Abstract
Among the genes that were found to be abundantly overexpressed in highly metastatic rat cell lines compared to poorly metastatic cell lines, we identified a completely novel complementary DNA (cDNA) without any homologous or related genes in the database in 1994. The full-length cDNA of this rat gene was cloned, sequenced, and named metastasis-associated gene 1 (mta1), and eventually, its human cDNA counterpart, MTA1, was also cloned and sequenced by our group. MTA1 has now been identified as one of the members of a gene family (MTA gene family) and the products of the MTA genes, the MTA proteins, are transcriptional co-regulators that function in histone deacetylation and nucleosome remodeling and have been found in nuclear histone remodeling complexes. Furthermore, MTA1 along with its protein product MTA1 has been repeatedly and independently reported to be overexpressed in a vast range of human cancers and cancer cell lines compared to non-cancerous tissues and cell lines. The expression levels of MTA1 correlate well with the malignant properties of human cancers, strongly suggesting that MTA1 and possibly other MTA proteins (and their genes) could be a new class of molecular targets for cancer diagnosis and therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metastasis-associated gene/protein (MTA/MTA) is a family of cancer progression-related genes and their encoded products [1–4]. MTA1 is the founding gene member, along with its protein product MTA1, of the MTA family [3–5]. The expressions of MTA1 and MTA1 have been repeatedly reported to correlate with various malignant properties of tumors, including prognosis, in a wide range of human cancers [3–5].
The MTA1 protein has been localized to the nucleosome remodeling and histone deacetylation (NuRD) complex, and its essential function is now recognized as a transcriptional co-repressor [4–6]. In addition, the NuRD complex, which contains MTA1, deacetylates non-histone proteins, such as tumor suppressor protein p53 and hypoxia-inducible factor-1α (HIF-1α). Notably, p53 is deacetylated and inactivated by both MTA1 and MTA2 proteins, leading to inhibition of growth arrest and apoptosis [7, 8]. Moreover, HIF-1α is also deacetylated and stabilized by MTA1 protein, resulting in stimulation of angiogenesis [9, 10]. On the other hand, MTA1 protein has been shown to also function as a transcriptional co-activator with RNA polymerase III for several genes related to cancer and inflammation [11]. Recent studies have shown that the MTA family plays important roles in epithelial-mesenchymal transitions and DNA damage response [5, 12, 13]. MTA proteins, especially MTA1, probably represent a set of master co-regulatory molecules involved in the carcinogenesis and progression of various malignant tumors. Thus, MTA proteins have been proposed to be potential new tools for clinical applications in cancer diagnosis and treatment [3–5].
During an attempt to identify candidate metastasis-associated genes in rat mammary adenocarcinoma systems in 1994, we first identified mta1 (rat homologue) complementary DNA (cDNA) as a completely novel gene [14]. Subsequently, we cloned the human homologue of mta1, MTA1 [15], and investigated the expression of human MTA1 messenger RNA (mRNA) in surgically resected human cancer tissues. We found significant positive correlations between the expression levels of MTA1 mRNA and several clinicopathological factors related to malignant potential [16, 17]. As the discoverers of the mta1/MTA1 genes, we have discussed the identification and characterization of mta1/MTA1 genes and their encoded proteins (Mta1/MTA1) in the other chapter of this issue. In this chapter, we will briefly introduce the further information reported before the discoveries showing the involvement of MTA1 in the nuclear NuRD complex and an epoch study by the Kumar Laboratory in 2001 that directly demonstrated the relationship between MTA1 protein in the NuRD complex and invasion/metastasis of cancer cells [18]. In addition, we will summarize the studies that show that overexpression of MTA1 gene/protein in human cancer tissues is commonly found and briefly discuss its significance.
2 Subcellular localization of the MTA1 protein
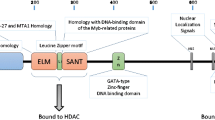
The MTA1 protein contains basic nuclear localization signals [14, 15]. To determine the subcellular localization of the MTA1 protein, the myc-epitope-tagged MTA1 recombinant protein was expressed in COS-7 cells, and immuno-fluorescence staining was performed using an anti-myc monoclonal antibody (9E10) [19]. The intact MTA1 protein (tagged with myc-epitope) was clearly localized in the nucleus of transfected cells. When a region of the carboxyl-terminal 102 amino acid residues containing three putative nuclear localization signals and an SH3 binding domain was expressed in COS-7 cells, this fusion protein was also localized in the nucleus. However, when the fusion protein was expressed without a nuclear localization signal, the protein was localized in the cytoplasm. These data suggested that the MTA1 protein is a nuclear protein, and the nuclear localization signals at the carboxyl-terminus of the protein are probably important for its localization in the nucleus [19] (Fig. 1). The MTA1 protein was also shown localized to the nucleus in many cancer cells [3–5].
Subcellular localization of MTA1 protein. The structural domains or motifs of Mta1/MTA1 protein are shown in the most upper line: BAH bromo-adjacent homology domain, ELM egl-27 and MTA1 homology domain, L leucine-zipper motif, SANT domain similar to the DNA binding domain of myb-related proteins, Zn GATA-type zinc-finger motif, NLS nuclear localization signal, SH3B src-homology 3 domain-binding motif. Plasmids pBJ-myc containing the full open reading frame (fragment A), the middle part of the MTA1 protein (fragment B: amino acids residue number 261–369), or the C-terminal 102 amino acids (fragment C: amino acid residue number 613–715) were transfected into COS-7 cells and immunofluorescence staining was done. Panels 1–3: anti-myc monoclonal antibody (MoAb), Panel 4: anti-hemagglutinin (HA) MoAb as a negative control. This figure has been modified from Toh et al. [19]
Recently, Liu et al. [20] reported the results of experiments designed to study the subcellular distribution of MTA1 protein [19]. They confirmed the localization of MTA1 protein using multiple molecular technologies, including immunohistochemistry, immunofluorescence, GFP tag tracking, Western blot analysis, immunoprecipitation, in situ proximity ligation, and immunoelectron microscopy. These multiple different techniques demonstrated that MTA1 protein is primarily localized to the nucleus in most normal human adult tissues and to the cytoplasm of embryonic tissues (mouse). Although the MTA1 protein was primarily found in the nucleus of most cells, it was also detected in both the nucleus and cytoplasm in colon cancer tissues [20]. Furthermore, they showed that the MTA1 protein found in the cytoplasm is localized to microtubules, components of the cytoskeleton. These results support our previous data that showed that MTA1 protein directly interacts with the cytoplasmic protein endophilin 3. This protein is involved in endocytosis regulation, in which microtubules play essential roles [21].
3 BLAST search of Mtal/MTA1 amino acid sequences 5 years after identification of the original mta1 cDNA
When we first reported on the amino acid sequence of the rat Mtal protein in 1994, there were no similar or homologous protein sequences in the databases, suggesting that mtal was a completely new, novel gene [14]. By 1999, however, similar genes or genes with homologous amino acid sequences to Mtal/MTA1 protein appeared in the databases. These include (1) er1: This gene was identified as a novel immediate-early gene in Xenopus embryos whose transcription was activated during mesoderm induction by fibroblast growth factor [22]. The ER1 protein contains a SH3-binding domain and a DNA-binding SANT domain [23] and has been shown to be a potent transcription factor; (2) egl-27: This gene was identified as a gene that controls cell polarity and cell migration during the establishment of embryonic patterns in Caenorhabditis elegans [24, 25]. The EGL-27 protein also contains a SANT domain, a GATA-like zinc finger motif, a leucine-zipper motif, and two other similar regions to the MTA1 protein and is involved in the Wnt signaling pathway; (3) ZG29p: The ZG29p gene encodes a protein that has been identified as a novel zymogen granule protein [26]. The MTA1 and ZG29p proteins are apparently differentially expressed from the same gene by alternative transcription initiation; and (4) MTA1–L1: This gene was isolated as an MTA l gene homologue in the human genome project [27]. MTAl-L1 is highly homologous to MTA1 and includes a leucine-zipper motif, a GATA-like zinc finger motif, and a SANT domain. It has been shown to be identical to the MTA2 gene. Even though similar or homologous genes had begun to emerge in the database, the functions of MTA1 were still unknown at this time.
4 MTA1 is a component of a NuRD complex
The first clues on the molecular and biochemical functions of MTA1 were obtained by four different groups from 1998 to 1999 [28–32]. In these studies, two disparate chromatin-modifying activities, ATP-dependent nucleosome remodeling activity and histone deacetylation, were discovered to be functionally and physically linked in the same protein complex. The original complex was named the NuRD complex, and it contained histone deacetylase (HDAC)1, HDAC2, the histone binding proteins RbAp46/48, and the dermatomyositis-specific autoantigen Mi-2, which has been shown to have transcription repressing activity [29–32]. Xue et al. [30] reported that MTA1 protein was found in a NuRD complex, and this complex had strong transcription-repressing activities. Subsequently, Zhang et al. [31] reported that a protein similar to MTA1 (identified as MTA2) was also a component of a NuRD complex and that MTA2 was highly expressed in rapidly dividing cells. Later, MTA3 was identified as an estrogen-inducible gene product that binds to and forms a distinct NuRD complex [33]. We also reported the physical interaction between MTA1 and HDAC1 in what turned out to be a NuRD complex [19]. Additional NuRD complexes, moreover, will likely be isolated that have subtle differences in their compositions, activities, and functions.
The biochemical functions of the MTA family members appear to be exerted through NuRD complexes that have chromatin remodeling and histone deacetylating properties. Although all MTA family proteins are found in NuRD complexes, three MTA members have now been found in mutually exclusive NuRD complexes with distinct and, in some cases, antagonistic activities [34]. The studies mentioned here and above suggest that a family of NuRD complexes that are important in chromatin remodeling, gene regulation, and possibly other activities exist and that MTA proteins are important components of these nuclear complexes.
In addition to the MTA1/MTA2 proteins, NuRD complexes containing several components and factors have been reported to be related to malignancy. For example, in the HDAC1/2 complex, the core components are known to be linked to oncogenesis, and their specific inhibitors are now being used to develop new cancer treatments [35]. Mi-2β, the largest component found in a NuRD complex, has also been described as an autoantigen found in dermatomyositis. It has been reported that patients with this autoimmune disease have a significantly increased risk for a wide variety of neoplasms, including breast, ovarian, lung, pancreatic, stomach, and colorectal cancers (up to 25 % of dermatomyositis patients have such cancers) [36]. Further, Zhang et al. showed that one of the methyl-CpG-binding domain-containing proteins, MBD3, is another component of the NuRD complex [31]. MDB3 is closely related to MBD2, and MBD2 has also been identified as a colon cancer antigen [37]. All of these data strongly suggest a link between the NuRD complex (including MTA1, MTA2, and possibly others) and malignancy. To determine the biologic function of MTA1, we had to wait for the epoch study by the Kumar Laboratory where evidence that directly demonstrated the relationship between MTA1 protein in the NuRD complex and invasion/metastasis of cancer cells was obtained [18]. They reported that forced expression of the MTA1 protein in the human breast cancer cell line MCF-7 is accompanied by enhancement of the ability of these cells to invade an artificial extracellular matrix and to grow in an anchorage-independent manner. They also showed that the enhancement is associated with the interaction between MTA1 protein and HDAC1, resulting in a repression of estrogen receptor (ER)-mediated transcription.
More recently, Alqarni et al. [38] reported that the MTA1 acts as a scaffold for NuRD complex assembly. The physical interactions between MTA proteins and the members of the NuRD complex are likely to be important for a wide variety of the functions of NuRD complex and its role in oncogenesis. This will be discussed in more detail elsewhere in this same issue.
5 Expression and relevance of MTA1/MTA1 gene/protein in human cancers
The MTA1 gene and its protein product MTA1 have been found to be overexpressed in a number of animal and human tumors of differing malignant potentials [3, 4]. After identification of the human MTA1 cDNA, we examined the significance of its overexpression in human cancer specimens. First, we collected 36 colorectal and 34 gastric cancer tissues along with their corresponding normal mucosal tissues and evaluated the relevance of the expression of this gene to human carcinoma progression [16]. Because anti-MTA1 antibodies that could be used for clinical samples were not available at that time, the expression of MTA1 mRNA was estimated by a semiquantitative reverse-transcription polymerase chain reaction (RT-PCR) method, and the results were compared with clinicopathological data. The relative overexpression of MTA1 mRNA (tumor/normal ratio ≥2) was observed in 14 of 36 (38.9 %) colorectal carcinomas and 13 of 34 (38.2 %) gastric carcinomas [16]. Clinicopathological correlations of MTA1 gene expression demonstrated that in colorectal carcinomas overexpressing MTA1 mRNA, the high-MTA1-expressing tumors exhibited significantly deeper wall invasion and a higher rate of metastasis to lymph nodes. They also tended to be at a more advanced Dukes’ stage with frequent lymphatic involvement.
In gastric carcinomas, the tumors overexpressing MTA1 mRNA showed significantly higher rates of serosal invasion and lymph node metastasis and tended to have higher rates of vascular involvement. These data suggested that overexpression of the MTA1 gene correlates with tumor invasion and the presence of metastases and that high expression of MTA1 mRNA could be a potential indicator for assessing the malignant potential of colorectal and gastric carcinomas [16].
Results similar to those obtained in colorectal and gastric carcinomas were observed in esophageal squamous cell carcinomas (ESCC). The relative overexpression of MTA1 mRNA (tumor/normal ratio ≥2) was observed in 16 out of 47 (34.0 %) ESCC. Esophageal tumors overexpressing MTA1 mRNA showed significantly higher frequencies of adventitial invasion (p < 0.05) and lymph node metastasis (p < 0.05) and tended to have a higher rate of lymphatic involvement than the remaining tumors [17].
To confirm the results discussed above, we developed anti-MTA1 antibodies usable for immunohistochemistry [39]. Using immunohistochemistry, the expression levels of MTA1 protein were examined in 70 cases of surgically resected ESCC. The intensities of immunostaining of MTA1 protein in carcinoma tissues (Ca) were compared to normal epithelium (N) contained in the same section. Thirty of 70 cases (42.9 %) displayed overexpression of MTA1 protein (N < Ca). Cancers overexpressing MTA1 protein invaded deeper into the esophageal wall (p < 0.005) and showed significantly higher degrees of lymph node metastasis (p < 0.01), higher pathological stage, more lymphatic involvement, and thus poorer prognosis than the remaining cases (p < 0.05). This was the first report to show the prognostic significance of MTA1 protein expression in human cancers. In this study, we also examined the acetylation levels of histone H4 by immunohistochemistry. The acetylation levels of histone H4 inversely correlated with the depth of cancer invasion and pathological stage (p < 0.05), and thus, the patients with higher level of histone H4 acetylation had a better prognosis (p < 0.005).
In addition, immunostaining patterns of MTA1 protein and acetylated histone H4 were inversely correlated (p < 0.001), demonstrating the relationship of deacetylation of histone H4 in MTA1-overexpressing carcinomas. Thus, the data suggest that the overexpression of MTA1 protein and acetylation level of histone H4 protein, both of which are closely related, might be useful predictors of malignant potential of ESCC [39].
Since we showed for the first time that the upregulation of MTA1 mRNA and higher expression of MTA1 protein were significantly correlated to the malignant properties of human gastric and colorectal cancers [16] as well as of ESCC [17, 39], other researchers from independent laboratories have been investigating the expression levels of MTA family members, especially MTA1, in various human cancers. More than 40 publications on the clinicopathological significance of MTA1 expression in human cancer tissues have appeared up to 2014. These publications have been listed up with their observed clinicopathological implications in Table 1. Apparently, MTA1 is upregulated in a vast range of malignant human tumors, and the results obtained in completely different studies were very similar, regardless of the types of cancers investigated. In general, overexpression of MTA1 closely relates to cancer invasion and metastasis and thus to the unfavorable prognosis.
Recently, Luo et al. [40] reported the results of a meta-analysis of cohort studies that focused on the prognostic significance of MTA1 expression in solid tumors, as detected by immunohistochemistry. They found 16 papers on this subject that were eligible for the meta-analysis. The types of solid tumors included were ESCC, breast cancer, non-small-cell lung cancer, cervical cancer, colon cancer, nasopharyngeal cancer, ovarian cancer, and gastric cancer. The meta-analysis confirmed that MTA1 overexpression was an independent predictor of poor prognosis. Furthermore, multivariate analysis demonstrated that MTA1 overexpression was significantly associated with tumor size, tumor stage, depth of invasion, and lymph node metastasis. These clinicopathological correlative studies mentioned above have been reinforced by additional experiments that show the biological relevance of MTA1 protein overexpression to carcinogenesis and cancer progression. These studies will be introduced in the other reviews of this issue.
6 Conclusions: MTA1 protein as a possible new molecular target for cancer diagnosis and therapy
Recent advances in molecular biology have resulted in the discovery of a wide variety of new molecules involved in carcinogenesis and cancer progression. However, in order to be clinically useful as molecular targets for the diagnosis and treatment of human cancers, the new molecules must fulfill two major requirements. The first is that abnormalities in expression or structure of molecules of interest and their clinical relevance must be definitively demonstrated in a variety of human cancers by independent studies. The second is the underlying molecular mechanisms necessary for the molecules to exert their functions in carcinogenesis or cancer progression must be determined.
Considering the numerous independent reports showing the clinical relevance of the expression of MTA1 mRNA and its encoded protein MTA1 in a wide variety of human cancers as well as definitive studies showing the molecular and biochemical mechanisms of MTA protein actions, which will be introduced in the other sections of this issue, it is likely that MTA proteins, especially MTA1, probably represent master co-regulatory molecules directly involved in the carcinogenesis and progression of various malignant tumors. Ultimately, this could lead to additional clinical applications of the MTA1 protein as a new class of molecular targets for cancer diagnosis and therapy. The success of future studies may yet determine whether targeting MTA1 protein is clinically effective or not.
The first studies that suggested the possibility of targeting MTA1 for cancer therapy were reported by our laboratory [15, 41]. We used antisense phosphorothioate oligonucleotides against MTA1 mRNA and found a growth inhibitory effect on human metastatic breast cancer cell lines. Since these reports, others have shown that inhibition of MTA1 expression can result in the inhibition of the malignant phenotypes of various cancers, and these results will be discussed elsewhere in this issue.
Various techniques have been used to regulate MTA1 expression. Using RNA interference (RNAi), Qian et al. inhibited MTA1 expression in a human ESCC cell line and demonstrated significant inhibition of in vitro invasion and migration properties of the cancer cells [42]. The same group further examined the therapeutic value of targeting MTA1 overexpression in malignant melanoma cells and demonstrated that downregulation of MTA1 by RNAi successfully suppressed the growth properties in vitro and experimental metastasis of mouse B16-F10 melanoma cells in vivo, suggesting a promising use of the MTA1 gene as a target for cancer gene therapy [43].
The functional roles of MTA proteins are thought to be transcriptional co-repressors that act through chromatin remodeling and histone deacetylation. Repression of ER-α transactivation function by MTA1 protein through deacetylation of estrogen responsive element (ERE) of the ER-responsive genes has been the most extensively investigated, and the data clearly demonstrate that MTA1 expression results in tumor formation in mammary glands and renders breast cancer cells phenotypically more aggressive [44]. In addition, MTA proteins deacetylate non-histone proteins [2, 3, 5]. For example, the tumor suppressor p53 protein is deacetylated and inactivated by both MTA1 and MTA2 proteins, resulting in inhibition of growth arrest and apoptosis [7, 8]. HIF-1α is also deacetylated and stabilized by MTA1, leading to angiogenesis [9, 10].
Inhibition of MTA1 expression or function may decrease the aggressiveness of cancer cells and enhance their chemosensitivity by restoring tumor suppressor function of p53 or changing other properties of malignant cells. For example, it may inhibit tumor angiogenesis by destabilizing the angiogenesis-promoting function of HIF-1α. Moreover, MTA proteins may cooperate with HDAC inhibitors, which are now being considered as a new class of anticancer agents [35]. Ideally, prior to actual clinical applications of MTA1 modulation therapy, understanding the physiological functions of MTA proteins will be absolutely necessary. MTA1 gene or MTA protein expression should also be clinically useful for the prediction of the malignant potentials of various human cancers and the prognosis of patients with these cancers.
In conclusion, MTA proteins, especially MTA1, are undoubtedly excellent candidates for determining the diagnosis and prognosis of human cancers and may be useful for developing new therapeutic strategies for treatments directed against highly malignant cancer cells. These topics should continue to be intensively studied for these and other possible clinical applications.
References
Kumar, R., Wang, R. A., & Bagheri-Yarmand, R. (2003). Emerging roles of MTA family members in human cancers. Seminars in Oncology, 30(Suppl 16), 30–37.
Manavathi, B., Singh, K., & Kumar, R. (2007). MTA family of coregulators in nuclear receptor biology and pathology. Nuclear Receptor Signaling, 5, 1–8.
Toh, Y., & Nicolson, G. L. (2009). The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clinical and Experimental Metastasis, 26(3), 215–227.
Toh, Y., & Nicolson, G. L. (2011). MTA1 of the MTA (metastasis-associated) gene family and its encoded proteins: molecular and regulatory functions and its role in human cancer progression. Atlas of Genetics and Cytogenetics in Oncology and Haematology, 15(3), 303–315.
Toh, Y., & Nicolson, G. L. (2013). Signaling pathways of MTA family proteins as regulators of cancer progression and metastasis. In R. R. Resende & H. Ulrich (Eds.), Trends in stem cell proliferation and cancer research (pp. 251–275). Dordrecht: Springer.
Manavathi, B., & Kumar, R. (2007). Metastasis tumor antigens, an emerging family of multifaceted master coregulators. Journal of Biological Chemistry, 282(3), 1529–1533.
Luo, J., Su, F., Chen, D., Shiloh, A., & Gu, W. (2000). Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature, 408(6810), 377–381.
Moon, H. E., Cheon, H., & Lee, M. S. (2007). Metastasis-associated protein 1 inhibits p53-induced apoptosis. Oncology Reports, 18(5), 1311–1314.
Moon, H. E., Cheon, H., Chun, K. H., Lee, S. K., Kim, Y. S., Jung, B. K., et al. (2006). Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncology Reports, 16(4), 929–935.
Yoo, Y. G., Kong, G., & Lee, M. O. (2006). Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. EMBO Journal, 25(6), 1231–1241.
Li, D. Q., Pakala, S. B., Nair, S. S., Eswaran, J., & Kumar, R. (2012). Metastasis-associated protein 1/nucleosome remodeling and histone deacetylase complex in cancer. Cancer Research, 72(2), 387–394.
Li, D. Q., & Kumar, R. (2010). Mi-2/NURD complex making inroads into DNA-damage response pathway. Cell Cycle, 9(11), 2071–2079.
Pakala, S. B., Reddy, S. D., Bui-Nguyen, T. M., Rangparia, S. S., Bommana, A., & Kumar, R. (2010). MTA1 coregulator regulates LPS response via MyD88-dependent signaling. Journal of Biological Chemistry, 285(43), 32787–32792.
Toh, Y., Pencil, S. D., & Nicolson, G. L. (1994). A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. Journal of Biological Chemistry, 269(37), 22958–22963.
Nawa, A., Nishimori, K., Lin, P., Maki, Y., Moue, K., Sawada, H., et al. (2000). Tumor metastasis-associated human MTA1 gene: its deduced protein sequence, localization, and association with breast cancer cell proliferation using antisense phosphorothioate oligonucleotides. Journal of Cell Biochemistry, 79(2), 202–212.
Toh, Y., Oki, E., Oda, S., Tokunaga, E., Ohno, S., Maehara, Y., et al. (1997). Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. International Journal of Cancer, 74(4), 459–463.
Toh, Y., Kuwano, H., Mori, M., Nicolson, G. L., & Sugimachi, K. (1999). Overexpression of metastasis-associated MTA1 mRNA in invasive oesophageal carcinomas. British Journal of Cancer, 79(11–12), 1723–1726.
Mazumdar, A., Wang, R. A., Mishra, S. K., Adam, L., Bagheri-Yarmand, R., Mandal, M., et al. (2001). Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nature Cell Biology, 3(1), 30–37.
Toh, Y., Kuninaka, S., Endo, K., Oshiro, T., Ikeda, Y., Nakashima, H., et al. (2000). Molecular analysis of a candidate metastasis-associated gene, MTA1: possible interaction with histone deacetylase 1. Journal of Experimental and Clinical Cancer Research, 19(1), 105–111.
Liu, J., Xu, D., Wang, H., Zhang, Y., Chang, Y., Zhang, J., et al. (2014). The subcellular distribution and function of MTA1 in cancer differentiation. Oncotarget, 5(13), 5153–5164.
Aramaki, Y., Ogawa, K., Toh, Y., Ito, T., Akimitsu, N., Hamamoto, H., et al. Direct interaction between metastasis-associated protein 1 and endophilin 3. FEBS Letter, 579(17), 3731–3736
Paterno, G. D., Li, Y., Luchman, H. A., Ryan, P. J., & Gillespie, L. L. (1997). cDNA cloning of a novel, developmentally regulated immediate early gene activated by fibroblast growth factor and encoding a nuclear protein. Journal of Biological Chemistry, 272(41), 25591–25595.
Paterno, G. D., Mercer, F. C., Chayter, J. J., Yan, G. X., Yan, G. X., Robb, J. D., et al. (1998). Molecular cloning of human er1 cDNA and its differential expression in breast tumours and tumour-derived cell lines. Gene, 222(1), 77–82.
Herman, M. A., Ch’ng, Q., Hettenbach, S. M., Ratliff, T. M., Kenyon, C., & Herman, R. K. (1999). EGL-27 is similar to a metastasis-associated factor and controls cell polarity and cell migration in C. elegans. Development, 126(5), 1055–1064.
Solari, F., Bateman, A., & Ahringer, J. (1999). The Caenorhabditis elegans genes egl-27 and egr-1 are similar to MTA1, a member of a chromatin regulatory complex, and are redundantly required for embryonic patterning. Development, 126(11), 2483–2494.
Kleene, R., Zdzieblo, J., Wege, K., & Kern, H. F. (1999). A novel zymogen granule protein (ZG29p) and the nuclear protein MTA1p are differentially expressed by alternative transcription initiation in pancreatic acinar cells of the rat. Journal of Cell Science, 112(Pt15), 2539–2548.
Futamura, M., Nishimori, H., Shiratsuchi, T., Saji, S., Nakamura, Y., & Tokino, T. (1999). Molecular cloning, mapping, and characterization of a novel human gene, MTA1-L1, showing homology to a metastasis-associated gene, MTA1. Journal of Human Genetics, 44(1), 52–56.
Bowen, N. J., Fujita, N., Kajita, M., & Wade, P. A. (2004). Mi-2/NuRD: multiple complexes for many purposes. Biochimica et Biophysica Acta, 1677(1–3), 52–57.
Tong, J. K., Hassig, C. A., Schnitzler, G. R., Kingston, R. E., & Schreiber, S. L. (1998). Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature, 395(6705), 917–921.
Xue, Y., Wong, J., Moreno, G. T., Young, M. K., Cote, J., & Wang, W. (1998). NuRD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Molecular Cell, 2(6), 851–861.
Zhang, Y., Ng, H. H., Erdjument-Bromage, H., Tempst, P., Bird, A., & Reinberg, D. (1999). Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes and Development, 13(15), 1924–1935.
Wade, P. A., Gegonne, A., Jones, P. L., Ballesta, R. E., Aubry, F., & Wolffe, A. P. (1999). Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nature Genetics, 23(1), 62–66.
Fujita, N., Jaye, D. L., Kajita, M., Geigerman, C., Moreno, C. S., & Wade, P. A. (2003). Mta3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell, 113(2), 207–219.
Basta, J., & Rauchman, M. (2014). The nucleosome remodeling and deacetylase complex in development and disease. Translational Research. doi:10.1016/j.trsl.2014.05.003.
Kelly, T. K., De Carvalho, D. D., & Jones, P. A. (2010). Epigenetic modifications as therapeutic targets. Nature Biotechnology, 28(10), 1069–1078.
Ramirez, J., & Hagman, J. (2009). The Mi-2/NuRD complex: a critical epigenetic regulator of hematopoietic development, differentiation and cancer. Epigenetics, 4(8), 532–536.
Scanlan, M. J., Chen, Y. T., Williamson, B., Gure, A. O., Stockert, E., Gordan, J. D., et al. (1998). Characterization of human colon cancer antigens recognized by autologous antibodies. International Journal of Cancer, 76(5), 652–658.
Alqarni, S. S., Murthy, A., Zhang, W., Przewloka, M. R., Silva, A. P., Watson, A. A., et al. (2014). Insight into the architecture of the NuRD complex: structure of the RbAp48-MTA1 subcomplex. Journal of Biological Chemistry, 289(32), 21844–21855.
Toh, Y., Ohga, T., Endo, K., Adachi, E., Kusumoto, H., Haraguchi, M., et al. (2004). Expression of the metastasis-associated MTA1 protein and its relationship to deacetylation of the histone H4 in esophageal squamous cell carcinomas. International Journal of Cancer, 110(3), 362–367.
Luo, H., Li, H., Yao, N., Hu, L., & He, T. (2014). Metastasis-associated protein 1 as a new prognostic marker for solid tumors: a meta-analysis of cohort studies. Tumour Biology, 35(6), 5823–5832.
Nicolson, G. L., Nawa, A., Toh, Y., Taniguchi, S., Nishimori, K., & Moustafa, A. (2003). Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation. Clinical and Experimental Metastasis, 20(1), 19–24.
Qian, H., Lu, N., Xue, L., Liang, X., Zhang, X., Fu, M., et al. (2005). Reduced MTA1 expression by RNAi inhibits in vitro invasion and migration of esophageal squamous cell carcinoma cell line. Clinical and Experimental Metastasis, 22(8), 653–662.
Qian, H., Yu, J., Li, Y., Wang, H., Song, C., Zhang, X., et al. (2007). RNA interference against metastasis-associated gene 1 inhibited metastasis of B16F10 melanoma cell in c57BL/6 model. Biology of the Cell, 99, 573–581.
Singh, R. R., & Kumar, R. (2007). MTA family of transcriptional metaregulators in mammary gland morphogenesis and breast cancer. Journal of Mammary Gland Biology and Neoplasia, 12(2–3), 115–125.
Jang, K. S., Paik, S. S., Chung, H., Oh, Y. H., & Kong, G. (2006). MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancers. Cancer Science, 97(5), 374–379.
Martin, M. D., Fischbach, K., Osborne, C. K., Mohsin, S. K., Allred, D. C., & O’Connell, P. (2001). Loss of heterozygosity events impeding breast cancer metastasis contain the mta1 gene. Cancer Research, 61(9), 3578–3580.
Martin, M. D., Hilsenbeck, S. G., Mohsin, S. K., Hopp, T. A., Clark, G. M., Osborne, C. K., et al. (2006). Breast tumors that overexpress nuclear metastasis-associated 1 (MTA1) protein have high recurrence risks but enhanced responses to systemic therapies. Breast Cancer Research and Treatment, 95(1), 7–12.
Sharma, G., Mirza, S., Parshad, R., Srivastava, A., Gupta, S. D., Pandya, P., et al. (2011). Clinical significance of Maspin promoter methylation and loss of its protein expression in invasive ductal breast carcinoma: correlation with VEGF-A and MTA1 expression. Tumour Biology, 32(1), 23–32.
Cheng, C. W., Liu, Y. F., Yu, J. C., Wang, H. W., Ding, S. L., Hsiung, C. N., et al. (2012). Prognostic significance of cyclin D1, beta-catenin, and MTA1 in patients with invasive ductal carcinoma of the breast. Annals of Surgical Oncology, 19(13), 4129–4139.
Mao, X. Y., Chen, H., Wang, H., Wei, J., Liu, C., Zheng, H. C., et al. (2012). MTA1 expression correlates significantly with ER-alpha methylation in breast cancer. Tumour Biology, 33(5), 1565–1572.
Li, S. H., Wang, Z., & Liu, X. Y. (2009). Metastasis-associated protein 1 (MTA1) overexpression is closely associated with shorter disease-free interval after complete resection of histologically node-negative esophageal cancer. World Journal of Surgery, 33(9), 1876–1881.
Li, S. H., Tian, H., Yue, W. M., Li, L., Gao, C., Li, W. J., et al. (2012). Metastasis-associated protein 1 nuclear expression is closely associated with tumor progression and angiogenesis in patients with esophageal squamous cell cancer. World Journal of Surgery, 36(3), 623–631.
Song, L., Wang, Z., & Liu, X. (2013). MTA1: a prognosis indicator of postoperative patients with esophageal carcinoma. Thoracic and Cardiovascular Surgery, 61(6), 479–485.
Miyatani, T., Kurita, N., Mikami, C., Kashihara, H., Higashijima, J., Yoshikawa, K., et al. (2011). Malignant potential of Barrett’s esophagus: special reference to HDAC-1 and MTA-1 expression. Hepato-Gastroenterology, 58(106), 472–476.
Deng, X., Du, L., Wang, C., Yang, Y., Li, J., Liu, H., et al. (2013). Close association of metastasis-associated protein 1 overexpression with increased angiogenesis and poor survival in patients with histologically node-negative gastric cancer. World Journal of Surgery, 37(4), 792–798.
Giannini, R., & Cavallini, A. (2005). Expression analysis of a subset of coregulators and three nuclear receptors in human colorectal carcinoma. Anticancer Research, 25(6B), 4287–4292.
Du, B., Yang, Z. Y., Zhong, X. Y., Fang, M., Yan, Y. R., Qi, G. L., et al. (2011). Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World Journal of Gastroenterology, 17(9), 1219–1226.
Higashijima, J., Kurita, N., Miyatani, T., Yoshikawa, K., Morimoto, S., Nishioka, M., et al. (2011). Expression of histone deacetylase 1 and metastasis-associated protein 1 as prognostic factors in colon cancer. Oncology Reports, 26(2), 343–348.
Kidd, M., Modlin, I. M., Mane, S. M., Camp, R. L., Eick, G. N., Latich, I., et al. (2006). Utility of molecular genetic signatures in the delineation of gastric neoplasia. Cancer, 106(7), 1480–1488.
Kidd, M., Modlin, I. M., Mane, S. M., Camp, R. L., Eick, G., & Latich, I. (2006). The role of genetic markers—NAP1L1, MAGE-D2, and MTA1—in defining small-intestinal carcinoid neoplasia. Annals of Surgical Oncology, 13(2), 253–262.
Modlin, I. M., Kidd, M., Latich, I., Zikusoka, M. N., Eick, G. N., Mane, S. M., et al. (2006). Genetic differentiation of appendiceal tumor malignancy: a guide for the perplexed. Annals of Surgery, 244(1), 52–60.
Miyake, K., Yoshizumi, T., Imura, S., Sugimoto, K., Batmunkh, E., Kanemura, H., et al. (2008). Expression of hypoxia-inducible factor-1alpha, histone deacetylase 1, and metastasis-associated protein 1 in pancreatic carcinoma: correlation with poor prognosis with possible regulation. Pancreas, 36(3), e1–e9.
Hofer, M. D., Chang, M. C., Hirko, K. A., Rubin, M. A., & Nose, V. (2009). Immunohistochemical and clinicopathological correlation of the metastasis-associated gene 1 (MTA1) expression in benign and malignant pancreatic endocrine tumors. Modern Pathology, 22(7), 933–939.
Hamatsu, T., Rikimaru, T., Yamashita, Y., Aishima, S., Tanaka, S., Shirabe, K., et al. (2003). The role of MTA1 gene expression in human hepatocellular carcinoma. Oncology Reports, 10(3), 599–604.
Moon, W. S., Chang, K., & Tarnawski, A. S. (2004). Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: relationship to vascular invasion and estrogen receptor-alpha. Human Pathology, 35(4), 424–429.
Ryu, S. H., Chung, Y. H., Lee, H., Kim, J. A., Shin, H. D., Min, H. J., et al. (2008). Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology, 47(3), 929–936.
Lee, S. H., Chung, Y. H., Kim, J. A., Lee, D., Jin, Y. J., Shim, J. H., et al. (2012). Single nucleotide polymorphisms associated with metastatic tumour antigen 1 overexpression in patients with hepatocellular carcinoma. Liver International, 32(3), 457–466.
Jin, Y. J., Chung, Y. H., Kim, J. A., Park, W. H., Lee, D., Seo, D. D., et al. (2012). Factors predisposing metastatic tumor antigen 1 overexpression in hepatitis B virus associated hepatocellular carcinoma. Digestive Diseases and Sciences, 57(11), 2917–2923.
Roepman, P., de Jager, A., Groot Koerkamp, M. J., Kummer, J. A., Slootweg, P. J., & Holstege, F. C. (2006). Maintenance of head and neck tumor gene expression profiles upon lymph node metastasis. Cancer Research, 66(23), 11110–11114.
Kawasaki, G., Yanamoto, S., Yoshitomi, I., Yamada, S., & Mizuno, A. (2008). Overexpression of metastasis-associated MTA1 in oral squamous cell carcinomas: correlation with metastasis and invasion. International Journal of Oral and Maxillofacical Surgery, 37(11), 1039–1046.
Li, W. F., Liu, N., Cui, R. X., He, Q. M., Chen, M., Jiang, N., et al. (2012). Nuclear overexpression of metastasis-associated protein 1 correlates significantly with poor survival in nasopharyngeal carcinoma. Journal of Translational Medicine, 10, 78.
Deng, Y. F., Zhou, D. N., Ye, C. S., Zeng, L., & Yin, P. (2012). Aberrant expression levels of MTA1 and RECK in nasopharyngeal carcinoma: association with metastasis, recurrence, and prognosis. Annals of Otology, Rhinology and Laryngology, 121(7), 457–465.
Park, J. O., Jung, C. K., Sun, D. I., Joo, Y. H., & Kim, M. S. (2011). Relationships between metastasis-associated protein (MTA) 1 and lymphatic metastasis in tonsil cancer. European Archives of Otorhinolaryngology, 268(9), 1329–1334.
Sasaki, H., Moriyama, S., Nakashima, Y., Kobayashi, Y., Yukiue, H., Kaji, M., et al. (2002). Expression of the MTA1 mRNA in advanced lung cancer. Lung Cancer, 35(2), 149–154.
Zhu, X., Guo, Y., Li, X., Ding, Y., & Chen, L. (2010). Metastasis-associated protein 1 nuclear expression is associated with tumor progression and clinical outcome in patients with non-small cell lung cancer. Journal of Thoracic Oncology, 5(8), 1159–1166.
Yu, Y., Wang, Z., Zhang, M. Y., Liu, X. Y., & Zhang, H. (2011). Relation between prognosis and expression of metastasis-associated protein 1 in stage I non-small cell lung cancer. Interactive Cardiovascular and Thoracic Surgery, 12(2), 166–169.
Li, S. H., Tian, H., Yue, W. M., Li, L., Li, W. J., Chen, Z. T., et al. (2011). Overexpression of metastasis-associated protein 1 is significantly correlated with tumor angiogenesis and poor survival in patients with early-stage non-small cell lung cancer. Annals of Surgical Oncology, 18(7), 2048–2056.
Sasaki, H., Yukiue, H., Kobayashi, Y., Nakashima, Y., Kaji, M., Fukai, I., et al. (2001). Expression of the MTA1 mRNA in thymoma patients. Cancer Letter, 174(2), 159–163.
Yi, S., Guangqi, H., & Guoli, H. (2003). The association of the expression of MTA1, nm23H1 with the invasion, metastasis of ovarian carcinoma. Chinese Medical Sciences Journal, 18(2), 87–92.
Dannenmann, C., Shabani, N., Friese, K., Jeschke, U., Mylonas, I., & Bruning, A. (2008). The metastasis-associated gene MTA1 is upregulated in advanced ovarian cancer, represses ERbeta, and enhances expression of oncogenic cytokine GRO. Cancer Biology and Therapy, 7(9), 1460–1467.
Prisco, M. G., Zannoni, G. F., De Stefano, I., Vellone, V. G., Tortorella, L., Fagotti, A., et al. (2012). Prognostic role of metastasis tumor antigen 1 in patients with ovarian cancer: a clinical study. Human Pathology, 43(2), 282–288.
Liu, T., Yang, M., Yang, S., Ge, T., Gu, L., & Lou, G. (2013). Metastasis-associated protein 1 is a novel marker predicting survival and lymph nodes metastasis in cervical cancer. Human Pathology, 44(10), 2275–2281.
Hofer, M. D., Kuefer, R., Varambally, S., Li, H., Ma, J., Shapiro, G. I., et al. (2004). The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Research, 64(3), 825–829.
Dias, S. J., Zhou, X., Ivanovic, M., Gailey, M. P., Dhar, S., Zhang, L., et al. (2013). Nuclear MTA1 overexpression is associated with aggressive prostate cancer, recurrence and metastasis in African Americans. Scientific Reports, 3, 2331.
Hofer, M. D., Tapia, C., Browne, T. J., Mirlacher, M., Sauter, G., & Rubin, M. A. (2006). Comprehensive analysis of the expression of the metastasis-associated gene 1 in human neoplastic tissue. Archives of Pathology and Laboratory Medicine, 130(7), 989–996.
Acknowledgments
The authors acknowledge the support from members of the Department of Gastroenterological Surgery, National Kyushu Cancer Center, Japan, and foundation and private donations to the Institute for Molecular Medicine.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toh, Y., Nicolson, G.L. Properties and clinical relevance of MTA1 protein in human cancer. Cancer Metastasis Rev 33, 891–900 (2014). https://doi.org/10.1007/s10555-014-9516-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9516-2