Abstract
Metastasis, especially bone metastasis, is a major cause of cancer-related deaths, which is associated with long-term pain due to skeletal-related events and poor quality of life. Tumor cells alter the bone microenvironment through aberrant activation of osteoclasts and osteoblasts which induces bone osteolysis and release of growth factors leading to cancer growth. Though this phenomenon has been well characterized, bone-targeted therapies have shown little improvement in patient survival. Recent evidence indicates a growing appreciation for the complex bone environment, in addition to bone-remodeling stromal cells, which includes an abundance of myeloid immune cells that can either protect against or contribute to the progression of the disease within the bone cavity. Additionally, myeloid cells are recruited into primary tumor sites, where they promote development of the pre-metastatic niche and also can regulate tumor progression within the tumor-bone microenvironment through a milieu of complex mechanisms and involving heterogeneous myeloid populations. In this review, we have highlighted the complex roles of myeloid immunity in bone metastasis and hope to bring attention to the potential of novel immunotherapeutic interventions for the elimination of bone metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metastasis occurs when cancer cells invade the surrounding tissue, intravasate into the lymphatics or blood circulation, successfully extravasate into distant tissues, and proliferate into secondary lesions. It is a substantially inefficient process requiring both intrinsic (i.e., genetic alterations) and extrinsic variables (i.e., stromal and immune cell interactions within the primary tumor and distant tissue microenvironments) that collectively facilitate tumor metastasis [1]. Due to its rich reservoir of growth factors, including insulin-like growth factor-1 (IGF-1) and transforming growth factor beta (TGFβ), the skeleton is an optimal tissue site for disseminated tumor cells [2, 3]. Despite its nutrient-rich environment, not all cancers will metastasize to the skeleton, a phenomenon described by Stephen Paget in 1918 in the “seed and soil hypothesis” which emphasizes the importance of specific seeds/cancer types thriving in select “soil” conditions/tissue environments [4, 5]. Importantly, successful establishment of disseminated cells and growth into secondary tumor lesions in the bone requires concerted efforts of the disseminated cells and stromal cells within the bone environment.
The bone consists of mineralized matrix that gets actively remodeled by bone-resorbing osteoclasts and bone-forming osteoblasts, to maintain skeletal integrity [6]. Skeletal micro-fractures result in the release of sclerostin produced by osteocytes, adult osteoblast cells embedded in the bone, which starts the resorption process mediated by osteoclasts, giant multi-nucleated cells derived from macrophages [7,8,9]. Osteoclasts degrade the bone through the secretion of acids, matrix-degrading enzymes, and hydrogen ions. TGFβ are released from the degraded bone matrix, which recruit osteoblastic precursor cells that differentiate in adult bone-forming osteoblasts which act to replace the resorbed bone [10,11,12,13]. Disseminated tumor cells promote the formation and, as a result, the hyperactivation of osteogenesis through secretion of bone remodeling factors, including parathyroid-related hormone (PthRP), that contributes to excessive bone degradation. The release of bone-derived factors promotes tumor proliferation, continued bone resorption, and the propagation of what has been characterized as a vicious cycle of tumor progression [14]. Our group and others have described this phenomenon in other review articles [14,15,16,17,18,19], and, for the sake of brevity, we will refrain from providing further detail on this topic.
Although current bone-targeted therapies, such as zoledronic acid and denosumab, significantly reduce skeletal lesions and skeletal-related events (SREs), they have little to no impact on patient survival [20, 21], demonstrating a significant need for novel therapeutic interventions for bone metastasis. In recent years, there has been an increased appreciation for the role of other cell types, including myeloid cells in bone metastasis and the growth of metastatic cells within the bone microenvironment [14, 22]. Common myeloid progenitors (CMPs) give rise to all myeloid cells, and each cell type has an essential role in tissue homeostasis and in bridging the innate and adaptive immunity response [23]. Myeloid cells and immature myeloid cells comprise ~ 30–40% of the bone marrow compartment compared to < 3% CD4 and CD8 lymphocytes, and more studies are identifying critical roles for myeloid cells in the progression of bone metastatic disease [24]. Tumor cells can promote myeloid cell production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα), as well as growth factors that promote proliferation and vessel formation, including TGFβ and vascular endothelial growth factor (VEGF), respectively [25]. Cancer cells specifically recruit myeloid cells into the primary tumor microenvironment where they can suppress anti-tumoral immunity and promote chronic inflammation resulting in tumor cell proliferation and dissemination [26]. Importantly, this can occur through cancer-mediated hematopoiesis and facilitation of the expansion and mobilization of tumor-promoting myeloid cells [23]. Pro-tumoral myeloid cells can contribute to the dissemination of tumor cells, but there is little evidence of how they contribute to the growth of secondary tumor lesions within the bone microenvironment.
Although the bone is a reservoir for myeloid cells, their roles and heterogeneous functions within the tumor-bone microenvironment are understudied; understanding myeloid cell contribution in the evolving tumor-bone microenvironment is necessary for the development of efficacious therapy. In this review, we have highlighted evidence demonstrating the importance of myeloid immunity in bone metastasis, including monocytes, macrophages, dendritic cells (DCs), granulocytes, and other CMP-derived cells (mast cells, platelets, and megakaryocytes), with a focus on specific cell populations and evidence of immunotherapeutic interventions.
2 Monocytes/macrophages within the tumor-bone microenvironment
Monocytes are generated in the bone marrow from CMPs and typically circulate in the bloodstream, patrolling for infections and/or tissue damage, where they are recruited into tissue sites of inflammation or pathogenic infections and stimulated to differentiate into macrophages [27]. In the bone microenvironment, monocytes are able to differentiate into tumor-associated macrophages (TAMs) as well as osteoclasts, both of which play vital roles in bone metastasis [28]. Macrophages are one of the main components of the mononuclear phagocyte system which includes blood monocytes and tissue macrophages. Blood monocytes migrate to tissues and differentiate to macrophages. In inflammation, macrophages have three major roles; phagocytosis, antigen presentation, and immunomodulation via production of various cytokines and growth factors [29]. They are activated during inflammation by multiple signals including cytokines (interferon γ, GM-CSF, and tumor necrosis factor-α (TNF-α), bacterial lipopolysaccharide (LPS), extracellular matrix proteins, and other chemical mediators. Deactivation is regulated by anti-inflammatory cytokines such as interleukin-10 (IL-10) and TGFβ [29,30,31,32]. TAMS are able to directly promote tumor progression, through secretion of inflammation-regulating molecules, and indirectly through suppression of antitumor immunity (Fig. 1) [33]. Although classically categorized into M1 and M2 subtypes, ongoing evidence has demonstrated a spectrum of macrophage subtypes that contribute to tumor progression. The M1, or pro-inflammatory macrophages, are activated by different factors such as interferon gamma (IFN γ) and LPS [34]. M1 is cytotoxic cells against tumor cells and intracellular microorganisms and produces nitric oxide, TNF, and reactive oxygen intermediates [35]. Anti-inflammatory M2 macrophages can be further classified into M2a, M2b, and M2c subtypes, depending on the signals from the microenvironment that determine their activation. M2c is considered as the most immunosuppressive of these subtypes [36]. M2a-like and M2b-like macrophages secrete chemokine (C-C motif) ligand 1 (CCL1) and IL-10 to support regulatory cell recruitment and to further shape a tolerogenic microenvironment [37].
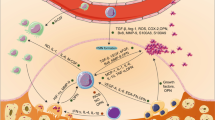
The impact of bone marrow myeloid immune cells on tumors in the bone. The representative figure shows factors secreted by myeloid-derived immune cells that can promote tumor progression in the bone. Osteoclasts (OCs), osteoblasts (OBs), myeloid-derived suppressor cell (MDSC), interleukins (IL-10, IL-1beta, IL-6), colony-stimulating factor (CSF), receptor activator of nuclear factor kappa-Β/ligand (RANK/RANK-L), parathyroid hormone-related protein (PTHrP), matrix metalloproteinase 9 (MMP9), fibroblast growth factor (bFGF), C-X-C chemokine receptor type 4 (CXCR4), C-C chemokine receptor type 2 (CCR2), transforming growth factor beta (TGFb), vascular endothelial growth factor (VEGF). Created with BioRender.com
The role of monocytes/macrophages in the tumor-bone microenvironment is largely attributed to macrophage differentiation into osteoclasts. However, recent studies have identified monocyte/macrophage function in bone tumors through chemokine and glycoprotein (colony-stimulating factor) signaling. Monocyte chemo-attractant protein 1 (MCP-1; CCL2) belongs to the CC chemokine family and is important for inflammatory cell recruitment, including monocytes and macrophages, via signaling through C-C chemokine receptor type 2 (CCR2) (Figure 1) [38]. The bone marrow is a major source of CCL2, and increased CCL2 secretion from bone marrow endothelial cells has been shown to recruit prostate cancer cells to the bone niche and promote tumor proliferation in bone [39]. Additionally, CCL2 has been shown to create a fertile environment for bone metastasis through TAM recruitment and promotion of osteoblast and osteoclast activity [40]. A previous study showed that CCL2 expression by bone metastatic PC3 cells resulted in the recruitment of TAMs into the primary tumor and osteoclast formation within the bone microenvironment. Specifically, CCL2 was expressed in luciferase-expressing PC3 cells; CCL2 had no impact on PC3 proliferation compared to control PC3. Conditioned medium of CCL2-overexpressing PC3 cells was shown to be a significant chemoattractant for mouse monocytes compared to control PC3 cells in vitro. This was verified using subcutaneous PC3 xenograft models as well, such that antibody treatment against CCL2 inhibited macrophage recruitment into PC3 primary tumors and, as a result, reduced the growth of PC3 tumors. Intracardiac bone metastasis models of PC3-CCL2 cells showed enhanced tumor growth in the bone; similar to the subcutaneous model findings, CCL2 inhibition inhibited tumor growth indirectly through reduced osteoclast formation and activity within the bone microenvironment. This study demonstrates that CCL2 increases tumor growth and bone metastasis through recruitment of macrophages and osteoclasts to the tumor in the bone [41].
Likewise, another study showed that depletion of monocytes/macrophages or reducing the infiltration of macrophages can significantly reduce the occurrence of bone metastasis [42, 43]. Other studies showed that infiltration of monocytes/macrophages facilitated bone metastases via stimulation of colony-stimulating factor 1 (CSF-1) and CSF-1 receptor [44, 45] supporting the extensive evidence that monocytes and macrophages can promote bone metastasis, albeit through different mechanisms.
In a prostate cancer (PCa) skeletal metastasis model, a previous study found a significant correlation between high levels of phagocytic CD68+ cells and Gleason score in human PCa samples [46]. Likewise, high numbers of M2-like macrophages (F4/80+CD206+) were observed in PCa bone tumors in mice. In in vitro co-culture experiments, soluble factors produced from efferocytic macrophages significantly increased the number of PC3, bone metastasis–derived prostate cancer cells. Trabectedin is a marine-derived alkaloid isolated from the Caribbean tunicate Ecteinascidia turbinata, shown to selectively deplete monocytes and macrophages in the blood and spleen of 4 different animal models. In vivo exposure to trabectedin was able to reduce M2-like (F4/80+CD206+) macrophages and reduced skeletal metastatic tumor growth after PC3 cell intracardiac inoculation. The same study also showed that trabectedin as a preventative therapy given 7 days prior to PC3 cell injection significantly reduced both bone tumor size and M2-like macrophages in the marrow. This indicates that M2-like macrophages mediate prostate cancer bone metastasis and that trabectedin treatment is able to prevent this process [46].
Finally, although macrophages have an established role in primary tumors, emerging evidence suggests that macrophages in the bone support cancers which preferentially metastasize to the skeleton.
A recent study revealed that CD137, a member of the TNF receptor superfamily, facilitates migration of monocytes/macrophages into the tumor niche through upregulation of Fra1, a transcriptional factor shown to promote cell migration and epithelial-mesenchymal transition (EMT) [47]. Further, CD137 was found to promote the differentiation of monocytes/macrophages into osteoclasts providing a suitable niche for colonization and growth of breast cancer cells in the bone. Using F4/80-targeted liposomal nanoparticles encapsulating the anti-CD137 blocking antibody, bone and lung metastases of breast cancer cells were significantly inhibited [47]. Similarly, in a mouse model of breast cancer, evidence showed that macrophage-stimulating protein (MSP) mediates osteolytic bone metastasis. MSP is a serum protein involved in macrophage accumulation and activation. MSP was also shown to stimulate mammary epithelial cells to invade extracellular matrix, indicating that these cells directly respond to MSP. Overexpression of MSP was associated with increasing metastatic bone foci in patients with breast cancer demonstrating a diversity of roles of macrophages in bone metastatic breast cancer [48].
It has been reported that breast cancer cells prefer the bone marrow and can remain dormant for decades [49]. Additionally, bone marrow metastasis of breast cancer can recur decades after the first diagnosis and treatment indicating the long-term survival of disseminated cancer cells in a dormant state. Although this phenomenon is understudied, emerging evidence suggests that macrophages can play a role in the “waking” of dormant breast cancer cells within the bone marrow. Specifically, a recent study showed that exosomes from macrophage subtypes showed differential regulation of breast cancer dormancy in bone [50]. Exosomes from M1 macrophages were able to bring breast cancer cells out of quiescence via activation of nuclear factor kappa beta (NFkB), whereas M2 macrophages formed gap junctions with breast cancer stem cells, inducing their quiescence. This suggests that macrophage subtypes within the bone marrow are critical mediators of the transition of dormant breast cancer to breast cancer progression into a secondary lesion in the bone microenvironment [51].
Macrophages have also been shown to contribute to progression of bone cancers, such as osteosarcoma. It was previously reported that M2-polarized TAMs were higher in patient’s tissue with osteosarcoma and facilitated cancer initiation as well as stemness of osteosarcoma cells [52, 53] . Treatment with all-trans retinoic acid (ATRA), an active metabolite of vitamin A, was able to attenuate TAM-induced osteosarcoma tumor formation via reduction of M2 polarization of TAMs, decreased colony formation and sphere-formation of osteosarcoma cells. Moreover, M2 macrophages enhanced an osteosarcoma stem cell phenotype through upregulation of CD133, CXCR4, Nanog, and Oct4, which was reversed by ATRA treatment [54]. Although bone cancer (i.e., oncogenesis of bone stromal cells) is markedly different from bone metastasis, it is possible that similarities exist in the tumor-bone macrophages that can be interrogated for potential therapeutic strategies. Collectively, these findings demonstrate that macrophages and monocytes contribute to metastasis to bone and tumor progression within the tumor-bone microenvironment.
3 Dendritic cells
Dendritic cells (DCs) are antigen-presenting cells (APCs) that act as liaison between the innate and adaptive immune systems [55] and were classically thought to be macrophages when first discovered by Dr. Ralph Steinman [56]. However, unlike macrophages, DCs show elongated cellular protrusions and lack phagocytic properties, though they share other common macrophage surface markers, such as CD68 and F4/80 [57, 58]. In addition to their role in after tissue injury, immature DCs capture antigen (Ag) and migrate to the lymphoid organs where they present Ag to CD4+ T helper cells to regulate Ag-specific immune cells, such as CD8+ cytotoxic T cells and B cells, and non-Ag-specific immune cells, such as macrophages, eosinophils, and natural killer (NK) cells [59, 60].
Although direct roles for DCs in bone metastasis is understudied, dendritic cells have been shown to contribute to bone destruction–associated diseases, including multiple myeloma. Specifically, it was reported that dendritic cells in the multiple myeloma bone environment displayed a high expression of osteoclast-related membrane receptors and were able to differentiate into osteoclasts which resulted in excessive bone destruction [61]. Additionally, osteoclasts released from bone destruction sites serve as an essential signal for activation and differentiation of immature dendritic cells through RANKL signaling. This suggests that both dendritic cells and osteoclasts can cooperate together to promote cancer-associated bone destruction and bone remodeling [62].
Defective dendritic cell (DC) function is dependent on high level of cytokines and adhesion molecules in bone marrow that can effect maturation and expansion of DCs; this was also previously shown in multiple myeloma (MM). Immature DCs (iDCs) co-cultured with myeloma cells showed clonogenic growth with osteoclasts-like phenotype. Activation of receptor activator of nuclear factor κB (RANK)–RANKL and CD47–thrombospondin (TSP)-I axes lead to iDCs undergoing OC-like trans-differentiation. This suggests that iDCs cooperate with malignant multiple myeloma cells to induce multiple myeloma osteoclastogenesis [61]. Further investigation is needed to understand the role of immature vs. mature DCs in bone metastasis.
Plasmacytoid DCs (pDCs) are a small population derived from hematopoietic stem cells found in the bone marrow, and they differentiate into the mature plasmacytic DCs in the marrow. However, there is limited information about their role in tumor progression [63]. Using metastatic models of breast cancer, (4T1 and PDCA-1), a study showed that plasmacytoid DCs can promote breast cancer growth and associated osteolysis. Upon intracardiac injection of 4T1 breast cancer cells, dissemination of breast cancer to the bone initiated the infiltration of macrophages and expansion of pDCs in the bone, triggering a DC-regulated Th2 immune response due to inflammatory factors such as, IL-15, CCL5/RANTES, and monocyte chemoattractant protein (MCP-1) that increased osteoclast differentiation and osteolysis [64]. The same study showed that pDC depletion led to a significant reduction of pro-inflammatory cytokines and increased CD8 T cell activity, which was associated with an absence of bone metastasis. These findings were further verified in IFNAR-/- mice which lack pDCs and showed reduced 4T1 breast cancer to bone metastasis. In addition, CXCL10, a member of the CXC chemokine family with a role in angiogenesis and tumor growth in the bone, was upregulated significantly in DCs-depleted mice [64], suggesting that DCs may control CXCL10 and its promotion of cancer (Fig. 1).
4 Granulocytes in the tumor-bone microenvironment
Granulocytes are the most abundant human leukocytes, accounting for ~ 70% of total leukocytes in the blood, and have a crucial role in the innate immune system. Granulocytes include neutrophils, eosinophils, and basophils; however, neutrophils are the most abundant population and are typically found in the bone marrow and spleen and in circulation [65]. Granulocytes are characterized by cytosolic granules, which are secreted into the extracellular space in response to pathogenic stimulation [66]. Although there is little evidence of granulocyte roles in bone metastasis, the most abundant findings are of neutrophils in bone metastasis so we have focused on those studies.
Polymorphonuclear leukocytes/neutrophils
Neutrophils are considered the first line of defense against pathogens [67]. In response to released chemoattractants, neutrophils are stimulated to mobilize from the bone marrow or circulation to sites of pathogenic infection where they can eliminate the invaders through different mechanisms, including: release of ROS in an oxidative burst, release of antibacterial enzymes, and production of neutrophil extracellular traps (NETs). Additionally, neutrophils will secrete pro-inflammatory cytokines and chemokines to recruit other myeloid cells, including macrophages [68, 69]. In the past decade, several studies have shown that neutrophils infiltrate tumor microenvironments (TME), where they can either promote or protect against tumor progression. However, their roles in tumors are still not fully clear [70, 71].
There are several factors in the tumor microenvironment which can affect neutrophil function, directing them to be either antitumor or pro-tumor tumor-associated neutrophils (TANs), such as TGF-β which promotes pro-tumor (N2) neutrophil phenotypes while IFN-β favors antitumor (N1) TAN [72, 73]. The neutrophil transition from anti-tumoral N1 and pro-tumoral N2 phenotypes is highly dependent on tissue context and microenvironmental stimuli. To date, the conversion of neutrophil function appears to be predominantly dependent on tumor type. A previous study inoculated either subcutaneous H22 hepatocarcinoma or B16 melanoma tumors with tumor-naïve neutrophils or neutrophils from the bone marrow of tumor-bearing mice (isolated 10 days after tumor inoculation). The tumor-naïve neutrophils suppressed growth of the primary tumor, whereas neutrophils from tumor-bearing mice promoted tumor growth suggesting that the primary tumor can regulate the conversion of neutrophil function within the bone marrow. In support of this idea, it was shown that IL-6 cooperated with G-CSF to modulate murine neutrophil function in the bone marrow before they entered the tumor milieu [74]. This led to increased STAT3 activation and resultant downregulation of interferon beta (IFN-β) expression in bone marrow macrophages by IL-6. High levels of STAT3 was important for upregulating the expression of Mmp9 and Bv8 genes and downregulating the expression of Trail and Rab27a genes in neutrophils, which correlated with attenuated exocytosis of neutrophil azurophilic granules, thus favoring tumor growth and tumor angiogenesis [74]. This study specifically demonstrated that neutrophil function was altered in the bone marrow to a phenotype that was maintained in circulation, after mobilization from the bone marrow, and after infiltration of the primary tumor. Further, another study showed similar results of neutrophil regulation within the bone marrow contributing to infiltration of pro-tumoral neutrophils in a breast cancer model [75]. However, it is unclear how these changes would impact growth of disseminated cells within the bone marrow.
Recently, our group revealed evidence showing that tumor-associated neutrophils have a role in bone metastatic prostate cancer and specifically protect against prostate cancer growth within the bone microenvironment. The majority of prostate cancer patients receive prostatectomy along with androgen deprivation therapy such that, in the event of development of bone metastatic disease, there is typically no longer a primary tumor present [76]. In a recently published data, we demonstrated that neutrophils are recruited to soluble factors from prostate cancer cells and localize to regions of prostate cancer lesions in patient bone biopsies. Bone marrow neutrophils were found to be cytotoxic and to induce apoptosis of bone metastatic prostate cancer (BM-PCa) cells, C42B, in vitro, such that depletion of neutrophils in in vivo intratibial bone models enhanced C42B tumor growth, by removing the suppressive neutrophil brake on growth [77]. This phenomenon appears to be linked to inhibition of STAT5, a transcription factor regulated by JAK/STAT activation and found to promote PCa progression [77]. Knockdown of STAT5 rendered the prostate cancer cells completely resistant to neutrophil killing suggesting STAT5 loss is a mechanism for cancer resistance to anti-tumoral neutrophils. Further, using co-culture killing assays of neutrophils isolated throughout tumor growth, we found that bone marrow neutrophils isolated from tumors 4 weeks after tumor inoculation (compared to one and 2 weeks after inoculation) lost their cytotoxicity against in vitro prostate cancer cells [77]. However, the tumor-derived neutrophils became less N1-like/anti-tumoral, though they did not demonstrate a switch to a pro-tumoral/N2-like response. This data further supports the findings that neutrophils within the tumor-bone microenvironment are initially anti-tumoral but lose their cytotoxic response through unknown mechanisms elicited by the advancing prostate tumor.
Therapy using neutrophils
With the emerging evidence of neutrophil involvement in cancer progression and metastasis, there are studies now beginning to investigate methods for therapeutic modulation of neutrophil function. Recently, it has been shown that remodeling of tumor microenvironments is a method used to enhance the delivery of nanoparticles (NPs). A study showed that direct priming of a tumor tissue using photosensitization rapidly activates neutrophil infiltration that mediates delivery of nanotherapeutics into the tumor. In photosensitization (PS), a photosensitizer absorbs the visible light in tissue and converts molecular oxygen to ROS, which induces acute inflammation and infiltration of neutrophils [78]. Chu et al. developed a photosensitization drug delivery method which consisted of nanoparticles coated with anti-CD11b antibodies to target activated neutrophils. The movement of nanoparticles coated with anti-CD11b (NPs-CD11b) was shown to be mediated by neutrophil infiltration induced through photosensitization. Neutrophil depletion was enough to abolish the nanoparticle tumor deposition suggesting that neutrophil tumor infiltration could be utilized to successfully deliver drugs using nanoparticle therapy [79]. Another study used nano-size neutrophil-mimicking drug delivery system (NM-NP) by coating neutrophil membranes on the surface with poly (latic-co-glycolic acid) nanoparticles (NPs). This NM-NP system displayed enhanced cellular association with circulating tumor cells (CTC) and high CTC capture efficiency in vivo compared with uncoated NP. In addition, reduced homing to the metastatic niche was noticed in this NM-NP system; with carfilzomib treatment, a second-generation proteasome inhibitor, the NM-NP was able to deplete circulating tumor cells in the blood which prevented early metastasis and, potentially, inhibited the progression of already-formed metastases. These findings suggest that inflammatory neutrophils possess both a CTC and niche-targeting property and this method of treatment may be important for reducing downstream formation of metastases [80]. The complexity of neutrophil subphenotypes must be considered in the development of neutrophil-mediated drug delivery and targeted neutrophil therapy [81].
5 The role of myeloid-derived suppressor cells (MDSC) in bone metastasis
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that suppress the activity of cytotoxic T lymphocytes (CTLs). Under non-disease conditions, immature myeloid cells (IMCs) originate in the bone marrow and can differentiate into granulocytes, macrophages, or dendritic cells. However, in response to molecular cues, partial blockage of IMC differentiation results in MDSCs which have been reported to be involved in various pathological conditions including cancer, trauma, stress, and chronic inflammatory state [82, 83]. In cancer, MDSCs have been demonstrated to suppress T cells through a number of mechanisms, including (Fig. 1) the following: (1) deprivation of arginine and cysteine amino acid, which are important for T cell function and proliferation [84] (2) production of ROS and nitric oxide (NO) which causes nitration of T cell receptors (TCR) and results in T cell and natural killer (NK) cell apoptosis [85, 86], (3) production of IL-10 and TGF-β1 to inhibit immune effector and cell functions [87], and (4) through increased programmed death-ligand 1 (PD-L1), an inhibitory signal [88].
MDSCs accumulate at primary and metastatic tumor sites, and this accumulation is associated with inhibition of antitumor immunity as well as tumor metastasis at distant tissue sites [89, 90]. Using the 4T1 mouse model of breast cancer bone metastasis, Sawant et al. showed that MDSCs from bone tumors can differentiate into functional osteoclasts in vitro and also in vivo when injected into the long bones of 4T1 tumor-bearing mice. Moreover, nitric oxide signaling was shown to be critical for differentiation of MDSCs into osteoclasts, suggesting cross-talk between myeloid progenitors and tumor cells through the process of osteoclast differentiation within the bone microenvironment [90]. Another study showed that leukocyte-derived MMP-9 resulted in the expansion of MDSCs within the bone marrow of MMTV Her2/neu mouse models. Elimination of MDSC numbers by treatment with bisphosphonates, standard of care therapy for osteoporosis and bone metastatic breast cancer [91], significantly reduced the tumor growth in the bone [92]. Based upon the role of bisphosphonates in targeting osteoclast function and survival, this finding is not surprising and demonstrates the similarities of monocytic myeloid populations and the potential for pan-myeloid therapies (e.g., against macrophages and osteoclasts) to target multiple cell populations in the bone microenvironment.
In the past decade, there has been more evidence demonstrating that MDSCs can be a heterogeneous population that can be further categorized into granulocytic/polymorphonuclear (PMN-MDSCs) or monocytic (M-MDSCs) and both subtypes suppress anti-tumor immunity and promote tumor growth [93]. Although there does not appear to be a specific subtype associated with bone metastases (in comparison to other metastatic sites), a study showed that PMN-MDSC recruitment into 4T1 mammary tumors promoted tumor progression to bone metastases [94]. A recent study showed an additional subtype in which granulocytes appeared to belong to the monocytic lineage and were named monocyte-like precursors of granulocytes (MLPGs). In contrast to steady-state conditions, these cells expanded significantly in tumor-bearing mice and differentiated to polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs). Downregulation of Rb1, tumor suppressor protein, has a role in the expansion of MLPGs which lead to abnormal myelopoiesis in cancer [95]. The importance of MDSC subtypes within the tumor-bone microenvironment is largely unknown despite the abundance of myeloid populations in the bone. However, more evidence will likely emerge soon based upon the developing results showing various subtypes involved in tumor metastasis.
Chemotherapy drugs have been shown to suppress MDSC expansion and numbers through effect on bone marrow cells [92]. Previously, the chemotherapeutic drug gemcitabine was reported to reduce MDSC numbers and, as result, caused rejection of established metastatic disease in the 4T1 mouse mammary model. Additionally, MDSC immunosuppression, including inhibition of T cell activation and interaction with macrophages to increase IL-10 and decrease IL-12 production was reversed by gemcitabine treatment [96]. Another study showed that gemcitabine-treated mice displayed less MDSCs and that breast cancer growth in the bone was also reduced. These studies collectively suggest that gemcitabine may be used not only as an anti-tumorigenic drug but also for decreasing bone destruction through regulation of MDSC populations in bone metastases [90].
6 Mast cells
Mast cells are immune cells found in all vertebrate animals [97] and are widely distributed in lymphatic vessels, stroma, epithelia, and blood [98]. Human mast cells originate in the bone marrow from CD34+, CD117+ (KIT) pluripotent hematopoietic stem cells [99]. However, mast cell progenitors mobilize into the bloodstream and complete their maturation in the tissue, where they participate in physiological and inflammatory processes including angiogenesis, lymphangiogenesis, wound healing, heart function, and tumorigenesis [100]. Additionally, mast cells have the ability to respond to immunologic insults such as injury, allergens, or other toxic products and starting inflammation process, which can be activated by IgE [101].
Mast cells in the tumor or tumor-associated mast cells (TAMCs) are common in solid and hematologic human tumor microenvironment [102], and, with regard to the bone environment, there is some evidence that TAMCs have a role in bone metastasis of multiple myeloma (MM). Previously, a high level of mast cells (MCs) was reported in the bone marrow and angiogenesis of 24 patients diagnosed with active MM compared with 34 patients with non-active MM. This might indicate that mast cells participate in angiogenesis and progression of MM [103]; however, this needs to be examined more closely. Another study that measured bone marrow mast cell density (MCD) in 52 patients diagnosed with active MM found MCD to be associated with high expression of RANKL and N-terminal propeptide of procollagen type I (Ntx). Furthermore, MCD correlated positively with high levels of immunohistochemical stain for tryptase, serum levels of MMP-9 and RANKL, along with urine levels of Ntx. This suggested that mast cells might participate in osteolytic activity and angiogenesis thereby promoting MM progression in the bone (Fig. 1) [104]. Further, TAMCs were shown to stimulate RANKL-dependent tumor-induced bone destruction and tumor growth and also stimulate RANKL-independent metastatic bone resorption. Therefore, targeting TAMCs could represent a potential approach to inhibit bone metastasis in gastric cancer patients [105, 106].
7 Megakaryocytes
Megakaryocytes (MKs) arise from common myeloid progenitor cells and are generated in the bone marrow from hematopoietic stem cell precursor cells and are also produced in the spleen, kidney, and liver [107]; mature MKs in response to stromal-derived factor-1 (SDF-1) produce and release platelets into circulation [108]. There are a small number of studies to demonstrate the importance of MKs in bone metastasis. Within the bone microenvironment, MKs have been shown to play a crucial role in bone metabolism and bone modulation and are able to inhibit osteoclast function and enhance osteoblast proliferation [109]. A study showed that MKs inhibit prostate cancer cell growth in the bone. Specifically, both K562 (human MK precursors) and primary MKs derived from mouse bone marrow was able to inhibit growth of prostate carcinoma PC3 cells in coculture. Direct growth inhibition occurred through cell cycle arrest via decreased cyclin D1 expression and stimulation of apoptosis via induction of apoptosis-associated specklike protein containing a CARD domain (ASC) and death-associated protein kinase 1 (DAPK1). Likewise, mice treated with recombinant thrombopoietin (TPO), a glycoprotein that promotes the proliferation and differentiation of MKs, showed reduced skeletal metastasis after intracardiac injection of PC3 cells, demonstration that MKs do not prevent dissemination into bone but are able to inhibit metastatic growth in the bone microenvironment [110]. These data suggest a protective role against MKs in bone metastatic prostate cancer in bone.
In support of this, increased megakaryopoiesis numbers were noticed at bone metastatic sites, but not primary tumor sites, in mice injected with metastatic mouse mammary carcinoma 4T1.2, (enhanced bone metastatic 4T1 cells) [111]. Similarly, TPO knockout BALB/cJ mice injected orthotopically with 4T1.2 cells displayed more aggressive metastasis to bone demonstrating the inhibitory effects of MKs. Clinically, the same study observed an increase in MKs in the bone marrow of 6 of 8 analyzed patient samples of metastatic breast cancer; however, this may suggest an increase of MK numbers in response to metastatic cells entering the bone marrow [111]. Collectively, these studies support a protective role of MKs against progression of bone metastatic disease within the bone compartment.
8 Platelets
Although we have primarily highlighted myeloid cell function within the tumor-bone microenvironment, we would be remiss to not acknowledge the importance of myeloid cells in dissemination to the bone. Platelets are the only CMP-derived immune cell shown to impact both dissemination to bone and growth into metastasis within the bone through interactions with bone stromal cells. Platelets are generated by MKs, play a crucial role in hemostasis and maintenance of blood barrier integrity, and produce a variety of factors that mediate inflammation and cancer progression [112]. Cancer cells have been shown to activate platelets to induce proteases and bioactive phospholipids that promote tumor angiogenesis [113]. With regard to bone metastasis, platelets have been shown to act in contrast to MKs and have been shown to promote bone metastasis via regulation of osteoclast activity. Beta integrins have been shown to contribute to tumor progression and metastasis [114, 115]. Platelets (and MKs) solely express αIIbβ3, whereas αvβ3 integrin is expressed on multiple cell types including osteoclasts, platelets, MKs, and endothelial cells. Based on the role of integrins in platelet recruitment, a previous study examined whether β3 integrins could contribute to platelet/osteoclast interactions and its role in bone metastasis [116]. Specifically, Bakewell et al. used a β3 integrin-depletion mouse model which showed osteolytic bone metastasis in 74% of β3+/+ mice compared to only 4% in β3-/- mice after intracardiac injection of B16 melanoma cells. Interestingly, β3-/- mice intratibially inoculated displayed a marrow replacement by tumor, but there was no associated trabecular bone resorption as seen in β3+/+ mice suggesting that β3 integrin is a critical mediator of bone resorption in the tumor-bone microenvironment [116]. To see the role of osteoclast versus platelet β3 integrin, the same study used osteoclast-defective Src-/- mice; Src-null mice were protected from tumor-associated bone destruction. However, metastasis to bone still occurred. Platelet aggregation inhibitor, a highly selective small molecule inhibitor of αIIbβ3 platelet function, prevented B16 melanoma metastasis to bone suggesting that αIIbβ3 on platelets regulate metastasis to bone and αvβ3 regulates metastatic growth in the bone through osteoclast activity and associated bone resorption [116]. Another study supported this evidence demonstrating that inhibition of platelet B3 integrin can reduce B16 melanoma metastasis to bone [117]. In a separate study, platelet-derived bioactive lipid, lysophosphatidic acid (LPA), enhanced bone metastasis. Treatment with integrilin, a platelet aggregation inhibitor, was able to reduce plasma levels of LPA and a decrease in osteolytic bone lesions from breast cancer [118](Fig. 1).
Autotaxin (ATX) is a lysophospholipase found in platelet granules and is important for basal levels of LPA in blood. ATX is elevated in many types of cancer including neuroblastoma, beta cell lymphoma, melanoma, and breast carcinomas; is associated with poor outcomes [119]; and has been shown to link platelets to cancer progression [120]. A study revealed high levels of ATX in murine breast carcinoma tissue. Mice inoculated with human breast cancer cells, MDA-B02, which do not express ATX, were treated with ATX inhibitor (BMP22) and showed a reduction in skeletal metastases and cancer cell colonization of bone [121, 122]. Collectively these findings demonstrate that platelets predominantly promote metastasis to bone. It is unclear whether platelets have predilection for promoting cancer metastasis to bone over other tissue sites though specific platelet integrin interactions with bone should be investigated for potential therapies.
9 Conclusion
Bone is a preferential site for homing of breast and prostate cancer disseminated cells but can be seen with other cancers as well, including but not limited to thyroid, bladder, and lung; however, breast and prostate display the most frequent incident of metastasis to bone compared to other cancers [123]. A critical component of cancer growth and progression within the bone marrow requires cancer-induced bone remodeling which provides a source of nutrients for metastatic cells and results in the well-characterized “vicious cycle” of tumor progression in bone [124]. However, the bone environment is comprised of ~ 5% of bone remodeling osteoblasts and osteoclasts whereas myeloid cells comprise about 30–40% of the marrow and have been emphasized in several studies to be mediators of tumor progression [125, 126]. Although there is an extensive evidence of myeloid cell’s roles in tumor progression and metastasis to the bone, we and others have identified that a number of myeloid-tumor interactions can either protect against or promote tumor growth within the bone suggesting the potential for myeloid-targeted immunotherapies for treating bone metastasis. It appears that specific myeloid cell types in the bone provide an amenable environment for cancer; however, it is unclear whether it differs based upon cancer type. Many of the studies highlighted in this review utilized preclinical bone metastatic cancers (such as breast, prostate, and multiple myeloma) and showed direct roles for myeloid cells in regulation of tumor growth in the bone, as well as dissemination to the bone. However, these findings do not explain whether myeloid cell function may differ in cancers that do not typically metastasize to the bone or if there are specific mechanisms of myeloid cells in the bone that may hinder growth or promote growth, for example, hindrance of a melanoma cell vs. promotion of a prostate cancer cell, such that there are specific cancer-induced microenvironmental changes within the bone. Further, it is possible that specific myeloid-tumor cell interactions regulate metastatic organotropism differently depending on cancer type. These considerations and additional studies are needed to fully define the importance of myeloid cells in bone metastasis and for the development of novel myeloid-targeted immunotherapy for treating bone metastatic cancers.
References
Valastyan, S., & Weinberg, R. A. (2011). Tumor metastasis: molecular insights and evolving paradigms. Cell, 147, 275–292.
Marie, P. (1997). Growth factors and bone formation in osteoporosis: roles for IGF-I and TGF-beta. Revue du Rhumatisme (English Ed.), 64, 44–53.
Linkhart, T. A., Mohan, S., & Baylink, D. J. (1996). Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone, 19, 1S–12S.
Ribatti, D., Mangialardi, G., & Vacca, A. (2006). Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clinical and Experimental Medicine, 6, 145–149.
Langley, R. R., & Fidler, I. J. (2011). The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. International Journal of Cancer, 128, 2527–2535.
Boskey, A. L., & Posner, A. S. (1984). Bone structure, composition, and mineralization. The Orthopedic Clinics of North America, 15, 597–612.
Kitaura, H., et al. (2020). Osteocyte-related cytokines regulate osteoclast formation and bone resorption. International Journal of Molecular Sciences, 21.
Udagawa, N., Koide, M., Nakamura, M., Nakamichi, Y., Yamashita, T., Uehara, S., Kobayashi, Y., Furuya, Y., Yasuda, H., Fukuda, C., & Tsuda, E. (2021). Osteoclast differentiation by RANKL and OPG signaling pathways. Journal of Bone and Mineral Metabolism, 39, 19–26.
McDonald, M. M., & Delgado-Calle, J. (2017). Sclerostin: an emerging target for the treatment of cancer-induced bone disease. Current Osteoporosis Reports, 15, 532–541.
Parfitt, A. M. (1976). The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone diseases. II. PTH and bone cells: bone turnover and plasma calcium regulation. Metabolism, 25, 909–955.
Hua, R., Zhang, J., Riquelme, M. A., & Jiang, J. X. (2021). Connexin gap junctions and hemichannels link oxidative stress to skeletal physiology and pathology. Current Osteoporosis Reports, 19, 66–74.
Karkache, I. Y., Damodaran, J. R., Molstad, D. H. H., & Bradley, E. W. (2021). Serine/threonine phosphatases in osteoclastogenesis and bone resorption. Gene, 771, 145362.
Rowe, P., Koller, A., & Sharma, S. (2021). Physiology, bone remodeling. In StatPearls. Treasure Island (FL).
Cook, L. M., Shay, G., Araujo, A., & Lynch, C. C. (2014). Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Reviews, 33, 511–525.
Cook, L. M., Araujo, A., Pow-Sang, J. M., Budzevich, M. M., Basanta, D., & Lynch, C. C. (2016). Predictive computational modeling to define effective treatment strategies for bone metastatic prostate cancer. Scientific Reports, 6, 29384.
Esposito, M., Guise, T., & Kang, Y. (2018). The biology of bone metastasis. Cold Spring Harbor Perspectives in Medicine, 8.
Coleman, R. E., Croucher, P. I., Padhani, A. R., Clézardin, P., Chow, E., Fallon, M., Guise, T., Colangeli, S., Capanna, R., & Costa, L. (2020). Bone metastases. Nature Reviews. Disease Primers, 6, 83.
Lin, S. C., Yu-Lee, L. Y., & Lin, S. H. (2018). Osteoblastic factors in prostate cancer bone metastasis. Current Osteoporosis Reports, 16, 642–647.
Yasuda, H. (2019). The mechanism of anti-RANKL antibody in the treatment of metabolic bone diseases including osteoporosis - possible applications of anti-RANKL antibody to the treatment of cancer patients. Nihon Yakurigaku Zasshi, 153, 11–15.
Frieling, J. S., Basanta, D., & Lynch, C. C. (2015). Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control, 22, 109–120.
Dionisio, M. R., et al. (2019). Clinical and translational pharmacology of drugs for the prevention and treatment of bone metastases and cancer-induced bone loss. British Journal of Clinical Pharmacology, 85, 1114–1124.
Ihle, C. L., & Owens, P. (2020). Integrating the immune microenvironment of prostate cancer induced bone disease. Molecular Carcinogenesis, 59, 822–829.
Wu, C., Hua, Q., & Zheng, L. (2020). Generation of myeloid cells in cancer: the spleen matters. Frontiers in Immunology, 11.
Zhao, E., Xu, H., Wang, L., Kryczek, I., Wu, K., Hu, Y., Wang, G., & Zou, W. (2012). Bone marrow and the control of immunity. Cellular & Molecular Immunology, 9, 11–19.
Awad, R. M., De Vlaeminck, Y., Maebe, J., Goyvaerts, C., & Breckpot, K. (2018). Turn back the TIMe: targeting tumor infiltrating myeloid cells to revert cancer progression. Frontiers in Immunology, 9, 1977.
Zamarin, D., et al. (2014). Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Science Translational Medicine, 6, 226ra232-226ra232.
Serbina, N. V., Jia, T., Hohl, T. M., & Pamer, E. G. (2008). Monocyte-mediated defense against microbial pathogens. Annual Review of Immunology, 26, 421–452.
Kylmaoja, E., et al. (2018). Peripheral blood monocytes show increased osteoclast differentiation potential compared to bone marrow monocytes. Heliyon, 4, e00780.
Fujiwara, N., & Kobayashi, K. (2005). Macrophages in inflammation. Current Drug Targets. Inflammation and Allergy, 4, 281–286.
Hart, P. H., & Whitty, G. A. (1988). Piccoli, D.S. & Hamilton, J.A. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. Increased TNF-alpha but not IL-1 activity. Journal of Immunology, 141, 1516–1521.
Randow, F., et al. (1997). In vitro prevention and reversal of lipopolysaccharide desensitization by IFN-gamma, IL-12, and granulocyte-macrophage colony-stimulating factor. Journal of Immunology, 158, 2911–2918.
Bundschuh, D. S., et al. (1997). Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. Journal of Immunology, 158, 2862–2871.
Cassetta, L., & Pollard, J. W. (2018). Targeting macrophages: therapeutic approaches in cancer. Nature Reviews Drug Discovery, 17, 887–904.
Gordon, S. (2003). Alternative activation of macrophages. Nature Reviews. Immunology, 3, 23–35.
Zhu, J., Zhi, Q., P. Zhou, B., Tao, M., Liu, J., & Li, W. (2016). The role of tumor associated macrophages in the tumor nicroenvironment: mechanism and functions. Anti-Cancer Agents in Medicinal Chemistry, 16, 1133–1141.
Rogers, T. L., & Holen, I. (2011). Tumour macrophages as potential targets of bisphosphonates. Journal of Translational Medicine, 9, 177.
Bianchini, R., Karagiannis, S. N., Jordakieva, G., & Jensen-Jarolim, E. (2020). The Role of IgG4 in the fine tuning of tolerance in IgE-mediated allergy and cancer. International Journal of Molecular Sciences, 21.
Lu, Y., Cai, Z., Xiao, G., Keller, E. T., Mizokami, A., Yao, Z., Roodman, G. D., & Zhang, J. (2007). Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Research, 67, 3646–3653.
Loberg, R. D., Day, L. S. L., Harwood, J., Ying, C., St. John, L. N., Giles, R., Neeley, C. K., & Pienta, K. J. (2006). CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia, 8, 578–586.
Craig, M. J., & Loberg, R. D. (2006). CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Reviews, 25, 611–619.
Mizutani, K., Sud, S., McGregor, N. A., Martinovski, G., Rice, B. T., Craig, M. J., Varsos, Z. S., Roca, H., & Pienta, K. J. (2009). The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia, 11, 1235–1242.
Hiraoka, K., Zenmyo, M., Watari, K., Iguchi, H., Fotovati, A., Kimura, Y. N., Hosoi, F., Shoda, T., Nagata, K., Osada, H., Ono, M., & Kuwano, M. (2008). Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Science, 99, 1595–1602.
Roelofs, A. J., Thompson, K., Ebetino, F. H., Rogers, M. J., & Coxon, F. P. (2010). Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Current Pharmaceutical Design, 16, 2950–2960.
Panni, R. Z., Linehan, D. C., & DeNardo, D. G. (2013). Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy, 5, 1075–1087.
Kaur, S., et al. (2017). Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. In Seminars in cell & developmental biology (Vol. 61, pp. 12–21). Elsevier.
Jones, J. D., Sinder, B. P., Paige, D., Soki, F. N., Koh, A. J., Thiele, S., Shiozawa, Y., Hofbauer, L. C., Daignault, S., Roca, H., & McCauley, L. K. (2019). Trabectedin reduces skeletal prostate cancer tumor size in association with effects on M2 macrophages and efferocytosis. Neoplasia, 21, 172–184.
Jiang, P., Gao, W., Ma, T., Wang, R., Piao, Y., Dong, X., Wang, P., Zhang, X., Liu, Y., Su, W., Xiang, R., Zhang, J., & Li, N. (2019). CD137 promotes bone metastasis of breast cancer by enhancing the migration and osteoclast differentiation of monocytes/macrophages. Theranostics, 9, 2950–2966.
Welm, A. L., Sneddon, J. B., Taylor, C., Nuyten, D. S. A., van de Vijver, M. J., Hasegawa, B. H., & Bishop, J. M. (2007). The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proceedings of the National Academy of Sciences, 104, 7570–7575.
Patel, S. A., Ramkissoon, S. H., Bryan, M., Pliner, L. F., Dontu, G., Patel, P. S., Amiri, S., Pine, S. R., & Rameshwar, P. (2012). Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Scientific Reports, 2, 906.
Walker, N. D., Elias, M., Guiro, K., Bhatia, R., Greco, S. J., Bryan, M., Gergues, M., Sandiford, O. A., Ponzio, N. M., Leibovich, S. J., & Rameshwar, P. (2019). Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death & Disease, 10, 59.
Walker, N. D., et al. (2019). Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death & Disease, 10, 1–16.
Raggi, C., Mousa, H. S., Correnti, M., Sica, A., & Invernizzi, P. (2016). Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene, 35, 671–682.
Noy, R., & Pollard, J. W. (2014). Tumor-associated macrophages: from mechanisms to therapy. Immunity, 41, 49–61.
Shao, X.-J., et al. (2019). Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacologica Sinica, 40, 1343–1350.
Steinman, R. M. (2006). Linking innate to adaptive immunity through dendritic cells. Novartis Foundation Symposium, 279, 101–109; discussion 109-113, 216-109.
Bashyam, H. (2007). Ralph Steinman: dendritic cells bring home the Lasker. The Journal of Experimental Medicine, 204, 2245–2248.
Guilliams, M., Ginhoux, F., Jakubzick, C., Naik, S. H., Onai, N., Schraml, B. U., Segura, E., Tussiwand, R., & Yona, S. (2014). Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature Reviews. Immunology, 14, 571–578.
Segerer, S., Heller, F., Lindenmeyer, M. T., Schmid, H., Cohen, C. D., Draganovici, D., Mandelbaum, J., Nelson, P. J., Gröne, H. J., Gröne, E. F., Figel, A. M., Nössner, E., & Schlöndorff, D. (2008). Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney International, 74, 37–46.
Ardavin, C. (2004). Amigorena, S. & Reis e Sousa, C. Dendritic cells: immunobiology and cancer immunotherapy. Immunity, 20, 17–23.
Lambrecht, B. N., Salomon, B., Klatzmann, D., & Pauwels, R. A. (1998). Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. Journal of Immunology, 160, 4090–4097.
Tucci, M., Stucci, S., Strippoli, S., Dammacco, F., & Silvestris, F. (2011). Dendritic cells and malignant plasma cells: an alliance in multiple myeloma tumor progression? Oncologist, 16, 1040–1048.
Wang, B., Dong, Y., Tian, Z., Chen, Y., & Dong, S. (2020). The role of dendritic cells derived osteoclasts in bone destruction diseases. Genes & Diseases.
Veglia, F., & Gabrilovich, D. I. (2017). Dendritic cells in cancer: the role revisited. Current Opinion in Immunology, 45, 43–51.
Sawant, A., Hensel, J. A., Chanda, D., Harris, B. A., Siegal, G. P., Maheshwari, A., & Ponnazhagan, S. (2012). Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. Journal of Immunology, 189, 4258–4265.
Lin, A., & Lore, K. (2017). Granulocytes: new members of the antigen-presenting cell family. Frontiers in Immunology, 8, 1781.
Geering, B., Stoeckle, C., Conus, S., & Simon, H. U. (2013). Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends in Immunology, 34, 398–409.
Rosales, C., Lowell, C. A., Schnoor, M., & Uribe-Querol, E. (2017). Neutrophils: their role in innate and adaptive immunity 2017. Journal of Immunology Research, 2017, 9748345.
Amulic, B., Cazalet, C., Hayes, G. L., Metzler, K. D., & Zychlinsky, A. (2012). Neutrophil function: from mechanisms to disease. Annual Review of Immunology, 30, 459–489.
Liang, W., & Ferrara, N. (2016). The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunology Research, 4, 83–91.
Jablonska, J., Leschner, S., Westphal, K., Lienenklaus, S., & Weiss, S. (2010). Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation, 120, 1151–1164.
Liu, Y., O'Leary, C. E., Wang, L. C. S., Bhatti, T. R., Dai, N., Kapoor, V., Liu, P., Mei, J., Guo, L., Oliver, P. M., Albelda, S. M., & Worthen, G. S. (2016). CD11b+ Ly6G+ cells inhibit tumor growth by suppressing IL-17 production at early stages of tumorigenesis. Oncoimmunology, 5, e1061175.
Fridlender, Z. G., Sun, J., Kim, S., Kapoor, V., Cheng, G., Ling, L., Worthen, G. S., & Albelda, S. M. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN. Cancer cell, 16, 183–194.
Andzinski, L., Kasnitz, N., Stahnke, S., Wu, C. F., Gereke, M., von Köckritz-Blickwede, M., Schilling, B., Brandau, S., Weiss, S., & Jablonska, J. (2016). Type I IFN s induce anti-tumor polarization of tumor associated neutrophils in mice and human. International Journal of Cancer, 138, 1982–1993.
Yan, B., Wei, J. J., Yuan, Y., Sun, R., Li, D., Luo, J., Liao, S. J., Zhou, Y. H., Shu, Y., Wang, Q., Zhang, G. M., & Feng, Z. H. (2013). IL-6 cooperates with G-CSF to induce protumor function of neutrophils in bone marrow by enhancing STAT3 activation. Journal of Immunology, 190, 5882–5893.
Casbon, A.-J., Reynaud, D., Park, C., Khuc, E., Gan, D. D., Schepers, K., Passegué, E., & Werb, Z. (2015). Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proceedings of the National Academy of Sciences, 112, E566–E575.
Kim, M., Song, C., Jeong, I. G., Choi, S. K., Park, M., Shim, M., Kim, Y. S., You, D., Hong, J. H., Kim, C. S., & Ahn, H. (2018). Androgen deprivation therapy during and after post-prostatectomy radiotherapy in patients with prostate cancer: a case control study. BMC Cancer, 18, 271.
Costanzo-Garvey, D. L., Keeley, T., Case, A. J., Watson, G. F., Alsamraae M., Yu Y., Su, K., Heim, C. E., Kielian T., Morrissey, C., Frieling, J. S., & Cook, L. M. (2020). Neutrophils are mediators of metastatic prostate cancer progression in bone. Cancer Immunology, Immunotherapy, 1–18.
Gollnick, S. O., Evans, S. S., Baumann, H., Owczarczak, B., Maier, P., Vaughan, L., Wang, W. C., Unger, E., & Henderson, B. W. (2003). Role of cytokines in photodynamic therapy-induced local and systemic inflammation. British Journal of Cancer, 88, 1772–1779.
Chu, D., Dong, X., Zhao, Q., Gu, J., & Wang, Z. (2017). Photosensitization priming of tumor microenvironments improves delivery of nanotherapeutics via neutrophil infiltration. Advanced Materials, 29.
Kang, T., Zhu, Q., Wei, D., Feng, J., Yao, J., Jiang, T., Song, Q., Wei, X., Chen, H., Gao, X., & Chen, J. (2017). Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano, 11, 1397–1411.
Keeley, T., Costanzo-Garvey, D. L., & Cook, L. M. (2019). Unmasking the many faces of tumor-associated neutrophils and macrophages: considerations for targeting innate immune cells in cancer. Trends Cancer, 5, 789–798.
Budhwar, S., Verma, P., Verma, R., Rai, S., & Singh, K. (2018). The yin and yang of myeloid derived suppressor cells. Frontiers in Immunology, 9, 2776.
Meirow, Y., Kanterman, J., & Baniyash, M. (2015). Paving the road to tumor development and spreading: myeloid-derived suppressor cells are ruling the fate. Frontiers in Immunology, 6, 523.
Raber, P., Ochoa, A. C., & Rodríguez, P. C. (2012). Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunological Investigations, 41, 614–634.
Bogdan, C. (2001). Nitric oxide and the immune response. Nature Immunology, 2, 907–916.
Parker, K. H., Beury, D. W., & Ostrand-Rosenberg, S. (2015). Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. In Advances in cancer research (Vol. 128, pp. 95–139). Elsevier.
Umansky, V., Blattner, C., Gebhardt, C., & Utikal, J. (2016). The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel), 4.
Chen, J., Sun, H. W., Yang, Y. Y., Chen, H. T., Yu, X. J., Wu, W. C., Xu, Y. T., Jin, L. L., Wu, X. J., Xu, J., & Zheng, L. (2021). Reprogramming immunosuppressive myeloid cells by activated T cells promotes the response to anti-PD-1 therapy in colorectal cancer. Signal Transduction and Targeted Therapy, 6, 4.
Morales, J. K., Kmieciak, M., Graham, L., Feldmesser, M., Bear, H. D., & Manjili, M. H. (2009). Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunology, Immunotherapy, 58, 941–953.
Sawant, A., Deshane, J., Jules, J., Lee, C. M., Harris, B. A., Feng, X., & Ponnazhagan, S. (2013). Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Research, 73, 672–682.
Kuznik, A., Pazdzierniok-Holewa, A., Jewula, P., & Kuznik, N. (2020). Bisphosphonates-much more than only drugs for bone diseases. European Journal of Pharmacology, 866, 172773.
Melani, C., Sangaletti, S., Barazzetta, F. M., Werb, Z., & Colombo, M. P. (2007). Amino-biphosphonate–mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Research, 67, 11438–11446.
Ostrand-Rosenberg, S., & Fenselau, C. (2018). Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. Journal of Immunology, 200, 422–431.
Condamine, T., Ramachandran, I., Youn, J. I., & Gabrilovich, D. I. (2015). Regulation of tumor metastasis by myeloid-derived suppressor cells. Annual Review of Medicine, 66, 97–110.
Mastio, J., Condamine, T., Dominguez, G., Kossenkov, A. V., Donthireddy, L., Veglia, F., Lin, C., Wang, F., Fu, S., Zhou, J., Viatour, P., Lavilla-Alonso, S., Polo, A. T., Tcyganov, E. N., Mulligan Jr., C., Nam, B., Bennett, J., Masters, G., Guarino, M., Kumar, A., Nefedova, Y., Vonderheide, R. H., Languino, L. R., Abrams, S. I., & Gabrilovich, D. I. (2019). Identification of monocyte-like precursors of granulocytes in cancer as a mechanism for accumulation of PMN-MDSCs. Journal of Experimental Medicine, 216, 2150–2169.
Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M., & Ostrand-Rosenberg, S. (2007). Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. Journal of Immunology, 179, 977–983.
Mulero, I., Sepulcre, M. P., Meseguer, J., García-Ayala, A., & Mulero, V. (2007). Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proceedings of the National Academy of Sciences, 104, 19434–19439.
Marone, G., Galli, S. J., & Kitamura, Y. (2002). Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends in Immunology, 23, 425–427.
Kirshenbaum, A. S., et al. (1999). Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13). Blood, The Journal of the American Society of Hematology, 94, 2333–2342.
Sammarco, G., Varricchi, G., Ferraro, V., Ammendola, M., de Fazio, M., Altomare, D. F., Luposella, M., Maltese, L., Currò, G., Marone, G., Ranieri, G., & Memeo, R. (2019). Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. International Journal of Molecular Sciences, 20, 2106.
Jiménez-Andrade, G. Y., Ibarra-Sánchez, A., González, D., Lamas, M., & González-Espinosa, C. (2013). Immunoglobulin E induces VEGF production in mast cells and potentiates their pro-tumorigenic actions through a Fyn kinase-dependent mechanism. Journal of Hematology & Oncology, 6, 1–14.
Siiskonen, H., Poukka, M., Bykachev, A., Tyynelä-Korhonen, K., Sironen, R., Pasonen-Seppänen, S., & Harvima, I. T. (2015). Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Research, 25, 479–485.
Ribatti, D., Vacca, A., Nico, B., Quondamatteo, F., Ria, R., Minischetti, M., Marzullo, A., Herken, R., Roncali, L., & Dammacco, F. (1999). Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. British Journal of Cancer, 79, 451–455.
Vyzoukaki, R., Tsirakis, G., Pappa, C. A., Devetzoglou, M., Tzardi, M., & Alexandrakis, M. G. (2015). The impact of mast cell density on the progression of bone disease in multiple myeloma patients. International Archives of Allergy and Immunology, 168, 263–268.
Leporini, C., Ammendola, M., Marech, I., Sammarco, G., Sacco, R., Gadaleta, C. D., Oakley, C., Russo, E., de Sarro, G., & Ranieri, G. (2015). Targeting mast cells in gastric cancer with special reference to bone metastases. World journal of gastroenterology: WJG, 21, 10493–10501.
Ammendola, M., Marech, I., Sammarco, G., Zuccalà, V., Luposella, M., Zizzo, N., Patruno, R., Crovace, A., Ruggieri, E., Zito, A., Gadaleta, C., Sacco, R., & Ranieri, G. (2015). Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. International Journal of Molecular Sciences, 16, 3237–3250.
Kaushansky, K. Thrombopoietin: the primary regulator of platelet production [see comments]. (1995).
Niswander, L. M., Fegan, K. H., Kingsley, P. D., McGrath, K. E., & Palis, J. (2014). SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood, 124, 277–286.
Kacena, M. A., Nelson, T., Clough, M. E., Lee, S. K., Lorenzo, J. A., Gundberg, C. M., & Horowitz, M. C. (2006). Megakaryocyte-mediated inhibition of osteoclast development. Bone, 39, 991–999.
Li, X., Koh, A. J., Wang, Z., Soki, F. N., Park, S. I., Pienta, K. J., & McCauley, L. K. (2011). Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. Journal of Bone and Mineral Research, 26, 125–134.
Jackson, W., et al. (2017). Role of megakaryocytes in breast cancer metastasis to bone. Cancer Research, 77, 1942–1954.
Leblanc, R., & Peyruchaud, O. (2016). Metastasis: new functional implications of platelets and megakaryocytes. Blood, The Journal of the American Society of Hematology, 128, 24–31.
English, D., Garcia, J. G., & Brindley, D. (2001). Platelet-released phospholipids link haemostasis and angiogenesis. Cardiovascular Research, 49, 588–599.
Desgrosellier, J. S., & Cheresh, D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews. Cancer, 10, 9–22.
Hamidi, H., & Ivaska, J. (2018). Every step of the way: integrins in cancer progression and metastasis. Nature Reviews. Cancer, 18, 533–548.
Bakewell, S. J., Nestor, P., Prasad, S., Tomasson, M. H., Dowland, N., Mehrotra, M., Scarborough, R., Kanter, J., Abe, K., Phillips, D., & Weilbaecher, K. N. (2003). Platelet and osteoclast β3 integrins are critical for bone metastasis. Proceedings of the National Academy of Sciences, 100, 14205–14210.
Uluçkan, Ö., Eagleton, M. C., Floyd, D. H., Morgan, E. A., Hirbe, A. C., Kramer, M., Dowland, N., Prior, J. L., Piwnica-Worms, D., Jeong, S. S., Chen, R., & Weilbaecher, K. (2008). APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. Journal of Cellular Biochemistry, 104, 1311–1323.
Boucharaba, A., Serre, C. M., Grès, S., Saulnier-Blache, J. S., Bordet, J. C., Guglielmi, J., Clézardin, P., & Peyruchaud, O. (2004). Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. The Journal of Clinical Investigation, 114, 1714–1725.
Leblanc, R., & Peyruchaud, O. (2015). New insights into the autotaxin/LPA axis in cancer development and metastasis. Experimental Cell Research, 333, 183–189.
Leblanc, R., Houssin, A., & Peyruchaud, O. (2018). Platelets, autotaxin and lysophosphatidic acid signalling: win-win factors for cancer metastasis. British Journal of Pharmacology, 175, 3100–3110.
Nontumoral, A. Interaction of platelet-derived autotaxin with tumor integrin aVb3 controls metastasis of breast cancer cells to bone. (2014).
Benesch, M. G., et al. (2014). Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. The FASEB Journal, 28, 2655–2666.
Macedo, F., et al. (2017). Bone Metastases: An Overview. Oncol Rev, 11, 321.
Ren, G., Esposito, M., & Kang, Y. (2015). Bone metastasis and the metastatic niche. Journal of Molecular Medicine (Berlin, Germany), 93, 1203–1212.
Florencio-Silva, R., Sasso, G. R., Sasso-Cerri, E., Simoes, M. J., & Cerri, P. S. (2015). Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Research International, 2015, 421746.
Schafer, R., et al. (2019). Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. Journal of Translational Medicine, 17, 115.
Funding
MA is supported by UNMC Diversity Funds. LMC is supported by a Research Scholar Grant (RSG-19-127-01-CSM) from the American Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alsamraae, M., Cook, L.M. Emerging roles for myeloid immune cells in bone metastasis. Cancer Metastasis Rev 40, 413–425 (2021). https://doi.org/10.1007/s10555-021-09965-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-021-09965-3