (10/30, 33%), and only two samples corresponded to locally advanced disease IIIA (2/30, 7%). Patients with stage IIIA were T1N2M0 and T2N2M0, and they were labeled with black dots in each graph where they were included. In 46.6% of the patients (14/30), there was at least one axillary lymph node with metastasis. Additional clinicopathologic characteristics of patients of the cohort analyzed are summarized in Table 1. Clinical follow-up was carried out in 26 patients over a mean of 27.4 months (6–35 months). During this period, there were no reports of recurrence or mortality.
Leukocyte infiltration
To determine if breast tumors in early stages are infiltrated by immune cells, we analyze the presence of leukocytes inside the tumor tissue. The identification of leukocytes was performed by the expression of CD45 analyzed by flow cytometry (Fig. 1a). Cell suspensions derived from tumor tissues contained a mixture of leukocytes CD45+ plus a fraction of CD45− cells that could correspond to tumor and stromal cells. Data showed that all samples analyzed have a considerable amount of leukocytes infiltrating the tumor bed (Fig. 1b). However, we could not find any relation with the clinicopathologic parameters examined, such as the size of the tumor, the stage, the grade, molecular subtype, or the involvement of lymph nodes. The follow-up time of the patients had less than 3 years; this time frame did not allow performing analysis of recurrence, metastasis, or mortality, because there were no reports of any of those conditions.
The frequency of leukocytes in peripheral blood and tumor tissue. Cell suspensions from blood and tumor tissue were analyzed by flow cytometry. a Gating strategy to identify leukocytes (CD45+ cells) and CD3+ T cells. Live cells were stained with anti-CD45 and anti-CD3 antibodies. Upper graphs represent cells stained with control isotype antibodies. Frequency of CD45+ (b) and CD3+ (c) cells in peripheral blood and tumor tissue
Tumor-infiltrating T cells reflected an immunosuppressive microenvironment
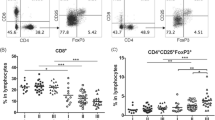
As shown in Fig. 1c, we found larger proportions of CD3+ T lymphocytes in the leukocyte fraction of tumor tissue (mean ± SD, 41 ± 20.5%) than those observed in peripheral blood (32.1 ± 23.2; P = 0.011 t test). However, the ratio of CD4 and CD8 T cells was very similar between both compartments (Fig. 2b), and the dominant population in both cases was the CD4+ T cell subset. When we measured the expression of CTLA-4, which is a negative regulatory molecule, we observed a higher expression in both CD4 and CD8 tumor-infiltrating lymphocytes in comparison with those of peripheral blood (Fig. 2c, d). Importantly, high percentages of Treg identified as CD3+CD4+FoxP3+CD127− cells (8.7 ± 5.4%) were observed into the tumors (Fig. 3a, b). Similar to conventional T cells, we also found a higher expression of CTLA-4 in Treg derived from tumor tissues than that observed in circulating Treg (Fig. 3c; P = 0.0011 Wilcoxon matched pairs test). Furthermore, tumor Treg expressed higher levels of the co-stimulatory molecule GITR than blood-derived Treg (Fig. 3d, P = 0.0093 Wilcoxon matched pairs test). The overexpression of both CTLA-4 and GITR indicates that tumor-derived Treg are more activated than blood Treg. Therefore, Treg are enriched and activated in the TME at early stages of the disease.
Tumor-infiltrating T cells overexpressed CTLA-4. CD3+/CD4+ and CD3+/CD8+ T lymphocytes in tumor tissue and peripheral blood were analyzed by flow cytometry. a Gating strategy. b The proportions of CD4 and CD8 T cells in blood and tumor tissue. c Expression of CTLA-4 in CD4 and CD8 T lymphocytes from the blood (gray line) and tumor (black line). d Expression levels of CTLA-4 in T cells derived from blood and tumor tissue. P values less than 0.05 were considered statistically significant (*P < 0.05)
Treg are enriched in the tumor microenvironment. a Analysis of cell suspensions by flow cytometry to identify Treg. Cells were selected on viable CD45+, and then Treg were identified as CD3 + CD4 + FoxP3 + CD127−. b Frequencies of Treg in peripheral blood and tumor tissue. Expression of CTLA-4 (c) and GITR (d) in Treg, measured as mean fluorescence intensity (MFI), are significantly higher in tumor-infiltrating cells. e Proportions of tumor Treg in patients stratified by tumor stage. f Percentages of tumor-infiltrating Treg between patients with or without lymph node metastasis. g Proportions of Treg according to the molecular subtype of breast tumors
The proportions of tumor-infiltrating Treg (TiTreg) were higher in stage IIB than stage IA. Although no significant differences were observed between stage IA and IIA, there was a tendency to be higher in more advanced stages (Fig. 3e). For stage III, there was only one sample analyzed; therefore, no conclusions can be made. Of note, the frequencies of TiTreg were significantly higher in patients with metastatic lymph nodes (Fig. 3f), but no differences were observed between molecular subtypes (Fig. 3g).
Because the ratio between CD8 T cells and Treg in the tumor microenvironment analyzed mainly by immunohistochemistry in several tumors including breast cancer has been associated with disease outcome, we analyzed if this ratio is associated with lymph node metastasis in our cohort of patients (Fig. 4). As expected, LN metastasis was higher in more advanced stages (Fig. 4a). Notably, the level of LN metastasis was different among the molecular subtypes of breast cancer, HER2+ tumors have the highest percentage of patients with positive LN metastasis, whereas ER+PR−HER2− has the lowest percentage (Fig. 4b). Importantly, the CD8/Treg ratio was significantly higher in patients without lymph node metastasis (Fig. 4c), but we did not find differences among molecular subtypes (Fig. 4d). We observed that stage IA has higher levels of CD8/Treg ratio than those observed in stages II and III (Fig. 4e), indeed there was a negative correlation between the proportions of tumor-infiltrating CD8 T cells and Treg (Fig. 4f).
Tumor-infiltrating CD8 to FOXP3 lymphocyte ratio correlates with lymph node metastasis. The percentages of patients with lymph node metastasis were analyzed by Fisher’s exact test according to tumor stage (a) and molecular subtype (b). Tumor-infiltrating CD8 to Treg lymphocyte ratio stratified by lymph node metastasis (c), molecular subtype (d) and tumor stage (e) were evaluated by t test. f Correlations between conventional T cells and Treg (Pearson). P values less than 0.05 were considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001)
Tumor-infiltrating DC in early breast tumors
The major subpopulations of DC are mDC and pDC, both populations have been identified in the leukocyte fraction that infiltrates breast cancer tumors; however, their frequency is little known in the early stages of the disease. We analyzed the presence of dendritic cells in cell suspensions derived from blood and tumor tissue; the gating strategy is shown in Fig. 5a. When we compared both populations in blood and tumor tissue (Fig. 5b), we found that mDC are present in both compartments in similar proportions (0.19 ± 0.25 vs. 0.29 ± 0.25% of CD45+ cells, respectively). Contrary to pDC that had much higher frequencies in peripheral blood than into the TME (1 ± 1.6 vs. 0.2 ± 0.22% of CD45+ cells, P = 0.001 Wilcoxon matched pairs test). Interestingly, it was observed that in the blood of patients, there was a clear predominance of pDC, whereas in the tumor tissue, this proportion was inverted with a dominance of mDC (Fig. 5b). Regarding the expression of the inducible costimulator ligand (ICOS-L), mDC expressed relatively low levels (Fig. 5a) and equivalent levels in the blood and tumor tissue (data not showed). Meanwhile, pDC has a high expression of ICOS-L, as observed in the histogram of Fig. 5a. Comparatively, tumor-infiltrating pDC expressed more elevated amounts of ICOS-L than blood-derived pDC (Fig. 5c; P = 0.019 Wilcoxon matched pairs test).
Early breast tumors are mainly infiltrated by mDC, which are positively correlated with Treg. Cell suspensions obtained from tumor tissue and blood were analyzed by flow cytometry. a Strategic analysis to identify pDC (CD45 + Lin-HLA-DR + CD123+) and mDC (CD45 + Lin-HLA-DR + CD11c+) in clinical samples. b The frequency of mDC and pDC among CD45+ cells in blood and tumor tissue. c Expression levels of ICOS-L in pDC from blood and tumor tissue. d Correlation between DC and Treg derived from breast tumors. Data were analyzed with the Spearman test (for mDC r = 0.7 and P = 0.005; and for pDC r = − 0.03 and P = 0.92). Proportions of mDC (e) and pDC (f) in patients stratified by the molecular subtype of breast tumors. P values less than 0.05 were considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001)
Because both pDC and mDC have been associated with the induction and activation of Treg, we evaluated if there was an association between the proportions of DC and Treg. Importantly, we found a positive correlation between the frequencies of tumor-infiltrating mDC and Treg (Spearman, P = 0.005, r = 0.7), but not with pDC (P = 0.92, r = − 0.03), as observed in Fig. 5d. Regarding the molecular subtypes, no differences were observed in the proportions of both mDC and pDCs amongst the different subtypes (Fig. 5e, f). Thus, early breast tumors are mainly infiltrated by mDC, and they have a positive correlation with the proportions of tumor Treg.
Cancer stem cells’ infiltration is associated with disease development and Treg frequencies
One subset of tumor cells that has been associated with the spread of the tumor is the cancer stem cells. Several markers have been defined to identify these cells; CD90 is one of them. Although CD90 is not exclusively expressed by CSC, it has been observed that CSC express high levels of this molecule and CD44; and together with the lack of CD45 and CD24 expression, it is possible to detect the presence of CSC in breast tumors [13]. We investigated the presence of these cells in our cohort of patients that were mainly at the early stages of the disease; the strategy of gating is displayed in Fig. 6a. We found that CSC were present in early breast tumors with a frequency of 0.2 ± 0.19% of the total of live cells isolated from tumor tissues. Only in 2 patients of 25 analyzed (8%), we did not detect the presence of this cell population. In peripheral blood, we did not identify the presence of CSC in most patients, only 3 of 25 (12%) had these cells with a frequency of 0.002 ± 0.009% (Fig. 6b). Notably, we observed a significant difference in the percentages of CSC between patients with or without metastasis in lymph nodes (LN). Patients with LN positive for metastasis (LN+) had a significantly higher proportion of CD45−CD24−CD90+CD44+ cells than those patients without LN metastasis (LN−), P = 0.02 Mann–Whitney test (Fig. 6c). However, we did not find clear differences in the proportions of CSC between different stages (Fig. 6d) or molecular subtypes (Fig. 6e). Finally, we observed a positive correlation between the percentages of CSC and Treg present in the tumor microenvironment (Spearman p = 0.0007, r = 0.83, Fig. 6f).
Cancer stem cells were associated with lymph node metastasis in breast cancer. a Gating strategy for CSC that were identified as live CD45−CD24−CD90highCD44+. b Frequencies of CSC in blood and tumor tissue. c The proportions of CSC analyzed by flow cytometry compared between patients with lymph nodes that were not affected by the tumor (LN−) with those who have at least one lymph node positive for tumor cells (LN+). Proportions of CSC in patients grouped by stage (d) or molecular subtype (e). f Correlation analysis (Spearman) between the frequencies of CSC and TiTreg. P values less than 0.05 were considered statistically significant (*P < 0.05)
Discussion
Although breast cancer has been considered poorly immunogenic, the presence of tumor-infiltrating leukocytes in breast tumors was documented more than 30 years ago [14]. In our cohort of patients, we observed that all the tumor samples contained a significant amount of CD45+ cells irrespective of breast cancer subtype, and even though most of the samples analyzed were at very early stages (I and II) of the disease. This observation confirms previous reports describing the presence of immune cells in the early stages, including carcinoma in situ and infiltrating ductal carcinoma [15]. Interestingly, we did not detect differences in the amount of CD45+ or CD3+ T cells between molecular subtypes of breast cancer, contrary to preceding reports stating that triple-negative tumors and HER2+ have the highest lymphocyte infiltration in comparison with luminal tumors [16, 17]. This discrepancy could be due to different methods used to evaluate the number of TILs because most of the previous studies are based on histopathology evaluations, while we used flow cytometry analysis. Histopathology and immunohistochemistry measure the numbers of cells over a specific area of tissue, while flow cytometry measures the proportions of cells within the total fragment of tissue analyzed, and can define cell subpopulations to a more detailed extent. Another important factor is the relatively small size of the cohort used in our study.
We also found that T cells are one of the main population infiltrating early tumors, and the dominant subset is CD4+ T cells. Of note, both CD4 and CD8 T cells infiltrating the tumor tissue showed an elevated expression of CTLA-4, a regulatory molecule associated with the down-regulation of the immune response and selectively expressed on TILs from different tumors [18, 19]. Indeed, CTLA-4 expression has been associated with poor prognosis and break down of anti-tumor immunity [20, 21]. The overexpression of this molecule on TILs could suggest that an immunosuppressive environment is present already at the early stages of the disease.
In addition to the tolerogenic phenotype of tumor-infiltrating T cells, we detected a high frequency of Treg in the tumor bed, which corroborates the suppressive tumor microenvironment. The presence of Treg in breast tumors has been reported previously, showing that the amount of these cells increased with the stage of the disease or the presence of metastatic lymph nodes [22, 23]. We found that the frequency of Treg was already enriched in early breast cancer, and they were more abundant in stage II compared with stage I. Patients with at least one lymph node with metastasis had significantly higher frequencies of Treg than patients without LN metastasis. Our results are in line with previous reports showing that Treg can be present in carcinoma in situ, and their frequency increases along with the disease development [23, 24]. These data highlight the role of this cell population in the early stages of breast tumor development.
We show that TiTreg expressed elevated levels of CTLA-4 and GITR in comparison with their counterpart in peripheral blood, these observations are in agreement with previous reports that showed that tumor Treg express high levels of suppressive molecules like programmed cell death protein 1 (PD-1) and CTLA-4 [22, 25], and they are highly suppressive [26]. Thus, the presence of CTLA-4+ T cells and activated Treg in early breast cancer confirms that the immunosuppressive environment in the tumor bed is established early in tumor development. Treg may be attracted and activated by CCL-22 produced in the TME, according to previous studies [27]. Our results are in agreement with several studies, mainly by immunohistochemistry, that have associated the presence of Treg with the prognosis of the disease and tumor development [28,29,30].
The ratio of cytotoxic CD8 T cells to Treg has been associated with favorable prognostic in some cancers including ovarian, colorectal cancer liver metastasis, oral squamous cell carcinoma and breast cancer [30, 31]. The majority of these studies were performed by immunohistochemistry analysis, now we confirmed by flow cytometry that this effector/suppressor ratio is associated with tumor status, and it may be a more important indicator of outcome than individual cell count.
Another population of tumor-infiltrating leukocytes associated with poor prognostic are pDC [3]; they are reported to accumulate in aggressive breast tumors [7]. We found a low infiltration of pDC in breast tumors at early stages, with a median of 0.04% of CD45+ cells, and 43% of the samples were negative for pDC. Interestingly in peripheral blood, the dominant subset of DC was pDC, this observation may be related to a previous report that shows that a high numbers of circulating pDC are an indicator of good prognosis [32], since most of the tumors in our cohort were in early stages of the disease and no reports of recurrence or mortality have been observed until now. Importantly, the expression of ICOS-L was up-regulated in tumor-derived pDC, as described in previous studies in melanoma, breast, liver, and ovarian cancer. The presence of ICOS-L + pDC has been associated with the induction of immunosuppressive T cells in the TME [1, 4, 8, 33]. However, in early breast tumors, the presence of this cell population was minimal, suggesting that the induction/activation of Treg may be due to other mechanisms independent of pDC. One possible mechanism could be mediated by mDC, because there was an infiltration of these cells in 87.5% of the samples analyzed with a frequency of about 0.3% of CD45+ cells. This infiltration was positively correlated with the presence of tumor-infiltrating Treg, suggesting that Treg may be induced or activated by the mDC present in the tumor bed, because it has been reported that tumor-derived mDC have tolerogenic properties [9, 34] and they colocalize with Treg in lymphoid infiltrates in breast cancer [27], but additional studies are necessary to confirm this idea. These findings suggest that mDC arrive at early stages and could be responsible for Treg induction/activation. Meanwhile, pDC arrive late to the TME in advanced stages when they play a pivotal role in tumor development and are required for tumor progression [35]. The analysis of DC subsets at different time points in the tumor microenvironment and the draining lymph node, and their correlation with immune responses may help clarify these issues.
In addition to immune cells, cancer cells are pivotal in determining the curse of tumor development, especially CSC, that have a high capacity to proliferate and migrate to other tissues. We identified the presence of CD45−CD24−CD90highCD44+ CSC in most of the tumors analyzed. The presence of these cells was associated with lymph node status. Patients with LN metastasis had higher percentages of these cells in comparison with patients that do not have metastasis in the lymph nodes, suggesting that they have an active role in the progression of the disease due to their migratory and proliferative capacities. Even though most studies on the functionality of these cells have been carried out in animal models, it has been observed an association between lymph node metastasis and the expression of some markers of CSC in patients with breast cancer [36]. Similarly, the expression of CD44 and aldehyde dehydrogenase 1 (ALDH1), markers of CSC, has been associated with an adverse prognosis and a high degree of metastasis [37, 38]. Indeed, in xenotransplanted mice with breast cancer cell lines, ALDH1+ cells have been related to tumor metastatic capacity [39]. Nevertheless, it will be necessary to determine their presence in the metastatic lymph nodes in future studies and confirms their functional properties. Furthermore, we found an association between the proportions of CSC and Treg present in the tumor microenvironment, suggesting possible crosstalk between these cell populations as it has been described in several models the interaction of CSC and immune cells [13, 40]. However, these mechanisms are not well known, guaranteeing the need for new studies aimed at characterizing the possible immunoregulatory functions of CSC.
In conclusion, our data, together with previous reports, show that TME in breast cancer has an active immune component from very early stages, and its compositions have dynamic changes along with tumor development. At early stages, mDC are the main population of DC, while pDC are low or absent. However, in the late stages, pDC have a significant role in tumor development and are associated with a bad prognosis. T cells are present in the tumor bed, and CD4+ T cells are dominant, from which a fraction corresponded to activated Treg. The positive correlation of mDC with Treg into the tumor tissue suggests an active interaction in which mDC could be inducing and activating Treg generating an immunosuppressive microenvironment. Furthermore, CSC are present in early breast tumors, and they are associated with lymph node metastasis and the frequency of Treg, suggesting an active role of these cells in the pathogenesis of early tumors. However, the interaction of this cell population with immune cells and other cells in the TME requires further investigations. The composition of leukocytes and the presence of CSC in early breast tumors should be considered for the development of new immunologic therapeutic approaches. Identification of specific antigens in CSC and immune checkpoint plays an important role in finding targets for immunotherapy. Reciprocal to the blockade of co-inhibitory receptors, strategies to directly stimulate the anti-tumor rejection like agonistic antibodies for co-stimulatory receptors and immunostimulatory cytokines would be necessary to induce an effective anti-tumor immunity. An example is the modulation of GITR because signaling through GITR enhances effectors CD4+ and CD8+ T cells and reduces Treg-mediated suppression. Additionally, strategies that can modify the ratio of Tregs or to CD8+ effector cells may be useful in improving outcomes in breast cancer.
References
Pedroza-Gonzalez A, Zhou G, Vargas-Mendez E, Boor PP, Mancham S, Verhoef C, et al. Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors. Oncoimmunology. 2015;4:e1008355.
Garcia-Romo GS, Garcia-Castillo KG, Diaz-Rodriguez A, Reyes-Hernandez D, Pedroza-Gonzalez A. Main immunoregulatory mechanisms that favor breast cancer development. Gac Med Mex. 2017;153:229–37.
Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–74.
Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012;72:6130–41.
Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–47.
Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–90.
Sisirak V, Faget J, Vey N, Blay JY, Menetrier-Caux C, Caux C, et al. Plasmacytoid dendritic cells deficient in IFNalpha production promote the amplification of FOXP3+ regulatory T cells and are associated with poor prognosis in breast cancer patients. Oncoimmunology. 2013;2:e22338.
Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012;72:5240–9.
Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 2017;20:558–71.
Al-Ejeh F, Smart CE, Morrison BJ, Chenevix-Trench G, Lopez JA, Lakhani SR, et al. Breast cancer stem cells: treatment resistance and therapeutic opportunities. Carcinogenesis. 2011;32:650–8.
Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N. Immune responses to human cancer stem-like cells/cancer-initiating cells. Cancer Sci. 2016;107:12–7.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8.
Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16:1105–17.
Whiteside TL, Miescher S, Hurlimann J, Moretta L, von Fliedner V. Clonal analysis and in situ characterization of lymphocytes infiltrating human breast carcinomas. Cancer Immunol Immunother. 1986;23:169–78.
Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–7.
Jang N, Kwon HJ, Park MH, Kang SH, Bae YK. Prognostic value of tumor-infiltrating lymphocyte density assessed using a standardized method based on molecular subtypes and adjuvant chemotherapy in invasive breast cancer. Ann Surg Oncol. 2018;25:937–46.
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–69.
Montler R, Bell RB, Thalhofer C, Leidner R, Feng Z, Fox BA, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunol. 2016;5:e70.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106.
Huang PY, Guo SS, Zhang Y, Lu JB, Chen QY, Tang LQ, et al. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget. 2016;7:13060–8.
Pedroza-Gonzalez A, Zhou G, Singh SP, Boor PP, Pan Q, Grunhagen D, et al. GITR engagement in combination with CTLA-4 blockade completely abrogates immunosuppression mediated by human liver tumor-derived regulatory T cells ex vivo. Oncoimmunology. 2015;4:e1051297.
Syed Khaja AS, Toor SM, El Salhat H, Faour I, Ul Haq N, Ali BR, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget. 2017;8:33159–71.
Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, et al. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol. 2013;36:224–31.
Wang ZK, Yang B, Liu H, Hu Y, Yang JL, Wu LL, et al. Regulatory T cells increase in breast cancer and in stage IV breast cancer. Cancer Immunol Immunother. 2012;61:911–6.
Pedroza-Gonzalez A, Verhoef C, Ijzermans JN, Peppelenbosch MP, Kwekkeboom J, Verheij J, et al. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology. 2013;57:183–94.
Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–34.
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–9.
Melichar B, Studentova H, Kalabova H, Vitaskova D, Cermakova P, Hornychova H, et al. Predictive and prognostic significance of tumor-infiltrating lymphocytes in patients with breast cancer treated with neoadjuvant systemic therapy. Anticancer Res. 2014;34:1115–25.
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Investig. 2013;123:2873–92.
Sideras K, Galjart B, Vasaturo A, Pedroza-Gonzalez A, Biermann K, Mancham S, et al. Prognostic value of intra-tumoral CD8(+) /FoxP3(+) lymphocyte ratio in patients with resected colorectal cancer liver metastasis. J Surg Oncol. 2018;118:68–766.
Semeraro M, Adam J, Stoll G, Louvet E, Chaba K, Poirier-Colame V, et al. The ratio of CD8(+)/FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology. 2016;5:e1218106.
Kini Bailur J, Gueckel B, Pawelec G. Prognostic impact of high levels of circulating plasmacytoid dendritic cells in breast cancer. J Transl Med. 2016;14:151.
Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–15.
Gervais A, Leveque J, Bouet-Toussaint F, Burtin F, Lesimple T, Sulpice L, et al. Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005;7:R326–R335335.
Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–65.
Wei W, Hu H, Tan H, Chow LW, Yip AY, Loo WT. Relationship of CD44+CD24-/low breast cancer stem cells and axillary lymph node metastasis. J Transl Med. 2012;10(Suppl 1):S6.
Sawant A, Ponnazhagan S. Role of plasmacytoid dendritic cells in breast cancer bone dissemination. Oncoimmunology. 2013;2:e22983.
Ma F, Li H, Li Y, Ding X, Wang H, Fan Y, et al. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Medicine (Baltimore). 2017;96:e6561.
Li W, Ma H, Zhang J, Zhu L, Wang C, Yang Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep. 2017;7:13856.
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA. 2011;108:12425–300.
Acknowledgements
This study was supported by the program: “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) de la Dirección General de Asuntos del Personal Académico (DGAPA)” from the National Autonomous University of Mexico (UNAM). Project numbers IA204515/RA204515 and IA208717. Diana Reyes Hernandez and Alvaro Diaz Rodriguez were fellows of the project PAPIIT IA204515/RA204515. We thank Santiago Cristobal Sigrist Flores, Karen Guadalupe Garcia Castillo, Maria Fernanda Diaz Quiroz, Alejandro Gallardo Flores and Estanislao Antonio Calixto for the technical assistant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were following the ethical standards of the institutional research committee of the IMSS and the FES Iztacala and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from each patient before blood and tissue donation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Solis-Castillo, L.A., Garcia-Romo, G.S., Diaz-Rodriguez, A. et al. Tumor-infiltrating regulatory T cells, CD8/Treg ratio, and cancer stem cells are correlated with lymph node metastasis in patients with early breast cancer. Breast Cancer 27, 837–849 (2020). https://doi.org/10.1007/s12282-020-01079-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01079-y