Abstract
Purpose
The role of Forkhead Box Protein 3 (Foxp3) expressing regulatory T cells (Tregs) in breast cancer remains unclear. We examined the abundance and localisation of total T cells, B cells and Tregs within samples from triple-negative breast cancers (TNBCs) and asked whether these parameters were associated with clinicopathological features of the cancer or clinical outcomes.
Methods
Samples from TNBCs diagnosed between 2003 and 2010 in Singapore were divided into “high” and “low” intra-tumoural or stromal groups, based on whether they had higher or lower than median densities of specific tumour-infiltrating lymphocyte populations (CD3+ total T cells, Foxp3+CD3+ Tregs, or CD20+ B cells) in the intra-tumoural space or stroma.
Results
Of the 164 samples, patients bearing tumours with high Tregs within their intra-tumoural, but not stromal, areas experienced significantly longer overall and disease-free survival compared to individuals with low Treg densities. These “high intra-tumoural Treg” tumours were also characterised by relatively higher frequencies of CD8+ T cells and CD20+ B cells, and expressed significantly higher levels of some genes associated with inflammation, immune cell functions and trafficking, altogether consistent with a more “immune-activated” tumour microenvironment, in contrast to tumours bearing lower densities of Tregs.
Conclusions
In summary, the combination of high densities of intra-tumoural Tregs, CD8+ T cells and CD20+ B cells represents a favourable prognostic panel in TNBCs. These data also indicate new avenues for further investigation on the interaction between immune cell types within the tumour microenvironment and highlight the potential of Treg density and localisation within tumours to affect clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancers (TNBCs), defined by the absence of oestrogen receptor (ER), progesterone receptor (PR) and c-erbB2 (HER2), account for between 9 and 17% of all breast cancers [1–3]. Patients frequently present with advanced disease, high incidence of metastasis and recurrence, and have significantly poorer prognosis [4–8]. Due to the lack of therapeutic targets, almost half of TNBC patients do not survive more than 5 years [5], warranting a better understanding of the underlying cellular processes in order to rationally design effective therapeutics against TNBC.
Recent studies indicate that immune cell activation is a particularly prominent feature of TNBCs, and the extent and type of immune cell infiltration can predict favourable responses to chemotherapy [9–13]. The immune cells are critical in tumour initiation, progression and metastasis [14–16], and the interaction between different types of immune cells is also an important determinant of outcome [17]. In breast cancer, a high frequency of tumour-infiltrating lymphocytes (TILs) is associated with poorer survival in patients with ER+ breast tumours, while in TNBCs, the same is linked to significantly longer survival [4, 18, 19], indicating that mere presence of TILs is insufficient to reliably predict their influence, warranting thorough characterization of the TILs in the context of TNBC.
Among the TILs, of particular interest is the role played by regulatory T cells (Tregs). Forkhead Box Protein 3 (Foxp3)-expressing Tregs are a sub-population of CD4+ T cells, preferentially recruited to tumour sites where they render effector T cells unable to control further growth of the cancerous cells. While Tregs typically comprise approximately 4% of circulating CD4+ lymphocytes, they can represent up to 25% of CD4+ TILs [20], and such high frequencies of Tregs have been linked with poor prognosis in a range of cancers [21, 22]. However, a recent study showed that while tumour-infiltrating Tregs are poor prognostic indicators in ER+ breast cancer, their presence has a favourable prognostic influence in the ER− HER2+ subtype [23], suggesting a link between Foxp3+ Tregs with ER and HER2 expression status. Moreover, the concurrent presence of relatively high frequencies of CD8+ TILs with Tregs may be important in driving a favourable outcome, as indicated in ER− [24] and ER− HER2+ breast cancers [23], as well as some TNBCs [25].
Taken together, the current literature establishes the importance of Tregs and their interactions with other immune cell types in determining clinical outcomes in many cancers, and highlights the dearth of knowledge surrounding the roles of these cell types in TNBCs. Here we report findings from our evaluation of T cells, in particular Tregs, abundance and localisation within TNBCs. We also aimed to establish how these immune cell parameters were linked with tumour clinicopathological features and their impact on clinical outcome.
Materials and methods
Patients and tumours
This study was approved by the SingHealth Centralized Institutional Review Board, CIRB Ref: 2013/664/F and 2015/2199. Archival specimens from 164 TNBCs diagnosed between 2003 and 2010 at the Division of Pathology, Singapore General Hospital, were retrieved. Clinicopathological parameters including patient age, tumour size, histologic growth pattern, grade and subtype, associated ductal carcinoma in situ, lymphovascular invasion and axillary lymph node status were reviewed (Table 1).
Tissue microarray (TMA) construction
Haematoxylin and eosin (H&E)-stained tumour slides were used for the construction of the TMA, before three representative tumour areas of 1 mm diameter were transferred from donor formalin-fixed paraffin-embedded (FFPE) tissue blocks to recipient blocks using a MTA-1 Manual Tissue Arrayer (Beecher Instruments, Sun Prairie, WI, USA), as previously described [6].
Immunohistochemistry (IHC) analysis of TMA cores
TMA sections of 4 µm thickness were incubated with antibodies specific for CD3, CD8, CD20 and Foxp3, as well as ER, PR and HER2. Antibodies specific for epidermal growth factor receptor (EGFR), cytokeratins (CK) 14 and 34βE12 were used to identify TNBCs with basal-like phenotype, according to previously published protocols [6, 26]. Details of antibodies, labelling patterns and dilution factors can be found in Table 2. Appropriate positive and negative controls were included. Scoring of antibody-labelled sections was carried out for nuclear ER and PR, membrane HER2 and EGFR, and cytoplasmic CK14 and 34βE12 positivity. All scoring was performed by manual scoring by two observers (AAT and JY). The core identified as exhibiting the highest labelling score for the antibody-of-interest (out of the 3 cores tested) was selected for data analysis. For ER, PR, CK14, EGFR and 34βE12, a positive result was defined by the presence of ≥1% of tumour cells displaying any intensity of unequivocal staining [27, 28]. For HER2, tumour positivity was defined by >10% of tumour cells exhibiting 3+ membrane staining [29].
TILs expressing CD3, Foxp3, CD8 and CD20 were identified within the stromal and intra-tumoural TILs populations separately. Intra-tumoural TILs were defined as lymphocytes within cancer cell nests and in direct contact with tumour cells [30]. Similarly, stromal TILs are defined as lymphocytes within the tumour stroma and not in direct contact with tumour cells. Tumoural and stromal areas for quantification were identified and marked manually by the observers. Quantification of TILs was determined by the percentage of the intra-tumoural or stromal areas occupied by the respective TIL population [30, 31]. Tumours were then divided into “high” and “low” with respect to a particular TIL population, when the % of the intra-tumoural or stromal areas occupied by cells labelled for the TIL marker (CD3 for total T cells, Foxp3 and CD3 for Tregs, and CD20 for B cells) was on/above or below the median, respectively (Multiple cut-off points in addition to median value were also analysed (supplementary Table 3 and 4.). After exclusion of cores lacking either stromal/tumour regions, and samples lost through sectioning and IHC processing, IHC analysis was performed on 159 samples for Foxp3 and CD3 labelling, and 142 cases for CD20 labelling.
Multiplex immunofluorescence (IF)
Multiplex IF was performed using an Opal Multiplex fIHC kit (PerkinElmer, Waltham, MA, USA) as described [32–35], on FFPE tissue sections processed according to the standard IHC protocol above. Slides were incubated with primary antibodies for Foxp3 and CD3, or CD20 followed by secondary antibodies, before application of the fluorophore (FITC, Cy3 or Cy5)-conjugated tyramides signal amplification (TSA) buffer (PerkinElmer, Waltham, MA, USA). DAPI was used as a nuclear counterstain, and images were acquired using a Vectra 3 pathology imaging system microscope (PerkinElmer, Waltham, MA, USA).
RNA extraction, NanoString gene expression and analysis
Eleven “high” and 11 “low” intra-tumoural Treg TNBCs identified by the IHC scoring, and four benign breast lesions were included as a reference standard for gene expression analysis. Unlabelled FFPE standard sections of 10 µm thickness were prepared from each sample. RNA was extracted using the RNeasy FFPE kit (Qiagen, Hilden, Germany) on a QIAcube automated sample preparation system (Qiagen, Hilden, Germany) and was quantified by an Agilent 2100 Bioanalyser system (Agilent, Santa Clara, CA, USA). A total of 100 ng of functional RNA (>300 nucleotides) was assayed on the nCounter PanCancer Progression Panel (NanoString Technologies, Seattle, WA, USA). Positive control probes and housekeeping genes were used to normalise NanoString counts (Online Resource: Table S1). The count data were then logarithmically transformed prior to further analysis. Genes that were significantly differentially expressed between the three sample groups (high intra-tumoural Treg, low intra-tumoural Treg and benign lesion) were identified using one-way ANOVA in R version 3.1.2. Multiple testing corrections were applied using the method of Benjamini and Hochberg. Significantly differentially expressed genes (DEG) after correction were subjected to post hoc analysis using pairwise t tests with multiple testing corrections using the Bonferroni method. p values <0.05 were deemed to be statistically significant.
Ingenuity pathway analysis (IPA)
DEG between high and low intra-tumoural Treg tumours was subjected to IPA core analysis using the entire list of genes on the NanoString cartridge as the background. Significant enrichment was determined by a p value < 0.05.
Validation dataset
A TNBC microarray experiment (GSE76124) was selected and extracted from NCBI GEO as the validation dataset [36]. 2251 DEG out of 20,283 unique genes were identified between the basal-like immune-activated (BLIA) and basal-like immune-suppressed (BLIS) subsets [36] of the samples using Limma. The hypergeometric distribution was used to determine the significance of the overlap between the DEG in our data and those of GSE76124.
Follow-up and statistical analysis
Follow-up data were obtained from medical records. Disease-free survival (DFS) and overall survival (OS) were defined as the time from diagnosis to recurrence or death/date of last follow-up, respectively. Statistical analysis was performed using SPSS for Windows, Version 18. The relationship between clinicopathological parameters and the frequency of various immune cell types was tested using χ 2 and Fisher’s exact tests. Survival outcomes were estimated with the Kaplan–Meier analysis and compared between groups with the log-rank statistics. Multivariate Cox regression was carried out to evaluate the effect of Tregs status with survival adjusted to the effects of tumour size, nuclear grade and patient age. Statistical significance was defined by a p value < 0.05.
Results
Patients with tumours exhibiting high intra-tumoural Treg density survived significantly longer than those with fewer intra-tumoural Tregs
The median numbers of Tregs, total T and B cells are shown in Table 3. Amongst total T cells (CD3+), 52.8% of the TNBC samples were designated as high intra-tumoural, Fig. 1 and 52.2% were high stromal, while 57.9% of samples were designated high intra-tumoural Treg (CD3 and Foxp3 co-expression), and 56.6% were high stromal Treg (Fig. 1, Table 3). Univariate analyses of the clinicopathological features revealed that high stromal Treg tumours were more likely to be of higher tumour grade (p = 0.025) and lymph node status (p = 0.039) (Table 1). According to Kaplan–Meier survival analysis (Fig. 2a–d), high intra-tumoural or stromal T cell TNBCs exhibited significantly longer DFS (intra-tumoural: p = 0.019 and stromal: p = 0.019) and OS (intra-tumoural: p = 0.005 and stromal: p = 0.018), in agreement with our previous findings [37]. A high intra-tumoural Treg TNBC was also associated with significantly longer DFS (p = 0.001, Fig. 2e); this was further confirmed by multivariate survival analysis (Hazard Ratio: 0.33, 95% CI 0.165–0.659, p = 0.002; Table 4). Multivariate analysis also indicated significant OS improvement in high intra-tumoural Treg tumours compared to low intra-tumoural cancers (Hazard Ratio: 0.487, 95% CI 0.251–0.947, p = 0.034).
CD3+ T cells and CD3+Foxp3+ Tregs infiltrate the tumour nest and stroma in TNBCs. Representative IHC images showing cells expressing cytoplasmic/membranous CD3 and nuclear Foxp3, both in the tumour nest (a) and the stroma (b) of TNBCs. These findings were confirmed by IF on tumour sections, showing the total T cell and Treg infiltrate (c–d)
Patients bearing high T cell or high Treg TNBCs survived significantly longer than those with low T cell or Treg tumours. Women with high intra-tumoural or stromal CD3+ total T cell tumours had significantly longer DFS (a–b) and OS (c–d) than that did women bearing low T cell tumours. Women with high intra-tumoural Tregs similarly had significantly longer DFS (e) and OS (f) than women bearing low Treg tumours
Expression of immune response-associated genes is significantly higher in high intra-tumoural Treg TNBCs
Given the strong association between intra-tumoural Treg density and patient survival, we compared gene expression profiles of a panel of 770 cancer-associated genes [15] in samples from 11 high intra-tumoural Treg and 11 low intra-tumoural Tregs by utilising the nCounter PanCancer Progression Panel [38–46]. Samples from four benign breast tumours were also included in this analysis. One-way ANOVA followed by post hoc t tests revealed that 31 genes were significantly differentially expressed between the high and low intra-tumoural Treg TNBCs (Table 5, Fig. 3).
High and low intra-tumoural Treg TNBCs exhibit distinct gene expression signatures. Heat map of the 31 significantly differentially expressed genes (p < 0.05) showing specific expression profiles in high and low intra-tumoural Tregs (iTregs), sorted by increasing gene expression. The heat map is coloured by the log10 normalised counts with the highest expression in red and the lowest expression in green. The genes are ordered by a hierarchical clustering using Euclidean distance
IPA was used to decipher the biological relationships of the 31 DEG (Online Resource: Table S2). This identified significant functional enrichment in expression of genes related to inflammatory response (APOH, CXCR3, FASLG, PLA2g2D, S1PR1 and SERPINE1), haematological system development and function (APOH, CXCR3, EGF, FASLG, ITGB7, PLA2g2D, RORA, S1PR1 and SERPINE1), immune cell trafficking (APOH, CXCR3, FASLG, ITGB7, PLA2g2D, S1PR1 and SERPINE1) and cell-mediated immune response (CXCR3, ITGB7, RORA and S1PR1) between the high and low intra-tumoural Treg TNBCs. Taken together, these four pathways share nine genes which are APOH, CXCR3, EGF, FASLG, ITGB7, PLA2g2D, RORA, S1PR1 and SERPINE1 (Online Resource: Table S3).
A recent study by Burstein et al. [36] used gene expression analysis to define distinct subtypes of tumours in TNBC that were associated with different prognoses: the BLIS and BLIA groups exhibited the worst and best survival characteristics, respectively. Given our observed association between high intra-tumoural Treg TNBCs and increased survival, we used the publicly available gene expression data from their study and found that seven of the 31 genes that were significantly differentially expressed between high and low intra-tumoural Treg TNBCs were also differentially expressed between BLIA and BLIS subtypes (p = 0.028) (Online Resource: Fig. S1; “Materials and methods” section: “Validation dataset” section). All seven genes were highly expressed in both the BLIA and high intra-tumoural Treg TNBC subtypes, suggesting that TNBC with high intra-tumoural Tregs are likely to possess an “immune-activated” tumour microenvironment, which is associated with significantly prolonged survival in both our study and Burstein et al.’s dataset.
High intra-tumoural Treg density correlates with high densities of CD8+ T cells and CD20+ B cells, which are independently and individually associated with better clinical outcome in TNBCs
Our data indicate that TNBCs with high intra-tumoural Tregs also expressed significantly higher levels of some genes associated with inflammation, immune cell functions and trafficking, altogether leading to the more “immune-activated” tumour microenvironment which is associated with longer patient survival. A recent report showed that high densities of CD25+ Tregs in ER− breast tumours were frequently accompanied by high numbers of CD8+ T cells, leading to an inflammatory gene expression profile consistent with a robust anti-tumour immune response, and increased survival [24]. We also found evidence of CD8+ T cell association with Tregs in our samples: the density of intra-tumoural Tregs was positively correlated with CD8+ T cells in both the intra-tumoural (p < 0.0001, r = 0.5926) and stromal compartments (p < 0.0001, r = 0.5051) (Fig. 4a–b).
The density of intra-tumoural Tregs correlates with the density of CD8+ T cells and CD20+ B cells in TNBCs. IHC scoring revealed a significant positive correlation between the densities of intra-tumoural Tregs and intra-tumoural CD8+ T cells (a) and CD20+ B cells (c). Similarly, significant correlations existed between densities of Tregs and CD8+ T cells (b) and CD20+ B cells (d) in the stroma. Pearson R correlation and p values are shown
Similarly, the density of CD20+ TILs was positively correlated with the density of Foxp3+ Tregs in both the intra-tumoural (p < 0.0001, r = 0.3819) and stromal compartments (p < 0.0001, r = 0.3655) (Fig. 4c–d). Genes associated with Tregs, CD8+ T cells and CD20+ B cells are more abundantly expressed in BLIA and Mesenchymal subtypes, compared to BLIS and Luminal-Androgen Receptor subtypes. In many previous studies, IL2RA (CD25) transcription was used as a marker for Tregs; hence, we analysed the dataset by Burstein et al. [36] and found that IL2RA expression level was also positively correlated with that of CD8A (p < 0.0001, r = 0.5586) and CD19 (p < 0.0001, r = 0.5603) (Online Resource: Fig. S2b–c), which is associated with CD8+ T cells and CD20+ B cells, respectively.
IF imaging of TNBC sections, which allowed us to observe more than two immune cell populations, further revealed that Foxp3+ Tregs were located in close proximity to CD8+ T cells and CD20+ B cells, both in the intra-tumoural (Fig. 5) and the stromal compartments (Online Resource: Fig. S3).
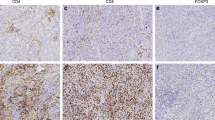
Tregs associate with CD8+ T cells and CD20+ B cells in the intra-tumoural compartment of TNBCs. H&E, multiplex IF and IHC labelling on five consecutive sections from a representative TNBC tissue sample show that Tregs, CD8+ T cells and CD20+ B cells are aggregated in close proximity within the intra-tumoural microenvironment. a H&E staining. b Multiplex IF labelling for Foxp3 (red), CD8 (green), CD20 (white) and DAPI (blue). c–f Higher magnification of the same region from image (a–b) shows single IHC labelling of Foxp3 in (c). Multiplex IF labelling of Foxp3 (red), CD8 (green) and CD20 (white) in (d). Double IHC labelling of CD8 and Foxp3, with the Tregs (Foxp3+, red) circled in (e), and double IHC labelling of CD20 and Foxp3 with the Tregs (Foxp3+, red) circled in (f)
The association between the density of CD8+ T cells and CD20+ B cells and clinical outcome was also examined. In the case of CD20+ B cells, 57.8% of samples were designated high intra-tumoural CD20+ B cell and 62.7% were high stromal CD20+ B cell. Kaplan–Meier analysis showed that high CD20+ B cell in both intra-tumoural (DFS: p = 0.015, OS: p = 0.020) and stromal (DFS: p = 0.012, OS: p = 0.031) compartments were associated with better clinical outcome (Fig. 6). Our own recently published study also showed that high densities of intra-tumoural CD8+ T cells are similarly associated with better clinical outcome in the same cancer patient cohort [37].
Discussion
In this study, we demonstrated that TNBC patients with high intra-tumoural Tregs were likely to survive significantly longer than those with low Treg density. The former group exhibited a distinct gene expression signature characterised by inflammatory and immune response-related genes and were also more likely to contain higher densities of CD8+ T cells and CD20+ B cells. A similar association between high tumour-infiltrating Tregs and improved prognosis has been reported in several recent studies in ER− breast cancers; Lee et al. reported that having greater than 15 Foxp3 Tregs per 10 fields of peri-tumoural area was linked with better survival in TNBCs [47]. On the other hand, using a 0% cut-off (Supplementary Tables 3 and 4), we also observed a similar trend of better clinical outcome comparable to that obtained using a median cut-off value. However, many studies have found that increased tumour-infiltrating Tregs are linked with poor outcome in breast cancers [48–53]. The fact that mere presence of Tregs is insufficient evidence to predict their effect on disease outcome has been confirmed in a recent meta-analysis: Jiang et al. found that the likelihood of finding high Treg numbers was affected by HER2, ER and PR status, and that an abundant Treg infiltrate had opposing prognostic significance in hormone receptor-negative and hormone receptor-positive tumours [54], hinting at possible interactions of hormone receptor-mediated and immune-regulatory pathways.
Burstein et al. defined four subtypes of TNBC based on distinct molecular profiles, two of which were dominated by specific immune-related gene expression signatures: BLIS TNBCs exhibit relatively lower numbers of B and T cells, accompanied by down-regulated expression of genes related to natural killer cell, immune-regulating and stimulatory cytokine pathway; in contrast, BLIA TNBCs exhibit an abundant B and T cell infiltrate and an immune-stimulatory expression profile and were associated with improved prognosis [36]. Our analyses of the dataset generated by Burstein et al. further revealed high levels of expression of Treg-associated genes in the BLIA subtype, thereby strengthening the evidence for an association between abundant Treg infiltrate and an active anti-tumoural immune response with the potential to significantly prolong survival of TNBC patients.
High tumour-infiltrating CD8+ T cells have long been considered as a good prognostic factor in breast cancers [16, 17, 24, 55]. A recent study by West et al. in ER− breast tumours showed that high CD8A gene expression was associated with high IL2RA gene expression, a commonly used Treg marker. The authors also noted that cytotoxicity of CD8+ T cell as well as pro-inflammatory cytokines were increased in CD25-high tumours relative to CD25-low tumours, suggesting that the anti-tumour immunity could be active in ER− breast tumours in spite of the presence of Tregs [24]. We therefore asked whether the same mechanisms might be active in TNBC: adopting CD25 as a Treg marker, we interrogated the dataset published by Burstein et al. [36] and found that the extent of CD25 gene expression was closely correlated with that of CD8A and the B cell marker CD19 (Online Resource: Fig. S2).
IPA of the 31-gene signature observed in the high Treg group of TNBCs showed nine genes in particular that are associated with inflammatory response, immune cell trafficking and cell-mediated immune response (Online Resource: Table S2). Some of these genes have been known to be critical in tumour control; for example, RORA has been identified as a potential tumour suppressor in breast cancers [56, 57]. These nine genes are highly expressed in the high intra-tumoural Treg compared to low intra-tumoural Treg TNBCs, further supporting our notion that this group is associated with a higher level of immune response within the tumour microenvironment, which is inherently enriched in cytotoxic T cells and B cells. We speculate that the particular microenvironment of TNBC may attenuate the Tregs’ immunosuppressive functions. It is also possible that the Tregs maintain their suppressive function, but not sufficiently to suppress the anti-tumour activity of CD8+ cytotoxic T cells and CD20+ B cells. In this case, depletion of Tregs from TNBCs could further enhance anti-tumour immunity beyond the pre-existing anti-tumoural microenvironment. Our data suggest that the favourable prognostic effect of Tregs in TNBCs may primarily be due to (a) the concomitant infiltration of CD8+ T cells; (b) physical contact with other T cells thereby exerting immune suppression [58] and (c) multiple factors involved in anti-tumour immunity, e.g. suppression of tumour-associated macrophages.
The interaction between T cells and B cells is known to be critical in several cancers including colorectal, lung, pancreatic and hepatocellular carcinomas [35, 59, 60]. Tumour-infiltrating B cells often co-localise with tumour-infiltrating T cells, especially in lymphoid aggregates, boosting T cell role in long-term immune response [61, 62]. Recently, Garnelo et al. demonstrated that the density of CD3+ T cells and CD20+ B cells was associated with better clinical outcome and reduced tumour aggressiveness in hepatocellular carcinoma [35], while the presence of tumour-infiltrating B cells has been linked with good prognosis in breast cancers [63]. Genetic studies support these findings which show a significant association between high densities of infiltrating B cells and favourable prognosis in TNBCs [64]. Interestingly, a single marker, immunoglobulin κC, has recently been shown to have similar predictive and prognostic value in breast cancers as the entire B cell metagene [65]. We also observed a positive association of B cells with favourable survival outcomes in our cohort, warranting further studies to examine the link between Tregs and B cells in TNBCs.
The presence of various immune cell populations within the tumour microenvironment, for example, a tumour microenvironment containing antigen-presenting cells (i.e. B cells or dendritic cells) and effector cells (i.e. T cells) is associated with better survival than cases where a single immune cell population is present [60, 61, 66]. In this study, we observed the presence and close proximity of Tregs with CD8+ T cells and CD20+ B cells. Since CD8+ T cells and Tregs share expression of numerous chemokine receptors (e.g. CCR5, CXCR3 and CXCR6) involved in extravasation, it may not be surprising that they co-infiltrate breast tumours [67].
In conclusion, our study demonstrates that a higher density of intra-tumoural Foxp3+ Tregs in TNBC is significantly positively associated with better clinical outcome. These findings highlight the need for a more integrated and comprehensive approach to phenotypic and functional profiling of Tregs within tumours in order to understand whether this cell population is likely to be playing a beneficial, deleterious or bystander role in any particular situation. Moreover, given the evidence for the influence of hormone receptor status upon the prognostic direction of Treg abundance in breast cancers, further mechanistic studies of this interaction are warranted. Greater understanding of the roles of Tregs and the diverse influences and interactions determining their effector function will be necessary in order to serve as a prognostic marker and to guide the development of novel therapeutic interventions.
References
Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH (2009) Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol 23:123–133
Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA (2015) DIfferences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the united states. JAMA 313:165–173. doi:10.1001/jama.2014.17322
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948. doi:10.1056/NEJMra1001389
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, Fralick M, Kumar R, Clemons M (2009) Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 9:29–33. doi:10.3816/CBC.2009.n.005
Thike AA, Yong-Zheng Chong L, Cheok PY, Li HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J, Tan PH (2014) Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol 27:352–360. doi:10.1038/modpathol.2013.145
Cheng CL, Thike AA, Tan SY, Chua PJ, Bay BH, Tan PH (2015) Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat 151:99–111. doi:10.1007/s10549-015-3371-x
Matsumoto H, Koo SL, Dent R, Tan PH, Iqbal J (2015) Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol. doi:10.1136/jclinpath-2015-202944
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, Andre F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kummel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33:983–991. doi:10.1200/JCO.2014.58.1967
Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, Fujiwara Y (2012) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 132:793–805. doi:10.1007/s10549-011-1554-7
Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, Andre F (2015) Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 26:1518. doi:10.1093/annonc/mdv241
Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, Watanabe G, Tada H, Suzuki A, Ohuchi N, Ishida T (2015) Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 17:124. doi:10.1186/s13058-015-0632-x
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, Piccart MJ, Loibl S, Denkert C, Smyth MJ, Joensuu H, Sotiriou C (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550. doi:10.1093/annonc/mdu112
Hainaut P, Plymoth A (2013) Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr Opin Oncol 25:50–51. doi:10.1097/CCO.0b013e32835b651e
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE 7:e50946. doi:10.1371/journal.pone.0050946
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1:54–67. doi:10.1158/2159-8274.CD-10-0028
Stagg J, Allard B (2013) Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol 5:169–181. doi:10.1177/1758834012475152
Anders CK, Deal AM, Miller CR, Khorram C, Meng H, Burrows E, Livasy C, Fritchie K, Ewend MG, Perou CM, Carey LA (2011) The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer 117:1602–1611. doi:10.1002/cncr.25746
Oleinika K, Nibbs RJ, Graham GJ, Fraser AR (2013) Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol 171:36–45. doi:10.1111/j.1365-2249.2012.04657.x
Joshi Nikhil S, Akama-Garren Elliot H, Lu Y, Lee D-Y, Chang Gregory P, Li A, DuPage M, Tammela T, Kerper Natanya R, Farago Anna F, Robbins R, Crowley Denise M, Bronson Roderick T, Jacks T (2015) Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43:579–590. doi:10.1016/j.immuni.2015.08.006
Nishikawa H, Sakaguchi S (2010) Regulatory T cells in tumor immunity. Int J Cancer 127:759–767
Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO (2014) Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 16:432. doi:10.1186/s13058-014-0432-8
West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH (2013) Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 108:155–162. doi:10.1038/bjc.2012.524
Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, Onoda N, Tanaka S, Ohsawa M, Hirakawa K (2016) Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 103:845–854. doi:10.1002/bjs.10127
Thike AA, Iqbal J, Cheok PY, Tse GM, Tan PH (2013) Ductal carcinoma in situ associated with triple negative invasive breast cancer: evidence for a precursor-product relationship. J Clin Pathol 66:665–670. doi:10.1136/jclinpath-2012-201428
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134:1543–2165
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: american Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271. doi:10.1093/annonc/mdu450
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. doi:10.1038/nature14011
Stack EC, Wang C, Roman KA, Hoyt CC (2014) Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70:46–58. doi:10.1016/j.ymeth.2014.08.016
Abel EJ, Bauman TM, Weiker M, Shi F, Downs TM, Jarrard DF, Huang W (2014) Analysis and validation of tissue biomarkers for renal cell carcinoma using automated high-throughput evaluation of protein expression. Hum Pathol 45:1092–1099
Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21:998–1009
Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V (2015) Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 15:2015–310814
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK, Hilsenbeck SG, Chang JC, Mills GB, Lau CC, Brown PH (2015) Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 21:1688–1698. doi:10.1158/1078-0432.CCR-14-0432
Matsumoto H, Thike AA, Li H, Yeong J, Koo SL, Dent RA, Tan PH, Iqbal J (2016) Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat 156:237–247. doi:10.1007/s10549-016-3743-x
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26:317–325. doi:10.1038/nbt1385
Felsenstein KM, Saunders LB, Simmons JK, Leon E, Calabrese DR, Zhang S, Michalowski A, Gareiss P, Mock BA, Schneekloth JS Jr (2016) Small molecule microarrays enable the identification of a selective, quadruplex-binding inhibitor of MYC expression. ACS Chem Biol 11:139–148. doi:10.1021/acschembio.5b00577
Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak H, Langer R (2011) Forty-year journey of angiogenesis translational research. Sci Transl Med. doi:10.1126/scitranslmed.3003149
Gilkes DM, Semenza GL, Wirtz D (2014) Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14:430–439. doi:10.1038/nrc3726
Artacho-Cordon F, Rios-Arrabal S, Lara PC, Artacho-Cordon A, Calvente I, Nunez MI (2012) Matrix metalloproteinases: potential therapy to prevent the development of second malignancies after breast radiotherapy. Surg Oncol 21:e143–e151. doi:10.1016/j.suronc.2012.06.001
Scheel C, Weinberg RA (2012) Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol 22:396–403. doi:10.1016/j.semcancer.2012.04.001
Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66:8319–8326. doi:10.1158/0008-5472.CAN-06-0410
Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, Thiery JP (2014) Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med 6:1279–1293. doi:10.15252/emmm.201404208
Cook LM, Hurst DR, Welch DR (2011) Metastasis suppressors and the tumor microenvironment. Semin Cancer Biol 21:113–122. doi:10.1016/j.semcancer.2010.12.005
Lee S, Cho EY, Park YH, Ahn JS, Im YH (2013) Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol 52:73–81. doi:10.3109/0284186X.2012.731520
Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, Koura K, Takahashi R, Otsuka H, Takahashi H, Iwakuma N, Nakagawa S, Fujii T, Sasada T, Yamaguchi R, Yano H, Shirouzu K, Kage M (2013) FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol 1:625–632. doi:10.3892/mco.2013.107
Lal A, Chan L, Devries S, Chin K, Scott GK, Benz CC, Chen YY, Waldman FM, Hwang ES (2013) FOXP3-positive regulatory T lymphocytes and epithelial FOXP3 expression in synchronous normal, ductal carcinoma in situ, and invasive cancer of the breast. Breast Cancer Res Treat 139:381–390. doi:10.1007/s10549-013-2556-4
Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T (2009) Regulatory T cells: how do they suppress immune responses? Int Immunol 21:1105–1111. doi:10.1093/intimm/dxp095
Chatila TA (2009) Regulatory T cells: key players in tolerance and autoimmunity. Endocrinol Metab Clin North Am 38:265–272. doi:10.1016/j.ecl.2009.01.002
Mellanby RJ, Thomas DC, Lamb J (2009) Role of regulatory T-cells in autoimmunity. Clin Sci (Lond) 116:639–649. doi:10.1042/CS20080200
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192. doi:10.1200/JCO.2008.18.7229
Jiang D, Gao Z, Cai Z, Wang M, He J (2015) Clinicopathological and prognostic significance of FOXP3 + tumor infiltrating lymphocytes in patients with breast cancer: a meta-analysis. BMC Cancer 15:727. doi:10.1186/s12885-015-1742-7
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH (2011) Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 13:R126. doi:10.1186/bcr3072
Xiong G, Wang C, Evers BM, Zhou BP, Xu R (2012) RORalpha suppresses breast tumor invasion by inducing SEMA3F expression. Cancer Res 72:1728–1739. doi:10.1158/0008-5472.CAN-11-2762
Du J, Xu R (2012) RORalpha, a potential tumor suppressor and therapeutic target of breast cancer. Int J Mol Sci 13:15755–15766
Shevach EM (2009) Mechanisms of foxp3 + T regulatory cell-mediated suppression. Immunity 30:636–645. doi:10.1016/j.immuni.2009.04.010
Driessens G, Kline J, Gajewski TF (2009) Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev 229:126–144. doi:10.1111/j.1600-065X.2009.00771.x
Linnebacher M, Maletzki C (2012) Tumor-infiltrating B cells: the ignored players in tumor immunology. Oncoimmunology 1:1186–1188. doi:10.4161/onci.20641
Nelson BH (2010) CD20 + B cells: the other tumor-infiltrating lymphocytes. J Immunol 185:4977–4982. doi:10.4049/jimmunol.1001323
Pimenta EM, Barnes BJ (2014) Role of tertiary lymphoid structures (tls) in anti-tumor immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers 6:969–997. doi:10.3390/cancers6020969
Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, Lehr HA, Hengstler JG, Kolbl H, Gehrmann M (2008) The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 68:5405–5413. doi:10.1158/0008-5472.CAN-07-5206
Hanker LC, Rody A, Holtrich U, Pusztai L, Ruckhaeberle E, Liedtke C, Ahr A, Heinrich TM, Sanger N, Becker S, Karn T (2013) Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res Treat 137:407–416
Schmidt M, Micke P, Gehrmann M, Hengstler JG (2012) Immunoglobulin kappa chain as an immunologic biomarker of prognosis and chemotherapy response in solid tumors. Oncoimmunology 1:1156–1158. doi:10.4161/onci.21653
Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH (2009) Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 4:e6412. doi:10.1371/journal.pone.0006412
Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M (2012) CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 72:4351–4360. doi:10.1158/0008-5472.CAN-12-0579
Funding
This study was funded by the SingHealth Duke-NUS Pathology Academic Clinical Program Budding Clinician-Scientist grant (ACP PATH BCS 14 001), A*STAR Biomedical Research Council, National Medical Research Council Stratified Medicine Programme Office (SMPO201302) awarded to Dr. Puay Hoon Tan and a Transition Award from the Singapore National Medical Research Council (NMRC/TA/0041/2015) awarded to Dr. Jabed Iqbal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The SingHealth Centralized Institutional Review Board (CIRB) approved the authors’ request for waiver of informed consent based on ethical consideration (Ref: 2013/664/F and 2015/2199). The SingHealth CIRB operates in accordance with the ICH/Singapore Guideline for Good Clinical Practices and with the applicable regulatory requirement(s).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yeong, J., Thike, A.A., Lim, J.C.T. et al. Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat 163, 21–35 (2017). https://doi.org/10.1007/s10549-017-4161-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4161-4