Abstract
Purpose
The purpose of the study was to evaluate protein expression of PD-L1 and CD20 as prognostic biomarkers of patient outcome in inflammatory breast cancer (IBC) samples.
Methods
PD-L1 and CD20 protein expression was measured by immunohistochemistry in 221 pretreatment IBC biopsies. PD-L1 was assessed in tumor cells (PD-L1+ tumor cells) and tumor stromal infiltrating lymphocytes (PD-L1+ TILs); CD20 was scored in tumor-infiltrating B cells. Kaplan–Meier curves and Cox proportional hazard models were used for survival analysis.
Results
PD-L1+ tumor cells, PD-L1+ TILs, and CD20+ TILs were found in 8%, 66%, and 62% of IBC, respectively. PD-L1+ tumor cells strongly correlated with high TILs, pathological complete response (pCR), CD20+ TILs, but marginally with breast cancer-specific survival (BCSS, P = 0.057). PD-L1+ TILs strongly correlated with high TILs, CD20+ TILs, and longer disease-free survival (DFS) in all IBC and in triple-negative (TN) IBC (P < 0.035). IBC and TN IBC patients with tumors containing both CD20+ TILs and PD-L1+ TILs (CD20+TILs/PD-L1+TILs) showed longer DFS and improved BCSS (P < 0.002) than patients lacking both, or those with either CD20+ TILs or PD-L1+ TILs alone. In multivariate analyses, CD20+TILs/PD-L1+TILs status was an independent prognostic factor for DFS in IBC (hazard ratio (HR): 0.53, 95% CI 0.37–0.77) and TN IBC (HR: 0.39 95% CI 0.17–0.88), and for BCSS in IBC (HR: 0.60 95% CI 0.43–0.85) and TN IBC (HR: 0.38 95% CI 0.17–0.83).
Conclusion
CD20+TILs/PD-L1+TILs status represents an independent favorable prognostic factor in IBC and TN IBC, suggesting a critical role for B cells in antitumor immune responses. Anti-PD-1/PD-L1 and B cell-activating immunotherapies should be explored in these settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory breast cancer (IBC) is a rare, poorly understood, and highly aggressive form of breast cancer. It accounts for less than 3% of all breast cancers, but is responsible for ~ 10% of all breast cancer-related deaths [1]. While multidisciplinary approaches incorporating neoadjuvant chemotherapy (NACT), surgery, and radiation therapy have modestly improved survival of IBC patients [2, 3], IBC has worse survival than stage-matched non-IBC with a 5-year survival of ~ 30% [4,5,6]. Hence, there is an urgent unmet clinical need to develop more effective therapies for IBC, and to develop prognostic and predictive biomarkers that identify patients who will potentially benefit from various treatment interventions.
Immune checkpoint inhibitors targeting the programmed cell death receptor 1 (PD-1) and/or its ligand (PD-L1) have shown clinical efficacy in various tumors [7, 8], including breast cancer [9]. PD-L1 is expressed on tumor-infiltrating immune cells and tumor cells where it plays a major role in immune suppression by binding to its receptors, PD-1 and B7.1 (CD80). This interaction results in suppression of T cell activation, induction of T cell apoptosis, and promotion of tumor immune escape [10, 11]. Blocking PD-L1 and PD-1 can relieve this inhibition and increase tumor-specific T cell immunity. Several antibodies specific for PD-1/PD-L1 have shown positive clinical responses in metastatic triple-negative (TN) breast cancer patients [9, 12]. Antibody blockade of the PD-1/PD-L1 axis therefore represents an attractive therapeutic approach for IBC. The protein levels of PD-L1 and the cells expressing PD-L1 in IBC, however, are unknown.
While there are several studies associating PD-L1 with favorable outcome in breast cancer [13,14,15,16], the prognostic role of B cells has not been well studied. A few reports identify CD20+ B cells within the tumor microenvironment as an important and clinically relevant factor that is strongly associated with response to NACT [17] and better patient outcome [18]. The clinical and prognostic role of B cells (identifiable by constitutive CD20 expression) in IBC is unknown. The objective of this study was to determine the clinical value of PD-L1 and CD20 as prognostic factors of disease-free (DFS) and breast cancer-specific survival (BCSS) in a large well-characterized cohort of IBC specimens.
Methods
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Committee for the Protection of Human Subjects at Dartmouth College (STUDY00029655), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Clinical specimens
Formalin-fixed paraffin embedded tissues (FFPETs) collected before initiation of neoadjuvant chemotherapy from 221 primary IBC patients diagnosed and treated at the Pierre et Marie Curie Cancer Center (PMCCC, Algiers, Algeria) between 2005 and 2009 were utilized in this study. This cohort has previously been partially described [19]. IBC was clinically defined according to the international consensus criteria [20]: Rapid onset (less than 6 months) of breast erythema, edema, and/or “peau d’orange,” and/or warm breast, with or without an underlying palpable mass. IBC samples were diagnostic biopsies (AJCC stages 3–4). The histological grading of the tumors was performed in accordance with the Bloom-Richardson classification. Pathological Complete Response (pCR) was defined as the absence of any residual invasive cancer in the breast and the absence of any metastatic cells in the regional lymph nodes (ypT0/is, ypN0) following completion of NACT [21]. Clinical and pathologic patient parameters are summarized in Supplementary Table S1. To establish the basal levels of CD20 expression in normal tissues, we used 50 breast tissue samples obtained from patients who underwent surgery for non-cancer-related treatments at the PMCCC during the same time period.
Evaluation of tumor-infiltrating lymphocytes (TILs)
Standard hematoxylin and eosin-stained full sections of pretreatment tumor tissue were used to evaluate the presence of TILs per international guidelines [22]. Briefly, stromal lymphocytic infiltration was defined as the percent of stromal areas containing mononuclear cells including lymphocytes, plasma cells and macrophages, and stratified using a median cut-point (with infiltration in ≥ 15% tumor stroma area defined as high TILs).
Immunohistochemistry and scoring of CD20 and PD-L1 expression
CD20 and PD-L1 protein expression levels were evaluated and reported following REMARK guidelines [23]. FFPETs were used to build tissue microarrays (TMAs; two 1.5 mm cores per case) as described elsewhere [19]. CD20 and PD-L1 protein expression was determined by IHC staining at the University of New Mexico Cancer Center and Johns Hopkins Hospital, respectively. IHC for CD20 (mouse monoclonal antibody; clone L26 at a concentration of 0.16 µg/mL for 20 min at room temperature; Dako, Carpintería, CA, USA) was performed on the Ventana’s BenchMark Ultra automatic stainer (Ventana Medical Systems, Tucson, AZ) per manufacturer’s instructions. IHC for PD-L1 was performed with the anti-PD-L1 monoclonal antibody SP142 (Spring Bioscience, Pleasanton, CA) at a concentration of 0.096 µg/mL, and antigen retrieval was performed in a citrate buffer, pH 6.0, as previously described in detail [24]. A biotinylated anti-rabbit IgG (Becton–Dickinson Biosciences, San Jose, CA) was used as a secondary antibody. Signal was developed using the ABC kit (Vector Elite PK-6100, Burlingame, CA) followed by amplification with the Perkin Elmer (Waltham, MA) tyramide signal amplification plus biotin kit (dilution 1:50). Samples were visualized using Streptavidin-HRP at a 1:300 dilution in TBST (Dako, Carpintería, CA) followed by DAB chromogen (Sigma, St. Louis, MO) and a hematoxylin counterstain. Breast tissues with known CD20+TILs and PD-L1+ status were used as positive controls; the same tissues, incubated with an isotype-matched antibody, were used as negative controls. Immunostaining was scored by board-certified pathologists (CD20 by NJ and PD-L1 by ACM) blinded to patient clinicopathologic characteristics. The percentages of membranous PD-L1 immunostaining in invasive carcinoma (epithelial) cells (PD-L1+ tumor cells) and in TILs (PD-L1+ TILs) were scored separately. PD-L1 positivity was defined as ≥ 5% of TILs or tumor cells expressing PD-L1, and staining was scored as an average percentage across all tissue microarray spots. The ≥ 5% cut-off point has been reported to be associated with clinical response to anti-PD-1 therapy [8]. Membranous CD20 immunostaining in ≥ 1% TILs was considered positive; this cut-off point has been associated with patient outcome in breast cancer [25]. Immunostaining and scoring for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) on this cohort were described previously [19].
Chemo-, hormone, and radiotherapy
Neoadjuvant anthracycline and taxane-based chemotherapy was administered to 95% of IBC patients. HER2 + patients (3 + IHC) received trastuzumab starting in 2008 when the drug became available in the Algerian cancer center. A combination of tamoxifen and goserelin was provided to 39% of IBC patients. Aromatase inhibitors were provided to 25% of IBC. Ninety-seven percent of IBC patients underwent mastectomy, while the remaining 3% of patients died before surgery could be performed. Radiotherapy was provided to 84% of IBC patients.
Statistics
Primary outcomes were BCSS and DFS. BCSS was calculated from the date of diagnosis with death from breast cancer scored as an event and censoring of other patients at the date of last follow-up or non-disease-related death. The DFS interval was calculated from the date of diagnosis to development of first recurrence. Patients without recurrence were censored at the time of last follow-up or death. Ten stage IV IBC samples were excluded from the DFS analysis. Chi-square and Fisher’s exact tests were used to compare demographic, clinical, and pathological data between IBC patients and PD-L1+ and CD20+ results between samples. For the analysis of PD-L1+, CD20+TILs, and combined CD20+ TILs and PD-L1+ TILs, patients were divided into two groups (positive and negative) as described above. BCSS and DFS for the groups defined by PD-L1+, CD20+TILs, and CD20+TILs/PD-L1+TILs and other variables (age, size, tumor grade, pCR, lymph node status, lymphovascular invasion (LVI), TILs, ER, PR, HER2, TN, CD + 20, PD-L1+ tumor cells, PD-L1+ TILs, and CD20+TILs/PD-L1+TILs) were plotted using Kaplan–Meier curves and compared using the log-rank test. The Cox proportional hazard model with single covariates was used to obtain the hazard ratios (HRs) and associated 95% confidence intervals (CIs) for the groups compared. Primary multivariable analyses were performed using the Cox model, with candidate variables of age (< 50, ≥ 50 years), number of positive nodes (≤ 3, ≥ 4), LVI (yes, no), nuclear grade (2 vs. 3), and ER, PR, HER2, CD20, PD-L1 (positive vs. negative), and the presence of CD20+TILs/PD-L1+TILs (presence of both vs. others) statuses. Variables found to be statistically significant in univariate analyses were considered for inclusion in the multivariate model. Multivariate Cox proportional models were built to examine the effect of CD20+TILs, PD-L1+, and CD20+TILs/PD-L1+TILs statuses on BCSS and DFS adjusted by the effect of age, size, tumor grade, pCR, lymph node status, lymphovascular invasion, TILs, ER, PR, HER2, and TN as covariates. Final multivariate models were obtained by a Cox stepwise procedure and verified by backward elimination [26]. For each ordinal variable, the lowest value was used as the reference in computing hazard ratios (HR). Survival rates and HRs are presented with their 95% CIs. Wald tests were used to test for significance of HRs. Two-tailed P values less than 0.05 were considered statistically significant. Statistical analyses were carried out using SAS (version 9.3) and GraphPad Prism (version 7.02) software.
Results
Increased stromal TILs correlates with PD-L1 and CD20 expression
A majority (66%) of IBCs contained PD-L1+ TILS, whereas 8% of IBCs showed PD-L1 expression on tumor cells (Fig. 1a, and Table S1). Sixty-two percent of IBCs contained CD20 + TILs; none of the tumors expressed CD20 on the malignant epithelial cells. The CD20+ TILs showed both diffuse and aggregate localization patterns, with higher numbers of B cells generally observed within the stroma rather than within tumor cell nests (Fig. 1b).
Immunostaining for PD-L1 and CD20 in inflammatory breast cancer (IBC) samples. (a) PD-L1 + TILs in the tumor stromal area, but not in tumor cells. (b) The same case also displays scattered CD20 + B cells. (c) There is a greater distribution of percentage PD-L1 + TILs compared to percentage PD-L1 + tumor cells across IBC cases. (d) Similarly, there is a greater distribution of percentage of CD20 + B cells across IBC cases compared to normal breast tissue. The percentage of positive cells is shown on the y-axis. Data are mean ± SD of PD-L1 or CD20 expression. Analysis was carried out using the Student’s t-test. *, P < 0.001 for all
Overall, there was a strong correlation between the presence of PD-L1+ tumor cells and PD-L1+ TILs (P = 0.037; data not shown). PD-L1 expression levels in TILs were heterogeneous, with a wider range relative to tumor cells. In particular, the distribution of PD-L1+ TIL levels across the 221 tumors was statistically higher than tumor PD-L1+ (Fig. 1c). A similar range of CD20 expression levels was observed in IBC samples compared to normal non-cancer-related breast tissues (Fig. 1d).
There was a statistically significant association between high stromal TILs and the presence of either CD20+ TILs, PD-L1+ tumor cells, or PD-L1+ TILs (P < 0.005; Table S2). When tumors were stratified as lymphocyte predominant breast cancer (LPBC; ≥50% tumor stromal area occupied by TILs), 15% of IBC patients were identified as LPBC (Table S1). As with high stromal TILs, LPBC status also significantly correlated with the presence of CD20+ TILs, PD-L1+ tumor cells, and PD-L1+ TILs (P < 0.02; Table S2).
Tumor cell PD-L1 and TILs PD-L1 expression differentially correlates with clinical variables and outcome in IBC
Univariate analysis showed that the presence of PD-L1+ tumor cells was significantly associated with higher survival and pCR rates, TN status, and the presence of CD20+ TILs (Table 1); the presence of PD-L1+ TILs was significantly associated with higher survival rates and the presence of CD20+ TILs (Table 1). In addition, the presence of PD-L1+ TILs was significantly associated with histologic grade 3 tumors (75% vs. 60% in grade 2 tumors; P = 0.030; data not shown). These correlations persisted when the presence of PD-L1+ TILs was analyzed as a continuous variable (Wilcoxon Rank sum test; data not shown). The presence of PD-L1+ tumor cells was marginally associated with improved BCSS (Fig. 2a), but not with DFS (P = 0.122; data not shown). In contrast, the presence of PD-L1+ TILs was significantly associated with better DFS (Fig. 2b), but not with BCSS (P = 0.108; data not shown). Further, in TN IBC patients, PD-L1+ TILs showed a trend towards better DFS (Fig. 2c) and significantly improved BCSS (Fig. 2d).
PD-L1+ tumor cells and PD-L1+ TILs are associated with improved outcome in IBC and TN IBC patients. Kaplan–Meier survival estimates of BCSS (a, d) and DFS (b, c) in patients with PD-L1+ tumor cells (a) or PD-L1+ TILs (b-d) compared to negative (< 5%) PD-L1 + tumor cells or PD-L1 + TILs. The number of patients at risk of death and/or relapse from IBC are shown at 0, 12, 24, 36, 48, 72, 84, and 96 months below the x-axis
CD20 TILs expression prognosticates DFS in IBC patients
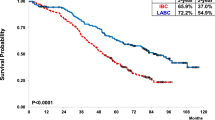
The presence of CD20+ TILs was significantly associated with higher survival rates, absence of relapses, and higher pCR (Table 1). The presence of CD20+ TILs was also associated with improved DFS, but not with BCSS in IBC patients (Fig. S1).
Co-existence of CD20+ TILs and PD-L1+ TILs shows a synergistic effect on clinical variables and improved outcome in IBC patients
Given the significant association of CD20+ TILs, PD-L1+ TILs, and PD-L1+ tumor cells with clinical outcomes (Table 1), we next analyzed the effect of the presence of CD20+ TILs plus PD-L1+ TILs, and CD20+ TILs plus PD-L1+ tumor cells on clinical variables. We found that the presence of CD20+ TILs with PD-L1+ tumor cells was significantly associated with high survival rates, high pCR rates, high TILs, and TN IBC (Table 2). The presence of CD20+ TILs and PD-L1+ TILs was associated with high survival rates, absence of relapses, high pCR, and high TILs (Table 2). Next, we analyzed the effect of the presence of CD20+ TILs and PD-L1+ TILs on BCSS and DFS. We found that combining CD20+ TILs with PD-L1+ TILs resulted in a highly significant improvement in both DFS (Fig. 3a) and BCSS (Fig. 3b). As illustrated in Fig. 2b, a 50% DFS rate was observed at ~ 54 months for patients with PD-L1+ TILs irrespective of CD20 status, whereas the same DFS rate was observed at ~ 62 months for patients with both CD20+ TILs and PD-L1+ TILs (compare Fig. 2b (P = 0.035) with 3a (P = 0.0005)). Further, while the DFS curves are nearly parallel for patients with PD-L1+ TILs until ~ 50 months (Fig. 2b), the survival curve for patients with CD20+ TILs and PD-L1+ TILs separates as early as ~ 30 months and remains higher for the period analyzed (Fig. 3a). This pattern was striking in BCSS for the combination of the two biomarkers. In particular, the presence of PD-L1+ TILs was not associated with BCSS (P = 0.108; data not shown), but patients with both CD20+ TILs and PD-L1+ TILs showed an improved BCSS as early as 30 months (P = 0.002; Fig. 3b). Further, subset analysis in TN IBC patients showed that while the presence of PD-L1+ TILs was not associated with DFS (Fig. 2c), the presence of CD20+ TILs and PD-L1+ TILs was significantly associated with improved DFS (Fig. 3c), and with BCSS (Fig. 3d).
Co-existence of CD20+ TILs and PD-L1+ TILs prognosticates better outcome in IBC and TN IBC patients. Kaplan–Meier survival estimates of DFS (a, c) and BCSS (b, d) for patients with CD20+ TILs and PD-L1+ TILs (positive) in IBC (a, b) and TN IBC (c, d) compared to patients without both CD20+ TILs and PD-L1+ TILs (negative). The number of patients at risk of death and/or relapse from TN IBC are shown at 0, 12, 24, 36, 48, 72, 84, and 96 months below the x-axis
Expression of tumor cell PD-L1, TILs PD-L1, and TILs CD20, as well as the co-existence of CD20 and PD-L1 expression, by tumor subtype, is shown in Supplementary Table S3. Other than the associations with TN IBC described above, there were no significant associations with ER, PR, and HER2 status.
Multivariate analysis
Multivariate analysis, using a stepwise evaluation, and verified by backward and subset variable analyses, determined that the presence of both CD20+ TILs and PD-L1+ TILs was the best predictor tested as a significant favorable prognostic factor of DFS and BCSS in the entire IBC cohort and among TN IBC patients (Table 3).
Discussion
To our knowledge, this is the largest cohort used to characterize immune infiltrates in IBC, and the first study to examine both an immune checkpoint inhibitor and CD20 in IBC. The strong correlation between PD-L1 expression, independent of the cellular location, with the presence of high stromal TILs, suggests the existence of a pre-existing immune-active microenvironment suppressed by PD-L1 [7], and strongly supports the exploration of anti-PD-1/PD-L1 therapy in IBC patients. As part of standard neoadjuvant therapy similar to that administered in the United States, Algerian IBC patients receive doxorubicin, cyclophosphamide, and taxanes, all of which are bona fide inducers of immunogenic cell death [27]. Hence, one could expect that pre-existing immunity could be activated with these agents and further amplified with anti-PD1/PD-L1 treatment to result in efficacious treatments in IBC patients whose tumors contain PD-L1+ TILs.
Only a few prior studies have examined PD-L1 expression in IBC [28, 29]. A study of 112 IBCs by Bertucci, et al. reported high PD-L1 mRNA in pretreatment IBC samples, a strong association between PD-L1 mRNA with pCR, TILs, basal and HER2 tumor types, and a gene signature characteristic of a strong cytotoxic response involving T cells, dendritic cells, and B cells [28]. He et al. analyzed 68 post-neoadjuvant IBC tumor samples and report that worse overall survival was significantly associated with positive PD-L1 status [29]. We found that PD-L1 in either tumor or TILs was associated with pCR and high TILs. However, unlike Bertucci, et al. and He et al., our study found significant associations between PD-L1+ tumor cells and improved BCSS, and between PD-L1+ TILs and longer DFS. The prognostic role of PD-L1 in breast cancer remains unclear, with studies often describing contradicting results. As He et al. summarize in their recent report, PD-L1 expression has been shown to be both a favorable and an adverse variable [29]. The discrepancy in outcome correlations amongst studies may be the result of variations in cohort size, specimen types, IHC techniques, and cut-off values. The divergence in cell type expressing PD-L1 and BCSS vs. DFS points to potential immune differences in cellular or cytokine variables that induce PD-L1 on tumors vs. on TILs. It is possible that the improved DFS association with expression of PD-L1 on TILs may imply a more systemic antitumor immune response.
The study reported here is the first to highlight the clinical relevance of CD20+ B cells in IBC. Our findings suggest that, in addition to T cell-mediated immunity, B cells play an important role in antitumor immunity in IBC. It has been shown that CD20+ TILs were strongly associated with breast cancer response to NACT [17] and with improved BCSS and longer DFS [18]. Of note, in both of these studies, the prognostic role of CD20+ TILs was independent of CD8+ TILs, underscoring the biological relevance of B cell immunity in conjunction with the cellular immune response in breast cancer [17, 18].
It has been proposed that there is a clonally restricted, antigen-directed B cell antitumor response, and the presence of somatic hypermutation sequences is suggestive of antigen-experienced B cells in the tumor microenvironment [30]. The positive prognostic synergy observed in IBC tumors with both CD20+ TILs and PD-L1+ TILs might be the result of cooperation between the cellular and humoral immune responses. While cellular injury and immunogenic cell death induced by chemotherapy can lead to formation of new immunogenic epitopes, chemokine and cytokine secretion, antigen cross-presentation, activation of dendritic cells, and induction of tumor-specific cytotoxic T cells (cellular response) [31], the presence of B cells might enhance such T cell responses by producing antibodies against breast tumor antigens, stimulating cytokine and chemokine secretion, serving as local antigen-presenting cells, and organizing the formation of tertiary lymphoid structures that sustain long-term immunity (humoral response) [32]. As immunotherapy for breast cancer advances, it will be important to evaluate B cell presence, antigen specificity, and activity to determine the extent of B cell modulation and its relationship to patient response. Additional studies, including validation in a second cohort, are warranted to confirm the synergistic relationship between T cells and B cells in IBC.
In addition to the large number of IBC samples evaluated, this study has a few other strengths that should be noted. First, the diagnosis of IBC in all patients was confirmed using standard clinical and histopathological methods [20]. Second, the IBC database is well annotated and from the main cancer center in Algeria, and is likely well representative of the general population in Algeria. Due to socialized health care in Algeria, the IBC patients received similar standard of care treatments, which reduces treatment variability when analyzing clinical outcomes. Third, the CD20 and PD-L1 labeling were characterized with validated antibodies, and PD-L1 scoring was performed separately on tumor cells and TILs in each cancer sample providing a unique opportunity to evaluate the individual effect of PD-L1 expression on clinical associations and patient outcome.
By identifying IBC as a disease likely to benefit from immunomodulatory treatments, this study provides a rationale for the use of targeted immunotherapies. Antibodies that block the PD-1/PD-L1 axis and immunomodulatory therapies supporting B cell responses are potentially valuable therapeutic approaches targeting T and B cell infiltrated tumors in IBC, which currently has poor prognosis with the currently available treatment strategies.
Abbreviations
- IBC:
-
Inflammatory breast cancer.
- TN:
-
Triple-negative.
- PD-1:
-
Programmed cell death 1.
- PD-L1:
-
Programmed cell death ligand 1.
- TILs:
-
Tumor-infiltrating lymphocytes.
- pCR:
-
Pathological complete response.
- BCSS:
-
Breast cancer-specific survival.
- DFS:
-
Disease-free survival.
- HR:
-
Hazard ratio.
- CI:
-
Confidential interval.
- NACT:
-
Neoadjuvant chemotherapy.
- FFPETs:
-
Formalin-fixed paraffin embedded tissues.
References
Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH (2005) Trends in Inflammatory Breast Carcinoma Incidence and Survival: The Surveillance, Epidemiology, and End Results Program at the National Cancer Institute. J Natl Cancer Inst 97(13):966–975. https://doi.org/10.1093/jnci/dji172
Dawood S, Lei X, Dent R, Gupta S, Sirohi B, Cortes J, Cristofanilli M, Buchholz T, Gonzalez-Angulo AM (2014) Survival of women with inflammatory breast cancer: a large population-based study. Ann Oncol 25(6):1143–1151. https://doi.org/10.1093/annonc/mdu121
Pierga J-Y, Petit T, Delozier T, Ferrero J-M, Campone M, Gligorov J, Lerebours F, Roché H, Bachelot T, Charafe-Jauffret E, Pavlyuk M, Kraemer S, Bidard F-C, Viens P (2012) Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol 13(4):375–384. https://doi.org/10.1016/S1470-2045(12)70049-9
Schlichting JA, Soliman AS, Schairer C, Schottenfeld D, Merajver SD (2012) Inflammatory and non-inflammatory breast cancer survival by socioeconomic position in the Surveillance, Epidemiology, and End Results database, 1990–2008. Breast Cancer Res Treat 134(3):1257–1268. https://doi.org/10.1007/s10549-012-2133-2
Dawood S, Ueno N, Valero V, Woodward W, Buchholz T, Hortobagyi G, Gonzalez-Angulo A, Cristofanilli M (2011) Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer 117(9):1819–1826
Gonzalez-Angulo AM, Hennessy BT, Broglio K, Meric-Bernstam F, Cristofanilli M, Giordano SH, Buchholz TA, Sahin A, Singletary SE, Buzdar AU, Hortobagyi GN (2007) Trends for Inflammatory Breast Cancer: Is Survival Improving? Oncologist 12(8):904–912. https://doi.org/10.1634/theoncologist.12-8-904
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. https://doi.org/10.1038/nature14011
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New Engl J Med 366(26):2443–2454. https://doi.org/10.1056/NEJMoa1200690
Nanda R, Chow LQM, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 34(21):2460–2467. https://doi.org/10.1200/jco.2015.64.8931
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med 8(8):793–800
Chen Daniel S, Mellman I (2013) Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012
Emens LA (2018) Breast Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res 24(3):511–520. https://doi.org/10.1158/1078-0432.ccr-16-3001
Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD (2014) Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 146(1):15–24. https://doi.org/10.1007/s10549-014-2988-5
Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett D, Kennedy CW, Gluch L, Carmalt H, Mak C, Warrier S, Gee HE, Chan C, McLean A, Walker E, McNeil CM, Beith JM, Swarbrick A, Scolyer RA, O’Toole SA (2016) Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 69(1):25–34. https://doi.org/10.1111/his.12904
Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL (2014) In Situ Tumor PD-L1 mRNA Expression Is Associated with Increased TILs and Better Outcome in Breast Carcinomas. Clin Cancer Res 20(10):2773–2782. https://doi.org/10.1158/1078-0432.ccr-13-2702
Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6(7):5449–5464
Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V (2014) Multiplexed Quantitative Analysis of CD3, CD8, and CD20 Predicts Response to Neoadjuvant Chemotherapy in Breast Cancer. Clin Cancer Res 20(23):5995–6005. https://doi.org/10.1158/1078-0432.ccr-14-1622
Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, Ellis IO, Green AR (2011) Tumor-Infiltrating CD8 + Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol 29(15):1949–1955. https://doi.org/10.1200/jco.2010.30.5037
Chaher N, Arias-Pulido H, Terki N, Qualls C, Bouzid K, Verschraegen C, Wallace AM, Royce M (2012) Molecular and epidemiological characteristics of inflammatory breast cancer in Algerian patients. Breast Cancer Res Treat 131(2):437–444. https://doi.org/10.1007/s10549-011-1422-5
Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A, Krishnamurthy S, Robertson FM, Woodward WA, Yang WT, Ueno NT, Cristofanilli M (2010) International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol 22:515–523. https://doi.org/10.1093/annonc/mdq345
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Altman DG, McShane LM, Sauerbrei W, Taube SE (2012) Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLOS Medicine 9(5):e1001216. https://doi.org/10.1371/journal.pmed.1001216
Sunshine JC, Nguyen PL, Kaunitz GJ, Cottrell TR, Berry S, Esandrio J, Xu H, Ogurtsova A, Bleich KB, Cornish TC, Lipson EJ, Anders RA, Taube JM (2017) PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin Cancer Res 23(16):4938–4944. https://doi.org/10.1158/1078-0432.ccr-16-1821
Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR (2012) The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 132(2):545–553. https://doi.org/10.1007/s10549-011-1620-1
Lachin JB Methods. John Wiley and Sons, NY, pp 272–2852000
Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, Bracci L, Breckpot K, Brough D, Buqué A, Castro MG, Cirone M, Colombo MI, Cremer I, Demaria S, Dini L, Eliopoulos AG, Faggioni A, Formenti SC, Fučíková J, Gabriele L, Gaipl US, Galon J, Garg A, Ghiringhelli F, Giese NA, Guo ZS, Hemminki A, Herrmann M, Hodge JW, Holdenrieder S, Honeychurch J, Hu H-M, Huang X, Illidge TM, Kono K, Korbelik M, Krysko DV, Loi S, Lowenstein PR, Lugli E, Ma Y, Madeo F, Manfredi AA, Martins I, Mavilio D, Menger L, Merendino N, Michaud M, Mignot G, Mossman KL, Multhoff G, Oehler R, Palombo F, Panaretakis T, Pol J, Proietti E, Ricci J-E, Riganti C, Rovere-Querini P, Rubartelli A, Sistigu A, Smyth MJ, Sonnemann J, Spisek R, Stagg J, Sukkurwala AQ, Tartour E, Thorburn A, Thorne SH, Vandenabeele P, Velotti F, Workenhe ST, Yang H, Zong W-X, Zitvogel L, Kroemer G, Galluzzi L (2014) Consensus guidelines for the detection of immunogenic cell death. OncoImmunology 3(9):e955691. https://doi.org/10.4161/21624011.2014.955691
Bertucci F, Finetti P, Colpaert C, Mamessier E, Parizel M, Dirix L, Viens P, Birnbaum D, van Laere S (2015) PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 6(15):13506–13519
He J, Huo L, Ma J, Zhao J, Bassett RL, Sun X, Ueno NT, Lim B, Gong Y (2018) Expression of Programmed Death Ligand 1 (PD-L1) in Posttreatment Primary Inflammatory Breast Cancers and Clinical Implications. Am J Clin Pathol 149(3):253–261. https://doi.org/10.1093/ajcp/aqx162
Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM, Serody JS (2014) Prognostic B-cell Signatures Using mRNA-Seq in Patients with Subtype-Specific Breast and Ovarian Cancer. Clin Cancer Res 20(14):3818–3829. https://doi.org/10.1158/1078-0432.ccr-13-3368
Pelekanou V, Carvajal-Hausdorf DE, Altan M, Wasserman B, Carvajal-Hausdorf C, Wimberly H, Brown J, Lannin D, Pusztai L, Rimm DL (2017) Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Res 19(1):91. https://doi.org/10.1186/s13058-017-0884-8
Nelson BH (2010) CD20 + B Cells: The Other Tumor-Infiltrating Lymphocytes. J Immunol 185(9):4977–4982. https://doi.org/10.4049/jimmunol.1001323
Acknowledgements
This study was supported in part by the GlaxoSmithKline Oncology Ethnic Research Initiative Grant (Drs. Arias-Pulido and Chaher; no grant number), and the UICC ICRETT fellowship (Dr. Chaher; ICR/09/043), and the National Institutes of Health (Dr. Prossnitz; CA163890). We would like to thank the PMCCC Human Tissue Repository for providing tissue samples and clinical data (Algiers, Algeria); Karen Buehler (Tricore, NM) for technical support with IHC. The authors thank Dr. J. Louise Lines of the Department of Microbiology and Immunology, Norris Cotton Cancer Center Geisel School of Medicine at Dartmouth for critical reading of this manuscript and insightful comments, Donald Fitzpatrick (Computing and Media Services; Dartmouth Biomedical Libraries) for help with the graphs, and Mrs. Kathleen Bryar for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LAE receives research funding from Genentech, Roche, EMD Serono, Merck, Astra Zeneca, and Corvus. She has served on advisory boards for Astrazeneca, Medimmune, Syndax, Bayer, and Abbvie. ACM receives research funding from Bristol-Myers Squibb. The remaining authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2018_4834_MOESM1_ESM.tif
Fig. S1. The presence of CD20+ TILs is associated with improved DFS, but not BCSS in IBC patients. Kaplan-Meier survival estimates of DFS (a) and BCSS (b) in IBC patients with positive (≥1%) CD20+ TILs compared to negative (<1%) CD20+ TILs. The number of patients at risk of death and/or relapse from IBC are shown at 0, 12, 24, 36, 48, 72, 84, and 96 months below the x axis. Supplementary material 1 (TIFF 3187 KB)
Rights and permissions
About this article
Cite this article
Arias-Pulido, H., Cimino-Mathews, A., Chaher, N. et al. The combined presence of CD20 + B cells and PD-L1 + tumor-infiltrating lymphocytes in inflammatory breast cancer is prognostic of improved patient outcome. Breast Cancer Res Treat 171, 273–282 (2018). https://doi.org/10.1007/s10549-018-4834-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4834-7